Abstract
This study used the transcranial magnetic stimulation/motor evoked potential (TMS/MEP) technique to pinpoint when the automatic tendency to mirror someone else's action becomes anticipatory simulation of a complementary act. TMS was delivered to the left primary motor cortex corresponding to the hand to induce the highest level of MEP activity from the abductor digiti minimi (ADM; the muscle serving little finger abduction) as well as the first dorsal interosseus (FDI; the muscle serving index finger flexion/extension) muscles. A neuronavigation system was used to maintain the position of the TMS coil, and electromyographic (EMG) activity was recorded from the right ADM and FDI muscles. Producing original data with regard to motor resonance, the combined TMS/MEP technique has taken research on the perception-action coupling mechanism a step further. Specifically, it has answered the questions of how and when observing another person's actions produces motor facilitation in an onlooker's corresponding muscles and in what way corticospinal excitability is modulated in social contexts.
Keywords: Behavior, Issue 82, action observation, transcranial magnetic stimulation, motor evoked potentials, corticospinal excitability
Introduction
Over the past ten years neuroscience research has largely modified the traditional view of the motor system. A considerable amount of data suggests that observing someone else's body movements activates motor representations in the onlooker's brain (e.g.1-3). These studies showed that the motor cortex of an observer dynamically replicates actions being observed as if those were being executed by the onlooker himself. Transcranial magnetic stimulation (TMS) is useful to assess corticospinal (CS) excitability with a relatively high temporal resolution in order to track excitability changes while someone observes someone else performing an action.
The fundamental principle of TMS functioning is that a changing primary electric current in a stimulation coil produces a changing magnetic field, which in turn induces a secondary flow of electric current in nearby conductors- in this case, cortical tissue- as prescribed by Faraday's law4. The brain is an inhomogeneous conductor consisting of white matter, gray matter and cerebral spinal fluid with conductivities 0.48, 0.7, and 1.79 S/m, respectively5. Analysis shows that for the purposes of magnetic stimulation, the brain can be treated as a homogenous conductor5. Depolarization of neurons is produced by virtue of the induced current. At the heart of the process is the transfer of charge across the nerve membrane commensurate to raise its intracellular potential about 30-40 mV. At the point that positive ions are driven into a nerve cell, its intracellular potential will rise, and if the rise is sufficient, an action potential results5. Priori and colleagues6 were the first to show that a weak current could modulate the excitability of the human motor cortex, as measured by the amplitude of the motor-evoked potential (MEP) from TMS. Much of the work involving magnetic stimulation of the human motor cortex has, indeed, focused on EMG responses in intrinsic hand muscles7. In 2004 Uozomi and colleagues8 uncovered that spTMS over area 44 could easily interrupt target-oriented hand movements and produced motor evoked potential from hand muscles. Human area 44 has facilitatory and inhibitory effects over both tonic and phasic finger movements9-10, and has direct fast-conducting corticospinal projections.
The first evidence that CS excitability is modulated not only during voluntary movements but also during action observation was produced by Fadiga and colleagues in 19953. TMS was applied to the hand areas of the primary motor cortices (M1) and MEPs were recorded from contralateral hand muscles while a volunteer was instructed to watch transitive and intransitive movements (the former are goal directed, the latter are not). The amplitude of MEPs recorded from opponens pollicis (OP) and FDI muscles was found to be increased during observation of grasping actions with respect to that registered in control conditions. The question thus arose: are the muscles that are facilitated during action observation the same ones utilized during action execution? EMG responses in hand muscles recorded while an object was being grasped and during arm lifting movements were all found to exactly replicate the pattern of MEPs elicited by TMS during action observation. Some research groups have been able to repeat these same experiments and have designed others11-16.
During action observation, the observer's motor system in practice "resonates" with the observed movements and simulates under threshold those actions in a strictly congruent fashion. As the muscles involved in the observer are the same as those being used by the person carrying out the action, they are temporally coupled with the dynamics of the observed action. In 2001 Gangitano and colleagues demonstrated that the execution-observation matching system is linked to the observed action even in terms of its temporal coding17. MEP amplitudes become larger as the finger aperture increases and smaller during the closure phase. Clark et al.18 set out to assess the specificity of corticospinal (CS) facilitation while participants watched, were asked to imagine, or observed actions that they were told they would later have to carry out. Those investigators reported that there did not seem to be any statistically significant differences in these three conditions.
There are at least two hypotheses explaining MEP facilitation induced by action observation. According to the first one, the enhancement of M1 excitability is produced through excitatory cortico-cortical connections. According to the second, TMS reveals, through CS descending volleys, a facilitation of motoneurons (MNs). Modulations in MEP amplitudes caused by variations in M1 or MNs excitability cannot be distinguished. As Baldissera et al.19 wanted to investigate spinal cord excitability linked to MEP facilitation, they decided to measure the amplitude of the Hoffmann reflex (evoked by stimulating the afferent fibers in peripheral nerves) in finger flexor forearm muscles while volunteers observed goal-directed hand actions. They reported that while modulation of cortical excitability closely imitated the movements being observed as if these were being performed by the observer him/herself, spinal cord excitability appeared to be reciprocally modulated. Those investigators considered the effect an expression of a mechanism blocking the overt execution of observed actions. Modulation of motor potentials evoked by TMS during action observation3,20,21 appears to be specific, then, for the muscles involved in executing an action3 and follows, in an anticipatory fashion22, the same temporal activation pattern17,23. Along these lines, Urgesi and colleagues24,25 recently found that observation of start and middle phases of grasp actions engendered a significantly higher motor facilitation than observing their final postures. Motor facilitation was maximal for the snapshots evoking ongoing but incomplete actions. The results provide compelling evidence that the frontal component of the observation-execution matching system plays an important role in the predictive coding of others' motor behaviors.
It is, however, undeniable that successful interaction in the real world often requires complementary rather than emulative actions26 and that imitation is not always an effective or appropriate response to action observation. In those cases in which, for example, someone hands someone else a mug being held by its handle, we all know that the receiver will, without thinking, grab the mug with a whole hand gesture (the only one that would be appropriate in this situation). Little is known regarding how the inflexible tendency to match observed actions onto our motor system can be reconciled with the request to prepare nonidentical responses. In this respect, some researchers showed that the automatic effects of mirroring can be abolished following incompatible training: mirror and counter-mirror responses seem to follow the same timecourse27,28. Interestingly, in contrast to previous studies, MEPs induced by spTMS were recently used to assess spontaneous corticospinal activation while video-clips evoking emulative or nonidentical complementary gestures were being simply observed29,30. Results showed a natural switch from an emulative to a context-related action in corticospinal activity. A matching mechanism at the beginning of an action sequence turned into a complementary one if a request for a reciprocal action became evident.
Capitalizing on those results, the present study was designed to specifically determine, using the combined TMS/MEP technique, at what stage the spontaneous shift from emulation to reciprocity takes places when action observation evokes a complementary response. MEPs were then recorded at five different moments of the sequence from the FDI and ADM hand muscles. We hypothesize that MEPs recorded at the time the observer initially perceive a whole-hand grasp might elicit both ADM and FDI muscles facilitation because such muscles are usually recruited for such a grip. Conversely, when the observed gesture elicit a nonidentical complementary gesture (i.e. a PG) in the observer, only MEPs recorded from the FDI muscle should reveal a pronounced increase in activation. This is because PG does not imply the recruitment of the ADM muscle. We also predict that when the observed action does not convey any social meaning, simple symmetrical facilitation effects should emerge during all the action sequence.
Protocol
1. Preparation of the Video Stimuli
- Commission a model to perform four action sequences.
- In the first two action sequences, seat the model at a table facing the camera. Place three mugs on the table near to her and a fourth one further away on the other side of the table in the foreground. Instruct the model to start her action by reaching towards and grasping a sugar spoon.
- Instruct the model to start her action by pouring sugar into the three mugs. When she has finished pouring sugar into the third mug, instruct the model to move her wrist as if she intends to pour sugar into the 4th one as well.
- Instruct the model to start her action by pouring sugar into the three mugs. When she has finished pouring sugar into the third mug, have the model move her wrist to bring it back to its original position.
- In the last two action sequences, seat the model once again at a table facing the camera. Place three espresso coffee cups on the table near to her and a fourth one further away from her on other side of the table in the foreground. Instruct the model to start her action by reaching towards and grasping a thermos.
- Instruct the model to start her action by pouring coffee into the three espresso coffee cups. When she has finished pouring coffee into the third cup, have the model move her wrist as if she intends to pour coffee into the fourth cup as well.
- Instruct the model to start her action by pouring coffee into the three espresso coffee cups. When she has finished pouring coffee into the third cup, have the model move her wrist to bring it back to its original position.
Instruct the model to pick up and hold the sugar spoon using a precision grip (PG; i.e. the opposition of the thumb with the index finger) and to pick up and hold the thermos in a natural way using a whole-hand grasp (WHG; i.e. the opposition of the thumb with the other fingers).
- At the beginning of each video-clip, instruct the model to show that her hand is in a prone position resting on the table.
- Arrange for the model to begin a reach-to-grasp movement approximately 900 msec later.
- Arrange for the model's fingers to make contact with the first object approximately 450 msec later.
- Have the model begin to move her hand to perform the second action step 5,000 msec later.
- Use a digitizing technique to perform a post-hoc kinematic analysis of the model's movements
- Mark each movement, frame by frame, by manually assigning a marker to the model's wrist.
- Track the model's movements. Pinpoint the Trajectory Deviation: the moment when the hand trajectory begins to diversify for social and nonsocial conditions. Lock the most salient kinematic events characterizing the action sequence with TMS stimulation timing.
2. Instrument Preparation
Connect four sintered Ag/AgCl bipolar and one monopolar surface electrodes (15 kΩ, 1.5 mm touch-proof safety socket) with a sensor area (9 mm diameter) to an isolated portable ExG input box linked to the main EMG amplifier. A twin fiber optic cable for signal transmission is recommended, but not compulsory.
Manage a script for individual resting motor threshold (rMT) assessment, presentation of video stimuli and TMS stimulation synchronized with EMG registration by E-Prime presentation software running on a PC with a monitor (resolution 1,280 x 1,024 pixels, refresh frequency 75 Hz, background luminance of 0.5 cd/m2) set at eye level.
Attain an animation effect by selecting a series of single frames (30 msec each, 30 fps) and the first and last frames lasting, respectively, 500 and 1,000 msec.
3. Participant Recruitment
Recruit only right handed participants with normal or corrected-to-normal vision. Check for handedness using the Standard Handedness Inventory questionnaire31.
- Verify if any of the candidates have contraindications to TMS32,33.
- Exclude subjects with higher than normal seizure risk (based on personal/family history of epilepsy, neurosurgery, brain injury) or receiving neuroactive medication in view of the fact that the principal known health risk of TMS is seizure induction.
- Exclude pregnant women as the risks of TMS to an unborn fetus are unknown.
Give basic information about the study to all the participants and ask them to sign written informed consent forms.
Possibly perform experiments in a sound-attenuated Faraday room: this is recommended, but not compulsory.
Have the participant sit in a comfortable armchair.
Position his/her right arm on a full arm-support.
Fix the participant's head on a head rest. Eye distance from the screen should be determined on the basis of the size of the stimulus presentation.
Ask the participant to remove all metal objects (earrings, necklaces, etc.) and objects sensitive to magnetic fields (mobile phones, credit cards) as the rapid rate of change of current in the coil is capable of inducing a changing magnetic field.
Instruct the participants to watch the visual stimuli carefully and to maintain a good level of attention; explain that they will be questioned later about the contents.
4. TMS Stimulation and MEP Recording
Determine where the electrodes should be positioned over the ADM and the FDI muscles by palpation during maximum voluntary muscle activation. Clean the skin for all electrode locations (also for the ground). Apply an abrasive skin prepping gel to the entire site using a gauze pad. Rub it into the skin lightly and remove any excess with a clean gauze pad.
- Place two surface electrodes, each containing a small amount of water soluble EEG conductive paste, over each muscle and attach them to the skin using self-adhering pads.
- Perform a belly-tendon montage by placing the active electrodes over the muscle bellies of the right ADM and FDI and the reference electrodes over the ipsilateral metacarpophalangeal joint. Attach a single ground electrode containing conductive paste on the participant's left wrist.
- Connect the electrodes to the common input of the ExG input box and check impedance values. In the event they are above the threshold (>5 Ω), prepare the skin again.
- Deliver single-pulse TMS to the scalp overlying the left primary motor cortex (M1) corresponding to the hand region using a 70 mm figure-of-eight coil connected to a Magstim 200 stimulator. Note: A basic TMS stimulator is composed by a power source, an energy storage element and a high-power switch precisely controlled by a processor that accepts control input from the equipment operator. The fundamental operating mechanism of a TMS stimulator is to create a changing magnetic field that can induce a current in adjacent conductive material (such as cortical tissue). Tissue stimulation is provoked by inducing a current of sufficient density in the tissue, which is proportional to the time rate of change of the magnetic flux density34. With a figure-of-eight coil, the isopotential lines of the induced electric field form an oval, whose long axis is parallel to the direction of current flow at the coil junction35.
- Place the coil at a 45° angle with respect to the interhemispheric fissure and position it perpendicularly with respect to the central sulcus: the lowest motor threshold is achieved when the induced electric current in the brain is flowing approximately perpendicular to the central sulcus36,37.
- Have the handle pointing laterally and caudally to induce a posterior-anterior brain current through the precentral gyrus38. At low, but supra-threshold, stimulation intensities, the TMS-induced current excites preferentially axons of interneurons which project directly or indirectly on corticospinal neurons. Both inhibitory and excitatory synapses are activated, but at such stimulation intensities the net effect is that of an excitatory post-synaptic potential in corticospinal neurons.
- Locate the optimal scalp position (OSP) over the pars opercularis of the inferior frontal gyrus. Stimuli of a slightly suprathreshold intensity on the OSP invariably produce the highest levels of MEP activity from the contralateral ADM and FDI muscles.
- Use a 10-20 International System (the stimulated site corresponding to the C3 location) to establish the OSP for eliciting motor-evoked potentials (MEPs) in the hand muscles, then move the intersection of the coil in approximately 0.5 cm steps around the target area and deliver TMS pulses at constant intensity.
- After the target area has been correctly identified, stabilize the coil using a mechanical support to maintain consistent positioning.
- Use a neuronavigation system to maintain constant coil-positioning throughout the entire experiment and prevent any bias due to small movements of the participant's head during data collection.
- Apply passive spherical markers both on the coil and on the participant's head.
- Record marker positions using an optical digitizer in order to reproduce them on the computer screen.
- Detect any difference in spatial coil location and orientation and adopt a tolerance of 2-3 mm for each of the Cartesian coordinates.
- Utilize three-dimensional online information with regard to the initial and actual coil placements to allow for the exact repositioning of the TMS coil in real time during the experimental session, when needed.
To determine the "individual resting motor threshold" (rMT) for each participant on the OSP, detect the minimum stimulation intensity necessary to produce reliable MEPs (≥50 μV peak-to-peak amplitude) in a relaxed muscle in five out of ten consecutive trials. Determine the OSP and the rMT for the higher threshold muscle to avoid the loss of any differential modulations involving the less excitable muscle.
Keep the stimulation intensity at a fixed value (i.e. 110% of the rMT) during the entire recording session.
Use a bandpass filter (20 Hz-1 kHz) to record the raw myographic signals. After amplification digitize the signals (5 kHz sampling rate) and store in the computer for offline analysis.
Record 10 MEPs while the participant passively watches a white-colored fixation cross on a black background on the computer screen at the beginning of the experimental session.
Record 10 more MEPs at the end of the experimental session.
- Record EMG data from the right ADM and FDI muscles after the TMS pulse at one of the five possible time points (Figure 1) and that is:
- When the model's hand first makes contact with the sugar spoon or the thermos (T1).
- When the model finishes pouring sugar/coffee into the third cup/mug (T2).
- When the model begins to pull her hand away from the third cup/mug (T3).
- When the model's arm begins to return to the starting position or starts moving towards the fourth cup/mug (respectively, the nonsocial and social conditions) (T4).
- When the model's arm returns to the starting point or when it reaches the fourth cup/mug (respectively, the nonsocial and social conditions) (T5).
Insert a 10 sec rest interval between the videos. Have a message appear during the first five seconds of the rest interval reminding the participants to keep their hands resting quietly and fully relaxed. Once the message disappears arrange for a fixation cross to appear for the remaining five seconds.
5. Debriefing
Provide the participants with detailed information about the experimental design at the end of the session.
6. Data Analysis
- Perform a post-hoc kinematical analysis.
- Set a frame of reference identifying x- and y-axes as horizontal and vertical directions and analyze the video sample frame-by-frame.
- Use a known length in the camera's field of view and in the movement's plane as the reference unit measurement.
- Assign a marker to the model's wrist to measure arm kinematics.
- Define the starting position as the time the model's right hand is resting in a prone position on the table. Track the wrist trajectory in space and time, extract the trajectory path, and identify the salient kinematic events characterizing the model's double-step action.
- Analyze EMG data.
- Segment the EMG tracing for each muscle into different segments (epochs) of the same length relative to a reference marker (TMS stimulus). Set the time window at 100 msec before TMS pulses are delivered and 200 msec after TMS pulses. This will allow you to check for possible background activity.
- In each channel of the EMG select a precise range of a time frame (e.g. 10-40 msec) to search for peaks in all the segments.
- Apply an algorithm which take into account the positive and negative peaks within each segment and calculate the maximum amplitude of the EMG curve in μV from peak to peak.
- Eliminate trials with background EMG activity greater than 100 µV to avoid contaminating MEP measurements by background activity.
Calculate the average peak-to-peak MEP amplitudes separately from the ADM and FDI muscles for each condition excluding those deviating more than 2 standard deviations from the mean (outliers).
Compare the two series of MEP amplitudes recorded from each muscle in each participant during the fixation cross baseline trials at the beginning and at the end of the experimental session to check for corticospinal excitability variations related to TMS per se. The average amplitude of the two series also allow to set the individual baseline value for data normalization procedures in each muscle separately39.
Compute ratio values using the participant's individual baseline value (MEP ratio = MEPobtained/MEPbaseline) 39.
Representative Results
The efficacy of the TMS/MEP technique in assessing CS excitability during action observation depends upon locating the optimal scalp position for both the ADM and FDI muscles. Surface electrodes in belly-tendon montages should be applied and must conform to regular single-pulse stimulation patterns.
In this study, results have been obtained on a sample of thirty participants (22 females and 8 males: age = 21±5 years), all right handed according to a Standard Handedness Inventory31 and with normal or corrected-to-normal vision. None had any contraindication to TMS32,33 nor experienced discomfort during the experiment. The experimental procedures outlined here were granted ethical approval (Ethics Committee of the University of Padova) in accordance with the principles of the 1964 Declaration of Helsinki and all of the participants gave written informed consent.
According to our hypothesis, the MEPs recorded when the need for a complementary action becomes apparent should be modulated depending on the object placed in the foreground. When the 4th coffee cup evokes a tendency to perform a PG, only the FDI muscle should be activated. But when the model's action towards the 4th mug evokes a WHG, then both ADM and FDI muscles should be found activated. As FDI was recruited for both the PG and WHG, no MEP modulation was expected in terms of the type of grasp observed. MEPs being recorded from the observer's hand at the time the model initially grasps the thermos should, moreover, show, for example, motor facilitation in both ADM and FDI muscles, that is the muscles classically involved in a WHG. On the contrary, observing the model as her hand moves towards the fourth coffee cup should produce only FDI muscle facilitation as only that muscle (and not the ADM) is involved in a PG.
Observing a two-step action sequence implicitly containing a request for a complementary movement caused a switch from emulation to responsiveness in the onlooker's corticospinal activity and indicated, in the trials carried out, exactly when the changeover took place (Figure 2).
A variation heralding a reciprocal action took place in the observer's ADM MEPs the moment the model's wrist started to move towards the fourth mug (the social condition). Inversely, a variation heralding an emulative action took place in the observer's MEPs the moment the model's wrist started to return to its original position (the nonsocial condition, see Figure 3). FDI, as expected, was actively involved in all observed movements and simulated actions (see Figures 4 and 5). It would seem, then, from these results that humans can code an action as social or nonsocial even before it becomes explicit. It can be concluded that observers are attuned to advance movement information provided by subtle kinematic cues and that they are able to use it to anticipate a future course of action. During the experimental sessions described here the participants showed that they were capable of discriminating between actions prompted by social or nonsocial conditions simply by observing nearly imperceptible kinematic cues. During the experiments carried out, modulation of corticospinal excitability was a reliable, indirect measure of the capacity to activate appropriate motor programs in an interactive context.
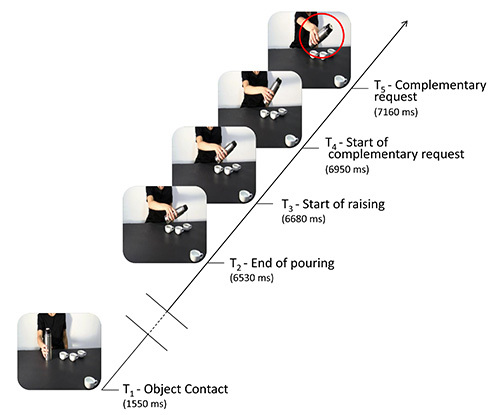 Figure 1.Here the sequence of events taking place during each trial is schematized. The continuous oblique line represents the entire video-clip presentation. Horizontal lines denote the time points when single TMS pulses were delivered: at T1 (when the model's hand makes contact with the cup/mug), T2 (when the model finishes pouring sugar/coffee), T3 (when the model begins to move her hand away from the third cup/mug), T4 (when the model's hand starts to return to its original position or to move towards the fourth cup/mug – considered the onset of a cue for a complementary gesture), and T5 (when the model's arm is clearly returning to its original position or moving towards the fourth cup/mug – considered the end of the cue for a complementary gesture). The frames not shown in the figure (the time between the model's action of making contact with the sugar spoon/thermos and the action of pouring sugar/coffee was completed) are represented by double oblique bars.
Figure 1.Here the sequence of events taking place during each trial is schematized. The continuous oblique line represents the entire video-clip presentation. Horizontal lines denote the time points when single TMS pulses were delivered: at T1 (when the model's hand makes contact with the cup/mug), T2 (when the model finishes pouring sugar/coffee), T3 (when the model begins to move her hand away from the third cup/mug), T4 (when the model's hand starts to return to its original position or to move towards the fourth cup/mug – considered the onset of a cue for a complementary gesture), and T5 (when the model's arm is clearly returning to its original position or moving towards the fourth cup/mug – considered the end of the cue for a complementary gesture). The frames not shown in the figure (the time between the model's action of making contact with the sugar spoon/thermos and the action of pouring sugar/coffee was completed) are represented by double oblique bars.
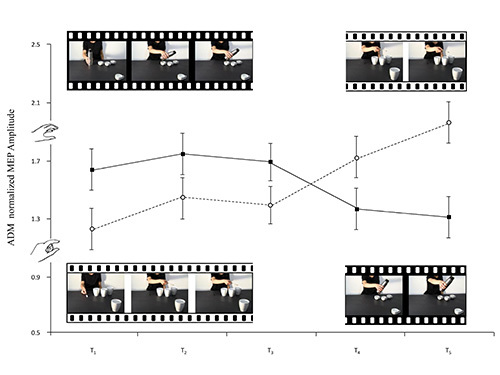 Figure 2.The frames extracted from the video-clips filmed for this study accompany the lines of the graph which represent the means of the ADM normalized MEP amplitudes. Social whole-hand grasp movements requiring a PG and social precision grip movements requiring a WHG are illustrated (black and white, respectively). Bars represent the standard error of means.
Figure 2.The frames extracted from the video-clips filmed for this study accompany the lines of the graph which represent the means of the ADM normalized MEP amplitudes. Social whole-hand grasp movements requiring a PG and social precision grip movements requiring a WHG are illustrated (black and white, respectively). Bars represent the standard error of means.
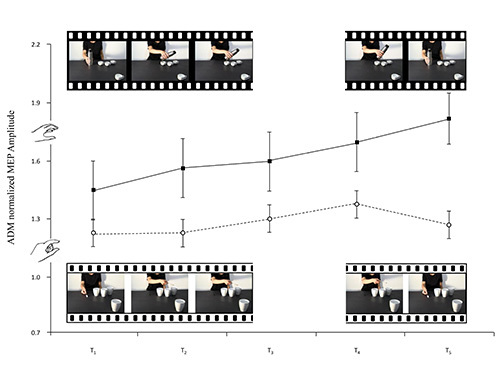 Figure 3.The frames extracted from the video-clips filmed for this study accompany the lines of the graph which represent the means of the ADM normalized MEP amplitudes. Nonsocial whole-hand grasp movements requiring a PG and nonsocial precision grip movements requiring a WHG are illustrated (black and white, respectively). Bars represent the standard error of means.
Figure 3.The frames extracted from the video-clips filmed for this study accompany the lines of the graph which represent the means of the ADM normalized MEP amplitudes. Nonsocial whole-hand grasp movements requiring a PG and nonsocial precision grip movements requiring a WHG are illustrated (black and white, respectively). Bars represent the standard error of means.
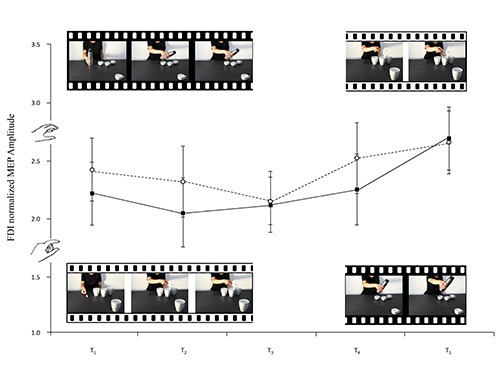 Figure 4.The frames extracted from the video-clips filmed for this study accompany the lines of the graph which represent the means of the FDI normalized MEP amplitudes. Social whole-hand grasp movements requiring a PG and social precision grip movements requiring a WHG are illustrated (black and white, respectively). Bars represent the standard error of means.
Figure 4.The frames extracted from the video-clips filmed for this study accompany the lines of the graph which represent the means of the FDI normalized MEP amplitudes. Social whole-hand grasp movements requiring a PG and social precision grip movements requiring a WHG are illustrated (black and white, respectively). Bars represent the standard error of means.
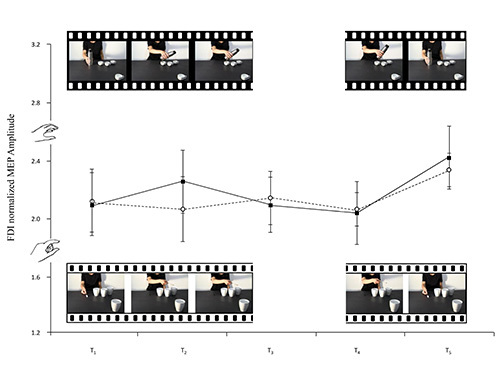 Figure 5.The frames extracted from the video-clips filmed for this study accompany the lines of the graph which represent the means of the FDI normalized MEP amplitudes. Nonsocial whole-hand grasp movements requiring a PG and nonsocial precision grip movements requiring a WHG are illustrated (black and white, respectively). Bars represent the standard error of means.
Figure 5.The frames extracted from the video-clips filmed for this study accompany the lines of the graph which represent the means of the FDI normalized MEP amplitudes. Nonsocial whole-hand grasp movements requiring a PG and nonsocial precision grip movements requiring a WHG are illustrated (black and white, respectively). Bars represent the standard error of means.
Discussion
The most critical steps in measuring modulation in CS excitability in humans during action observation are: 1) designing/filming video clips that induce an action tendency in an observer anticipating both emulative and complementary responses; 2) determining the kinematic events characterizing the various phases of the model's actions to time-lock TMS stimulation accordingly; 3) identifying the optimal scalp position for each hand muscle and maintaining consistent positioning throughout the experiment; 4) properly registering EMG activity from the stimulated muscles.
Previous studies utilizing the TMS/MEP technique have shown that corticospinal activation resulting from action observation does not invariably possess an imitative bias but, depending on contextual factors, can also prime motor activation for complementary actions29,30. Single-pulse TMS studies have demonstrated that observing a two-step action sequence in which a complementary request is embedded prompts a switch from emulation to responsiveness in the participants' corticospinal activity. This study goes a step further by showing when exactly the switch takes place and demonstrates that humans are able to anticipate the social intent of an action by observing precocious kinematic cues signaling the need/request for a complementary response. Advance movement information is, indeed, sufficient for an observer to make inferences about the intention behind it. Mechanisms underlying action observation seem then to be malleable, prompt, and sensitive to complex requests embedded in social contexts. Future research will go on to analyze if processing is serial or parallel. Neuroimaging studies employing paradigms such as the one used here will be able to further clarify this process, delineating the cortical networks underlying the ability to shift from emulation to reciprocity.
These results will also point the way for future applications of TMS/EMG techniques to study CS excitability and motor system plasticity. Numerous studies have already shown that TMS measurements of motor cortex function are safe, reliable, and potentially useful in the clinical setting40.46. Longitudinal comparisons of MEP amplitude could, in fact, provide a direct assessment of motor cortical plasticity effects.
Recent studies have reported that action observation has a positive effect on post-stroke rehabilitation of motor deficits and can be beneficially utilized to reactivate motor areas in individuals needing to rehabilitate motor control47. A complementary action observation therapy strategy could, thus, be developed which makes use of observation of complementary gestures to reactivate impaired motor skills. If, as it seems, motor behavior is the result of both internal and external factors, action observation should be included in training protocols aiming to rehabilitate this type of patient. Observation of everyday actions together with physical practice could pave the way to a more efficacious rehabilitation strategy. Until now, moreover, only indirect measures such as functional or subjective scales have been used to assess clinical improvement; in the future TMS/EMG assessment can be utilized to measure functional improvement in these patients.
In conclusion, this study delineates how and when observing another person's actions produces motor facilitation in an onlooker's corresponding muscles and in what way corticospinal excitability is modulated in social contexts. It also confirms that motor potentials evoked by TMS are safe, reliable indicators of CS excitability and modulation during action observation.
Disclosures
There is nothing to disclose.
Acknowledgments
Luisa Sartori was supported by a grant from Università degli Studi di Padova, Bando Giovani Studiosi 2011, L. n.240/2010.
References
- Aglioti SM, Cesari P, Romani M, Urgesi C. Action anticipation and motor resonance in elite basketball players. Nat. Neurosci. 2008;11:1109–1116. doi: 10.1038/nn.2182. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bolognini N, Malavita A, Aglioti SM. Somatic and motor components of action simulation. Curr. Bio. 2007;17:2129–211235. doi: 10.1016/j.cub.2007.11.045. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J. Neurophysiol. 1995;73:2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Epstein CM. Electromagnetism. In: Wasserman E, Epstein C, Ziemann U, Walsh V, Paus T, Lisanby S, editors. Oxford Handbook of Transcranial Stimulation. ed Wasserman. University Press; 2008. [Google Scholar]
- Davey K. Magnetic field stimulation: the brain as a conductor. In: Oxford Handbook of Transcranial Stimulation. Oxford University Press; 2008. [Google Scholar]
- Priori A, Berardelli A, Rona S, Accornero N&, Manfredi Manfredi. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;15:2257–2260. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Johansson RS, Westling G. Corticospinal control during reach, grasp, and precision lift in man. J. Neurosci. 1995;15:6145–6156. doi: 10.1523/JNEUROSCI.15-09-06145.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozumi T, Tamagawa A, Hashimoto T, Tsuji S. Motor hand representation in cortical area 44. Neurology. 2004;62:757–761. doi: 10.1212/01.wnl.0000113731.75479.25. [DOI] [PubMed] [Google Scholar]
- Kraskov A, Dancause N, Quallo MM , Shepherd S, RN Lemon. Corticospinal neurons in macaque ventral premotor cortex with mirror properties: a potential mechanism for action suppression. Neuron. 2009;64:922–930. doi: 10.1016/j.neuron.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J. Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard G, Tremblay F. Corticomotor facilitation associated with observation, imagery and imitation of hand actions: a comparative study in young and old adults. Exp. Brain Res. 2007;177:167–175. doi: 10.1007/s00221-006-0657-6. [DOI] [PubMed] [Google Scholar]
- Tremblay F, Leonard G, Tremblay L. Corticomotor facilitation associated with observation and imagery of hand actions is impaired in Parkinson's disease. Exp. Brain Res. 2008;185:249–257. doi: 10.1007/s00221-007-1150-6. [DOI] [PubMed] [Google Scholar]
- Liepert J. Neveling N. Motor excitability during imagination and Observation of foot dorsiflexions. J. Neural Transm. 2009;116:1613–161609. doi: 10.1007/s00702-009-0287-9. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD , Rothwell JC , Day BL , Thompson PD , Ferbert A, et al. Corticocortical inhibition in human motor cortex. J. Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurra M, Bianco G, Polizzotto NR , Innocenti I, Rossi A, Rossi S. Cortico-Cortical Connectivity between Right Parietal and Bilateral Primary Motor Cortices during Imagined and Observed Actions: A Combined TMS/tDCS Study. Front Neural Circuits. 2011;5 doi: 10.3389/fncir.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo L, Caruana F, Jezzini A, Rizzolatti G. Representation of goal and movements without overt motor behavior in the human motor cortex: a Transcranial magnetic stimulation study. J. Neurosci. 2009;29:11134–11138. doi: 10.1523/JNEUROSCI.2605-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangitano M, Mottaghy FM, Pascual-Leone A. Phase-specific modulation of cortical motor output during movement observation. NeuroReport. 2001;12:1489–1492. doi: 10.1097/00001756-200105250-00038. [DOI] [PubMed] [Google Scholar]
- Clark S, Tremblay F, Ste-Marie D. Differential modulation of corticospinal excitability during observation, mental imagery and imitation of hand actions. Neuropsychologia. 2004;42(1):105–112. doi: 10.1016/s0028-3932(03)00144-1. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Cavallari P, Craighero L, Fadiga L. Modulation of spinal excitability during observation of hand actions in humans. Eur. J. Neurosci. 2001;13:190–194. doi: 10.1046/j.0953-816x.2000.01368.x. [DOI] [PubMed] [Google Scholar]
- Maeda F, Chang VY, Mazziotta J, Iacoboni M. Experience-dependent modulation of motor corticospinal excitability during action observation. Exp. Brain. Res. 2001;140:241–244. doi: 10.1007/s002210100827. [DOI] [PubMed] [Google Scholar]
- Paus Strafella AP, Paus T. Modulation of cortical excitability during action observation: a transcranial magnetic stimulation study. NeuroReport. 2000;11:2289–2292. doi: 10.1097/00001756-200007140-00044. [DOI] [PubMed] [Google Scholar]
- Borroni P, Montagna M, Cerri G, Baldissera F. Cyclic time course of motor excitability modulation during observation of hand actions in humans. Eur. J. Neurosci. 2005;13:190–194. [Google Scholar]
- Montagna M, Cerri G, Borroni P, Baldissera F. Excitability changes in human corticospinal projections to muscles moving hand and fingers while viewing a reaching and grasping action. Eur. J. Neurosci. 2005;22:1513–1520. doi: 10.1111/j.1460-9568.2005.04336.x. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Maieron M, Avenanti A, Tidoni E, Fabbro F, Aglioti SM. Simulating the future of actions in the human corticospinal system. Cereb. Cortex. 2010;20:2511–2521. doi: 10.1093/cercor/bhp292. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Moro V, Candidi M. Aglioti SM. Mapping implied body actions in the human motor system. J. Neurosci. 2006;26:7942–7949. doi: 10.1523/JNEUROSCI.1289-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebanz N, Bekkering H, Knoblich G. Joint action: Bodies and minds moving together. Trends Cogn. Sci. 2006;10:70–76. doi: 10.1016/j.tics.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Cavallo A, Heyes C, Becchio C, Bird G, Catmur C. Timecourse of mirror and counter-mirror effects measured with transcranial magnetic stimulation. SocCogn Affect Neurosci. 2013. [DOI] [PMC free article] [PubMed]
- Cattaneo L, Barchiesi G. Transcranial Magnetic Mapping of the Short-Latency Modulations of Corticospinal Activity from the Ipsilateral Hemisphere during Rest. Front Neural Circuits. 2011;5 doi: 10.3389/fncir.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori L, Cavallo A, Bucchioni G, Castiello U. Corticospinal excitability is specifically modulated by the social dimension of observed actions. Exp. Brain. Res. 2011;211(3-4):3–4. doi: 10.1007/s00221-011-2650-y. [DOI] [PubMed] [Google Scholar]
- Sartori L, Cavallo A, Bucchioni G, Castiello U. From simulation to reciprocity: The case of complementary actions. Soc. Neurosci. 2011;7(2):146–158. doi: 10.1080/17470919.2011.586579. [DOI] [PubMed] [Google Scholar]
- Briggs GG, Nebes RD. Patterns of hand preference in a student population. Cortex. 1975;11:230–238. doi: 10.1016/s0010-9452(75)80005-0. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation. Electroen. Clin. Neur. 1996;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice. Clin. Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehl M. TMS stimulator design. In: Riehl M, Wasserman E, Ziemann U, Walsh V, Paus T, Lisanby S, editors. Oxford Handbook of Transcranial Stimulation. Oxford University Press; 2008. [Google Scholar]
- Epstein CM. In: TMS stimulation coils. InOxford Handbook of Transcranial Stimulation. Wasserman E, Epstein C, Ziemann U, Walsh V, Paus T, Lisanby S, editors. Oxford University Press; 2008. [Google Scholar]
- Brasil-Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J. Clin. Neurophysiol. 1992;9:132–136. [PubMed] [Google Scholar]
- Mills KR, Boniface SJ, Schubert M. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr. Clin. Neurophysiol. 1992;85:17–21. doi: 10.1016/0168-5597(92)90096-t. [DOI] [PubMed] [Google Scholar]
- Sommer M, Paulus W. TMS waveform and current direction. In: Wasserman E, Epstein C, Ziemann U, Walsh V, Paus T, Lisanby S, editors. Oxford Handbook of Transcranial Stimulation. 2008. Oxford University Press; 2008. [Google Scholar]
- Lepage JF, Tremblay S, Théoret H. Early non-specific modulation of corticospinal excitability during action observation. Eur. J. Neurosci. 2010;31:931–937. doi: 10.1111/j.1460-9568.2010.07121.x. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Riek S, Carson RG. Reliability of the input-output properties of the cortico-spinal pathway obtained from transcranial magnetic and electrical stimulation. J. Neurosci. Meth. 2001;112:193–202. doi: 10.1016/s0165-0270(01)00468-x. [DOI] [PubMed] [Google Scholar]
- Malcolm MP, Triggs WJ, Light KE, Shechtman O, Khandekar G, Gonzalez Rothi LJ. Reliability of motor cortex transcranial magnetic stimulation in four muscle representations. Clin. Neurophysiol. 2006;117:1037–1046. doi: 10.1016/j.clinph.2006.02.005. [DOI] [PubMed] [Google Scholar]
- McMillan AS, Watson C, Walshaw D. Transcranial magnetic-stimulation mapping of the cortical topography of the human masseter muscle. Arch. Oral Biol. 1998;43:925–931. doi: 10.1016/s0003-9969(98)00081-8. [DOI] [PubMed] [Google Scholar]
- Miranda PC, de Carvalho M, Conceiço I, Luis ML, Ducla-Soares E. A new method for reproducible coil positioning in transcranial magnetic stimulation mapping. Electroencephalogr. Clin. Neurophysiol. 1997;105(2):116–123. doi: 10.1016/s0924-980x(97)95720-9. [DOI] [PubMed] [Google Scholar]
- Mortifee P, Stewart H, Schulzer M, Eisen A. Reliability of transcranial magnetic stimulation for mapping the human motor cortex. Electroencephalogr. Clin. Neurophysiol. 1994;93:131–137. doi: 10.1016/0168-5597(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Uy J, Ridding MC, Miles TS. Stability of maps of human motor cortex made with transcranial magnetic stimulation. Brain Topogr. 2002;14:293–297. doi: 10.1023/a:1015752711146. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47(1):61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Ertelt D, et al. Action observation has a positive impact on rehabilitation of motor deficits after stroke. Neuroimage. 2007;36:164–173. doi: 10.1016/j.neuroimage.2007.03.043. [DOI] [PubMed] [Google Scholar]


