ABSTRACT
Infections with the beta genus of human papillomaviruses (β-HPVs) may contribute to the development of nonmelanoma skin cancers. However, β-HPV genomes are found at too low a copy number in tumors for the virus to be necessary for tumor maintenance. Instead, they are hypothesized to destabilize the host genome by allowing the persistence of mutations that can drive tumorigenesis independently of the viral genome. Supporting this premise is our previous finding that the expression of some β-HPV E6 proteins can attenuate p53 signaling in response to DNA damage. We show that β-HPV E6 proteins can prevent the stabilization of p53 in response to two types of genome-destabilizing events, aberrant mitosis and dysregulated centrosome duplication. The inability to stabilize p53 in response to these stimuli allows cells expressing HPV5, HPV8, or HPV38 E6 to remain proliferatively active, leading to further genome deterioration in a proportion of these cells. These phenotypes are lost by the introduction of a mutation into the p300 binding domain of HPV8 E6 or by the transfection of mutated p300 that is resistant to the degradation mediated by HPV5 or HPV8 E6. These findings expand the understanding of the role played by p300 in promoting the faithful resolution of mitotic figures as well as proper centrosome duplication. Finally, we describe a phenomenon by which binucleated cells are resolved via cytokinesis into two cells, each with one nucleus. These data support the hypothesis that β-HPV infections may promote tumorigenesis via genome destabilization.
IMPORTANCE The work described in this report provides support for the hypothesis that β-HPV infections may contribute to nonmelanoma skin cancer by increasing the likelihood that tumorigenic mutations are introduced into the host cell's genome. We demonstrate that expression of the E6 proteins from some of these viruses increases the tolerance of two genome-destabilizing events, aberrant cell division and dysregulated centrosome duplication. Typically, these mutagenic occurrences elicit the stabilization of the tumor suppressor p53, which prevents further propagation of cells containing these errors. We show that the expression of β-HPV E6 restricts this stabilization of p53, leading not only to continued cellular proliferation but also to further accumulation of similar mutagenic events. Finally, in addition to supporting a role for β-HPV infections in certain skin cancers, we present studies with a mutated β-HPV E6 protein suggesting that the histone acetyltransferase p300 plays a role in promoting genome stability during replication.
INTRODUCTION
Human papillomaviruses (HPVs) are a large family of double-stranded DNA viruses that infect the cutaneous and mucosal epithelia of humans. Sequence homology allows the HPV family to be divided into five genera (1). Although infections by some members of the beta-, mu-, and gammapapillomavirus genera can result in a range of clinically important outcomes, members of the alpha genus of HPVs (α-HPVs) receive the bulk of scientific attention due to the ability of certain α-HPVs to cause anogenital carcinomas (2, 3). Because only some α-HPVs are associated with increased cancer risks, members of the genus Alphapapillomavirus are often grouped on the basis of the relative likelihood that a viral infection will lead to a carcinoma (high-risk [HR] and low-risk α-HPVs) (4). Members of the beta genus of HPVs (β-HPVs) may also play a role in tumorigenesis, specifically in the development of nonmelanoma skin cancer (NMSC) (5–9). As a result, our lab and others have become interested in understanding the mechanisms that might connect β-HPV infections to NMSC development. This endeavor is complicated by the fact that unlike high-risk α-HPV genomes, β-HPV genomes are not found at a high enough copy number in tumors to be necessary for tumor maintenance. Instead, β-HPV infection may destabilize the genome of the host cell in ways that increase the cell's carcinogenic potential without requiring the continued presence of the viral genome.
Because the high-risk α-HPV E6 and E7 proteins are the primary viral oncogenes, our work has focused on their counterparts in β-HPVs, particularly β-HPV E6. In support of a connection between β-HPV infections and NMSC development, our group and others have shown that the expression of the E6 proteins from some β-HPVs hinders the ability of cells to repair UVB-induced DNA damage (10). Specifically, our lab has shown that the expression of some β-HPV E6 proteins (HPV5, HPV8, and HPV38 E6 proteins) attenuates p53 phosphorylation and ubiquitination in response to UVB exposure, resulting in less-efficient repair of the resulting DNA damage (10). Mechanistically, this blunted reaction is dependent on the binding and destabilization of the histone acetyltransferase p300 by β-HPV E6 (11). Reduced p300 protein levels attenuate p53 activation both directly and indirectly (10). While p300 increases the DNA binding capability of p53 by acetylating the tumor suppressor (12), we have also shown that p300 is necessary for the optimal expression of two phosphatidylinositol (PI) 3-kinases involved in p53 stabilization, ATM and ATR (10, 13). Accordingly, the β-HPV E6-mediated attenuation of ATM and ATR expression is lost when the p300 binding/destabilization domain of HPV8 E6 is mutated (HPV Δ8 E6) (10, 13).
In addition to induction by DNA damage, p53 signaling is induced in response to genomic instability. The resulting increase in p53 stability occurs specifically in response to both supernumerary centrosomes (>2 centrosomes per cell) and polyploidy (the presence of one or more extra sets of chromosomes) (14–17). Polyploidy, which can be a precursor to aneuploidy, can occur through failed cytokinesis following mitosis (18). Similarly, the duplication of centrosomes is also closely linked to mitosis, since the presence of more than 2 centrosomes in a mitotic cell hinders the proper segregation of its chromosomes. The stabilization of p53 in response to these stimuli prevents cells from actively progressing through the cell cycle (19, 20). Underlying the role of p53 in maintaining the integrity of the genome, the tolerance of both supernumerary centrosomes and binucleation is significantly increased in the absence of p53 (15, 16, 21).
p53-independent mechanisms also limit the impact of genome-destabilizing events. As cells age, their growth rates slow and their telomeres shorten, inducing senescence (22, 23). Together, these processes act as a mechanism to limit the propagation of mutations and otherwise destabilized genomes that arise from extensive divisions. Indeed, after a certain number of divisions, cells permanently exit the cell cycle (24). Many viruses, including HPVs, require actively cycling cells to replicate their own genomes. To meet this need, HR α-HPV E6 promotes the propagation of late-passage cells in two ways. First, by activating telomerase, the expression of the viral oncogene staves off senescence (25). Second, HR α-HPV E6 increases proliferation rates by promoting the hyperphosphorylation of pRB (26).
Since β-HPVs and HR α-HPVs have similar requirements for replication, they may also contribute to the tolerance of genomic instability through p53-independent mechanisms, namely, by allowing cells to avoid the aging-associated reduction in proliferative potential. Additionally, because some β-HPV E6 proteins can attenuate p53 signaling in response to UVB exposure, we hypothesized that the expression of these β-HPV E6 proteins would also increase the tolerance of genomic instability in p53-dependent ways. Specifically, we propose that the expression of HPV5, HPV8, or HPV38 E6 will inhibit the stabilization of p53 in response to dysregulated centrosome duplication as well as failed cytokinesis and that a lack of p53 accumulation allows these cells to remain proliferatively active.
In support of these hypotheses, we present data demonstrating that expression of the E6 proteins of the β-HPVs HPV5, HPV8, and HPV38 allows cells with polyploid genomes and supernumerary centrosomes to accumulate in late-passage human foreskin keratinocytes (HFKs) by preventing the stabilization of p53 and consequently allowing these cells to continue their progression through the cell cycle. We also show that these phenotypes are dependent on the residues of HPV8 E6 that are responsible for decreasing the steady-state levels of p300, ATR, and ATM, enzymes involved in the activation of p53 in response to multiple stimuli (10, 13). Furthermore, we show that the E6 proteins of the β-HPVs HPV5, -8, and -38 can significantly increase the growth of late-passage primary keratinocytes independently of their ability to disrupt p53 signaling. Together, the data presented in this paper support the hypothesis that β-HPV infection may act as an initiation factor in the development of NMSC by creating a cellular environment favoring oncogenesis after the infection has cleared.
MATERIALS AND METHODS
Size fractionation.
HFKs (carrying a control vector or expressing HPV5 E6, HPV8 E6, HPV8 E6 with a mutation in the p300 binding domain [HPV Δ8 E6], HPV38 E6, or HPV16 E6) growing in 10-cm plates were trypsinized and were resuspended in 5 ml of warmed medium. By using a Sterifil filter system from Millipore that had been autoclaved and held a sterile nylon net filter (diameter, 47 mm; pore size, 20 μm; catalog no. NY2004700; Millipore), the 5-ml cell suspension was pulled through the filter with the vacuum in the tissue culture (TC) hood. The flowthrough was collected, spun down, resuspended in medium, and plated for observation. The filter apparatus was washed carefully by pulling 5 ml of phosphate-buffered saline (PBS) through the filter 3 times to concentrate the larger cells by washing through the smaller cells. The apparatus was then opened; the filter was carefully turned over; and the apparatus was reassembled. Five milliliters of warmed medium was pulled through the filter twice by vacuum. The cell suspension was collected, and the cells were counted and were plated onto a 6-well dish at 1.0 × 105 cells per well.
Senescence-associated β-galactosidase staining.
At a designated passage, HFKs were seeded onto a 6-well plate and were grown for 24 h. Following the growth period, the cells were fixed, permeabilized, and stained for senescence-associated β-galactosidase expression according to the manufacturer's protocol by using the Senescence β-galactosidase staining kit from Cell Signaling Technology.
IncuCyte microscopy.
The IncuCyte kinetic imaging system from Essen BioScience is a live-cell imaging system that can be programmed to take images of cells as they grow in the incubator. Size fractionation was used to increase the frequency of larger cells (more likely to be multinucleated). These cells were counted and were plated at 1.0 × 105 per well in 6-well dishes. The dishes were loaded into the IncuCyte module in an incubator, and each well was marked with 9 separate fields. Bright-field phase-contrast images (magnification, ×20) of each field were taken every half hour for 72 h. The resulting images were compiled into movies and were analyzed.
Bright-field microscopy.
HFKs (carrying a control vector or expressing HPV5 E6, HPV8 E6, HPV Δ8 E6, HPV38 E6, or HPV16 E6) were counted, plated at 1.0 × 105 cells per well in a 6-well plate, and grown in EpiLife medium for 2 days. Before trypsinization, the plates were photographed at ×20 magnification on an inverted Nikon TE 3000 microscope. The pictures were captured as tagged image files (TIFs) using the bright-field setting in MetaMorph, version 7.1, a microscopy software package from Molecular Devices.
Immunofluorescence microscopy.
HFKs (carrying a control vector or expressing HPV5 E6, HPV8 E6, HPV Δ8 E6, HPV38 E6, or HPV16 E6) were seeded on coverslips and were grown overnight. These cells were washed once with PBS and were fixed with 4% paraformaldehyde–PBS for 15 min. Following fixation, the coverslips were washed 3 times with PBS and were permeabilized with 0.5% Triton X–PBS for 15 min. The coverslips were washed again 3 times and were then blocked with blocking buffer (3% bovine serum albumin [BSA], 0.1% Tween 20, PBS) for 1 h at room temperature. The coverslips were then stained with a fluorescein isothiocyanate (FITC)-conjugated rabbit anti-Ki67 antibody (clone SP6; dilution, 1:500; catalog no. ab27619; Abcam), a mouse monoclonal antibody (MAb) against α-tubulin (clone TU-01; dilution, 1:500; catalog no. 138000; Life Technologies), a rabbit polyclonal antibody (PAb) against α-tubulin (dilution, 1:500; catalog no. ab18251; Abcam), a mouse MAb against γ-tubulin (clone GTU-88; dilution, 1:500; catalog no. T5326-200; Sigma-Aldrich), an Alexa Fluor 555-conjugated rabbit MAb against p53 (dilution, 1:500; catalog no. 5395S; Cell Signaling), and rabbit polyclonal IgG against p300 (dilution, 1:200; catalog no. sc-8981; Santa Cruz Biotechnology).
The primary antibodies were added to the coverslips in blocking buffer and were incubated at 4°C overnight. The next day, the coverslips were washed 3 times with PBS and were stained with appropriate Alexa Fluor-conjugated secondary antibodies from Life Technologies and with Hoechst 33342 (dilution, 1:10,000; catalog no. H3570; Life Technologies) in blocking buffer for 1 h at 37°C.
Microscopy quantifications.
For each independent repeat of the quantifications from both bright-field and immunofluorescent microscopy, a minimum of 3 images, each containing at least 10 cells, were evaluated. More frequently, the images from bright-field microscopy contained >100 cells.
DNA constructs.
All HPV E6 DNA constructs were made by cloning HPV E6 DNA into the pLXSN vector. HPV E6 DNA was generated by PCR from full-length HPV DNA. The mutated p300 expression vectors (p300E and p300A), as well as the HPV E6 expression vectors, have been described previously (11, 27, 28).
Tissue culture.
Primary HFKs were derived from neonatal human foreskins and were grown in EpiLife medium supplemented with calcium chloride (60 μM), human keratinocyte growth supplement (Cascade Biologics, Portland, OR), and penicillin-streptomycin. Multiple cell lines were derived from several donors for this work. Following viral transduction, selection, and expansion, expression of HPV E6 proteins was confirmed by reverse transcription-PCR (RT-PCR). Further confirmation of HPV E6 expression was obtained by performing Western blot analysis to look at the previously characterized reductions in ATR, ATM, and p300 levels in cells expressing HPV5 and HPV8 E6 proteins, as well as reduced p53 levels in HPV16 E6-expressing HFKs. To aid in the retroviral transduction of HPV E6 genes, we used 293T cells. These cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS).
Briefly, HPV genes cloned into an LXSN vector were cotransfected with vesicular stomatitis virus glycoprotein (VSV-G) helper plasmids into 293T cells using Fugene 6 (Roche), and medium containing retroviruses was collected at 12, 24, 36, and 48 h posttransfection. The viruses in the collected medium were then concentrated using ultracentrifugation and were used to infect HFKs in the presence of Polybrene (8 μg/ml; Sigma-Aldrich). Four hours after infection, HFKs were washed with PBS and were returned to the growth medium. The cells were then expanded when confluent and were placed under neomycin/G418 selection (50 mg/liter) for 7 days. Following selection, cells were grown in growth medium. When confluent, each cell line was passaged and uniformly reseeded. In this manner, each HFK line was followed from passage 5 to passage 25.
All p300A and p300E transfections were done using TransIT keratinocyte transfection reagent (Mirus), and cells were assessed 48 h later for multinucleation, as well as p53 accumulation and loss of Ki67 expression in response to either aberrant mitosis or centrosome division. The transient expression of p300 was confirmed by immunofluorescence microscopy 48 h posttransfection.
Passaging of HFKs.
In parallel, control and HPV E6-expressing HFKs were grown until one of the cell lines reached approximately 90% confluence. Once one of the six cell lines became confluent, all of the cell lines were trypsinized and collected. These cells were then quantified using the TC10 automated cell counter (Bio-Rad), and 500,000 cells of each cell line were plated onto a new 10-cm dish. For the purposes of this investigation, a passage consists of one cycle beginning with the seeding of newly trypsinized/harvested cells and ending at the point at which one of the six cell lines becomes confluent. This process was repeated until the cells reached passage 25.
Fluorescence-activated cell sorter (FACS) analysis.
At designated passage intervals, cells were harvested by trypsinization and were then pelleted by centrifugation at 4°C. The resulting pellets were then resuspended in PBS–2% FBS before being pelleted again by centrifugation at 4°C. During vortexing, the pellets were resuspended in 3 ml of cold 95% ethanol. Once thoroughly resuspended, the cells were covered in foil and were kept at 4°C overnight. The cells were then pelleted by centrifugation at 4°C, and the supernatant was aspirated. The resulting pellet was then resuspended with continuous vortexing in 2 ml IFA (150 mM NaCl, 4% fetal bovine serum, 0.15 sodium azide) plus 5% Tween 20. The cells were once again pelleted by centrifugation at 4°C, and the supernatant was removed. The resulting pellet was resuspended in 250 μl of 5-μg/ml RNase dissolved in PBS and was transferred to 12- by 75-mm snap cap tubes with filter caps. Following 30 min of incubation at 37°C, 250 μl of 100-μg/ml propidium iodide (in PBS) was added. The samples were then analyzed for DNA content using a BD FACSCanto instrument and FlowJo software.
Five-day proliferation assay.
HFKs (carrying a control vector or expressing HPV5 E6, HPV8 E6, HPV Δ8 E6, HPV38 E6, or HPV16 E6) growing in EpiLife medium were counted, and 3.0 × 104 cells were plated into 5 wells per line on 2- to 24-well tissue culture dishes. One well per line was counted by trypsinizing, resuspending in 1 ml of medium, and running 10 μl of the cell suspension through a Bio-Rad TC10 cell counter every day for 5 days.
Immunofluorescence intensity measurements.
Cells were plated onto coverslips and were grown overnight at 37°C. The next day, cells were fixed as described above and were stained for various proteins. The coverslips were mounted, and images were obtained using a DeltaVision Elite confocal microscope. Ten separation fields per line with 25 Z sections per image were captured at ×40 magnification on the DeltaVision Elite confocal microscope located in our Scientific Imaging Facility. The images were deconvolved and were processed using the SoftWoRx program, version 4.1.2. The images were normalized, saved as separate 16-bit-channel TIFs, and further analyzed for image intensity values using the Cell Scoring Application located within the MetaMorph image-processing program (version 7.7) from Molecular Devices.
Statistical analysis.
Unless otherwise noted, statistical significance was determined by a paired Student t test and was confirmed when appropriate by two-way analysis of variance (ANOVA) with Bonferroni's correction. Only P values less than or equal to 0.05 were reported as significant.
Growth index calculations.
Microsoft Excel software was used to fit growth curve data to an exponential growth curve. As cell counts were set to 100 at day zero, the y-intercepts of these best-fit curves were held constant at 100. This results in the formula y = 100e^ax, where y is the relative number of cells, x is the time in days, and a is the growth index for each set of data. Growth curves were performed in triplicate and were fit to this exponential growth curve. Figure 2A reports the mean growth index from these three experiments.
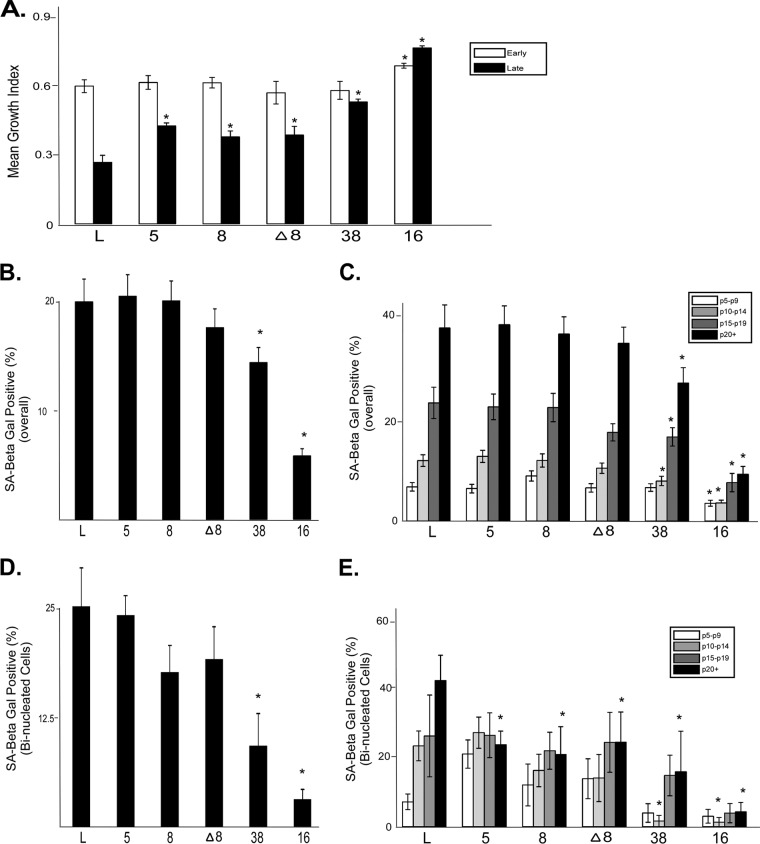
FIG 2.
β-HPV E6 expression promotes late-passage proliferation. (A) Equal numbers of cells from each indicated passage for each cell line were seeded, and proliferation rates were recorded over a 5-day period. Bars represent the mean growth index scores (from ≥3 independent experiments) for each of the cell lines. Early-passage cells were at passage 5, while late-passage cells were at passage 20. (B) Overall (passages 5 through 25) mean percentage (from ≥60 independent experiments) of cells staining positive for senescence-associated β-galactosidase (SA-Beta Gal). (C) Mean percentages (from ≥15 independent experiments) of cells staining positive for senescence-associated β-galactosidase for five passage intervals between p5 and p25. (D) Overall (passages 5 through 25) mean percentage (from ≥60 independent experiments) of binucleated cells staining positive for senescence-associated β-galactosidase. (E) Mean percentages (from ≥15 independent experiments) of binucleated cells staining positive for senescence-associated β-galactosidase for five passage intervals between p5 and p25. Error bars show the standard errors of the means. Asterisks indicate data points that are significantly different (P, ≤0.05) from the result for the corresponding vector control cell line.
RT-PCR.
RNA was isolated with TRIzol reagent (Invitrogen). Briefly, 1 ml of TRIzol was added to each 10-cm plate; cells were incubated for 5 min; and 200 μl of chloroform was added. The aqueous phase was transferred to another tube, mixed with an equal volume of isopropanol, incubated for 10 min, and pelleted at 12,000 rpm for 10 min at 4°C. After being washed with 75% ethanol, the samples were again pelleted at 9,000 rpm for 5 min and were resuspended in RNase-free water. One microgram of total RNA was reverse transcribed to generate cDNA by using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Semiquantitative PCR amplification was then performed to identify 100-bp amplicons with E6 and 36B4 primers as described previously (27).
RESULTS
β-HPV E6 expression increases tolerance of failed cytokinesis.
Our previous publications have shown that the expression of certain β-HPV E6 proteins leads to attenuated p53 signaling, suggesting that these β-HPV proteins might similarly weaken the p53 response to mitotic irregularities, increasing the frequency of abnormalities such as the failure to undergo cytokinesis following replication (10, 13). To test this hypothesis, we generated human foreskin keratinocytes (HFKs) carrying a control vector (LXSN) or expressing HPV5, HPV8, or HPV38 E6. In order to reflect the expression of HPV E6 seen in natural infections, a weak long-terminal-repeat (LTR) promoter was used to drive transcription of the HPV E6 genes in these cells. Commercial antibodies against β-HPV E6 proteins are not available, so we confirmed expression by semiquantitative RT-PCR (Fig. 1A). Because the presence of more than one nucleus in a cell is indicative of a failed cleavage furrow resection (18), costaining with β-actin (for the cytoplasm), lamin B (for the nuclear membrane), and 4′,6-diamidino-2-phenylindole (DAPI) (for the nucleus) was used to identify these abnormal cells. Cells with more than one nucleus were observed in each of our cell lines (data not shown). To determine if the expression of HPV E6 proteins altered the frequency of failed cleavage furrow resection, the proportion of cells with two or more nuclei was quantified. This analysis found similar overall occurrences of cells with abnormal numbers of nuclei among HFKs carrying the control vector (LXSN) and those expressing HPV E6 (Fig. 1B). The frequency of these abnormal cells was not increased by HPV16 E6, a protein that is known to disrupt p53 signaling directly by promoting the degradation of the tumor suppressor (Fig. 1B).
FIG 1.
β-HPV E6 expression increases multinucleation. (A) Representative RT-PCR confirming HPV E6 expression. 36B4 was used as a loading control (L). Note that since different PCR primers were used to amplify each E6 protein, relative expression can only be confirmed using this approach; the results may not accurately reflect the relative expression of HPV E6 among the cell lines. (B) Overall (passages 5 through 25) mean percentage (from ≥60 independent experiments) of cells with two or more nuclei for each cell line. (C) Overall (passages 5 through 25) mean percentage (from ≥60 independent experiments) of cells with an abnormal number of nuclei (≥2) that are multinucleated (≥3 nuclei) for each cell line. (D) Mean percentage (from ≥15 independent experiments) of cells that are multinucleated in four passage (p) intervals. (E) Mean percentage of cells with an abnormal number of nuclei (≥2) that are multinucleated (≥3 nuclei) for HFKs expressing the vector control (L), HPV5 E6, or HPV8 E6. Filled bars, cells transfected with the p300A expression vector; open bars, cells transfected with the p300E expression vector. (F) Relative percentages of cells with >4N DNA content, a measure of polyploidy. The values for HFKs carrying the control LXSN vector are set to 1. Early-passage cells have been cultured for <15 passages, while late-passage cells have been grown for >15 passages. Error bars show the standard errors of the means. Asterisks indicate data points that are significantly different (P, ≤0.05) from the result for the corresponding vector control cell line.
While a binucleated cell (a cell with 2 nuclei) can form from a single failed cytokinesis, the presence of a multinucleated cell (a cell with 3 or more nuclei) indicates that a binucleated cell has entered mitosis and once again has not divided. While the overall appearance of binucleated cell formation was not altered by β-HPV E6 expression, improper p53 activation in cells expressing these viral proteins may allow binucleated cells to remain proliferatively active more frequently and thus be more likely to become multinucleated cells. Among cells that had at least 2 nuclei, the percentage that were multinucleated (3 or more nuclei) (Fig. 1C) was determined. Expression of HPV5, HPV8, or HPV38 E6 increased the likelihood that cells would have 3 or more nuclei. In support of the link to p53 signaling, HPV16 E6 expression caused a similarly significant increase in the frequency of multinucleated cells (Fig. 1C). Likely reflecting the more complete disruption of p53 signaling, significant buildup of multinucleated cells occurred in cells expressing HPV16 E6 before the accumulation of these aberrant cells was observed in β-HPV E6-expressing cells.
Our data suggest that unlike HFKs expressing the vector control, HFKs expressing HPV5, HPV8, or HPV38 E6 that have undergone an initial round of replication absent cytokinesis can remain cycling and are thus able to undergo further mitosis. Increased tolerance of binucleation in cells expressing these β-HPV E6 proteins implies that the number of multinucleated cells will increase more rapidly over time among these cells than among control cells. To test this hypothesis, the frequency of multinucleated cells as a function of passaging was measured in control cells as well as in cells expressing HPV5, -8, or -38 E6 between passages 5 and 25. While these β-HPV E6 proteins did not significantly alter the frequency of multinucleated cells from passage 5 to passage 19, once the cells had been grown for 20 or more passages, HPV5, -8, or -38 E6 expression significantly increased the prevalence of cells with 3 or more nuclei (Table 1; Fig. 1D). To determine whether the increased frequency of multinucleation in cells expressing β-HPV E6 was dependent on the ability to bind/destabilize p300 and, as a result, to attenuate p53 signaling, a mutant form of HPV8 E6 (HPV Δ8 E6) that is incapable of binding p300 was expressed in HFKs (10). There was no significant increase in the frequency of multinucleation in cells expressing HPV Δ8 E6 over that in control cells (Fig. 1D) (10, 13). The hypothesis that the increased frequency of multinucleated cells with β-HPV E6 expression results, at least in part, from an attenuated p53 response in these cells is supported by the findings for HPV16 E6-expressing HFKs. HPV16 E6 both reduces p53 signaling in these cells and also leads to a higher frequency of multinucleated cells at early passages (Fig. 1D).
TABLE 1.
The frequency of cells with 3+ nuclei increases with passaging
| Vector | % Change in frequency of HFKs with 3+ nuclei (early vs late passage)a |
|---|---|
| LXSN | 1.7 |
| HPV5 E6 | 4.5* |
| HPV8 E6 | 5.0* |
| HPV Δ8 E6 | 2.0 |
| HPV38 E6 | 1.7 |
| HPV16 E6 | 1.7 |
Early passage, passages 5 through 9; late passage, passage 20+. An asterisk indicates that the data point is significantly different (P, ≤0.05) from that for the vector control (LXSN).
β-HPV E6 expression destabilizes p300 by blocking its phosphorylation by AKT (11). The introduction of a phospho-dead mutation (p300 S1834A [mutant referred to here as p300A]) in the AKT phosphorylation site of p300 abrogates p300 activity (29). However, the introduction of a phosphomimetic mutation in this AKT phosphorylation site (p300 S1834E [mutant referred to here as p300E]) abrogates the abilities of HPV5 and HPV8 E6 proteins to destabilize the histone acetyltransferase (11). These mutants were used to further validate the connection between the increased tolerance of binucleation by cells expressing β-HPV E6 and the reduced p300 levels in these cells. Late-passage control HFKs, as well as HPV5 and HPV8 E6-expressing HFKs, were transfected with p300E or with p300A as a negative control. Following transfection, the percentage of multinucleated cells among those cells with at least 2 nuclei was assessed. As expected, transfection with p300A did not hinder the ability of HPV5 or HPV8 E6 to increase the prevalence of multinucleated cells in this population (Fig. 1E). However, expression of HPV5 or HPV8 E6 was unable to alter this frequency when combined with p300E transfection (Fig. 1E).
To further confirm that the expression of HPV5, HPV8, or HPV38 E6 increases the tolerance of cells that have bypassed division following replication, we determined the frequency of cells with >4N content by FACS analysis of propidium iodide-stained early-passage (passage <15 [p ≤15]) and late-passage (p ≥15) HFKs. Compared to that for control HFKs, expression of HPV5, -8, or -38 E6 significantly increased the number of late-passage cells with >4N DNA (Fig. 1F). Again, this increase is at least partially dependent on the residues of E6 responsible for destabilizing p300 and attenuating p53 signaling, as evidenced by the fact that HFKs expressing HPV Δ8 E6 had significantly fewer cells with >4N content than HFKs expressing HPV8 E6 (1.2% versus 1.8%) (Fig. 1F). Finally, the frequency of cells with polyploid genomes was greatly increased by the expression of HPV16 E6 at both early and late passages, correlating with the role of p53 activation in preventing the accumulation of cells that have replicated their DNA but failed to divide properly (Fig. 1F).
β-HPV E6 expression increases the proliferation rates of late-passage HFKs independently of p53.
The difference in the frequency of multinucleated cells between control and β-HPV E6-expressing cells becomes most striking at late passages (Fig. 1D). Because proliferation is required for the formation of multinucleated cells, differences in growth rates could contribute to these late-passage differences. To determine if β-HPV E6 expression was able to increase the growth rates of late-passage HFKs, we measured the proliferation of cells over a 5-day period at early (p5) and late (p20) passages (data not shown). The resulting growth curves were then fit to a regression line and were used to determine a growth index for each cell line. Although the proliferation rates of control and β-HPV E6-expressing HFKs were similar at early passages, HPV5, -8, or -38 E6-expressing HFKs grew significantly faster than control HFKs at late passages (Fig. 2A). In support of previously published work (26), the expression of HPV16 E6 significantly increased the growth rate of late-passage cells (Fig. 2A).
HPV16 E6 has been reported to increase the proliferation rate of cells in a p53-independent manner (25). To test if β-HPV E6 expression might be increasing late-passage growth rates independently of its ability to attenuate p53 signaling by destabilizing p300, the growth rates of HFKs expressing HPV8 E6 or HPV Δ8 E6 were compared to those of control cells. In agreement with the hypothesis that β-HPV E6 expression increases late-passage growth rates independently of the destabilization of p300, HPV Δ8 E6-expressing HFKs (like HPV8 E6-expressing HFKs) grew faster than control cells at passage 20 (Fig. 2A). Unlike the increased tolerance of failed cytokinesis, the increased growth of late-passage cells is independent of p300 destabilization, suggesting that differences in proliferation are not a major factor in the observed β-HPV E6-associated increases in multinucleation.
HPV5 and HPV8 E6 expression does not reduce senescence in late-passage HFKs.
The proliferation of late-passage primary cells is prevented in part by the induction of senescence due to critically shortened telomeres (30). Previous work has shown that β-HPV E6 expression can weakly activate telomerase, the ribonucleoprotein that extends telomeres (22, 28). Importantly, there is a threshold of telomerase activation required to prevent senescence (31), but it has not been determined whether the induction of telomerase activation by β-HPV E6 expression exceeds this cutoff (28, 30). Because delayed senescence might explain the increased proliferation rates of late-passage β-HPV E6-expressing HFKs, control and HPV E6-expressing HFKs were stained for the expression of a senescence-associated β-galactosidase to measure the frequency of senescence as a function of passage number (32). As reported previously, HPV16 E6 expression significantly decreased the percentage of senescence-associated β-galactosidase-positive HFKs from that for control cells, both overall and at late passages (Fig. 2B and C) (26). HPV38 E6 expression also significantly reduced the percentage of senescent cells both overall and at late passages (Fig. 2B and C). In contrast, neither HPV5 E6 nor HPV8 E6 expression significantly diminished senescence from that for control cells (Fig. 2B and C). These data suggest that although HPV5 and HPV8 E6 expression is capable of feebly activating telomerase, this activation is not robust enough to prevent senescence. Indeed, the presence of senescent cells correlated inversely with the magnitude of reported telomerase activation (Table 2) (28, 31).
TABLE 2.
Reported hTERT activity correlates inversely with senescence-associated β-galactosidase staininga
| Vectorb | hTERT activationc | SA-Beta Gald staining in late-passage cells: |
|
|---|---|---|---|
| Overall | With 2+ nuclei | ||
| LXSN | − | +++ | +++ |
| HPV5 E6 | +/− | +++ | ++ |
| HPV8 E6 | + | +++ | ++ |
| HPV Δ8 E6 | ND | ++ | ++ |
| HPV38 E6 | ++ | + | + |
| HPV16 E6 | +++ | − | − |
Results are presented as follows: −, only background levels of staining/activity; +/−, a non-statistically significant increase in staining or activity; +, a small but significant increase in activity, or low-level staining; ++, a significant and moderate increase in staining, or intermediate activity; +++, a large and statistically significant increase in staining, or a high level of staining activity; ND, not determined.
SA-Beta Gal, senescence-associated β-galactosidase.
To determine if the propensity to undergo senescence differed in binucleated cells, the levels of senescence-associated β-galactosidase were measured in these cells. There was no significant difference in senescence-associated β-galactosidase staining between control cells that were binucleated and those that were not (Fig. 2B and D). As with the total population of cells, only HPV38 and HPV16 E6 expression significantly reduced senescence from that for control cells (Fig. 2D). Binucleated HFKs expressing HPV38 or HPV16 E6 were similarly less likely to undergo senescence in middle (p10 to p14) and late (p20+) passages (Fig. 2E). In contrast to the findings for the total population of cells, expression of HPV5 or HPV8 E6 significantly reduced the number of late-passage (p20+) binucleated keratinocytes that were β-galactosidase positive relative to that for control cells (Fig. 2E). As was the case with growth rates, HPV Δ8 E6-expressing cells did not differ significantly from HPV8 E6-expressing cells with respect to their propensity to senesce (Fig. 2B to E). Finally, as with the overall population of HFKs, the presence of senescent binucleated HPV E6-expressing HFKs also correlated inversely with the ability of the HPV protein to activate telomerase (Table 2). These data suggest that while the activation of human telomerase reverse transcriptase (hTERT) by β-HPV E6 expression may play a role in the increased late-passage proliferation of β-HPV E6-expressing cells, it is not the primary driver of the enhanced growth potential.
β-HPV E6 expression leads to abnormal centrosome duplication.
Because β-HPV E6 expression increases the tolerance of failed cytokinesis, an event that typically elicits a p53 response, it may also increase the prevalence of other, similarly regulated types of genomic instability, such as supernumerary centrosomes. Specifically, the expression of β-HPV E6 proteins may increase the frequency of cells with more than two centrosomes. To test this, control and HPV E6-expressing HFKs were stained for γ-tubulin (a central component of the centrosome [33]) as a means of determining the numbers of centrosomes in these cells. So that the quantification of centrosomes was not complicated by bi- or multinucleated cells, only cells with a single nucleus were used for this analysis. Additionally, to enable the observation of centrosomes in distinct cells, the cytoplasm of cells was marked by staining for α-tubulin. The frequency of cells with supernumerary centrosomes was significantly elevated by HPV5, HPV8, or HPV38 E6 expression (Fig. 3A and C). Not only were HPV5, -8, and -38 E6-expressing HFKs more often found to have more than 2 centrosomes, but the expression of these viral proteins also significantly increased the average number of centrosomes (Fig. 3B). More specifically, the expression of HPV5, -8, or -38 E6 significantly increased the likelihood that a cell would have 5 or more centrosomes (Fig. 3C). In agreement with previous reports, the frequency of supernumerary centrosomes and the abundance of centrosomes per cell were significantly increased by HPV16 E6 expression (Fig. 3A to C) (34).
FIG 3.
β-HPV E6 expression increases the frequency of cells with abnormal numbers of centrosomes. (A) Representative images of cells stained with α-tubulin (red) and γ-tubulin (indicating centrosomes) (green) for each cell line. (Insets) Enlargements of areas positively stained with γ-tubulin. (B) Mean number of centrosomes (from ≥3 independent experiments) per cell in each cell line. (C) Distribution of centrosomes in cells. At least three independent experiments were performed. Error bars show the standard errors of the means. Asterisks indicate data points that are significantly different (P, ≤0.05) from the result for the corresponding vector control cell line.
To determine if the tolerance of dysregulated centrosome duplication in β-HPV E6-expressing cells is dependent on the binding/destabilization of p300 and the subsequent attenuation of p53 signaling, the average number of centrosomes in HFKs expressing HPV Δ8 E6 was determined. HFKs expressing HPV Δ8 E6 were significantly less likely to have supernumerary centrosomes than HPV8 E6-expressing HFKs (Fig. 3A and B). Likewise, HPV Δ8 E6 expression resulted not only in a number of average centrosomes per cell similar to that in control HFKs but also in a comparable frequency of cells with at least 5 centrosomes (Fig. 3C).
Nuclear and centrosomal abnormalities fail to induce a robust p53 response in β-HPV E6-expressing HFKs.
These data demonstrate that the tolerance of events that typically induce p53-dependent growth arrest is also largely dependent on the residues of HPV E6 responsible for destabilizing p300, suggesting that p53 stabilization in response to the nuclear and centrosomal abnormalities may be abrogated. To test this hypothesis, binucleated HFKs, as well as HFKs with supernumerary centrosomes, were stained for p53. Levels of p53 were elevated in nearly 80% of binucleated control HFKs (Fig. 4A and B). In contrast, p53 levels were elevated in significantly fewer HPV5, -8, and -38 E6-expressing binucleated HFKs (Fig. 4A and B). Further, as expected, p53 staining was more intense in binucleated control cells than in the overall population of control cells (Fig. 4C). In contrast, HPV5, -8, and -38 E6-expressing binucleated HFKs had significantly less intense p53 staining than similar control cells (Fig. 4C).
FIG 4.
β-HPV E6-expressing cells fail to stabilize p53 in response to binucleation. (A) Representative images of binucleated cells with nuclei (blue) and p53 (pink) stained. (B) Percentage of binucleated cells that are positive for p53 in each cell line. At least three independent experiments were performed. Error bars show the standard errors of the means. Asterisks indicate data points that are significantly different (P, ≤0.05) from the result for the corresponding vector control cell line. (C) Mean intensity of p53 staining in binucleated cells and overall p53 staining for each cell line. At least 15 independent experiments were performed. (D) Representative images of multinucleated cells with nuclei (blue) and p53 (pink) stained. (E) Representative images of multinucleated cells transfected with either p300A or p300E as indicated. Nuclei (blue) and p53 (pink) were stained.
In support of the hypothesis that β-HPV E6 expression reduces p53 stabilization in response to failed cytokinesis by reducing the amount of p53-stabilizing enzymes in cells, p53 stabilization did not differ significantly between control and HPV Δ8 E6-expressing cells with respect to frequency or intensity (Fig. 3A to C). Although multinucleated control cells were not observed during our p53 staining analysis, a limited number of multinucleated HPV E6-expressing cells were seen. In agreement with the idea that β HPV E6 expression prevents both binucleation and multinucleation from triggering a p53 response, HPV5, HPV8, and HVP38 E6-expressing multinucleated HFKs did not have elevated p53 levels (Fig. 4D). However, p53 protein levels were increased in each of the multinucleated cells expressing HPV Δ8 E6 that we observed by immunofluorescence microscopy (Fig. 4D). As a control for these experiments, p53 levels were measured in bi- and multinucleated HFKs expressing HPV16 E6. As expected, none of these cells showed evidence of p53 stabilization (Fig. 4).
To further support the hypothesis that by reducing p300 (and as a result attenuating the expression of the p53-stabilizing enzymes ATM and ATR), β-HPV E6 expression reduces p53 stabilization in response to binucleation, transfections with p300E were used to restore p300 expression in cells expressing HPV5 or HPV8 E6. Following transfection with this p300 mutant, which is not destabilized by β-HPV E6 expression, p53 accumulation was seen in binucleated cells expressing HPV5 or HPV8 E6 (Fig. 4E). In contrast, expression of an inactivated p300 mutant (p300A) did not hinder β-HPV E6 expression from attenuating p53 accumulation in binucleated cells (Fig. 4E).
β-HPV E6 expression may similarly allow cells with an abnormal number of centrosomes to persist by preventing the stabilization of p53 that would typically accompany the presence of more than 2 centrosomes. Elevated p53 levels were detected in HFKs with more than 2 centrosomes. As with the initial quantification of centrosomes, both bi- and multinucleated cells were ignored in this analysis in order to avoid complications that might arise from two stimuli promoting the stabilization of p53. As anticipated, the presence of an irregular number of centrosomes induced robust stabilization of p53 in control cells (Fig. 5). Approximately 80% of mononucleated control HFKs with supernumerary centrosomes had increased p53 levels (Fig. 5B). Similarly, the intensity of p53 staining in these cells was double the overall mean p53 intensity of control cells (Fig. 5C). As was the case with binucleated cells, the expression of HPV5, HPV8, or HPV38 E6 in cells with more than 2 centrosomes was associated with significantly muted p53 stabilization (Fig. 5). In agreement with our hypothesis, p53 stabilization was robust in HPV Δ8 E6-expressing HFKs containing supernumerary centrosomes (Fig. 5). Additionally, p53 levels in HPV16 E6-expressing HFKs with more than 2 centrosomes were undetectable (Fig. 5).
FIG 5.
β-HPV E6 expression abrogates p53 stabilization in mononucleated cells with ≥3 centrosomes. (A) Representative images of cells with more than 2 centrosomes. Nuclei were stained with DAPI (blue). Immunofluorescence was used to detect p53 (pink) and γ-tubulin (indicating centrosomes) (green). (Insets) Magnifications of the γ-tubulin-stained portions of the images. (B) Percentage of cells with more than 2 centrosomes that stained positive for p53 in each cell line. At least three independent experiments were performed. Error bars show the standard errors of the means. Asterisks indicate data points that are significantly different (P, ≤0.05) from the result for the corresponding vector control cell line. (C) Mean intensity (from ≥15 independent experiments) of p53 staining in cells with 3 or more centrosomes and overall p53 staining intensity in each cell line. (D) Representative images of cells with more than 2 centrosomes transfected with p300A or p300E as indicated. Nuclei were stained with DAPI (blue). Immunofluorescence was used to detect p53 (pink) and γ-tubulin (indicating centrosomes) (green). (Insets) Magnifications of the γ-tubulin-stained portions of the images.
To further connect the lack of p53 accumulation in HPV5 or HPV8 E6-expressing cells with more than 2 centrosomes to the destabilization of p300 in these cells, control and HPV E6-expressing HFKs were transfected with p300 mutant expression vectors. Expression of an inactivated form of p300 (p300A) did not alter p53 accumulation in either control cells or cells expressing HPV5 or HPV8 E6 that had supernumerary centrosomes. In contrast, transfection with a p300E expression vector resulted in elevated p53 levels in cells with more than 2 centrosomes, regardless of whether these cells expressed HPV E6 or not.
HPV E6-expressing binucleated cells remain proliferatively active.
Typically, the stabilization of p53 in response to abnormally nucleated cells prevents these cells from proliferating (20). The diminished p53 response in binucleated HFKs expressing β-HPV E6 suggests that these cells may also remain proliferatively active and therefore may be capable of undergoing a second round of mitosis without cytokinesis, potentially explaining the increased prevalence of multinucleated β-HPV E6-expressing HFKs. To determine if β-HPV E6 expression allows some binucleated cells to avoid exiting the cell cycle, HFKs were stained for the proliferative marker Ki67. Although Ki67 expression is not an indication of how rapidly cells are moving through the cell cycle, it is expressed only in actively cycling cells (35). While Ki67 staining was observed in nearly all single-nucleated LXSN HFKs (data not shown), Ki67 was absent from all binucleated LXSN cells (Fig. 6A and B). In contrast, approximately half of the binucleated HFKs expressing HPV5, HPV8, or HPV38 E6 continued to express Ki67 (Fig. 6A and B). As a control, and in agreement with previously published observations (21), Ki67 was observed in binucleated cells expressing HPV16 E6 (Fig. 6A and B).
FIG 6.
Binucleated cells expressing β-HPV E6 continue to proliferate. (A) Representative images of binucleated cells stained with anti-α-tubulin (cytoplasm) and anti-Ki67 (proliferation) immunofluorescent antibodies. Nuclei were stained with DAPI. (B) Percentage of binucleated cells that were positive for Ki67 in each cell line. At least three independent experiments were performed. Error bars show the standard errors of the means. (C) Mean intensity (from ≥15 independent experiments) of Ki67 staining in binucleated cells as well as in the total cell population for each cell line. Asterisks indicate data points that are significantly different (P, ≤0.05) from the result for the corresponding vector control cell line. (D) Representative images of binucleated cells transfected with p300A or p300E as indicated and stained with anti-α-tubulin (cytoplasm) and anti-Ki67 (proliferation) immunofluorescent antibodies. Nuclei were stained with DAPI.
To determine if the ability of HPV8 E6 to prevent binucleated HFKs from exiting the cell cycle was dependent on its ability to attenuate the steady-state levels of enzymes that modify p53 in response to genomic instability, binucleated cells expressing HPV Δ8 E6 were stained for Ki67. In support of this explanation, no binucleated HFKs expressing HPV Δ8 E6 expressed Ki67 (Fig. 6A and B). Additionally, while the mean intensity of Ki67 staining in binucleated HFKs expressing HPV5, HPV8, or HPV38 E6 was significantly higher than that in vector control cells, this was not the case for binucleated HPV Δ8 E6 cells (Fig. 6C).
To further connect β-HPV E6-mediated p300 reduction to increased tolerance of binucleation, control HFKs and HFKs expressing HPV5 or HPV8 E6 were transfected with mutant p300 expression vectors (p300A or p300E). No Ki67 staining was seen in control binucleated cells transfected with either vector (Fig. 6D). As anticipated, HPV5 and HPV8 E6-expressing cells transfected with the inactivated form of p300 (p300A) continued to express Ki67 even if they contained more than one nucleus. However, if p300 expression was restored in cells expressing HPV5 or HPV8 E6 by transfection with p300E, Ki67 staining was no longer seen in binucleated HFKs expressing HPV5 or HPV8 E6.
HFKs expressing β-HPV E6 remain proliferatively active despite abnormal numbers of centrosomes.
As is the case with cells that fail to undergo cytokinesis, dysregulated centrosome duplication also results in the stabilization of p53, which removes cells from the cell cycle (20). Since the expression of HPV5, HPV8, and HPV38 E6 proteins abrogates the accumulation of p53, these cells may continue to progress through the cell cycle. To evaluate this possibility, Ki67 staining was observed in HFKs with more than 2 centrosomes. As anticipated, in control cells with an abnormal number of centrosomes, Ki67 expression was reduced (Fig. 7A and B). In contrast, the expression of HPV5, -8, or -38 E6 significantly increased the frequency of cells that stained positive for Ki67 despite the presence of supernumerary centrosomes (Fig. 7A and B). Furthermore, the intensity of Ki67 staining was significantly reduced in control cells with more than 2 centrosomes, while Ki67 staining remained robust in cells expressing HPV5, -8, or -38 E6 (Fig. 7C). Finally, the ability of HFKs expressing β-HPV E6 to remain proliferatively active despite aberrant centrosome duplication is likely dependent on the ability to disrupt p53 stabilization, since no HPV Δ8 E6-expressing cells with more than 2 centrosomes expressed Ki67 (Fig. 6). As a control and in confirmation of previous reported data (21), levels of Ki67 in HPV16 E6-expressing cells that had undergone dysregulated centrosome duplication were found to be elevated.
FIG 7.
β-HPV E6 expression allows cells with more than 2 centrosomes to proliferate. (A) Representative images of cells with supernumerary centrosomes. DNA was stained with DAPI. Anti-γ-tubulin (centrosomes) and anti-Ki67 (proliferation) immunofluorescent antibodies were used to mark centrosomes. (Insets) Higher magnifications of the portions of the images stained with anti-γ-tubulin (centrosomes). (B) Frequency of positive staining for Ki67 expression among cells with 3+ centrosomes. At least 15 independent experiments were performed. Error bars show standard errors of the means. Asterisks indicate data points that are significantly different (P, ≤0.05) from the result for the corresponding vector control cell line. (C) Mean Ki67 staining intensity (from ≥15 independent experiments) both in the total cell population and in cells with supernumerary centrosomes. (D) Representative images of normal mitotic cells (cells carrying LXSN alone or expressing HPV Δ8 E6) and multipolar mitotic cells (cells expressing HPV5, -8, -38, or -16 E6). (E) Representative images of cells with supernumerary centrosomes transfected with p300A or p300E as indicated. DNA was stained with DAPI. Anti-γ-tubulin (centrosomes) and anti-Ki67 (proliferation) immunofluorescent antibodies were used to mark centrosomes. (Insets) Higher magnifications of the portions of the images stained with anti-γ-tubulin (centrosomes).
The presence of supernumerary centrosomes in proliferating cells can lead to multipolar mitosis, almost guaranteeing unequal segregation of chromosomes between daughter cells (36). Because HPV5, HPV8, and HPV38 E6 expression allows cells with more than 2 centrosomes to continue active progression through the cell cycle, β-HPV E6 expression may result in the formation of multipolar mitotic figures. To test this premise, β-HPV E6-expressing cells with more than 2 centrosomes that were undergoing mitosis were observed. While no multipolar control cells were observed, a fraction of the HPV5, -8, and -38 E6-expressing cells that contained supernumerary centrosomes formed multipolar mitotic figures (5 to 10%) (Fig. 7D and data not shown). Furthermore, because HPV Δ8 E6-expressing HFKs do not remain proliferatively active following aberrant centrosome duplication, HFKs expressing this mutant HPV8 E6 are not expected to undergo multipolar mitosis. As predicted, no multipolar mitotic figures were observed in HPV Δ8 E6-expressing cells (Fig. 7D). Further supporting the hypothesis that the ability to prevent p53 stabilization was at the root of multipolar mitosis in cells expressing β-HPV E6 proteins, we observed multipolar mitotic figures in a proportion of HPV16 E6-expressing cells containing more than 2 centrosomes (Fig. 7D).
To provide more support for the hypothesis that β-HPV E6 expression increases the tolerance of supernumerary centrosomes by reducing the expression of p53-stabilizing enzymes through the destabilization of p300, control HFKs and HFKs expressing HPV5 or HPV8 E6 were transfected with one of two mutant p300 expression vectors (p300A and p300E). p300 expression was confirmed by immunofluorescence microscopy (data not shown). While control cells with more than 2 centrosomes did not exhibit Ki67 staining when they were transfected with either expression vector, HPV5 and -8 E6-expressing HFKs with more than 2 centrosomes continued to express Ki67 when transfected with an inactive mutant of p300 (p300A) (Fig. 7E). In contrast, these β-HPV E6-expressing cells with supernumerary centrosomes did not express Ki67 when transfected with a p300 mutant that is resistant to β-HPV E6-mediated destabilization (p300E) (Fig. 7E).
Binucleated HFKs expressing β-HPV E6 can undergo multiple rounds of mitosis absent cytokinesis.
The reduced p53 protein levels and increased Ki67 protein levels in binucleated cells expressing HPV E6 suggest that these cells continue to proliferate, increasing the probability that a binucleated cell becomes a multinucleated cell. To directly test whether a binucleated cell expressing HPV E6 could undergo a second round of replication without dividing, a size filtration system was used to obtain a population of HFKs enriched for larger binucleated cells. These cells were then observed for 4 days using the IncuCyte live-cell imaging system, which obtained images of multiple fields every half hour without removal of the cells from the incubator. These images were then combined to generate time lapse videos of our enriched populations of binucleated cells.
The videos showed >700 binucleated cells for each cell line (HFKs carrying LXSN alone or expressing HPV5, HPV8, HPV38, or HPV16 E6), and <2% of these cells entered mitosis (as determined by mitotic figure formation [data not shown]). For the 12 control binucleated HFKs that did enter mitosis, all of the mitotic figures resolved into two cells, each with a single nucleus (Table 3). While the majority of the mitotic figures formed by binucleated HFKs expressing HPV E6 likewise resolved into two single-nucleated cells, a portion of these cells underwent an additional round of replication but once again failed to divide, becoming multinucleated cells (Table 3). Mitotic figures formed by binucleated cells expressing HPV5, -38, or -16 E6 also on occasion resolved into two binucleated cells (Table 3).
TABLE 3.
Replication of binucleated cellsa
| Vector | No. of mitotic figures with the indicated outcome/no. analyzed (%) |
||
|---|---|---|---|
| 2 Single nucleated cells | 2 Binucleated cells | Failed cytokinesisb | |
| LXSN | 12/12 (100) | 0/12 (0) | 0/12 (0) |
| HPV5 E6 | 13/19 (68) | 4/19 (21) | 2/19 (11) |
| HPV8 E6 | 3/4 (75) | 0/4 (0) | 1/4 (25) |
| HPV Δ8 E6 | 12/12 (100) | 0/12 (0) | 0/12 (0) |
| HPV38 E6 | 7/9 (77) | 1/9 (11) | 1/9 (11) |
| HPV16 E6 | 18/43 (42) | 8/43 (19) | 17/43 (39) |
From analysis of a video of live binucleated cells that formed mitotic figures.
Mitotic figures were resolved into a single cell, often containing visibly more nucleic material than normal.
The ability of some binucleated HFKs expressing HPV8 E6 to form multinucleated cells can be explained by the ability of HPV8 E6 expression to abrogate p53 stabilization and, as a result, to bypass the checkpoint that typically prevents binucleated cells from progressing through the cell cycle. To test this hypothesis, the resolution of mitotic figures in binucleated HFKs expressing HPV Δ8 E6 was observed. As with the other cell lines, very few (<2%) binucleated cells expressing HPV Δ8 E6 formed mitotic figures (data not shown). Like control cells, when binucleated HPV Δ8 E6-expressing cells did form mitotic figures, they were resolved into two single-nucleated cells (Table 3).
DISCUSSION
β-HPVs may play a role in the development of some skin cancers (5–9), but since few viral genomes are found in these tumors, they are not required for tumor maintenance. Instead, β-HPV infections may destabilize the host genome, increasing the carcinogenic potential of the cell and allowing a tumor to develop in the absence of the viral genome. In support of this hypothesis, we have shown previously that by binding and destabilizing p300, certain β-HPV E6 proteins are capable of attenuating p53 phosphorylation and acetylation in response to damaged DNA (10, 11, 13). The reduction in p53 signaling then leads to inefficient DNA damage repair. Interestingly, although the stabilizing modifications of p53 are reduced in cells expressing β-HPV E6, p53 stabilization remains largely unperturbed in response to UV damage. In contrast, we show here that the expression of HPV5, HPV8, and HPV38 E6 proteins prevents robust stabilization of p53 in response to failed cytokinesis and dysregulated centrosome duplication (Fig. 4 and 5). Lack of p53 stabilization correlates with increased tolerance of the resulting abnormalities by HFKs, allowing these cells to remain proliferatively active; the abnormalities accumulate with passaging and increase the likelihood of a second round of aberrant mitosis (Fig. 1D and E, 3C, 6, and 7; Table 3). This increased tolerance is dependent on the ability of β-HPV E6 to disrupt p53 signaling by promoting p300 degradation, since mutations either of the viral protein or of p300 that prevent p300 destabilization by β-HPV E6 expression abolish the ability of HPV8 E6 to alter p53 stabilization or cellular tolerance in response to binucleation/supernumerary centrosomes (Fig. 4 to 7). Although the increased multinucleation in HFKs expressing β-HPV E6 is greatest at high passages, this is not due to proliferation differences between these cells and control cells, since HPV Δ8 E6 expression induces late-passage proliferation but does not result in increased multinucleation. Further, the accelerated growth associated with β-HPV E6 expression is also largely independent of delayed senescence, since the ability of HPV E6 expression to postpone senescence does not correlate with the viral protein's ability to promote proliferation (Fig. 2A to C). Together, these data add to the understanding of the mechanisms of β-HPV-associated genomic instability.
β-HPV E6 expression and genomic instability.
Here we present data demonstrating that β-HPV E6 expression is destabilizing to the host genome in at least two ways: by increased tolerance of failed cleavage furrow regression and of supernumerary centrosomes. Work by Shi and King (18) shows that chromosome disjunction and cytokinesis are closely tied and that as a result, binucleated cells are most often formed as a result of chromosome nondisjunction. Further, they suggest that nondisjunction/furrow regression can serve as a precursor to aneuploidy should these tetraploid cells undergo multipolar mitosis. Indeed, our data corroborate their findings, since we see a correlation between an increased frequency of cells that have undergone these irregular mitoses and the prevalence of cells with >4N DNA content (Fig. 1). Further, these observations support the hypothesis that β-HPV infections can promote the potentially oncogenic destabilization of the host cell's genome, since both polyploidy and aneuploidy are forerunners to carcinogenesis. Not only does β-HPV E6 expression increase the frequency of polyploid cells; our data demonstrate that cells expressing β-HPV E6 can also undergo multipolar mitosis. The importance of this observation stems from the fact that the presence of supernumerary centrosomes does not necessitate multipolar mitosis, since centrosomes can cluster during the mitosis of cancer cells and, as a result, avoid this deleterious division (37).
β-HPV E6 expression attenuates p53 stabilization in response to binucleation and supernumerary centrosomes.
Although β-HPV E6 expression can attenuate p53 modifications in response to DNA damage, our lab and others have shown that p53 is still robustly stabilized in response to DNA lesions (27, 38). In contrast, here we present data demonstrating that the stabilization of p53 in response to either failed cytokinesis or dysregulated centrosome duplication can be abrogated by HPV5, HPV8, and HPV38 E6 expression. Further, the inability to stabilize p53 in response to these abnormalities has functional consequences; these cells continue to proliferate and therefore undergo further genome-destabilizing events. The attenuation of p53 stabilization in these cases is dependent on the destabilization of p300 by β-HPV E6 expression, since mutations of either HPV8 E6 or p300 that prevent the viral protein from degrading p300 also inhibit its ability to abrogate both p53 stabilization and the removal of the cells from active cycling. Therefore, our data show that the ability of β-HPV E6 expression to abrogate or attenuate p53 signaling/stabilization differs depending on the stimulus that is inducing p53 activation. Further, the implications of these data extend past HPV biology and suggest that the responses of p53-activating enzymes, such as p300, ATM, and ATR, may differ depending on the stimulus. Specifically, since low levels of these enzymes remain in β-HPV E6-expressing cells, our data suggest that close to optimal expression of the proteins is required for responses to certain events (cleavage furrow regression or dysregulated centrosome duplication), while a minimal amount of the enzymes can support p53 stabilization in response to other events (DNA damage).
p300 plays a key role in protecting the human genome by maintaining mitotic integrity.
It has been shown previously that a p53-dependent checkpoint protects cells from binucleation and aberrant centrosome duplication (15, 17). Supporting this hypothesis are the observations by Duensing et al. that expression of HR α-HPV E6 can abrogate these checkpoints by targeting p53 for degradation (21). Here, using a mutant HPV8 E6 (HPV Δ8 E6), we infer that other cellular signaling enzymes, namely, ATR, ATM, and p300, are also important for proper checkpoint activation in response to polyploidy as well as supernumerary centrosomes. Our report expands previous work by Ha et al. connecting p300 to the regulation of mitosis by demonstrating that partial reductions in the levels of three enzymes (ATM, ATR, and p300) significantly increase the tolerance of failed cytokinesis and dysregulated centrosome duplication (39). Because we have shown previously that p300 is required for optimal ATM and ATR expression, this work extends the importance of p300 for promoting genomic stability beyond its role in DNA damage repair (10, 13).
Further highlighting the importance of p300 for maintaining the integrity of the genome, we found that binucleated cells can be “eliminated” by division into two single cells, each with one nucleus (Table 3). While these elimination events occur in control HFKs as well as in cells expressing HPV5, -8, or -38 E6, they occur less frequently when p300 protein levels are reduced by β-HPV E6 expression (Table 3). Indeed, all of the control and HPV Δ8 E6-expressing binucleated HFKs that formed mitotic figures resolved these figures into two cells with one nucleus apiece. Conversely, approximately 25% of the mitotic figures formed by binucleated HPV5, -8, and -38 E6-expressing HFKs were not resolved in this manner. While our live-cell imaging data cannot address the ploidy of the cells derived from such an elimination event, they do suggest that p300 plays a role in a mechanism that reduces the frequency of binucleated cells. It should be noted that in some assays (Fig. 1D), an HPV8 E6 protein unable to destabilize p300 was nevertheless capable of increasing the frequency of polyploidy, suggesting that the viral protein can contribute to genomic instability in a p300-independent manner.
β-HPV infection may drive the proliferation of old cells, leading to an elevated cancer risk.
The reduced ability of older cells to proliferate minimizes the propagation of the genomic instability that cells accumulate as they age. We show that β-HPV E6 expression increases the growth rate of these late-passage cells. If a β-HPV infected a cell that would otherwise have a diminished ability to divide due to advanced age, the infection could potentially drive the otherwise quiescent cell back into active cell cycle progression (Fig. 2). The renewed propagation of this cell would spread any potentially oncogenic mutations the cell had acquired to its daughter cells, providing another way in which the expression of β-HPV E6 can destabilize the human genome. Despite our previous reports that β-HPV E6 can weakly activate hTERT, we found that the same β-HPV E6 proteins were unable to delay aging-associated senescence (28). Further, the growth advantages of older cells expressing β-HPV E6 are not dependent on the viral protein's ability to destabilize p300; a mutant abrogating this ability had no significant effect on the growth of late-passage cells. Finally, the ability to promote cellular proliferation also further confirms previous reports that deletion of the p300 binding site in HPV8 E6 does not result in gross misfolding or inactivation of the viral protein (41).
Together, these data provide new insight into how β-HPV infections, while not persisting in tumors, might contribute to the development of nonmelanoma skin cancers by introducing/propagating mutations that create a cellular environment conducive to tumorigenesis in the absence of the viral genome. Previously, we reported that p53 phosphorylation and ubiquitination, but not stabilization, in response to UVB-induced DNA damage were attenuated by some β-HPV E6 proteins (10). Here we show that HPV5, -8, or -38 E6 expression significantly reduces the stabilization of p53 in response to binucleation and supernumerary centrosomes (Fig. 4 and 5). The resulting increased tolerance of these abnormalities significantly increases the occurrence of polyploidy (Fig. 1 and 3; Table 3). Further, the elevated frequency of supernumerary centrosomes in cells that express β-HPV E6 proteins suggests an increased likelihood of aneuploidy from multipolar mitotic events such as those we describe. Ultimately, these novel genome-destabilizing phenotypes associated with β-HPV E6 expression support the hypothesis that β-HPV infections may be able to contribute to the development of skin cancers by creating an environment conducive to tumor formation.
ACKNOWLEDGMENTS
We acknowledge and thank Julio Vazquez Lopez and the members of the FHCRC Scientific Imaging Facility for assistance with our immunofluorescence imaging. Furthermore, Andrew Berger and the staff of the FHCRC Flow Cytometry Facility were very helpful with our FACS analysis. Additionally, we thank Toshi Taniguchi and members of his lab for helpful suggestions and advice over the course of this project. We also thank the current and former members of the Galloway lab for assistance, encouragement, and advice during the implementation of this research.
Finally, this work would not have been possible without financial support from P01 CA 042792 (to D.A.G.) and NIH research training grant T32 CA009657 (to N.A.W.).
Footnotes
Published ahead of print 19 March 2014
REFERENCES
- 1.Bernard HU, Burk RD, Chen Z, van Doorslaer K, Hausen H, de Villiers EM. 2010. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 401:70–79. 10.1016/j.virol.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.zur Hausen H. 1999. Papillomaviruses in human cancers. Proc. Assoc. Am. Physicians 111:581–587. 10.1046/j.1525-1381.1999.99723.x [DOI] [PubMed] [Google Scholar]
- 3.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F. 2005. Carcinogenicity of human papillomaviruses. Lancet Oncol. 6:204. 10.1016/S1470-2045(05)70086-3 [DOI] [PubMed] [Google Scholar]
- 4.Lorincz AT, Reid R, Jenson AB, Greenberg MD, Lancaster W, Kurman RJ. 1992. Human papillomavirus infection of the cervix: relative risk associations of 15 common anogenital types. Obstet. Gynecol. 79:328–337. 10.1097/00006250-199203000-00002 [DOI] [PubMed] [Google Scholar]
- 5.Orth G, Jablonska S, Jarzabek-Chorzelska M, Obalek S, Rzesa G, Favre M, Croissant O. 1979. Characteristics of the lesions and risk of malignant conversion associated with the type of human papillomavirus involved in epidermodysplasia verruciformis. Cancer Res. 39:1074–1082 [PubMed] [Google Scholar]
- 6.Bouwes Bavinck JN, Feltkamp M, Struijk L, ter Schegget J. 2001. Human papillomavirus infection and skin cancer risk in organ transplant recipients. J. Investig. Dermatol. Symp. Proc. 6:207–211. 10.1046/j.0022-202x.2001.00048.x [DOI] [PubMed] [Google Scholar]
- 7.Lutzner MA, Blanchet-Bardon C, Orth G. 1984. Clinical observations, virologic studies, and treatment trials in patients with epidermodysplasia verruciformis, a disease induced by specific human papillomaviruses. J. Investig. Dermatol. 83:18s–25s. 10.1111/1523-1747.ep12281128 [DOI] [PubMed] [Google Scholar]
- 8.Pfister H. 1992. Human papillomaviruses and skin cancer. Semin. Cancer Biol. 3:263–271 [PubMed] [Google Scholar]
- 9.Akgül B, Cooke JC, Storey A. 2006. HPV-associated skin disease. J. Pathol. 208:165–175. 10.1002/path.1893 [DOI] [PubMed] [Google Scholar]
- 10.Wallace NA, Robinson K, Howie HL, Galloway DA. 2012. HPV 5 and 8 E6 abrogate ATR activity resulting in increased persistence of UVB induced DNA damage. PLoS Pathog. 8:e1002807. 10.1371/journal.ppat.1002807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howie HL, Koop JI, Weese J, Robinson K, Wipf G, Kim L, Galloway DA. 2011. Beta-HPV 5 and 8 E6 promote p300 degradation by blocking AKT/p300 association. PLoS Pathog. 7:e1002211. 10.1371/journal.ppat.1002211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu W, Roeder RG. 1997. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90:595–606. 10.1016/S0092-8674(00)80521-8 [DOI] [PubMed] [Google Scholar]
- 13.Wallace NA, Gasior SL, Faber ZJ, Howie HL, Deininger PL, Galloway DA. 2013. HPV 5 and 8 E6 expression reduces ATM protein levels and attenuates LINE-1 retrotransposition. Virology 443:69–79. 10.1016/j.virol.2013.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinmura K, Bennett RA, Tarapore P, Fukasawa K. 2007. Direct evidence for the role of centrosomally localized p53 in the regulation of centrosome duplication. Oncogene 26:2939–2944. 10.1038/sj.onc.1210085 [DOI] [PubMed] [Google Scholar]
- 15.Tarapore P, Fukasawa K. 2002. Loss of p53 and centrosome hyperamplification. Oncogene 21:6234–6240. 10.1038/sj.onc.1205707 [DOI] [PubMed] [Google Scholar]
- 16.Tarapore P, Horn HF, Tokuyama Y, Fukasawa K. 2001. Direct regulation of the centrosome duplication cycle by the p53–p21Waf1/Cip1 pathway. Oncogene 20:3173–3184. 10.1038/sj.onc.1204424 [DOI] [PubMed] [Google Scholar]
- 17.Stukenberg PT. 2004. Triggering p53 after cytokinesis failure. J. Cell Biol. 165:607–608. 10.1083/jcb.200405089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Q, King RW. 2005. Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature 437:1038–1042. 10.1038/nature03958 [DOI] [PubMed] [Google Scholar]
- 19.Shen Y, White E. 2001. p53-dependent apoptosis pathways. Adv. Cancer Res. 82:55–84. 10.1016/S0065-230X(01)82002-9 [DOI] [PubMed] [Google Scholar]
- 20.Agarwal ML, Agarwal A, Taylor WR, Stark GR. 1995. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 92:8493–8497. 10.1073/pnas.92.18.8493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duensing S, Duensing A, Crum CP, Munger K. 2001. Human papillomavirus type 16 E7 oncoprotein-induced abnormal centrosome synthesis is an early event in the evolving malignant phenotype. Cancer Res. 61:2356–2360 http://cancerres.aacrjournals.org/content/61/6/2356.long [PubMed] [Google Scholar]
- 22.Greider CW. 1990. Telomeres, telomerase and senescence. Bioessays 12:363–369. 10.1002/bies.950120803 [DOI] [PubMed] [Google Scholar]
- 23.Moorad JA, Promislow DE. 2008. A theory of age-dependent mutation and senescence. Genetics 179:2061–2073. 10.1534/genetics.108.088526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayflick L, Moorhead PS. 1961. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25:585–621. 10.1016/0014-4827(61)90192-6 [DOI] [PubMed] [Google Scholar]
- 25.Malanchi I, Accardi R, Diehl F, Smet A, Androphy E, Hoheisel J, Tommasino M. 2004. Human papillomavirus type 16 E6 promotes retinoblastoma protein phosphorylation and cell cycle progression. J. Virol. 78:13769–13778. 10.1128/JVI.78.24.13769-13778.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klingelhutz AJ, Foster SA, McDougall JK. 1996. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 380:79–82. 10.1038/380079a0 [DOI] [PubMed] [Google Scholar]
- 27.Underbrink MP, Howie HL, Bedard KM, Koop JI, Galloway DA. 2008. E6 proteins from multiple human betapapillomavirus types degrade Bak and protect keratinocytes from apoptosis after UVB irradiation. J. Virol. 82:10408–10417. 10.1128/JVI.00902-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bedard KM, Underbrink MP, Howie HL, Galloway DA. 2008. The E6 oncoproteins from human betapapillomaviruses differentially activate telomerase through an E6AP-dependent mechanism and prolong the lifespan of primary keratinocytes. J. Virol. 82:3894–3902. 10.1128/JVI.01818-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang WC, Chen CC. 2005. Akt phosphorylation of p300 at Ser-1834 is essential for its histone acetyltransferase and transcriptional activity. Mol. Cell. Biol. 25:6592–6602. 10.1128/MCB.25.15.6592-6602.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harley CB, Vaziri H, Counter CM, Allsopp RC. 1992. The telomere hypothesis of cellular aging. Exp. Gerontol. 27:375–382. 10.1016/0531-5565(92)90068-B [DOI] [PubMed] [Google Scholar]
- 31.Klingelhutz AJ, Barber SA, Smith PP, Dyer K, McDougall JK. 1994. Restoration of telomeres in human papillomavirus-immortalized human anogenital epithelial cells. Mol. Cell. Biol. 14:961–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. U. S. A. 92:9363–9367. 10.1073/pnas.92.20.9363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiese C, Zheng Y. 2006. Microtubule nucleation: gamma-tubulin and beyond. J. Cell Sci. 119:4143–4153. 10.1242/jcs.03226 [DOI] [PubMed] [Google Scholar]
- 34.Duensing S, Munger K. 2002. Human papillomaviruses and centrosome duplication errors: modeling the origins of genomic instability. Oncogene 21:6241–6248. 10.1038/sj.onc.1205709 [DOI] [PubMed] [Google Scholar]
- 35.Scholzen T, Gerdes J. 2000. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 182:311–322. [DOI] [PubMed] [Google Scholar]
- 36.Gergely F, Basto R. 2008. Multiple centrosomes: together they stand, divided they fall. Genes Dev. 22:2291–2296. 10.1101/gad.1715208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quintyne NJ, Reing JE, Hoffelder DR, Gollin SM, Saunders WS. 2005. Spindle multipolarity is prevented by centrosomal clustering. Science 307:127–129. 10.1126/science.1104905 [DOI] [PubMed] [Google Scholar]
- 38.Jackson S, Harwood C, Thomas M, Banks L, Storey A. 2000. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev. 14:3065–3073. 10.1101/gad.182100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ha GH, Kim HS, Lee CG, Park HY, Kim EJ, Shin HJ, Lee JC, Lee KW, Lee CW. 2009. Mitotic catastrophe is the predominant response to histone acetyltransferase depletion. Cell Death Differ. 16:483–497. 10.1038/cdd.2008.182 [DOI] [PubMed] [Google Scholar]
- 40.Liu L, Scolnick DM, Trievel RC, Zhang HB, Marmorstein R, Halazonetis TD, Berger SL. 1999. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol. Cell. Biol. 19:1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Müller-Schiffmann A, Beckmann J, Steger G. 2006. The E6 protein of the cutaneous human papillomavirus type 8 can stimulate the viral early and late promoters by distinct mechanisms. J. Virol. 80:8718–8728. 10.1128/JVI.00250-06 [DOI] [PMC free article] [PubMed] [Google Scholar]