Abstract
The associations of pulmonary function with cardiovascular disease (CVD) independent of diabetes mellitus (DM) and metabolic syndrome have not been examined in a population-based setting. We examined prevalence and incidence CVD in relation to lower pulmonary function in the Strong Heart Study 2nd examination (1993-1995) in 352 CVD and 2873 non-CVD adults free of overt lung disease (mean age 60 years). Lung function was assessed by standard spirometry. Participants with metabolic syndrome or DM with or without CVD had lower pulmonary function than participants without these conditions after adjustment for hypertension, age, gender, abdominal obesity, smoking, physical activity index and study field center. CVD participants with DM had significantly lower FVC than participants with CVD alone. Significant associations were observed between reduced pulmonary function, preclinical CVD and prevalent CVD after adjustment for multiple CVD risk factors. During follow-up (median 13.3 years), pulmonary function did not predict CVD incidence, it predicted CVD mortality. Among 3225 participants, 412 (298 without baseline CVD) died from CVD by the end of 2008. In models adjusted for multiple CVD risk factors, DM, metabolic syndrome and baseline CVD, compared to highest quartile of lung function, lower lung function predicted CVD mortality (RR up to 1.5, 95% CI 1.1-2.0, p<0.05). In conclusion, a population with a high prevalence of DM and metabolic syndrome, lower lung function was independently associated with prevalent clinical and preclinical CVD, and its impairment predicted CVD mortality. Additional research is needed to identify mechanisms linking metabolic abnormalities, low lung function and CVD.
Keywords: lung function, cardiovascular diseases, diabetes mellitus, metabolic syndrome
Epidemiologic studies have shown that CVD is the leading cause of mortality and morbidity in American Indian population 1-3, which also has the highest prevalences of obesity and diabetes mellitus (DM) 4, 5. The aims of this study were to test the hypotheses that reduced lung function is independently associated with prevalent CVD, and also predicts subsequent incident CVD and CVD mortality in this population.
Methods
The Strong Heart Study is a multicenter, population-based, prospective study of CVD and its risk factors among American Indian adults that enrolled 4549 men and women 45-74 years old at the 1st examination in 1989 to 1992. The study design, survey methods, and laboratory techniques have been described previously 6, 7. The study population comprises members of 13 tribes who reside in the study communities in Arizona, North and South Dakota and Oklahoma. The present analysis utilized lung function assessment by standard spirometry at the 2nd examination (1993-1995). Approval was obtained from relevant institutional review boards, and all participants gave written informed consent.
Incident CVD events included fatal and nonfatal CVD events which occurred between the 2nd examination (1993-1995) and December 31, 2008. Fatal CVD events included fatal myocardial infarction, sudden death presumed due to coronary heart disease, fatal congestive heart failure, other fatal coronary heart disease, and fatal stroke. Deaths occurring between 2nd examination and December 31, 2008 were confirmed through tribal and Indian Health Service hospital records and through direct contact with participants’ families or other informants by study personnel, as reported previously 1, 6, 7. Non-fatal CVD events included definite myocardial infarction, coronary heart disease, congestive heart failure and stroke, either identified by participant contact and medical record review or electrocardiograms obtained at subsequent examinations 1, 6, 7.
Individuals were classified as having diabetes mellitus (DM) according to 1997 American Diabetes Association criteria; fasting glucose level at least 7.0 mmol/L (126 mg/dL); current use of anti-diabetes medication; or on renal dialysis / kidney transplant with a positive response to the question “Has a medical person ever told you that you had diabetes?”. This group included adults with DM - primarily type2, but also some with type 1 DM.
Metabolic syndrome (MS) in participants without DM was defined according to the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) guidelines 8 as having at least three of the following five conditions: abdominal obesity (waist circumference >102 cm in men and >88 cm in women), increased triglycerides (≥150 mg/dL), reduced high-density lipoprotein (HDL) cholesterol (<40 mg/dL in men and <50 mg/dL in women), elevated blood pressure (≥130/≥85 mmHg), and high fasting glucose (100-125 mg/dL).
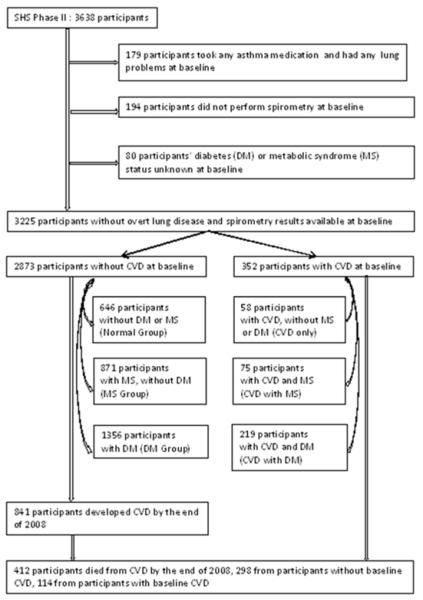
The following criteria were used to exclude participants from the analysis population: (1) any self-reported lung problems and use of asthma medications (N=179), (2) missing pulmonary function results (N=194), and (3) missing data on DM or MS status (N=80). The final study sample consisted of 3225 adults of which 352 had CVD at the 2nd examination. The 2873 without CVD (CVD-free) included: 646 without MS, DM or CVD (Normal group), 871 without DM or CVD but with MS (MS group), and 1356 without CVD but with DM (DM group). The 352 with prevalent CVD included: 58 CVD only, 75 CVD with MS and 219 CVD with DM. These six groups of participants were mutually exclusive. CVD-free (N=2873) participants were used for the prediction of incident CVD (Figure 1).
Figure1.
Flowchart of participant selection for the current analysis.
Spirometry was performed by centrally trained and certified nurses and technicians. The study-specific forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) were predicted using the equations developed by Marion et al. for healthy Strong Heart Study participants using age, sex, and height 9. Before the analysis, crude data on FVC and FEV1 were divided by predicted FVC and FEV1, respectively, to yield FVC % predicted and FEV1 % predicted.
The ankle brachial index is the ratio of the blood pressure in the lower legs to the blood pressure in the arms. A higher ankle brachial index suggests calcification of the walls of the arteries and incompressible vessels, reflecting possible peripheral vascular disease. The method of measuring ankle brachial index in the Strong Heart Study has been previously published 10. At the study examination, right arm blood pressure and bilateral ankle blood pressure measured by handheld Doppler were taken by a trained certified nurse with the participants supine. The means of the 2 measurements for each leg and for the arm were used to calculate ankle brachial index, and the lower of the 2 values was used to define ankle brachial index for each individual. The aforementioned Strong Heart Study publication found a U-shaped association between peripheral arterial disease and mortality risk through the measurement of ankle brachial index. In this study, abnormal ankle brachial index was defined as ankle brachial index <0.90 or ankle brachial index >1.40 10.
The left ventricular (LV) ejection fraction represents the volumetric fraction of blood pumped out of ventricle with each heartbeat. The LV ejection fraction was calculated from LV linear dimensions, as shown to be prognostically relevant to cardiovascular mortality 11. Partition values were used to separate the Strong Heart Study participants with normal LV ejection fraction from those with LV dysfunction (ejection fraction <54%) 11, 12.
LV hypertrophy, increased LV myocardial mass and most commonly a reaction to CVD or high blood pressure, is a strong marker for heart disease. The LV mass index was estimated by echocardiography using an anatomically validated formula, and indexed by a method that detects both disease-related and obesity-related LV hypertrophy 13, 14. LV hypertrophy was defined as LV mass index >49.2 g/m2.7 in men, and >46.7 g/m 2.7 in women in the Strong Heart Study 15.
LV wall movement abnormalities have recognized prognostic significance in patients with CVD; echocardiographic LV wall movement abnormalities in Strong Heart Study participants without overt CVD are also associated with 2.4 to 3.4-fold higher risks of CVD morbidity and mortality 16. Segmental LV wall movement abnormalities were considered present if present in 2 contiguous segments in a coronary territory 16. All echocardiogram for the Strong Heart Study were evaluated at a central reading center (Weill Cornell Medical Center, New York, NY).
The definitions and methods used for other measurements (age, education level, physical activity index, height, body mass index, hypertension) have been reported previously 17. Cigarette smokers were defined as persons who smoked at least 100 cigarettes during their lifetime. The homeostasis model assessment –estimated insulin resistance was calculated according to the following formula: (fasting insulin in uU/mL X fasting glucose in mg/dL)/405.
Characteristics of Normal, MS, DM, and CVD (CVD alone, CVD with MS, and CVD with DM) groups were compared using general linear models for continuous variables and chi-square tests for categorical variables; p-values were calculated between MS vs. Normal, DM vs. Normal, and CVD vs. Normal. Kruskal-Wallis analysis of variance by ranks was used to compare total triglycerides and urine albumin/creatinine because of skewed distributions. Multiple linear regression models were used to describe the cross-sectional associations between lung function and MS, DM, or CVD after adjusting for potential demographic confounding variables including age, sex, abdominal obesity, height, physical activity index, education level, smoking status, Strong Heart Study center; as well as CVD risk factors (hypertension, high non-HDL cholesterol and albuminuria). High non-HDL cholesterol was defined as values ≥175 mg/dL, the highest quartile of this measurement; albuminuria was defined as urinary albumin/creatinine ratio ≥30 mg/g. The same models were also fitted to describe the cross-sectional associations between lung function and cardiovascular abnormalities with adjustment for potential confounding variables.
Cox proportional-hazard models were used to analyze associations between incident CVD and pulmonary function, controlling for confounding variables. Since measurements of FVC and FEV1 as % predicted values were not affected by factors other than pulmonary function such as age and body size, these two measurements were used for the prediction of CVD incidence. For the incidence prediction, the 352 with prevalent CVD at baseline were excluded. The follow-up time for CVD incidence was the time between the 2nd examination and the first episode of CVD events, fatal or non-fatal. For participants without a CVD event, the time was between the 2nd examination (1993-1995) and December 31, 2008. In the CVD mortality analysis, the follow-up time for CVD/non-CVD death was from the 2nd examination to death. Survivors were censored at the end of 2008. All tests of significance were two-tailed, with an alpha level of 0.05. All analyses were performed using version 9.2 of the SAS statistical software package (SAS Institute, Cary, NC).
Results
The characteristics of the study cohort are display in Table 1. Mean age of these 3225 participants was 59.8 ± 7.9 years (61% female; 35% from Arizona, 33% from Oklahoma, 32% from Dakotas), 70% were smokers (current smokers and ex-smokers). Prevalent hypertension and prevalent DM was present in 47% and 49% of the cohort, respectively. Mean body mass index and waist circumferences were 31.2 ± 0.1 kg/m2, and 106.6 ± 14.5 centimeters, respectively. CVD participants with MS or DM had higher inflammatory marker (fibrinogen) and they were also more likely to be less active, more hypertensive, and presence of albuminuria compare with participants with MS or DM alone (Table 1). The clinical measurements of the excluded group due to missing DM, MS or pulmonary function test status were similar to those of the study group, except they were older and had lower mean body mass index (data not shown).
Table 1.
Baseline characteristics of the participants
| No CVD Normal (n=646) |
MS (n=871) | DM (n=1356) | CVD No MS/DM (n=58) |
MS (n=75) | DM (n=219) | |
|---|---|---|---|---|---|---|
| Arizona | 110 | 247 | 659 | 13 | 12 | 84 |
| Oklahoma | 265 | 326 | 373 | 24 | 25 | 58 |
| Dakotas | 271 | 298 | 324 | 21 | 38 | 77 |
| Male | 341 | 300 | 441 | 19 | 35 | 122 |
| Female | 305 | 571 | 915 | 39 | 40 | 97 |
| Mean age (years) | 59.0 (8.0) | 59.5 (8.1) | 59.6 (7.6) | 63.9 (8.3)* | 63.4 (8.7)* | 62.4 (7.7)* |
| High school graduate | 58% (54-62) | 60% (57-63) | 49% (46-52)* | 47% (34-59)* | 55% (43-66)* | 38% (31-44)* |
| Cigarette smoking | ||||||
| Current smoker | 47% (43-51) | 31% (28-34)* | 24% (22-27)* | 43% (30-56) | 30% (19-40)* | 18% (13-23)* |
| Ex-smoker | 29% | 38% | 42% | 36% | 48% | 54% |
| Never smoker | 24% | 31% | 34% | 21% | 23% | 28% |
| Leisure activity in past year (MET hours/ week) |
31.8 (44.4) | 27.4 (43.1) | 22.2 (35.1)* | 34.9 (48.5) | 21.5 (28.5)* | 19.7 (30.9)* |
| Waist circumference (cm) | 95.6 (12.5) | 109.0 (13.0)* | 110.4 (14.0)* | 97.3 (13.8) | 108.2 (12.5)* | 107.9 (13.3)* |
| Body Mass Index (kg/m2) | 27.0 (5.0) | 32.5 (5.9)* | 32.5 (6.4)* | 27.6 (6.2) | 32.1 (5.9)* | 31.3 (6.3)* |
| Hypertension | 22% (18-25) | 44% (41-47)* | 54% (52-57)* | 43% (30-56)* | 73% (63-83)* | 74% (69-80)* |
| HDL†-cholesterol (mg/dL) | 50.5 (15.7) | 39.0 (11.3)* | 38.7 (11.6)* | 47.1 (13.5) | 39.4 (11.3)* | 35.8 (11.2)* |
| Total triglyceride‡ (mg/dL) | 92.5 (66, 120) | 145 (103, 195)† | 148 (104, 214)† | 93 (68, 123) | 128 (96, 175)† | 153 (101, 253)† |
| % Hemoglobin A1C | 5.2 (0.9) | 5.4 (0.9) | 8.6 (2.4)* | 5.5 (1.2) | 5.6 (1.1) | 8.3 (2.3)* |
| Albuminuria | ||||||
| Macroalbuminuria | 2% (1-3) | 3% (2-4) | 24% (21-26) | 9% (2-16) | 7% (1-13) | 35% (29-42) |
| Microalbuminuria | 11% | 11% | 34% | 13% | 23% | 31% |
| No albuminuria | 87% | 86% | 42%* | 79% | 70%* | 34%* |
| Fibrinogen (mg/dL) | 333.6 (64.1) | 345.0 (66.0)* | 384.1 (92.5)* | 362.9 (62.0)* | 364.0 (84.6)* | 395.0 (87.6)* |
| HOMA-IR§ | 2.6 (2.6) | 5.3 (3.8)* | 14.1 (12.9)* | 3.0 (2.4) | 5.4 (4.3)* | 13.5 (13.0)* |
MS: metabolic syndrome; DM: diabetes mellitus; CVD: cardiovascular disease. Data in parentheses are 1 SD for continuous variables and 95% CI for percentages unless otherwise indicated. MET: metabolic equivalent.
For continuous variables, general linear models were used; for categorical variables, χ2 tests were used to test significant difference for MS, DM or CVD vs. Normal.
HDL, high-density-lipoprotein.
Median, first quartile, and third quartile; Kruskal-Wallis tests were used to test differences.
Homeostasis model assessment – estimated insulin resistance.
Participants with MS or DM with or without CVD had lower pulmonary function than participants without these conditions after adjustment for hypertension, age, gender, abdominal obesity, education level, physical activity index, height, smoking status and study field center. Within CVD participants, CVD participants with DM had significantly lower FVC than participants with CVD alone (p<0.05; Table 2). The effect modifications for MS, DM, CVD and smoking in relation to pulmonary function were tested. The results indicated that there were no interactions among these groups (P>0.05).
Table 2.
Unadjusted and adjusted spirometry results for six groups of participants
| FVC (ml) | FEV1 (ml) | FVC % predicted | FEV1 % predicted | |
|---|---|---|---|---|
| Normal, MS, DM, CVD: Unadjusted models | ||||
| Normal (n=646) | 3,724 (3,651-3,797) | 2,732 (2,676-2,787) | 99.1 (97.7-100.4) | 95.2 (93.9-96.6) |
| MS, no CVD (n=871) | 3,326 (3,263-3,388) | 2,513 (2,466-2,561) | 94.1 (92.9-95.2) | 92.9 (91.7-94.1) |
| DM, no CVD (n=1356) | 3,117 (3,067-3,167) | 2,381 (2,343-2,419) | 90.3 (89.4-91.3) | 90.0 (89.1-91.0) |
| CVD only (n=58) | 3,451 (3,208-3,693) | 2,489 (2,304-2,673) | 92.5 (88.0-97.1) | 90.0 (85.4-94.6) |
| CVD, MS (n=75) | 3,275 (3,062-3,488) | 2,439 (2,277-2,601) | 90.5 (86.5-94.5) | 89.6 (85.5-93.6) |
| CVD, DM (n=219) | 2,995 (2,870-3,119) | 2,255 (2,160-2,350) | 85.5 (83.1-87.8) | 85.0 (82.6-87.3) |
| MS: Adjusted models† | ||||
| MS, No CVD vs. Normal | −108 (−202 to −14)* | −55 (−133 to 22) | −2.9 (−5.3 to −0.4)* | −1.9 (−4.5 to 0.7) |
| MS, CVD vs. Normal | --283 (−490 to −76)* | −157 (−326 to 13) | −6.1 (−11.6 to −0.6)* | −4.0 (−9.7 to 1.8) |
| DM: Adjusted models† | ||||
| DM, No CVD vs. Normal | −190 (−277 to −103)* | −97 (−167 to −28)* | −4.7 (−7.1 to −2.2)* | −3.1 (−5.4 to −0.7)* |
| DM, CVD vs. Normal | −396 (−531 to −261)* | −240 (−348 to −132)* | −10.6 (−14.3 to −6.8)* | −8.6 (−12.3 to −4.9)* |
| CVD: Adjusted models† | ||||
| CVD only vs. Normal | −234 (−489 to 21) | −169 (−373 to 35) | −4.4 (−10.9 to 2.1) | −2.8 (−9.5 to 3.8) |
| CVD, MS vs. Normal | −284 (−534 to −34)* | −171 (−372 to 29) | −5.9 (−12.3 to 0.4) | −4.4 (−10.9 to 2.2) |
| CVD, DM vs. Normal | −360 (−536 to −185)* | −227 (−368 to −86)* | −9.8 (−14.3 to −5.4)* | −8.6 (−13.2 to −4.0)* |
| Within CVD Group: Unadjusted models | ||||
| CVD, MS vs. CVD only | −176 (−550 to 199) | −50 (−339 to 240) | −2.0 (−9.9 to 5.9) | −0.4 (−8.6 to 7.7) |
| CVD, DM vs. CVD only | −456 (−772 to −139)* | −234 (−478 to 11) | −7.1 (−13.7 to −0.4)* | −5.0 (−12.0 to 1.9) |
MS: metabolic syndrome; DM: diabetes mellitus; CVD: cardiovascular disease; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 second. General linear models were used to calculate the p- values between MS vs. Normal, DM vs. Normal, and CVD vs. Normal;
indicates the difference was statistically significant between two groups.
Models were adjusted for hypertension and demographic factors include age, sex, abdominal obesity, height, physical activity index, education level, smoking status, and Strong Heart Study center.
The prevalence of preclinical CVD among study participants was measured. Participants with CVD were more likely to have cardiovascular abnormalities; participants with DM, with or without CVD were more likely to have an abnormal ankle brachial index and higher LV mass index compared to their normal counterparts (Supplementary Table 1).
There was a significant reduction in FVC and FEV1 % predicted in participants with markers of cardiovascular abnormality measured by ankle-brachial index, LV eject fraction or by LV mass index after adjustment for MS, DM, hypertension and demographic factors (Table 3); the results for ejection fraction remained significant after additional adjustment for CVD risk factors (data not shown). LV wall movement abnormality was significantly related to FEV1 reduction after adjustment for MS, DM, hypertension and demographic factors. The effect modifications for preclinical CVD, MS, DM and smoking in relation to pulmonary function were also tested. The results indicated that there were no interactions among these groups (P>0.05).
Table 3.
Spirometry and prevalence of preclinical CVD measurements among Strong Heart Study participants
| FVC (ml) | FEV1 (ml) | FVC % predicted | FEV1 % predicted | |
|---|---|---|---|---|
| Measured by ankle brachial index (ABI) | ||||
| Unadjusted model | ||||
| Normal ABI | 3,366 (3,329-3,402) | 2,535 (2,508-2,562) | 94.1 (93.4-94.7) | 92.6 (91.9-93.3) |
| Abnormal ABI† | 2,955 (2,837-3,074) | 2,208 (2,119-2,297) | 87.5 (85.3-89.7) | 86.8 (84.6-89.0) |
| Adjusted model | ||||
| Abnormal ABI vs. Normal ABI | −106 (−201 to −11)* | −58 (−134 to 17) | −4.0 (−6.6 to −1.4)* | −2.9 (−5.5 to −0.3)* |
| Measured by LV ejection fraction (EF) | ||||
| Unadjusted model | ||||
| Normal EF | 3,280 (3,242-3,318) | 2,482 (2,454-2,511) | 93.8 (93.1-94.6) | 92.8 (92.1-93.5) |
| Reduced EF‡ | 3,391 (3,296-3,485) | 2,507 (2,436-2,578) | 88.7 (87.0-90.5) | 86.6 (84.9-88.3) |
| Adjusted model | ||||
| Reduced EF vs. Normal EF | −107 (−182 to −32)* | −106 (−164 to −48)* | −3.2 (−5.3 to −1.2)* | −4.1 (−6.1 to −2.1)* |
| Measured by LV mass index | ||||
| Unadjusted model | ||||
| No LV hypertrophy | 3,447 (3,408-3,487) | 2,598 (2,568-2,628) | 94.3 (93.6-95.1) | 93.0 (92.2-93.7) |
| LV hypertrophy§ | 2,844 (2,777-2,912) | 2,151 (2,100-2,202) | 89.5 (88.2-90.8) | 88.7 (87.4-90.0) |
| Adjusted model | ||||
| LV hypertrophy vs. No LV hypertrophy | −121 (−184 to −58)* | −83 (−132 to −34)* | −2.5 (−4.2 to −0.7)* | −1.9 (−3.6 to −0.2)* |
| Measured by LVWM abnormality¶ (ABN) | ||||
| Unadjusted model | ||||
| No LVWM ABN | 3,280 (3,245-3,315) | 2,478 (2,452-2,505) | 92.8 (92.2-93.5) | 91.8 (91.1-92.4) |
| LVWM ABN | 3,430 (3,280-3,580) | 2,461 (2,348-2,574) | 93.0 (90.2-95.7) | 88.0 (85.3-90.8) |
| Adjusted model | ||||
| WMABN vs. No WMABN | −4 (−118 to 111) | −101 (−191 to −11)* | 0.7 (−2.4 to 3.8) | −3.1 (−6.2 to 0.0)* |
General linear models were used to calculate the p- values between normal and abnormal groups; adjusted models were adjusted for MS, DM, hypertension and demographic factors which include age, sex, abdominal obesity, height, physical activity index, education level, smoking status, and Strong Heart Study center;
indicates the difference was statistically significant between two groups.
Abnormal ABI was defined as ratio <0.90 or ratio>1.40.
Reduced EF was defined as value <0.54.
The Strong Heart Study indicated that LV hypertrophy was defined as LV mass index >49.2 g/m2.7 in men, and >46.7 g/m2.7 in women.
Left ventricular wall movement abnormality.
During follow-up (median 13.3 years), among 2873 participants without CVD at baseline, CVD developed in 841 (29.3%) participants (27.7 per 1000 person-years); males (31.5 per 1000 person-years, 95 C.I. 28.3-35.0) were higher than females (25.6 per 1000 person-years, 95 C.I. 23.5-28.0), p<0.05. In unadjusted analyses, FVC and FEV1% both predicted CVD incidence (data not shown). When multiple CVD risk factors including MS and DM were considered, pulmonary function did not predict CVD incidence, DM participants had 2- fold higher risks of CVD incidence compared to their normal counterparts (Table 4, top part).
Table 4.
Cox Proportional-Hazard models for the prediction of CVD incidence or CVD mortality, based on metabolic syndrome, diabetes, CVD status and quartiles of pulmonary function tests
| Model | Variable | Relative risk (95% CI) | p Value | Covariates |
|---|---|---|---|---|
| Predict CVD incidence* | FVC% predicted (>=103.8%) | 1.0 | age, center, sex, smoking, hypertension, | |
| Forced Vital Capacity | FVC% predicted (93.4-103.8%) | 1.0 (0.8-1.2) | 0.75 | albuminuria, high non-HDL cholesterol |
| FVC% predicted (82.8-93.4%) | 1.1 (0.9-1.4) | 0.27 | ||
| FVC %predicted (<82.8%) | 1.2 (0.9-1.4) | 0.19 | ||
| MS vs. Normal | 1.0 (0.8-1.3) | 0.84 | ||
| DM vs. Normal | 1.9 (1.6-2.4) | <0.01 | ||
| Forced Expiratory | FEV1% predicted (>=103.4%) | 1.0 | age, center, sex, smoking, hypertension, | |
| Volume in 1 second | FEV1% predicted (92.9-103.4%) | 1.0 (0.8-1.2) | 0.76 | albuminuria, high non-HDL cholesterol |
| FEV1% predicted (81.6-92.9%) | 1.1 (0.9-1.4) | 0.28 | ||
| FEV1 %predicted (<81.6%) | 1.1 (0.9-1.4) | 0.19 | ||
| MS vs. Normal | 1.0 (0.8-1.3) | 0.78 | ||
| DM vs. Normal | 2.0 (1.6-2.5) | <0.01 | ||
| Predict CVD mortality* | FVC% predicted (>=103.4%) | 1.0 | age, sex, smoking, education, hypertension, | |
| Forced Vital Capacity | FVC% predicted (92.8-103.4%) | 0.9 (0.7-1.3) | 0.65 | albuminuria, high non-HDL cholesterol, center |
| FVC% predicted (81.7-92.8%) | 1.1 (0.8-1.5) | 0.52 | ||
| FVC %predicted (<81.7%) | 1.4 (1.1-1.9) | 0.02 | ||
| MS vs. Normal | 0.8 (0.5-1.2) | 0.20 | ||
| DM vs. Normal | 1.5 (1.0-2.3) | 0.03 | ||
| CVD vs. Normal | 3.0 (2.0-4.5) | <0.01 | ||
| Forced Expiratory | FEV1% predicted (>=103.0%) | 1.0 | age, sex, smoking, education, hypertension, | |
| Volume in 1 second | FEV1% predicted (92.5-103.0%) | 0.8 (0.6-1.1) | 0.14 | albuminuria, high non-HDL cholesterol, center |
| FEV1% predicted (80.8-92.5%) | 1.2 (0.9-1.6) | 0.20 | ||
| FEV1 %predicted (<80.8%) | 1.5 (1.1-2.0) | <0.01 | ||
| MS vs. Normal | 0.8 (0.5-1.2) | 0.26 | ||
| DM vs. Normal | 1.7 (1.1-2.4) | 0.01 | ||
| CVD vs. Normal | 3.1 (2.1-4.7) | <0.01 |
Models were reduced by stepwise selection; pulmonary function, DM, MS were forced into the model.
Covariates considered were demographic factors, hypertension, high non-HDL cholesterol, albuminuria; all covariates were candidates for removal; covariates remained significant (p≤0.05) shown in the table.
In addition to covariates considered in the incidence models, CVD status was also included in the mortality models.
Among 3225 participants retained in this analysis, 412 (298 without baseline CVD) died from CVD by the end of 2008. The results indicated that lower FVC and FEV1 both predicted CVD mortality even when multiple CVD risk factors (age, sex, abdominal obesity, height, physical activity index, education level, smoking status, Strong Heart Study center, hypertension, high non-HDL cholesterol, albuminuria, DM, MS and CVD status at the study 2nd examination) were considered (Table 4, bottom). The relative risks of 1.5 (95% CI, 1.1-2.0, p<0.01) were observed for the lowest quartiles of FEV1 % predicted; the lowest quartile of FVC% also predicted CVD mortality (relative risk=1.4, 95%CI 1.1-1.9, p=0.02). DM participants had 1.5 to 1.7-fold higher risks of CVD mortality compared to the Normal group, CVD participants had 3-fold higher risks of CVD mortality compared to their normal counterparts (relative risks up to 3.1, 95%CI 2.1-4.7, p<0.01).
Additional analyses were carried out to examine the prediction of incidence for each component of CVD (congestive heart failure, coronary heart disease, myocardial infarction and stroke) separately by pulmonary function. In the unadjusted analyses, FEV1% predicted incident coronary heart disease; however, when multiple CVD risk factors including MS and DM were considered, pulmonary function did not predict incident coronary heart disease (Supplementary Table 2, top part). When incident congestive heart failure was considered as outcome, both FVC and FEV1% predicted incident congestive heart failure, after adjustment of multiple CVD risk factors; relative risks up to 2.2 (95% CI, 1.5-3.4, p<0.01) were observed (Supplementary Table 2, bottom). Both FVC% and FEV1% did not predict incident myocardial infarction or incident stroke in this population (data not shown).
Discussion
Reduced lung function has been reported to be a significant predictor of CVD, including CVD mortality 18-21. A recent study using the same Strong Heart Study population identified possible associations of obesity, metabolic syndrome and diabetes with pulmonary function impairment 22. Similar results were found in the present study that adults with MS or DM had significantly lower FVC and FEV1; this study also found that lower pulmonary function predicted CVD mortality. Very few studies have reported the association of lower lung function in CVD patients with MS or those with DM. In the present study, CVD participants who also had DM had significantly lower FVC than participants with CVD alone, a result that has not been reported before.
Reduced lung function has been reported to be associated with different type of preclinical CVD, MS or DM separately 23-26, very few studies have considered reduced lung function and all these conditions. In the present study, there was a significant reduction in FVC or FEV1 % predicted in participants with markers of cardiovascular abnormality measured by ankle-brachial index, ejection fraction, LV mass index or by LV wall movement after adjustment for multiple CVD risk factors including hypertension, MS and DM, a result that has not been reported before.
This study found that DM was a significant predictor for CVD incidence and for CVD mortality, very similar results to other studies. This study also discovered that both FEV1 and FVC were significantly related to reduced LV ejection fraction which is an important marker for congestive heart failure; very consistent results were also found that lower lung function predicted congestive heart failure. The results suggest that reduced lung function may be used to predict cardiovascular risk fairly precisely at a very early stage.
While an association between reduced pulmonary function and increased risk of vascular events was also reported by other study, the mechanisms underlying this association are unknown 27. Data from animal and human studies suggest that adipose tissue in obesity leads to a systemic pro-inflammatory state 28, producing the metabolic and cardiovascular complications of obesity and insulin resistance29, but whether this inflammatory milieu also modulates airway pathophysiology, and leads to reduced lung function, is not yet known 28, 29. Alternatively, systemic inflammation as a manifestation of chronic obstructive pulmonary disease may worsen metabolic and cardiovascular disease. The mechanisms linking metabolic abnormalities, airway disease and cardiovascular disease require further investigation.
The major strengths of this study include the community-based sample, standardized spirometric techniques, extensive data on potential confounders, systematic CVD outcome data with minimal loss to follow-up, and a large sample size that increased precision and permitted multiple statistical adjustments; also, this report is one of very few to study lung function in a population with very high prevalence of DM. Limitations include limited data on pulmonary function, a relatively small number of CVD participants and the inclusion of a single ethnic group which might limit generalizability to other populations. However, given the rising tides of obesity and DM in other populations, it is plausible to speculate that our findings could be generalized to the general population.
Supplementary Material
Acknowledgments
The authors acknowledge the assistance and cooperation of the participating tribes and the Indian Health Service facilities serving those tribes. The authors also thank the study participants and the directors of the Strong Heart Study clinics and their staffs. Without their contributions this research would not be possible. The opinions expressed in this paper are those of the author(s) and do not necessarily reflect the views of the Indian Health Service.
Funding Sources: The Strong Heart Study was supported by cooperative agreement U01-HL41642, U01-HL41652, U01-HL41654 and U01-HL65521 from the National Heart, Lung, and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee ET, Cowan LD, Welty TK, Sievers M, Howard WJ, Oopic A, Wang W, Yeh J, Devereux RB, Rhoades ER, Fabsitz RR, Go O, Howard VB. All-cause mortality and cardiovascular disease mortality in three American Indian populations, aged 45-74 years, 1984-1988. The Strong Heart Study. Am J Epidemiol. 1998;147:995–1008. doi: 10.1093/oxfordjournals.aje.a009406. [DOI] [PubMed] [Google Scholar]
- 2.Howard BV, Lee ET, Cowan LD, Devereux RB, Galloway JM, Go OT, Howard WJ, Rhoades ER, Robbins DC, Sievers ML, Welty TK. Rising tide of cardiovascular disease in American Indians. The Strong Heart Study. Circulation. 1999;99:2389–2395. doi: 10.1161/01.cir.99.18.2389. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed April 2014];Morbidity and Mortality : 2012 Chart Book on cardiovascular, lung, and blood disease. National Heart Lung and Blood Institute of Health. 2012 :16–7. http://www.nhlbi.nih.gov/resources/docs/2012_ChartBook_508.pdf.
- 4. [Accessed April 2014];Summary Statistics for U.S. Adults: National Health Interview Survey, 2011. Vital and Health Statistics. 2011 10:106. http://www.cdc.gov/nchs/data/series/sr_10/sr10_256.pdf. [Google Scholar]
- 5.National Diabetes Fact Sheet, United States, 2011. Centers for Disease Control and Prevention (CDC); [Accessed April 2014]. 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. [Google Scholar]
- 6.Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, Cucchiara AJ, Savage PJ, Howard BV. The Strong Heart Study: A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132:1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 7.Best LG, Zhang Y, Lee ET, Yeh JL, Cowan L, Palmieri V, Roman M, Devereux RB, Fabsitz RR, Robbins TD, Davidson M, Ahmed A, Howard BV. C-reactive protein as a predictor of cardiovascular risk in a population with a high prevalence of diabetes: the Strong Heart Study. Circulation. 2005;112:1289–1295. doi: 10.1161/CIRCULATIONAHA.104.489260. [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM, Brewer HB, Jr., Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 9.Marion MS, Leonardson GR, Rhoades ER, Welty TK, Enright PL. Spirometry reference values for American Indian adults: results from the Strong Heart Study. Chest. 2001;120:489–495. doi: 10.1378/chest.120.2.489. [DOI] [PubMed] [Google Scholar]
- 10.Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, Howard BV. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–739. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 11.Devereux RB, Roman MJ, Palmieri V, Liu JE, Lee ET, Best LG, Fabsitz RR, Rodeheffer RJ, Howard BV. Prognostic implications of ejection fraction from linear echocardiographic dimensions: the Strong Heart Study. Am Heart J. 2003;146:527–534. doi: 10.1016/S0002-8703(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 12.Roman MJ, Pickering TG, Schwartz JE, Pini R, Devereux RB. Association of carotid atherosclerosis and left ventricular hypertrophy. J Am Coll Cardiol. 1995;25:83–90. doi: 10.1016/0735-1097(94)00316-i. [DOI] [PubMed] [Google Scholar]
- 13.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 14.de Simone G, Kizer JR, Chinali M, Roman MJ, Bella JM, Best LG, Lee ET, Devereux RB. Normalization for body size and population-attributable risk of left ventricular hypertrophy: the Strong Heart Study. Am J Hypertens. 2005;18:191–196. doi: 10.1016/j.amjhyper.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 15.Palmieri V, Devereux RB, Hollywood J, Bella JN, Liu JE, Lee ET, Best LG, Howard BV, Roman MJ. Association of pulse pressure with cardiovascular outcome is independent of left ventricular hypertrophy and systolic dysfunction: the Strong Heart Study. Am J Hypertens. 2006;19:601–607. doi: 10.1016/j.amjhyper.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Cicala S, de Simone G, Roman MJ, Best LG, Lee ET, Wang W, Welty TK, Galloway JM, Howard BV, Devereux RB. Prevalence and prognostic significance of wall-motion abnormalities in adults without clinically recognized cardiovascular disease: the Strong Heart Study. Circulation. 2007;116:143–150. doi: 10.1161/CIRCULATIONAHA.106.652149. [DOI] [PubMed] [Google Scholar]
- 17.Welty TK, Lee ET, Yeh J, Cowan LD, Go O, Fabsitz RR, Le NA, Oopok AJ, Robbins DC, Howard BV. Cardiovascular disease risk factors among American Indians. The Strong Heart Study. Am J Epidemiol. 1995;142:269–287. doi: 10.1093/oxfordjournals.aje.a117633. [DOI] [PubMed] [Google Scholar]
- 18.Kannel WB, Hubert H, Lew EA. Vital capacity as predictor of cardiovascular disease: the Framingham study. Am Heart J. 1983;105:311–315. doi: 10.1016/0002-8703(83)90532-x. [DOI] [PubMed] [Google Scholar]
- 19.Lee HM, Le H, Lee BT, Lopez VA, Wong ND. Forced vital capacity paired with Framingham risk score for prediction of all-cause mortality. Eur Respir J. 2010:1002–1006. doi: 10.1183/09031936.00042410. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal SK, Heiss G, Barr RG, Chang PP, Loehr LR, Chambless LE, Shahar E, Kitzman DW, Rosamond WD. Airflow obstruction, lung function, and risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) study. Eur J Heart Fail. 2012;14:414–422. doi: 10.1093/eurjhf/hfs016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HM, Chung SJ, Lopez VA, Wong ND. Association of FVC and total mortality in US adults with metabolic syndrome and diabetes. Chest. 2009;136:171–176. doi: 10.1378/chest.08-1901. [DOI] [PubMed] [Google Scholar]
- 22.Yeh F, Dixon AE, Marion S, Schaefer C, Zhang Y, Best LG, Calhoun D, Rhoades ER, Lee ET. Obesity in adults is associated with reduced lung function in metabolic syndrome and diabetes: the Strong Heart Study. Diabetes Care. 2011;34:2306–2313. doi: 10.2337/dc11-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schroeder EB, Welch VL, Evans GW, Heiss G. Impaired lung function and subclinical atherosclerosis. The ARIC Study. Atherosclerosis. 2005;180:367–373. doi: 10.1016/j.atherosclerosis.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Eguchi K, Boden-Albala B, Jin Z, Rundek T, Sacco R, Homma S, Di Tullio MR. Association between diabetes mellitus and left ventricular hypertrophy in a multiethnic population. Am J Cardiol. 2008;101:1787–1791. doi: 10.1016/j.amjcard.2008.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chinali M, Devereux RB, Howard BV, Roman MJ, Bella JN, Liu JE, Resnick HE, Lee ET, Best LG, de Simone G. Comparison of cardiac structure and function in American Indians with and without the metabolic syndrome (the Strong Heart Study) Am J Cardiol. 2004;93:40–44. doi: 10.1016/j.amjcard.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 26.de Simone G, Devereux RB, Chinali M, Roman MJ, Lee ET, Resnick HE, Howard BV. Metabolic syndrome and left ventricular hypertrophy in the prediction of cardiovascular events: the Strong Heart Study. Nutr Metab Cardiovasc Dis. 2009;19:98–104. doi: 10.1016/j.numecd.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Crim C, Willits LR, Yates JC, Vestbo J. Cardiovascular events in patients with COPD: TORCH study results. Thorax. 2010;65:719–725. doi: 10.1136/thx.2010.136077. [DOI] [PubMed] [Google Scholar]
- 28.O’Donnell CP, Holguin F, Dixon AE. Pulmonary physiology and pathophysiology in obesity. J Appl Physiol. 2010;108:197–198. doi: 10.1152/japplphysiol.01208.2009. [DOI] [PubMed] [Google Scholar]
- 29.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.