ABSTRACT
The human T-cell leukemia virus type 1 (HTLV-1) is a complex human retrovirus that causes adult T cell leukemia and of HTLV-associated myelopathy/tropical spastic paraparesis. The mRNA of some complex retroviruses, including the human and simian immunodeficiency viruses (HIV and SIV), can initiate translation using a canonical cap-dependent mechanism or through an internal ribosome entry site (IRES). In this study, we present strong evidence showing that like HIV-1 and SIV, the 5′-untranslated region (5′UTR) of the HTLV-1 full-length mRNA harbors an IRES. Cap-independent translational activity was evaluated and demonstrated using dual luciferase bicistronic mRNAs in rabbit reticulocyte lysate, in mammalian cell culture, and in Xenopus laevis oocytes. Characterization of the HTLV-1 IRES shows that its activity is dependent on the ribosomal protein S25 (RPS25) and that its function is highly sensitive to the drug edeine. Together, these findings suggest that the 5′UTR of the HTLV-1 full-length mRNA enables internal recruitment of the eukaryotic translation initiation complex. However, the recognition of the initiation codon requires ribosome scanning. These results suggest that, after internal recruitment by the HTLV-1 IRES, a scanning step takes place for the 40S ribosomal subunit to be positioned at the translation initiation codon.
IMPORTANCE The mechanism by which retroviral mRNAs recruit the 40S ribosomal subunit internally is not understood. This study provides new insights into the mechanism of translation initiation used by the human T-cell lymphotropic virus type 1 (HTLV-1). The results show that the HTLV-1 mRNA can initiate translation via a noncanonical mechanism mediated by an internal ribosome entry site (IRES). This study also provides evidence showing the involvement of cellular proteins in HTLV-1 IRES-mediated translation initiation. Together, the data presented in this report significantly contribute to the understanding of HTLV-1 gene expression.
INTRODUCTION
Translation initiation of the vast majority of eukaryotic mRNAs occurs by a cap-dependent mechanism. This mechanism involves the recognition of the 5′cap structure (m7GpppN) by eukaryotic translation initiation factors (eIFs), recruitment of the 40S ribosomal subunit, and ribosome scanning of the 5′-untranslated region (5′UTR) in a 5′ to 3′ direction until the initiation codon is recognized (1, 2). The cap-binding protein eIF4E, together with the ATP-dependent RNA helicase eIF4A and translation initiation factor eIF4G, forms the protein complex eIF4F, which binds to the 5′cap structure. The eIF4F complex bridges the 5′cap structure with the poly(A) tail through its interaction with the poly(A)-binding protein (PABP) and with the 40S ribosomal subunit via its interaction with eIF3 (1, 2). The 40S ribosomal subunit is recruited to the mRNA as a 43S initiation complex composed of the 40S subunit, eIF2/GTP/Met-tRNAi (ternary complex), eIF1A, eIF1, and eIF3. Upon binding to the mRNA, the 43S complex scans in a 5′-to-3′ direction toward the AUG initiation codon. Scanning stops when the initiation codon is recognized by the anticodon of the initiator Met-tRNAi and GTP is hydrolyzed. Finally, the 60S subunit joins the 40S subunit resulting in the formation of a protein synthesis-competent 80S ribosome in which initiator Met-tRNAi is positioned in the peptidyl (P) site of the ribosome (1, 2). An alternative mechanism of initiation exists, in which the 40S ribosomal subunit is directly recruited to an internal site within the 5′UTR in a cap-independent fashion (1, 2). This alternative mechanism of ribosomal recruitment, initially described for the uncapped mRNAs from the Picornaviridae family, is dependent on an internal ribosomal entry site (IRES) (3, 4). Functionally, IRESs were identified by inserting the poliovirus (PV) or encephalomyocarditis virus (EMCV) 5′UTR into the intercistronic spacer of a bicistronic construct encoding two proteins (3, 4). Expression of the second cistron was dependent upon the ability of the inserted sequence to promote internal ribosome binding and translation independent of the first cistron (5–7). In general, IRES-mediated translation is independent of the nature of the extreme 5′ end of the RNA since it does not require the 5′cap structure to be functional. In the artificial bicistronic mRNA model, translation of the downstream cistron occurs even when translation of the upstream cistron is abolished (5–7). The mRNAs of many viruses from other families, including several members of the Retroviridae family, have been shown to also initiate translation via an IRES (6, 8). Identification of IRESs within retroviruses has been the subject of intense debates over the past several years (8, 9). This controversy is exemplified by the studies focused on understanding the mechanism of translation initiation of the HIV-1 full-length mRNA (from here on referred to as HIV-1 mRNA) (8, 9). Initial studies defined translation initiation of this viral mRNA to be strictly cap dependent when assayed in the rabbit reticulocyte (RRL) system (10). Such a hypothesis was later revisited with data showing the presence of functional IRES elements both within the 5′UTR and the gag coding region of the HIV-1 mRNA (11, 12). A different study later showed that scanning can also take place on the HIV-1 5′UTR in cultured cells (13). More recently, the presence of an IRES within the 5′UTR of the HIV-1 mRNA has been reconfirmed (14–17). Taken together, these reports suggest that both mechanisms (cap- and IRES-driven) are used by the HIV-1 mRNA to drive the synthesis of the viral Gag protein (8, 9, 18, 19).
Another interesting and as-yet-unresolved question relates to the mechanism of translation initiation used by the human T-cell lymphotropic virus type 1 (HTLV-1) full-length mRNA (HTLV-1 mRNA). An early study of the mechanism of translation initiation of the HTLV-1 mRNA suggested the presence on an IRES within its 5′UTR (20). This IRES was successfully used by others in the construction of tricistronic viral vectors (21). When these data were reexamined by others the existence of an IRES was dismissed, suggesting that translation initiation of the HTLV-1 mRNA was exclusively cap dependent (22).
In the present study, new and compelling evidence is presented showing that, akin to other retroviruses (8), the HTLV-1 mRNA harbors an IRES within its 5′UTR. In addition, evidence is provided demonstrating that the HTLV-1 IRES is dependent on ribosomal protein S25 for full activity. The HTLV-1 IRES is sensitive to edeine, a drug that inhibits the initiation step of protein synthesis by impeding the recognition of the initiation codon by scanning 40S ribosomal subunits (23–25), suggesting that upon internal recruitment, the initiation complex follows a scanning mechanism leading to the recognition of the initiation codon of the HTLV-1 Gag open reading frame.
(This research was conducted by Eduardo Olivares in partial fulfillment of the requirements for a Ph.D. from the Programa de Doctorado en Microbiología, Facultad de Química y Biología, Universidad de Santiago de Chile, Santiago, Chile.)
MATERIALS AND METHODS
Cell culture and cell extracts.
HeLa cells (CCL-2TM) were cultured at 37°C in a 5% CO2 atmosphere, in Dulbecco modified Eagle medium (HyClone Laboratories, Inc., Logan, UT) containing 10% bovine fetal serum (HyClone), with 2.5 μg of Fungizone (HyClone)/ml, 100 U of penicillin (HyClone)/ml, and 100 μg of streptomycin (HyClone)/ml. To generate cell extracts, HeLa cells were grown to 60% confluence in standard media (nonsynchronized) or media supplemented with 400 ng of nocodazole (Sigma-Aldrich, St. Louis, MO)/ml for 16 h to enrich cells in the G2/M phase of the cell cycle, as previously described (12, 14). Cell cycle arrest was confirmed by flow cytometry and cytoplasmic cell extracts (S10) were prepared, following a previously described protocol (12, 14). The HeLaShV and HeLaShS25 cells were generated as previously described (26) and were cultures as described above.
Plasmid constructions.
For the generation of the bicistronic vector dl HTLV-1 5′UTR, the 5′UTR of HTLV-1 (nucleotides [nt] 1 to 449) was recovered by PCR from plasmid K30 (accession number L03561; kindly provided by B. Barbeau, Université du Québec, Montréal, Québec, Canada) using primers described in Table 1. Plasmid K30, which encodes a biologically active HTLV-1 genomic clone, has been previously described (27). A series of bicistronic dl vector harboring HTLV-1 5′UTR deletion mutants (nt 1 to 423, nt 1 to 390, nt 1 to 267, and nt 268 to 449) were also constructed by PCR using primers described in Table 1. In all constructs, two cytosines residues were added to the original sequence to allow in-frame cloning into the dual luciferase vector by creating a NcoI restriction site as previously described (12). The amplicons was digested with EcoRI and NcoI (both restriction sites added by PCR) and used to replace the HIV IRES sequence in the intercistronic region of dl HIV-1 IRES plasmid (12). Briefly, a three-piece ligation was performed using the EcoRI-XbaI (1,655-bp) and NcoI-XbaI (5,252-bp) fragments from the dl HIV-1 IRES plasmid along with the EcoRI-NcoI fragment containing the HTLV-1 5′UTR using T4 DNA ligase (Fermentas). The final vectors were verified by sequence analysis (Macrogen Corp., Rockville, MD). The dl ΔEMCV (kindly provided by P. Sarnow, Stanford University, Stanford, CA), dl HIV-1 IRES and dl PV IRES (kindly provided by N. Sonenberg, McGill University, Montreal, Quebec, Canada), and dl HCV IRES plasmids were previously described (12, 28–30). To generate plasmids ΔSV40 dl HTLV-1 5′UTR and ΔSV40 dl ΔEMCV, the dl HTLV-1 5′UTR and dl ΔEMCV vectors were digested with HindIII and MluI and ligated using T4 DNA ligase (Fermentas), resulting in a deletion of the simian virus 40 (SV40) promoter. The ΔEMCV region was removed from the dl HTLV-1 5′UTR by digesting with EcoRI and XhoI and religating the plasmid to generate the dl ΔΔEMCV HTLV-1 5′UTR plasmid. All vectors were verified by sequence analysis (Macrogen Corp.).
TABLE 1.
Primers used to generate the dl HTLV-1 bicistronic vectors
| HTLV-1 5′UTR (nt)a | Sequence (5′–3′) |
|
|---|---|---|
| Sense | Antisense | |
| 1–449 | GAATTCGGCTCGCATCTCTCCTTC | CCATGGTGCCTAGGGAATAAAGGGG |
| 1–423 | GAATTCGGCTCGCATCTCTCCTTC | CCATGGATCCCGGACGAGCCC |
| 1–390 | GAATTCGGCTCGCATCTCTCCTTC | CCATGGTCTCTCCTGGAGAGTGC |
| 1–267 | GAATTCGGCTCGCATCTCTCCTTC | CCATGGGATCTGTAGCGGCGCAG |
| 268–449 | GAATTCGAAAGTTCCACCCCTTTCC | CCATGGTGCCTAGGGAATAAAGGGG |
Nucleotide (nt) numbering is with respect to HTLV-1 plasmid K30 (accession number L03561).
In vitro transcription.
The dual luciferase plasmids were digested with XbaI (Fermentas) and used as a template to generate bicistronic RNAs. Capped RNAs were synthesized using the mMESSAGE mMACHINE kit (Applied Biosystems/Ambion, Austin, TX) according to the manufacturer's protocol. The poly(A) tailing kit (Applied Biosystems/Ambion) was used to add poly(A) tail to the in vitro-synthesized mRNAs according to the manufacturer's specifications. Bicistronic mRNAs with a nonfunctional “Acap” were synthesized in the presence of 10 mM ApppG cap-analog (New England Biolabs, Ipswich, MA) using T7 RNA polymerase according to the manufacturer's specifications (T7 RiboMAX; Promega Corp., Madison, WI). The template DNA was degraded with DNase I, and the RNA was precipitated with 2.5 M LiCl. The RNA was resuspended in nuclease-free water (Integrated DNA Technologies [IDT], Coralville, IA). RNA concentrations were determined spectrophotometrically (NanoDrop Technology, Wilmington, DE), and RNA integrity was assessed by electrophoresis on denaturing agarose gels.
In vitro translation.
In vitro translations were carried out in nuclease-treated rabbit reticulocyte lysate (RRL; Promega Corp., Madison, WI) at 35% (vol/vol), supplemented with 20 mM amino acids (Promega), 0.8 U of Ribolock RNase inhibitor (Fermentas)/μl, and 1 ng of capped RNA/μl. Where indicated, the concentrations of potassium acetate (KOAc) and magnesium acetate (MgOAc) were varied. For the FMDV L protease assays, capped and polyadenylated mRNA of the FMDV L protease was generated by in vitro transcription of XbaI (Fermentas)-linearized pLb plasmid (kindly provided by G. Belsham, Institute for Animal Health, Pirbright, United Kingdom) (31). The RNA was translated in nuclease-treated RRL as previously described (32). The l-protease–RRL was diluted 1:5 or 1:10 in nuclease-free water (IDT) and added (to a final concentration of 3 and 6% [vol/vol]) to the fresh nuclease-treated 35% (vol/vol) RRL and preincubated for 15 min at 30°C, prior to the addition of the bicistronic mRNA. When HeLa cell extracts (S10) were used, bicistronic mRNA was preincubated for 10 min with the cell extracts prior to the addition of the RRL translation mix. For in vitro translation reactions conducted in the presence of edeine, kindly provided by I. Brierley (Division of Virology, Department of Pathology, University of Cambridge, Cambridge, United Kingdom), RRL was incubated at 30°C for 10 min in the presence (ranging from 0.125 to 0.5 μM) or absence of edeine, prior to the addition of bicistronic mRNAs used for in vitro translation assay as previously described (33). The optimal salt concentrations used to in vitro translate the dl HCV IRES (100 mM KCl and 0.75 mM MgCl2), dl HIV IRES (120 mM KOAc and 0.5 mM MgOAc), and dl Polio IRES (80 mM KOAc and 0.25 mM MgOAc) mRNAs were as previously described (12, 14, 29, 34). All translation reaction mixtures were incubated at 30°C for 90 min. Renilla luciferase (RLuc) and firefly luciferase (FLuc) activities were measured using a DLR assay system (Promega) according to the manufacturer's instructions on a Sirius single-tube luminometer (Lumat 9507; Berthold Detection Systems GmbH, Pforzheim, Germany) or an FB12 luminometer (Berthold Detection Systems GmbH).
Western analysis.
eIF4GI cleavage by FMDV l-protease was assessed by 5 to 15% gradient SDS-PAGE of RRL (10 μl), followed by transfer to a 0.45-μm-pore-size polyvinylidene difluoride (PVDF) membrane (Thermo Scientific, Inc., Rockford, IL), and eIF4GI was detected using an eIF4G-polyclonal antibody (kindly provided by L. Carrasco, Centro de Biología Molecular Severo Ochoa, Madrid, Spain) (35, 36).
DNA transfection.
HeLa cells were seeded at 1 × 105 cells/well in 12-well culture plates, or HeLaShV and HeLaShS25 cells were seeded at 5 × 104 cells per well in a 24-well plate on the day prior to transfection. For the bicistronic reporter assays, DNA transfections were performed on cells that were 60 to 80% confluent by the JetPei system (Polyplus-Transfection SA, Illkirch, France) or Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocols. Cells were transfected with 200 ng of bicistronic DNA plasmids together with 50 ng of pcDNA 3.1-LacZ plasmid, encoding the β-galactosidase enzyme, used as a control for the transfection efficiency. For the RPS25 knockdown and rescue, 400 ng of bicistronic DNA, 400 ng of pcDNA 3.1-LacZ plasmid, and 400 ng of either the hS25 rescue plasmid (26) or the empty vector pcDNA3.1 (Invitrogen) were cotransfected. At 24 h posttransfection, the cells were washed once with phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4·7H2O, 1.4 mM KH2PO4 [pH 7.4]) and lysed with 100 μl of lysis buffer (100 mM potassium phosphate [pH 7.8], 0.2% Triton X-100 [Applied Biosystems]). Luciferase activities were measured as described above. The activity of β-galactosidase was measured from the same lysates using the Beta-Glo assay system (Promega) according to the manufacturer's protocols or a Galacto-light Plus kit (Applied Biosystems) according to the manufacturer's protocols.
RNA extraction, DNA extraction, PCR, and reverse transcription-PCR (RT-PCR).
Transfected cells (see above) were washed three times with phosphate-buffered saline at 4°C. The cells were lysed in 200 μl of RLNa buffer (10 mM Tris-HCl [pH 8.0], 10 mM NaCl, 3 mM MgCl2, 1 mM dithiothreitol, 0.5% NP-40, 10 U of RiboLock RNase inhibitor [Fermentas]/μl) for 2 min on ice. The mixture was centrifuged (16,000 × g for 5 min at 4°C) using an Hermle Labortechnik GmbH Z216 MK refrigerated microcentrifuge, and the supernatant was directly mixed with 1 ml of TRIzol reagent (Invitrogen). From this step on, the total RNA was extracted according to the manufacturer's instructions (Invitrogen). The total RNA was resuspended in 20 μl of nuclease-free water (IDT) and treated using the DNA-free kit (Applied Biosystems/Ambion) according to the manufacturer's protocol. The RNA integrity was assessed by electrophoresis on denaturing agarose gels and quantified by spectrophotometry (NanoDrop Technology). DNA was extracted from the cells by using an EZNA kit (Omega Bio-Tek, Inc., Norcross, GA) according to the manufacturer's protocol. DNA concentration was determined by spectrophotometry (NanoDrop Technology).
The RT-PCR assay was carried out using a SuperScript III one-step RT-PCR system with a Platinum Taq DNA polymerase kit (Invitrogen) according to the manufacturer's protocol, using 400 ng of total RNA and the primers p2anti (5′-TCTCTTCATAGCCTTATGCAGTTG-3′) and Pforluc (5′-CATGACTTCGAAAGTTTATGATC-3′) as previously described (15). The PCR assay was conducted using the primers p2anti and Pforluc, 400 ng of total DNA, and the GoTaq Green Master mix (Promega), according to the manufacturers' protocol. The no-RT [(−)RT] control assay was performed with 100 ng of total RNA under the same conditions.
siRNA transfection.
HeLaShV cells were seeded at 5 × 104 cells per well in a 24-well plate on the day prior to transfection. On the day of transfection, 400 ng of bicistronic plasmid DNA was cotransfected with 20 nM either Silencer Select 1 scrambled control short interfering RNA (siRNA; Ambion, catalogue no. 4390844) or one of two siRNAs targeting the RLuc open reading frame (Renilla siRNA 1, 5′-UGGACGACGAACUUCACCUUUCUCUUU-3′; Renilla siRNA 2, 5′-UAUAAGAACCAUUACCAGAUUUGCCUG-3′ [IDT]) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocols. The RLuc and FLuc activities were measured at 24 h posttransfection as described above.
Oocyte harvesting and RNA microinjection.
Oocytes were isolated from Xenopus laevis ovarian fragments by manual dissection as previously described (32). Oocytes were incubated at 15°C for 24 h in standard Barth's solution supplemented with 10 IU of penicillin-streptomycin/liter and 2 mM pyruvate (32). To evaluate the viral IRES activity in oocytes, 6.25 ng of in vitro-transcribed capped and polyadenylated RNA was microinjected into each oocyte with glass micropipettes calibrated to deliver a final volume of 50 nl (32, 37). When HeLa cell extracts were used, oocytes were first microinjected with mRNAs as described and 15 min later microinjected with 0.2 μg of HeLa cell extracts. After 24 h, the oocytes were lysed in 1× passive lysis buffer (Promega) and centrifuged at 16,000 × g for 5 min; the protein concentrations were then determined (NanoDrop Technology), and 2.5 μg of total protein was used in the detection assay using the kit DLR assay system (Promega).
Statistical analysis and sequence alignments.
The statistical data analysis and graphics were evaluated using the GraphPad v5.03 program (GraphPad, La Jolla, CA), and the BioEdit v7.0.9 (Ibis Biosciences, Carlsbad, CA) and Vector NTI v11 (Invitrogen Life Technologies) programs were used for sequence alignments and analysis.
RESULTS
The HTLV-1 5′UTR drives translation of the second cistron in a bicistronic mRNA.
The full-length mRNA of some retroviruses can initiate protein synthesis through both a cap-dependent and a cap-independent mechanism (8, 18, 19). At present, an IRES element is defined solely by performing a functional assay and cannot be predicted by the presence of characteristic RNA sequences or structural motifs (7, 38). Thus, to evaluate whether an IRES element exists within the HTLV-1 mRNA, the entire 5′UTR (nt 1 to 449) from the mRNA of the K30 HTLV-1 infectious clone (27), was inserted into a dual luciferase (dl) reporter containing an upstream Renilla luciferase gene (RLuc) and a downstream firefly luciferase gene (FLuc). To ensure that the two cistrons were translated independently, a highly structured defective encephalomyocarditis virus IRES sequence (ΔEMCV), that is known to inhibit ribosome reinitiation and readthrough (12, 28), was inserted upstream of the HTLV-1 5′UTR, generating the dl HTLV-1 5′UTR plasmid used for the production of the dl HTLV-1 5′UTR RNA (Fig. 1A). A similar strategy has been successfully used in previous studies (12, 15, 16, 32). In this context the translational activity of the HTLV-1 5′UTR was measured by using FLuc activity as the readout, while the RLuc reporter gene was translated by a cap-dependent mechanism and serves as an internal control. In the first series of experiments, the putative IRES activity of the HTLV-1 5′UTR was assessed by programming the nuclease-treated rabbit reticulocyte lysate system (RRL) with the dl HTLV-1 5′UTR-capped in vitro-transcribed RNA. The dl HCV IRES RNA (29), harboring the well-characterized HCV 1b genotype IRES, was used as a positive control for IRES activity (Fig. 1A). A dl RNA containing only the ΔEMCV in the intercistronic region (28) was used as negative control for IRES activity (Fig. 1A). The results show that cap-dependent translation from the first cistron (RLuc) was comparable for all bicistronic RNAs (Fig. 1B, left panel). However, translation of the second cistron (FLuc), driven by the HTLV 5′UTR was significantly higher (P < 0.05) than background (Fig. 1B, right panel; compare ΔEMCV to dlHTLV-1 5′UTR) and only 2-fold lower than that exhibited by the HCV IRES (Fig. 1B, right panel). The IRES activity is normalized to cap-dependent translation, and the results are also expressed as the relative translation activity (RTA), which corresponds, to the FLuc/RLuc ratio (Fig. 1C). The mean translation efficiency of the dl HTLV-1 5′UTR RNA was set to 100% (± the standard error of the mean [SEM]). Results show that in vitro, the HTLV-1 5′UTR is capable of driving translation of the second cistron. This first assay strongly suggests that the 5′UTR of the HTLV-1 full-length mRNA harbors an IRES.
FIG 1.
The HTLV-1 5′UTR in the context of a bicistronic mRNA drives translation initiation in RRL. (A) Schematic representation of the in vitro-transcribed RNAs used in the in vitro translation studies. dl ΔEMCV and dl HCV IRES RNA have been previously described (28, 29). The HTLV-1 5′UTR amplified from the K30 plasmid (27) was cloned into dual luciferase bicistronic (dl) vectors harboring Renilla luciferase (RLuc), a defective encephalomyocarditis virus (ΔEMCV) IRES, followed by the firefly luciferase (FLuc) to generate the dl HTLV-1 5′UTR vector, which is used to produce the dl HTLV-1 5′UTR RNA. (B) Capped dl HTLV-1 5′UTR, dl ΔEMCV, and dl HCV IRES RNAs were generated by in vitro transcription and used to program nuclease-treated rabbit reticulocyte lysate (RRL). The RLuc (left side, white bars) and FLuc (right side, black bars) luciferase activities (in relative light units [RLU]) were measured. (C) FLuc/RLuc ratio, expressed as the relative translation activity (RTA [%]) relative to the HTLV-1 5′UTR, which has been set to 100%. The values shown are means ± the SEM for three independent experiments, each conducted in duplicate. Statistical analysis was performed by a one-way analysis of variance (ANOVA), followed by a Tukey's multiple test comparison. *, P < 0.05.
Optimization of the conditions of HTLV-1 IRES activity in vitro.
In vitro, IRES-mediated translation initiation is highly dependent on the concentration of potassium (K+) and magnesium (Mg2+) ions present within the translation mixture (39). To evaluate how the salt concentration impacts translation initiation driven by the HTLV-1 5′UTR, RRL was programmed with either the dl ΔEMCV or dl HTLV-1 5′UTR bicistronic in vitro-transcribed RNAs using a range of potassium acetate (KOAc) (Fig. 2A) or magnesium acetate (MgOAc) concentrations (Fig. 2B). The results are presented relative to the RLuc and FLuc activities obtained in RRL (40 mM KOAc and 0.25 mM MgOAc) without additional salt supplementation, which is set to 100% (± the SEM). When RRL is programmed with the HTLV-1 5′UTR bicistronic mRNA translation was significantly enhanced (P < 0.05) at 60 to 80 mM KOAc (Fig. 2A, right panel), but there was no advantage to increasing the concentrations of MgOAc (Fig. 2B, right panel). Interestingly, and in keeping with what has been previously described (12, 32), both of the cistrons, RLuc (left panel) and FLuc (right panel), showed different responses to various K+ and Mg2+ concentrations (Fig. 2A and B), supporting the notion that in vitro translation of the RLuc and FLuc cistrons are independent of one another. Taken together, the data presented in Fig. 1 and 2, indicate that the HTLV-1 5′UTR harbors an IRES element that is active in RRL.
FIG 2.
Effect of potassium and magnesium acetate on translation of the Renilla (RLuc) or firefly luciferase (FLuc) open reading frames. The dl HTLV-1 5′UTR (black bars) and dl ΔEMCV (white bars) in vitro transcripts were translated RRL in the presence of various concentrations of KOAc (A) or MgOAc (B). The RLuc and FLuc activities obtained using RRL without additional salt supplementation was set to 100% (± the SEM). The values shown are means ± the SEM for three independent experiments, each conducted in triplicate. Statistical analysis was performed by a one-way ANOVA test, followed by a Tukey's multiple test comparison. *, P < 0.05.
Translation of FLuc is not due to the reconstitution of a chimeric EMCV/HTLV-1 RNA element with artificial IRES activity.
A recent report suggests that in the case of the HIV-1 IRES its activity is the result of the contribution of different structural motifs that act in a concerted fashion to provide IRES activity (16). This observation raises the possibility that other retroviral IRESs might function in the same way. If so, it is plausible that the combination of the ΔEMCV sequence with the HTLV-1 5′UTR might give rise to an artificial IRES element. This possibility is supported by studies that showed that the sum of small structured RNA modules can give rise to artificial IRES activity (40). Furthermore, when the R region of the HTLV-1 5′UTR is attached to other genetic elements, it is known to stimulate gene expression (41, 42). To rule out the possibility that the ΔEMCV sequence together with the HTLV-1 5′UTR could artificially exhibit IRES activity, the luciferase activities from bicistronic reporters containing the ΔEMCV sequences (dl HTLV-1 5′UTR) or not (dl ΔΔEMCV HTLV-1 5′UTR) were measured (Fig. 3A). Salt-optimized RRL (Fig. 2) was programmed with in vitro-transcribed RNA harboring either a functional 5′m7GpppG (cap) or an ApppG cap-analog (Acap). The Acap is not recognized by eIF4E and therefore does not support cap-dependent translation initiation (43), while IRES-mediated translation should be the same regardless of which cap is used. The results of this experiment are presented as relative luciferase activities (RLA) with the RLuc and FLuc activities obtained in RRL programmed with the capped dl HTLV-1 5′UTR mRNA set to 100% (± the SEM) (Fig. 3B). As expected, translation of RLuc, the cap-dependent cistron, was reduced when the RNA had an Acap (Fig. 3B, left panel). In contrast, the HTLV-1 5′UTR directed translation either in the presence or the absence of a functional 5′cap structure, indicating that translation of the second cistron is independent of translation from the first cistron, RLuc (Fig. 3B). Translation from the second cistron increased in the absence of the ΔEMCV element (Fig. 3B). However, this increase was observed only when the bicistronic mRNA had a functional 5′cap structure, which is consistent with a termination reinitiation event or readthrough of the RLuc stop codon. These observations validate the use of the ΔEMCV element to prevent ribosomes translating the first cistron from contributing to expression of the second cistron in a bicistronic mRNA (28). In addition, these findings confirm that in vitro the HTLV-1 5′UTR by itself can drive cap-independent translation initiation when in the context of a bicistronic mRNA.
FIG 3.
Effect of ΔEMCV element on translation of the firefly luciferase (FLuc) open reading frame. (A) Schematic representation of the in vitro-transcribed RNAs used in the in vitro translation studies. (B) The dl HTLV-1 5′UTR RNA, containing ΔEMCV element, or the dl ΔΔEMCV HTLV-1 5′UTR RNA, lacking the ΔEMCV element, were in vitro transcribed with either a functional 5′m7GpppG cap structure (white bars) or a nonfunctional 5′ApppG cap analog (Acap; black bars) (43). Salt-optimized RRL (60 mM KOAc and 0.25 mM MgOAc) was programmed with the RNAs, and the luciferase activity was measured. The RLuc and FLuc activities are shown relative to the capped dl HTLV-1 5′UTR RNA, which was set to 100% (± the SEM). The values shown are means ± the SEM for three independent experiments, each conducted in duplicate. Statistical analysis was performed by a one-way ANOVA test, followed by a Tukey's multiple test comparison.
The HTLV-1 5′UTR directs translation in RRL when cap-dependent translation initiation is inhibited by the FMDV L protease.
An interesting feature, one common to many viruses that are known to harbor an IRES, is that a virally encoded protease cleaves the translation initiation factor eIF4G (44, 45). Cleavage of eIF4G leads to the inhibition of cap-dependent but not IRES-mediated translation. Interestingly, the HTLV-1 protease cleaves the initiation factor eIF4GI both in intact cells and in cell-free systems, inhibiting cap-dependent translation initiation (45). Therefore, we evaluated the translational activity of the HTLV-1 5′UTR in salt-optimized RRL when cap-dependent translation initiation was inhibited by cleavage of eIF4G. Since we lacked the HTLV-1 protease, in vitro reactions were conducted in the presence of the well-characterized foot-and-mouth disease virus (FMDV) leader protease (FMDV L protease) (46–48). The FMDV L protease cleaves eIF4G into an N-terminal (one-third of the protein) and a C-terminal (two-thirds of the protein) domain (46, 47, 49). The proteolytic cleavage of eIF4G results in the specific inhibition of cap-dependent translation initiation, whereas the IRES activity remains unaffected (46, 49, 50). FMDV L protease was synthesized in RRL (32, 49), and 3 or 6% (vol/vol) of the L protease RRL reaction was added to fresh RRL. Western analysis demonstrated that this results in efficient cleavage of eIF4G (Fig. 4A). In vitro-transcribed dl HCV IRES (Fig. 4B) and dl HTLV-1 5′UTR (Fig. 4C) RNAs were translated in RRL in the presence or absence of FMDV l-protease. The presence of FMDV l-protease significantly reduced cap-dependent translation (RLuc) from the dl HCV IRES RNA (Fig. 4B, white bars, left panel), whereas synthesis of the second cistron (FLuc) was unaffected (Fig. 4B, black bar, left panel). To better evidence IRES activity, the results are also expressed as the relative translation activity (RTA) that corresponds to the FLuc/RLuc ratio, a direct index of IRES-mediated translation initiation (Fig. 4B, right panel). The mean translation efficiency of the dl HCV IRES RNA translated in the absence of FMDV l-protease was set to 100% (± the SEM). The results confirm that the activity from the HCV IRES in RRL is resistant to eIF4G cleavage (Fig. 4B, right panel). The data for the dl HTLV-1 RNA (Fig. 4C) in general recapitulate those described for the dl HCV IRES RNA. The presence of FMDV l-protease significantly reduces cap-dependent translation (RLuc) from the dl 5′UTR HTLV-1 RNAs (Fig. 4C, white bars, left panel); however, both cistrons seem to be reduced at 6% (vol/vol) FMDV l-protease. Nonetheless, when IRES activity is directly examined (see RTA in Fig. 4C, right panel), it is evident that the activity of the HTLV-1 IRES is maintained in the presence of the FMDV l-protease. Collectively, these data strongly suggest that in vitro the 5′UTR of the HTLV-1 full-length mRNA drives IRES-dependent translation initiation.
FIG 4.
Proteolytic cleavage of eIF4G by the FMDV L protease negatively impacts cap-dependent translation initiation but not translation driven by the HTLV-1 5′UTR. (A) In vitro translation RRL reactions were supplemented with 3 or 6% (vol/vol) RRL that was programmed with FMDV L protease RNA template (lanes 2 and 3) or not (lane 1). Western analysis was performed using polyclonal antibodies against eIF4GI (35). The positions of the molecular mass standards (in kDa) are shown. The cleavage product (CP) has been previously characterized (47). (B and C) In vitro-transcribed dl HCV IRES (B) or dl HTLV-1 5′UTR RNA (C) were translated in salt-optimized RRL in the absence (−) or presence of 3 or 6% (vol/vol) RRL programmed with FMDV-L protease. RLuc (white bars) and FLuc (black bars) activities are shown for each RNA relative to the translation reaction conducted in the absence of FMDV-L protease, which was set to 100% (± the SEM) (left panel). The results are also shown as the RTA (right panel) that corresponds to the FLuc/RLuc ratio for the data presented in the left panel (right panel). The values shown are means ± the SEM for five independent experiments, each conducted in triplicate.
Translational activity of the HTLV-1 5′UTR is stimulated by cell extracts in RRL.
Optimal activity of the HIV-1 IRES in RRL requires supplementation of the in vitro translation system with HeLa cell extracts (14). Therefore, we evaluated whether translation initiation driven by the HTLV-1 5′UTR in RRL could be improved by the addition of HeLa cell extracts to the in vitro translation system. RRL programmed with dl HTLV-1 5′UTR RNA or control dl HIV-1 IRES RNA (Fig. 5) was supplemented with increasing amounts of cytoplasmic HeLa cell extracts generated from nonsynchronized (NS) or nocodazole-G2/M-arrested cells as previously described (14). The dl HIV-1 IRES RNA harbors the 5′UTR of the HIV-1 pNL4.3 infectious clone between the RLuc and FLuc reporter genes (12, 14). Knowing that the activity of the HIV-1 IRES is poor in RRL (14), results obtained in the presence of 0.1 μg of NS cell extracts were arbitrarily set to 100% (± the SEM) (Fig. 5A; left panel). As previously reported (14), the HIV-1 IRES is not functional in RRL (compare FLuc activity for dl HIV-1 IRES and the dl ΔEMCV), but supplementation with either NS or G2/M HeLa cell extract enhances IRES (FLuc) activities (Fig. 5A, left panel black bars) but has no effect on cap-dependent translation (Fig. 5A, left panel white bars). To better appreciate IRES activity, data are also shown as the RTA (Fig. 5A, right panel), with the HIV-1 IRES activity in the presence of 0.1 μg of NS cell extracts arbitrarily set to 100% (± the SEM). The highest HIV-1 IRES activity was obtained when RRL was supplemented with extracts (0.2 μg) generated from G2/M-arrested cells (Fig. 5A). These results indicate that the HeLa cell extracts are enhancing HIV-1 IRES activity as previously reported (14). To determine whether translation from the dl HTLV-1 5′UTR RNA is enhanced by HeLa cell extracts, the same nonsynchronized or G2/M HeLa cell extracts used in Fig. 5A were added to RRL programmed with the dl HTLV-1 5′UTR RNA. In contrast to the HIV-1 IRES (Fig. 5A), the HTLV-1 IRES is active in RRL; thus, the RLA of the HTLV-1 IRES activity obtained in the absence (−) of cell extracts was arbitrarily set to 100% (± the SEM) (Fig. 5B, left panel). As before, cap-dependent translation (RLuc) was largely unaffected (Fig. 5B, left panel, white bars), while expression of the second cistron (FLuc), which is dependent on the HTLV-1 5′UTR, significantly increased (P < 0.05) when RRL was supplemented with HeLa cell extracts (Fig. 5B, left panel, black bars). To better visualize the IRES activity, the data are also presented as the RTA (Fig. 5B, right panel). Again, the RTA of the HTLV-1 IRES activity obtained in the absence of cell extracts (−) was arbitrarily set to 100% (± the SEM) (Fig. 5B, right panel). Together, the results show that in contrast to what is observed for the HIV-1 IRES (Fig. 5A) (14), both NS and G2/M extracts stimulated translation initiation from the HTLV-1 5′UTR equally (Fig. 5B). This observation suggests that in RRL the IRES activity of the HTLV-1 5′UTR has different protein requirements than that of the HIV-1 IRES (14).
FIG 5.
HeLa cell extract stimulates translation driven by the HTLV-1 5′UTR in RRL. In vitro translations were conducted in RRL alone or supplemented with different amounts (μg of total protein) of cytoplasmic extracts (CE) generated from nonsynchronized (NS) or G2/M-arrested HeLa cells. RRL was programmed with the dl HIV-1 IRES (A) or dl HTLV-1 5′UTR RNA (B), and the RLuc (white bars) and FLuc (black bars) activities were determined. The RLuc and FLuc activities are shown relative to RRL supplemented with 0.1 μg of NS cell extract, which was set to 100% (left panel). The results are also shown as the RTA (right panel) that corresponds to the FLuc/RLuc ratio for the data presented in the left panel. The values shown are means ± the SEM for five independent experiments each conducted in duplicate. Statistical analysis was performed by a one-way ANOVA test, followed by a Tukey's multiple test comparison. *, P < 0.05.
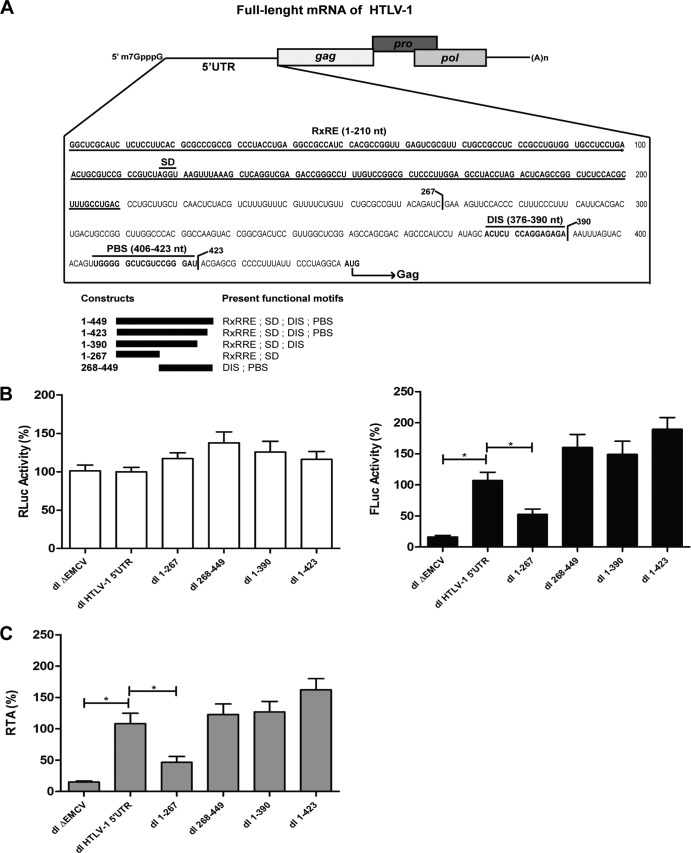
Mapping the HTLV-1 IRES in RRL.
Next, we sought to identify the region of the HTLV-1 5′UTR that has IRES activity. Unfortunately, no RNA structural information encompassing the full-length HTLV-1 5′UTR is currently available. Thus, RNA structure analysis could not be used to map the IRES activity of the HTLV-1 5′UTR. Therefore, we used well-characterized functional domains of the HTLV-1 5′UTR to guide the design of a series of deletion mutants. These domains include the Rex protein response element (RexRE), the major splice donor signal (SD), the RNA dimerization signal (DIS), the primer binding site (PBS), and the Gag protein initiation codon (Fig. 6A). This strategy was successfully used to characterize other retroviral IRESs (12, 51). Dual luciferase vectors were constructed that contained the following HTLV-1 sequences in the intercistronic region (Fig. 6A): 1-423 (deletion of the region between the base of the PBS and the Gag initiation codon), 1-390 (deletion of the PBS), 1-267 (20), or 268-449. Salt-optimized RRL was programmed with in vitro-transcribed capped RNAs corresponding to dl HTLV-1 5′UTR, dl 1-423, dl 1-390, dl 1-267, or dl 268-449. The dl ΔEMCV was used as a negative control for IRES activity. The results are presented as RLA with the RLuc and FLuc activities obtained in RRL programmed with the capped dl HTLV-1 5′UTR mRNA set to 100% (± the SEM). The region comprising nt 1 to 267 exhibited poor IRES activity, since no statistical difference was observed when we compared the FLuc activity from the dl ΔEMCV and the dl 1-267 RNAs (Fig. 6B). This suggests that the region comprising the RxRE and SD domains is unable to drive translation in the context of a bicistronic mRNA in RRL. This finding is consistent with the work of Bolinger et al. (22). Further analysis of the deletion mutants revealed that segments 1-423, 1-390, and dl 268-449 all exhibited cap-independent translation initiation (Fig. 6B). Therefore, the addition of 123 nt (i.e., nt 1 to 390) was sufficient to enable IRES activity from the 1-267 segment of the HTLV-1 5′UTR.
FIG 6.
Mapping the HTLV-1 IRES in RRL. (A) Nucleotide sequence of the 5′UTR of the full-length HTLV-1 mRNA (nt 1 to 449). Numbering of the HTLV-1 5′UTR follows that of the K30 plasmid (27) (accession number L03561). The positions of the Rex responsive element (RxRE), splice donor (SD), dimerization signal (DIS), primer binding site (PBS), and Gag initiation codon are indicated. Deletion fragments are schematized, indicating the functional domains they harbor. (B) Capped dl HTLV-1 5′UTR, dl ΔEMCV, dl 1-267, dl 268-449, dl 1-390, and dl 1-423 RNAs were generated by in vitro transcription and used to program RRL. The RLuc (left panel, white bars) and FLuc (right panel, black bars) luciferase activities in RLU were measured. (C) FLuc/RLuc ratios, indicated as the RTA (%), are expressed relative to the dl HTLV-1 5′UTR, which has been set to 100%. The values shown are means ± the SEM for four independent experiments, each conducted in duplicate. Statistical analysis was performed by a one-way ANOVA test, followed by a Tukey's multiple test comparison. *, P < 0.05.
Intrigued by these results, we next evaluated the possibility that a factor(s) missing from the RRL would be required to enable IRES activity from segment 1-267. Therefore, we evaluated whether translation initiation driven by the 1-267 segment in RRL could be improved by the addition of HeLa cell extracts to the in vitro translation system. RRL was programmed with dl 1-267 (Fig. 7A) or dl 268-449 (Fig. 7B) RNAs and supplemented with increasing amounts of HeLa NS or G2/M cell extracts as previously described for Fig. 5. dl HTLV-1 5′UTR RNA was used as a reference for the baseline translational activity of the HTLV-1 5′UTR, while dl ΔEMCV RNA was used as a negative control for IRES activity. The results show that translation driven by the 1-267 region of the HTLV-1 5′UTR was not impacted by the presence of HeLa cell extracts (Fig. 7A). In contrast, when in the presence of HeLa cell extracts, the 268-449 region was enhanced similar to the full-length HTLV-1 5′UTR in Fig. 5B (Fig. 7B). In summary, the results presented in Fig. 6 and 7 show that HTLV-1 IRES activity is confined to an RNA segment of 181 nt encompassing the DIS and PBS domains (nt 268 to 449) of the HTLV-1 5′UTR.
FIG 7.
HeLa cell extract does not stimulate translation driven by the nt 1 to 267 in RRL. In vitro translation was conducted in RRL alone (−) or supplemented with different amounts (μg of total protein) of cytoplasmic extracts (CE) generated from nonsynchronized (NS) or G2/M-arrested HeLa cells. RRL was programmed with the dl 1-267 (A) or dl 268-449 (B) RNAs, and the RLuc (white bars) and FLuc (black bars) activities were determined. The control dl HTLV-1 5′UTR RNA and dl ΔEMCV were translated in nonsupplemented RRL. The RLuc and FLuc activities are expressed relative to the expression of both reporter proteins obtained from the dl HTLV-1 5′UTR RNA, which was set to 100%. The values shown are means ± the SEM for three independent experiments. Statistical analysis was performed by a one-way ANOVA test, followed by a Tukey's multiple test comparison. *, P < 0.05.
The HTLV-1 5′UTR mediates cap-independent translation initiation in HeLa cells.
To evaluate whether the HTLV-1 IRES has activity in cells, the dl HTLV-1 5′UTR, dl HIV-1 IRES, and dl ΔEMCV plasmids (Fig. 8A), were transfected into HeLa cells. Even though HeLa cells are not the natural target of HTLV-1, they were selected for these assays since they are easy to handle and modify (see the RPS25 data presented below), efficiently transfected, known to support IRES activity from other retroviral mRNAs, including that of the HIV-1 IRES (12, 14, 15), and HeLa cell extracts enhance HTLV-1 IRES activity (Fig. 5B). The RLuc and FLuc activities were measured 24 h after transfection. The results are presented as the RTA (%) relative to the HIV-1 IRES activity, which is set to 100% (Fig. 8B). The dl ΔEMCV reporter was again used as a negative control, exhibiting no IRES activity (12, 15, 28). Consistent with the in vitro data (Fig. 1 through 7), the 5′UTR of the HTLV-1 was capable of driving translation of the second cistron in the context of a bicistronic reporter in HeLa cells (Fig. 8B). In addition, in HeLa cells the 5′UTR of HTLV-1 exhibited significantly (P < 0.05) more translational activity than the HIV-1 pNL4.3 IRES (Fig. 8B) (12). This observation strongly supports the notion that the 5′UTR of the HTLV-1 infectious clone recovered from the K30 plasmid harbors an active IRES that is functional in HeLa cells.
FIG 8.
HTLV 5′UTR drives translation initiation in HeLa cells. (A) Schematic representation of the dual luciferase bicistronic DNA reporters used in the transfection assays. The bicistronic reporters are expressed from the SV40 promoter and receive a poly(A) tail by cleavage and polyadenylation at the SV40 poly(A) signal (SV40pA). (B) The dlΔEMCV, HIV-1 IRES, or dl HTLV-1 5′UTR vectors were cotransfected into HeLa cells with the pcDNA3.1 lacZ (50 ng) vector that expresses β-galactosidase from a cap-dependent transcript (12, 28). Cotransfection of β-galactosidase serves as an internal control for transfection efficiency. RLuc and FLuc activities were measured and normalized to the β-galactosidase activity. Values are expressed as the RTA (%) relative to the dl HIV-1 IRES (12), which was set to 100%. The values shown are means ± the SEM for three independent experiments, each performed in triplicate. Statistical analysis was performed by a one-way ANOVA test, followed by a Tukey's multiple test comparison. *, P < 0.05.
Studies that use translation of bicistronic reporter plasmids transfected into cells in order to identify IRESs have received strong criticism (52, 53). A commonly cited caveat to the bicistronic reporter assays has been the potential for false-positive findings caused by alternative splicing or cryptic promoter activity, which can result in expression of the second cistron by a cap-dependent mechanism rather than an IRES (52, 53). The firefly luciferase reporter gene is known to exhibit cryptic promoter activity that is detectable in both yeast and mammalian cells (54). The identified promoter lies within the FLuc coding sequence and generates short mRNAs that do not code for a functional luciferase enzyme (54). An earlier study suggests that expression driven by the HTLV-1 5′UTR is associated with the presence of aberrant transcripts (22). However, that study failed to examine whether the additional short RNA transcript indeed contributed to the overall measured luciferase activity (22). The in vitro data (Fig. 1 through 7) using RNA bicistronic reporters, which are not subject to alternative splicing or cryptic promoter activity, clearly suggest the presence of an IRES within the 5′UTR of the HTLV-1 mRNA. Therefore, we wanted to establish whether possible aberrant transcripts are contributing to the amount of luciferase activity obtained from cells transfected with DNA plasmids (Fig. 8B). To determine the contribution of firefly luciferase activity that is not from the bicistronic reporter, we removed the SV40 promoter generating the ΔSV40 vectors (Fig. 9A). The promoterless vectors will allow for the detection of luciferase activity from any transcripts that are generated by cryptic promoter activity (52). The promoterless bicistronic vectors were transfected into HeLa cells and, 24 h later, DNA, RNA, and protein were isolated. PCR analysis, using primers that hybridize to the RLuc and the FLuc sequences (15, 32), confirmed that the cells were transfected with the dl vectors (Fig. 9B). RNA analysis by RT-PCR (15, 32), also using primers that hybridize to the RLuc and the FLuc sequences, confirmed that the bicistronic (RLuc-FLuc) RNA was expressed only in cells that were transfected with the constructs that contained an SV40 promoter (Fig. 9C). Importantly, the RT-PCR product was dependent on the RT demonstrating that the amplified product was from RNA and not from contaminating DNA (Fig. 9D). This result confirmed that no synthesis of the bicistronic reporter RNA was observed in the absence of the SV40 promoter. Next, we determined the luciferase activities from the transfected cells. The RLuc and FLuc activities are shown relative to the activities obtained from the dl HTLV-1 5′UTR plasmid. When the SV40 promoter was not present, the RLuc activity was not above the background (Fig. 9E, left panel), even though PCR analysis confirmed that the plasmids were transfected into the cells (Fig. 9B). Interestingly, some FLuc activity was observed in the cells that were transfected with the ΔSV40 dl HTLV-1 5′UTR vector (Fig. 9E, right panel), suggesting that ca. 20% of the translational activity ascribed to the HTLV-1 5′UTR could be attributed to short monocistronic transcripts generated from cryptic promoter activity. Since there was not any FLuc activity observed for the dl ΔEMCV vector, the cryptic promoter probably lies within the HTLV-1 5′ leader (Fig. 9E, right panel). This observation is consistent with the data reported by Bolinger et al. (22) that describe the generation of smaller RNAs from a bicistronic construct harboring the R region of the HTLV-1 5′UTR in cells.
FIG 9.
IRES-mediated translation accounts for most of the FLuc activity in HeLa cells. (A) Schematic representation of the bicistronic constructs. The SV40 promoter from dl ΔEMCV or dl HTLV-1 5′UTR was removed to generate the promoterless (ΔSV40) vectors. SV40pA represents the SV40 polyadenylation signal. (B) HeLa cells were transfected with DNA (200 ng) corresponding to the vectors depicted in panel A, together with the pcDNA3.1 lacZ (50-ng) plasmid. Total DNA was extracted at 24 h posttransfection from the HeLa cells, and the presence of the transfected plasmids in HeLa cells was confirmed by PCR. A schematic representation of the experimental procedure showing the primers and the size of the expected amplicons is presented (top panel). As positive controls for amplification, purified dl HTLV-1 5′UTR (lane 3) or dl ΔEMCV (lane 4) plasmid DNA was used. Lane 1 corresponds to the molecular marker (M), while lane 2 contains the negative control in which no DNA was added. Lanes 5 and 6 correspond to the amplification product of DNA harvested from cells transfected with the SV40 promoter containing vectors, while lanes 7 and 8 correspond to the cells transfected with promoterless (ΔSV40) vectors. (C) A one-step RT-PCR was conducted using total RNA extracted from HeLa cells transfected with either the dl HTLV-1 5′UTR (lane 5), dl ΔEMCV (lane 6), ΔSV40-dl ΔEMCV (lane 8), or ΔSV40-dl HTLV-1 5′UTR (lane 7). In vitro-transcribed RNAs generated from the dl HTLV-1 5′UTR (lane 3) or dl ΔEMCV (lane 4) plasmids were used as positive controls (+). Lane 1 corresponds to the molecular marker (M), and lane 2 contains the negative RT-PCR control, in which no template RNA was included. RT-PCR products were visualized by separation on a 0.7% denaturing agarose gel stained with SYBR Green I nucleic acid gel stain (Invitrogen). (D) A no-RT control was performed on total RNA isolated from dl HTLV-1 5′UTR or the dl ΔEMCV-transfected HeLa cells. RNA was either reversed transcribed (+)RT; lanes 3 and 4) or not [(−) RT; lanes 5 and 6] prior to PCR amplification. (E) HeLa cells were cotransfected with the pcDNA3.1 lacZ (50 ng) plasmid, along with the indicated plasmid. β-Galactosidase served as a transfection control. RLuc (left panel) and FLuc (right panel) activities were determined and normalized to the β-galactosidase activity. The RLuc/β-galactosidase and FLuc/β-galactosidase activities obtained for the dl HTLV-1 5′UTR vector were set to 100%. The values shown are means ± the SEM for three independent experiments, each performed in triplicate.
If a monocistronic transcript encoding an active firefly luciferase enzyme is indeed generated from a cryptic promoter or through RNA splicing, as suggested from the data presented above (Fig. 9), then knockdown of the full-length bicistronic RNA using a siRNA that targets the Renilla luciferase coding region should knock down the bicistronic RNA without affecting the expression levels of a monocistronic firefly luciferase transcript. Therefore, to confirm or disregard our previous observations (Fig. 9), the dl HTLV-1 5′UTR vector was cotransfected in HeLa cells together with an siRNA that targeted the RLuc coding sequence (55). Two different siRNAs targeting different regions of RLuc were used in independent experiments (Fig. 10A). If RLuc and FLuc are indeed translated from a bicistronic mRNA, both RLuc and FLuc activities should be reduced following RLuc encoding mRNA knockdown. However, if FLuc is expressed exclusively from a monocistronic mRNA as previously proposed (22), then FLuc activity should be unaffected by the presence of siRNAs that specifically target RLuc. The results of this experiment are expressed as the RLA with the RLuc and FLuc activity in the absence of the siRNAs set to 100% (± the SEM). When the siRNAs were present, RLuc expression (white bars) was reduced to background levels, while ca. 20% of the FLuc activity (black bars) remained (Fig. 10B). Similar results were obtained for both siRNAs. These findings are consistent with what was observed with the promoterless bicistronic plasmids (Fig. 9E) and suggest that an alternate transcript may exist in HeLa cells transfected with dl HTLV-1 5′UTR construct. Nonetheless, in contrast to what has been previously proposed (22), this alternative transcript, generated by a cryptic promoter activity present within the HTVL-1 5′UTR (Fig. 9E), only accounts for ca. 20% of the documented FLuc activity (Fig. 9E and 10B). Therefore, the results indicate that 80% of the FLuc expression from the bicistronic vector in HeLa cells was due to an IRES present within the HTLV-1 5′UTR. Consequently, translation initiation from the HTLV-1 5′UTR is not exclusively cap dependent.
FIG 10.

Targeting Renilla luciferase in the bicistronic reporter using siRNA. (A) Schematic representation of the bicistronic constructs highlighting the two siRNA (siRNA1 or siRNA2) targets over the RLuc coding region. (B) Scrambled control siRNA, or one of the different siRNAs that targets the Renilla luciferase open reading frame, was cotransfected with the dl HTLV-1 5′UTR plasmid. RLuc (white bars) and FLuc (black bars) activities were measured at 24 h posttransfection and are expressed relative to the scrambled control siRNA set to 100% (i.e., the relative luciferase activity [RLA]). A representative experiment of three independent siRNA knockdown experiments is shown. The values shown are means ± the SEM for a single experiment performed in triplicate.
HTLV-1 5′UTR drives cap-independent translation initiation in Xenopus laevis oocytes.
Next, we evaluated whether a bicistronic mRNA harboring the HTLV-1 5′UTR could drive IRES-dependent translation when microinjected in Xenopus laevis oocytes—a translation system that has proven useful for the study of IRESs, including those present within retroviral mRNAs, such as the HIV-1 IRES (14, 32, 56–58). In vitro-synthesized, capped, and polyadenylated bicistronic dl RNAs containing the ΔEMCV, HIV-1 IRES, or HTLV-1 5′UTR were microinjected into the cytoplasm of Xenopus laevis oocytes. RLuc (first cistron) and FLuc (second cistron) activities were measured at 24 h postinjection (14, 15, 32). The RLuc levels from the dl ΔEMCV, dl HIV-1 IRES, and the dl HTLV-1 mRNAs were comparable, suggesting that a similar amount of each RNA was microinjected (Fig. 11A, left panel). As expected (14, 15, 32), only background levels of expression were observed from the second cistron of the dl ΔEMCV reporter RNA (Fig. 11A, right panel). FLuc from the dl HIV-1 IRES and dl HTLV-1 5′UTR RNAs were expressed well in excess of dl ΔEMCV background levels (Fig. 11A, right panel), demonstrating that in the context of a synthetic bicistronic RNA the HTLV-1 5′UTRs is certainly capable of driving cap-independent translation. The translational activity of the HTLV-1 5′UTRs in Xenopus laevis oocytes was significantly (P < 0.05) greater than that of the control HIV-1 IRES (Fig. 11A, right panel). As an additional control, dl ΔEMCV, dl HIV-1 IRES, and dl HTLV-1 5′UTR DNA plasmids were microinjected into the cytoplasm of Xenopus laevis oocytes. In all cases, neither RLuc nor FLuc activity could be detected, eliminating the possibility of de novo RNA synthesis from a DNA template (data not shown). Taken together, these findings suggest that the HTLV-1 5′UTR contains an IRES, since expression of the second cistron is observed for an in vitro-transcribed bicistronic RNA injected into Xenopus oocytes. This excludes the possibility that expression of the second cistron could be due to a splicing event or from the generation of a monocistronic transcript, or readthrough of the stop codon of the first cistron, which was also ruled out by the 4G cleavage and the Acap experiments (Fig. 3 and 4).
FIG 11.
Assessment of HTLV-1 IRES activity using in vitro-transcribed RNA in Xenopus laevis oocytes. (A) In vitro-transcribed capped and polyadenylated RNA generated from dl ΔEMCV, dl HIV IRES, or dl HTLV-1 5′UTR vectors were microinjection into the cytoplasm of Xenopus laevis oocytes. At 24 h after the microinjection, RLuc (left panel) and FLuc (right panel) activities were determined in RLU from at least three oocytes obtained from different animals. (B) Xenopus laevis oocytes were comicroinjected with dl ΔEMCV, dl HIV IRES, or dl HTLV-1 5′UTR mRNAs and together with protein extraction buffer (−), NS, or G2/M HeLa cell extracts. The RLuc (left panel) and FLuc activities (right panel) are shown. RLuc and FLuc activities were measured at 24 h postmicroinjection. The values shown are means ± the SEM for at least three oocytes. Statistical analysis was performed by a one-way ANOVA test, followed by a Tukey's multiple test comparison. *, P < 0.05.
Considering that translational activity of some IRESs in Xenopus laevis oocytes can vary in the presence of cell extracts (14, 37), we next evaluated whether cell extracts could modulate HTLV-1 IRES activity in this system. To this end, the control dl ΔEMCV, dl HIV-1 IRES, or dl HTLV-1 5′UTR RNAs were microinjected into Xenopus oocytes either alone or with HeLa cell extracts generated from either nonsynchronized (NS) or G2/M-arrested HeLa cells (Fig. 11B). In agreement with previous reports (14, 15, 32), no effect was observed for cap-dependent (RLuc) translation in the presence of HeLa cell extracts (Fig. 11B, left panel). This also indicates that there was no change in RNA stability in Xenopus laevis oocytes when HeLa cell extracts were injected (14, 32, 37). As expected (14), FLuc activity driven by the HIV-1 5′UTR increased significantly in Xenopus laevis oocytes in the presence of G2/M HeLa cell extracts (Fig. 11B, right panel). Consistent with what was observed in RRL (Fig. 5B), in Xenopus laevis oocytes the IRES activity of the HTLV-1 5′UTR increased in the presence of HeLa cell extracts; however, only G2/M HeLa cell extracts significantly enhanced translation initiation from the HIV-1 5′UTR (Fig. 11B, right panel). The HeLa cell extracts differentially impacted on RLuc and FLuc expression in Xenopus laevis oocytes. This observation strengthens the notion that translation initiation from the second cistron in the dl HTLV-1 5′UTR RNA is independent from translation initiation of the first cistron in Xenopus laevis oocytes.
Together, the results obtained using Xenopus laevis oocytes provide substantial evidence to support the presence of an IRES within the HTLV-1 5′UTR. Furthermore, these findings are in agreement with previous studies (14, 32) and imply that additional viral proteins are not required for HTLV-1 IRES activity in Xenopus laevis oocytes. However, they also indicate that a protein(s) present in HeLa cell extracts can stimulate HTLV-1 IRES activity in Xenopus laevis oocytes, suggesting that this translation system might prove useful in the future for identification of IRES transacting factors for the HTLV-1 IRES.
HTLV-1 IRES activity is dependent of ribosomal protein S25.
Several retroviral mRNAs have been shown to harbor IRES activity (reviewed in reference 8). However, little is known about the mechanism by which the retroviral mRNAs recruit the translation initiation complex internally. The mechanism of ribosome recruitment by various viral IRESs differs greatly (59, 60). Therefore, it is impossible to predict how a newly described IRES such as the HTLV-1 IRES will interact with the translation initiation complex. A recent report suggests that despite structural and functional differences between IRESs they share a mechanism that utilizes ribosomal protein S25 (RPS25) for cap-independent initiation (26). Although both viral and cellular IRESs utilize RPS25 for initiation, RPS25 has no effect on cap-dependent initiation (61). These findings strongly suggest that RPS25 might play a critical role in a common mechanism that is used by IRESs to manipulate the host ribosome for cap-independent initiation (26). To test whether RPS25 plays a role in HTLV-1 IRES-mediated translation initiation, the dl HTLV-1 5′UTR DNA plasmid was transfected into previously described stable HeLa cells lines that were generated by lentiviral transduction to constitutively express an shRNA against RPS25 (HeLaShS25) or a control empty vector (HeLaShV) (26, 61). As an additional control, cells were transfected with a pcDNA control plasmid or with a vector expressing a previously described shRNA-resistant RPS25 mRNA (pS25A). A Western analysis demonstrating undetectable RPS25 levels in the stable HeLaShS25 cells and recovery of RPS25 expression from a plasmid has been published previously (see Fig. 2C in reference 26). The results are expressed as the RTA, with the FLuc/RLuc ratio obtained in HeLaShV cells set to 100% (Fig. 12). Expression pS25A in HeLaShV cells increased translation driven by the HTLV-1 5′UTR in ca. 40% (Fig. 12), suggesting that the overexpression of RPS25 stimulates HTLV-1 IRES activity. Conversely, knockdown of RPS25 significantly reduced HTLV-1 IRES activity (54%) (Fig. 12). It is noteworthy that 20% of FLuc activity from the bicistronic DNA is expected to be cap dependent, as established in Fig. 9 and 10, and therefore should not be affected by RPS25 knockdown. Thus, in this assay, reduction of HTLV-1 IRES activity is clearly being underestimated. It is intriguing that the observed reduction in the translational activity of the HTLV-1 5′UTR in the RPS25 knockdown HeLa cells is similar to what has been previously described for the poliovirus (PV) and the EMCV IRESs (26). Importantly, cotransfection of a plasmid pS25A, resulted in a complete rescue (96%) of translational activity for the HTLV-1 IRES in HeLaShS25 cells (Fig. 12). These results further confirm that the HTLV-1 5′UTR harbors an IRES and indicate that optimal HTLV-1 IRES activity is dependent on RPS25.
FIG 12.

HTLV-1 IRES activity is reduced in cells when RPS25 is knocked down. A HeLa cell line stably expressing an RPS25 shRNA (HeLaShS25) or not (HeLaShV) were cotransfected with the dl HTLV-1 5′UTR plasmid, pcDNA3.1 lacZ, and either an empty vector (pcDNA) or the rescue vector expressing a version of RPS25 that is resistant to the shRNA (pS25) (26). The luciferase activities were measured at 24 h posttransfection and were normalized to the β-galactosidase activities here used as a transfection control (Luc/β-galactosidase). The values are presented relative to the HeLaShV transfected with the empty vector (pcDNA), which is set to 100%. The values shown are means ± the SEM for five independent experiments performed in triplicate. Statistical analysis was performed by a one-way ANOVA test, followed by a Tukey's multiple test comparison. *, P < 0.05.
Translation initiation driven by the HTLV-1 5′UTR is sensitive to edeine.
Ribosome recruitment to the IRES can occur either upstream or directly at the initiation codon (59, 60). The PV IRES, for example, recruits the 40S ribosomal subunit upstream from the initiation codon and the 40S-ternary complex (eIF2-GTP/Met-tRNAi) scans the 5′UTR in a 5′ to 3′ direction until the initiation codon is recognized. In contrast, the HCV IRES recruits the 40S ribosomal subunit directly to the initiation codon without scanning. Therefore, we used edeine, a strongly basic, linear oligopeptide antibiotic that interferes with initiation codon (AUG) recognition by the scanning 40S-ternary complexes (24, 25, 62, 63), to determine whether the 40S ribosomal subunit was recruited by the HTLV-1 IRES directly at the initiation codon or upstream of it. At low concentrations, edeine inhibits translation initiation mediated by scanning 40S ribosomal subunits in RRL, with no effect on polypeptide elongation (25, 64). To validate our experimental setup, RRL was programmed with the dl PV IRES or dl HCV IRES bicistronic mRNAs, harboring the IRES elements from PV or HCV (29, 30, 33), respectively. In vitro translation reactions were conducted in the absence or the presence of increasing concentrations of edeine (33). As predicted, for both biscistronic mRNAs cap-dependent translation (RLuc) was inhibited in the presence of 0.25 and 0.5 μM edeine (Fig. 13A and B, white bars). Expression of the downstream cistron mediated by the PV IRES was also inhibited (Fig. 13A, black bars), consistent the requirement of scanning ternary complexes for the recognition of the initiation codon. In contrast, translation initiation mediated by the HCV IRES remained unaffected at 0.25 μM edeine (Fig. 13B, black bars). These results are consistent with the ability of the HCV IRES to directly recruit 40S ribosomal subunits to the initiation codon without scanning (65, 66). Inhibition of HCV IRES activity at 0.5 μM edeine has been previously described (33) and likely reflects inhibition of elongation, which is observed at high concentrations of edeine (66). Next, we sought to evaluate the effect of edeine on translation driven by the HTLV-1 5′UTR. Translation of a bicistronic dl HTLV-1 5′UTR mRNA in RRL with increasing amounts of edeine resulted in inhibition of translation of both RLuc (Fig. 13C, white bars) and FLuc (Fig. 13C, black bars), suggesting that upon recruitment of the initiation complex by the HTLV-1 IRES the 40S subunit scans the mRNA to reach the initiation codon.
FIG 13.

Translation initiation from the HTLV-1 5′UTR is sensitive to edeine in RRL. The capped dl PV IRES (A), dl HCV-1 IRES (B), and dl HTLV-1 5′UTR RNA (C) were in vitro translated in RRL in the absence (−) or presence of increasing concentrations of edeine (0.125, 0.25, or 0.5 μM). The RLuc (black bars) and FLuc (white bars) activities were measured. The RLuc and FLuc activities for each bicistronic reporter are shown relative to the luciferase activity obtained in the absence (−) of edeine, which was set to 100%. The values shown are means ± the SEM for four independent experiments.
DISCUSSION
Much like eukaryotic mRNAs, retroviral mRNAs have a 5′ cap and a 3′ poly(A) tail. Interestingly, the presence of IRES elements within the viral mRNA has been described in a large number of retroviruses and retrotransposons (reviewed in reference 8). In general, retroviral IRESs have been identified by using heterologous bicistronic mRNAs. Nevertheless, the real requirement for IRES activity in the context of the full-length viral mRNA during the viral replication cycle still remains highly controversial (8). This is evident upon analysis of disparate reports regarding the mechanism of translation initiation of the HIV-1 mRNA (10, 12). Conflict has also extended to other retroviruses such as HTLV-1 (20, 22). In an early study, Takebe et al. (41) show that, when it is attached to the SV40 early gene promoter, the HTLV-1 R region stimulated gene expression (41). Takebe et al. showed that increased gene expression was controlled at the level of mRNA translation. This observation led Attal et al. (20) to evaluate whether the HTLV-1 R region harbored an IRES. These researchers concluded that RNA fragments containing the complete R region and the beginning of the U5 region from the HTLV-1 mRNA (nt 1 to 267) were sufficient to stimulate translation initiation of the second cistron of a bicistronic mRNA. These data were, however, disregarded in the report of Bolinger et al. (22), which used almost the same RNA segment (nt 1 to 263) to establish the generation of aberrant shorter transcripts from the bicistronic reporter and concluded that translation initiation of the HTLV-1 mRNA was exclusively cap dependent (22). Since 1995, a number of reports claim the presence of IRES activity within the 5′UTR of retroviral mRNAs (reviewed in reference 8). In contrast to the reports of Attal et al. (20) and Bolinger et al. (22), most studies use the full-length 5′UTR of the retroviral mRNA to determine IRES activity (12, 15). Some even extend the region used to include part of the Gag protein open reading frame (16, 17). Once the existence of an IRES is confirmed within the full-length 5′UTR, some studies conduct 5′ and 3′ truncations to map the minimal region required for IRES activity (12, 16). The studies of Attal et al. (20) and Bolinger et al. (22) left out from their analysis 182 nt of the HTLV-1 5′UTR that are located directly upstream of the HTLV-1 Gag initiation codon (20, 22). This, plus the notion that many retroviral mRNAs harbor IRESs (8, 9), prompted us to reassess whether the 5′UTR of the HTLV-1 full-length mRNA harbored an IRES. Therefore, we evaluated here the ability of the full-length HTLV-1 5′UTR (nt 1 to 449) to drive cap-independent translation initiation. Our results definitively show that the 5′UTR of the full-length HTLV-1 mRNA harbors an IRES. Furthermore, the data show that the IRES activity is confined to a RNA segment of 181 nt encompassing the DIS and the PBS domains (nt 268 to 449), a fragment that was left out in both previous studies (20, 22). In agreement with the previous work of Bolinger et al. (22), negligible IRES translation activity is contributed by nt 1 to 267. Translational activity of the HTLV-1 IRES was demonstrated in three different experimental systems: RRL, Xenopus laevis oocytes, and mammalian cells. In addition, our results show that in HeLa cells the dl HTLV-1 5′UTR exhibits a small amount of cryptic promoter activity, which is likely located within the viral 5′UTR (Fig. 9 and 10). At this point it cannot be concluded whether the presence of this cryptic promoter is exclusively associated with the artificial bicistronic DNA used in these assays or whether it is a feature also used by the virus during its replication cycle. It is noteworthy that two primate lentiviruses—namely, SIVmac239 and HIV-2—are known to generate shorter mRNAs, alternative to the full-length mRNA, encoding the Gag protein in a strategy believed to increase viral protein expression (67, 68). Although the presence of a cryptic promoter does not necessarily mean that an RNA element does not contain an IRES, it does suggest that transfection of a DNA bicistronic reporter cannot be used exclusively to establish IRES activity (or not). Importantly, in vitro translation assays (Fig. 1 through 5) and RNA injections in Xenopus laevis oocytes experiments (Fig. 11), both of which would not be subject to splicing or cryptic promoter activities, strongly suggest that the 5′UTR of the HTLV-1 full-length mRNA contains an IRES. In agreement with this, the monocistronic cap-dependent transcript generated following DNA transfections into mammalian cells accounts for ca. 20% of the total amount of expression of the second cistron (Fig. 9 and 10). Nonetheless, the results clearly indicate that in HeLa cells 80% of the second cistron expression is IRES mediated (Fig. 9 and 10). Therefore, our data do not support the conclusion of others suggesting that translation initiation from the full-length HTLV-1 mRNA is exclusively cap dependent (22), nor do they indicate that translation initiation from the full-length HTLV-1 mRNA is exclusively IRES dependent. Several studies have shown that primate lentiviruses exhibit a redundancy in the mechanism of initiation of protein synthesis (reviewed in reference 8), suggesting that these mRNAs can initiate translation using both a cap-dependent and an IRES-dependent mechanism (18, 19). Based on the evidence and on the apparent conservation of functions among the Retroviridae (8), we expect the HTLV-1 full-length mRNA to also use dual cap- and IRES-mediated mechanisms of translation initiation. Furthermore, since the translation initiation process was evaluated in the absence of viral proteins, we know that these are not required for HTLV-1 IRES-mediated translation initiation in RRL, HeLa cells, or in Xenopus laevis oocytes.
As for most retroviral IRES elements, the biological significance of the HTLV-1 IRES within the viral context remains obscure. However, it is tempting to speculate that the presence of an IRES within the 5′UTR of the HTLV-1 full-length mRNA confers this transcript with a clear recruitment advantage over cellular mRNAs during the viral replication cycle. In the case of HIV-1, alternative initiation has been proposed to allow the viral mRNA to bypass the constraints of global cellular translational repression that results from the inhibition of cap-dependent translation initiation (8, 9). This proposal is given credence by experimental evidence showing that the HIV-1 IRES supports translation initiation during osmotic stress (17, 69) and during the G2/M phase of the cell cycle (12), two conditions that are induced during HIV-1 replication in cells. Under these conditions, cap-dependent translation initiation of cellular mRNAs is globally suppressed (70). IRES-mediated translation initiation may also ensure synthesis of HIV-1 structural proteins during the late stages of the replication cycle, when the eukaryotic initiation factor eIF4G and the poly(A)-binding protein, which are both required for cap-dependent translation initiation, are cleaved by the viral protease (reviewed in reference 9). It is less clear why the HTLV-1 full-length mRNA would have maintained a functional IRES. Nonetheless, close analysis of several published reports highlight a striking similarity in the regulation of viral gene expression between HTLV-1 and other retroviruses known to harbor an IRES. For example, the HTLV-1 protease cleaves eIF4G in a process that is expected to hinder cap-dependent translation initiation (45).
Upon infection HTLV-1 enters into a latent state, rendering the infected individuals asymptomatic seropositive carriers, and only a small percentage of these carriers eventually develop ATL or TSP/HAM (71). A number of studies suggest that cellular stress conditions and cellular stress response mechanisms are capable of activating latent HTLV-1 by stimulating LTR-induced transcription of viral mRNA (72, 73). Upon HTLV-1 activation, the viral accessory proteins p13 participates in the maintenance and further stimulation of a cellular stress environment required for HTLV-1 replication (74). During cellular stress responses, transcription of the HTLV-1 mRNA is enhanced (75, 76). Andrews et al. (77) showed by Western analysis of proteins extracted from HTLV-1-infected cells that viral proteins are expressed at 48 h after the induction of cellular stress response mechanisms using Na arsenite. Their results demonstrate a clear increase in all proteins associated with the HTLV-1 full-length mRNA, including the polyproteins GagPol (p65) and Gag (p55), as well as the processed protein products p19 and p24 (77). The same report indicates that similar observations are made 24 h after the induction of cellular stress responses (77). This and a subsequent study from the same group (78) conclude that the increase in viral protein expression under cellular stress conditions is regulated mostly at the level of mRNA translation. Strikingly, most of the described cell stress conditions known to stimulate HTLV-1 mRNA transcription and translation are also predicted to reduce cap-dependent translation initiation (1, 2, 79–81). Thus, it is plausible that under stress conditions known to activate both HTLV-1 mRNA synthesis and viral protein expression (72, 73), translation initiation of the full-length HTLV-1 mRNA occurs via a cap-independent mechanism.
The mechanism by which retroviral mRNAs recruit the 40S ribosomal subunit internally is not understood. In the present study, we provide the first evidence showing the participation of RPS25 in retroviral IRES-mediated translation initiation (Fig. 12). In general, both viral and cellular IRESs utilize various mechanisms to recruit the 40S ribosomal subunit. However, IRESs appear to rely on RPS25 for a step that is downstream of 40S recruitment in initiation (26). Several alternative possibilities have been proposed to explain the role of RPS25 in IRES-mediated translation, such as mRNA loading into the mRNA binding channel of the 40S ribosomal subunit, start codon recognition, and 60S subunit joining (26). Although the findings described here do not contribute to the understanding of the role of RPS25 in IRES-mediated translation initiation, they do demonstrate that, as with other viral and cellular IRESs, translation initiation driven by the HTLV-1 5′UTR is RPS25 dependent (Fig. 12). Further experiments are required to fully determine how RPS25 and the HTLV-1 IRES interact during the initiation of protein synthesis. Nonetheless, these results show that the HTLV-1 IRES activity has distinct requirements compared to cellular cap-dependent translation initiation, such as the requirement for RPS25 for full IRES activity. This simple observation raises the possibility that IRES-mediated translation initiation of HTLV-1 mRNA can be targeted for the development of a therapeutic that specifically inhibits IRES activity without hindering cellular cap-dependent translation initiation.
The success in showing that RPS25 played a role in HTLV-1 IRES activity pushed us to evaluate other aspects of the mechanism by which the viral RNA is recruited to the 40S ribosomal subunit internally. By using the drug edeine, which inhibits the initiation step of protein synthesis by blocking the recognition of the initiation codon by scanning ribosomes (23, 24, 62, 66), we provide insight into the strategy used by the HTLV-1 IRES to recruit the initiation complex (Fig. 13). Translation of the HTLV-1 IRES in the presence of edeine suggests that the 40S ribosomal subunit is recruited upstream of the HTLV-1 gag AUG and scans in a 5′-to-3′ direction until it reaches the initiation codon. This would imply that upon recruitment, the initiation complex scans the HTLV-1 5′UTR in a 5′ to 3′ direction until the start codon is recognized by the codon anticodon interaction with Met-tRNAi.
ACKNOWLEDGMENTS
We are grateful to G. Belsham (Institute for Animal Health, Pirbright, United Kingdom), N. Sonenberg (McGill University, Montreal, Quebec, Canada), P. Sarnow (Stanford University, Stanford, CA), and B. Barbeau (Université du Québec à Montréal, Montréal, Québec, Canada) for kindly providing the plasmids used in this study. We thank L. Carrasco (Centro de Biología Molecular Severo Ochoa, Madrid, Spain) for kindly providing the polyclonal antibodies against eIF4GI and I. Brierley (University of Cambridge, Cambridge, United Kingdom) for sharing the drug edeine.
This study was supported by the Comision Nacional de Investigacion Cientifica y Tecnologica, Gobierno de Chile (CONICYT), through grant FONDECYT 1130270 and by Proyecto P09/016-F de la Iniciativa Cientifica Milenio del Ministerio de Economia, Fomento y Turismo to M.L.L. J.P.H.-T. was funded by CARE, program projects PFB 12/29007 and FONDECYT 1110672. S.R.T. was supported by National Institutes of Health grant R01GM084547 and a UAB Cancer Center HIV-Associated Malignancy pilot research grant (UAB Comprehensive Cancer Center core support grant P30 CA13148). E.O. was supported by UCH0604 MECESUP-USACH doctoral fellowships.
E.O., D.M.L., C.J.C., K.P., F.R., and C.N. performed the experiments. E.O. and M.L.L. designed experiments performed in RRL and in HeLa cells. E.O., C.J.C., and F.R. conducted experiments using RRL. E.O. and K.P. performed experiments in cells. E.O., J.P.H.-T., and M.L.L. designed the experiments using Xenopus laevis oocytes. E.O. and C.N. conducted the experiments in X. laevis oocytes. D.M.L. and S.R.T. designed and conducted the experiments using siRNA targeting Renilla luciferase, as well as the experiments performed to establish the role of ribosomal protein S25 on HTLV-1 IRES activity. E.O., D.M.L., S.R.T., and M.L.L. assisted with manuscript preparation and editing. S.R.T. and M.L.L. wrote the manuscript. All authors read and approved the final manuscript.
Footnotes
Published ahead of print 12 March 2014
REFERENCES
- 1.Jackson RJ, Hellen CU, Pestova TV. 2010. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell. Biol. 11:113–127. 10.1038/nrm2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sonenberg N, Hinnebusch AG. 2009. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136:731–745. 10.1016/j.cell.2009.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jang SK, Krausslich HG, Nicklin MJ, Duke GM, Palmenberg AC, Wimmer E. 1988. A segment of the 5′ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J. Virol. 62:2636–2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelletier J, Sonenberg N. 1988. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 334:320–325. 10.1038/334320a0 [DOI] [PubMed] [Google Scholar]
- 5.Jackson RJ, Hunt SL, Reynolds JE, Kaminski A. 1995. Cap-dependent and cap-independent translation: operational distinctions and mechanistic interpretations. Curr. Top. Microbiol. Immunol. 203:1–29 [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Lastra M, Ramdohr P, Letelier A, Vallejos M, Vera-Otarola J, Valiente-Echeverria F. 2010. Translation initiation of viral mRNAs. Rev. Med. Virol. 20:177–195. 10.1002/rmv.649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson SR. 2012. So you want to know if your message has an IRES? Wiley Interdiscip. Rev. RNA 3:697–705. 10.1002/wrna.1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balvay L, Lopez Lastra M, Sargueil B, Darlix JL, Ohlmann T. 2007. Translational control of retroviruses. Nat. Rev. Microbiol. 5:128–140. 10.1038/nrmicro1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Breyne S, Soto-Rifo R, Lopez-Lastra M, Ohlmann T. 2013. Translation initiation is driven by different mechanisms on the HIV-1 and HIV-2 genomic RNAs. Virus Res. 171:366–381. 10.1016/j.virusres.2012.10.006 [DOI] [PubMed] [Google Scholar]
- 10.Miele G, Mouland A, Harrison GP, Cohen E, Lever AM. 1996. The human immunodeficiency virus type 1 5′ packaging signal structure affects translation but does not function as an internal ribosome entry site structure. J. Virol. 70:944–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buck CB, Shen X, Egan MA, Pierson TC, Walker CM, Siliciano RF. 2001. The human immunodeficiency virus type 1 gag gene encodes an internal ribosome entry site. J. Virol. 75:181–191. 10.1128/JVI.75.1.181-191.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brasey A, Lopez-Lastra M, Ohlmann T, Beerens N, Berkhout B, Darlix JL, Sonenberg N. 2003. The leader of human immunodeficiency virus type 1 genomic RNA harbors an internal ribosome entry segment that is active during the G2/M phase of the cell cycle. J. Virol. 77:3939–3949. 10.1128/JVI.77.7.3939-3949.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berkhout B, Arts K, Abbink TE. 2011. Ribosomal scanning on the 5′-untranslated region of the human immunodeficiency virus RNA genome. Nucleic Acids Res. 39:5232–5244. 10.1093/nar/gkr113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallejos M, Deforges J, Plank TD, Letelier A, Ramdohr P, Abraham CG, Valiente-Echeverria F, Kieft JS, Sargueil B, Lopez-Lastra M. 2011. Activity of the human immunodeficiency virus type 1 cell cycle-dependent internal ribosomal entry site is modulated by IRES trans-acting factors. Nucleic Acids Res. 39:6186–6200. 10.1093/nar/gkr189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallejos M, Carvajal F, Pino K, Navarrete C, Ferres M, Huidobro-Toro JP, Sargueil B, Lopez-Lastra M. 2012. Functional and structural analysis of the internal ribosome entry site present in the mRNA of natural variants of the HIV-1. PLoS One 7:e35031. 10.1371/journal.pone.0035031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plank TD, Whitehurst JT, Kieft JS. 2013. Cell type specificity and structural determinants of IRES activity from the 5′ leaders of different HIV-1 transcripts. Nucleic Acids Res. 41:6698–6714. 10.1093/nar/gkt358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gendron K, Ferbeyre G, Heveker N, Brakier-Gingras L. 2011. The activity of the HIV-1 IRES is stimulated by oxidative stress and controlled by a negative regulatory element. Nucleic Acids Res. 39:902–912. 10.1093/nar/gkq885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricci EP, Soto Rifo R, Herbreteau CH, Decimo D, Ohlmann T. 2008. Lentiviral RNAs can use different mechanisms for translation initiation. Biochem. Soc. Trans. 36:690–693. 10.1042/BST0360690 [DOI] [PubMed] [Google Scholar]
- 19.Monette A, Valiente-Echeverria F, Rivero M, Cohen EA, Lopez-Lastra M, Mouland AJ. 2013. Dual mechanisms of translation initiation of the full-length HIV-1 mRNA contribute to Gag synthesis. PLoS One 8:e68108. 10.1371/journal.pone.0068108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Attal J, Theron MC, Taboit F, Cajero-Juarez M, Kann G, Bolifraud P, Houdebine LM. 1996. The RU5 (‘R') region from human leukaemia viruses (HTLV-1) contains an internal ribosome entry site (IRES)-like sequence. FEBS Lett. 392:220–224. 10.1016/0014-5793(96)00815-0 [DOI] [PubMed] [Google Scholar]
- 21.Douin V, Bornes S, Creancier L, Rochaix P, Favre G, Prats AC, Couderc B. 2004. Use and comparison of different internal ribosomal entry sites (IRES) in tricistronic retroviral vectors. BMC Biotechnol. 4:16. 10.1186/1472-6750-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolinger C, Yilmaz A, Hartman TR, Kovacic MB, Fernandez S, Ye J, Forget M, Green PL, Boris-Lawrie K. 2007. RNA helicase A interacts with divergent lymphotropic retroviruses and promotes translation of human T-cell leukemia virus type 1. Nucleic Acids Res. 35:2629–2642. 10.1093/nar/gkm124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozak M, Shatkin AJ. 1978. Migration of 40 S ribosomal subunits on messenger RNA in the presence of edeine. J. Biol. Chem. 253:6568–6577 [PubMed] [Google Scholar]
- 24.Dinos G, Wilson DN, Teraoka Y, Szaflarski W, Fucini P, Kalpaxis D, Nierhaus KH. 2004. Dissecting the ribosomal inhibition mechanisms of edeine and pactamycin: the universally conserved residues G693 and C795 regulate P-site RNA binding. Mol. Cell 13:113–124. 10.1016/S1097-2765(04)00002-4 [DOI] [PubMed] [Google Scholar]
- 25.Obrig T, Irvin J, Culp W, Hardesty B. 1971. Inhibition of peptide initiation on reticulocyte ribosomes by edeine. Eur. J. Biochem. 21:31–41. 10.1111/j.1432-1033.1971.tb01436.x [DOI] [PubMed] [Google Scholar]
- 26.Hertz MI, Landry DM, Willis AE, Luo G, Thompson SR. 2013. Ribosomal protein S25 dependency reveals a common mechanism for diverse internal ribosome entry sites and ribosome shunting. Mol. Cell. Biol. 33:1016–1026. 10.1128/MCB.00879-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavanagh MH, Landry S, Audet B, Arpin-Andre C, Hivin P, Pare ME, Thete J, Wattel E, Marriott SJ, Mesnard JM, Barbeau B. 2006. HTLV-1 antisense transcripts initiating in the 3′LTR are alternatively spliced and polyadenylated. Retrovirology 3:15. 10.1186/1742-4690-3-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson JE, Powell MJ, Hoover SE, Sarnow P. 2000. Naturally occurring dicistronic cricket paralysis virus RNA is regulated by two internal ribosome entry sites. Mol. Cell. Biol. 20:4990–4999. 10.1128/MCB.20.14.4990-4999.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barria MI, Gonzalez A, Vera-Otarola J, Leon U, Vollrath V, Marsac D, Monasterio O, Perez-Acle T, Soza A, Lopez-Lastra M. 2009. Analysis of natural variants of the hepatitis C virus internal ribosome entry site reveals that primary sequence plays a key role in cap-independent translation. Nucleic Acids Res. 37:957–971. 10.1093/nar/gkn1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poulin F, Gingras AC, Olsen H, Chevalier S, Sonenberg N. 1998. 4E-BP3, a new member of the eukaryotic initiation factor 4E-binding protein family. J. Biol. Chem. 273:14002–14007. 10.1074/jbc.273.22.14002 [DOI] [PubMed] [Google Scholar]
- 31.Medina M, Domingo E, Brangwyn JK, Belsham GJ. 1993. The two species of the foot-and-mouth disease virus leader protein, expressed individually, exhibit the same activities. Virology 194:355–359. 10.1006/viro.1993.1267 [DOI] [PubMed] [Google Scholar]
- 32.Vallejos M, Ramdohr P, Valiente-Echeverria F, Tapia K, Rodriguez FE, Lowy F, Huidobro-Toro JP, Dangerfield JA, Lopez-Lastra M. 2010. The 5′-untranslated region of the mouse mammary tumor virus mRNA exhibits cap-independent translation initiation. Nucleic Acids Res. 38:618–632. 10.1093/nar/gkp890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vera-Otarola J, Solis L, Soto-Rifo R, Ricci EP, Pino K, Tischler ND, Ohlmann T, Darlix JL, Lopez-Lastra M. 2012. The Andes hantavirus NSs protein is expressed from the viral small mRNA by a leaky scanning mechanism. J. Virol. 86:2176–2187. 10.1128/JVI.06223-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dorner AJ, Semler BL, Jackson RJ, Hanecak R, Duprey E, Wimmer E. 1984. In vitro translation of poliovirus RNA: utilization of internal initiation sites in reticulocyte lysate. J. Virol. 50:507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aldabe R, Feduchi E, Novoa I, Carrasco L. 1995. Efficient cleavage of p220 by poliovirus 2Apro expression in mammalian cells: effects on vaccinia virus. Biochem. Biophys. Res. Commun. 215:928–936. 10.1006/bbrc.1995.2553 [DOI] [PubMed] [Google Scholar]
- 36.Gradi A, Svitkin YV, Imataka H, Sonenberg N. 1998. Proteolysis of human eukaryotic translation initiation factor eIF4GII, but not eIF4GI, coincides with the shutoff of host protein synthesis after poliovirus infection. Proc. Natl. Acad. Sci. U. S. A. 95:11089–11094. 10.1073/pnas.95.19.11089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gamarnik AV, Andino R. 2006. Exploring RNA virus replication in Xenopus oocytes. Methods Mol. Biol. 322:367–378. 10.1007/978-1-59745-000-3_26 [DOI] [PubMed] [Google Scholar]
- 38.Baird SD, Turcotte M, Korneluk RG, Holcik M. 2006. Searching for IRES. RNA 12:1755–1785. 10.1261/rna.157806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paulous S, Malnou CE, Michel YM, Kean KM, Borman AM. 2003. Comparison of the capacity of different viral internal ribosome entry segments to direct translation initiation in poly(A)-dependent reticulocyte lysates. Nucleic Acids Res. 31:722–733. 10.1093/nar/gkf695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chappell SA, Edelman GM, Mauro VP. 2000. A 9-nt segment of a cellular mRNA can function as an internal ribosome entry site (IRES) and when present in linked multiple copies greatly enhances IRES activity. Proc. Natl. Acad. Sci. U. S. A. 97:1536–1541. 10.1073/pnas.97.4.1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takebe Y, Seiki M, Fujisawa J, Hoy P, Yokota K, Arai K, Yoshida M, Arai N. 1988. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol. Cell. Biol. 8:466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Attal J, Theron MC, Kann G, Bolifraud P, Puissant C, Houdebine LM. 2000. The stimulation of gene expression by the R region from HTLV-1 and BLV. J. Biotechnol. 77:179–189. 10.1016/S0168-1656(99)00221-7 [DOI] [PubMed] [Google Scholar]
- 43.Bergamini G, Preiss T, Hentze MW. 2000. Picornavirus IRESes and the poly(A) tail jointly promote cap-independent translation in a mammalian cell-free system. RNA 6:1781–1790. 10.1017/S1355838200001679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lloyd RE. 2006. Translational control by viral proteinases. Virus Res. 119:76–88. 10.1016/j.virusres.2005.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alvarez E, Menendez-Arias L, Carrasco L. 2003. The eukaryotic translation initiation factor 4GI is cleaved by different retroviral proteases. J. Virol. 77:12392–12400. 10.1128/JVI.77.23.12392-12400.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borman AM, Kirchweger R, Ziegler E, Rhoads RE, Skern T, Kean KM. 1997. elF4G and its proteolytic cleavage products: effect on initiation of protein synthesis from capped, uncapped, and IRES-containing mRNAs. RNA 3:186–196 [PMC free article] [PubMed] [Google Scholar]
- 47.Lamphear BJ, Kirchweger R, Skern T, Rhoads RE. 1995. Mapping of functional domains in eukaryotic protein synthesis initiation factor 4G (eIF4G) with picornaviral proteases: implications for cap-dependent and cap-independent translational initiation. J. Biol. Chem. 270:21975–21983 [DOI] [PubMed] [Google Scholar]
- 48.Kirchweger R, Ziegler E, Lamphear BJ, Waters D, Liebig HD, Sommergruber W, Sobrino F, Hohenadl C, Blaas D, Rhoads RE, et al. 1994. Foot-and-mouth disease virus leader proteinase: purification of the Lb form and determination of its cleavage site on eIF-4 gamma. J. Virol. 68:5677–5684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohlmann T, Rau M, Morley SJ, Pain VM. 1995. Proteolytic cleavage of initiation factor eIF-4 gamma in the reticulocyte lysate inhibits translation of capped mRNAs but enhances that of uncapped mRNAs. Nucleic Acids Res. 23:334–340. 10.1093/nar/23.3.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohlmann T, Rau M, Pain VM, Morley SJ. 1996. The C-terminal domain of eukaryotic protein synthesis initiation factor (eIF) 4G is sufficient to support cap-independent translation in the absence of eIF4E. EMBO J. 15:1371–1382 [PMC free article] [PubMed] [Google Scholar]
- 51.Ohlmann T, Lopez-Lastra M, Darlix JL. 2000. An internal ribosome entry segment promotes translation of the simian immunodeficiency virus genomic RNA. J. Biol. Chem. 275:11899–11906. 10.1074/jbc.275.16.11899 [DOI] [PubMed] [Google Scholar]
- 52.Han B, Zhang JT. 2002. Regulation of gene expression by internal ribosome entry sites or cryptic promoters: the eIF4G story. Mol. Cell. Biol. 22:7372–7384. 10.1128/MCB.22.21.7372-7384.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kozak M. 2003. Alternative ways to think about mRNA sequences and proteins that appear to promote internal initiation of translation. Gene 318:1–23. 10.1016/S0378-1119(03)00774-1 [DOI] [PubMed] [Google Scholar]
- 54.Vopalensky V, Masek T, Horvath O, Vicenova B, Mokrejs M, Pospisek M. 2008. Firefly luciferase gene contains a cryptic promoter. RNA 14:1720–1729. 10.1261/rna.831808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Eden ME, Byrd MP, Sherrill KW, Lloyd RE. 2004. Demonstrating internal ribosome entry sites in eukaryotic mRNAs using stringent RNA test procedures. RNA 10:720–730. 10.1261/rna.5225204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keiper BD, Rhoads RE. 1997. Cap-independent translation initiation in Xenopus oocytes. Nucleic Acids Res. 25:395–402. 10.1093/nar/25.2.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Velden AW, Los A, Voorma HO, Thomas AA. 2000. Sequence and translation initiation properties of the xenopus TGFβ5, PDGF-A, and PDGF-alpha receptor 5′ untranslated regions. Int. J. Dev. Biol. 44:851–859 [PubMed] [Google Scholar]
- 58.Gamarnik AV, Andino R. 1996. Replication of poliovirus in Xenopus oocytes requires two human factors. EMBO J. 15:5988–5998 [PMC free article] [PubMed] [Google Scholar]
- 59.Fitzgerald KD, Semler BL. 2009. Bridging IRES elements in mRNAs to the eukaryotic translation apparatus. Biochim. Biophys. Acta 1789:518–528. 10.1016/j.bbagrm.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kieft JS. 2008. Viral IRES RNA structures and ribosome interactions. Trends Biochem. Sci. 33:274–283. 10.1016/j.tibs.2008.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Landry DM, Hertz MI, Thompson SR. 2009. RPS25 is essential for translation initiation by the Dicistroviridae and hepatitis C viral IRESs. Genes Dev. 23:2753–2764. 10.1101/gad.1832209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hunter AR, Jackson RJ, Hunt T. 1977. The role of complexes between the 40-S ribosomal subunit and Met-tRNA-Met-f in the initiation of protein synthesis in the wheat-germ system. Eur. J. Biochem. 75:159–170. 10.1111/j.1432-1033.1977.tb11513.x [DOI] [PubMed] [Google Scholar]
- 63.Kozak M. 1987. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 15:8125–8148. 10.1093/nar/15.20.8125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hunt T. 1974. The control of globin synthesis in rabbit reticulocytes. Ann. N. Y. Acad. Sci. 241:223–231. 10.1111/j.1749-6632.1974.tb21880.x [DOI] [PubMed] [Google Scholar]
- 65.Pestova TV, Shatsky IN, Fletcher SP, Jackson RJ, Hellen CU. 1998. A prokaryotic-like mode of cytoplasmic eukaryotic ribosome binding to the initiation codon during internal translation initiation of hepatitis C and classical swine fever virus RNAs. Genes Dev. 12:67–83. 10.1101/gad.12.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lancaster AM, Jan E, Sarnow P. 2006. Initiation factor-independent translation mediated by the hepatitis C virus internal ribosome entry site. RNA 12:894–902. 10.1261/rna.2342306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Viglianti GA, Rubinstein EP, Graves KL. 1992. Role of TAR RNA splicing in translational regulation of simian immunodeficiency virus from rhesus macaques. J. Virol. 66:4824–4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strong CL, Lanchy JM, Dieng-Sarr A, Kanki PJ, Lodmell JS. 2009. A 5′UTR-spliced mRNA isoform is specialized for enhanced HIV-2 Gag translation. J. Mol. Biol. 391:426–437. 10.1016/j.jmb.2009.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Monette A, Ajamian L, Lopez-Lastra M, Mouland AJ. 2009. Human immunodeficiency virus type 1 (HIV-1) induces the cytoplasmic retention of heterogeneous nuclear ribonucleoprotein A1 by disrupting nuclear import: implications for HIV-1 gene expression. J. Biol. Chem. 284:31350–31362. 10.1074/jbc.M109.048736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pyronnet S, Dostie J, Sonenberg N. 2001. Suppression of cap-dependent translation in mitosis. Genes Dev. 15:2083–2093. 10.1101/gad.889201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cook LB, Elemans M, Rowan AG, Asquith B. 2013. HTLV-1: persistence and pathogenesis. Virology 435:131–140. 10.1016/j.virol.2012.09.028 [DOI] [PubMed] [Google Scholar]
- 72.Torgeman A, Ben-Aroya Z, Grunspan A, Zelin E, Butovsky E, Hallak M, Lochelt M, Flugel RM, Livneh E, Wolfson M, Kedar I, Aboud M. 2001. Activation of HTLV-1 long terminal repeat by stress-inducing agents and protection of HTLV-1-infected T cells from apoptosis by the viral tax protein. Exp. Cell Res. 271:169–179. 10.1006/excr.2001.5363 [DOI] [PubMed] [Google Scholar]
- 73.Schavinsky-Khrapunsky Y, Gold E, Ben-Aroya Z, Torgeman A, Aboud M, Huleihel M. 2003. Activation of HTLV-1 long terminal repeat by apoptosis inducing agents: mechanism and implications for HTLV-1 pathogenicity. Int. J. Mol. Med. 11:3–11. 10.3892/ijmm.11.1.3 [DOI] [PubMed] [Google Scholar]
- 74.Silic-Benussi M, Biasiotto R, Andresen V, Franchini G, D'Agostino DM, Ciminale V. 2010. HTLV-1 p13, a small protein with a busy agenda. Mol. Aspects Med. 31:350–358. 10.1016/j.mam.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andrews JM, Newbound GC, Lairmore MD. 1997. Transcriptional modulation of viral reporter gene constructs following induction of the cellular stress response. Nucleic Acids Res. 25:1082–1084. 10.1093/nar/25.5.1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andrews JM, Newbound GC, Oglesbee M, Brady JN, Lairmore MD. 1997. The cellular stress response enhances human T-cell lymphotropic virus type 1 basal gene expression through the core promoter region of the long terminal repeat. J. Virol. 71:741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andrews JM, Oglesbee MJ, Trevino AV, Guyot DJ, Newbound GC, Lairmore MD. 1995. Enhanced human T-cell lymphotropic virus type I expression following induction of the cellular stress response. Virology 208:816–820. 10.1006/viro.1995.1218 [DOI] [PubMed] [Google Scholar]
- 78.Andrews JM, Oglesbee M, Lairmore MD. 1998. The effect of the cellular stress response on human T-lymphotropic virus type I envelope protein expression. J. Gen. Virol. 79(Pt 12):2905–2908 [DOI] [PubMed] [Google Scholar]
- 79.Lasfargues C, Martineau Y, Bousquet C, Pyronnet S. 2012. Changes in translational control after proapoptotic stress. Int. J. Mol. Sci. 14:177–190. 10.3390/ijms14010177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liwak U, Faye MD, Holcik M. 2012. Translation control in apoptosis. Exp. Oncol. 34:218–230 [PubMed] [Google Scholar]
- 81.Cully M, Downward J. 2009. Translational responses to growth factors and stress. Biochem. Soc. Trans. 37:284–288. 10.1042/BST0370284 [DOI] [PubMed] [Google Scholar]