ABSTRACT
Chikungunya virus (CHIKV) causes a major public health problem. In 2004, CHIKV began an unprecedented global expansion and has been responsible for epidemics in Africa, Asia, islands in the Indian Ocean region, and surprisingly, in temperate regions, such as Europe. Intriguingly, no local transmission of chikungunya virus (CHIKV) had been reported in the Americas until recently, despite the presence of vectors and annually reported imported cases. Here, we assessed the vector competence of 35 American Aedes aegypti and Aedes albopictus mosquito populations for three CHIKV genotypes. We also compared the number of viral particles of different CHIKV strains in mosquito saliva at two different times postinfection. Primarily, viral dissemination rates were high for all mosquito populations irrespective of the tested CHIKV isolate. In contrast, differences in transmission efficiency (TE) were underlined in populations of both species through the Americas, suggesting the role of salivary glands in selecting CHIKV for highly efficient transmission. Nonetheless, both mosquito species were capable of transmitting all three CHIKV genotypes, and TE reached alarming rates as high as 83.3% and 96.7% in A. aegypti and A. albopictus populations, respectively. A. albopictus better transmitted the epidemic mutant strain CHIKV_0621 of the East-Central-South African (ECSA) genotype than did A. aegypti, whereas the latter species was more capable of transmitting the original ECSA CHIKV_115 strain and also the Asian genotype CHIKV_NC. Therefore, a high risk of establishment and spread of CHIKV throughout the tropical, subtropical, and even temperate regions of the Americas is more real than ever.
IMPORTANCE Until recently, the Americas had never reported chikungunya (CHIK) autochthonous transmission despite its global expansion beginning in 2004. Large regions of the continent are highly infested with Aedes aegypti and Aedes albopictus mosquitoes, and millions of dengue (DEN) cases are annually recorded. Indeed, DEN virus and CHIK virus (CHIKV) share the same vectors. Due to a recent CHIK outbreak affecting Caribbean islands, the need for a Pan-American evaluation of vector competence was compelling as a key parameter in assessing the epidemic risk. We demonstrated for the first time that A. aegypti and A. albopictus populations throughout the continent are highly competent to transmit CHIK irrespective of the viral genotypes tested. The risk of CHIK spreading throughout the tropical, subtropical, and even temperate regions of the Americas is more than ever a reality. In light of our results, local authorities should immediately pursue and reinforce epidemiological and entomological surveillance to avoid a severe epidemic.
INTRODUCTION
Chikungunya virus (CHIKV) is an alphavirus in the family Togaviridae that is transmitted by mosquitoes, mainly Aedes aegypti and Aedes albopictus within an urban cycle. Since 2004, CHIKV has reemerged in Indian Ocean islands and has caused severe epidemics in several countries in tropical and subtropical regions in Africa and Asia, as well as in temperate Mediterranean areas in Europe (1).
Aedes aegypti is widespread in the Americas, where it is the only confirmed natural dengue virus (DENV) vector (2). Although its geographical distribution is more limited, A. albopictus is considered a potential vector in the Americas due to the high level of vector competence of local populations for DENV (3, 4). More than 2 million dengue (DEN) cases are annually reported in the American continent each year (5). The most critical epidemiological situation is that described for South America, which reported more than 1.5 million dengue cases in 2013, with an incidence rate of more than 650 cases/100,000 inhabitants in the South Cone alone (6). Such an epidemiological scenario points to the weakness of mosquito control activities and the high receptivity to introduction and spread of other arboviruses transmitted by both mosquito species like CHIKV in other parts of the continent (1, 7, 8). In fact, as CHIKV and DENV share the same mosquito vector species, epidemic waves caused by both viruses affect the same regions, and human coinfections may occur (9, 10). Moreover, the intensification of intercontinental travel with recurrent returns of dozens of viremic CHIKV cases from affected areas—which may bypass the surveillance systems due to the clinical similarities to other viruses circulating in the Americas—exemplifies the vulnerability of this continent to CHIKV epidemics (11, 12). Indeed, Brazil, Canada, the United States, French Guiana, and the French West Indies (Guadeloupe and Martinique) have reported several imported cases of CHIKV since its reemergence in 2004 (6, 13).
Intriguingly, until December 2013, autochthonous CHIKV transmission had never been reported in the Americas, a continents in which all of the conditions are apparently suitable for its establishment: (i) the Americas are a virgin continent for CHIKV, (ii) the main mosquito vectors of CHIKV, A. aegypti and A. albopictus, are present at high densities in most areas, (iii) imported cases are annually reported in periods of high mosquito density and activity, and (iv) temperature and environmental conditions of large tropical and subtropical zones are favorable to mosquito development and activity as well as to viral replication in the vector (11, 14). In early December 2013, two laboratory-confirmed autochthonous CHIKV cases were reported in the French territory of Saint-Martin Island in the Caribbean (6). Very rapidly, an epidemic was established on the island, with almost 2,030 clinical cases and more than 765 confirmed cases, and subsequently, some CHIKV cases were detected in Martinique, Guadeloupe, Saint-Barthelemy, and also French Guiana (15). Therefore, CHIKV is progressively spreading, putting at high epidemic risk the vast areas of the Americas infested with A. aegypti and A. albopictus.
To achieve efficient transmission, numerous factors regarding the invertebrate and the vertebrate hosts, the virus, and the environmental conditions must ideally converge (16). Concerning the mosquito host, vector competence is considered to be unique and characteristic for each virus-vector pair. Indeed differences of vector competence can be found between different populations belonging to a single insect vector species (17). Vector competence is a quantitative phenotypic parameter controlled by genetic characteristics of both vector and virus, which in turn is influenced by environmental conditions (18–20). Mosquito vector competence to CHIKV and DENV seems to be determined by genotype-by-genotype interactions, in which successful transmission depends on some specific combination of mosquito and viral genetic characteristics (21–26). CHIKV has four major lineages: East-Central-South Africa (ECSA), West Africa, Asian, and the Indian Ocean, a monophyletic lineage descendant from the ECSA group (27). The CHIKV lineages have displayed distinct transmission efficiencies in mosquito vector species and populations (25, 28, 29). Throughout the 2005-2006 CHIKV epidemic in the Indian Ocean region, a CHIKV lineage strain harboring a substitution of an alanine to valine at position 226 of the E1 envelope glycoprotein (E1-A226V) was better transmitted by A. albopictus (22, 25, 30). It was later shown that other positions in the E2 glycoprotein exert epistatic effects on the position E1-226V (23, 24), and some substitutions can block the adaptation of E1-226V to A. albopictus. These epistatic interactions are lineage specific.
Determination of vector competence of mosquito populations is a key parameter in evaluating the risk of CHIKV transmission and spread. Given the alarming epidemiological situation due to the very recent chikungunya outbreak affecting the Caribbean islands, the need for evaluation of the vector competence of American mosquito populations is compelling. Until now, studies were only limited to mosquitoes from the United States and the French Caribbean (31–34). With the aim of understanding the factors that may influence CHIKV emergence in the Americas and the risk of a CHIKV epidemic spreading throughout the continent, we carried out a comprehensive Pan-American evaluation of vector competence of 35 A. aegypti and A. albopictus populations from 10 countries toward three CHIKV isolates belonging to two distinct lineages.
MATERIALS AND METHODS
Ethics statement.
The Institut Pasteur animal facility has received accreditation from the French Ministry of Agriculture to perform experiments on live animals in compliance of the French and European regulations on care and protection of laboratory animals. This study was approved by the Institutional Animal Care and Use Committee (IACUC) at the Institut Pasteur. No specific permits were required for the described field studies in locations that are not protected in any way and did not involve endangered or protected species.
Mosquitoes.
Thirty-five mosquito populations collected in 10 countries from North, Central, and South America were used: 22 populations of A. aegypti and 13 of A. albopictus (Fig. 1; Table 1). The mosquitoes were field collected in 2012 with ovitraps (10 to 58 per collection site). The mosquito collection sites were strategically chosen in order to essentially represent the diverse climates, environments, ecotopes, and dengue epidemiological history across the American continent. The field-collected eggs were immersed in water for hatching; larvae were split by 100 to 150 individuals per pan and fed with yeast tablets. Emerging adults were maintained in cages at 28 ± 1°C with a 14-h-light/10-h-dark cycle, 80% relative humidity, and supplied with a 10% sucrose solution. The F1 generation was used for all infection assays.
FIG 1.
Mosquito populations tested. The color code indicates localities where only A. aegypti (red), only A. albopictus (blue), and both A. aegypti and A. albopictus (green) mosquitoes were collected. TYS, Tyson, MO; VRB, Vero Beach, FL; MXC, Chiapas, Mexico; PAN, Panamá, Panama; DEL, Delta Amacuro, Venezuela; TUM, Tumbes, Peru; PUM, Punchana, Peru; MAN, Manaus, Brazil; STR, Santarém, Brazil; PNM, Parnamirim, Brazil; CAB, Campos Belos, Brazil; CPG, Campo Grande, Brazil; JRB, Jurujuba, Brazil; PAQ, Paquetá, Brazil; VAZ, Vaz Lobo, Brazil; BEL, Belford Roxo, Brazil; SAN, Santos, Brazil; BMA, Monteagudo, Bolivia; SDG, Salto del Guairá, Paraguay; ASU, Asunción, Paraguay; SAL, Salto, Uruguay; MIA, Misiones, Argentina; ACO, Corrientes, Argentina; BUE, Buenos Aires, Argentina.
TABLE 1.
Mosquito populations used in this study by country of collection from north to south
| Mosquito population | Collection site | Country | Coordinates | Mosquito species used | Climate | Dominant vegetation | Environment | History of dengue incidencea |
|---|---|---|---|---|---|---|---|---|
| TYS | Tyson, MO | United States | 38°31′N, 90°33′W | A. albopictus | Temperate | Temperate grassland | Suburban | F |
| VRB | Vero Beach, FL | United States | 27°35′N, 80°22′W | A. aegypti, A. albopictus | Humid subtropical | Subtropical evergreen forest | Suburban | F |
| MXC | Tapachula | Mexico | 14°53′N, 92°15′W | A. aegypti, A. albopictus | Tropical wet and dry | Tropical deciduous forest | Suburban | M |
| PAN | Panamá/Colon | Panama | 08°59′N, 79°30′W/09°21′N, 79°53′W | A. aegypti, A. albopictus | Tropical wet and dry | Savanna | Urban and suburban | L |
| DEL | Delta Amacuro, Tucupita | Venezuela | 09°03′N, 62°02′W | A. aegypti | Tropical wet and dry | Savanna | Suburban | L |
| PUM | Punchana, Iquitos | Peru | 03°43′S, 73°15′W | A. aegypti | Tropical wet and dry | Amazon forest | Urban | H |
| TUM | Tumbes, Huaquillas | Peru | 03°29′S, 80°15′W | A. aegypti | Arid | Desert | Suburban | L |
| MAN | Manaus | Brazil | 03°06′S, 60°03′W | A. aegypti, A. albopictus | Tropical wet | Amazon forest | Suburban | H |
| STR | Santarém | Brazil | 02°25′S, 54°42′W | A. aegypti, A. albopictus | Tropical wet | Amazon forest | Suburban | M |
| PNM | Parnamirim | Brazil | 05°54′S, 35°16′W | A. aegypti, A. albopictus | Semiarid | Transitional tropical rainforest | Suburban | H |
| CAB | Campos Belos | Brazil | 13°02′S, 46°46′W | A. aegypti | Tropical wet and dry | Savanna | Urban | L |
| BEL | Belford Roxo, Rio de Janeiro | Brazil | 22°45′S, 43°24′W | A. aegypti, A. albopictus | Tropical wet and dry | Atlantic rain forest | Suburban | H |
| VAZ | Vaz Lobo, Rio de Janeiro | Brazil | 22°51′S, 43°19W | A. aegypti, A. albopictus | Tropical wet and dry | Atlantic rain forest | Urban | H |
| JRB | Jurujuba, Rio de Janeiro | Brazil | 22°55′S, 43°07′W | A. aegypti, A. albopictus | Tropical wet and dry | Atlantic rain forest | Suburban | L |
| PAQ | Paquetá, Rio de Janeiro | Brazil | 22°45′S, 43°06′W | A. aegypti, A. albopictus | Tropical wet and dry | Atlantic rain forest | Suburban island | M |
| SAN | Santos | Brazil | 23°57′S, 46°20′W | A. aegypti, A. albopictus | Tropical wet and dry | Atlantic rain forest | Suburban | M |
| CPG | Campo Grande | Brazil | 20°27′S, 54°37′W | A. aegypti | Tropical wet and dry | Savanna | Urban | H |
| BMA | Monteagudo | Bolivia | 19°48′S, 63°57′W | A. aegypti | Tropical wet and dry | Mountain forest | Urban | L |
| ASU | Asunción | Paraguay | 25°18′S, 57°37′W | A. aegypti | Tropical wet and dry | Chaco | Urban | M |
| SDG | Salto del Guairá | Paraguay | 24°03′S, 54°18′W | A. aegypti | Humid subtropical | Savanna | Suburban | L |
| MIA | Misiones | Argentina | 25°36′S, 54°34′W | A. albopictus | Humid subtropical | Paranaense forest | Rural | L |
| ACO | Corrientes | Argentina | 27°28′S, 58°50′W | A. aegypti | Humid subtropical | Humid Chaco | Urban | M |
| BUE | Buenos Aires | Argentina | 34°35′S, 58°22W | A. aegypti | Temperate | Pampas | Urban | L |
| SAL | Salto | Uruguay | 31°23′S, 57°58′W | A. aegypti | Temperate | Pampa | Urban | F |
F, free; L, low; M, mediun; H, high.
Viral strains.
Three CHIKV isolates belonging to two distinct lineages were used: two CHIKV isolates from La Réunion and one from New Caledonia. The isolates from La Réunion were the strains (i) CHIKV 05.115 (CHIKV_115) and (ii) CHIKV 06.21 (CHIKV_0621), both isolated in 2005 (35) and provided by the French National Reference Center for Arboviruses at the Institut Pasteur in Paris. The amino acid consensus sequences of these strains differed by only a single substitution: CHIKV_115 has an alanine at position 226 of the E1 envelope glycoprotein (E1-226A), whereas CHIKV_0621 harbors a valine at the same position (E1-226V). It has been shown the E1-A226V substitution is located in a region known to be involved in viral entry via fusion with endosomal membranes (36). Both strains have an alanine at position 98 of the E1 glycoprotein (E1-98A) that has been shown to exert no negative epistatic effects on position E1-226; the position E1-98 is located at the base of the fusion loop and presumably modulates the kinetics of the pH-dependent conformational changes and fusion reaction in the endosomal compartment (37). The viral titer estimated by serial 10-fold dilutions on Vero cells was 109 PFU/ml for both CHIKV_115 and CHIKV_0621. Both strains were isolated on A. albopictus C6/36 cells from human serum or viral stocks and were produced following three passages on A. albopictus C6/36 cells and then harvested and stored at −80°C until used for the mosquito experimental infection assays. The New Caledonia CHIKV strain referenced as NC/2011-568 (CHIKV_NC), was isolated in 2011 (28, 37) and provided by the Institut Pasteur of New Caledonia. Phylogenetic analysis using the complete CHIKV_NC genome nucleotide sequence demonstrated that CHIKV_NC belongs to the Asian lineage, displaying 98.1% nucleotide identity to other isolates of the Asian cluster of CHIKV phylogeny. The CHIKV_NC strain has an alanine at position E1-226 (E1-226A) and a threonine at position E1-98 (E1-98T). It has been shown that in contrast with the ECSA genotype, the substitution E1-98T exerts a negative epistatic interaction leading to blocking the ability of Asian CHIKV strains to adapt to A. albopictus via the E1-A226V substitution (24). The whole genome sequence of CHIKV_NC is available in GenBank under accession no. HE806461. CHIKV_NC 2nd passage was used for the experimental infections of mosquitoes. The titer of CHIKV_NC stocks was 108.1 PFU/ml.
Mosquito oral infections.
Five- to 7-day-old females were fed on an infectious blood meal containing 2 ml of washed rabbit erythrocytes and 1 ml of viral suspension supplemented with a phagostimulant (ATP) at a final concentration of 5 mM. The titer of all performed infectious blood-meals was 107.5 PFU/ml. Mosquito feeding was limited to 50 min. After the infectious blood meal, nonengorged females were discarded. Fully engorged females were transferred in cardboard containers and maintained with 10% sucrose at 28°±1°C. All 35 mosquito populations were challenged with the CHIKV_0621 strain (13 A. albopictus and 22 A. aegypti populations), whereas 22 populations (9 A. albopictus and 13 A. aegypti) were challenged with the CHIKV_115 strain and 6 populations (3 A. albopictus and 3 A. aegypti) with CHIKV_NC. Mosquito populations from the same location were simultaneously tested with the CHIKV_0621 and CHIKV_115 strains.
Dissemination and transmission analysis.
Batches of ∼30 mosquitoes of each combination of mosquito population and virus strain were analyzed at days 7 and 10 postinfection (p.i.) for all CHIKV strains tested. Days p.i. were defined according to the kinetics of CHIKV dissemination and transmission efficiencies (DE and TE, respectively) in A. albopictus mosquitoes from Paquetá, Rio de Janeiro, Brazil (maximum at day 7 p.i. and slight decrease by day 10) (Fig. 2). To estimate viral dissemination, heads were removed from mosquitoes and ground in 250 μl of Leibovitz L15 medium (Invitrogen) supplemented with 2% fetal bovine serum (FBS) for further inoculation onto A. albopictus C6/36 cell culture in 96-well plates. After incubation at 28°C for 3 days, plates were stained using hyperimmune ascetic fluid specific to CHIKV as the primary antibody. Alexa Fluor 488 goat anti-mouse IgG was used as the second antibody (Life Technologies).
FIG 2.

Dissemination (A) and transmission (B) efficiencies of two CHIKV isolates and two clones of the respective viral isolates in A. albopictus mosquitoes from Paquetá, Rio de Janeiro, Brazil. At days 1, 2, 3, 7, and 10 after an infectious blood meal, mosquitoes were sacrificed, and heads and saliva were collected for determination of their infectious status. Mosquito heads were individually ground in 250 μl Leibovitz L15 medium supplemented with 4% FBS, following inoculation onto an A. albopictus C6/36 cell monolayer in 96-well plates and incubation at 28°C for 3 days. Plates were fixed with 3.6% formaldehyde, washed three times with PBS, and analyzed by indirect immunofluorescence assay (IFA). For saliva collection, each mosquito had the wings and legs removed, and the proboscis was inserted into a 20-μl tip containing 5 μl of FBS. After 45 min of salivation, FBS containing saliva was expelled into 45 μl of Leibovitz L15 medium and inoculated onto an A. albopictus C6/36 cell monolayer in 96-well plates. Plates were incubated and stained (IFA) as described in Materials and Methods. Dissemination efficiency corresponds to the proportion of mosquito females with disseminated virus in the head among the tested mosquitoes. Transmission efficiency corresponds to the proportion of mosquitoes with infectious saliva among the tested mosquitoes. CHIKV_0621 is a strain isolated from La Réunion (E1-226V substitution), CHIKV_115 is a strain isolated from La Réunion (E1-226A), CHIKV_0621 (V) is a clone corresponding to a single virus isolated from CHIKV_0621, and CHIKV_115 (A) is a clone corresponding to a single virus isolated from CHIKV_115. Clones were provided by C. Arias-Goeta, Institut Pasteur, Paris, France.
To estimate viral transmission, saliva was collected from individual mosquitoes as described in reference 38. For collection, the wings and legs were removed from each mosquito and the proboscis was inserted into a 20-μl tip containing 5 μl of FBS. After 45 min of salivation, FBS containing saliva was expelled into 45 μl of Leibovitz L15 medium for titration. One limitation of this technique is that the volume of saliva delivered by females could not be estimated.
Dissemination efficiency (DE) corresponds to the proportion of mosquitoes with virus detected in heads among tested ones (i.e., engorged mosquitoes which have survived until the day of examination). Transmission efficiency corresponds to the proportion of mosquitoes with virus in the saliva among tested ones (i.e., surviving females, including females unable to disseminate the virus and those able to disseminate). The number of infectious particles per saliva sample was estimated by titration using a focus fluorescent assay on A. albopictus C6/36 cells. Samples were serially diluted and inoculated onto C6/36 cells in 96-well plates, following incubation at 28°C for 3 days, and then the plates were stained as explained above.
Statistical analysis.
Statistical analyses were performed with STATISTICA 8 software (StatSoft, Inc., Tulsa, OK). The numbers of infectious particles in saliva were compared using the Kruskal-Wallis test. Dissemination and transmission efficiencies were compared using the chi-square test. Kruskal-Wallis Z multiple-comparison test was used to compare more than 5 dissemination and transmission efficiency rates.
RESULTS
DE.
To measure the ability of American A. aegypti and A. albopictus mosquitoes to allow CHIKV to overcome the midgut barrier, dissemination efficiency (DE) was assessed for each pairing of mosquito population and virus strain at days 7 and 10 p.i. (Tables 2 and 3).
TABLE 2.
Dissemination efficiency of three CHIKV isolates in 22 A. aegypti and 13 A. albopictus populations from 10 American countries at day 7 postinfection
| Country | Mosquito populationa | % dissemination efficiency (no. of mosquitoes)b |
|||||
|---|---|---|---|---|---|---|---|
| CHIKV_0621 |
CHIKV_115 |
CHIKV_NC |
|||||
| A. aegypti | A. albopictus | A. aegypti | A. albopictus | A. aegypti | A. albopictus | ||
| United States | TYS | ND | 96.7 (30) | ND | 83.3(30) | ND | ND |
| VRB | 100 (30) | 93.3 (30) | 100 (18) | 73.3 (30)* | ND | ND | |
| Mexico | MXC | 96.7 (30) | 73.3 (30)* | 96.7 (30) | 66.7 (30)* | ND | ND |
| Panama | PAN | 96.7 (30) | 96.7 (30) | 96.7 (30) | 93.3 (30) | 100 (30) | 96.7 (30) |
| Venezuela | DEL | 100 (23) | ND | 100 (28) | ND | ND | ND |
| Peru | TUM | 100 (30) | ND | ND | ND | ND | ND |
| PUM | 100 (30) | ND | 100 (29) | ND | ND | ND | |
| Brazil | MAN | 100 (30) | 96.7 (30) | ND | 90.3 (31) | 100 (30) | 90 (30) |
| STR | 100 (30) | 100 (30) | ND | 88.4 (26) | ND | ND | |
| PNM | 100 (30) | 93.3 (30) | ND | ND | ND | ND | |
| CAB | 100 (30) | ND | ND | ND | ND | ND | |
| CPG | 100 (30) | ND | 100 (30) | ND | ND | ND | |
| JRB | 100 (30) | 100 (30) | 100 (30) | ND | ND | ND | |
| PAQ | 100 (30) | 87.1 (31) | 100 (30) | 96.9 (29) | ND | ND | |
| VAZ | 100 (30) | 91.3 (23) | ND | ND | ND | ND | |
| BEL | 100 (30) | 90.9 (22) | ND | ND | ND | ND | |
| SAN | 93.3 (30) | 100 (30) | ND | 87.5 (8) | ND | ND | |
| Bolivia | BMA | 100 (30) | ND | 100 (30) | ND | ND | ND |
| Paraguay | SDG | 100 (30) | ND | ND | ND | ND | ND |
| ASU | 100 (30) | ND | 96.7 (30) | ND | ND | ND | |
| Uruguay | SAL | 100 (30) | ND | 100 (30) | ND | ND | ND |
| Argentina | MIA | ND | 60 (30) | ND | 66.7 (26) | ND | 93.3 (30) |
| ACO | 100 (30) | ND | 100 (30) | ND | ND | ND | |
| BUE | 100 (30) | ND | 96.6 (29) | ND | 96.9 (33) | ND | |
Mosquito populations (from north to south): TYS, Tyson, MO; VRB, Vero Beach, FL; MXC, Chiapas, Mexico; PAN, Panamá, Panama; DEL, Delta Amacuro, Venezuela; TUM, Tumbes, Peru; PUM, Punchana, Peru; MAN, Manaus, Brazil; STR, Santarém, Brazil; PNM, Parnamirim, Brazil; CAB, Campos Belos, Brazil; CPG, Campo Grande, Brazil; JRB, Jurujuba, Brazil; PAQ, Paquetá, Brazil; VAZ, Vaz Lobo, Brazil; BEL, Belford Roxo, Brazil; SAN, Santos, Brazil; BMA, Monteagudo, Bolivia; SDG, Salto del Guairá, Paraguay; ASU, Asunción, Paraguay; SAL, Salto, Uruguay; MIA, Misiones, Argentina; ACO, Corrientes, Argentina; BUE, Buenos Aires, Argentina.
Dissemination efficiency corresponds to the proportion of mosquitoes with disseminated virus in heads among tested ones. The numbers of analyzed mosquitoes are shown in parentheses. The titer of infectious blood meals was 107.5 PFU/ml. CHIKV_0621 was isolated from La Réunion (ECSA genotype, E1-226V and E1-98A substitutions), CHIKV_115 was isolated from La Réunion (ECSA genotype, E1-226A and E1-98A substitutions), and CHIKV_NC was isolated from New Caledonia (Asian genotype, E1-226A and E1-98T substitutions). ND, not determined. Statistically significant differences in dissemination efficiency between the two mosquito species for a given virus are shown by asterisks (P < 0.05).
TABLE 3.
Dissemination efficiency of three CHIKV isolates in 22 A. aegypti and 13 A. albopictus populations from 10 American countries at day 10 postinfection
| Country | Mosquito populationa | % dissemination efficiency (no. of mosquitoes)b |
|||||
|---|---|---|---|---|---|---|---|
| CHIKV_0621 |
CHIKV_115 |
CHIKV_NC |
|||||
| A. aegypti | A. albopictus | A. aegypti | A. albopictus | A. aegypti | A. albopictus | ||
| United States | TYS | ND | 93.3 (30) | ND | 63.6 (11) | ND | ND |
| VRB | 100 (30) | 85.7 (7)* | ND | 96.7 (30) | ND | ND | |
| Mexico | MXC | 93.3 (30) | 70.0 (30)* | 100 (30) | 53.3 (30)*** | ND | ND |
| Panama | PAN | 100 (30) | 96.7 (30) | 96.7 (30) | 83.3 (30) | 100 (30) | 96.7 (30) |
| Venezuela | DEL | 100 (10) | ND | 100 (15) | ND | ND | ND |
| Peru | TUM | 100 (30) | ND | ND | ND | ND | ND |
| PUM | 100 (29) | ND | 100 (30) | ND | ND | ND | |
| Brazil | MAN | 100 (30) | 100 (36) | ND | 97.1 (34) | 100 (30) | 93.3 (30) |
| STR | 100 (30) | 100 (20) | ND | ND | ND | ND | |
| PNM | 100 (30) | 90 (30) | ND | ND | ND | ND | |
| CAB | 100 (30) | ND | ND | ND | ND | ND | |
| CPG | 100 (30) | ND | 100 (29) | ND | ND | ND | |
| JRB | 100 (30) | 100 (30) | 100 (30) | ND | ND | ND | |
| PAQ | 100 (30) | 87.5 (32)* | 100 (30) | ND | ND | ND | |
| VAZ | 96.7 (30) | 100 (32) | ND | ND | ND | ND | |
| BEL | 100 (30) | 88.9 (27) | ND | ND | ND | ND | |
| SAN | 100 (29) | 100 (30) | ND | ND | ND | ND | |
| Bolivia | BMA | 100 (30) | ND | 100 (30) | ND | ND | ND |
| Paraguay | SDG | 100 (30) | ND | ND | ND | ND | ND |
| ASU | 100 (30) | ND | 93.3 (30) | ND | ND | ND | |
| Uruguay | SAL | 100 (30) | ND | 100 (30) | ND | ND | ND |
| Argentina | MIA | ND | 93.3 (30) | ND | 80 (30) | ND | 96.7 (30) |
| ACO | 100 (30) | ND | 96.7 (30) | ND | ND | ND | |
| BUE | 96.7 (30) | ND | 100 (30) | ND | 90 (30) | ND | |
Mosquito populations (from north to south): TYS, Tyson, MO; VRB, Vero Beach, FL; MXC, Chiapas, Mexico; PAN, Panamá, Panama; DEL, Delta Amacuro, Venezuela; TUM, Tumbes, Peru; PUM, Punchana, Peru; MAN, Manaus, Brazil; STR, Santarém, Brazil; PNM, Parnamirim, Brazil; CAB, Campos Belos, Brazil; CPG, Campo Grande, Brazil; JRB, Jurujuba; Brazil; PAQ, Paquetá, Brazil; VAZ, Vaz Lobo, Brazil; BEL, Belford Roxo, Brazil; SAN, Santos, Brazil; BMA, Monteagudo, Bolivia; SDG, Salto del Guairá, Paraguay; ASU, Asunción, Paraguay; SAL, Salto, Uruguay; MIA, Misiones, Argentina; ACO, Corrientes, Argentina; BUE, Buenos Aires, Argentina.
Dissemination efficiency corresponds to the proportion of mosquitoes with disseminated virus in heads among tested ones. Numbers of analyzed mosquitoes are shown in parentheses. CHIKV_0621 was isolated from La Réunion (ECSA genotype, E1-226V and E1-98A substitutions), CHIKV_115 was isolated from La Réunion (ECSA genotype, E1-226A and E1-98A substitutions), and CHIKV_NC was isolated from New Caledonia (Asian genotype, E1-226A and E1-98T substitutions). ND, not determined. Statistically significant differences in dissemination efficiencies between the two mosquito species for a given virus are shown by asterisks (*, P < 0.05; ***, P < 0.001).
All A. aegypti and A. albopictus populations showed similar DE values at days 7 and 10 p.i. for the three CHIKV isolates (chi-square test, P > 0.05). For CHIKV_0621, DE at day 7 p.i. ranged from 60% to 100% for A. albopictus and from 93.3% to 100% for A. aegypti. For CHIKV_115, DE at day 7 varied from 66.7% to 96.9% for A. albopictus and from 96.6% to 100% for A. aegypti, while for CHIKV_NC, DE ranged from 90% to 96.7% for A. albopictus and from 96.9% to 100% for A. aegypti. The A. aegypti populations tested displayed similar DE values of around 100% for the three CHIKV isolates (chi-square test, P > 0.05). Likewise, DE values obtained for A. albopictus were extensively high, although rates were significantly heterogeneous for CHIKV_0621 (chi-square test, P < 0.05) and CHIKV_115 (chi-square test, P < 0.05). Thus, when comparing DE values for a given virus between the two mosquito species sampled in a same location, no significant difference was found, except for MXC in Mexico when infected with CHIKV_0621 (chi-square test, P < 0.05) and CHIKV_115 (chi-square test, P < 0.05) and for VRB in the United States when infected with CHIKV_115 (chi-square test, P < 0.05). In these last three cases, A. aegypti exhibited a higher DE than A. albopictus collected in the same site whatever the viral strain. In addition, no difference was observed in DE values between the three A. aegypti and A. albopictus populations challenged with the CHIKV_NC isolate (chi-square test, P > 0.05).
TE.
In order to determine the ability of American A. aegypti and A. albopictus mosquitoes to sustain CHIKV transmission, we assessed transmission efficiency (TE) at days 7 and 10 p.i. Only TE values at day 7 p.i are presented in Fig. 3 and 4. (For TE values at day 10 p.i., see Table S1 in the supplemental material.) The TE values obtained for A. aegypti and A. albopictus were highly heterogeneous and lower than the DE values.
FIG 3.
Transmission efficiency of three CHIKV isolates in 35 A. albopictus and A. aegypti populations from 10 American countries at day 7 postinfection. After an infectious blood meal, mosquitoes were sacrificed, and saliva was collected from individual mosquitoes and titrated by focus fluorescent assay on A. albopictus C6/36 cells to determine infectious status. Transmission efficiency corresponds to the proportion of mosquitoes with infectious saliva among those tested. Viral strains are as follows: CHIKV_0621 was isolated from La Réunion (ECSA genotype, E1-226V and E1-98A substitutions), CHIKV_115 was isolated from La Réunion (ECSA genotype, E1-226A and E1-98A substitutions), and CHIKV_NC was isolated from New Caledonia (Asian genotype, E1-226A and E1-98T substitutions). Mosquito populations are as follows (from north to south): TYS, Tyson, MO; VRB, Vero Beach, FL; MXC, Chiapas, Mexico; PAN, Panamá, Panama; DEL, Delta Amacuro, Venezuela; TUM, Tumbes, Peru; PUM, Punchana, Peru; MAN, Manaus, Brazil; STR, Santarém, Brazil; PNM, Parnamirim, Brazil; CAB, Campos Belos, Brazil; CPG, Campo Grande, Brazil; JRB, Jurujuba, Brazil; PAQ, Paquetá, Brazil; VAZ, Vaz Lobo, Brazil; BEL, Belford Roxo, Brazil; SAN, Santos, Brazil; BMA, Monteagudo, Bolivia; SDG, Salto del Guairá, Paraguay; ASU, Asunción, Paraguay; SAL, Salto, Uruguay; MIA, Misiones, Argentina; ACO, Corrientes, Argentina; and BUE, Buenos Aires, Argentina. Error bars show 95% confidence intervals.
FIG 4.
Transmission efficiency of CHIKV_0621 and CHIKV_115 isolates in 35 A. aegypti and A. albopictus populations from 10 American countries at day 7 postinfection. Transmission efficiency corresponds to the proportion of mosquitoes with infectious saliva among those tested. The color code indicates different degrees of transmission efficiency (TE): yellow, mosquito strains with TE ≤ 30% (low TE); pale orange, strains with 30% < TE < 70% (moderate TE); red, strains with TE ≥ 70% (high TE). The viral strains are as follows: CHIKV_0621 was isolated from La Réunion (ECSA genotype, E1-226V substitution) and CHIKV_115 isolated from La Réunion (ECSA genotype, E1-226A substitution). The mosquito populations are as follows (from north to south): TYS, Tyson, MO; VRB, Vero Beach, FL; MXC, Chiapas, Mexico; PAN, Panamá, Panama; DEL, Delta Amacuro, Venezuela; TUM, Tumbes, Peru; PUM, Punchana, Peru; MAN, Manaus, Brazil; STR, Santarém, Brazil; PNM, Parnamirim, Brazil; CAB, Campos Belos, Brazil; CPG, Campo Grande, Brazil; JRB, Jurujuba, Brazil; PAQ, Paquetá, Brazil; VAZ, Vaz Lobo, Brazil; BEL, Belford Roxo, Brazil; SAN, Santos, Brazil; BMA, Monteagudo, Bolivia; SDG, Salto del Guairá, Paraguay; ASU, Asunción, Paraguay; SAL, Salto, Uruguay; MIA, Misiones, Argentina; ACO, Corrientes, Argentina; BUE, Buenos Aires, Argentina.
When mosquitoes were exposed to CHIKV_0621, TE values ranged from 13.3% to 96.7% at day 7 p.i. and 6.7% to 85.2% at day 10 p.i. A. albopictus better transmitted CHIKV_0621 than A. aegypti at day 7 p.i. (mean ± confidence interval [CI], 44.7% ± 7.8% for A. aegypti and 55.8% ± 12.3% for A. albopictus) and at day 10 p.i. (mean ± CI, 33.1% ± 6.2% for A. aegypti and 55.5% ± 12.0% for A. albopictus). Within the same mosquito species, TE values were significantly different (chi-square test, P < 0.05) at days 7 and 10 p.i. When considering each of the 10 populations where the two species coexist (VRB, MXC, PAN, MAN, PNM, JRB, PAQ, VAZ, BEL, and SAN), A. albopictus exhibited a higher TE than A. aegypti when infected with CHIKV_0621, except for the VRB population from Florida (Fig. 3 and 4; see Table S1 in the supplemental material).
When mosquitoes were infected with CHIKV_115, TE values comprised between 11.1% and 82.1% at day 7 p.i. and 10% and 76.7% at day 10 p.i. A. aegypti better transmitted CHIKV_115 than A. albopictus at day 7 p.i. (mean ± CI, 49.5% ± 10.3% for A. aegypti and 49.5% ± 13.6% for A. albopictus). Within the same mosquito species, TE values were significantly different (chi-square test, P < 0.05) at days 7 and 10 p.i. When considering each of the four populations where the two species coexist (VRB, MXC, PAN, and PAQ), one species did not present a clear-cut advantage over the other to transmit CHIKV_115 (Fig. 3 and 4; see Table S1 in the supplemental material).
Interestingly, among the eight A. albopictus populations simultaneously challenged with CHIKV_0621 and CHIKV_115, four showed unexpected lower TE for CHIKV_115 and one displayed equal rates (Fig. 3; see Table S1 in the supplemental material). Remarkably, TE rates were heterogeneous even between A. albopictus populations geographically close, i.e., from Rio de Janeiro, Brazil (JRB, PAQ, BEL, and VAZ), when exposed to the same CHIKV_0621 isolate (Fig. 3 and 4).
Finally, when mosquitoes were exposed to the CHIKV_NC strain, TE values varied from 30% to 83.3% at day 7 p.i. and from 26.7% to 53.3% at day 10 p.i. A. aegypti better transmitted CHIKV_NC than A. albopictus at day 7 p.i. (mean ± CI, 64.5% ± 20.7% for A. aegypti and 48.9% ± 25.1% for A. albopictus). Within the same mosquito species, TE values were significantly different (chi-square test, P < 0.05) at day 7 and not at day 10 p.i. (chi-square test, P > 0.05) (see Table S1 in the supplemental material).
We also found that 23% to 56% of mosquitoes collected in temperate regions, A. albopictus TYS (Tyson, MO) and A. aegypti SAL (Salto, Uruguay) and BUE (Buenos Aires, Argentina) were able to efficiently transmit CHIKV_0621. Moreover, A. aegypti mosquitoes from the last two sites of the Southern Cone were also competent to efficiently transmit CHIKV_0115 and CHIKV_NC at day 7 p.i., respectively (SAL, 70% for CHIKV_115; BUE, 48.3% for CHIKV_115 and 63.6% for CHIKV_NC).
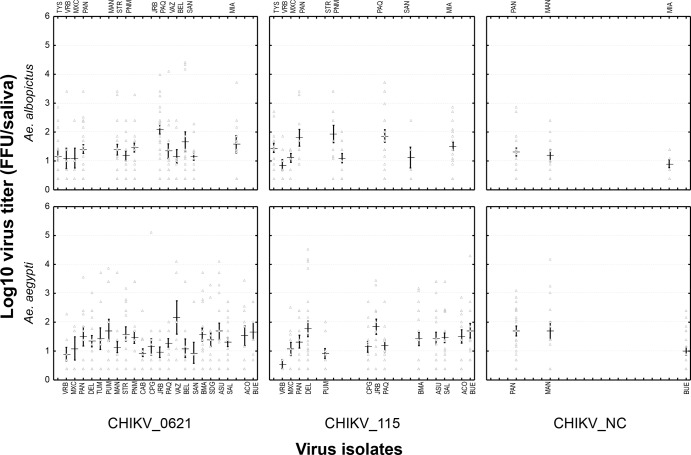
Intensity of transmission.
The intensity of viral transmission can be calculated by estimating the viral load in saliva collected from mosquitoes. When infected with the CHIKV_0621 isolate, the number of viral particles in saliva ranged from 0.4 to 4.4 log10 particles for A. albopictus and from 0.4 to 5.1 log10 particles for A. aegypti. Concerning mosquitoes infected with the CHIKV_115 isolate, the number of viral infectious particles varied from 0.4 to 4.7 log10 for A. albopictus and from 0.4 to 5.0 log10 for A. aegypti. For mosquitoes exposed to CHIKV_NC, the viral load in saliva ranged from 0.4 to 2.9 log10 particles for A. albopictus and from 0.4 to 4.2 log10 particles for A. aegypti (Fig. 5). Viral loads of the three tested CHIKV strains were equivalent in A. aegypti populations, whereas A. albopictus displayed a slightly lower titer when challenged with CHIKV_NC in comparison to CHIKV_0621 and CHIKV_115, both at day 7 p.i. Viral loads were highly heterogeneous between individuals belonging to the same population and infected with a given viral strain, but the means calculated for each mosquito population were roughly similar overall. Indeed, when comparing viral loads in saliva between mosquito strains for a given virus at days 7 and 10 p.i. (Fig. 5; see Fig. S1 in the supplemental material), no significant differences were found for either A. aegypti or A. albopictus (Kruskal-Wallis test, P > 0.05), except for A. albopictus challenged with CHIKV_115.
FIG 5.
Viral loads of three CHIKV isolates in saliva of A. albopictus and A. aegypti mosquitoes from 35 populations from the Americas at day 7 postinfection. At day 7 after an infectious blood meal, mosquitoes were sacrificed, and saliva was collected from individual mosquitoes and titrated by focus fluorescent assay on A. albopictus C6/36 cells. The viral strains are as follows: CHIKV_0621 was isolated from La Réunion (ECSA genotype, E1-226V and E1-98A substitutions), CHIKV_115 was isolated from La Réunion (ECSA genotype, E1-226A and E1-98A substitutions), and CHIKV_NC was isolated from New Caledonia (Asian genotype, E1-226A and E1-98T substitutions). The mosquito populations are as follows (from north to south): TYS, Tyson, MO; VRB, Vero Beach, FL; MXC, Chiapas, Mexico; PAN, Panamá, Panama; DEL, Delta Amacuro, Venezuela; TUM, Tumbes, Peru; PUM, Punchana, Peru; MAN, Manaus, Brazil; STR, Santarém, Brazil; PNM, Parnamirim, Brazil; CAB, Campos Belos, Brazil; CPG, Campo Grande, Brazil; JRB, Jurujuba, Brazil; PAQ, Paquetá, Brazil; VAZ, Vaz Lobo, Brazil; BEL, Belford Roxo, Brazil; SAN, Santos, Brazil; BMA, Monteagudo, Bolivia; SDG, Salto del Guairá, Paraguay; ASU, Asunción, Paraguay; SAL, Salto, Uruguay; MIA, Misiones, Argentina; ACO, Corrientes, Argentina; BUE, Buenos Aires, Argentina. Error bars refer to the standard error of the mean titer for each pairing of mosquito population and virus strain.
DISCUSSION
All 35 populations of A. aegypti and A. albopictus mosquitoes collected throughout the Americas were susceptible to CHIKV infection by all three tested genotypes. Thus, temperate as well as tropical and subtropical North, Central, and South American Aedes mosquitoes are efficient CHIKV vectors. A. albopictus better transmitted the epidemic CHIKV_0621 strain isolated on La Réunion Island in 2006 (35) than A. aegypti, whereas the latter species was more capable at transmitting the original strain, CHIKV_115, both belonging to the ECSA genotype (39). The Asian genotype represented by the CHIKV_NC strain (28) was better transmitted by A. aegypti, although it was also efficiently transmitted by A. albopictus.
Most American Aedes mosquitoes are highly susceptible to CHIKV.
More than 60% of mosquitoes per population were able to disseminate CHIKV after crossing the midgut barrier (i.e., entry in epithelial cells, viral replication, and release of virions from the midgut basal lamina). Thus, after being ingested with a blood meal provided at a titer of 107.5 PFU/ml, CHIKV succeeded in disseminating within the mosquito hemocele, which is an essential prerequisite for transmission. It has been shown that a titer of ∼104 PFU/ml in monkeys was sufficient to infect mosquitoes (40). CHIKV transmission was highly heterogeneous in American mosquitoes, ranging from 11.1% to 96.7% at day 7 p.i. when considering all CHIKV strains. It should be underlined that we are not able to provide a control of salivation, and we hypothesize that a CHIKV-negative saliva sample did not correspond to mosquitoes unable to salivate but to mosquitoes delivering noninfected saliva. As expected from previous studies (22, 25, 30, 41), A. albopictus better transmitted the epidemic strain CHIKV_0621 of the ECSA genotype than A. aegypti, even in cases where both mosquito species cohabit. A. aegypti transmitted preferentially CHIKV_115 and also the Asian genotype CHIKV_NC in accordance with previous findings (28). CHIKV Asian strains have a particular E1-98T substitution that constrains CHIKV adaptation to A. albopictus via E1-A226V mutation (24). A. aegypti mosquitoes are more abundant in the Americas than A. albopictus mosquitoes, and the E1-98T substitution of CHIKV viral strains does not have a negative effect on CHIKV interaction with A. aegypti. Thus, CHIKV Asian strains together with the CHIKV ECSA strains represent a real danger to the Americas. Intriguingly, the CHIKV strain isolated during the last outbreak in the Caribbean also belongs to the Asian genotype (42) primarily transmitted in the past by A. aegypti. Although the intensity of transmission is highly variable between mosquitoes, the mean numbers of viral particles delivered by mosquitoes were quite similar for each combination of mosquito strain and viral strain.
Mosquitoes collected in tropical Latin America, Panama, Venezuela, Brazil, Bolivia, Paraguay, Argentina, and Uruguay showed the highest transmission efficiency, with up to 10,000 viral particles detected in mosquito saliva. Interestingly, mosquitoes from the main Brazilian city of Rio de Janeiro showed high transmission efficiencies. For example, 96.7% of A. albopictus JRB mosquitoes were able to transmit CHIKV_0621 (see Table S1 in the supplemental material). Moreover, the extrinsic incubation periods of CHIKV (i.e., the time necessary for the virus to be detected in saliva ready for transmission after being ingested with the blood meal [43]), in both mosquito species are quite short (38). Indeed, an A. albopictus population from Rio de Janeiro (PAQ) was able to transmit infectious viral particles as rapidly as 2 days p.i. (Fig. 2). Therefore, the risk of CHIKV establishment in densely populated cities, such as Rio de Janeiro, hosting more than 6 million people and infested by anthropophilic Aedes mosquitoes, should be considered very high.
Mosquitoes from temperate regions of the Americas are potentially capable of sustaining CHIKV transmission.
The ability of CHIKV to extend its natural range of distribution to include temperate regions was exemplified by the Italian outbreak in 2007 and the French local, autochthonous cases in 2010 (44, 45). In the Americas, more than 100 imported CHIKV cases were detected in the United States between 1995 and 2009 (11). Some of them developed a viremia high enough to infect mosquitoes. We found that 56.7% of A. albopictus TYS mosquitoes from Tyson, MO, and 83.3% of A. aegypti SAL mosquitoes from Salto, Uruguay, were able to transmit CHIKV_0621 at day 7 p.i. (see Table S1 in the supplemental material). Transmission efficiencies were lower for A. aegypti BUE from Buenos Aires, Argentina (i.e., 23.3%) (Fig. 3; see Table S1), but were higher when infected with the CHIKV_NC Asian genotype (i.e., 63.6%) (Fig. 3; see Table S1). Therefore, the establishment of CHIKV in temperate American countries is not simply a fiction, even if less than 30% of both mosquito species collected in the southern part of the United States (VRB from Florida) were able to transmit CHIKV_0621. It has been found that A. albopictus mosquitoes from Florida are more competent vectors of CHIKV than A. aegypti (31–33). Outbreaks of DENV, also transmitted by Aedes mosquitoes, have occurred in Texas and Florida in the past few years (46), reinforcing the risk of epidemics due to imported arboviruses in the United States. Local transmission of CHIKV could be maintained if the virus is introduced in the right place at the right time. Taken together, these findings underline the high variation of susceptibility to CHIKV of American mosquitoes, calling for the inclusion of other factors (biological and environmental) in assessing potential risk of transmission (47). Moreover, the mosquitoes' genetic structure should be promptly investigated. Phylogenetic analysis of both mosquito species should bring additional information on the colonization history of A. aegypti and A. albopictus in the different countries of the Americas (48, 49). A. aegypti was most likely introduced in North America during the slave trade (50), while A. albopictus was established in 1985 in the United States (51), probably introduced in shipments of used tires from Japan (52), and in Brazil in 1986 (53), probably arriving from tropical Asia (52).
The fear becomes a reality.
Still absent until very recently, CHIKV was detected for the first time in the Americas in late December 2013. Currently, among the 2,030 suspected CHIKV cases from the island of Saint-Martin in the Caribbean, more than 765 were confirmed positive for CHIKV by serology (15). The virus then spread to neighboring islands: Saint-Barthelemy with 380 cases, Martinique with 3,940 cases, and Guadeloupe with 1,460 cases. Until now, 10 autochthonous cases have been reported in French Guiana, which maintains a daily air link with the two other French Overseas Territories of Guadeloupe and Martinique. We previously showed that A. aegypti mosquitoes from French Guiana and French West Indies were highly competent to disseminate CHIKV and that mosquito populations collected in dense housing environments exhibited the highest susceptibility (34). Thus, the risk of CHIKV spread and establishment is real and should concern all areas in the Americas where the vector mosquitoes are present.
Cocirculation of CHIKV and DENV could have great implication for human health.
Interestingly, DENV is still circulating in the Caribbean, together with CHIKV. Cases of DENV-CHIKV coinfection in patients were first reported in 1967 (54), and since the emergence of CHIKV, reports of coinfections have been increasing (10, 55–63). Both viruses are transmitted by the same mosquito vectors, A. aegypti and A. albopictus. Coinfection of a mosquito vector by two viruses can occur after two successive infectious blood meals taken from two different viremic hosts or after a single blood meal taken from a coinfected host. It has been shown that CHIKV and DENV can be delivered together in one mosquito bite (64). As coinfections are a quite common phenomenon, consequences for the clinical presentation of the disease are expected.
Finally, the assessment of vector competence should be considered a prerequisite to better evaluate the potential risk of CHIKV outbreaks once the virus is introduced from regions of endemicity. The numerous imported CHIKV viremic cases presaged the potential importance of this emerging arbovirus for the Americas, where both mosquito species are well established. In light of epidemics now starting in the Caribbean, it remains imperative to pursue and reinforce epidemiological and entomological surveillance actions and control against mosquitoes of the species A. aegypti and A. albopictus.
Supplementary Material
ACKNOWLEDGMENTS
We thank Anayansi C Valderrama, Carlos Eduardo Borda, Darío Vezzani, Daniel Sánchez-Guillén, Eduardo A. Lestani, Fátima Domingos, Gabriel Sylvestre, Gabriela Willat, Glenda Velásquez, Ima Braga, José Bento Lima, L. Phil Lounibos, Lorenzo Carceres, Marcelo Celestino dos Santos, Márcia Gonçalves de Castro, Maria de Lourdes Macoris, Mário Navarro, Nidia Martínez Acosta, Maria C. Carrasquilla, Maria Ignez L. Bersot, Mirian G. Palomino Salcedo, Mirko Rojas-Cortez, Oscar D. Salomon, Rafael Maciel-de-Freitas, Romeo Humberto Montoya, Sérgio L. Bessa Luz, Steve Juliano, Tamara Chávez Espada, Teresa F. Silva-do-Nascimento, and Yasmin Rubio-Palis for help with mosquito sampling in the field and/or mosquito rearing; Marie Vazeille and Laurence Mousson for technical advice; Myrielle Dupont-Pouzeyrol for providing CHIKV_NC; and Peter Sahlins and Henri Jupille for correcting the manuscript.
This work was funded by CNPq (grants 202106/2011-0 and 306340/2009-7), Instituto Oswaldo Cruz, Institut Pasteur (ACIP grant A-03-2012), and the French Government's Investissement d'Avenir program, Laboratoire d'Excellence “Integrative Biology of Emerging Infectious Diseases” (grant no. ANR-10-LABX-62-IBEID). A.V.-R. was supported by the French Ministry of Superior Education and Research and K.Z. by the European Community's Seventh Framework Programme (FP7/2007-2 013) under the project “VECTORIE,” EC grant agreement no. 261466, and the Foundation Inkermann (Fondation de France).
The authors declare that they have neither competing interests nor conflicts of interest related to this article.
R.L.-D.-O. and A.-B.F. conceived the study. R.L.-D.-O., A.V.-R., and K.Z. carried out experimental infections of mosquitos and performed titration assays. A.V.-R., R.L.-D.-O., and A.-B.F. drafted the manuscript. K.Z. and R.G. helped to draft and to revise the manuscript. All authors read and approved the final version of the manuscript.
Footnotes
Published ahead of print 26 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00370-14.
REFERENCES
- 1.Staples JE, Breiman RF, Powers AM. 2009. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin. Infect. Dis. 49:942–948. 10.1086/605496 [DOI] [PubMed] [Google Scholar]
- 2.Lourenço-de-Oliveira R, Vazeille M, Filipis AMB, Failloux A-B. 2004. Aedes aegypti in Brazil: genetically differentiated populations with high susceptibility to dengue and yellow fever viruses. Trans. R. Soc. Trop. Med. Hyg. 98:43–54. 10.1016/S0035-9203(03)00006-3 [DOI] [PubMed] [Google Scholar]
- 3.Lourenço-de-Oliveira R, Vazeille M, de Filippis AM, Failloux AB. 2003. Large genetic differentiation and low variation in vector competence for dengue and yellow fever viruses of Aedes albopictus from Brazil, the United States, and the Cayman Islands. Am. J. Trop. Med. Hyg. 69:105–114 http://www.ajtmh.org/content/69/1/105.long [PubMed] [Google Scholar]
- 4.Benedict MQ, Levine RS, Hawley WA, Lounibos LP. 2007. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 7:76–85. 10.1089/vbz.2006.0562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Organización Panamericana de la Salud. Dengue. http://new.paho.org/hq/index.php?option=com_content&view=article&id=264&Itemid=363&lang=es Accessed 6 January 2014
- 6.Organización Panamericana de la Salud. Epidemiological alert. Chikungunya fever, 9 December 2013. (http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&gid=23806+&Itemid=999999&lang=en) Accessed 6 January 2014
- 7.Figueiredo LT. 2007. Emergent arbovirus in Brazil. Rev. Soc. Bras. Med. Trop. 40:224–229. 10.1590/S0037-86822007000200016 [DOI] [PubMed] [Google Scholar]
- 8.Maciel-de-Freitas R, Aguiar R, Bruno RV, Guimarães MC, Lourenço-de-Oliveira R, Sorgine MH, Struchiner CJ, Valle D, O'Neill SL, Moreira LA. 2012. Why do we need alternative tools to control mosquito-borne diseases in Latin America? Mem. Inst. Oswaldo Cruz 107:828–829. 10.1590/S0074-02762012000600021 [DOI] [PubMed] [Google Scholar]
- 9.Mackenzie JS, Chua KB, Daniels PW, Eaton BT, Field HE, Hall RA, Halpin K, Johansen CA, Kirkland PD, Lam SK, McMinn P, Nisbet DJ, Paru R, Pyke AT, Ritchie SA, Siba P, Smith DW, Smith GA, Van den Hurk AF, Wang LF, Williams DT. 2012. Emerging viral diseases of Southeast Asia and the Western Pacific. Emerg. Infect. Dis. 7(Suppl 3):497–504. 10.3201/eid0707.017703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chahar HS, Bharaj P, Dar L, Guleria R, Kabra SK, Broor S. 2009. Co-infections with chikungunya virus and dengue virus in Delhi, India. Emerg. Infect. Dis. 15:1077–1080. 10.3201/eid1507.080638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibney KB, Fischer M, Prince HE, Kramer LD, St. George K, Kosoy OL, Laven JJ, Staples JE. 2011. Chikungunya fever in the United States: a fifteen year review of cases. Clin. Infect. Dis. 52:e121–e126. 10.1093/cid/ciq214 [DOI] [PubMed] [Google Scholar]
- 12.Chaves TS, Pellini AC, Mascheretti M, Jahnel MT, Ribeiro AF, Rodrigues SG, Vasconcelos PF, Boulos M. 2012. Travelers as sentinels for chikungunya fever, Brazil. Emerg. Infect. Dis. 18:529–530. 10.3201/eid1803.110838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. 2006. Chikungunya fever diagnosed among international travelers—United States, 2005–2006. MMWR Morb. Mortal. Wkly. Rep. 55:1040–1042 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5538a2.htm [PubMed] [Google Scholar]
- 14.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers F, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CIRE Antilles Guyane. Le chikungunya dans les Antilles_Guyane. Le point épidémiologique, n. 8, 17–23 February 2014. http://www.invs.sante.fr/fr/Publications-et-outils/Points-epidemiologiques/Tous-les-numeros/Antilles-Guyane/2014/Situation-epidemiologique-du-chikungunya-dans-les-Antilles.-Point-au-27-fevrier-2014 Accessed 27 February 2014
- 16.Lambrechts L, Failloux AB. 2012. Vector biology prospects in dengue research. Mem. Inst. Oswaldo Cruz 107:1080–1082. 10.1590/S0074-02762012000800022 [DOI] [PubMed] [Google Scholar]
- 17.Bennett KE, Olson KE, Muñoz ML, Fernández-Salas I, Farfán JA, Higgs S, Black WC, Beaty BJ. 2002. Variation in vector competence for dengue 2 virus among 24 collections of Aedes aegypti from Mexico and the United States. Am. J. Trop. Med. Hyg. 67:85–92 http://www.ajtmh.org/content/67/1/85.long [DOI] [PubMed] [Google Scholar]
- 18.Failloux AB, Vazeille M, Rodhain F. 2002. Geographic genetic variation in populations of the dengue virus vector Aedes aegypti. J. Mol. Evol. 55:653–663. 10.1007/s00239-002-2360-y [DOI] [PubMed] [Google Scholar]
- 19.Black WC, IV, Bennett KE, Gorrochótegui-Escalante N, Barrilas-Mury CV, Fernandez-Salas I, Munoz ML, Farfan-Ale JÁ, Olson KE, Beaty BJ. 2002. Flavivirus susceptibility in Aedes aegypti. Arch. Med. Res. 33:379–388. 10.1016/S0188-4409(02)00373-9 [DOI] [PubMed] [Google Scholar]
- 20.Tabachnick WJ. 2013. Nature, nurture and evolution of intra-species variation in mosquito arbovirus transmission competence. Int. J. Environ. Res. Public Health 10:249–277. 10.3390/ijerph10010249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosio CF, Fulton RE, Salasek ML, Beaty BJ, Black WC., IV 2000. Quantitative trait loci that control vector competence for dengue-2 virus in the mosquito Aedes aegypti. Genetics 156:687–698 http://www.genetics.org/content/156/2/687.long [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. 2007. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 3:e201. 10.1371/journal.ppat.0030201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsetsarkin KA, McGee CE, Volk SM, Vanlandingham DL, Weaver SC, Higgs S. 2009. Epistatic roles of E2 glycoprotein mutations in adaption of chikungunya virus to Aedes albopictus and Ae. aegypti mosquitoes. PLoS One 4:e6835. 10.1371/journal.pone.0006835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vazeille M, Moutailler S, Coudrier D, Rousseaux C, Khun H, Huerre M, Thiria J, Dehecq JS, Fontenille D, Schuffenecker I, Despres P, Failloux AB. 2007. Two chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS One 2:e1168. 10.1371/journal.pone.0001168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambrechts L. 2011. Quantitative genetics of Aedes aegypti vector competence for dengue viruses: towards a new paradigm? Trends Parasitol. 27:111–114. 10.1016/j.pt.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 26.Volk SM, Chen R, Tsetsarkin KA, Adams AP, Garcia TI, Sall AA, Nasar F, Schuh AJ, Holmes EC, Higgs S, Maharaj PD, Brault AC, Weaver SC. 2010. Genome-scale phylogenetic analyses of chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J. Virol. 84:6497–6504. 10.1128/JVI.01603-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dupont-Rouzeyrol M, Caro V, Guillaumot L, Vazeille M, D'Ortenzio E, Thiberge JM, Baroux N, Gourinat AC, Grandadam M, Failloux AB. 2012. Chikungunya virus and the mosquito vector Aedes aegypti in New Caledonia (South Pacific Region). Vector Borne Zoonotic Dis. 12:1036–1041. 10.1089/vbz.2011.0937 [DOI] [PubMed] [Google Scholar]
- 28.Vega-Rua A, Zouache K, Caro V, Diancourt L, Delaunay P, Grandadam M, Failloux AB. 2013. High efficiency of temperate Aedes albopictus to transmit chikungunya and dengue viruses in the Southeast of France. PLoS One 8:e59716. 10.1371/journal.pone.0059716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arias-Goeta C, Mousson L, Rougeon F, Failloux AB. 2013. Dissemination and transmission of the E1-226V variant of chikungunya virus in Aedes albopictus are controlled at the midgut barrier level. PLoS One 8:e57548. 10.1371/journal.pone.0057548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiskind MH, Pesko K, Westbrook CJ, Mores CN. 2008. Susceptibility of Florida mosquitoes to infection with chikungunya virus. Am. J. Trop. Med. Hyg. 78:422–425 http://www.ajtmh.org/content/78/3/422.full.pdf+html [PMC free article] [PubMed] [Google Scholar]
- 31.Richards SL, Anderson SL, Smartt CT. 2010. Vector competence of Florida mosquitoes for chikungunya virus. J. Vector Ecol. 35:439–443. 10.1111/j.1948-7134.2010.00105.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pesko K, Westbrook CJ, Mores CN, Lounibos LP, Reiskind MH. 2009. Effects of infectious virus dose and bloodmeal delivery method on susceptibility of Aedes aegypti and Aedes albopictus to chikungunya virus. J. Med. Entomol. 46:395–399. 10.1603/033.046.0228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Girod R, Gaborit P, Marrama L, Etienne M, Ramdini C, Rakotoarivony I, Dollin C, Carinci R, Issaly J, Dusfour I, Gustave J, Yp-Tcha MM, Yébakima A, Failloux AB, Vazeille M. 2011. High susceptibility to Chikungunya virus of Aedes aegypti from the French West Indies and French Guiana. Trop. Med. Int. Health 16:134–139. 10.1111/j.1365-3156.2010.02613.x [DOI] [PubMed] [Google Scholar]
- 34.Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney MC, Lavenir R, Pardigon N, Reynes JM, Pettinelli F, Biscornet L, Diancourt L, Michel S, Duquerroy S, Guigon G, Frenkiel MP, Bréhin AC, Cubito N, Desprès P, Kunst F, Rey FA, Zeller H, Brisse S. 2006. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 3:e263. 10.1371/journal.pmed.0030263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voss JE, Vaney MC, Duquerroy S, Vonrhein C, Girard-Blanc C, Crublet E, Thompson A, Bricogne G, Rey FA. 2010. Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography. Nature 468:709–712. 10.1038/nature09555 [DOI] [PubMed] [Google Scholar]
- 36.Tsetsarkin KA, Chen R, Leal G, Forrester N, Higgs S, Huang J, Weaver SC. 2011. Chikungunya virus emergence is constrained in Asia by lineage-specific adaptive landscapes. Proc. Natl. Acad. Sci. U. S. A. 108:7872–7877. 10.1073/pnas.1018344108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alibert A, Pfannstiel A, Grangeon JP. 2011. Chikungunya outbreak in New Caledonia in. Status report as at 22 August 2011. Informa'Action no. 34. https://www.spc.int/phs/ENGLISH/Publications/InformACTION/IA34/Status_report_Chikungunya_Outbreak_New_Caledonia-22Aug2011.pdf Accessed 7 January 2014
- 38.Dubrulle M, Mousson L, Moutailler S, Vazeille M, Failloux AB. 2009. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PLoS One 4:e5895. 10.1371/journal.pone.0005895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powers AM, Logue CH. 2007. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J. Gen. Virol. 88:2363–2377. 10.1099/vir.0.82858-0 [DOI] [PubMed] [Google Scholar]
- 40.Turell MJ, Beaman JR, Tammariello RF. 1992. Susceptibility of selected strains of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) to chikungunya virus. J. Med. Entomol. 29:49–53 [DOI] [PubMed] [Google Scholar]
- 41.Martin E, Moutailler S, Madec Y, Failloux AB. 2010. Differential responses of the mosquito Aedes albopictus from the Indian Ocean region to two chikungunya isolates. BMC Ecol. 10:8. 10.1186/1472-6785-10-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, de Lamballerie X. 2014. Chikungunya in the Americas. Lancet 383:514. 10.1016/S0140-6736(14)60185-9 [DOI] [PubMed] [Google Scholar]
- 43.Kramer LD, Ebel GD. 2003. Dynamics of flavivirus infection in mosquitoes. Adv. Virus Res. 60:187–232. 10.1016/S0065-3527(03)60006-0 [DOI] [PubMed] [Google Scholar]
- 44.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, Cordioli P, Fortuna C, Boros S, Magurano F, Silvi G, Angelini P, Dottori M, Ciufolini MG, Majori GC, Cassone A, CHIKV Study Group 2007. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet 370:1840–1846. 10.1016/S0140-6736(07)61779-6 [DOI] [PubMed] [Google Scholar]
- 45.Grandadam M, Caro V, Plumet S, Thiberge JM, Souares Y, Failloux AB, Tolou HJ, Budelot M, Cosserat D, Leparc-Goffart I, Desprès P. 2011. Chikungunya virus, southeastern France. Emerg. Infect. Dis. 17:910–913. 10.3201/eid1705.101873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention. 2010. Locally acquired dengue–Key West, Florida, 2009–2010 MMWR Morb. Mortal. Wkly. Rep. 59:577–581 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5919a1.htm [PubMed] [Google Scholar]
- 47.Fontenille D, Failloux A-B, Romi R. 2007. Should we expect chikungunya and dengue in Southern Europe?, p 69–184 In Takken W, Knols BGJ. (ed). Emerging pests and vector-borne diseases in Europe. Wageningen Academic Publishers, Wageningen, The Netherlands [Google Scholar]
- 48.Bracco JE, Capurro ML, Lourenço-de-Oliveira R, Sallum MA. 2007. Genetic variability of Aedes aegypti in the Americas using a mitochondrial gene: evidence of multiple introductions. Mem. Inst. Oswaldo Cruz 102:573–580. 10.1590/S0074-02762007005000062 [DOI] [PubMed] [Google Scholar]
- 49.Mousson L, Dauga C, Garrigues T, Schaffner F, Vazeille M, Failloux AB. 2005. Phylogeography of Aedes (Stegomyia) aegypti (L.) and Aedes (Stegomyia) albopictus (Skuse) (Diptera: Culicidae) based on mitochondrial DNA variations. Genet. Res. 86:1–11. 10.1017/S0016672305007627 [DOI] [PubMed] [Google Scholar]
- 50.Powell JR, Tabachnick WJ. 2013. History of domestication and spread of Aedes aegypti—a review. Mem. Inst. Oswaldo Cruz 108(Suppl I):11–17. 10.1590/0074-0276130395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sprenger D, Wuithiranyagool T. 1986. The discovery and distribution of Aedes albopictus in Harris County, Texas. J. Am. Mosq. Control Assoc. 2:217–219 [PubMed] [Google Scholar]
- 52.Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig GB., Jr 1987. Aedes albopictus in North America: probable introduction in used tires from northern Asia. Science 236:1114–1116. 10.1126/science.3576225 [DOI] [PubMed] [Google Scholar]
- 53.Consoli RAGB, Lourenço-de-Oliveira R. 1994. Principais mosquitos de importância sanitária no Brasil. Fiocruz, Rio de Janeiro, Brazil [Google Scholar]
- 54.Myers RM, Carey DE. 1967. Concurrent isolation from patient of two arboviruses, chikungunya and dengue type 2. Science 157:1307–1308. 10.1126/science.157.3794.1307 [DOI] [PubMed] [Google Scholar]
- 55.Nayar SK, Noridah O, Paranthaman V, Ranjit K, Norizah K, Chem Mustafa YKB, Chua KB. 2007. Co-infection of dengue virus and chikungunya virus in two patients with acute febrile illness. Med. J. Malaysia 62:335–336 http://www.e-mjm.org/2007/v62n4/Dengue_Virus_Chikungunya_Virus.pdf [PubMed] [Google Scholar]
- 56.Hapuarachchi HAC, Bandara KBAT, Hapugoda MD, Williams S, Abeyewickreme W. 2008. Laboratory confirmation of dengue and chikungunya co-infection. Ceylon Med. J. 53:104–105. 10.4038/cmj.v53.i3.252 [DOI] [PubMed] [Google Scholar]
- 57.Ratsitorahina M, Harisoa J, Ratovonjato J, Biacabe S, Reynes JM, Zeller H, Raoelina Y, Talarmin A, Richard V, Louis Soares J. 2008. Outbreak of dengue and chikungunya fevers, Toasmasina, Madagascar, 2006. Emerg. Infect. Dis. 14:1135–1137. 10.3201/eid1407.071521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schilling S, Emmerich P, Günter S, Schmidt-Chanasit J. 2009. Dengue and chikungunya virus co-infection in a German traveler. J. Clin. Virol. 45:163–164. 10.1016/j.jcv.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 59.Leroy EM, Nkoghe D, Olomo B, Nze-Nkogue C, Becquart P, Grard G, Pourrut X, Charrel R, Moureau G, Ndjoyi-Mbiguino A, De-Lamballerie X. 2009. Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg. Infect. Dis. 15:591–593. 10.3201/eid1504.080664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang SF, Su CL, Shu PY, Yang CF, Liao TL, Cheng CH, Hu HC, Huang JH. 2010. Concurrent isolation of chikungunya virus and dengue virus from a patient with coinfection resulting from a trip to Singapore. J. Clin. Microbiol. 48:4586–4589. 10.1128/JCM.01228-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalawat U, Sharma KK, Reddy SG. 2011. Prevalence of dengue and chickungunya fever and their co-infection. Indian J. Pathol. Microbiol. 54:844–846. 10.4103/0377-4929.91518 [DOI] [PubMed] [Google Scholar]
- 62.Kumar KJ, Manjunath VG, Shailashree M, Girish GN. 2012. Coinfection with dengue and chikungunya—a case report. J. Indian Med. Assoc. 110:749–752 [PubMed] [Google Scholar]
- 63.Caron M, Paupy C, Grard G, Becquart P, Mombo I, Nso BB, Kassa Kassa F, Nkoghe D, Leroy EM. 2012. Recent introduction and rapid dissemination of Chikungunya virus and Dengue virus serotype 2 associated with human and mosquito coinfections in Gabon, central Africa. Clin. Infect. Dis. 55:e45–e53. 10.1093/cid/cis530 [DOI] [PubMed] [Google Scholar]
- 64.Vazeille M, Mousson L, Martin E, Failloux AB. 2010. Orally co-infected Aedes albopictus from La Reunion Island, Indian Ocean, can deliver both dengue and chikungunya infectious viral particles in their saliva. PLoS Negl. Trop. Dis. 4:e706. 10.1371/journal.pntd.0000706 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.