Abstract
Nomifensine is a dopamine/norepinephrine reuptake inhibitor. Nomifensine and some of its structural analogues produce behavioral effects indicative of indirect dopaminergic agonist properties, such as hyperlocomotion. By contrast, the deaminated and demethylated nomifensine analogue 4-phenyl-1,2,3,4-tetrahydroisoquinoline (PTIQ) is reported to have amphetamine-antagonistic properties, as demonstrated by inhibition of methamphetamine (METH)-induced dopamine release in the nucleus accumbens and METH-induced hyperlocomotion in rats. In the present study, we examined the effect of PTIQ (10 mg/kg, i.p.) and nomifensine (3 mg/kg, i.p.) on METH (5 or 10 mg/kg ,i.p.)-induced stereotypical behavior in mice in order to determine whether PTIQ and nomifensine inhibit and augment, respectively, METH-induced stereotypical behavior. Unexpectedly, our observations demonstrated that both PTIQ and nomifensine significantly augmented METH-induced stereotypical behavior and locomotion in mice. This augmentation is likely the result of additive effects on dopaminergic function by METH in combination with PTIQ or nomifensine. These results suggest that, contrary to some reports, PTIQ may display dopaminergic agonist properties in mice.
Keywords: Nomifensine; 4-Phenyl-1,2,3,4-tetrahydroisoquinoline; Methamphetamine; Persistent locomotion; Stereotypical behavior
1. Introduction
Nomifensine (8-amino-2-methyl-4-phenyl-1,2,3,4-tetrahydroisoquinoline) is a dopamine/norepinephrine reuptake inhibitor (Hunt et al., 1974; Schacht and Heptner, 1974). Because of these properties, nomifensine was initially investigated as an antidepressant agent (Hoffmann, 1973; Brogden et al., 1979), but has been removed from medical use because of side effects in humans including hemolytic anemia (Bournerias and Nabibi, 1979; Prescott et al., 1980). In experimental animals, nomifensine itself induces stereotypical behavior (Gianutsos et al., 1982; Jolicoeur et al., 1983; Rupniak et al., 1984; Al-Khatib et al., 1995), hyperlocomotion (Gerhards et al., 1974; Gianutsos et al., 1982; Al-Khatib et al., 1995), and rewarding effects (Katz et al., 1977; Martin-Iverson et al., 1985; Sagara et al., 2008), which are all consistent with dopaminergic agonist properties. In addition to indirect dopamine agonism mediated by dopamine reuptake inhibition (Hunt et al., 1974; Church et al., 1987; Kuczenski and Segal, 1992; Meiergerd and Schenk, 1994; Garris et al., 2003), some portion of the effects of nomifensine may also be mediated by direct activation of postsynaptic dopamine receptors (Costall et al., 1975; Gianutsos et al., 1982), although not all studies have found that nomifensine acts as a dopaminergic agonist (Poat et al., 1978) and there has been some suggestion that nomifensine metabolites may mediate the direct dopamine agonist effects that have been observed in some studies.
Nomifensine analogues, including 4’-hydroxy-nomifensine, 8-amino-2-methyl-4-(3,4-dihydroxyphenyl)-1,2,3,4-tetrahydroisoquinoline and 8-amino-2-methyl-4-(3-hydroxy-4-methoxyphenyl)-1,2,3,4-tetrahydroisoquinoline, have also been suggested to possess dopaminergic agonist properties (Kruse et al., 1977; Costall and Naylor, 1978; Hafizi et al., 1992), and have also been shown to be nomifensine metabolites in vivo. However, other nomifensine analogues, which are not in vivo metabolites of nomifensine, such as the deaminated and demethylated nomifensine analogue 4-phenyl-1,2,3,4-tetrahydroisoquinoline (PTIQ) have been reported to inhibit methamphetamine (METH)-induced hyperlocomotion and dopamine release in the rat nucleus accumbens (Tateyama et al., 1993a, b). On the basis of these observations, it was hypothesized that PTIQ may inhibit, while pretreatment with nomifensine may augment, METH-induced stereotypical behavior.
2. Results
2.1. The effect of nomifensine on METH-induced stereotypy
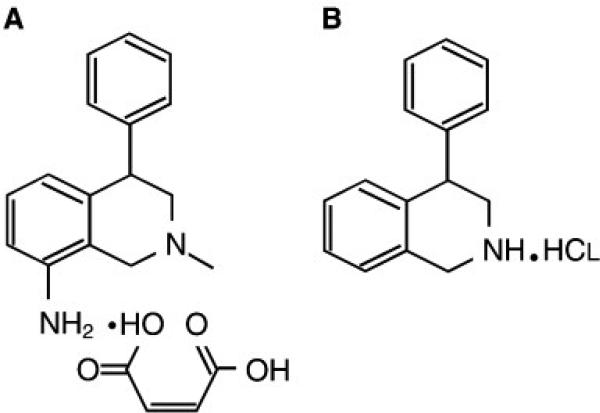
Chemical structures of nomifensine maleate (8-amino-2-methyl-4-phenyl-1,2,3,4-tetrahydroisoquinoline, maleate salt) and PTIQ (4-phenyl-1,2,3,4-tetrahydroisoquinoline hydrochloride) are shown in Fig. 1.
Fig. 1.
Chemical structures of nomifensine maleate (8-amino-2-methyl-4-phenyl-1,2,3,4-tetrahydroisoquinoline, maleate salt) (A) and PTIQ (4-phenyl-1,2,3,4-tetrahydroisoquinoline hydrochloride) (B).
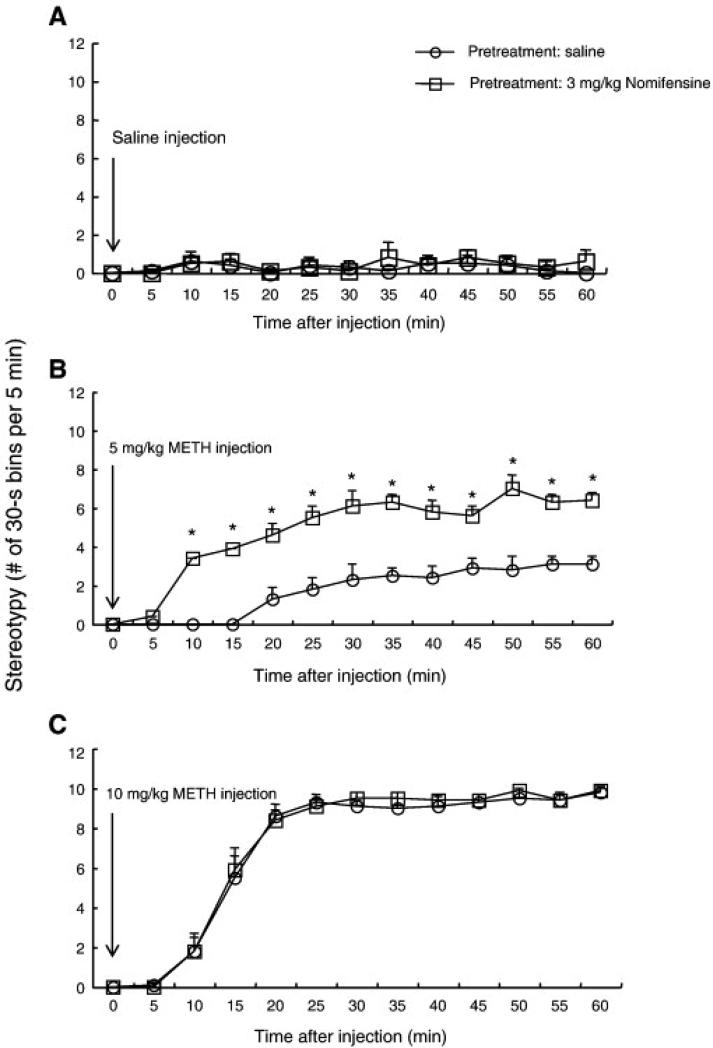
Figure 2 shows the time course of the frequency of all types of stereotypical behavior after METH (or saline vehicle) challenge in mice. There was an increase in the overall frequency of stereotypy in mice after 5 mg/kg METH challenge, as compared to that after saline challenge, beginning at 20 min post-injection. Pretreatment with 3 mg/kg nomifensine augmented the overall frequency of stereotypical behaviors displayed by 5 mg/kg METH-challenged mice, and the onset of stereotypy was sooner (10 min). There was an increase in the overall frequency of stereotypy in the mice after 10 mg/kg METH challenge, as compared to saline or 5 mg/kg METH challenge, beginning at 10 min post-injection, reaching a maximum at 20 min post-injection, and continuing unabated for the duration of the test session. Pretreatment with 3 mg/kg nomifensine did not affect the overall frequency of stereotypical behaviors displayed by 10 mg/kg METH- or saline-challenged mice. A repeated-measures ANOVA (pretreatment x time) applied to the saline data represented in Fig. 2A yielded no significant main effects of pretreatment (F(1,14) = 0.2, P = 0.63) or time (F(12,182) = 1.0, P = 0.46), nor a significant nomifensine pretreatment x time interaction (F(12,182) = 0.4, P = 0.95). ANOVA (pretreatment x time) applied to the data represented in Fig 2B yielded significant main effects of pretreatment (F(1,14) = 68.6, P < 0.0001) and time (F(12,182) = 23.8, P < 0.0001), and also yielded a significant nomifensine pretreatment x time interaction (F(12,182) = 3.5, P < 0.0001). Post-hoc pair-wise comparisons showed significant differences between saline and nomifensine pretreatment groups at each time point between 10 and 60 min (Bonferroni/Dunn test, P < 0.05). ANOVA (pretreatment x time) applied to the data represented in Fig 2C yielded a significant main effect of time (F(12,182) = 146.7, P < 0.0001) but no significant main effect of pretreatment (F(1,14) = 0.1, P = 0.73) nor any significant nomifensine pretreatment x time interaction (F(12,182) = 1.0, P = 0.999).
Fig. 2.
Frequencies of stereotypy after a single administration of saline (A), 5 mg/kg methamphetamine (B) and 10 mg/kg methamphetamine (C) in mice pretreated with 3 mg/kg nomifensine or vehicle (i.e. saline). Values are shown as the mean ± SEM (n = 8). METH, methamphetamine. *P < 0.05, compared with saline-pretreated mice (post-hoc Bonferroni/Dunn test).
Four individual categories of stereotypical behaviors and persistent locomotion were observed, and the frequency of each behavior is presented in Table 1. METH challenge and nomifensine pretreatment affected the incidence of each behavior and altered the distribution of behavioral output.
Table 1.
Effect of nomifensine pretreatment on methamphetamine-induced stereotypy and persistent locomotion in mice.
| Expression pattern of behavior after drug challenge (No. of 30-s bins for 1 h) |
|||||
|---|---|---|---|---|---|
| Head-bobbing | Circling | Sniffing | Biting | Persistent locomotion | |
| Challenge: saline | |||||
| Saline pretreatment | N.D. | 0.1 ± 0.1 | 0.9 ± 0.6 | 2.4 ± 2.0 | 0.1 ± 0.1 |
| Nomifensine pretreatment | N.D. | N.D. | 3.8 ± 1.6 | 1.1 ± 0.6 | 1.5 ± 1.4 |
| Challenge: 5 mg/kg METH | |||||
| Saline pretreatment | 1.4 ± 0.5 | 2.1 ± 1.4 | 18.5 ± 2.4†† | N.D. | 85.1 ± 3.5††† |
| Nomifensine pretreatment | 5.6 ± 0.6***,††† | 11.9 ± 1.1***,††† | 36.4 ± 3.0***,††† | 7.3 ± 0.9*** | 45.3 ± 2.6***,††† |
| Challenge: 10 mg/kg METH | |||||
| Saline pretreatment | 1.8 ± 0.9 | 1.5 ± 0.8 | 11.1 ± 5.4† | 76.0 ± 9.6†††,‡‡‡ | 13.8 ± 1.6†††,‡‡‡ |
| Nomifensine pretreatment | 1.6 ± 0.8‡‡‡ | 1.8 ± 0.6‡‡‡ | 13.0 ± 5.2‡‡‡ | 75.6 ± 8.9†††,‡‡‡ | 14.6 ± 2.4†††,‡‡‡ |
Mice were pretreated with 3 mg/kg of nomifensine or saline for 30 min followed by challenge of methamphetamine (5 or 10 mg/kg) or saline for 1 h. Values are expressed as no. of 30-s bins for 1 h (mean ± SEM, n = 8). METH, methamphetamine; N.D., not detected.
P < 0.001, compared with corresponding group pretreated with saline (one-way ANOVA followed by a post-hoc Fischer's PLSD test).
P < 0.05
P < 0.01
P < 0.001, compared with corresponding group challenged with saline (one-way ANOVA followed by a post-hoc Fischer's PLSD test).
P < 0.001, compared with corresponding group challenged with 5 mg/kg METH (one-way ANOVA followed by a post-hoc Fischer's PLSD test).
To analyze the effects of METH dose on individual components of stereotypy one-way ANOVA were applied separately for each pretreatment (i.e. saline or 3 mg/kg nomifensine pretreatment) as shown in Table 1. Firstly, in mice pretreated with saline ANOVA showed significant main effects of METH challenge for stereotypical sniffing (F(1,14) = 6.6, P < 0.01), biting (F(1,14) = 58.2, P < 0.0001), and persistent locomotion (F(1,14) = 414.2, P < 0.0001) but not for stereotypical head-bobbing (F(1,14) = 2.3, P = 0.12) or circling (F(1,14) = 1.2, P = 0.33). Stereotypical sniffing was significantly increased by both 5 and 10 mg/kg METH compared to saline-challenged mice, but stereotypical sniffing was decreased in the 10 mg/kg METH group compared to 5 mg/kg METH-challenge (Fisher's PLSD test, P < 0.05). Stereotypical biting was not affected by 5 mg/kg METH, but was increased by 10 mg/kg METH. Indeed, this became the predominant behavior at this dose. Thus, post-hoc comparisons indicated significant differences in the frequencies of the stereotypical biting between the saline-challenged and 10 mg/kg METH-challenged and between the 5 mg/kg METH-challenged and 10 mg/kg METH-challenged mice pretreated with saline (Fisher's PLSD test, P < 0.001 and P < 0.001, respectively). Persistent locomotion was increased by 5 mg/kg METH but this increase was reduced in 10 mg/kg METH-treated mice, so that post-hoc comparisons indicated significant differences in the frequencies of persistent locomotion between the saline-challenged mice and both METH-treated groups, and between 5 mg/kg METH-challenged and 10 mg/kg METH-challenged mice (Fisher's PLSD test, P < 0.001, P < 0.001 and P < 0.001, respectively).
In mice pretreated with nomifensine ANOVA showed significant main effects of METH challenge for stereotypical head-bobbing (F(1,14) = 23.6, P < 0.0001), circling (F(1,14) = 79.9, P < 0.0001), sniffing (F(1,14) = 22.0, P < 0.0001), biting (F(1,14) = 64.1, P < 0.0001), and persistent locomotion (F(1,14) = 104.4, P < 0.0001). Stereotypical head-bobbing was increased by 5 mg/kg METH-challenge, but reduced to near saline levels after 10 mg/kg METH, so that post-hoc comparisons indicated significant differences in the frequencies of the stereotypical head-bobbing between saline-challenged mice and 5 mg/kg METH-challenged and between the 5 mg/kg METH-challenged and 10 mg/kg METH-challenged mice (Fisher's PLSD test, P < 0.001 and P < 0.001, respectively). A similar pattern was seen for stereotypical circling, so that post-hoc comparisons indicated significant differences in the frequencies of stereotypical circling between the saline-challenged and 5 mg/kg METH-challenged and between the 5 mg/kg METH-challenged and 10 mg/kg METH-challenged mice (Fisher's PLSD test, P < 0.001 and P < 0.001, respectively). Stereotypical sniffing was increased by 5 and 10 mg/kg METH compared to saline, but again there were reductions in the frequency of stereotypical sniffing at the high dose compared to the low dose. Thus, post-hoc comparisons indicated significant differences in the frequencies of the stereotypical sniffing between the saline-challenged and 5 mg/kg METH-challenged and between the 5 mg/kg METH-challenged and 10 mg/kg METH-challenged mice (Fisher's PLSD test, P < 0.001 and P < 0.001, respectively). As in the saline-pretreated group, in the nomifensine-pretreated group stereotypical biting became the predominant behavior after challenge with 10 mg/kg METH, but there was also a slight increase in the behavior after treatment with 5 mg/kg METH, but far less than observed after the higher dose. Post-hoc comparisons indicated significant differences in the frequencies of stereotypical biting between the saline-challenged and 10 mg/kg METH-challenged and between the 5 mg/kg METH-challenged and 10 mg/kg METH-challenged mice (Fisher's PLSD test, P < 0.001 and P < 0.001, respectively). Persistent locomotion was again increased at the low dose, but this increase was reduced substantially in the high dose group. Thus, post-hoc comparisons indicated significant differences in the frequencies of the persistent locomotion between the saline-challenged and both METH-challenged groups, and between the 5 mg/kg METH-challenged and 10 mg/kg METH-challenged mice (Fisher's PLSD test, P < 0.001, P < 0.001 and P < 0.001, respectively).
As is obvious in Table 1, as well as in the analysis above, the effects of METH were different in saline- and nomifensine-pretreated mice. Therefore, the effects of nomifensine were analyzed separately in saline- and METH-challenged mice. Nomifensine pretreatment increased the frequency of each category of stereotypical behavior and persistent locomotion induced by 5 mg/kg METH (but not by 10 mg/kg METH), compared with saline pretreatment. One-way ANOVA (for nomifensine pretreatment) was applied separately to the data for each dose of METH shown in Table 1. In mice challenged with saline ANOVA showed no significant main effects of nomifensine pretreatment for stereotypical circling (F(1,14) = 1.0, P = 0.33), sniffing (F(1,14) = 2.8, P = 0.12), biting (F(1,14) = 0.4, P = 0.55), or persistent locomotion (F(1,14) = 1.0, P = 0.33). By contrast, in mice challenged with 5 mg/kg METH ANOVA showed significant main effects of nomifensine pretreatment for stereotypical head-bobbing (F(1,14) = 30.0, P < 0.0001), circling (F(1,14) = 29.9, P < 0.0001), sniffing (F(1,14) = 21.9, P < 0.001), biting (F(1,14) = 67.7, P < 0.0001), and persistent locomotion (F(1,14) = 82.3, P < 0.0001). Post-hoc comparisons identified significant increases in the frequencies of the four stereotypical behavior components but a decrease in the frequency of persistent locomotion between the saline-pretreated and nomifensine-pretreated mice challenged with 5 mg/kg METH (Fisher's PLSD test, P < 0.001). In mice challenged with 10 mg/kg METH ANOVA showed no significant main effects of nomifensine pretreatment for stereotypical head-bobbing (F(1,14) = 0.01, P = 0.92), circling (F(1,14) = 0.06, P = 0.81), sniffing (F(1,14) = 0.06, P = 0.81), biting (F(1,14) = 0.001, P = 0.98), or persistent locomotion (F(1,14) = 0.09, P = 0.77) In summary, these data indicate not just changes in the overall amounts of stereotypy, but effects of METH and nomifensine on the nature of this stereotypy.
2.2. The effect of PTIQ pretreatment on METH-induced stereotypy
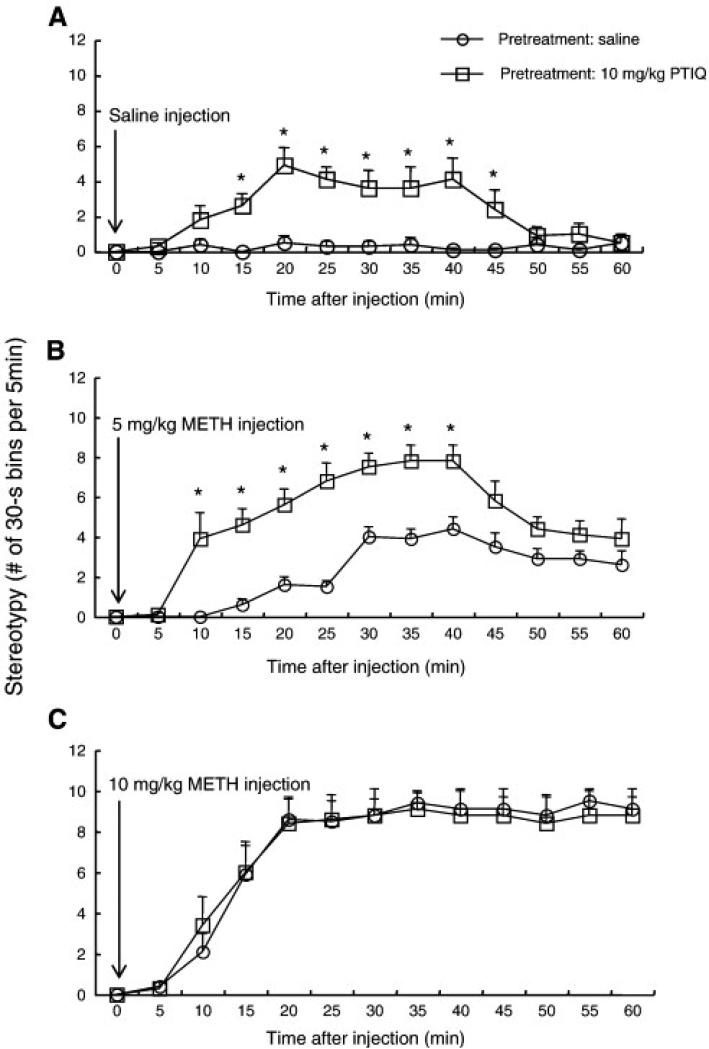
The effects of PTIQ on METH-induced stereotypies and persistent locomotion are shown in Fig. 3, which represents the time course of the frequency of all types of stereotypical behavior after METH- or saline-challenge in mice. As before, in saline-pretreated mice there was an increase in the overall frequency of stereotypy after 5 mg/kg METH challenge, as compared to that after saline challenge, beginning at 20 min post-injection. There was an increase in the overall frequency of stereotypy in the mice after 10 mg/kg METH challenge, as compared to that after saline challenge and 5 mg/kg METH, as well as an acceleration of the onset of stereotypy, beginning at 10 min post-injection (similar to the high dose of METH), reaching a maximum at 20 min post-injection, and continuing for the duration of the test session. Pretreatment with PTIQ augmented the overall frequency of stereotypical behaviors displayed by 5 mg/kg METH-challenged mice, but not 10 mg/kg METH-challenged mice. Mice pretreated with PTIQ also displayed a transient, but substantial, expression of stereotypy 15-45 min after saline challenge (of comparable magnitude to 5 mg/kg METH). A repeated-measures ANOVA (pretreatment x time) applied to the saline-challenge data represented in Fig. 3A yielded significant main effects of pretreatment (F(1,14) = 17.1, P < 0.01) and time (F(12,182) = 5.8, P < 0.0001). The analysis also yielded a significant PTIQ pretreatment x time interaction (F(12,182) = 5.2, P < 0.0001). Post-hoc pair-wise comparisons showed significant differences between saline and PTIQ pretreatment groups at each time point between 15 and 45 min (Bonferroni/Dunn test, P < 0.05). ANOVA (pretreatment x time) applied to the data represented in Fig. 3B yielded significant main effects of pretreatment (F(1,14) = 22.9, P < 0.001) and time (F(12,182) = 27.7, P < 0.0001). This analysis also yielded a significant PTIQ pretreatment x time interaction (F(12,182) = 5.0, P < 0.001). Post-hoc pair-wise comparisons showed significant differences between saline and PTIQ pretreatment groups at each time point between 10 and 40 min (Bonferroni/Dunn test, P < 0.05). ANOVA (pretreatment x time) applied to the data represented in Fig 3C yielded a significant main effect of time (F(12,182) = 62.7, P < 0.0001) but no significant main effect of pretreatment (F(1,14) = 0.009, P = 0.93) nor any significant PTIQ pretreatment x time interaction (F(12,182) = 0.3, P = 0.99).
Fig. 3.
Frequencies of stereotypy after a single administration of saline (A), 5 mg/kg methamphetamine (B) and 10 mg/kg methamphetamine (C) in mice pretreated with 10 mg/kg PTIQ or vehicle (i.e. saline). Values are shown as the mean ± SEM (n = 8). METH, methamphetamine; PTIQ, 4-phenyl-1,2,3,4-tetrahydroisoquinoline. *P < 0.05, compared with saline-pretreated mice (post-hoc Bonferroni/Dunn test).
Four categories of stereotypical behaviors and persistent locomotion were observed, and the frequency of each behavior is represented in Table 2. As in the previous experiment, METH-challenge increased these behaviors in a dose-dependent fashion. These effects were somewhat different in saline- and PTIQ-pretreated mice. To analyze these effects one-way ANOVA were applied separately for each pretreatment (i.e. saline or PTIQ pretreatment). In mice pretreated with saline ANOVA showed significant main effects of METH challenge for stereotypical sniffing (F(1,14) = 31.5, P < 0.0001), biting (F(1,14) = 43.6, P < 0.0001), and persistent locomotion (F(1,14) = 296.6, P < 0.0001), but not for stereotypical head-bobbing (F(1,14) = 1.9, P = 0.18) or circling (F(1,14) = 0.9, P = 0.42). Stereotypical sniffing was increased by 5 mg/kg METH compared to saline-challenge, but was reduced at 10 mg/kg METH compared to 5 mg/kg METH. Thus, post-hoc comparisons indicated significant differences in the frequencies of the stereotypical sniffing between the saline-challenged and 5 mg/kg METH-challenged groups and between the 5 mg/kg METH-challenged and 10 mg/kg METH-challenged mice (Fisher's PLSD test, P < 0.001 and P < 0.001, respectively). Stereotypical biting was unchanged after 5 mg/kg METH, but substantially increased after 10 mg/kg METH, such that it became the predominant behavior. Post-hoc comparisons indicated significant differences in the frequencies of the stereotypical biting between 10 mg/kg METH-challenged mice and both the saline- and 5 mg/kg METH-challenged groups (Fisher's PLSD test, P < 0.001 and P < 0.001, respectively). Regarding persistent locomotion, post-hoc comparisons indicated significant differences in the frequencies of the persistent locomotion between all groups of mice pretreated with saline (Fisher's PLSD test, P < 0.001). ANOVA showed significant main effects of METH challenge for stereotypical head-bobbing (F(1,14) = 6.6, P < 0.001), circling (F(1,14) = 11.4, P < 0.001), sniffing (F(1,14) = 10.1, P < 0.001), biting (F(1,14) = 24.1, P < 0.0001), and persistent locomotion (F(1,14) = 8.6, P < 0.05) observed in mice pretreated with 10 mg/kg PTIQ. Regarding stereotypical head-bobbing, post-hoc comparisons indicated significant differences in the frequencies of the stereotypical head-bobbing between the saline-challenged and 5 mg/kg METH-challenged mice pretreated with 10 mg/kg PTIQ (Fisher's PLSD test, P < 0.01). Regarding stereotypical circling, post-hoc comparisons indicated significant differences in the frequencies of the stereotypical circling between the saline-challenged and 5 mg/kg METH-challenged and between the 5 mg/kg METH-challenged and 10 mg/kg METH-challenged mice pretreated with 10 mg/kg PTIQ (Fisher's PLSD test, P < 0.001 and P < 0.01, respectively). Regarding stereotypical sniffing, post-hoc comparisons indicated significant differences in the frequencies of the stereotypical sniffing between the saline-challenged and 5 mg/kg METH-challenged and between the 5 mg/kg METH-challenged and 10 mg/kg METH-challenged mice pretreated with 10 mg/kg PTIQ (Fisher's PLSD test, P < 0.05 and P < 0.001, respectively). Regarding stereotypical biting, post-hoc comparisons indicated significant differences in the frequencies of the stereotypical biting between the saline-challenged and 10 mg/kg METH-challenged and between the 5 mg/kg METH-challenged and 10 mg/kg METH-challenged mice pretreated with 10 mg/kg PTIQ (Fisher's PLSD test, P < 0.001 and P < 0.001, respectively). Regarding persistent locomotion, post-hoc comparisons indicated significant differences in the frequencies of the persistent locomotion between the saline-challenged and both groups of METH-challenged mice pretreated with 10 mg/kg PTIQ (Fisher's PLSD test, P < 0.001 and P < 0.05, respectively).
Table 2.
Effect of PTIQ pretreatment on methamphetamine-induced stereotypy and persistent locomotion in mice.
| Expression pattern of behavior after drug challenge (No. of 30-s bins for 1 h) |
|||||
|---|---|---|---|---|---|
| Head-bobbing | Circling | Sniffing | Biting | Persistent locomotion | |
| Challenge: saline | |||||
| Saline pretreatment | N.D. | N.D. | 0.8 ± 0.5 | 2.3 ± 1.7 | 4.1 ± 2.7 |
| PTIQ pretreatment | N.D. | N.D. | 10.9 ± 3.7* | 18.9 ± 4.2*** | 0.6 ± 0.4 |
| Challenge: 5 mg/kg METH | |||||
| Saline pretreatment | 1.5 ± 0.2 | 0.9 ± 0.3 | 25.5 ± 1.6††† | N.D. | 76.3 ± 1.6††† |
| PTIQ pretreatment | 4.6 ± 0.6*,†† | 8.6 ± 1.7***,††† | 36.4 ± 6.2† | 12.5 ± 1.2*** | 28.5 ± 5.2***,††† |
| Challenge: 10 mg/kg METH | |||||
| Saline pretreatment | 2.3 ± 1.4 | 4.5 ± 4.4 | 7.3 ± 3.6‡‡‡ | 75.4 ± 11.1†††,‡‡‡ | 13.9 ±2.4†††,‡‡‡ |
| PTIQ pretreatment | 2.0 ± 1.1 | 2.4 ± 1.5‡‡ | 9.6 ± 4.0‡‡‡ | 73.9 ± 11.1†††,‡‡‡ | 15.0 ± 6.4† |
Mice were pretreated with 10 mg/kg of PTIQ or saline for 30 min followed by challenge of methamphetamine (5 or 10 mg/kg) or saline for 1 h. Values are expressed as no. of 30-s bins for 1 h (mean ± SEM, n = 8). METH, methamphetamine; N.D., not detected; PTIQ, 4-phenyl-1,2,3,4-tetrahydroisoquinoline.
P < 0.001, compared with corresponding group pretreated with saline (one-way ANOVA followed by a post-hoc Fischer's PLSD test).
P < 0.05
P < 0.01
P < 0.001, compared with corresponding group challenged with saline (one-way ANOVA followed by a post-hoc Fischer's PLSD test).
‡ P < 0.05
P < 0.01
P < 0.001, compared with corresponding group challenged with 5 mg/kg METH (one-way ANOVA followed by a post-hoc Fischer's PLSD test).
In mice challenged with saline, PTIQ pretreatment increased the frequencies of stereotypical sniffing and biting, but not other types of stereotypy, compared to mice pretreated with saline. In mice challenged with 5 mg/kg METH, PTIQ increased the frequency of each category of stereotypical behavior except sniffing and reduced persistent locomotion, compared to saline pretreatment. PTIQ pretreatment did not affect any category of stereotypical behavior or persistent locomotion displayed by mice challenged with 10 mg/kg METH, compared to saline pretreatment. To analyze these effects one-way ANOVA were applied separately for each dose of METH shown in Table 2. In mice challenged with saline ANOVA showed significant main effects of PTIQ pretreatment for stereotypical sniffing (F(1,14) = 7.5, P < 0.05) and biting (F(1,14) = 13.4, P < 0.01), but no significant effects for any of the other behaviors. Post-hoc comparisons indicated significant differences in the frequencies of stereotypical sniffing and biting between the saline-pretreated and PTIQ-pretreated mice challenged with saline (Fisher's PLSD test, P < 0.05 and P < 0.001 for sniffing and biting, respectively). In mice challenged with 5 mg/kg METH ANOVA showed significant main effects of PTIQ pretreatment for stereotypical head-bobbing (F(1,14) = 8.3, P < 0.05), circling (F(1,14) = 19.2, P < 0.001) and biting (F(1,14) = 115.1, P < 0.0001), and for persistent locomotion (F(1,14) = 77.7, P < 0.0001), but no significant main effect of PTIQ pretreatment for stereotypical sniffing (F(1,14) = 2.9, P = 0.11). Post-hoc comparisons indicated significant differences in the frequencies of the stereotypical head-bobbing, circling and biting components, and for persistent locomotion, between the saline-pretreated and PTIQ-pretreated mice challenged with 5 mg/kg METH (Fisher's PLSD test, P < 0.05). In mice challenged with 10 mg/kg METH ANOVA showed no significant main effects of PTIQ pretreatment for any of the behaviors measured.
2.3. The effect of nomifensine pretreatment on METH-induced locomotion
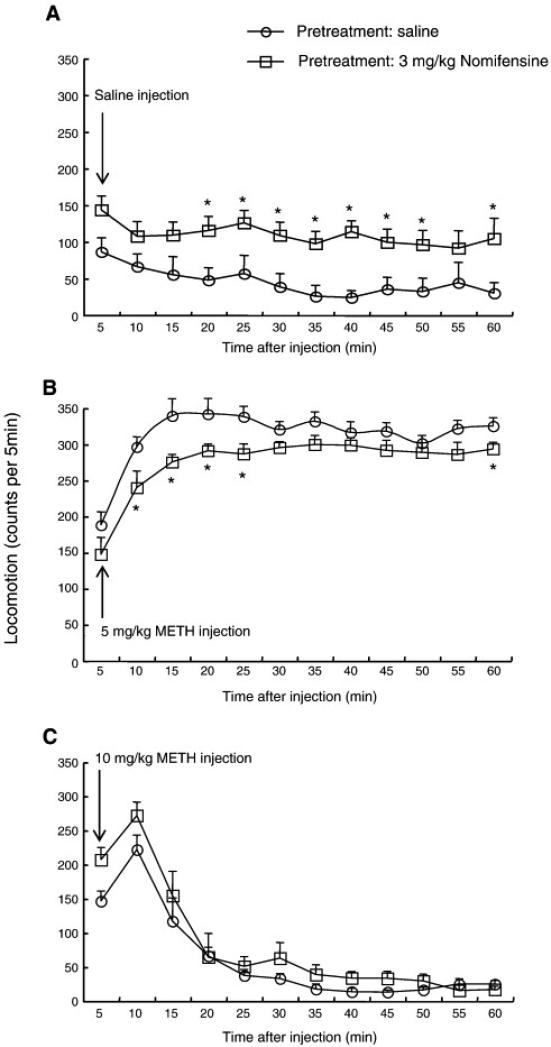
As shown in Fig. 4A, saline-challenged mice displayed modest locomotor activity during the 1 h observation period, and nomifensine pretreatment increased this locomotion. METH (5 mg/kg) treatment produced much more robust increases in horizontal locomotion, reaching a maximum at 15 min post-injection, and, in contrast to the effect in saline-treating animals, nomifensine pretreatment decreased locomotor activity, especially during the first 30 min (Fig. 4B). At a dose of 10 mg/kg, METH treatment induced a transient increase in horizontal locomotion with a peak at the time point of 10 min post-injection, following by a prolonged reduction in locomotion that lasted for the rest of the test session. This pattern of effects was not altered by nomifensine pretreatment (Fig. 4C). A repeated-measures ANOVA (pretreatment x time) applied to the data represented in Fig 4A yielded significant main effects of pretreatment (F(1,14) = 11.1, P < 0.01) and time (F(11,168) = 2.2, P < 0.05) but no significant nomifensine pretreatment x time interaction (F(11,168) = 0.9, P = 0.96). Post-hoc pair-wise comparisons found significant differences between saline and nomifensine pretreatment groups at each time point between 20 and 50 min and at 60 min (Bonferroni/Dunn test, P < 0.05). ANOVA (pretreatment x time) applied to the data represented in Fig 4B yielded significant main effects of pretreatment (F(1,14) = 7.5, P < 0.05) and time (F(11,168) = 23.2, P < 0.0001) but no significant nomifensine pretreatment x time interaction (F(11,168) = 0.8, P = 0.60). Post-hoc pair-wise comparisons found significant differences between saline and nomifensine pretreatment groups at each time point between 10 and 25 min and at 60 min (Bonferroni/Dunn test, P < 0.05). ANOVA (pretreatment x time) applied to the data represented in Fig 4C yielded a significant main effect of time (F(11,168) = 47.2, P < 0.0001) but no significant main effect of pretreatment (F(1,14) = 3.1, P = 0.10) or nomifensine pretreatment x time interaction (F(11,168) = 1.0, P = 0.46).
Fig. 4.
Horizontal locomotor activity after a single administration of saline (A), 5 mg/kg methamphetamine (B) and 10 mg/kg methamphetamine (C) in mice pretreated with 3 mg/kg nomifensine or vehicle (i.e. saline). Values are shown as the mean ± SEM (n = 8). METH, methamphetamine. *P < 0.05, compared with saline-pretreated mice (post-hoc Bonferroni/Dunn test).
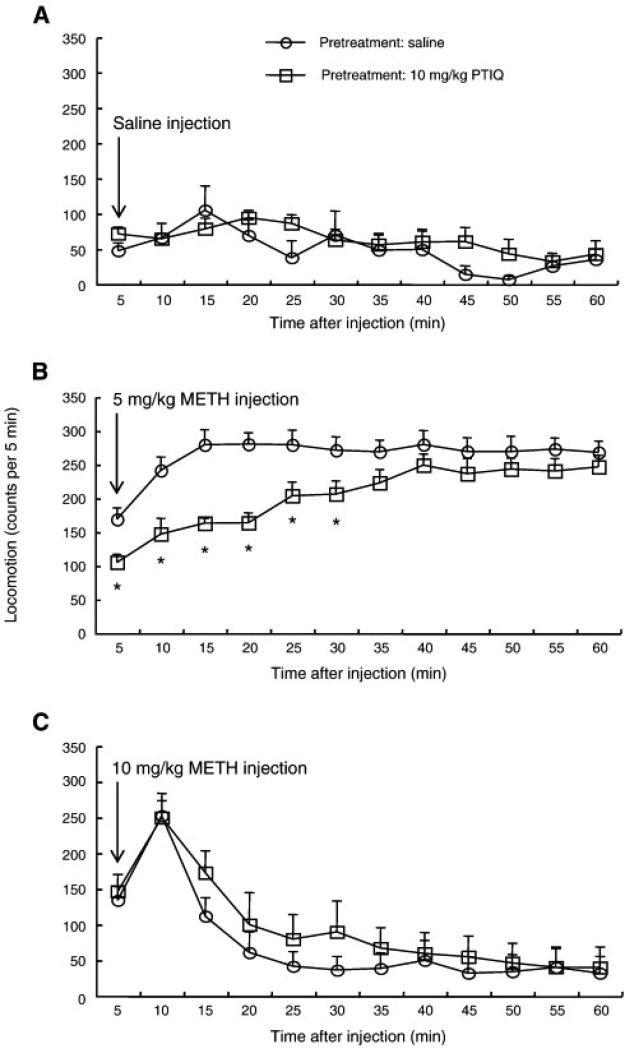
2.4. The effect of PTIQ pretreatment on METH-induced locomotion
PTIQ pretreatment did not affect horizontal locomotion displayed by mice challenged with saline (Fig. 5A) or 10 mg/kg METH (Fig. 5C), compared with saline pretreatment. As described in the previous section, METH (5 mg/kg) treatment increased horizontal locomotion, reaching a maximum at 15 min post-injection. PTIQ pretreatment decreased METH-induced locomotor activity, especially during the first 30 min (Fig. 5B). Repeated-measures ANOVA (pretreatment x time) applied to the data represented in Fig 5A yielded a significant main effect of time (F(11,168) = 3.4, P < 0.001) but no significant main effect of pretreatment (F(1,14) = 0.8, P = 0.38) nor a significant PTIQ pretreatment x time interaction (F(11,168) = 1.0, P = 0.42). ANOVA (pretreatment x time) applied to the data represented in Fig 5B yielded significant main effects of pretreatment (F(1,14) = 7.8, P < 0.05) and time (F(11,168) = 20.5, P < 0.0001), and also a significant PTIQ pretreatment x time interaction (F(11,168) = 4.7, P < 0.0001). Post-hoc pair-wise comparisons found significant differences between saline and PTIQ pretreatment groups at each time point between 5 and 30 min (Bonferroni/Dunn test, P < 0.05). ANOVA (pretreatment x time) applied to the data represented in Fig 5C yielded a significant main effect of time (F(11,168) = 18.2, P < 0.0001) but no significant main effect of pretreatment (F(1,14) = 0.7, P = 0.42) nor a significant nomifensine pretreatment x time interaction (F(11,168) = 0.5, P = 0.91).
Fig. 5.
Horizontal locomotor activity after a single administration of saline (A), 5 mg/kg methamphetamine (B) and 10 mg/kg methamphetamine (C) in mice pretreated with 10 mg/kg PTIQ or vehicle (i.e. saline). Values are shown as the mean ± SEM (n = 8). METH, methamphetamine; PTIQ, 4-phenyl-1,2,3,4-tetrahydroisoquinoline. *P < 0.05, compared with saline-pretreated mice (post-hoc Bonferroni/Dunn test).
3. Discussion
The main findings of the present study were that pretreatment with nomifensine (3 mg/kg) or PTIQ (10 mg/kg) significantly augmented METH-induced stereotypical behavior (Figs. 2B and 3B), and reduced METH-induced locomotion (Figs. 4B and 5B). These effects were only observed for the low METH dose (5 mg/kg). At the high METH dose (10 mg/kg), PTIQ or nomifensine no longer provided any additional component of stereotypical behavior (suggesting a ceiling effect; Figs. 2C and 3C). The augmentation of METH-induced stereotypical behavior seemed to affect a broad range of stereotypical behaviors (although see discussion below), suggesting that the augmentation of stereotypical behavior by nomifensine and PTIQ in combination with METH was most indicative of generally increased dopaminergic function.
There is a possibility that both nomifensine and its metabolites augment METH effects by inhibiting metabolism of METH. Of nomifensine analogues, 4’-hydroxy-nomifensine, 8-amino-2-methyl-4-(3,4-dihydroxyphenyl)-1,2,3,4-tetrahydroisoquinoline, and 8-amino-2-methyl-4-(3-hydroxy-4-methoxyphenyl)-1,2,3,4-tetrahydroisoquinoline have been suggested to possess dopaminergic agonist properties (Kruse et al., 1977; Costall and Naylor, 1978; Hafizi et al., 1992). These three nomifensine analogues are identified to be nomifensine metabolites in vivo. In contrast, PTIQ is not a nomifensine metabolite, although it is a structural analogue to nomifensine (Tateyama et al., 1993a, b). Nomifensine is a dopamine/norepinephrine reuptake inhibitor (Hunt et al., 1974; Schacht and Heptner, 1974). With this pharmacological property (but not the inhibition of METH metabolism) nomifensine is thought to augment METH effects. It is unclear what mechanisms underlie the additive effects of 4’-hydroxy-nomifensine, 8-amino-2-methyl-4-(3,4-dihydroxyphenyl)-1,2,3,4-tetrahydroisoquinoline, 8-amino-2-methyl-4-(3-hydroxy-4-methoxyphenyl)-1,2,3,4-tetrahydroisoquinoline, or PTIQ on METH actions but given that there is currently no evidence for inhibition of METH metabolism, and some evidence for direct dopamine agonist effects, this seems to be the most likely mechanism, although in either case, it remains a speculation. The pattern of behavioral effects observed in this study is also most consistent with this mechanism.
The systemic administration of amphetamines to rodents induces increased locomotor activity that is replaced by stereotypical behavior at higher doses (Randrup and Munkvad, 1967; Nishikawa et al., 1983; Rebec and Bashore, 1984). Similarly, nomifensine has been reported to induce both hyperlocomotion (Gerhards et al., 1974; Gianutsos et al., 1982; Al-Khatib et al., 1995), and stereotypical behavior (Gianutsos et al., 1982; Jolicoeur et al., 1983; Rupniak et al., 1984; Al-Khatib et al., 1995), and to transition from hyperlocomotion to stereotypical behavior in a dose dependent manner (Al-Khatib et al., 1995). The results of the present study could be explained from the dose-dependent transition between hyperlocomotion and stereotypy: PTIQ alone (or in combination with saline) induced relatively intense stereotypical behavior that would attenuate its increasing effects on locomotor activity and thereby no significant increase in locomotion; nomifensine at the dose (3 mg/kg) that produced hyperlocomotion without stereotypy augmented the intensity of stereotypy induced by METH, and this augmentation would result in reduced locomotor activity. These possibilities cannot be excluded. Like amphetamines (Kelley et al., 1975), these effects are dependent on the ventral and dorsal striatum, respectively (Al-Khatib et al., 1995). A similar dose-dependent transition has been reported for PTIQ (Costall and Naylor, 1978). However, PTIQ not only inhibits METH-induced hyperlocomotion but also dopamine release in the rat nucleus accumbens (Tateyama et al., 1993a, b), and the inhibition of METH-induced locomotion was demonstrated under several conditions that are unlikely to be the result of competing behavioral stereotypies, suggesting that it may have actions that are antagonistic to METH. Microinjection of PTIQ (20 μg) into the nucleus accumbens of the rat in combination with METH (10 μg) inhibited both the hyperlocomotion and dopamine release produced by METH, consistent with an antagonistic effect of PTIQ (Tateyama et al., 1993). These conditions obviously should not induce any stereotypy, but stereotypy was not reported in that study. Differences in drug doses used (5 mg/kg vs. 20 μg/animal), species (mouse vs. rat), pretreatment periods (30 min before METH injection vs. co-administration with PTIQ and METH), or drug injection routes (i.p. vs. nucleus accumbens i.c.) may account for these discrepancies. Clarification will require further studies, but there are certainly indications from the present study that the effects of nomifensine and PTIQ differ in at least some respects. PTIQ increased stereotypical behavior alone (e.g. after saline pretreatment; Fig. 3A) while nomifensine did not (Fig. 2A), while nomifensine alone increased horizontal locomotion (Fig. 4A) but PTIQ did not (Fig. 5A). Although this might be an issue of the relative doses of nomifensine and PTIQ, a possibility which cannot be completely excluded based on the current experiments that used single doses, other information may suggest otherwise, including PTIQ inhibition of METH-induced hyperlocomotion and dopamine release mentioned above (Tateyama et al., 1993a, b). The doses of nomifensine and PTIQ used here produced similar reductions in METH-induced horizontal locomotion and similar increases in stereotypy (compare Figs. 2B and 3B, and Figs. 4B and 5B), despite having very different effects in saline-treated subjects.
The augmentation of METH-induced stereotypy by nomifensine and PTIQ could be interpreted as a general enhancement of dopaminergic neurotransmission, but there were some differences in the type of stereotypy observed under some conditions. Pretreatment with PTIQ induced increases in incidence of stereotypical behavior, which was restricted to sniffing and biting in saline-challenged mice, compared with corresponding mice pretreated with saline alone (Table 2). The results suggested that increased dopaminergic function induced by PTIQ alone is sufficient for expression of stereotypical sniffing and biting, but not head-bobbing or circling. In rats, stereotypical head-bobbing is reported to appear after higher doses of nomifensine than that used here, and higher than that which produces other forms of stereotypy (Costall and Naylor, 1978; Al-Khatib et al., 1995). This may suggest that the doses of nomifensine and PTIQ were not quite equivalent, but the PTIQ-induced stereotypy in saline-challenged mice was transient (onset 45 min after PTIQ injection; Fig. 3A), while 8-amino-2-methyl-4-(3,4-dihydroxyphenyl)-1,2,3,4-tetrahydroisoquinoline-induced repetitive head movements lasted for 3.5-4 h (Costall and Naylor, 1978). Despite nomifensine alone having no effects on stereotypy, its effects on locomotion were immediate (that is, observed at the beginning of the locomotor test, 30 min post-injection) and long-lasting. It is uncertain whether these differences are due to an inherent difference in activity between the two compounds or rather to pharmacokinetic factors, but it is obvious that nomifensine and PTIQ have very different effects, with different time courses, in saline-challenged mice, suggesting a different basis. On the other hand the effects of nomifensine and PTIQ on METH-induced stereotypies appeared to be very similar, increasing focal stereotypies at the expense of persistent locomotion. At the highest METH dose no effects of either pretreatment were found, but at that dose the majority of the stereotypy was biting at the expense of all other behavioral categories, including locomotion.
The present study indicated that PTIQ, a deaminated and demethylated analogue of nomifensine, augmented METH-induced stereotypical behavior, suggesting that PTIQ might also possess dopaminergic agonist properties, although the basis of these may be slightly different from those of nomifensine.
4. Materials and Methods
4.1. Subjects
Male ICR mice (10-12 weeks old; Japan SLC, Shizuoka, Japan) were housed in groups of eight (cage size: 37 × 22 × 15 cm) in a temperature- (22 ± 2°C) and humidity- (50 ± 10%) controlled environment under a 12 h light/dark cycle (lights on at 07:00) with food and water available ad libitum, except during testing. The observation of stereotypical behavior was performed by trained observers (see Rating of stereotypical behavior). All animal care and use were conducted according to the Guide for the Care and Use of Laboratory Animals (7th edition, Institute of Laboratory Animal Resources-National Research Council, National Academy Press 1996), and all experiments were approved by, and under the supervision of, the Hyogo College of Medicine Institutional Animal Research Committee. Mice were used only once after at least one-week habituation in the facility (body weight on experimental day: 37-51 g, n = 96 total).
4.2. Reagents
METH hydrochloride was purchased from Dainippon Sumitomo Pharma Co., Ltd (Osaka, Japan). Nomifensine maleate (8-amino-2-methyl-4-phenyl-1,2,3,4-tetrahydroisoquinoline, maleate salt) (Fig. 1A) and PTIQ hydrochloride (Fig. 1B) were obtained from Sigma-Aldrich (St. Louis, MO, USA) and Tocris (Ellisville, MO, USA), respectively.
4.3. Treatment protocols
4.3.1. Preparation of reagents
All reagents were dissolved in sterile saline on the day of the experiment. The drug solutions were prepared in such a way that the necessary dose could be injected intraperitoneally (i.p.) in a volume of 0.1 ml/10 g of body weight. The doses of the reagents refer to the weight of salt, and were chosen based on previous reports (Gianutsos et al., 1982; Kitanaka et al., 2005, 2010; Ono et al., 1991; Scarponi et al., 1999). METH-induced stereotypy is dose-dependent; lower doses stimulate locomotion but do not produce stereotypical behavior. For instance, about only 30% of mice exhibited maximal expression levels of stereotypy when 5 mg/kg of METH was administered (authors’ unpublished observation). Since the intention of this study was to examine individual components of METH-induced stereotypy, a higher (i.e. 10 mg/kg) dose was used along with the intermediate dose (i.e. 5 mg/kg).
4.3.2. Effect of nomifensine pretreatment on METH-induced stereotypy
On the day of the experiment, mice (n = 48) were weighed and randomly divided into six groups (n = 8 per group). The subjects were pretreated with saline or nomifensine (3 mg/kg, i.p.) 30 min prior to METH (or saline control) treatment. This period of pretreatment was chosen because a significant increase in horizontal locomotion was observed at this time point in preliminary experiments (data not shown). After the pretreatment period, mice were challenged with saline or 5 or 10 mg/kg METH and then placed in the test apparatus immediately after the injection to assess stereotypic behavior for 1 h as described below.
4.3.3. Effect of PTIQ pretreatment on METH-induced stereotypy
On the day of the experiment, mice (n = 48) were weighed and randomly divided into six groups (n = 8 per group). The subjects were pretreated with saline, or 10 mg/kg (i.p.) PTIQ, followed by saline or 5 or 10 mg/kg METH 30 min later. This period of pretreatment was the same time as for nomifensine. After the challenge injection, all mice were placed in the test apparatus in order to assess stereotypic behavior for 1 h as described below.
4.4. Rating of stereotypical behavior
Test subjects were placed in a transparent acrylic box (30 × 30 × 35 cm) and observed for stereotypy for 1 h following METH administration by observers unaware of the treatments. Behavior was assessed in 30-s intervals, and the predominant behavior observed during each interval was recorded. Since individual stereotypical behaviors were unchanged for long periods (>30 s) after drug treatment, it was possible to record the observations by hand. The behaviors scored were inactive (awake and inactive, or sleeping), ambulation, rearing, persistent locomotion, head bobbing (up-and-down movements of the head), continuous sniffing, circling, and continuous nail and/or wood chip biting or licking, according to a method described previously (Kitanaka et al., 2010). Ambulation, rearing, and persistent locomotion were considered locomotor and exploratory behaviors, and the last four categories were considered stereotypies. Persistent locomotion was not classified as stereotypy because the mice scored as having “persistent locomotion” showed horizontal locomotor activity less than or equal to that displayed by mice showing “hyperlocomotion” induced by 1 mg/kg METH (which is not generally defined as a stereotypy) measured by Animex Auto (Kitanaka et al., 2005, 2010). The cumulative number of intervals within each 5 min period in which stereotypies were observed is shown as a time course below (maximal value = 10). Stereotypical cage climbing (Gianutsos et al., 1982) was not observed in our experimental system because of use of acrylic test chamber without a stainless steel grid top.
4.5. Measurement of locomotor activity
Locomotor activity was measured in a transparent acrylic test box (30 × 30 × 35 cm) with approximately 25 g of fresh wood chips spread on the floor of the chamber using an Animex Auto apparatus (System MK-110; Muromachi Kikai Co., Ltd., Tokyo, Japan) in a quiet room as described previously (Kitanaka et al., 2003, 2005, 2007). The apparatus detects changes in electrical capacitance (oscillation frequency) in an LC oscillator circuit system under the floor of the apparatus as an animal moves horizontally in an electric field. In this set of experiments, sensitivity parameter was set at 580 (Kitanaka et al., 2003). Under this criterion, the count of oscillation frequency parallels the degree of horizontal locomotion. The acrylic test boxes were cleaned and wiped dry between sessions for each animal. All experiments were conducted between 9:00 and 16:00.
4.6. Statistics
Data are presented as mean ± the standard error of the mean (SEM). Statistical analysis was performed using mixed factor analysis of variance (ANOVA) with or without repeated measures followed by Bonferroni/Dunn or Fisher's PLSD post-hoc tests (Statview 5.0 for Apple Macintosh, SAS Institute, Inc., Cary, NC, USA). P < 0.05 was considered statistically significant.
Acknowledgments
The authors are grateful to Ms. A. Yoshioka of the Department of Pharmacology, Hyogo College of Medicine, for preparing the animal study proposal. This research was supported, in part, by a Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 21790254 to N.K.) and intramural funding from the National Institute on Drug Abuse (USA, GRU and FSH).
Abbreviations
- ANOVA
analysis of variance
- i.p.
intraperitoneally
- METH
methamphetamine
- PTIQ
4-phenyl-1,2,3,4-tetrahydroisoquinoline
- s.c.
subcutaneously
- SEM
standard error of the mean
References
- Al-Khatib IMH, Dökmeci I, Fujiwara M. Differential role of nucleus accumbens and caudate-putamen in mediating the effect of nomifensine and methamphetamine on ambulation and rearing of rats in the open-field test. Jpn. J. Pharmacol. 1995;67:69–77. doi: 10.1254/jjp.67.69. [DOI] [PubMed] [Google Scholar]
- Bournerias F, Nabibi B. Nomifensine-induced immune haemolytic anaemia and impaired renal function. Lancet. 1979;2:95–96. doi: 10.1016/s0140-6736(79)90144-2. [DOI] [PubMed] [Google Scholar]
- Brogden RN, Heel RC, Speight TM, Avery GS. Nomifensine: a review of its pharmacological properties and therapeutic efficiency in depressive illness. Drugs. 1979;18:1–24. doi: 10.2165/00003495-197918010-00001. [DOI] [PubMed] [Google Scholar]
- Church WH, Justice JB, Jr., Byrd LD. Extracellular dopamine in rat striatum following uptake inhibition by cocaine, nomifensine and benztropine. Eur. J. Pharmacol. 1987;139:345–348. doi: 10.1016/0014-2999(87)90592-9. [DOI] [PubMed] [Google Scholar]
- Costall B, Kelly DM, Naylor RJ. Nomifensine: a potent dopaminergic agonist of antiparkinson potential. Psychopharmacologia. 1975;41:153–164. doi: 10.1007/BF00421073. [DOI] [PubMed] [Google Scholar]
- Costall B, Naylor RJ. Studies on the dopamine agonist properties of 8-amino-2-methyl-4-(3,4-dihydroxyphenyl)-1,2,3,4-tetrahydroisoquinoline, a derivative of nomifensine. J. Pharm. Pharmacol. 1978;30:514–516. doi: 10.1111/j.2042-7158.1978.tb13307.x. [DOI] [PubMed] [Google Scholar]
- Garris PA, Budygin EA, Phillips PE, Venton BJ, Robinson DL, Bergstrom BP, Rebec GV, Wightman RM. A role of presynaptic mechanisms in the actions of nomifensine and haloperidol. Neuroscience. 2003;18:819–829. doi: 10.1016/s0306-4522(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Gerhards HJ, Carenzi A, Costa E. Effect of nomifensine on motor activity, dopamine turnover rate and cyclic 3′,5′-adenosine monophosphate concentrations of rat striatum. Naunyn Schmiedeberg's Arch. Pharmacol. 1974;286:49–63. doi: 10.1007/BF00499104. [DOI] [PubMed] [Google Scholar]
- Gianutsos G, Morrow G, Light S, Sweeney MJ. Dopaminergic properties of nomifensine. Pharmacol. Biochem. Behav. 1982;17:951–954. doi: 10.1016/0091-3057(82)90478-6. [DOI] [PubMed] [Google Scholar]
- Hafizi S, Palij P, Stamford JA. Activity of two primary human metabolites of nomifensine on stimulated efflux and uptake of dopamine in the striatum: in vitro voltammetric data in slices of rat brain. Neuropharmacology. 1992;31:817–824. doi: 10.1016/0028-3908(92)90046-r. [DOI] [PubMed] [Google Scholar]
- Hoffmann I. 8-Amino-2-methyl-4-phenyl-1,2,3,4-tetrahydroisoquinoline, a new antidepressant. Arzneimittelforschung. 1973;23:45–50. [PubMed] [Google Scholar]
- Hunt P, Kannengiesser MH, Raynaud JP. Nomifensine: a new potent inhibitor of dopamine uptake into synaptosomes from rat brain corpus striatum. J. Pharm. Pharmacol. 1974;26:370–371. doi: 10.1111/j.2042-7158.1974.tb09294.x. [DOI] [PubMed] [Google Scholar]
- Jolicoeur FB, De Michele G, Barbeau A, St-Pierre S. Neurotensin affects hyperactivity but not stereotypy induced by pre and post synaptic dopaminergic stimulation. Neurosci. Biobehav. Rev. 1983;7:285–390. doi: 10.1016/0149-7634(83)90043-x. [DOI] [PubMed] [Google Scholar]
- Katz RJ, Baldrighi G, Carroll BJ. Effects of nomifensine (HOE 984) upon psychomotor activity and intracranial self-stimulation in the rat. Pharmacol. Biochem. Behav. 1977;7:269–272. doi: 10.1016/0091-3057(77)90144-7. [DOI] [PubMed] [Google Scholar]
- Kelley PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in rat following 6-OHDA lesions of nucleus accumbens and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Kitanaka J, Kitanaka N, Tatsuta T, Miyoshi A, Koumoto A, Tanaka K, Nishiyama N, Morita Y, Takemura M. Pretreatment with L-histidine produces a shift from methamphetamine-induced stereotypical biting to persistent locomotion in mice. Pharmacol. Biochem. Behav. 2010;94:464–470. doi: 10.1016/j.pbb.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Kitanaka N, Kitanaka J, Takemura M. Inhibition of methamphetamine-induced hyperlocomotion in mice by clorgyline, a monoamine oxidase-A inhibitor, through alteration of the 5-hydroxytryptamine turnover in the striatum. Neuroscience. 2005;130:295–308. doi: 10.1016/j.neuroscience.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Kruse H, Hoffmann I, Gerhards HJ, Leven M, Schacht U. Pharmacological and biochemical studies with three metabolites of nomifensine. Psychopharmacology (Berl.) 1977;51:117–123. doi: 10.1007/BF00431726. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Differential effects of amphetamine and dopamine uptake blockers (cocaine, nomifensine) on caudate and accumbens dialysate dopamine and 3-methoxytyramine. J. Pharmacol. Exp. Ther. 1992;262:1085–1094. [PubMed] [Google Scholar]
- Martin-Iverson MT, Ortmann R, Fibiger HC. Place preference conditioning with methylphenidate and nomifensine. Brain Res. 1985;332:59–67. doi: 10.1016/0006-8993(85)90389-0. [DOI] [PubMed] [Google Scholar]
- Meiergerd SM, Schenk JO. Kinetic evaluation of the commonality between the site(s) of action of cocaine and some other structurally similar and dissimilar inhibitors of the striatal transporter for dopamine. J. Neurochem. 1994;63:1683–1692. doi: 10.1046/j.1471-4159.1994.63051683.x. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Mataga N, Takashima M, Toru M. Behavioral sensitization and relative hyperresponsiveness of striatal and limbic dopaminergic neurons after repeated methamphetamine treatment. Eur. J. Pharmacol. 1983;88:195–203. doi: 10.1016/0014-2999(83)90006-7. [DOI] [PubMed] [Google Scholar]
- Ono H, Hasebe Y, Satoh M, Nagao T, Ohta S, Hirobe M, Fuduka H. Amphetamine-antagonistic properties of 4-phenyl-1,2,3,4-tetrahydroisoquinoline: inhibition of spinal reflex-enhancing effects of methamphetamine, phenylethylamine and nomifensine. Brain Res. 1991;564:319–322. doi: 10.1016/0006-8993(91)91469-h. [DOI] [PubMed] [Google Scholar]
- Poat JA, Woodruff GN, Watling KJ. Direct effect of a nomifensine derivative on dopamine receptors. J. Pharm. Pharmacol. 1978;30:495–497. doi: 10.1111/j.2042-7158.1978.tb13301.x. [DOI] [PubMed] [Google Scholar]
- Prescott LF, Illingworth RN, Critchley JA, Frazer I, Stirling ML. Acute haemolysis and renal failure after nomifensine overdosage. Br. Med. J. 1980;281:1392–1393. doi: 10.1136/bmj.281.6252.1392-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randrup A, Munkvad I. Stereotyped activities produced by amphetamine in several animal species and man. Psychopharmacologia (Berl.) 1967;11:300–310. doi: 10.1007/BF00404607. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Bashore TR. Critical issues in assessing the behavioral effects of amphetamine. Neurosci. Biobehav. Rev. 1984;8:153–159. doi: 10.1016/0149-7634(84)90030-7. [DOI] [PubMed] [Google Scholar]
- Rupniak NM, Jenner P, Marsden CD. Enhancement of stereotypy induced by nomifensine in rats during continuous chronic haloperidol treatment. Neurosci. Lett. 1984;50:63–65. doi: 10.1016/0304-3940(84)90463-4. [DOI] [PubMed] [Google Scholar]
- Sagara H, Kitamura Y, Sendo T, Araki H, Gomita Y. Motivational effect of nomifensine in the intracranial self-stimulation behavior using a runway method. Biol. Pharm. Bull. 2008;31:1036–1040. doi: 10.1248/bpb.31.1036. [DOI] [PubMed] [Google Scholar]
- Scarponi M, Bernardi G, Mercuri NB. Electrophysiological evidence for a reciprocal interaction between amphetamine and cocaine-related drugs on rat midbrain dopaminergic neurons. Eur. J. Neurosci. 1999;11:593–598. doi: 10.1046/j.1460-9568.1999.00482.x. [DOI] [PubMed] [Google Scholar]
- Schacht U, Heptner W. Effect of nomifensine (HOE 984), a new antidepressant, on uptake of noradrenaline and serotonin and on release of noradrenaline in rat brain synaptosomes. Biochem. Pharmacol. 1974;23:3413–3422. doi: 10.1016/0006-2952(74)90344-x. [DOI] [PubMed] [Google Scholar]
- Tateyama M, Nagao T, Ohta S, Hirose M, Ono H. 4-phenyltetrahydroisoquinoline, but not nomifensine or cocaine, inhibits methamphetamine-induced dopamine release. Eur. J. Pharmacol. 1993a;240:51–56. doi: 10.1016/0014-2999(93)90544-r. [DOI] [PubMed] [Google Scholar]
- Tateyama M, Ohta S, Nagao T, Hirose M, Ono H. Inhibitory effect of 4-phenyltetrahydroisoquinoline on locomotion and dopamine release induced by micro-injection of methamphetamine into the nucleus accumbens of the rat. Neuropharmacology. 1993b;32:761–766. doi: 10.1016/0028-3908(93)90184-5. [DOI] [PubMed] [Google Scholar]