Abstract
Biomaterials capable of providing localized and sustained presentation of bioactive proteins are critical for effective therapeutic growth factor delivery. However, current biomaterial delivery vehicles commonly suffer from limitations that can result in low retention of growth factors at the site of interest or adversely affect growth factor bioactivity. Heparin, a highly sulfated glycosaminoglycan, is an attractive growth factor delivery vehicle due to its ability to reversibly bind positively charged proteins, provide sustained delivery, and maintain protein bioactivity. This study describes the fabrication and characterization of heparin methacrylamide (HMAm) microparticles for recombinant growth factor delivery. HMAm microparticles were shown to efficiently bind several heparin-binding growth factors (e.g. bone morphogenetic protein-2 (BMP-2), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (FGF-2)), including a wide range of BMP-2 concentrations that exceeds the maximum binding capacity of other common growth factor delivery vehicles, such as gelatin. BMP-2 bioactivity was assessed on the basis of alkaline phosphatase (ALP) activity induced in skeletal myoblasts (C2C12). Microparticles loaded with BMP-2 stimulated comparable C2C12 ALP activity to soluble BMP-2 treatment, indicating that BMP-2-loaded microparticles retain bioactivity and potently elicit a functional cell response. In summary, our results suggest that heparin microparticles stably retain large amounts of bioactive BMP-2 for prolonged periods of time, and that presentation of BMP-2 via heparin microparticles can elicit cell responses comparable to soluble BMP-2 treatment. Consequently, heparin microparticles present an effective method of delivering and spatially retaining growth factors that could be used in a variety of systems to enable directed induction of cell fates and tissue regeneration.
Introduction
Recombinant growth factor delivery has been effective for a number of tissue engineering applications. In particular, bone morphogenetic proteins (BMPs), which are potent osteoinductive growth factors, have been used extensively to treat bone defects in both research and clinical settings [1–3]. However, current treatment strategies require supraphysiological levels of recombinant proteins, such as BMPs, in order to stimulate endogenous mechanisms of repair. This inefficient use of growth factor is largely due to the inability of biomaterial delivery vehicles to provide adequate sustained and localized presentation of growth factors necessary to stimulate repair over long periods of time. Current biomaterial delivery vehicles have major limitations, such as the rapid release of molecular cargo upon deployment, causing low retention of soluble factors at the site of interest [4–6], or alternatively, reliance upon growth factor tethering strategies that can significantly reduce growth factor bioactivity [7, 8]. Thus, materials with the ability to strongly, but reversibly, interact with their molecular payload are necessary, and may significantly decrease the amount of growth factor required for therapies, while improving physiological response.
Recently, glycosaminoglycan-containing biomaterials have become an attractive delivery method for recombinant growth factors, due to their ability to strongly bind a variety of growth factors in a reversible manner. Glycosaminoglycans (GAGs) are linear polysaccharide chains that bind positively charged growth factors primarily through their negatively charged sulfate groups and exist both as free chains and covalently-linked components of glycosylated proteins known as proteoglycans [9, 10]. GAGs such as heparin, heparan sulfate, and chondroitin sulfate are ubiquitous components of natural extracellular matrices (ECM) that are involved in sequestering and immobilizing growth factors within the cellular microenvironment [11–13]. Thus, GAG-based materials present the opportunity to harness the natural growth factor binding capacity of the ECM and deliver growth factors in a biomimetic manner with spatiotemporal control. Heparin, in particular, is highly negatively charged and has a strong affinity for a class of positively charged growth factors known as “heparin binding growth factors,” for which specific growth factor binding sequences on heparin chains have been identified [14–16]. The non-covalent, reversible interactions between heparin and heparin-binding growth factors ensure that binding occurs with minimal impact on growth factor structure. Heparin-binding growth factors such as transforming growth factor β (TGF-β), vascular endothelial growth factor (VEGF), fibroblast growth factors (FGFs), insulin-like growth factors (IGFs), and bone morphogenetic proteins (BMPs), are especially influential in many developmental and regeneration processes, and it is thought that heparin itself may play an influential role in the preservation and presentation of molecules through electrostatic interactions [17, 18].
The use of heparin and heparin-containing biomaterials for BMP-2 delivery, as well as the delivery of several other growth factors, including FGF-2, VEGF, and TGF-β2, has been widely explored in both in vitro and in vivo test beds [19–24]. Although several studies have investigated heparin-BMP-2 interactions, the effects of heparin-BMP-2 binding on protein bioactivity have been inconsistent and depend largely on the amount of heparin and method of heparin immobilization. Previous studies have demonstrated that co-delivery of soluble heparin with BMP-2 can enhance BMP-2-mediated osteogenesis or, contrastingly, interfere with BMP-2 and BMP receptor binding to inhibit osteogenesis, depending on the cell type and culture conditions [25–31]. Nevertheless, the addition of heparin to biomaterials, including microparticles and bulk gels, has previously resulted in improvement in growth factor retention and BMP-2-induced osteogenesis [32–36]. Heparin-mediated delivery of BMP-2 has also resulted in a wide range of effects in vivo, with studies demonstrating variable amounts of mineralization in both ectopic and orthotopic sites [25, 37–39], reflecting an inconsistent ability to form functional bone. Furthermore, the majority of these materials consist of relatively small amounts of heparin immobilized within a larger bulk material [23, 24, 40–43], which may attenuate heparin’s ability to effectively bind and present growth factors. As a result, previous reports on heparin-containing biomaterials may significantly underestimate the amount of BMP-2 that can be delivered via heparin binding. Thus, improving the growth factor binding ability of heparin-containing biomaterials may enable consistent delivery of highly localized BMP-2 concentrations necessary to stimulate more effective bone formation.
Herein, we present a method of fabricating pure heparin microparticles from a modified heparin methacrylamide species that can be thermally cross-linked. Physical and chemical characterization of heparin microparticles was performed, and growth factor binding and release were quantified with different BMP-2 loading concentrations. Additionally, growth factor bioactivity was evaluated by introducing BMP-2 laden heparin microparticles to cultures of C2C12 cells and measuring BMP-2-induced alkaline phosphatase activity, as well as changes in DNA content. Overall, this study marks a crucial first step in developing heparin microparticles as a versatile delivery vehicle and therapeutic platform for growth factor-stimulated tissue engineering, by investigating their capacity to efficiently capture and present BMP-2 to induce a potent functional cell response.
Materials and Methods
Heparin Methacrylamide Modification
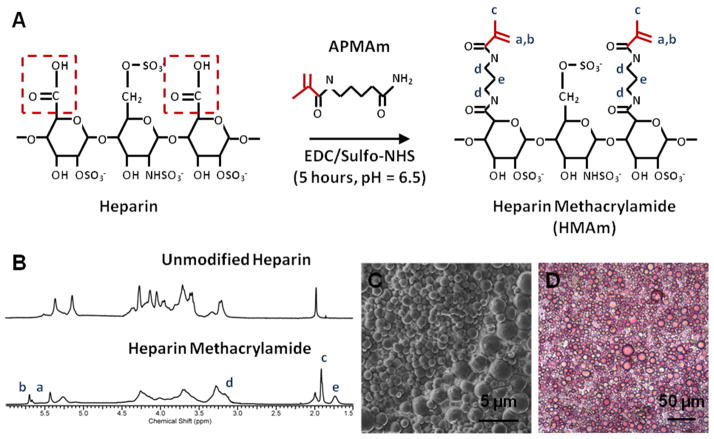
Heparin ammonium salt from porcine intestinal mucosa (17–19 kDa; Sigma-Aldrich, St. Louis, MO) was conjugated with N-(3-Aminopropyl)methacrylamide (APMAm; Polysciences, Warrington, PA) using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC; Thermoscientific, Rockford, IL) and N-hydroxysulfosuccinimide (Sulfo-NHS; Thermoscientific, Rockford, IL) as described in previous protocols [44, 45] (Figure 1A). EDC/Sulfo-NHS chemistry causes activation of the carboxyl groups on heparin and subsequent methacrylamide substitution via covalent bonds created with the primary amines on APMAm. Briefly, 325 mg of heparin ammonium salt was mixed with 10-fold molar excess of sulfo-NHS, EDC, and APMAm, in relation to heparin’s carboxyl groups, in a reaction volume of 200 mL at room temperature and pH 6.5. The total reaction time was 5 hours, with a second set of 10-fold molar excess of EDC and APMAm being added after the first 2.5 hours, to replace reactants that had undergone hydrolysis. The reaction volume was then immediately dialyzed against 2 L of water using dialysis tubing with a molecular weight cutoff of 3500 Da (Spectrum Laboratories, Rancho Dominguez, CA) for at least 48 hours to remove excess reactants; dialysis water was replaced every 12 hours. The remaining volume in the dialysis tubing (~200 mL) was lyophilized (Labconco, Kansas City, MO) for four days.
Figure 1. Characterization of Heparin Microparticles.
A) In the methacrylamide substitution reaction, heparin ammonium salt is combined with excess N-(3-Aminopropyl)methacrylamide hydrochloride (APMAm), N-hydroxysulfosuccinimide (sulfo-NHS), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) to produce heparin methacrylamide (HMAm). B) 1H-NMR results indicate that approximately 50% of carboxyl groups on heparin are substituted with methacrylamide groups. Degree of substitution was determined by comparing the integration regions of APMAm protons and unmodified heparin. C) Scanning electron microscopy image of heparin microparticles demonstrates the polydisperse and spherical nature of the microparticles. D) Image of heparin microparticles stained with 1,9-dimethylmethylene blue (DMMB), confirming the presence of heparin.
Heparin Methacrylamide Characterization
5 mg of modified or unmodified heparin was dissolved in 750 μL deuterium oxide (Cambridge Isotope Laboratories, Tewksbury, MA) and analyzed using a Bruker Avance III 400 MHz spectrometer (Bruker Biospin Corp, Billerica, MA) for proton nuclear magnetic resonance (1H-NMR) spectra analysis. 1H-NMR spectra were phase corrected, baseline subtracted, and integrated using ACD/NMR processor 12.0 software. For the determination of methacrylamide modification degree, protons located at the C1 position of heparin’s disaccharide units were integrated between the chemical shifts of δ=5.0 ppm and δ=5.75 ppm and assigned a reference value of 1. However, in modified heparin, the protons of the methacrylamide groups (δ=5.41 ppm and δ=5.66 ppm) have a similar chemical shift within the aforementioned heparin integration region. Therefore, an alternate region in the heparin spectra (δ=4.0 ppm and δ=4.33 ppm) that was not affected by the presence of methacrylamide signals was selected, also determined to have an integral of 1, and compared to the integral of the methacrylamide protons at δ=5.41 ppm and δ=5.66 ppm. The number of methacrylamide groups per heparin disaccharide unit was determined by taking the ratio of the integration regions of the methacrylamide protons (δ=5.41 ppm and δ=5.66 ppm) and the heparin (δ=4.0 ppm and δ=4.33 ppm).
Heparin Microparticle Fabrication
Heparin methacrylamide microparticles were fabricated using a water-in-oil emulsion followed by thermal cross-linking of the methacrylamide groups, similar to a previously developed protocol [46]. Briefly, 55.6 mg of heparin methacrylamide was dissolved in 440 μL of phosphate buffered saline (PBS; Corning Mediatech, Manassas, VA) and mixed with equimolar amounts (30 μL of a 0.3 M solution) of the free radical initiators ammonium persulfate (Sigma Aldrich) and tetramethylethylenediamine (Sigma Aldrich). Immediately after mixing, the solution was added dropwise to 60 mL of corn oil and 1 mL of polysorbate 20 (Promega, Madison, WI), and the entire mixture was homogenized on ice at 3000 rpm for 5 minutes using a Polytron PT3100 homogenizer (Kinematica, Switzerland) to create a water-in-oil emulsion. The emulsion was then submerged in a hot water bath (55°C) and stirred to activate free radical polymerization and thermal cross-linking of the methacrylamide groups. The reaction proceeded for 30 minutes under nitrogen purging to prevent depletion of free radicals by the presence of oxygen. The solution was centrifuged at 3000 rpm for at least 10 minutes and the corn oil phase was removed. The resulting pellet of microparticles was washed once in acetone and centrifuged again (3000 rpm, 10 minutes) before undergoing several water washes to remove excess oil or loosely cross-linked microparticles that did not settle with centrifugation. Prior to cell culture studies, microparticles were disinfected in a 70% ethanol solution for 30 minutes followed by three additional washes with sterile water. The microparticles were then lyophilized for two days and stored at 4°C until use.
Heparin Microparticle Characterization
To visualize the microparticles, lyophilized samples were incubated with 0.0016% (w/v) 1,9-dimethylmethylene blue (DMMB; Sigma Aldrich), which specifically stains sulfated GAGs, for 30 minutes at room temperature, and then washed several times with water before being imaged on a Nikon Eclipse TE2000-U inverted microparticle (Nikon Instruments, Melville, NY). Microparticle size distribution was determined by using CellProfiler image analysis software [47] to evaluate images of microparticle samples. For size analysis, microparticles were stained with 0.1% (w/v) Safranin O for 30 minutes for enhanced contrast prior to imaging on a Nikon Eclipse TE2000-U inverted microscope. Images were then analyzed using a custom script on CellProfiler to quantify individual microparticle sizes. Briefly, images were loaded into CellProfiler and converted to grayscale. Microparticles were identified using the Otsu Adaptive thresholding method and the cross-sectional area of each microparticle was measured in order to estimate microparticle diameter. The number of microparticles per mg of polymer was determined using a Z2 Coulter Particle Counter with a 100 μm aperture (Beckman Coulter, Fullerton, CA). A known weight of lyophilized microparticles was resuspended in 5 mL of Isoton II diluent (Beckman Coulter) for counting. For scanning electron microscopy (SEM), 0.1 mg of lyophilized microparticles were resuspended in 70% ethanol and placed on an SEM mounting stud covered in copper tape. The microparticle samples were left at room temperature overnight to allow the ethanol to evaporate. The studs were sputter coated with gold using a Hummer 5 Gold/Palladium Sputterer (Anatech, Union City, CA) at 140–160 mTorr and 25–30 mA. Images were taken on a FEI Nova Nanolab 200 Focused Ion Beam/Scanning Electron Microscope (FEI, Hillsboro, OR).
Growth Factor Binding and Release
The binding capacity of the microparticles for human VEGF (pI = 8.5), human FGF-2 (pI = 9.6), and human tumor necrosis factor alpha (TNF-α, pI = 5.3) [48–50] were investigated at a fixed growth factor concentration (10 ng/mL) and fixed microparticle amount (0.1 mg), while binding capacity for human BMP-2 (pI = 9.0) [51] was investigated for a broad range of different growth factor concentrations (0.1–5000 μg/mg of microparticles) and microparticle amounts (0.02–200 μg). For comparison with gelatin methacrylate microparticles, 0.1 mg of both heparin microparticles and gelatin microparticles were incubated with a single fixed concentration of BMP-2 (10 ng/mL). All growth factors were purchased from R&D Systems. Microparticles were incubated with a single growth factor in 1 mL of 0.1% (w/v) bovine serum albumin (BSA; Millipore Corporation, Billerica, MA) in PBS, and rotated at 4°C for 16 hours. The microparticles were then centrifuged, and the supernatant was removed and analyzed for growth factor content via enzyme-linked immunosorbant assay (ELISA; R&D Systems, Minneapolis, MN). Growth factor binding was determined by subtracting the amount of growth factor remaining in solution for microparticle-containing samples from growth factor solutions rotated at 4°C for 16 hours that lacked microparticles.
Passive release of BMP-2 into 0.1% (w/v) BSA in PBS was monitored over a period of 28 days at both 4°C and 37°C. Heparin microparticles (0.1 mg) were incubated with a range of BMP-2 concentrations to obtain growth factor loading between 25 and 1000 ng BMP-2 per mg of microparticles. For comparison with gelatin methacrylate microparticles, heparin microparticles and gelatin microparticles (0.1 mg) were incubated with 25 and 50 ng/mL of BMP-2 respectively, to obtain an equal loading of 200 ng BMP-2 per mg of microparticles. At various time points over 28 days, the microparticles were centrifuged at 3000 rpm for 5 minutes, and 300 μL samples of the supernatant were removed and replaced with an equivalent volume of fresh 0.1% (w/v) BSA in PBS. Release samples were then analyzed for growth factor content via ELISA.
Alkaline Phosphatase Activity
Skeletal myoblasts (C2C12; ATCC, Manassas, VA) were cultured in growth media consisting of DMEM (Corning Mediatech, Manassas, VA) supplemented with 16% fetal bovine serum (FBS; Thermo Scientific Hyclone, Logan, UT) and maintained at 37°C in a 5% CO2 humidified incubator. For the alkaline phosphatase (ALP) activity assay, C2C12 cells were seeded in 96-well plates at an initial density of 20,000 cells per well and allowed to attach for 6 hours under growth conditions (16% FBS in DMEM). Cells were then switched to low serum media (1% FBS in DMEM), and the following treatments were added: soluble BMP-2 (5–1500 ng/mL), loaded and unloaded heparin microparticles (0.02–0.5 mg), and soluble unmodified heparin (0.1 mg). For transwell experiments, cells were cultured on the bottom compartment of a 96-well transwell plate, and microparticles were added above the polycarbonate membrane (0.2 μm pore diameter) placed on top. After 72 hours of treatment, cells were washed twice with PBS and lysed using 100 μL of CelLytic M (Sigma Aldrich). Cell lysate was stored at −80°C prior to analysis.
ALP activity of C2C12 cultures was measured by incubating 50 μL of cell lysate with 50 μL of CelLytic M and 100 μL of a substrate solution consisting of 3.33 mM MgCl2 (VWR, West Chester, PA), 500 mM 2-amino-2-methyl-1-propanol (Sigma Aldrich), and 6.67 mM p-nitrophenyl phosphate (pNPP; Sigma Aldrich). The reaction was terminated with 0.2 M NaOH after 5 minutes, and absorbance measured at 405 nm on a Synergy H4 microplate reader (Bioteck, Winooski, VT). A standard curve for total ALP activity was generated using graded concentrations of the ALP product, 4-nitrophenol (0–1 nmol/μL; Sigma Aldrich).
DNA Quantification and Staining
DNA content of C2C12 cultures was measured using the QuantiFluor dsDNA kit (Promega, Madison, WI). Briefly, 20 μL of cell lysate was incubated with 80 μL of the assay buffer and 100 μL of the fluorescent DNA-binding dye for five minutes, protected from light. Fluorescence was then measured on a Synergy H4 microplate reader (Bioteck, Winooski, VT), using an excitation wavelength of 504 nm and emission wavelength of 519 nm. Total DNA content in each well was correlated to fluorescence by comparison to a lambda DNA standard curve (48.5 kb). Fold change in DNA was determined by dividing the final DNA content after 72 hours of culture by DNA content after 6 hours of initial cell attachment (Day 0).
C2C12 cultures were also fixed and stained with Hoechst for visualization of cell nuclei. Cells were washed twice with PBS and fixed with 4% paraformaldehyde in PBS (Alfa Aesar, Ward Hill, MA) for 15 minutes. Hoechst 33258 (50 mg/mL; Sigma Aldrich) was diluted 1:100 with 2% BSA and 0.1% polysorbate 20 in PBS and added to the cells for 15 minutes, followed by two additional PBS washes. Fixed cultures were imaged with a laser scanning confocal microscope (Zeiss LSM 700–405; Carl Zeiss Inc., Oberkochen, Germany).
Statistical Analysis
All data are reported as mean ± standard error of the mean, with a minimum of three replicates for each experimental group. Statistical significance was determined using one-way or two-way ANOVA, followed by Tukey’s post hoc analysis (Systat Software, Version 12); p < 0.05 was considered statistically significant.
Results
Heparin Methacrylamide Microparticle Characterization
1H NMR analysis indicated that approximately 50% of the carboxyl groups of heparin were conjugated with methacrylamide groups (Figure 1B). Heparin microparticle fabrication produced microparticles with an average diameter of 5.6 ± 4.0 μm (Figure S1), and approximately 1.9 × 107 microparticles per mg of heparin methacrylamide polymer. SEM images of the microparticles depicted a smooth, spherical morphology (Figure 1C), while brightfield images revealed that the microparticles stained positively with DMMB, confirming the presence of sulfated GAGs (Figure 1D).
Growth Factor Binding to Heparin Microparticles
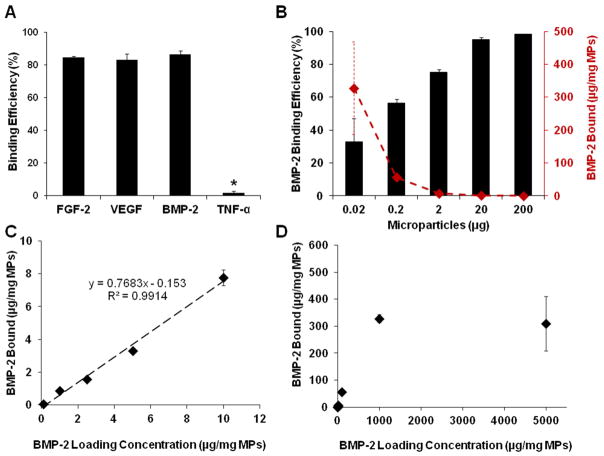
Growth factors that had a net positive charge at pH = 7.5, such as FGF-2 (pI = 9.6), VEGF (pI = 8.5), and BMP-2 (pI = 9.0), were significantly depleted from solution, and exhibited high binding to the negatively charged heparin microparticles (respectively, 84.5% ± 0.8%, 83.1% ± 3.8%, and 86.4% ± 2.0% of added growth factor; Figure 2A). In contrast, TNF-α (pI = 5.3), which is negatively charged at pH = 7.5, was not significantly depleted from solution, with only 1.9% ± 1.1% of the added growth factor bound to the microparticles (Figure 2A), revealing that growth factor binding to heparin microparticles was dependent on electrostatic charge interaction. Additionally, when compared to gelatin microparticles, heparin microparticles bound ~44% more BMP-2 than similarly cross-linked gelatin methacrylate microparticles (Figure S2A).
Figure 2. Growth Factor Binding Capacity of Heparin Microparticles.
Microparticles were incubated with various concentrations of growth factors in 0.1% BSA in PBS for 16 hours at 4°C; remaining unbound growth factor was measured via ELISA. A) 0.1 mg of heparin microparticles were incubated with 10 ng of FGF-2, VEGF, BMP-2, and TNF-α. Heparin microparticles depleted the majority of positively charged growth factors from solution (FGF-2, VEGF, BMP-2), while the negatively charged growth factor (TNF-α) did not bind. (* = p < 0.05 compared to all other conditions.) B) Different amounts of heparin microparticles were incubated with 20 ng of BMP-2. Percentage of BMP-2 depleted increased with the addition of more microparticles. Conversely, the amount of BMP-2 depleted on a per mg of microparticles basis increased with decreasing microparticle amounts. C) 0.2 μg of heparin microparticles were incubated with increasing amounts of BMP-2. Heparin microparticles depleted significantly more BMP-2 from solution as the loading mass of BMP-2 added was increased. D) 0.2 μg of heparin microparticles were incubated with increasing amounts of BMP-2. Heparin microparticles exhibited maximal BMP-2 binding at approximately 300 μg BMP-2/mg microparticles.
To further investigate the growth factor binding capacity of heparin microparticles, BMP-2 binding was measured with different amounts of microparticles (0.02–200 μg) and a fixed loading concentration and volume of BMP-2 (20 ng/mL in 1 mL; Figure 2B). As expected, the percentage of BMP-2 bound to the microparticles increased in a dose-dependent manner with the addition of more microparticles, and nearly all of the BMP-2 was captured with >20 μg of microparticles, which bound >95% of the added BMP-2. Conversely, the amount of BMP-2 bound per milligram of microparticles decreased with increasing numbers of microparticles, indicating a reduced amount of BMP-2 bound per microparticle.
The relationship between microparticle binding capacity and BMP-2 solution concentration was evaluated using a fixed amount of microparticles (0.2 μg) and range of BMP-2 concentrations (0.1–5000 μg/mg of microparticles) (Figure 2C, 2D). Heparin microparticles depleted significantly more BMP-2 from solution as the loading mass of BMP-2 was increased. At BMP-2 loading concentrations between 0.1–10 μg/mg of microparticles, the relationship between loading mass and BMP-2 bound was relatively linear, with approximately 80% of the added BMP-2 bound to the microparticles, regardless of loading concentration; however, as the BMP-2 loading mass increased beyond 10 μg/mg of microparticles, the percentage of BMP-2 bound decreased to <55% of the loading mass. Maximum BMP-2 binding was observed at a concentration of approximately 300 μg BMP-2/mg microparticles.
Growth Factor Release from Heparin Microparticles
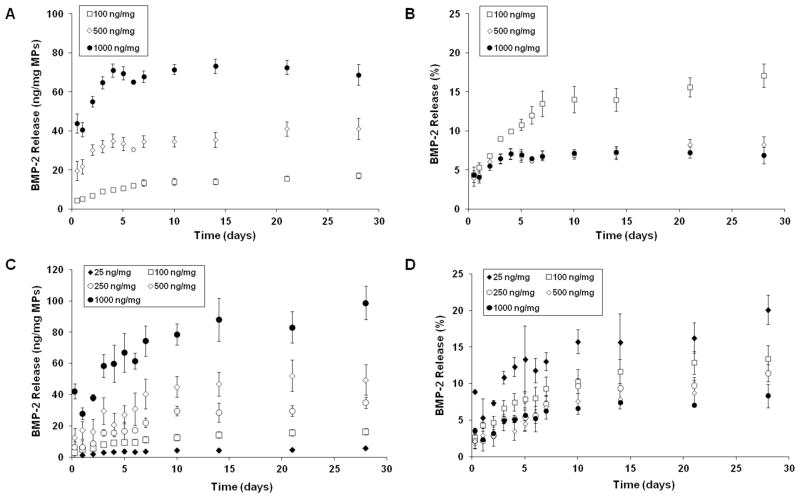
Release profiles of BMP-2 from heparin microparticles were investigated at different growth factor loading densities (25–1000 ng BMP-2/mg of microparticles) and temperatures (4°C, 37°C) (Figure 3). At both temperatures, microparticles exhibited a low burst release within the first 6 hours (<10%), followed by additional sustained release over the next 4 weeks. Heparin microparticles loaded with higher concentrations of BMP-2 exhibited a greater initial burst release and overall cumulative release over the 28 day period than microparticles loaded at lower BMP-2 concentrations; the differences in final cumulative release were significant for release profiles at both temperatures, while the differences in initial burst release were only significant at 37°C. Despite drastically different BMP-2 loading (ng BMP-2/mg microparticles), the cumulative percentage of BMP-2 released was independent of loading mass and similar for all growth factor loading concentrations at 4°C (Figure 3D). Total release of BMP-2 after 28 days was <20% of the loaded growth factor at 37°C, and <25% at 4°C, indicating that the majority of growth factor was retained by the microparticles at both temperatures. Release profiles of BMP-2 from heparin microparticles were also compared to BMP-2 release from gelatin methacrylate microparticles. When both types of microparticles were loaded with the same amount of BMP-2 (200 ng BMP-2/mg microparticles), the BMP-2 release from the gelatin microparticles at 37°C was significantly greater at each timepoint, with ~30% of the growth factor being released from the gelatin microparticles within the first seven days (Figure S2B).
Figure 3. BMP-2 Release Kinetics of Heparin Microparticles.
Heparin microparticles were loaded for 16 hours at 4°C with different amounts of BMP-2 to achieve BMP-2 binding between 25–1000 ng BMP-2/mg of microparticles. Microparticles were allowed to passively release BMP-2 into a 0.1% BSA in PBS solution over 28 days. A) Release curves at 37°C presented as cumulative mass of BMP-2 released per mg of microparticles demonstrate that microparticles loaded with more BMP-2 exhibited greater release over the 28-day period. (p < 0.05 for all groups at 6 hours of release and 28 days of release.) B) Release curves at 37°C presented as cumulative percent of loaded BMP-2 show that percentage of BMP-2 released is highest for the lowest loading amount investigated. C) Release curves at 4°C presented as cumulative mass of BMP-2 released per mg of microparticles also show that microparticles loaded with more BMP-2 exhibited greater release over the 28-day period. (p < 0.05 for 25 ng and 100 ng vs. 1000 ng at 6 hours of release and p < 0.05 for all groups except 250 ng vs. 500 ng at 28 days of release.) D) Release curves at 4°C presented as cumulative percent of loaded BMP-2 demonstrate that percentage of BMP-2 released was independent of loading mass and remained similar for all growth factor loading densities.
Microparticle-Mediated Effects on Alkaline Phosphatase Activity of C2C12 Cultures
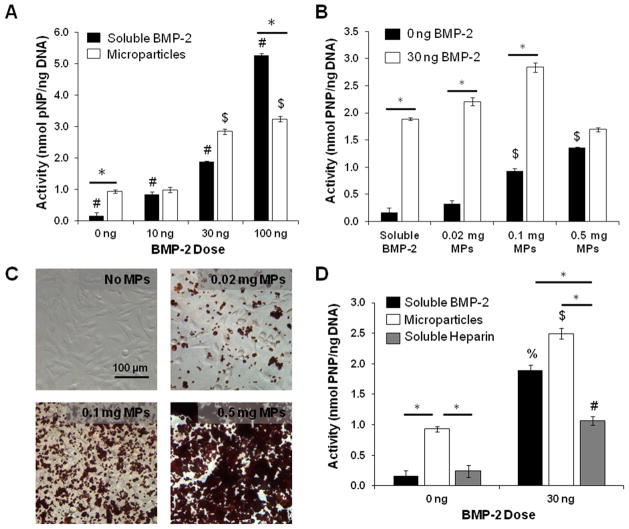
The bioactivity of BMP-2 bound to heparin microparticles was examined using an ALP activity assay on skeletal myoblasts (C2C12). C2C12 cells were chosen to evaluate BMP-2 bioactivity, since the addition of BMP-2 to low serum culture conditions has been shown to robustly inhibit C2C12 myogenesis and enhance expression of osteogenic markers, such as ALP [52]. A fixed amount of microparticles (0.1 mg) was loaded with several BMP-2 amounts (10, 30, 100 ng) that stimulate ALP activity within the dynamic range of the cells (Figure S3). When normalized to DNA content, resulting ALP activity was comparable between soluble BMP-2 and loaded microparticle groups at 10 and 30 ng, and lower for the loaded microparticle group at 100 ng (Figure 4A). Interestingly, unloaded heparin microparticles (without BMP-2) induced a 6-fold increase in ALP activity compared to no treatment, resulting in ALP activity that was comparable to that of 10 ng of soluble BMP-2 treatment (~1 nmol pNP/ng DNA).
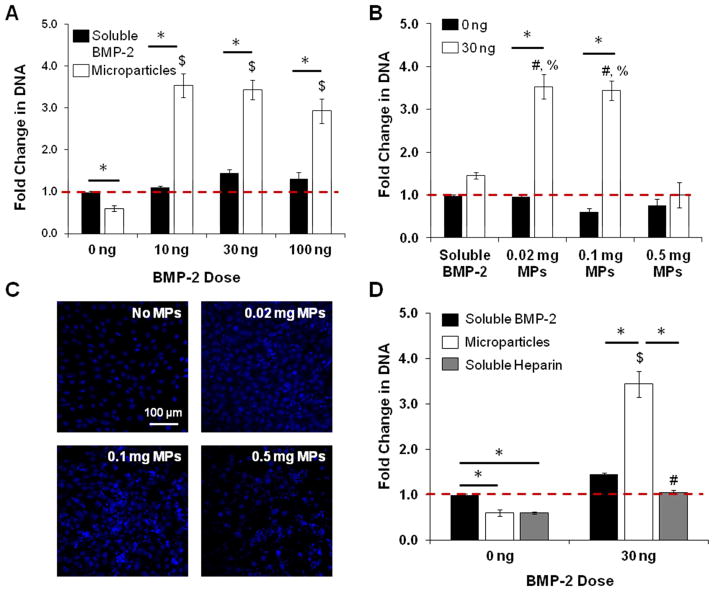
Figure 4. Effect of Heparin Microparticles Loaded with BMP-2 on Alkaline Phosphatase Activity in C2C12 Cultures.
C2C12 cells were cultured for 72 hours with each treatment. Cell lysate was incubated with p-nitrophenyl phosphate substrate solution to determine ALP activity and normalized to DNA content. A) 0.1 mg of microparticles pre-loaded with BMP-2 at three doses induced ALP activity in a dose-dependent manner comparable to that of soluble BMP-2 at 10 and 30 ng, and lower than soluble BMP-2 at 100 ng. The same mass of unloaded MPs also induced greater ALP activity than cells without treatment. (* = p < 0.05 as indicated; # = p < 0.05 for all conditions in group; $ = p < 0.05 compared to unloaded MPs (0 ng).) B) C2C12 cells were cultured with different doses of microparticles (0.02 mg, 0.1 mg, 0.5 mg) that were either unloaded or loaded with 30 ng of BMP-2. Loaded microparticles induced significantly higher ALP activity than unloaded microparticles in all cases except for 0.5 mg of microparticles. (* = p < 0.05 as indicated; $ = p < 0.05 compared to no treatment and 0.02 mg of unloaded MPs (0 ng).) C) Representative images of C2C12 cells cultured without microparticles and with 0.02 mg of microparticles, 0.1 mg of microparticles, and 0.5 mg of microparticles in a well of a 96-well plate. Microparticles were stained with Safranin O for enhanced contrast. D) 0.1 mg of soluble heparin did not induce the same increase in ALP activity as 0.1 mg of unloaded microparticles. Soluble heparin in the presence of BMP-2 inhibited ALP activity compared to BMP-2 delivered solubly and via microparticles. (* = p < 0.05 as indicated; % = p < 0.05 compared to no treatment; $ = p < 0.05 compared to unloaded MPs (0 ng); # = p < 0.05 compared to soluble heparin (0 ng).)
The effect of microparticle dose on ALP activity was also determined by culturing C2C12 cells with different amounts of microparticles (0.02, 0.1, 0.5 mg) loaded with a fixed BMP-2 dose (30 ng total; Figure 4B). Representative images of Safranin O-stained microparticles cultured with C2C12 cells after 6 hours of attachment show the differences in microparticle coverage area on a 96-well plate (Surface Area = 0.32 cm2) (Figure 4C). As expected, C2C12 cells cultured with 0.02 mg and 0.1 mg of microparticles loaded with BMP-2 stimulated higher ALP activity than no treatment and cells cultured with unloaded microparticles. However, 0.5 mg of loaded microparticles induced lower ALP activity than equivalent amounts of BMP-2 delivered solubly and with 0.02 and 0.1 mg of microparticles, with no significant difference in ALP expression between cultures containing 0.5 mg of unloaded and loaded microparticles. Interestingly, increasing the amount of unloaded microparticles dose-dependently increased ALP expression, and 0.1 and 0.5 mg of unloaded microparticles induced significantly higher ALP activity than no treatment.
In order to determine whether the stimulation of C2C12 ALP activity could simply be attributed to the presence of heparin in the cultures, C2C12 cells were treated with 0.1 mg of soluble modified heparin, with and without BMP-2 (Figure 4D). The presence of soluble heparin alone did not stimulate an increase in ALP activity compared to unloaded heparin microparticles. Additionally, when soluble heparin was incubated with 30 ng of BMP-2 beforehand and cultured with the cells, ALP activity was attenuated compared to soluble BMP-2 and an equivalent amount of loaded microparticles (1.07 ± 0.08 nmol pNP/ng DNA compared to 1.45 ± 0.09 nmol pNP/ng DNA and 2.84 ± 0.09 nmol pNP/ng DNA, respectively).
Microparticle-Mediated Effects on DNA Content of C2C12 Cultures
The DNA content of C2C12 cultures treated with a fixed number of BMP-2-loaded microparticles (0.1 mg) increased 3–4 fold over the three day culture period, while cultures treated with equivalent amounts of soluble BMP-2 did not display any significant change in DNA content (Figure 5A), suggesting that the presence of loaded microparticles increased cell number. At a fixed BMP-2 dose (30 ng), C2C12 cells cultured with fewer loaded microparticles (0.02 and 0.1 mg) at a higher BMP-2 loading density exhibited a ~3.5-fold increase in DNA content compared to unloaded microparticle controls, whereas cells cultured with 0.5 mg of microparticles exhibited no change in DNA content (Figure 5B). Comparable differences in cell number were observed with Hoechst staining of C2C12 cells cultured without microparticles and with various doses of microparticles in the presence of 30 ng of BMP-2 (Figure 5C). This result demonstrated that BMP-2 loading density of the microparticles may determine effects on DNA content, with higher loading densities stimulating larger DNA increases.
Figure 5. Effect of Heparin Microparticles Loaded with BMP-2 on DNA Content in C2C12 Cultures.
C2C12 cells were cultured for 72 hours with each treatment. Cells were lysed, and cell lysate was incubated with a fluorescent dsDNA-binding dye to determine DNA content. Fold change in DNA is presented as final Day 3 DNA content compared to initial Day 0 DNA content (baseline). A) Microparticles loaded with BMP-2 significantly increased DNA content compared to soluble BMP-2 controls. Unloaded microparticles did not increase DNA content. (* = p < 0.05 as indicated; $ = p < 0.05 compared to unloaded MPs.) B) Cells cultured with 0.02 mg and 0.1 mg of microparticles in the presence of BMP-2 both exhibited a significant increase in DNA content compared to unloaded microparticle controls, while cells cultured with 0.5 mg of microparticles did not. (* = p < 0.05 as indicated; # = p < 0.05 compared to soluble BMP-2 (30 ng); % = p < 0.05 compared to 0.5 mg MPs (30 ng).) C) Representative images of C2C12 cells cultured without microparticles and with 0.02 mg of microparticles, 0.1 mg of microparticles, and 0.5 mg of microparticles in the presence of 30 ng of BMP-2 for three days. Cell nuclei were stained with Hoechst. D) Cells cultured with soluble heparin without BMP-2 displayed similar DNA content compared to cells cultured without treatment and with unloaded microparticles. Cells cultured with soluble heparin in the presence of BMP-2 did not exhibit the same increase in DNA content observed with cells cultured with loaded microparticles. (* = p < 0.05 as indicated; $ = p < 0.05 compared to unloaded MPs; # = p < 0.05 compared to soluble heparin (0 ng).)
C2C12 cells cultured with 0.1 mg of soluble heparin and 0.1 mg of unloaded microparticles both exhibited similar decreases in DNA content over the culture period, indicating that the presence of heparin alone in the system reduced cell number (Figure 5D). Although BMP-2-loaded microparticles induced a significant increase in DNA content compared to no treatment, cells cultured with soluble heparin in the presence of BMP-2 (30 ng) did not exhibit any increase in DNA content.
Contact-Mediated Effects on Alkaline Phosphatase Activity and DNA Content of C2C12 Cultures
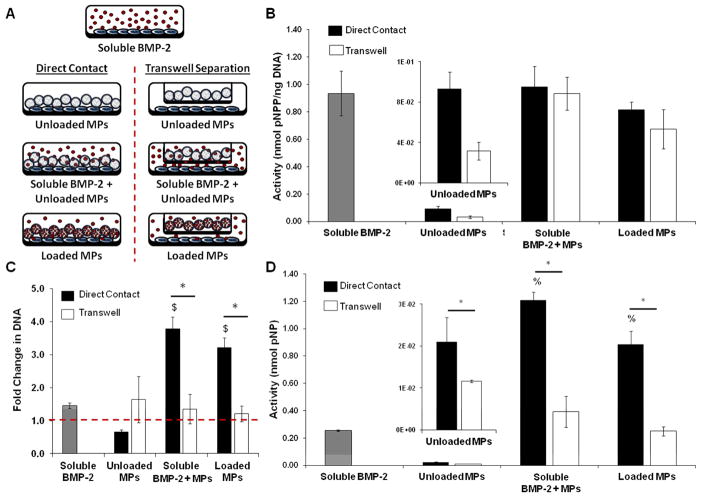
In order to determine whether the observed effects of heparin microparticles on C2C12 ALP activity and DNA content were contact-dependent, heparin microparticles were physically separated from the cells by a transwell membrane and added without BMP-2 or with 30 ng of BMP-2 (soluble or pre-loaded; Figure 6A). The presence of unloaded microparticles in direct contact with the cells significantly increased normalized and total ALP activity compared to both no treatment and physically separated unloaded microparticles (Figure 6B, 6D). In BMP-2 treatment groups, ALP activity normalized to DNA content was similar between cells in direct contact with the microparticles and physically separated from the microparticles, regardless of whether the BMP-2 was added solubly or loaded onto the microparticles (Figure 6B). However, total ALP activity of C2C12 cultures physically separated from the microparticles was significantly lower than direct contact cultures (Figure 6D). This was due to the fact that transwell separation of cells and microparticles in the presence of soluble or loaded BMP-2 did not affect DNA content over the culture period, while cultures in which the cells and microparticles were in direct contact exhibited significant DNA increases (3–4 fold; Figure 6C). This indicated that the changes in DNA content were a result of the cells being in direct contact with the microparticles. Similarly, while cells cultured in direct contact with unloaded microparticles exhibited a significant decrease in DNA content over three days, there was no change in DNA content when the cells and unloaded microparticles were physically separated from each other.
Figure 6. Contact-Mediated Effect of Heparin Microparticles on Alkaline Phosphatase Activity and DNA Content in C2C12 Cultures.
C2C12 cells were cultured for 72 hours with each treatment and then lysed. Cell lysate was incubated with a p-nitrophenyl phosphate substrate solution to determine ALP activity and incubated with a fluorescent dsDNA-binding dye to determine DNA content. Fold change in DNA is presented as final Day 3 DNA content compared to initial Day 0 DNA content (baseline). A) Schematic indicating the conditions investigated with cells and microparticles in direct contact and physically separated: unloaded microparticles, soluble BMP-2 and unloaded microparticles, and BMP-2-loaded microparticles. B) Normalized ALP activity of cells incubated with microparticles in direct contact and physically separated were comparable to each other and soluble BMP-2 treatment, regardless of whether the BMP-2 in the culture was added solubly or pre-loaded onto the microparticles. Normalized ALP activity of cells in direct contact with unloaded microparticles was higher than that of cells separated from unloaded microparticles. C) Cells cultured in the presence of both BMP-2 and microparticles physically separated from the cells by a transwell demonstrated significantly lower DNA content than cells cultured in direct contact with microparticles and in the presence of BMP-2. (* = p < 0.05 as indicated; $ = p < 0.05 compared to unloaded MPs.) D) Total ALP activity of cells in direct contact with the microparticles was significantly higher than that of cells that were separated from the microparticles via transwell insert and soluble BMP-2 treatment alone. Total ALP activity of cells in direct contact with unloaded microparticles was significantly higher than that of cells separated from unloaded microparticles. (* = p < 0.05 as indicated; % = p < 0.05 compared to soluble BMP-2.)
Discussion
In this study, the ability of heparin methacrylamide microparticles to bind, retain, and present bioactive growth factors in vitro was investigated. Heparin microparticles bound considerable amounts of several positively charged heparin-binding growth factors (BMP-2, VEGF, FGF-2), including high quantities of BMP-2 that exceeded the maximum reported growth factor binding capacity of other heparin-containing biomaterials by >1000-fold [23, 24, 36, 38, 53, 54] and surpassed the BMP-2 binding of gelatin methacrylate microparticles by nearly 50%. The majority of BMP-2 loaded onto heparin microparticles at various concentrations (25–1000 ng BMP-2/mg microparticles) was retained within the hydrogel matrix, with <25% of the bound growth factor being released over a period of 28 days. Loaded microparticles stimulated ALP activity in C2C12 cells comparable to soluble BMP-2 treatment at multiple BMP-2 and microparticle doses, demonstrating that microparticle-bound BMP-2 maintained its bioactivity under conditions of low release. Moreover, in addition to inducing ALP activity, BMP-2-loaded microparticles also stimulated a striking contact-mediated increase in DNA content over a three day culture period, that was inhibited when cells and microparticles were physically separated and absent when cells were cultured with an equivalent amount of heparin instead. Overall, this study illustrates that heparin microparticles can bind and present high amounts of BMP-2 that elicit a functional cell response and can therefore serve as a potent vehicle for the sustained delivery of bioactive growth factors.
Conventional heparin delivery strategies typically involve immobilization of soluble heparin onto other bulk biomaterials, such as collagen, PLGA, fibrin, or Sepharose [25, 33–35, 38, 39, 55]. However, the methacrylamide-mediated cross-linking reaction used here allows for the generation of hydrogel microparticles that consist purely of heparin and can incorporate large amounts of growth factor, facilitating the delivery of highly concentrated molecular cargo. Since heparin microparticles exhibit stable BMP-2 retention (>75%), these microparticles may be better suited for localized growth factor presentation than conventional delivery vehicles that exhibit substantial growth factor release and low retention (0–20%), such as polyethylene glycol (PEG) and gelatin biomaterials [5, 56–58]. The gelatin methacrylate microparticles used in this study were thermally cross-linked with free radical polymerization in a manner similar to heparin methacrylamide; since ~55% of the amines on the gelatin methacrylate microparticles were substituted with methacrylate groups, a similar cross-linking density to heparin microparticles could be achieved (data unpublished). Although gelatin also primarily binds positively charged growth factors through electrostatic interactions, heparin microparticles captured and retained significantly more BMP-2 than gelatin microparticles, due to their higher charge density [10, 11].
Previous studies investigating the co-delivery of heparin and BMP-2 have demonstrated both stimulatory and inhibitory effects on BMP-2-mediated osteogenesis [25–31]. In this study, the ALP activity induced in C2C12 cells by both soluble BMP-2 and microparticle-bound BMP-2 was comparable. Interestingly, since release curves of BMP-2 from heparin microparticles at 37°C indicated that the majority of BMP-2 was retained within the microparticles, ALP activity results suggested that the heparin-BMP-2 interaction formed by the microparticles effectively presented BMP-2 to cells and induced a functional cellular response without complete release of the growth factor. This finding agrees with several studies that have shown that co-delivery of heparin and BMP-2 promotes ALP secretion in several cell types, including C2C12 skeletal myoblasts, MC3T3 pre-osteoblasts, and mesenchymal stem cells [25, 26, 38, 59]. Although the specific mechanism by which heparin enhances BMP-2 signaling remains unclear, co-delivery of BMP-2 and heparin may enhance BMP-2 cell signaling by facilitating formation of growth factor-receptor complexes on the cell surface [14, 60] or by prolonging the half life of BMP-2 in culture [25, 31, 37]. Furthermore, this mechanism of action could also potentially explain the fact that unloaded heparin microparticles dose-dependently elicited an increase in ALP activity without the addition of exogenous BMP-2. Heparin microparticles could potentially sequester cell-secreted heparin-binding proteins, such as BMPs and other growth factors, thereby prolonging growth factor presentation and subsequently enhancing ALP activity compared to cultures that lacked microparticles [61].
Unlike microparticle delivery of BMP-2, co-delivery of soluble heparin and BMP-2 resulted in ALP activity that was significantly lower than that of soluble BMP-2 alone. This may suggest that soluble heparin binds to BMP-2 in solution, preventing its interaction with cell surface receptors and subsequently inhibiting further cell signaling [26, 27, 30]. Since BMP-2-loaded heparin microparticles induced significantly higher ALP activity than co-delivery of soluble heparin and BMP-2, microparticle delivery of heparin may be a more efficient method of concentrating and presenting BMP-2 to cells. Heparin microparticles may improve growth factor delivery by concentrating BMP-2 locally in the immediate proximity of the cells, where high densities of BMP-2 bound to the microparticles result in an increased local growth factor concentration. Similar effects have been observed in other systems containing surface-immobilized growth factors through direct growth factor tethering or indirect affinity-based growth factor-ECM interactions, in which the sustained presentation of immobilized growth factors stimulates enhanced cell signaling compared to similar concentrations of soluble growth factors [62–64]. Concentrated BMP-2 effects may be stronger when fewer microparticles are loaded with higher BMP-2 densities, as evidenced by the increased ALP activity observed with fewer microparticles loaded with the same total amount of BMP-2 (0.02 and 0.1 mg), while decreased ALP activity was observed with more microparticles (0.5 mg). Furthermore, total ALP activity was significantly decreased when cells and BMP-2-loaded microparticles were physically separated from each other, indicating that contact between cells and heparin materials are critical for efficient BMP-2 signaling. Ultimately, the ability to spatially concentrate growth factors using heparin microparticles may provide an advantage for numerous growth factor delivery applications, such as the regeneration of tissue defects, in which the local presentation of high concentrations of growth factor is often necessary to stimulate repair. This is similar to the native role played by cell membrane-bound GAG species during wound healing, in which signaling pathways involved in cell proliferation and migration, such as FGF signaling, are potentiated by direct GAG-growth factor contact [65].
In addition to inducing ALP activity, BMP-2-loaded microparticles also stimulated a striking increase in DNA content in C2C12 cultures. Both heparin and heparan sulfate have been shown to increase cell proliferation by binding to endogenous mitogens in the system, such as basic fibroblast growth factor (FGF-2), and facilitating their interactions with cell surface receptors [66, 67]. In studies with mesenchymal stem cells, delivering soluble BMP-2 with selectively desulfated heparin increased the number of mesenchymal stem cells in in vitro cultures, while native heparin did not [38]. This is similar to the effects observed herein, in which the combined presence of both heparin microparticles and BMP-2 (soluble or pre-loaded) increased DNA content. The effects of heparin microparticles on DNA content appear to be both contact-mediated and specific to the microparticle delivery platform, since significant changes in DNA content were not observed when soluble heparin was delivered or when C2C12 cells were physically separated from the microparticles. Taken together, these results indicate that BMP-2 laden heparin microparticles may simultaneously initiate cell signaling events related to both cell proliferation and ALP production, and that these effects may involve multiple pathways, such as Smad 1/5/8-dependent and Smad 1/5/8-independent pathways, which have both previously been observed in BMP-2-stimulated C2C12 cells [25, 68, 69].
Conclusions
The results of this study demonstrate that heparin microparticles can be used to sequester and retain large amounts of bioactive BMP-2, and that sustained presentation of BMP-2 via heparin microparticles can elicit a comparable cellular response to soluble BMP-2 treatment. Heparin microparticles offer a versatile platform for growth factor delivery, as loaded microparticles can be directly injected and retained in a tissue defect site, providing a much higher surface area for efficient growth factor presentation and contact with infiltrating cells. Consequently, heparin microparticles may be an effective method of delivering and spatially retaining growth factors for a variety of applications, including both in vitro growth factor-mediated induction of cell fates and in vivo tissue regeneration therapies.
Supplementary Material
Acknowledgments
This work was supported by a Transformative Research Award from the National Institutes of Health (TR01 AR062006) and a grant from the National Science Foundation (NSF DMR 1207045). MHH is supported by funding from the Natural Science and Engineering Research Council (NSERC) of Canada. The authors would like to thank Ms. Shalini Saxena for her assistance with scanning electron microscopy, and Ms. Marissa Cooke for her assistance with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vaibhav B, Nilesh P, Vikram S, Anshul C. Bone morphogenic protein and its application in trauma cases: a current concept update. Injury. 2007;38:1227–35. doi: 10.1016/j.injury.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302:58–66. doi: 10.1001/jama.2009.956. [DOI] [PubMed] [Google Scholar]
- 3.Gautschi OP, Frey SP, Zellweger R. Bone morphogenetic proteins in clinical applications. ANZ J Surg. 2007;77:626–31. doi: 10.1111/j.1445-2197.2007.04175.x. [DOI] [PubMed] [Google Scholar]
- 4.Ennett AB, Kaigler D, Mooney DJ. Temporally regulated delivery of VEGF in vitro and in vivo. J Biomed Mater Res A. 2006;79:176–84. doi: 10.1002/jbm.a.30771. [DOI] [PubMed] [Google Scholar]
- 5.van de Wetering P, Metters AT, Schoenmakers RG, Hubbell JA. Poly(ethylene glycol) hydrogels formed by conjugate addition with controllable swelling, degradation, and release of pharmaceutically active proteins. J Control Release. 2005;102:619–27. doi: 10.1016/j.jconrel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Uludag H, D’Augusta D, Palmer R, Timony G, Wozney J. Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in the rat ectopic model. J Biomed Mater Res. 1999;46:193–202. doi: 10.1002/(sici)1097-4636(199908)46:2<193::aid-jbm8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Tao L, Liu J, Xu J, Davis TP. Synthesis and bioactivity of poly(HPMA)-lysozyme conjugates: the use of novel thiazolidine-2-thione coupling chemistry. Org Biomol Chem. 2009;7:3481–5. doi: 10.1039/b907061c. [DOI] [PubMed] [Google Scholar]
- 8.Al-Azzam W, Pastrana EA, King B, Méndez J, Griebenow K. Effect of the covalent modification of horseradish peroxidase with poly (ethylene glycol) on the activity and stability upon encapsulation in polyester microspheres. J Pharm Sci. 2005;94:1808–19. doi: 10.1002/jps.20407. [DOI] [PubMed] [Google Scholar]
- 9.Lindahl U, Hook M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- 10.Miller T, Goude MC, McDevitt TC, Temenoff JS. Molecular engineering of glycosaminoglycan chemistry for biomolecule delivery. Acta Biomater. 2014;10:1705–19. doi: 10.1016/j.actbio.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capila I, Linhardt RJ. Heparin–protein interactions. Angew Chem Int Ed Engl. 2002;41:390–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.Schönherr E, Hausser H-J. Extracellular matrix and cytokines: a functional unit. Dev Immunol. 2000;7:89–101. doi: 10.1155/2000/31748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deepa SS, Umehara Y, Higashiyama S, Itoh N, Sugahara K. Specific molecular interactions of oversulfated chondroitin sulfate E with various heparin-binding growth factors: Implications as a physiological binding partner in the brain and other tissues. J Biol Chem. 2002;277:43707–16. doi: 10.1074/jbc.M207105200. [DOI] [PubMed] [Google Scholar]
- 14.Ruppert R, Hoffmann E, Sebald W. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur J Biochem. 1996;237:295–302. doi: 10.1111/j.1432-1033.1996.0295n.x. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi NS, Mancera RL. Prediction of heparin binding sites in bone morphogenetic proteins (BMPs) Biochim Biophys Acta. 2012;1842:1374–81. doi: 10.1016/j.bbapap.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Guimond SE, Turnbull JE. Fibroblast growth factor receptor signalling is dictated by specific heparan sulphate saccharides. Curr Biol. 1999;9:1343–6. doi: 10.1016/s0960-9822(00)80060-3. [DOI] [PubMed] [Google Scholar]
- 17.Slack J, Darlington B, Heath J, Godsave S. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987;326:197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- 18.Damon DH, Lobb RR, D’Amore PA, Wagner JA. Heparin potentiates the action of acidic fibroblast growth factor by prolonging its biological half-life. J Cell Physiol. 1989;138:221–6. doi: 10.1002/jcp.1041380202. [DOI] [PubMed] [Google Scholar]
- 19.Ye L, Wu X, Mu Q, Chen B, Duan Y, Geng X, et al. Heparin-conjugated PCL scaffolds fabricated by electrospinning and loaded with fibroblast growth factor 2. J Biomater Sci Polym Ed. 2011;22:389–406. doi: 10.1163/092050610X487710. [DOI] [PubMed] [Google Scholar]
- 20.Liu LS, Ng CK, Thompson AY, Poser JW, Spiro RC. Hyaluronate-heparin conjugate gels for the delivery of basic fibroblast growth factor (FGF-2) J Biomed Mater Res. 2002;62:128–35. doi: 10.1002/jbm.10238. [DOI] [PubMed] [Google Scholar]
- 21.Pike DB, Cai S, Pomraning KR, Firpo MA, Fisher RJ, Shu XZ, et al. Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials. 2006;27:5242–51. doi: 10.1016/j.biomaterials.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Schroeder-Tefft J, Bentz H, Estridge T. Collagen and heparin matrices for growth factor delivery. J Control Release. 1997;49:291–8. [Google Scholar]
- 23.Zhao J, Luo C, Chen Y, Wu D, Shen C, Han W, et al. Preparation, structure and BMP-2 controlled release of heparin-conjugated hyaluronan microgels. Carbohyd Polym. 2011;86:806–11. [Google Scholar]
- 24.Xu X, Jha AK, Duncan RL, Jia X. Heparin-decorated, hyaluronic acid-based hydrogel particles for the controlled release of bone morphogenetic protein 2. Acta Biomater. 2011;7:3050–9. doi: 10.1016/j.actbio.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao B, Katagiri T, Toyoda H, Takada T, Yanai T, Fukuda T, et al. Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. J Biol Chem. 2006;281:23246–53. doi: 10.1074/jbc.M511039200. [DOI] [PubMed] [Google Scholar]
- 26.Kanzaki S, Ariyoshi W, Takahashi T, Okinaga T, Kaneuji T, Mitsugi S, et al. Dual effects of heparin on BMP-2-induced osteogenic activity in MC3T3-E1 cells. Pharmacol Rep. 2011;63:1222–30. doi: 10.1016/s1734-1140(11)70642-9. [DOI] [PubMed] [Google Scholar]
- 27.Kanzaki S, Takahashi T, Kanno T, Ariyoshi W, Shinmyouzu K, Tujisawa T, et al. Heparin inhibits BMP-2 osteogenic bioactivity by binding to both BMP-2 and BMP receptor. J Cell Physiol. 2008;216:844–50. doi: 10.1002/jcp.21468. [DOI] [PubMed] [Google Scholar]
- 28.Hausser HJ, Brenner RE. Low doses and high doses of heparin have different effects on osteoblast-like Saos-2 cells in vitro. J Cell Biochem. 2004;91:1062–73. doi: 10.1002/jcb.20007. [DOI] [PubMed] [Google Scholar]
- 29.Yang L, Butcher M, Simon RR, Osip SL, Shaughnessy SG. The effect of heparin on osteoblast differentiation and activity in primary cultures of bovine aortic smooth muscle cells. Atherosclerosis. 2005;179:79–86. doi: 10.1016/j.atherosclerosis.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 30.Jiao X, Billings PC, O’Connell MP, Kaplan FS, Shore EM, Glaser DL. Heparan sulfate proteoglycans (HSPGs) modulate BMP2 osteogenic bioactivity in C2C12 cells. J Biol Chem. 2007;282:1080–6. doi: 10.1074/jbc.M513414200. [DOI] [PubMed] [Google Scholar]
- 31.Takada T, Katagiri T, Ifuku M, Morimura N, Kobayashi M, Hasegawa K, et al. Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins. J Biol Chem. 2003;278:43229–35. doi: 10.1074/jbc.M300937200. [DOI] [PubMed] [Google Scholar]
- 32.Bhakta G, Rai B, Lim ZX, Hui JH, Stein GS, van Wijnen AJ, et al. Hyaluronic acid-based hydrogels functionalized with heparin that support controlled release of bioactive BMP-2. Biomaterials. 2012;33:6113–22. doi: 10.1016/j.biomaterials.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeon O, Song SJ, Kang S-W, Putnam AJ, Kim B-S. Enhancement of ectopic bone formation by bone morphogenetic protein-2 released from a heparin-conjugated poly (L-lactic-co-glycolic acid) scaffold. Biomaterials. 2007;28:2763–71. doi: 10.1016/j.biomaterials.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 34.Yang HS, La W-G, Bhang SH, Jeon J-Y, Lee JH, Kim B-S. Heparin-conjugated fibrin as an injectable system for sustained delivery of bone morphogenetic protein-2. Tissue Eng Part A. 2010;16:1225–33. doi: 10.1089/ten.TEA.2009.0390. [DOI] [PubMed] [Google Scholar]
- 35.Kang S-W, La W-G, Kang JM, Park J-H, Kim B-S. Bone morphogenetic protein-2 enhances bone regeneration mediated by transplantation of osteogenically undifferentiated bone marrow-derived mesenchymal stem cells. Biotechnol Lett. 2008;30:1163–8. doi: 10.1007/s10529-008-9675-8. [DOI] [PubMed] [Google Scholar]
- 36.Teixeira S, Yang L, Dijkstra P, Ferraz M, Monteiro F. Heparinized hydroxyapatite/collagen three-dimensional scaffolds for tissue engineering. J Mater Sci Mater Med. 2010;21:2385–92. doi: 10.1007/s10856-010-4097-2. [DOI] [PubMed] [Google Scholar]
- 37.Bramono DS, Murali S, Rai B, Ling L, Poh WT, Lim ZX, et al. Bone marrow-derived heparan sulfate potentiates the osteogenic activity of bone morphogenetic protein-2 (BMP-2) Bone. 2012;50:954–64. doi: 10.1016/j.bone.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ratanavaraporn J, Tabata Y. Enhanced osteogenic activity of bone morphogenetic protein-2 by 2-O-desulfated heparin. Acta Biomater. 2012;8:173–82. doi: 10.1016/j.actbio.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 39.Johnson MR, Boerckel JD, Dupont KM, Guldberg RE. Functional restoration of critically sized segmental defects with bone morphogenetic protein-2 and heparin treatment. Clin Orthop Relat Res. 2011;469:3111–7. doi: 10.1007/s11999-011-2012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui C, Schwendeman SP. One-step surface modification of poly (lactide-co-glycolide) microparticles with heparin. Pharm Res. 2007;24:2381–93. doi: 10.1007/s11095-007-9378-1. [DOI] [PubMed] [Google Scholar]
- 41.Denizli A. Heparin-immobilized poly(2-hydroxyethylmethacrylate)-based microspheres. J Appl Polym Sci. 1999;74:655–62. [Google Scholar]
- 42.Abbah SA, Liu J, Goh JCH, Wong H-K. Enhanced control of in vivo bone formation with surface functionalized alginate microbeads incorporating heparin and human bone morphogenetic protein-2. Tissue Eng Part A. 2012;19:350–9. doi: 10.1089/ten.tea.2012.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hannink G, Geutjes PJ, Daamen WF, Buma P. Evaluation of collagen/heparin coated TCP/HA granules for long-term delivery of BMP-2. J Mater Sci Mater Med. 2013;24:325–32. doi: 10.1007/s10856-012-4802-4. [DOI] [PubMed] [Google Scholar]
- 44.Seto SP, Casas ME, Temenoff JS. Differentiation of mesenchymal stem cells in heparin-containing hydrogels via coculture with osteoblasts. Cell Tissue Res. 2012;347:589–601. doi: 10.1007/s00441-011-1265-8. [DOI] [PubMed] [Google Scholar]
- 45.Park YD, Tirelli N, Hubbell JA. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials. 2003;24:893–900. doi: 10.1016/s0142-9612(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 46.Lim JJ, Hammoudi TM, Bratt-Leal AM, Hamilton SK, Kepple KL, Bloodworth NC, et al. Development of nano-and microscale chondroitin sulfate particles for controlled growth factor delivery. Acta Biomater. 2011;7:986–95. doi: 10.1016/j.actbio.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamprecht MR, Sabatini DM, Carpenter AE. CellProfiler: free, versatile software for automated biological image analysis. Biotechniques. 2007;42:71–5. doi: 10.2144/000112257. [DOI] [PubMed] [Google Scholar]
- 48.Ferrara N, Houck KA, Jakeman LB, Winer J, Leung DW. The vascular endothelial growth factor family of polypeptides. J Cell Biochem. 1991;47:211–8. doi: 10.1002/jcb.240470305. [DOI] [PubMed] [Google Scholar]
- 49.Gospodarowicz D, Ferrara N, Schweigerer L, Neufeld G. Structural characterization and biological functions of fibroblast growth factor. Endocr Rev. 1987;8:95–114. doi: 10.1210/edrv-8-2-95. [DOI] [PubMed] [Google Scholar]
- 50.Aggarwal BB, Kohr WJ, Hass PE, Moffat B, Spencer SA, Henzel WJ, et al. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985;260:2345–54. [PubMed] [Google Scholar]
- 51.Uludag H, D’Augusta D, Golden J, Li J, Timony G, Riedel R, et al. Implantation of recombinant human bone morphogenetic proteins with biomaterial carriers: a correlation between protein pharmacokinetics and osteoinduction in the rat ectopic model. J Biomed Mater Res. 2000;50:227–38. doi: 10.1002/(sici)1097-4636(200005)50:2<227::aid-jbm18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 52.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–66. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeon O, Powell C, Solorio LD, Krebs MD, Alsberg E. Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J Control Release. 2011;154:258–66. doi: 10.1016/j.jconrel.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jha AK, Yang W, Kirn-Safran CB, Farach-Carson MC, Jia X. Perlecan domain I-conjugated, hyaluronic acid-based hydrogel particles for enhanced chondrogenic differentiation via BMP-2 release. Biomaterials. 2009;30:6964–75. doi: 10.1016/j.biomaterials.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.La WG, Kwon SH, Lee TJ, Yang HS, Park J, Kim BS. The effect of the delivery carrier on the quality of bone formed via bone morphogenetic protein-2. Artif Organs. 2012;36:642–7. doi: 10.1111/j.1525-1594.2011.01420.x. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto M, Ikada Y, Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J Biomater Sci Polym Ed. 2001;12:77–88. doi: 10.1163/156856201744461. [DOI] [PubMed] [Google Scholar]
- 57.Kawai K, Suzuki S, Tabata Y, Ikada Y, Nishimura Y. Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials. 2000;21:489–99. doi: 10.1016/s0142-9612(99)00207-0. [DOI] [PubMed] [Google Scholar]
- 58.Zhao Y, Liu Z, Pan C, Li Z, Zhou J, Wang J, et al. Preparation of gelatin microspheres encapsulated with bFGF for therapeutic angiogenesis in a canine ischemic hind limb. J Biomater Sci Polym Ed. 2011;22:665–82. doi: 10.1163/092050610X489880. [DOI] [PubMed] [Google Scholar]
- 59.Mathews S, Mathew SA, Gupta PK, Bhonde R, Totey S. Glycosaminoglycans enhance osteoblast differentiation of bone marrow derived human mesenchymal stem cells. J Tissue Eng Regen Med. 2014;8:143–52. doi: 10.1002/term.1507. [DOI] [PubMed] [Google Scholar]
- 60.Kuo W-J, Digman MA, Lander AD. Heparan sulfate acts as a bone morphogenetic protein coreceptor by facilitating ligand-induced receptor hetero-oligomerization. Mol Biol Cell. 2010;21:4028–41. doi: 10.1091/mbc.E10-04-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Terada K, Misao S, Katase N, Nishimatsu S, Nohno T. Interaction of Wnt signaling with BMP/Smad signaling during the transition from cell proliferation to myogenic differentiation in mouse myoblast-derived cells. Int J Cell Biol. 2013;2013:616294. doi: 10.1155/2013/616294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ito Y, Shu Qin L, Imanishi Y. Enhancement of cell growth on growth factor-immobilized polymer film. Biomaterials. 1991;12:449–53. doi: 10.1016/0142-9612(91)90141-v. [DOI] [PubMed] [Google Scholar]
- 63.Mann BK, Schmedlen RH, West JL. Tethered-TGF-β increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22:439–44. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 64.Hudalla GA, Kouris NA, Koepsel JT, Ogle BM, Murphy WL. Harnessing endogenous growth factor activity modulates stem cell behavior. Integr Biol (Camb) 2011;3:832–42. doi: 10.1039/c1ib00021g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–62. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 66.Dombrowski C, Song SJ, Chuan P, Lim X, Susanto E, Sawyer AA, et al. Heparan sulfate mediates the proliferation and differentiation of rat mesenchymal stem cells. Stem Cells Dev. 2009;18:661–70. doi: 10.1089/scd.2008.0157. [DOI] [PubMed] [Google Scholar]
- 67.Furue MK, Na J, Jackson JP, Okamoto T, Jones M, Baker D, et al. Heparin promotes the growth of human embryonic stem cells in a defined serum-free medium. Proc Natl Acad Sci U S A. 2008;105:13409–14. doi: 10.1073/pnas.0806136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boergermann JH, Kopf J, Yu PB, Knaus P. Dorsomorphin and LDN-193189 inhibit BMP-mediated Smad, p38 and Akt signalling in C2C12 cells. Int J Biochem Cell Biol. 2010;42:1802–7. doi: 10.1016/j.biocel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gallea S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, et al. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone. 2001;28:491–8. doi: 10.1016/s8756-3282(01)00415-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.