ABSTRACT
Because of its very low human seroprevalence, vesicular stomatitis virus (VSV) has promise as a systemic oncolytic agent for human cancer therapy. However, as demonstrated in this report, the VSV infectious titer drops by 4 log units during the first hour of exposure to nonimmune human serum. This neutralization occurs relatively slowly and is mediated by the concerted actions of natural IgM and complement. Maraba virus, whose G protein is about 80% homologous to that of VSV, is relatively resistant to the neutralizing activity of nonimmune human serum. We therefore constructed and rescued a recombinant VSV whose G gene was replaced by the corresponding gene from Maraba virus. Comparison of the parental VSV and VSV with Maraba G substituted revealed nearly identical host range properties and replication kinetics on a panel of tumor cell lines. Moreover, in contrast to the parental VSV, the VSV with Maraba G substituted was resistant to nonimmune human serum. Overall, our data suggest that VSV with Maraba G substituted should be further investigated as a candidate for human systemic oncolytic virotherapy applications.
IMPORTANCE Oncolytic virotherapy is a promising approach for the treatment of disseminated cancers, but antibody neutralization of circulating oncolytic virus particles remains a formidable barrier. In this work, we developed a pseudotyped vesicular stomatitis virus (VSV) with a glycoprotein of Maraba virus, a closely related but serologically distinct member of the family Rhabdoviridae, which demonstrated greatly diminished susceptibility to both nonimmune and VSV-immune serum neutralization. VSV with Maraba G substituted or lentiviral vectors should therefore be further investigated as candidates for human systemic oncolytic virotherapy and gene therapy applications.
INTRODUCTION
We are interested in developing an oncolytic therapy for systemic treatment of multiple myeloma (MM), an incurable disseminated cancer of antibody-secreting plasma cells primarily localized in the bone marrow, which is characterized by the development of progressive and destructive osteolytic bone disease (1–4). Despite advances in diagnosis and treatment and improvements in patient survival, MM remains an incurable disease (2, 5–9). Therefore, development of novel alternative approaches, such as virotherapy, is needed for treatment of relapsed or refractory multiple myeloma.
Oncolytic viruses have shown potential for the treatment of a variety of cancers (10–16), and we are developing vesicular stomatitis virus (VSV) as an oncolytic agent for treatment of MM. VSV is a Vesiculovirus of the family Rhabdoviridae with a negative-sense RNA genome (16, 17). VSV is a preferred candidate as a platform for oncolytic virus development against a variety of cancers (10, 16–27), primarily due to its very broad tropism infecting a wide variety of animals and different cells, its short replication cycle, and high sensitivity to host interferon-mediated antiviral activity (28–35). Tumor-selective tropism can be further enhanced by mutating the M protein or engineering the virus to encode beta interferon (IFN-β). These engineered versions of VSV are highly effective in certain mouse cancer models, showing a good therapeutic ratio with efficacy at doses not associated with neurotoxicity, even with an intravenous route of administration (10, 18, 24, 31–34, 36, 37).
However, previous reports have claimed that VSV is neutralized by nonimmune human serum (38, 39). This could potentially diminish or negate the benefit of systemic therapy for human cancer. We therefore sought to better characterize the phenomenon, looking at the VSV-neutralizing capacities of nonimmune sera from nonhuman species, the kinetics of virus neturalization, the mechanism of infectivity neutralization, and the relative susceptibilities of VSV and Maraba virus, a closely related vesiculovirus family member that has also demonstrated oncolytic potential (40, 41).
Here, we show that nonimmune serum of human, mouse, or dog origin neutralizes VSV. Using human and/or mouse serum, we show that the serum-mediated anti-VSV activity depends on IgM antibody and complement components of serum. In addition, we show that serum samples from cancer patients differ in their levels of anti-VSV neutralizing activity, e.g., MM patient serum possesses lower VSV-neutralizing activity than sera from healthy subjects or ovarian cancer patients. Interestingly, we also show that when pseudotyped with Maraba virus G glycoprotein, VSV retains its parental cell tropism and growth kinetics. More importantly, in contrast to the parental VSV, the pseudotyped VSV demonstrated considerable resistance to neutralization by nonimmune serum.
MATERIALS AND METHODS
Reagents.
Monoclonal antibodies against low-density lipoprotein receptor (LDLR) (6E2) were kind gifts from Ross Milne, Diabetes and Atherosclerosis Laboratory, University of Ottawa Heart Institute, Ottawa, Canada. Monoclonal antibodies against CD46 were kind gifts from Roberto Cattaneo, Department of Molecular Medicine, Mayo Clinic, Rochester, MN.
Viruses.
VSV expressing green fluorescent protein (VSV-GFP) (strain Indiana), constructed by insertion of the GFP gene at XhoI/NheI restriction sites between the G and L viral genes, was provided by Glen N. Barber (University of Miami School of Medicine, Miami, FL) (31). The Mayo Clinic vector core manufactured a preclinical-grade oncolytic VSV-GFP. Maraba virus that expresses GFP, inserted between the G and L viral genes, was obtained from David Stojdl (Children's Hospital of Eastern Ontario Research Institute, Ontario, Canada) (41). VSV/Maraba G-GFP, VSV with its G gene replaced with the G gene of Maraba virus (this study), and measles virus encoding GFP (MV-GFP) were also used.
Cells.
Human 293T cells (ATCC CRL-1573) and African green monkey kidney Vero cells (ATCC CCL-81) were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO) for 293T cells or 5% FBS for Vero cells and with penicillin and streptomycin (Gibco). MPC-11 murine myeloma (ATCC CCL-167), TRAMP-C1 (ATCC CRL-2730), and 4T1 (ATCC CRL-2539) cells were obtained from the American Type Culture Collection (Manassas, VA). LM-1 murine ovarian cancer cells were obtained from A. Al-Hendy, University of Saskatchewan. All of the above-mentioned cells were cultured in DMEM (Mediatech, Herndon, VA) supplemented with 10% FBS. K562 human erythroleukemia cells were a kind gift from S. Blystone (Upstate Medical University, Syracuse, NY). K562 cells were maintained in Iscove's modified Dulbecco's medium (IMDM) (Life Technologies, NY) supplemented with 10% FBS, 0.5 U/liter penicillin-streptomycin, and 2 mM l-glutamine.
Recombinant VSV generation.
Recombinant VSV/Maraba G was generated as follows. First, Maraba G was amplified from Maraba virus with primers VSV/Mar-F, 5′ GAGATCGATCTGTTTACGCGTCACTAT 3′, and VSV/Mar-1R, 5′ AATCTGTTGTGCAGGATTTGAGTTATT 3′. Also, the VSV intergenic region was amplified from VSV using VSV/Mar-2F, 5′ GAGTCGATTGGGAAATAAATAACTCAA 3′, and GFP68R, 5′ GCTGAACTTGTGGCCGTTTA 3′. An overlapping PCR using primers VSV/Mar-F and GFP68R was performed. The overlapping-PCR product was double digested with MluI and AvrII and cloned into a VSV-GFP plasmid vector, replacing VSV G with Maraba G. Positive clones were selected and confirmed by sequencing analysis for proper insertion of Maraba G in place of VSV G.
Recombinant VSV/Maraba G production.
Recovery of recombinant VSV was performed following the method of Lawson et al. (42). Briefly, BHK cells were plated at a density of 1 × 106 cells/well in 6-well plates. The cells were infected with vaccinia virus encoding T7 polymerase at a multiplicity of infection (MOI) of 10. After an hour, excess vaccinia virus was removed, and the cells were transfected with 1 μg pVSV/Maraba G or parental pVSV, 0.5 μg pN, 0.4 μg pP, and 0.2 μg pL (the N, P, and L plasmids were constructed in the pCI vector) using 6.25 μl of Lipofectamine LTX transfection reagent (Life Technologies, Grand Island, NY) according to the manufacturer's instructions. The cells were incubated for 48 h at 37°C in Opti-MEM Reduced-Serum Medium (Gibco). After 48 h, the culture medium was harvested, filtered twice through a 0.2-μm filter, and overlaid onto new BHK cells in a 6-well plate. Forty-eight hours later, the culture medium was harvested, subjected to low-speed centrifugation, filtered through a 0.2-μm filter, titrated on fresh Vero cells, and stored at −80°C.
Growth curves.
For virus growth curves, Vero cells were incubated with VSV or VSV/Maraba G at an MOI of 1.0 for 1 h at 37°C. Following this incubation, the supernatant was removed, the monolayer was washed, and fresh growth medium was added. Supernatant was collected at predetermined time points (0, 4, 8, 12, 24, and 48 h) and subjected to low-speed centrifugation, filtered through a 0.2-μm filter, and titrated on Vero cells. For virus titration, Vero cells were grown on 96-well plates and infected with serially diluted virus stocks. GFP expression was considered an indicator of infection. Fifty percent tissue culture infectious dose (TCID50) values were determined by the Spearman-Karber equation.
Virus neutralization assays.
Virus neutralization was performed as previously described (39). VSV-GFP, VSV/Maraba G, or a recombinant Maraba virus encoding GFP (Maraba-GFP) (5-μl volume with 1 × 108 TCID50) was incubated with 100 μl (20 times the virus volume) nonimmune human serum (fresh sera collected from 4 or 5 healthy volunteers or a purchased serum product [Valley Biomedical; human serum AB; lot J91774]) or naive mouse serum with or without 10% (by volume) standard guinea pig complement (Cedarelane; CL500) at 37°C for 1 h (or at specified time points), followed by virus titer (TCID50/ml) determination on Vero cells (1 × 104 cells/well). Heat inactivation of serum was performed at 56°C for 30 min. The role of IgM antibody in serum VSV neutralization was analyzed by preincubation of the serum for 30 min at room temperature with 50 μg of anti-IgM antibody (Bethyl Laboratories, Inc.; A80-100) or with addition of 15 μg purified IgM protein from normal serum (Cederalane) to complement. For analysis of complement involvement in nonimmune-serum-mediated VSV neutralization, we assessed VSV neutralization by C1q-depleted human serum (Quidel; A509) or C1q-depleted serum reconstituted with 15 μg purified Clq protein (Cedarlane; A098-5) or by reconstitution of heat-inactivated sera with guinea pig complement. As a control, virus was incubated with medium only. The reaction mixture was overlaid on Vero cells. The neutralization assay result was read 48 h after infection with virus that was treated or not with serum. All virus neutralization assay experiments were conducted in triplicate. A similar approach was employed to investigate the anti-VSV neutralization activity of patient serum samples from ovarian and multiple myeloma cancer patients obtained following Mayo Clinic guidelines approved by the Institutional Review Board.
Binding assay.
A virus binding assay was performed by blocking LDLR with a specific monoclonal antibody against human LDLR. K562-avβ3 cells were incubated with anti-LDLR monoclonal antibody or anti-CD46 monoclonal antibody for 1 hour at 37°C. Then, VSV, VSV/Maraba G, Maraba virus, or measles virus (103 TCID50) was added and incubated for 30 min at 37°C; the cells were washed twice with phosphate-buffered saline (PBS); growth medium was added; and 16 h or 48 h (measles virus) later, the infected cells were analyzed for GFP expression.
Statistical analysis.
GraphPad Prism software (GraphPad Software, CA) was used for data analysis. A two-tailed Student's t test was used to compare mean values. A P value of <0.05 was considered statistically significant.
RESULTS
VSV is neutralized by nonimmune sera from multiple species.
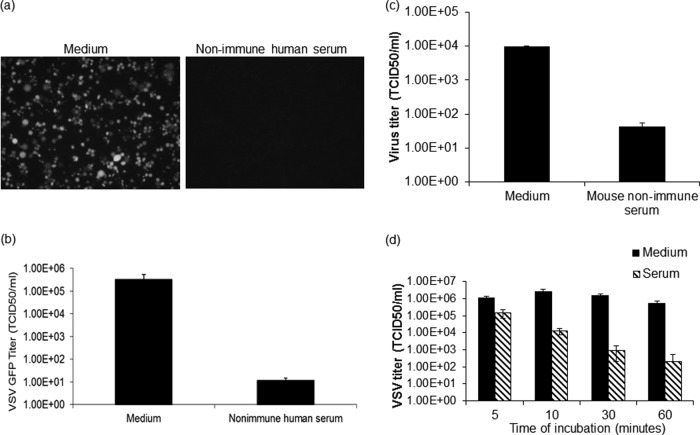
VSV-GFP was propagated on Vero cells, harvested, titrated on Vero cells, and frozen in aliquots, which were thawed and used immediately for the experiments described below. Sera were harvested from multiple donors, heat treated to inactivate complement proteins, and confirmed by plaque reduction neutralization (PRN) assay to be negative for neutralizing activity (IgG antibodies) (see Fig. 2, 4, and 5). In addition, treatment of nonimmune sera with mercaptoethanol, which preferentially destroys IgM (43), inactivates the VSV-neutralizing activity of nonimmune sera but not that of VSV-immune sera (data not shown). Non-heat-treated human serum (20-fold) from these nonimmune seronegative donors was next incubated for 1 h at 37°C with VSV-GFP in the presence of fresh guinea pig complement, which was added to compensate for the variable losses of complement activity that are known to occur after freezing and thawing of serum samples. We have established that fresh nonimmune human serum has potent VSV-neutralizing activity with or without guinea pig complement added (39) (see Fig. 2b). As shown in Fig. 1a and b, the infectious VSV titer was reduced approximately 10,000-fold after 1 h of incubation with nonimmune human serum, confirming previous findings (38, 39). Since the preclinical studies showing that VSV is a potent systemic antimyeloma therapy were conducted in immunocompetent mice, we next sought to determine whether nonimmune mouse serum can also neutralize VSV. As shown in Fig. 1c, VSV-GFP is efficiently neutralized by complement-rich serum from nonimmune mice, but the kinetics of neutralization are slower than with human serum, so that the infectious titer is reduced 100-fold (as opposed to 10,000-fold) after a 60-min exposure time. Because of its broad species tropism, VSV is being considered for comparative oncology studies in which its anticancer activity will be meaningfully evaluated in companion dogs with spontaneously arising malignancies that need to be treated. We therefore tested the VSV-neutralizing potency of nonimmune dog sera with added guinea pig complement and also determined how fast the VSV was being neutralized upon exposure to nonimmune dog serum by performing the time course study shown in Fig. 1d. Neutralization occurred with relatively slow kinetics, in which only 10-fold of the virus was neutralized after 5 min of serum exposure. The nonimmune dog serum neutralized VSV-GFP with speed and efficiency similar to those of human serum (39).
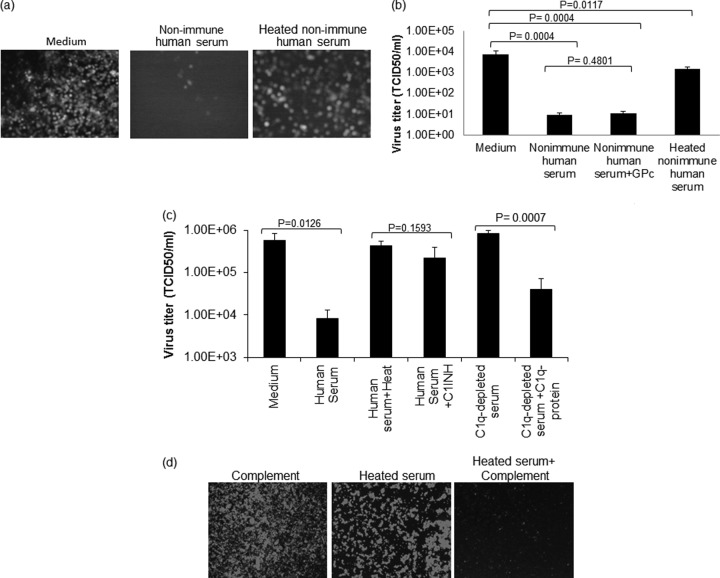
FIG 2.
Neutralization of VSV with nonimmune human serum depends on serum complement. (a and b) VSV-GFP was incubated with nonimmune human serum with or without addition of 10% standard guinea pig complement (GPc), heat-treated (56°C for 30 min) human serum, or medium control. (a) The virus-serum or virus-medium mixture was incubated for 1 h at 37°C. Following incubation, the mixture was overlaid on 12-well plates of Vero cells, and micrographs were taken 24 h postinfection. (b) The virus-serum mixture was diluted 10-fold and titrated on 96-well plates of overnight-plated Vero cells for a TCID50/ml determination. (c) VSV-GFP was incubated for 1 h at 37°C with nonimmune human serum, C1INH-treated nonimmune human serum, Clq-depleted human serum, or Clq-depleted human serum that was constituted with purified Clq protein or, as a control, with medium only. The virus titer was determined as for panel b. (d) VSV-GFP (1 × 104 TCID50) was incubated for 1 h at 37°C with medium containing 10% guinea pig complement, heated nonimmune serum, or heated nonimmune serum reconstituted with 10% guinea pig complement, followed by plating the mixture on monolayers of Vero cells on 96-well plates. Micrographs were taken 24 h postinfection. All virus neutralization assay experiments were conducted in triplicate, and the error bars show standard deviations.
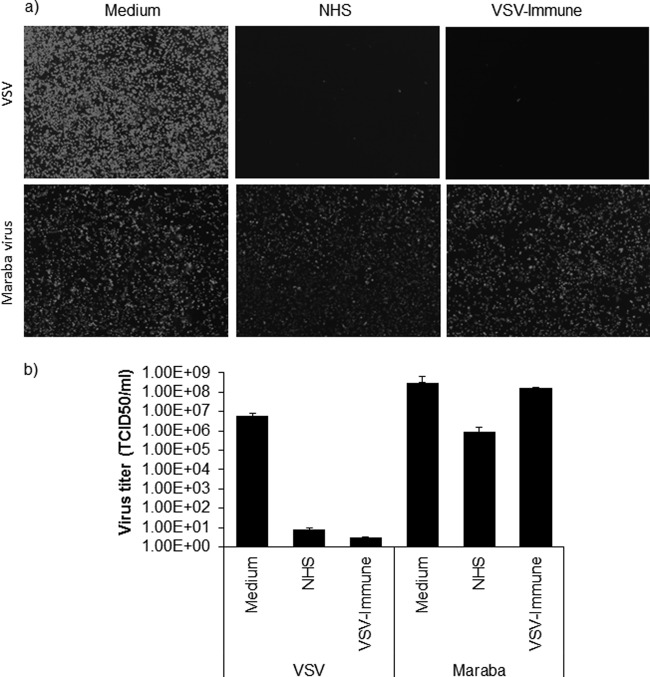
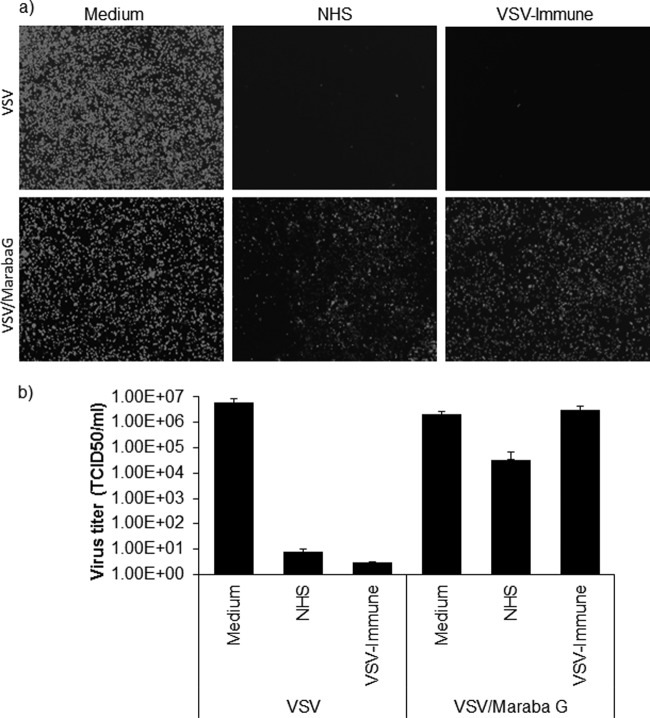
FIG 4.
Maraba virus is more resistant to serum neutralization. (b) VSV-GFP or Maraba-GFP (a 5-μl volume at 1 × 108 TCID50) was incubated with 100 μl undiluted nonimmune human serum (NHS) that contained 10% standard guinea pig complement or heat-inactivated VSV-immune serum at 37°C for 1 h. As a control, virus was incubated with medium only. This was followed by determination of the virus titer (TCID50/ml) on Vero cells (1 × 104 cells/well). The neutralization assay result was read at 48 h after infection with virus that was treated or not with serum. (a) Alternatively, virus treated in the presence or absence of serum was plated on 12-well plates of Vero cells (1 × 105 cells/well), and fluorescent images were taken at 24 h postinfection for each condition. All virus neutralization assay experiments were conducted in triplicate, and the error bars show standard deviations.
FIG 5.
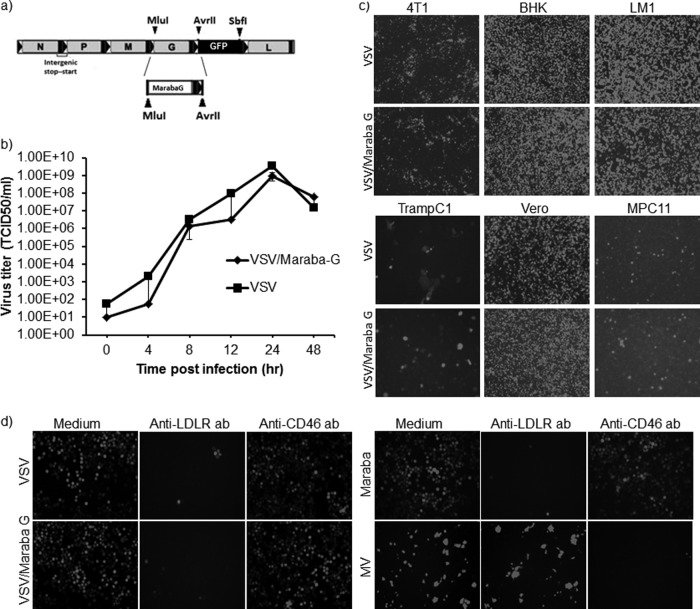
Cloning and characterization of Maraba virus G protein-pseudotyped VSV. (a) Generation of chimeric VSV containing Maraba G in place of VSV G. (b) For virus growth curves, Vero cells were incubated with VSV or VSV/Maraba G at an MOI of 1.0 for 1 h at 37°C. Following this incubation, excess virus was removed by washing the cells with PBS three times, followed by replacing the medium with fresh growth medium. Supernatant was collected at predetermined time points (0, 4, 8, 12, 24, and 48 h) and subjected to low-speed centrifugation, filtered through a 0.2-μm filter, and titrated on Vero cells. TCID50 values were determined by the Spearman-Karber equation. The error bars indicate standard deviations. (c) The infectivity of VSV was compared with that of VSV/Maraba G on different cell lines. (d) LDLR was blocked with a specific monoclonal antibody against human LDLR. K562-avβ3 cells were incubated with anti-LDLR monoclonal antibody for an hour at 37°C or with a control anti-CD46 antibody. Then, VSV, VSV/Maraba G, Maraba virus, or, as a control, measles virus (103 TCID50) was added and incubated for 30 min at 37°C; the cells were washed twice with PBS, and the medium was replaced with fresh growth medium. Sixteen hours (for vesiculovirus-infected cells) or 48 h (for MV-infected cells) postinfection, GFP expression was analyzed.
FIG 1.
Nonimmune serum neutralizes VSV. VSV-GFP was incubated with nonimmune human serum (a and b), nonimmune mouse serum (c), or nonimmune dog serum (d) containing 10% guinea pig complement or, as a control, virus was incubated with medium (a to d) for 1 h (a, b, and c) or multiple time intervals (d) at 37°C. (a) Following incubation, the virus-serum mixture was overlaid on 12-well plates of overnight-plated Vero cells, and micrographs were taken at 24 h postinfection. (b, c, and d) The virus-serum mixture was diluted 10-fold and titrated on 96-well plates of overnight-plated Vero cells for a TCID50/ml determination. All virus neutralization assay experiments were conducted in triplicate, and the error bars show standard deviations.
Neutralization is mediated by the concerted actions of IgM and complement.
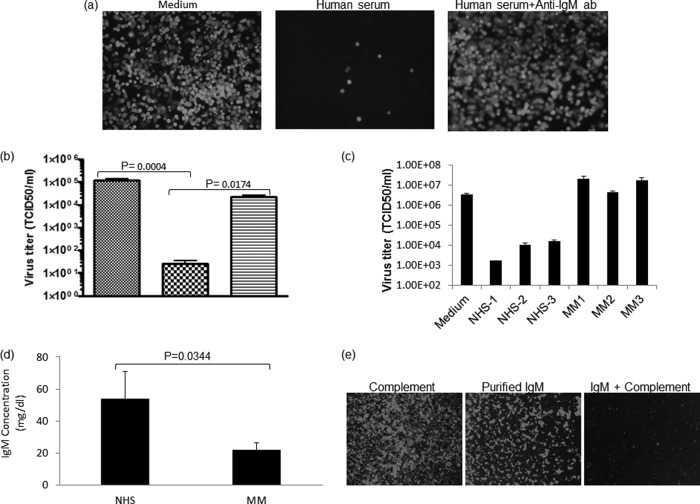
As shown in Fig. 2a to d, heat inactivation destroys the VSV-neutralizing activity of serum from VSV-naive subjects. To determine whether the critical heat-labile VSV-neutralizing serum factor(s) is a component of the complement system, we performed additional complement restoration and depletion studies. As shown in Fig. 2d, the VSV-neutralizing activity of heat-inactivated serum could be fully restored by adding guinea pig complement. To further confirm the role of complement proteins as mediators of VSV neutralization, we tested sera that had been treated with (i) a C1 inhibitor (C1INH), (ii) commercially available C1q-depleted serum, or (iii) C1q-depleted serum that had been reconstituted with purified C1q protein (Fig. 2c). These experiments show conclusively that complement proteins are required for neutralization of VSV by nonimmune human serum. To determine whether natural IgM antibodies work in concert with complement proteins to neutralize infectious VSVs, we next blocked IgM of human serum samples by preincubating them with an anti-IgM antibody preparation. As shown in Fig. 3a and b, blocking IgM with ant-IgM antibody completely destroyed the VSV-neutralizing activity of nonimmune serum. We also show that the VSV-neutralizing activity of complement can be restored by addition of purified IgM protein (Fig. 3e). To further investigate this finding, we tested the neutralizing activities of sera from 3 healthy subjects and 3 myeloma patients. As shown in Fig. 2c, compared to normal nonimmune human sera or ovarian cancer sera (data not shown), the IgM-depleted myeloma sera (Fig. 2d) had considerably diminished virus-neutralizing activity. Taken together, these data show that the neutralization of VSV by nonimmune serum is due to the concerted actions of natural IgM antibodies and complement.
FIG 3.
Neutralization of VSV with nonimmune human serum depends on serum IgM antibodies. (a and b) VSV-GFP was incubated with nonimmune human serum or nonimmune human serum that was treated with anti-IgM antibody (30 min at room temperature) or, as a control, with medium only. The virus-serum or virus-medium mixture was incubated for 1 h at 37°C. Following incubation, the mixture was overlaid on a 12-well plate of overnight-plated Vero cells and micrographs were taken 24 h postinfection (a) or the mixture was diluted 10-fold and titrated on 96-well plates of overnight-plated Vero cells for a TCID50/ml determination (hatched, medium; checked, normal serum; stippled, normal serum plus anti-IgM antibody) (b). (c) VSV-GFP was incubated with nonimmune MM patient serum or nonimmune normal serum, or as a control, virus was incubated with medium for 1 h at 37°C. Following incubation, the virus-serum mixture was diluted 10-fold and titrated on 96-well plates of overnight-plated Vero cells for a TCID50/ml determination as for panel b. (d) IgM concentrations of nonimmune human serum (NHS) (n = 3) and a multiple myeloma patient sample (n = 3) were analyzed, and the averages are shown. (e) VSV-GFP (1 × 104 TCID50) was incubated for 1 h at 37°C with media containing 10% guinea pig complement, 15 μg of purified IgM protein, or 15 μg purified IgM protein reconstituted with 10% (by volume) guinea pig complement, followed by plating the mixture on monolayers of 96-well plates of Vero cells. Micrographs were taken 24 h postinfection. All virus neutralization assay experiments were conducted in triplicate, and the error bars show standard deviations.
Maraba virus is relatively resistant to nonimmune serum.
Maraba virus, a vesiculovirus closely related to VSV, was isolated in the early 1980s from phlebotomine sandflies in the Amazon basin of Brazil (40, 41, 44). The natural mammalian host of the virus has not yet been identified, but its pathogenicity in mice is similar to that of VSV-Indiana and VSV-New Jersey. At the amino acid level, the G protein of Maraba virus is about 80% homologous to the G protein of VSV (reference 40 and data not shown). To confirm that Maraba virus and VSV are correctly classified as distinct vesiculovirus serotypes, Maraba-GFP and VSV-GFP were incubated with serial dilutions of sera harvested from VSV-immunized mice. As shown in Fig. 4a and b, the sera harvested from VSV-immunized mice had minimal cross-neutralizing activity against Maraba virus. We therefore decided to investigate whether the Maraba virus and VSV G proteins are similarly susceptible to neutralization by nonimmune serum. Maraba-GFP was therefore incubated with nonimmune human serum (20-fold) for 1 h at 37°C in the presence of standard guinea pig complement (10%). As shown in Fig. 4a and b, the infectious titer of Maraba-GFP was reduced only 100-fold after 1 h of incubation with nonimmune human serum, retaining a titer close to 106 TCID50/ml. The titer of the VSV-GFP stock exposed in parallel to the same nonimmune human serum was reduced to less than 10 TCID50/ml. Thus, compared to VSV, Maraba virus is approximately 100-fold more resistant to the neutralizing activity of nonimmune human serum. Our data show that, while VSV is very sensitive to nonimmune human sera, Maraba virus shows extensive but not complete resistance to nonimmune sera, which is in line with previous studies that showed, using a complement fixation assay, that VSV and Maraba virus show about 34% cross-reactivity (40).
A recombinant VSV with Maraba G substituted propagates as efficiently as parental VSV.
Since the G protein is known to be the primary target of VSV-neutralizing antibodies (16), we next sought to determine whether VSV would be resistant to the neutralizing activity of nonimmune human serum if pseudotyped with the Maraba G protein. To this end, we constructed a recombinant VSV-GFP genome in which the G cistron from VSV was replaced by the corresponding G cistron from Maraba virus (Fig. 5a). The new recombinant virus was easily rescued and propagated to high titer on Vero cells. One-step growth curves of the parental VSV and VSV/Maraba G were next compared on Vero cells and found to be almost superimposable (Fig. 5b). Since the host range properties of Maraba virus have not been extensively studied and its natural host species has not been identified, there is considerable uncertainty as to whether the Maraba virus and VSV G proteins bind to the same receptor. We therefore compared the infectivities of the parental and Maraba G-pseudotyped viruses on a panel of tumor cell lines. As shown in Fig. 5c, there was no discernible difference in the infectivities of the two viruses for any of the tumor cell lines that were tested, suggesting that the two G proteins do indeed use the same receptor. To further investigate this question, we performed an additional antibody-blocking study. It was recently shown that the primary VSV receptor on mammalian cells belongs to the LDL receptor family and that VSV entry can be inhibited by preincubating target cells with an anti-LDLR antibody (45). We therefore tested the infectivities of VSV, Maraba virus, and VSV/Maraba G on K562-avβ3 cells that had been pretreated with this VSV receptor-blocking antibody or control measles virus receptor binding antibody (anti-CD46 antibody). As shown in Fig. 5d, the entry of all viruses except MV was equally impacted by the anti-LDLR antibody, but treatment with anti-CD46 antibody had no effect on the vesiculoviruses while it blocked MV infection, confirming that the VSV and Maraba G proteins do use the same cellular receptor.
VSV with Maraba G substituted is resistant to nonimmune human serum.
Since VSV and VSV/Maraba G have indistinguishable cell tropisms and replication kinetics (Fig. 5b and c), we anticipate that the oncolytic potency of the Maraba G-pseudotyped VSV will be at least equivalent to that of the parental VSV. However, the goal of generating the VSV/Maraba G pseudotype virus was to introduce the serum-resistant phenotype of Maraba virus into the oncolytic VSV platform. VSV and VSV/Maraba G were therefore incubated for 1 h at 37°C with nonimmune human serum (20-fold) supplemented with standard guinea pig complement. As shown in Fig. 6a and b, the infectious titer of VSV/Maraba G was at least 1,000-fold higher after this treatment than that of the parental VSV. Thus, as was observed for the parental Maraba virus, the VSV/Maraba G pseudotype virus is highly resistant to the neutralizing activity of nonimmune human serum.
FIG 6.
Maraba G-pseudotyped VSV escapes serum neutralization. VSV-GFP or VSV/Maraba G (5-μl volume with 1 × 108 TCID50) was incubated with 100 μl undiluted nonimmune human serum that contained 10% standard guinea pig complement (Cedarlane) or heat-inactivated VSV-immune serum at 37°C for 1 h, followed by determination of the virus titer (TCID50/ml) on Vero cells (1 × 104 cells/well). As a control, virus was incubated with medium only and plated on Vero cells. The neutralization assay result was read at 48 h after infection with virus that was treated or not with serum. Alternatively, virus treated in the presence or absence of serum was plated on 12-well plates of Vero cells (1 × 105 cells/well) and fluorescence images were taken at 24 h postinfection for each condition. All virus neutralization assay experiments were conducted in triplicate. The error bars represent standard deviations.
DISCUSSION
We are developing VSV as an oncolytic agent for systemic treatment of multiple myeloma. VSV is a negative-sense RNA virus that can infect a wide variety of animals and cells and possesses a rapid lytic replication cycle but demonstrates dramatic sensitivity to the host interferon response (16, 30, 32, 33). The high sensitivity of VSV to innate interferon responses and its lack of pathogenicity to humans have made it an attractive oncolytic platform that has been extensively investigated and has been shown to kill cancer cells selectively, particularly when they have a disrupted interferon response (28–34). Preclinical studies show that VSV is promising for the treatment of a variety of human cancers (10, 16–26). However, efficient intravenous delivery will be critical for its successful clinical application. Neutralization of VSV by human serum is therefore an important area of study.
The objective of the study reported here was to characterize the VSV-neutralizing activity of nonimmune serum and to engineer a recombinant VSV no longer susceptible to nonimmune serum. We first demonstrated that VSV is neutralized by nonimmune sera from human, mouse, dog (Fig. 1a to d), and monkey (data not shown), reducing its infectivity by up to 4 log units within 1 h. We then showed that this neutralizing activity is mediated by the concerted actions of IgM antibody and complement (Fig. 2 and 3). These results are in line with previously published studies showing that nonimmune human serum neutralizes VSV via the combined actions of a heat-labile factor(s) and IgM (38, 39). We further analyzed the complement dependence of human serum by using C1q-depleted serum or serum treated with a C1INH, both of which abrogated the VSV neutralization ability of nonimmune human serum (Fig. 2c). Moreover, addition of purified C1q protein to the Clq-depleted serum restored most of its VSV-neutralizing activity (Fig. 2c). As a further indication of the role of IgM in this neutralizing activity, complement-rich sera from patients with multiple myeloma had reduced VSV-neutralizing activity compared to sera from healthy subjects or patients with ovarian cancer (Fig. 3c and data not shown), and this correlated with the reduced IgM antibody concentration in the multiple myeloma cancer patient serum samples (Fig. 3d). Furthermore, addition of purified IgM protein to complement was sufficient to restore its VSV-neutralizing activity (Fig. 3e).
Several approaches can be contemplated to address the slow inactivation of VSV by nonimmune human serum, which, at least in theory, may be a significant barrier to the success of VSV as an intravenously administered oncolytic agent (14, 46, 47) (see Discussion below). Previous studies have explored the use of virus-infected cells as carriers to protect and transport the virus to sites of tumor growth (48–51) or synthetic polymers to coat and protect the virus from antibody neutralization (39, 52) or have employed G protein evolution strategies to generate neutralization-resistant variants of VSV G (53). However, each of these approaches has its limitations. We therefore chose in the current study to explore the effect of switching the VSV surface glycoprotein (G) with that of Maraba virus, a closely related but serologically distinct member of the family Rhabdoviridae (16, 40).
We first determined that, in contrast to VSV, Maraba virus is relatively resistant to nonimmune human serum (Fig. 4a and b) and that this resistance could be efficiently transferred to VSV by replacing VSV G with Maraba virus G (Fig. 5a and 6a and b). Interestingly, VSV with Maraba G substituted demonstrated cell tropism and growth kinetics similar to those of the parental VSV, and its infectivity is similarly neutralized by an anti-LDL receptor antibody, indicating that the Maraba G protein probably interacts with the same cellular receptor as VSV G (Fig. 5b, c, and d). Another potentially useful characteristic of VSV with Maraba G substituted is that it is also resistant to neutralization by sera obtained from VSV-immunized mice (Fig. 6). Considering that VSV G and Maraba virus G possess about 80% amino acid homology (reference 40 and data not shown), it may prove possible by site-directed mutagenesis studies to pinpoint the VSV G residue(s) that plays a role in both serum-IgM and serum-IgG interactions.
It is interesting that the VSV-neutralizing activity of nonimmune human serum is not completely destroyed by heat inactivation or by complement depletion (Fig. 2a, b, and c), suggesting that IgM may have some limited neutralizing activity even in the absence of complement. Whether this residual complement-independent IgM-dependent neutralization activity is due to virus aggregation or direct blockade of G protein-receptor interactions, as also suggested for herpes simplex virus IgM-dependent serum neutralization (54), will be further explored in future studies. Similarly, the level of neutralization by C1q-reconstituted sera is not the same as that of normal nonimmune sera, which suggests the presence of an alternative pathway, or it could be that we are unable to optimally reconstitute the C1q protein level to that of normal sera. It should also be noted that Maraba virus and VSV with Maraba G substituted are not completely resistant to the neutralizing effect of nonimmune human serum, suggesting that they are still bound at some lower level by natural human IgM. Similarly, compared to nonimmune human serum, nonimmune mouse serum has considerably less VSV-neutralizing activity, suggesting that natural mouse IgM may bind to the virus less efficiently than natural human IgM. In light of these variable interactions and the variable evolutionary pressures that have been experienced by the various known serotypes of VSV and related viruses (16, 40, 55), there are several additional rhabdovirus G glycoproteins that could be evaluated to determine whether they afford more complete protection from serum neutralization than Maraba virus G.
Neutralization of VSV by IgM and complement in nonimmune serum is relatively slow, so it takes several minutes to achieve even a 10-fold reduction in virus titer (Fig. 2) while we have reported limited mouse experimental data that show 2- to 3-log-unit reduction in VSV titer within 1 min after injection (39). Thus, it is not certain to what extent this neutralizing activity can negatively impact the antitumor efficacy of a systemically administered oncolytic VSV. The kinetics of virus extravasation and tumor cell infection at sites of tumor growth have not yet been subjected to detailed study after bolus administration or intravenous infusion of VSV. Likewise, the kinetics with which circulating VSV is sequestered by antigen-processing cells in liver and spleen have not yet been determined. If substantial extravasation and tumor cell infection occur within the first few minutes of virus administration, when circulating concentrations of infectious virus are at their highest level, there may be little negative impact of the serum-neutralizing activity that has been studied here. Future studies are therefore planned to better elucidate this question. Unfortunately, the process cannot be meaningfully studied in mice because of their low blood volume and blood draw restrictions. We therefore plan initially to conduct pharmacokinetics (PK) studies in the context of an ongoing veterinary clinical trial in which an oncolytic VSV is administered intravenously to companion dogs with lymphoma or multiple myeloma requiring treatment. This will allow us to determine the rate at which VSV infectivity disappears from the bloodstream in this highly meaningful animal model, following which we will be better equipped to determine whether the rates of decay of circulating infectivity are different for the unmodified VSV and VSV with Maraba G substituted and whether this can impact intratumoral virus extravasation and antitumor efficacy. Such animal model studies will also provide us with an opportunity to evaluate the effect of treatment with complement cascade inhibitors or incorporation of complement-antagonizing proteins into the virus surface on the stability of VSV in circulation and its antitumor efficacy.
In summary, we have shown that the VSV infectious titer drops by 4 log units during the first hour of exposure to nonimmune human serum due to the concerted actions of natural IgM and complement. We have further shown that a VSV with Maraba G substituted is relatively resistant to this neutralizing activity. VSV with Maraba G substituted should therefore be further investigated as a candidate for human systemic oncolytic virotherapy applications.
Footnotes
Published ahead of print 19 March 2014
REFERENCES
- 1.Edwards CM, Zhuang J, Mundy GR. 2008. The pathogenesis of the bone disease of multiple myeloma. Bone 42:1007–1013. 10.1016/j.bone.2008.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. 2007. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat. Rev. 7:585–598. 10.1038/nrc2189 [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Rajkumar SV. 2004. Multiple myeloma. N. Engl. J. Med. 351:1860–1873. 10.1056/NEJMra041875 [DOI] [PubMed] [Google Scholar]
- 4.Vande Broek I, Vanderkerken K, Van Camp B, Van Riet I. 2008. Extravasation and homing mechanisms in multiple myeloma. Clin. Exp. Metastasis 25:325–334. 10.1007/s10585-007-9108-4 [DOI] [PubMed] [Google Scholar]
- 5.Cook R. 2008. Economic and clinical impact of multiple myeloma to managed care. J. Manag. Care Pharm. 14(Suppl):19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyle RA, Rajkumar SV. 2009. Treatment of multiple myeloma: a comprehensive review. Clin. Lymphoma Myeloma 9:278–288. 10.3816/CLM.2009.n.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laubach JP, Mahindra A, Mitsiades CS, Schlossman RL, Munshi NC, Ghobrial IM, Carreau N, Hideshima T, Anderson KC, Richardson PG. 2009. The use of novel agents in the treatment of relapsed and refractory multiple myeloma. Leukemia 23:2222–2232. 10.1038/leu.2009.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Horner MJ, Howlader N, Eisner MP, Reichman M, Edwards BK. (ed). 2007. SEER cancer statistics review, 1975–2004. National Cancer Institute, Bethesda, MD [Google Scholar]
- 9.Schwartz RN, Vozniak M. 2008. Current and emerging treatments for multiple myeloma. J. Manag. Care Pharm. 14:S12–S18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lichty BD, Stojdl DF, Taylor RA, Miller L, Frenkel I, Atkins H, Bell JC. 2004. Vesicular stomatitis virus: a potential therapeutic virus for the treatment of hematologic malignancy. Hum. Gene Ther. 15:821–831. 10.1089/hum.2004.15.821 [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Russell SJ, Peng K-W. 2010. Systemic therapy of disseminated myeloma in passively immunized mice using measles virus-infected cell carriers. Mol. Ther. 18:1155–1164. 10.1038/mt.2010.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu TC, Galanis E, Kim D. 2007. Clinical trial with oncolytic virotherapy: a century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 4:101–117. 10.1038/ncponc0736 [DOI] [PubMed] [Google Scholar]
- 13.Russell S. 2002. RNA viruses as virotherapy agents. Cancer Gene Ther. 9:961–966. 10.1038/sj.cgt.7700535 [DOI] [PubMed] [Google Scholar]
- 14.Russell SJ, Peng K-W, Bell JC. 2012. Oncolytic virotherapy. Nat. Biotechnol. 30:658–670. 10.1038/nbt.2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaha-Koskela MJ, Heikkila JE, Hinkkanen AE. 2007. Oncolytic viruses in cancer therapy. Cancer Lett. 254:178–216. 10.1016/j.canlet.2007.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner RR, Rose JK. 1996. Rhabdoviridae: the viruses and their replication, p 1121–1135 In Fields BN, Knipe DM, Howley PM. (ed), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, PA [Google Scholar]
- 17.Lichty BD, Power AT, Stojdl DF, Bell JC. 2004. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol. Med. 10:210–216. 10.1016/j.molmed.2004.03.003 [DOI] [PubMed] [Google Scholar]
- 18.Ahmed M, Cramer SD, Lyles DS. 2004. Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses. Virology 330:34–49. 10.1016/j.virol.2004.08.039 [DOI] [PubMed] [Google Scholar]
- 19.Bergman I, Griffin JA, Gao Y, Whitaker-Dowling P. 2007. Treatment of implanted mammary tumors with recombinant vesicular stomatitis virus targeted to Her2/neu. Int. J. Cancer 121:425–430. 10.1002/ijc.22680 [DOI] [PubMed] [Google Scholar]
- 20.Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, Valdes M, Barber G, Vile RG. 2007. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 67:2840–2848. 10.1158/0008-5472.CAN-06-3974 [DOI] [PubMed] [Google Scholar]
- 21.Ebert O, Harbaran S, Shinozaki K, Woo SL. 2005. Systemic therapy of experimental breast cancer metastases by mutant vesicular stomatitis virus in immune-competent mice. Cancer Gene Ther. 12:350–358. 10.1038/sj.cgt.7700794 [DOI] [PubMed] [Google Scholar]
- 22.Ebert O, Shinozaki K, Huang TG, Savontaus MJ, Garcia-Sastre A, Woo SL. 2003. Oncolytic vesicular stomatitis virus for treatment of orthotopic hepatocellular carcinoma in immune-competent rats. Cancer Res. 63:3605–3611 [PubMed] [Google Scholar]
- 23.Huang TG, Ebert O, Shinozaki K, Garcia-Sastre A, Woo SL. 2003. Oncolysis of hepatic metastasis of colorectal cancer by recombinant vesicular stomatitis virus in immune-competent mice. Mol. Ther. 8:434–440. 10.1016/S1525-0016(03)00204-1 [DOI] [PubMed] [Google Scholar]
- 24.Lun X, Senger DL, Alain T, Oprea A, Parato K, Stojdl D, Lichty B, Power A, Johnston RN, Hamilton M, Parney I, Bell JC, Forsyth PA. 2006. Effects of intravenously administered recombinant vesicular stomatitis virus (VSV (deltaM51)) on multifocal and invasive gliomas. J. Natl. Cancer Inst. 98:1546–1557. 10.1093/jnci/djj413 [DOI] [PubMed] [Google Scholar]
- 25.Shinozaki K, Ebert O, Kournioti C, Tai YS, Woo SL. 2004. Oncolysis of multifocal hepatocellular carcinoma in the rat liver by hepatic artery infusion of vesicular stomatitis virus. Mol. Ther. 9:368–376. 10.1016/j.ymthe.2003.12.004 [DOI] [PubMed] [Google Scholar]
- 26.Sung CK, Choi B, Wanna G, Genden EM, Woo SL, Shin EJ. 2008. Combined VSV oncolytic virus and chemotherapy for squamous cell carcinoma. Laryngoscope 118:237–242. 10.1097/MLG.0b013e3181581977 [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Lun X, Zhou H, Wang L, Sun B, Bell JC, Barrett JW, McFadden G, Biegel JA, Senger DL, Forsyth PA. 2008. Oncolytic efficacy of recombinant vesicular stomatitis virus and myxoma virus in experimental models of rhabdoid tumors. Clin. Cancer Res. 14:1218-1227. 10.1158/1078-0432.CCR-07-1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belkowski LS, Sen GC. 1987. Inhibition of vesicular stomatitis viral mRNA synthesis by interferon. J. Virol. 61:653–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carey BL, Ahmed M, Puckett S, Lyles DS. 2008. Early steps of the virus replication cycle are inhibited in prostate cancer cells resistant to oncolytic vesicular stomatitis virus. J. Virol. 82:12104–12115. 10.1128/JVI.01508-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moerdyk-Schauwecker M, Shah NR, Murphy AM, Hastie E, Mukherjee P, Grdzelishvili VZ. 2013. Resistance of pancreatic cancer cells to oncolytic vesicular stomatitis virus: role of type I interferon signaling. Virology 436:221–234. 10.1016/j.virol.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obuchi M, Fernandez M, Barber GN. 2003. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. Virology 77:8843–8856. 10.1128/JVI.77.16.8843-8856.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saloura V, Wang LC, Fridlender ZG, Sun J, Cheng G, Kapoor V, Sterman DH, Harty RN, Okumura A, Barber GN, Vile RG, Federspiel MJ, Russell SJ, Litzky L, Albelda SM. 2010. Evaluation of an attenuated vesicular stomatitis virus vector expressing interferon-β for use in malignant pleural mesothelioma: heterogeneity in interferon responsiveness defines potential efficacy. Hum. Gene Ther. 21:51–64. 10.1089/hum.2009.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stojdl DE, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, Bell JC. 2000. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 6:821–825. 10.1038/77558 [DOI] [PubMed] [Google Scholar]
- 34.Stojdl DF, Lichty BD, ten Oever BR, Paterson JM, Power AT, Knowles S, Marius R, Reynard J, Poliquin L, Atkins H, Brown EG, Durbin RK, Durbin E, Hiscott J, Bell JC. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4:263–275. 10.1016/S1535-6108(03)00241-1 [DOI] [PubMed] [Google Scholar]
- 35.Critchley-Thornea RJ, Simonsa DL, Yana N, Miyahiraa AK, Dirbasc FM, Johnsonc DL, Swetterd SM, Carlsone RW, Fishere GA, Koongf A, Holmesb S, Leea PP. 2009. Impaired interferon signaling is a common immune defect in human cancer. Proc. Natl. Acad. Sci. U. S. A. 106:9010–9015. 10.1073/pnas.0901329106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naik S, Nace R, Federspiel MJ, Barber GN, Peng KW, Russell SJ. 2012. Curative one-shot systemic virotherapy in murine myeloma. Leukemia 26:1870–1878. 10.1038/leu.2012.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naik S, Nace R, Federspiel MJ, Barber GN, Russell SJ. 2012. Potent systemic therapy of multiple myeloma utilizing oncolytic vesicular stomatitis virus coding for interferon-β. Cancer Gene Ther. 19:443–450. 10.1038/cgt.2012.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beebe DP, Cooper NR. 1981. Neutralization of vesicular stomatitis virus (VSV) by human complement requires a natural IgM antibody present in human serum. J. Immunol. 126:1562–1568 [PubMed] [Google Scholar]
- 39.Tesfay MZ, Kirk AC, Hadac EM, Griesmann GE, Federspiel MJ, Barber GN, Henry SM, Peng KW, Russell SJ. 2013. PEGylation of vesicular stomatitis virus extends virus persistence in blood circulation of passively immunized mice. J. Virol. 87:3752–3759. 10.1128/JVI.02832-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pauszek SJ, Barrera JC, Goldberg T, Allende R, Rodriguez LL. 2011. Genetic and antigenic relationships of vesicular stomatitis viruses from South America. Arch. Virol. 10.1007/s00705-011-1081-1 [DOI] [PubMed] [Google Scholar]
- 41.Brun J, McManus D, Lefebvre C, Hu K, Falls T, Atkins H, Bell JC, McCart JA, Mahoney D, Stojdl DF. 2010. Identification of genetically modified Maraba virus as an oncolytic rhabdovirus. Mol. Ther. 18:1440–1449. 10.1038/mt.2010.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawson ND, Stillmans EA, Whit MA, Rose JK. 1995. Recombinant vesicular stomatitis viruses from DNA (rhabdovirus/viral replication/viral assembly). Proc. Natl. Acad. Sci. U. S. A. 92:4477–4481. 10.1073/pnas.92.10.4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hosono M, Muramatsu S. 1972. Use of 2-mercaptoethanol for distinguishing between IgM and IgG antibody-producing cells of mice immunized with bovine globulin. J. Immunol. 109:857–863 [PubMed] [Google Scholar]
- 44.Travassos da Rosa APA, Mather TN, Takeda T, Whitehouse CA, Shope RE, Popov VL, Guzman H, Coffey L, Araujo TP, Tesh RB. 2002. Two new rhabdoviruses (Rhabdoviridae) isolated from birds during surveillance for arboviral encephalitis, northeastern United States. Emerg. Infect. Dis. 8:614–618. 10.3201/eid0806.010384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finkelshtein D, Werman A, Novick D, Barak S, Rubinstein M. 2013. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc. Natl. Acad. Sci. U. S. A. 110:7306–7311. 10.1073/pnas.1214441110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brunner KT, Hurez D, McCluskey RT, Benacerraf B. 1960. Blood clearance of p32-labeled vesicular stomatitis and Newcastle disease viruses by the reticuloendothelial system in mice. J. Immunol. 85:99–105 [PubMed] [Google Scholar]
- 47.Ferguson MS, Lemoine NR, Wang Y. 31 January 2012. Systemic delivery of oncolytic viruses: hopes and hurdles. Adv. Virol. 10.1155/2012/805629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munguia A, Ota T, Miest T, Russell SJ. 2008. Cell carriers to deliver oncolytic viruses to sites of myeloma tumor growth. Gene Ther. 15:797–806. 10.1038/gt.2008.45 [DOI] [PubMed] [Google Scholar]
- 49.Power AT, Bell JC. 2008. Taming the Trojan horse: optimizing dynamic carrier cell/oncolytic virus systems for cancer biotherapy. Gene Ther. 15:772–779. 10.1038/gt.2008.40 [DOI] [PubMed] [Google Scholar]
- 50.Willmon C, Harrington K, Kottke T, Prestwich R, Melcher A, Vile R. 2009. Cell carriers for oncolytic viruses: Fed Ex for cancer therapy. Mol. Ther. 17:1667–1676. 10.1038/mt.2009.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Power AT, Wang J, Falls TJ, Paterson JM, Parato KA, Lichty BD, Stojd DF, Forsyth PAJ, Atkins H, Bell JC. 2007. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol. Ther. 15:123–130. 10.1038/sj.mt.6300039 [DOI] [PubMed] [Google Scholar]
- 52.Croyle MA, Callahan SM, Auricchio A, Schumer G, Linse KD, Wilson JA, Brunner LJ, Kobinger GP. 2004. PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. J. Virol. 78:912–921. 10.1128/JVI.78.2.912-921.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hwang BY, Schaffer DV. 2013. Engineering a serum-resistant and thermostable vesicular stomatitis virus G glycoprotein for pseudotyping retroviral and lentiviral vectors. Gene Ther. 20:807–815. 10.1038/gt.2013.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, Harsh GR, IV, Louis DN, Bartus RT, Hochberg FH, Chiocca EA. 1999. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat. Med. 5:881–887. 10.1038/11320 [DOI] [PubMed] [Google Scholar]
- 55.Trobridge GD, Wu RA, Hansen M, Ironside C, Watts KL, Olsen P, Beard BC, Kiem H-P. 2010. Cocal-pseudotyped lentiviral vectors resist inactivation by human serum and efficiently transduce primate hematopoietic repopulating cells. Mol. Ther. 18:725–733. 10.1038/mt.2009.282 [DOI] [PMC free article] [PubMed] [Google Scholar]