Abstract
Aim(s)
Insulin-like growth factor-1 receptor (IGF-1R) targeted therapies have become one of the intriguing areas in anticancer drug development during the last decade. As one of these therapies, anti-IGF-1R monoclonal antibodies (mAbs) are also advancing further in development. Our purpose was to conduct a systematic review of the adverse events (AEs) caused by anti-IGF-1R monoclonal antibodies in cancer therapy.
Methods
We searched the term‘IGF-1R monoclonal antibody’ in the Pubmed database and found 389 related articles. After elaborate selection, 15 clinical studies that satisfied our criteria were then adopted for further analysis. We extracted all the useful information about the AEs of mAbs from the enrolled studies. Every kind of reported AE as well as corresponding incidences were summed up and calculated. We compared AE incidence differences in two age groups, and analyzed toxicities of mAbs used as a single agent or combined with chemotherapies. Finally, the differences of AE profiles between individual mAbs were also valued.
Results
AEs were more severe in the lower age group and 13 of 19 AE incidences in the single-agent group were significantly lower than in the combination group (P < 0.05). R1507 seemed to show a worse AE profile than cixutumumab and figitumumab.
Conclusions
When anti-IGF-1R mAbs are used for cancer therapy, it is essential to choose the proper drug and combined chemotherapies to reduce AE occurrences. Also, administration of these mAbs to younger patients should be more carefully supervised. Furthermore, some more frequently observed AEs for specific mAb should be paid adequate attention.
Keywords: adverse events, R1507, anti–IGF-1R monoclonal antibody, cixutumumab, dalotuzumab, figitumumab
Introduction
Insulin-like growth factor-1 receptor (IGF-1R) targeted therapy has become one of the most investigated areas in anticancer drug development during the last decade 1. Experimental exploration and studies of clinical tumour biopsy specimens suggest that cancer progression is frequently associated with increased expression of the IGF-1R. There are a broad range of tumour types such as breast, colon, sarcoma, lung, prostate, thyroid and myeloma that aberrantly express IGF-1R. Therefore the strategy of blocking IGF-1R activity is of possibly great use in the treatment of various cancers 2. Monoclonal antibodies (mAbs) targeting IGF-1R are one of these strategies. Unfortunately, the result of phase III trial of carboplatin and paclitaxel with or without figitumumab was disappointing and patients who received figitumumab suffered from more severe adverse events (AEs) such as early fatal toxicities 3. Besides, Roche decided to suspend the development of R1507 for business reasons 4. Despite all these pitfalls, IGF-1R remains an attractive anticancer target and several ongoing trials are testing anti-IGF-1R mAbs or in combination with chemotherapy in patients with malignant tumours, such as pancreatic and ovarian cancer 5. In the current environment of similar IGF-1R-targeted agents competing for similar patient populations, differences in the frequency and intensity of AEs may be an important determinant as to whether a mAb will win out, although AEs caused by these mAbs were generally reported to be tolerable in early clinical trials 2,6. We conducted this systematic review to assess the AEs described in former clinical studies and hoped to provide significant information for further research of these mAbs.
Methods
Search methods and study selection
We searched ‘Pubmed’ using the search term ‘IGF-1R monoclonal antibody’. From 389 achieved articles published before April 20 2012, we selected the studies that met each of following criteria: 1) clinical studies were concerned with the use of any anti-IGF-1R mAbs for treatment of malignant tumours, 2) patients' demographics and baseline disease characteristics were clearly demonstrated, 3) most AEs occurred during a whole specific clinical study process were given in a clear fashion, and the baseline of AEs were 'AE with its incidence >5%' or 'AEs grade ≥2' (there were two exceptions, the baseline for studies 8 and 9 was ≥10%, but the two samples' sizes were small thus and this baseline was indeed acceptable), 4) AEs were graded using the same criteria: ‘National Cancer Institute Common Terminology Criteria for Adverse Events’ (version 3.0) and 5) AEs were counted in numbers of patients experiencing them, rather than the observed episodes of AEs.
Data extraction
Two independent investigators (Honghai Ma and Hongxin Cao) reviewed the publications and extracted the data. Every enrolled study was given a ‘study number’ and details are shown in Table 1. The following information was extracted from each article: 1) basic information from papers such as year of publication, journal name and authors' name, 2) characteristics of patients such as age, gender and tumour type, 3) information of study designation such as phase I/II and treatment protocol, 4) information on treatment such as treatment modality, dose of chemotherapy and 5) number of patients experiencing AEs during the whole clinical trial. Available information was extracted and recorded to a data collection form and entered into an electronic database.
Table 1.
Characteristics of studies included in the analysis
| Study number | Study | Number of patiens | Gender (male/female) | Median (range) age (years) | Tumour type | Study phase | Drugs | The baseline for including AEs |
|---|---|---|---|---|---|---|---|---|
| 1 | Kurzrock et al., 2008 14 | 35 | 23/12 | 48.3 (18–74) | Advanced solid tumours | I | R1507 | All |
| 2 | Tolcher et al., 2009 29 | 53 | 38/15 | 54 (22–84) | Refractory solid tumours | I | Ganitumab | All |
| 3 | Naing et al., 2011 30 | 42 | 19/23 | 53 (20–79) | Advanced cancer | I | CIX and Torisel | All |
| 4 | Molife et al., 2010 25 | 46 | 40/6 | 59.4 (25–79) | Advanced solid tumours | Ib | CP and DOC | All figitumumab related |
| 5 | Karp et al., 2009 22 | 42 | 24/18 | 60.5 (26–80) | Advanced cancer | Ib | CP and PAC and CB | >5% figitumumab related |
| 6 | Olmos et al., 2010 27 | 29 | 21/8 | 30 (12–63) | Sarcoma and Ewing's sarcoma | I | CP | All |
| 7 | Atzori et al., 2011 31 | 80 | 40/40 | 57 (19–81) | Advanced solid tumours | I | Dalotuzumab | All |
| 8 | Weickhardt et al., 2012 9 | 18 | 8/10 | 65 (48–77) | Advanced NSCLC | I/II | CIX and erlotinib | ≥10%, or ≥grade 3 |
| 9 | Quek et al., 2010 32 | 21 | 12/9 | 56 (25–77) | Advanced sarcomas and other solid tumours | I | CP and everolimus | ≥10% |
| 10 | Goto et al., 2011 33 | 19 | 12/7 | 57 (21–74) | Advanced NSCLC | I | CP,CB and PAC | All |
| 11 | Malempati et al., 2011 34 | 47 | 24/23 | 15 (4–28) | Paediatric patients with refractory solid tumours and Ewing sarcoma | I/II | CIX | ≥Grade 2 |
| 12 | Pappo et al., 2011 4 | 115 | 75/40 | 25 (8–78) | Recurrent or refractory Ewing sarcoma | II | R1507 | ≥5% |
| 13 | Ramalingam et al., 2011 11 | 114 | 77/37 | 62.5 | Advanced NSCLC | II | Erlotinib with placebo or R1507 | All |
| 14 | Reidy et al., 2010 10 | 64 | 33/31 | 61 (40–84) | Refractory metastatic colorectal cancer | II | CIX, with or without cetuximab | ≥Grade 2 |
| 15 | Lacy et al., 2008 35 | 47 | 30/17 | 61.3 (42–81) | Multiple myeloma | I | CP | ≥4% |
| Total: | 772 | 476/296 | 50 (4–84) | I/II | ||||
QW:once a week, Q3W:once every three weeks. CIX, cixutumumab; CP, figitumumab; DOC, docetaxel; NSCLC, non-small cell lung cancer; PAC, paclitaxel; CB, carboplatin; Torisel, temsirolimus.
Data analysis
The overall average incidence of every enrolled AE, every grade ≥3 AE with an incidence higher than 0.1%, every dose-limited toxicity (DLT) and death which occurred in the enrolled studies was summarized or calculated. From the AE profile we extracted from all the enrolled studies, we selected AEs with an overall incidence of higher than 1.5% and analyzed them in our review (in total 31 types of AEs).
We individually performed the (continuity adjusted) chi-square test to compare the AE profile differences between three drugs and the whole patient population. In three specific enrolled studies, mAbs were used in a lower age group compared with the other studies. We therefore analyzed the AE incidence differences between the two age groups, also using the (continuity-adjusted) chi-square test. Furthermore, toxicities with combined therapies were compared with those in single mAb therapies. Finally, we discussed the AE characteristics of every mAb.
Results
Clinical material
Fifteen studies satisfied our inclusion criteria and entered our analysis. Every enrolled study was given a study number from 1 to 15. Five different mAbs were included in these studies. The total number of patients treated with different mAbs was 772. The baseline for reported AEs was shown in Table 1.
Merging of synonymous or similar AEs
We noted that some AEs depicted in different words were indeed synonymous and we adopted one of these words in our review. In addition, some terms contained the meaning of some other words, so we selected and used the terms that have a more extensive meaning. Furthermore, some AEs were similar and sometimes they were reported together, but sometimes they were reported separately, depending on the different studies concerning them. We put these AEs together in such circumstance. Table 2 shows all terms that met the three situations discussed above.
Table 2.
Merging of synonymous or similar AEs
| Terms used in this systematic review | Terms found in adverse effects reporting tables of enrolled studies |
|---|---|
| Anaemia | Haemoglobin, anaemia |
| Albumin decreased | Hypoalbuminaemia, albumin decreased |
| Arthralgia | Joint pain, arthralgia |
| Decreased appetite | Anorexia, decreased appetite |
| Dizziness | Ocular flashes of light, dizziness |
| Dyspepsia | Dyspepsia, eructation |
| Elevated AST/ALT | AST-SGOT ALT-SGOT, AST, ALT, elevated AST/ALT |
| Fatigue | Asthenia, fatigue |
| Flushing | Hot flashes/flushes, flushing |
| Hyperuracaemia | Raised uric acid concentration, hyperuracaemia |
| Hypomagnesaemia | Potassium decreased, hypomagnesaemia |
| Infection | Opportunistic infection, infection |
| Leukopenia | Leukocytes, leukopenia |
| Lymphopenia | Lymphocyte count decrease, lymphopenia |
| Muco/stomatitis | Mucositis, stomatitis, mucosal inflammation |
| Musculoskeletal pain | Extremity limb pain, skeletal pain, joint pain, muscle pain |
| Nail changes | Onychoclasis, nail changes |
| Nausea and vomiting | Nausea, vomiting |
| Neuropathy | Neuropathy, peripheral sensory neuropathy |
| Neutropenia | Neutrophils, neutropenia |
| Pain | Chest, back, abdominal or not defined pain, pain |
| Pyrexia | Pyrexia, fever |
| Skin reaction | Rash, dermatitis, pruritis, erythematous, skin reaction |
| Taste alteration | Taste disturbance, taste alteration |
| Thrombocytopenia | Thrombocytopenia, platelets |
| Hyperglycaemia | Blood glucose elevation, type 2 diabetes mellitus, hyperglycaemia |
| Weight decreased | Weight loss, weight decreased |
Overall overview of major toxicities
There were six single agent trials and nine combined-agent trials, of which two trials were case control studies. Toxicities reported by more than 10 of the enrolled studies were hyperglycaemia (15), nausea and vomiting (14), fatigue (13), anorexia (13) and skin reaction (11). Toxicities reported in more than 10.0% of the total patients were fatigue (28.8%), skin reaction (20%), diarrhoea (18.1%), nausea/vomiting (17.6%), hyperglycaemia (14.9%), anorexia (12.5%), muco/stomatitis (12.3%) and thrombocytopenia (10.6%) (Table 3). Grade ≥3 toxicities reported in no less than 2.5% of the patients were (Table 4) fatigue (4.9%), thrombocytopenia (3.6%), neutropenia (2.9%), hyperglycaemia (2.6%) and pain (2.5%). Despite the fact that the definitions of DLTs were not quite accordant in our enrolled studies, all the reported DLTs were summed up and are shown in Table 5. The top three frequently observed DLTs were thrombocytopenia (5), fatigue (4) and acneiform rash (3). Nine deaths were mAb-related in our analysis.
Table 3.
Overall AE profile of all enrolled clinical studies
| AEs | Statistics from individual study (incidence, %) | TOTAL | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1* | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | AEs | Reporting studies | Incidence (%) | |
| Fatigue | 8.6 | 41.5 | 33.3 | 6.5 | 40.0 | 10.3 | 72.2 | 85.7 | 10.6 | 37.4 | 67.5 | 4.7 | 12.8 | 223 | 13 | 28.9 | ||
| Skin reaction | 5.7 | 24.5 | 40.5 | 13.7 | 1.3 | 55.6 | 52.4 | 2.1 | 7.0 | 72.8 | 4.3 | 152 | 11 | 19.7 | ||||
| Diarrhoea | 2.9 | 7.5 | 40.0 | 6.9 | 50.0 | 28.6 | 47.4 | 2.1 | 20.9 | 59.6 | 1.6 | 8.5 | 142 | 12 | 18.4 | |||
| Nausea and vomiting | 2.9 | 17.0 | 26.2 | 6.5 | 6.9 | 1.3 | 27.8 | 66.6 | 26.3 | 6.4 | 31.3 | 31.6 | 6.3 | 8.5 | 134 | 14 | 17.4 | |
| Hyperglycaemia | 2.9 | 9.4 | 71.4 | 4.3 | 4.8 | 17.2 | 18.8 | 16.7 | 61.9 | 5.3 | 10.6 | 19.1 | 5.3 | 4.7 | 6.4 | 116 | 15 | 15.0 |
| Decreased appetite | 5.7 | 13.2 | 33.3 | 6.9 | 1.3 | 27.8 | 42.9 | 73.7 | 6.4 | 20.9 | 11.4 | 1.6 | 95 | 12 | 12.3 | |||
| Muco/stomatitis | 5.7 | 45.2 | 1.3 | 44.4 | 100 | 2.1 | 35.1 | 93 | 7 | 12.0 | ||||||||
| Thrombocytopenia | 8.6 | 24.5 | 47.6 | 2.4 | 52.4 | 84.2 | 8.5 | 10.4 | 1.6 | 8.5 | 85 | 10 | 11.0 | |||||
| Anaemia | 2.9 | 11.3 | 6.9 | 52.4 | 68.4 | 21.3 | 15.7 | 6.1 | 1.6 | 14.9 | 76 | 10 | 9.8 | |||||
| Pain | 2.9 | 6.9 | 1.3 | 2.1 | 48.7 | 6.1 | 68 | 6 | 8.8 | |||||||||
| Elevated AST/ALT | 8.6 | 16.7 | 10.9 | 6.9 | 61.9 | 17.0 | 12.2 | 14.9 | 59 | 8 | 7.6 | |||||||
| Musculoskeletal pain | 2.9 | 15.1 | 9.5 | 6.9 | 2.5 | 52.4 | 25.2 | 1.6 | 58 | 8 | 7.5 | |||||||
| Hypertriglyceridaemia | 71.4 | 52.4 | 2.1% | 42 | 3 | 5.4 | ||||||||||||
| Neutropenia | 21.4 | 28.6 | 94.7 | 8.5 | 6.1 | 40 | 5 | 5.2 | ||||||||||
| Constipation | 11.1 | 18.3 | 13.2 | 3.1 | 40 | 4 | 5.2 | |||||||||||
| Headache | 2.9 | 10.3 | 19.0 | 2.1 | 22.6 | 4.7 | 38 | 6 | 4.9 | |||||||||
| Cough | 13.0 | 15.8 | 33 | 2 | 4.3 | |||||||||||||
| Pyrexia | 15.1 | 1.3 | 2.1 | 18.3 | 1.6 | 31 | 4 | 4.0 | ||||||||||
| Hypercholesterolaemia | 59.5 | 19.0 | 29 | 2 | 3.8 | |||||||||||||
| Dyspnoea | 15.7 | 7.9 | 27 | 2 | 3.5 | |||||||||||||
| Leukopenia | 28.6 | 94.7 | 4.3 | 26 | 3 | 3.4 | ||||||||||||
| Weight decreased | 2.9 | 6.9 | 23.8 | 6.4 | 11.3 | 24 | 5 | 3.1 | ||||||||||
| Muscle spasm | 6.5 | 6.9 | 11.1 | 13.2 | 4.3 | 24 | 5 | 3.1 | ||||||||||
| Dehydration | 2.9 | 11.1 | 4.3 | 10.5 | 17 | 4 | 2.2 | |||||||||||
| Creatinine elevation | 16.7 | 6.1 | 4.3 | 16 | 3 | 2.1 | ||||||||||||
| Neuropathy | 84.2 | 16 | 1 | 2.1 | ||||||||||||||
| Hypophosphataemia | 2.9 | 14.3 | 4.3 | 7.0 | 14 | 4 | 1.8 | |||||||||||
| Elevated ALK phosphatase | 2.9 | 2.1 | 9.6 | 14 | 3 | 1.8 | ||||||||||||
| Muscular weakness | 2.9 | 7.5 | 5.2 | 13 | 3 | 1.7 | ||||||||||||
| Dyspepsia | 2.9 | 9.6 | 12 | 2 | 1.6 | |||||||||||||
| Paronychia | 10.5 | 12 | 1 | 1.6 | ||||||||||||||
Study number.
Table 4.
All the grade ≥3 AEs (counted in numbers)
| AEs | Statistics from individual study (number of patients who experienced this AE) | TOTAL | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1* | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | Reporting Studies | Number | Incidence | |
| Number of patients | 35 | 53 | 42 | 46 | 42 | 29 | 80 | 18 | 21 | 19 | 47 | 115 | 114 | 64 | 47 | 772 | ||
| Fatigue | 1 | 2 | 1 | 1 | 4 | 2 | 6 | 19 | 1 | 1 | 10 | 38 | 4.9 | |||||
| Thrombocytopenia | 8 | 3 | 1 | 4 | 2 | 8 | 1 | 7 | 27 | 3.5 | ||||||||
| Neutropenia | 4 | 16 | 2 | 3 | 22 | 2.8 | ||||||||||||
| Hyperglycaemia | 4 | 2 | 1 | 1 | 1 | 1 | 3 | 6 | 1 | 1 | 10 | 21 | 2.7 | |||||
| Anaemia | 2 | 4 | 9 | 2 | 1 | 1 | 6 | 19 | 2.5 | |||||||||
| Pain | 1 | 1 | 17 | 3 | 19 | 2.5 | ||||||||||||
| Nausea and vomiting | 1 | 1 | 3 | 1 | 1 | 8 | 6 | 15 | 1.9 | |||||||||
| Diarrhoea | 1 | 1 | 1 | 1 | 2 | 7 | 6 | 13 | 1.7 | |||||||||
| Elevated AST/ALT | 3 | 2 | 2 | 2 | 3 | 1 | 6 | 13 | 1.7 | |||||||||
| Decreased appetite | 1 | 2 | 1 | 7 | 1 | 5 | 12 | 1.6 | ||||||||||
| Skin reaction | 3 | 8 | 2 | 11 | 1.4 | |||||||||||||
| Dyspnoea | 2 | 6 | 2 | 8 | 1.0 | |||||||||||||
| Deep venous thrombosis | 1 | 5 | 2 | 6 | 0.8 | |||||||||||||
| Hypophosphataemia | 2 | 1 | 1 | 3 | 4 | 0.5 | ||||||||||||
| Muco/tomatitis | 2 | 2 | 2 | 4 | 0.5 | |||||||||||||
| Constipation | 4 | 1 | 4 | 0.5 | ||||||||||||||
| Potassium decreased | 4 | 1 | 4 | 0.5 | ||||||||||||||
| Leukocytes | 4 | 1 | 4 | 0.5 | ||||||||||||||
| Dehydration | 1 | 2 | 2 | 3 | 0.4 | |||||||||||||
| Hyperuricaemia | 1 | 2 | 2 | 3 | 0.4 | |||||||||||||
| Elevated ALK phosphatase | 1 | 2 | 2 | 3 | 0.4 | |||||||||||||
| Hyponatraemia | 3 | 1 | 3 | 0.4 | ||||||||||||||
| Musculoskeletal pain | 1 | 1 | 2 | 2 | 0.3 | |||||||||||||
| Elevated γGT | 1 | 1 | 2 | 2 | 0.3 | |||||||||||||
| Cough | 2 | 1 | 2 | 0.3 | ||||||||||||||
| Dyspepsia | 2 | 1 | 2 | 0.3 | ||||||||||||||
| Bilirubin elevation | 2 | 1 | 2 | 0.3 | ||||||||||||||
Study number.
Table 5.
Number of DLTs
| AEs | Statistics from individual study (DLT number) | Tatal number | |||||
|---|---|---|---|---|---|---|---|
| 2† (Ganitumab) ‡ | 3 (CIX and Torisel) | 7 (dalotuzumab) | 8 (Erlotinib and CIX) | 10 (CP and CB and PAC) | 11 (CIX) | ||
| Thrombocytopenia | 2 | 1 | 1 | 1 | 5 | ||
| Fatigue | 4 | 4 | |||||
| Acneiform rash | 3 | 3 | |||||
| Febrile neutropenia | 1 | 1 | |||||
| Leukocytoclastic vasculitis | 1 | 1 | |||||
| Hyperuricaemia | *1 | 1 | |||||
| Hypermagnesaemia | *1 | 1 | |||||
| Hyponatraemia | *1 | 1 | |||||
| Hyperkalaemia | *1 | 1 | |||||
| Dehydration | 1 | 1 | |||||
DLT observed in the same patient;
study number;
drugs. CB, carboplatin; CIX, cixutumumab; CP, figitumumab; DLT, dose-limited toxicities; PAC, paclitaxel; Torisel, temsirolimus.
The differences of AE incidence in the two age groups
In three of our enrolled studies, mAbs were used in some prepubertal teenage patients (the tumour types were mainly sarcoma). We compared the average AE incidence differences between the lower age group which was composed of the above three studies and the higher age group which included the rest of the 12 studies, using the (continuity-adjusted) chi-square test. Results were shown in Table 6. Of the total 31 types of AEs, 12 AE incidences were significantly (P < 0.05) higher in the lower age group, while six AE incidences were significantly (P < 0.05) lower in the same group. The AE profile was obviously better in the higher age group, indicating that anti–IGF-1R mAbs might cause more serious side effects in prepubertal teenage patients. Such side effects included musculoskeletal pain, headache and pyrexia.
Table 6.
The differences in AE-incidences in the two age groups
| Median (range) age (years) | Lower age group incidence | Number of AEs | Higher age group incidences | Number of AEs | X2 | P |
|---|---|---|---|---|---|---|
| 23 (4–78) | 58 (18–84) | |||||
| Hyperglycaemia | 16.8 | 32 | 14.5 | 84 | 0.5935 | 0.4411 |
| Nausea and vomiting | 21.5 | 41 | 16.0 | 93 | 2.9864 | 0.0840 |
| Fatigue | 26.7 | 51 | 29.6 | 172 | 0.5895 | 0.4426 |
| Anorexia | 15.2 | 29 | 11.4 | 66 | 1.9473 | 0.1629 |
| Diarrhoea | 14.1 | 27 | 19.8 | 115 | 3.0649 | 0.0800 |
| Skin reaction | 6.8 | 13 | 23.9 | 139 | 26.6377 | 0.0000 |
| Anaemia | 15.7 | 30 | 7.9 | 46 | 9.8269 | 0.0017 |
| Thrombocytopenia | 8.4 | 16 | 11.9 | 69 | 1.7963 | 0.1802 |
| Musculoskeletal pain | 16.2 | 31 | 4.6 | 27 | 27.7561 | 0.0000 |
| Elevated AST/ALT | 12.6 | 24 | 6.0 | 35 | 8.7140 | 0.0032 |
| Pain | 30.9 | 59 | 1.5 | 9 | 154.0618 | 0.0000 |
| Muco/stomatitis | 0.5 | 1 | 15.8 | 92 | 31.8048 | 0.0000 |
| Headache | 15.7 | 30 | 1.4 | 8 | 63.0713 | 0.0000 |
| Weight decreased | 9.4 | 18 | 1.0 | 6 | 33.6032 | 0.0000 |
| Neutropenia | 5.8 | 11 | 5.0 | 29 | 0.1725 | 0.6779 |
| Muscle spasm | 1.0 | 2 | 3.8 | 22 | 3.5813 | 0.0584 |
| Dehydration | 1.0 | 2 | 2.6 | 15 | 0.9401 | 0.3322 |
| Pyrexia | 11.5 | 22 | 1.5 | 9 | 37.0658 | 0.0000 |
| Constipation | 11.0 | 21 | 3.3 | 19 | 17.4583 | 0.0000 |
| Hypophosphataemia | 5.2 | 10 | 0.7 | 4 | 14.2358 | 0.0002 |
| Muscular weakness | 3.1 | 6 | 1.2 | 7 | 2.1914 | 0.1388 |
| Dyspnoea | 9.4 | 18 | 1.5 | 9 | 26.4126 | 0.0000 |
| Hypertriglyceridaemia | 0.5 | 1 | 7.1 | 41 | 11.9265 | 0.0006 |
| Elevated ALK phoshatase | 6.3 | 12 | 0.3 | 2 | 25.2321 | 0.0000 |
| Leukopenia | 1.0 | 2 | 4.1 | 24 | 4.2001 | 0.0404 |
| Creatinine elevation | 3.7 | 7 | 1.5 | 9 | 2.2139 | 0.1368 |
| Hypercholesterolaemia | 0.0 | 0 | 5.0 | 29 | 9.9057 | 0.0016 |
| Cough | 7.9 | 15 | 3.1 | 18 | 7.9437 | 0.0048 |
| Dyspepsia | 0.0 | 0 | 2.1 | 12 | 2.7711 | 0.0960 |
| Neuropathy | 0.0 | 0 | 2.8 | 16 | 4.1000 | 0.0429 |
| Paronychia | 0.0 | 0 | 2.1 | 12 | 2.7711 | 0.0960 |
Toxicities of mAbs used as a single agent or combined with chemotherapy (inhibitors of epidermal growth factor receptor (EGFR)) and cytotoxic chemotherapy)
For figitumumab, it was used as a single agent in two enrolled studies, while it was used combined with carboplatin & paclitaxel in other two studies. We analyzed the differences in the AE profile in the two groups. Results showed that most of the AEs in the single-agent group had significantly lower incidences than in the combined-agent group (13 of 19 AEs, P < 0.05) (Table 7). Surprisingly, the incidence of elevated AST/ALT was higher in the single-agent group. Furthermore, it was reported that most patients treated with figitumumab in single agent studies did not develop severe hyperglycaemia 7,8. In our review, as was shown in Table 4, there were in total three patients (2.34%) who experienced grade ≥3 hyperglycaemia in the combined-agent group, while only one patient (1.32%) experienced grade ≥3 hyperglycaemia in the single-agent group (difference not significant, P = 0.99).
Table 7.
Comparation of AE-incidences between combination groups and single agent groups of figitumumab
| AEs with a higher incidence in the combination group | A* (in total 61 patients) | % | B (in total 76 patients) | % | X2 | P |
|---|---|---|---|---|---|---|
| Decreased appetite | 14 | 0.23 | 2 | 0.03 | 13.5448 | 0.0002 |
| Diarrhoea | 22 | 0.36 | 6 | 0.08 | 16.5150 | 0.0000 |
| Thrombocytopenia | 17 | 0.28 | 4 | 0.05 | 13.3236 | 0.0003 |
| Neutropenia | 18 | 0.30 | 0 | 0.00 | 25.8184 | 0.0000 |
| Leukocytes | 18 | 0.30 | 0 | 0.00 | 25.8184 | 0.0000 |
| Neuropathy | 16 | 0.26 | 0 | 0.00 | 22.5704 | 0.0000 |
| AEs with a higher incidence in the single-agent group | ||||||
| Elevated AST/ALT | 0 | 0.00 | 9 | 0.12 | 5.9226 | 0.0149 |
| AEs with a similar incidence in the two groups | ||||||
| Hyperglycaemia | 3 | 0.05 | 8 | 0.11 | 0.7819 | 0.3766 |
| Nausea and vomiting | 5 | 0.08 | 6 | 0.08 | 0.0633 | 0.8013 |
| Fatigue | 13 | 0.21 | 9 | 0.12 | 2.2511 | 0.1335 |
| Skin reaction | 0 | 0.00 | 6 | 0.08 | 3.3276 | 0.0681 |
| Anaemia | 13 | 0.21 | 9 | 0.12 | 2.2511 | 0.1335 |
| Musculoskeletal pain | 4 | 0.07 | 2 | 0.03 | 0.4843 | 0.4865 |
| Pain | 0 | 0.00 | 2 | 0.03 | 0.3133 | 0.5757 |
| Headache | 0 | 0.00 | 3 | 0.04 | 0.9637 | 0.3262 |
| Weight decreased | 0 | 0.00 | 2 | 0.03 | 1.6290 | 0.2018 |
| Muscle spasm | 0 | 0.00 | 4 | 0.05 | 3.3071 | 0.0690 |
| Creatinine elevation | 0 | 0.00 | 2 | 0.03 | 0.3133 | 0.5757 |
A, number of AEs in combination group; B, number of AEs in single agent group.
In study 8, the combination of erlotinib and cixutumumab had a relatively high level of EGFR-related side effects including acneiform rash and diarrhoea. The combined-agent patient group also showed a significant association with grades 3 and 4 fatigue 9. Neither the randomized trial of cixutumumab in combination with the EGFR mAb cetuximab (Imclone) nor the randomized trial of R1507 with erlotinib reported any significantly higher increased incidence of rash or fatigue 10,11.
Analysis on each drug
There were five anti-IGF-1R monoclonal antibodies enrolled in our review, namely R1507, cixutumumab (IMC-A12), figitumumab (CP-751 871), dalotuzumab (MK-0646) and ganitumab (AMG-479).
R1507
There were three studies in our analysis concerning this drug. This mAb seemed to show a worse AE profile than cixutumumab and figitumumab. The top three frequently observed AEs for this drug were fatigue (46.6%), diarrhoea (35.2%) and skin reaction (35.2%). Compared with the whole patient population, patients who used this drug reported 16 kinds of AEs that showed significantly (P < 0.05) higher incidence. (Table 8). Most of them also showed significantly (P < 0.05) higher incidences than for the figitumumab and cixutumumab groups (including all patients using figitumumab or cixutumumab), except muco/stomatitis dehydration and muscle spasm. Meanwhile, the incidence of hyperglycaemia, thrombocytopenia, neutropenia, hypertriglyceridaemia, leukocytes, hypercholesterolaemia and neuropathy were all significantly (P < 0.05) lower than the overall incidence in the whole population (thrombocytopenia, neutropenia and hypertriglyceridaemia were also significantly lower than in the figitumumab and cixutumumab groups). Other AEs which showed no significant differences are also shown in Table 8.
Table 8.
Comparison of AE-incidences between specific mAbs and the whole patient population
| Incidence for specific mAb | Number of AEs | Incidence in whole population | Total number of AEs | X2 | P | |
|---|---|---|---|---|---|---|
| AEs with lower incidences in mAb groups | ||||||
| R1507 | ||||||
| Hyperglycaemia | 11.0 | 29 | 15.0 | 116 | 16.9961 | 0.0000 |
| Thrombocytopenia | 5.7 | 15 | 11.0 | 85 | 13.4768 | 0.0002 |
| Neutropenia | 2.7 | 7 | 5.2 | 40 | 4.4013 | 0.0359 |
| Hypertriglyceridaemia | 0.0 | 0 | 5.4 | 42 | 16.1373 | 0.0001 |
| Leukocytes | 0.0 | 0 | 3.4 | 26 | 9.1201 | 0.0025 |
| Hypercholesterolaemia | 0.0 | 0 | 3.8 | 29 | 10.7523 | 0.0010 |
| Neuropathy | 0.0 | 0 | 2.1 | 16 | 4.4628 | 0.0346 |
| Cixutumumab | ||||||
| Fatigue | 20.5 | 35 | 28.9 | 223 | 4.9917 | 0.0255 |
| Diarrhoea | 6.4 | 11 | 18.4 | 142 | 14.7348 | 0.0001 |
| Musculoskeletal pain | 0.6 | 1 | 7.5 | 58 | 11.4566 | 0.0007 |
| Pain | 0.6 | 1 | 8.8 | 68 | 13.9597 | 0.0002 |
| Constipation | 2.3 | 4 | 5.2 | 40 | 4.2062 | 0.0403 |
| Pyrexia | 1.2 | 2 | 4.0 | 31 | 4.3009 | 0.0381 |
| Cough | 0.0 | 0 | 4.3 | 33 | 8.5063 | 0.0035 |
| Figitumumab | ||||||
| Hyperglycaemia | 12.7 | 26 | 15.0 | 116 | 16.2414 | 0.0001 |
| Fatigue | 21.1 | 43 | 28.9 | 223 | 4.9612 | 0.0259 |
| Skin reaction | 8.3 | 17 | 19.7 | 152 | 14.5335 | 0.0001 |
| Pain | 1.0 | 2 | 8.8 | 68 | 14.8512 | 0.0001 |
| Muco/stomatitis | 10.3 | 21 | 12.0 | 93 | 10.2296 | 0.0014 |
| Constipation | 0.0 | 0 | 5.2 | 40 | 12.2793 | 0.0005 |
| Pyrexia | 0.0 | 0 | 4.0 | 31 | 9.2086 | 0.0024 |
| Cough | 0.0 | 0 | 4.3 | 33 | 9.8750 | 0.0017 |
| AEs with higher incidences in mAb groups | ||||||
| R1507 | ||||||
| Nausea and vomiting | 27.7 | 73 | 17.4 | 134 | 13.0384 | 0.0003 |
| Fatigue | 46.6 | 123 | 28.9 | 223 | 27.7232 | 0.0000 |
| Diarrhoea | 35.2 | 93 | 18.4 | 142 | 31.7855 | 0.0000 |
| Skin reaction | 35.2 | 93 | 19.7 | 152 | 26.3049 | 0.0000 |
| Pain | 24.2 | 64 | 8.8 | 68 | 42.1505 | 0.0000 |
| Muco/stomatitis | 15.2 | 40 | 12.0 | 93 | 10.7145 | 0.0011 |
| Headache | 10.2 | 27 | 4.9 | 38 | 10.5165 | 0.0012 |
| Constipation | 13.6 | 36 | 5.2 | 40 | 21.4960 | 0.0000 |
| Muscle spasm | 5.7 | 15 | 3.1 | 24 | 4.0809 | 0.0434 |
| Pyrexia | 8.0 | 21 | 4.0 | 31 | 7.1672 | 0.0074 |
| Dyspnoea | 10.2 | 27 | 3.5 | 27 | 18.0338 | 0.0000 |
| Cough | 12.5 | 33 | 4.3 | 33 | 22.6890 | 0.0000 |
| Dyspepsia | 4.5 | 12 | 0.6 | 5 | 16.1279 | 0.0001 |
| Elevated ALK phosphatase | 4.5 | 12 | 1.8 | 14 | 6.1119 | 0.0134 |
| Dehydration | 4.9 | 13 | 2.2 | 17 | 5.3829 | 0.0203 |
| Paronychia | 4.5 | 12 | 1.6 | 12 | 7.8280 | 0.0051 |
| Cixutumumab | ||||||
| Hyperglycaemia | 24.0 | 41 | 15.0 | 116 | 23.3586 | 0.0000 |
| Thrombocytopenia | 14.6 | 25 | 11.0 | 85 | 10.0313 | 0.0015 |
| Elevated AST/ALT | 8.8 | 15 | 7.6 | 59 | 4.1693 | 0.0412 |
| Muco/stomatitis | 16.4 | 28 | 12.0 | 93 | 12.2882 | 0.0005 |
| Hypertriglyceridaemia | 18.1 | 31 | 5.4 | 42 | 32.1086 | 0.0000 |
| Hypercholesterolaemia | 14.6 | 25 | 3.8 | 29 | 30.5818 | 0.0000 |
| Figitumumab | ||||||
| Anaemia | 16.2 | 33 | 9.8 | 76 | 6.5211 | 0.0107 |
| Thrombocytopenia | 15.7 | 32 | 11.0 | 85 | 11.1615 | 0.0008 |
| Elevated AST/ALT | 13.2 | 27 | 7.6 | 59 | 9.6979 | 0.0018 |
| Neutropenia | 11.8 | 24 | 5.2 | 40 | 12.6551 | 0.0004 |
| Leukocytes | 11.8 | 24 | 3.4 | 26 | 23.4071 | 0.0000 |
| Neuropathy | 7.8 | 16 | 2.1 | 16 | 16.9329 | 0.0000 |
Cixutumumab
There were four studies in our analysis concerning this drug. Its AEs profile seemed to be better than R1507 and figitumumab as shown in Table 8. The top three frequently observed AEs for this drug were hyperglycaemia (24.0%), fatigue (20.5%) and hypertriglyceridaemia (18.6%). Comparing with the overall incidence of the whole population, six kinds of AEs showed significantly (P < 0.05) higher incidences (Table 8). These AEs were mainly laboratory abnormalities (hyperglycaemia, hypertriglyceridaemia, hypercholesterolaemia, elevated AST/ALT, muco/stomatitis and thrombocytopenia), most of which also showed a significantly (P < 0.05) higher incidences than the figitumumab and R1507 groups, except for thrombocytopenia, elevated AST/ALT and muco/stomatitis. Seven kinds of AEs showed significantly (P < 0.05) lower incidences than the whole population, including musculoskeletal pain, which also maintained a lower incidence than in the figitumumab and R1507 groups.
Dalotuzumab
There was only one study concerning this drug in our analysis. From the single study; we observed that every AE incidence was below 2.5% except hyperglycaemia (18.8%) but there was inadequate evidence to affirm that this drug had a better AE profile than the other drugs.
Figitumumab
The highest number of studies in our review was concerning this drug (six studies). This is the only fully human IgG2 antibody (the others have a IgG1 backbone) in our review and has the longest t1/2 of approximately 20 days 12. The top three frequently observed AEs for this drug were fatigue (21.1%), diarrhoea (16.7%) and anaemia (16.2%). Six kinds of AEs showed significantly (P < 0.05) higher incidences than the whole population, most of which also showed significantly (P < 0.05) higher incidences than the R1507 and cixutumumab groups (Table 6), except thrombocytopenia and neutropenia. Eight kinds of AEs showed significantly (P < 0.05) lower incidences than in the whole population, including skin reaction, which also showed a significantly (P < 0.05) lower incidence than in the R1507 and cixutumumab groups.
Ganitumab
There was also only one study concerning this drug in our analysis. In the single study, the top three frequently observed AEs for this drug were fatigue (41.5%), thrombocytopenia (24.5%) and skin reaction (24.5%).
Discussion
The identification of specific AEs related to anti-IGF-1R mAbs
Of all the AEs reported in clinical studies, many were often related to the IGF-1R mAbs. Still it was significant to identify specific AEs related to anti-IGF-1R mAbs. To overcome this problem, in this review we tried to focus our attention on a limited number of AEs with high frequencies and may relate to the mechanism of action of the mAbs. Four important AEs that may relate to mAbs were discussed in the following context. From the whole AEs profile we extracted from all the enrolled studies, we selected AEs with an overall incidence of higher than 1.5% and discussed them in our review (in total 31 kinds of AEs).
The characteristics of some important AEs related to anti-IGF-1R mAbs and possible mechanism/explanation of hyperglycaemia and hypoglycaemia
Hyperglycaemia and hypoglycaemia
The highest incidence of hyperglycaemia was reported in study 3 (cixutumumab and temsirolimus) as 71.4%. Patients with previous glucose intolerance or treated with concomitant steroids were more susceptible to developing hyperglycaemia 12. The overall incidence of hyperglycaemia in our analysis was 14.4%, frequently observed but lower than the data reported by two former reviews (25% 13, 20% 12). It was reported in a single study that approximately 50% of patients showed abnormal glucose tolerance throughout that trial 14, so hyperglycaemia was an obviously common and mechanism-based toxicity. On the other hand, it appears usually to be easily controllable with metformin and other anti-diabetic agents. Interestingly, in a former review 12, it was reported that the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) was not suited for evaluating toxicities of targeted drugs, because it considered a serum glucose concentration above 250 mg dl–1 as grade 3, which might result in defining it as a dose-limiting toxicity while being clinically insignificant. The authors declared that the NCI-CTCAE should be reviewed or specific guidelines for the management of tolerable, mechanism-based toxicities should be developed.
The mechanism of hyperglycaemia is still unclear. There was a theory indicating that the cross reactivity of the anti-IGF-1R mAbs with the insulin receptor (IR) leads to hyperglycaemia, but it is not convincing enough because there is strong evidence to show that these mAbs can all act to spare the IR. Furthermore, although some antibodies do target, IR/IGF-1R hybrids', these hybrid receptors do not play a predominant role in the regulation of glycaemia, and they are more sensitive to IGFs than to insulin 15–17. More complicated mechanisms (shown in Figure 1) to explain hyperglycaemia are as follows: besides tumour cells, IGF-IR monoclonal antibodies also act on normal tissues. Most of all, IGF-IRs in the hypothalamic-pituitary axis that is involved in homeostatic feedback control are also targeted. This reduces the feedback inhibition of growth hormone secretion, thus lead to elevation in growth hormone (GH). Elevation in GH can cause insulin resistance in classic insulin target organs, increased gluconeogenesis, and thus leading to elevations in glucose concentrations. This in turn results in increased insulin secretion which commonly corrects hyperglycemia to some extent. Furthermore, the elevated GH concentrations stimulate increased IGF-I production by the liver, accounting for the observed IGF-I elevations in the circulation 15,18.
Figure 1.
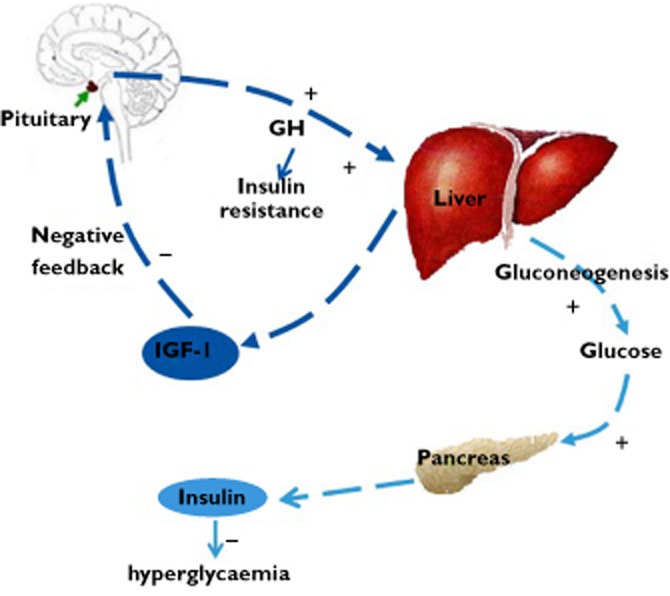
Mechanisms of hyperglycaemia. IGF-IR mAbs also act on normal tissues besides tumour cells. They block IGF-IRs that control the homeostasis of the GH-IGF-I axis as well. This reduces the feedback inhibition of GH secretion, which in turn leads to elevation in GH. Elevation in GH can lead to insulin resistance in classic insulin target organs, and increased gluconeogenesis and subsequent elevation in glucose. Glucose elevation in turn cause increased insulin secretion, which commonly corrects hyperglycaemia. Furthermore, the elevated GH concentrations stimulate increased IGF-I production by the liver, leading to IGF-I elevation in the circulation. IGF-IR, insulin-like growth factor type I receptor; GH, growth hormone; IR, insulin receptor
Finally, besides hyperglycaemia, hypoglycaemia was also observed in another clinical trial, although it was not severe and no treatment was required 19. We speculated that hypoglycaemia might be caused by elevated IGF-I concentrations in the circulation, since it was reported that IGF-I could reduce hepatic glucose production and increases peripheral glucose uptake 20,21. Moreover, the inverse effect of elevated GH mentioned above is not sufficient to reverse the effect of IGF-1 elevation.
Thrombocytopenia
In study 9 (Figitumumab & Everolimus), thrombocytopenia was mentioned as the most commonly observed reason for treatment delay. In the present review, thrombocytopenia was rare when figitumumab was given as single agent (0% and 8.5%), as similarly described in study 5 22. This is important because haematological toxicity has been shown to be dose limiting for anti-IGF-1R antibodies with an IgG1 backbone 23. Figitumumab is a fully human IgG2 antibody and, consequently, is expected to be a poor stimulator of antibody mediated cytotoxicity and complement fixation 24, so the haematological toxicities related to this drug were supposed to be lower than the other IgG1 backbone mAbs. In our review, the overall incidence of thrombocytopenia in patients who received figitumumab was 15.7%, higher than patients who received R1507 (5.7%, P = 0.00), cixutumumab (14.6%, P = 0.77), dalotuzumab (single study, 0%) and the whole patient population (11.0%, P = 0.07). Only the incidence for ganitumab (single study, 24.5%, P = 0.13) was higher, but possibly this result might also indicate that obviously more haematological toxicities arose when figitumumab is combined with chemotherapies.
Neutropenia
It was reported in study 6 (figitumumab) that there was no apparent effect of figitumumab on the frequency or severity of observed neutropenia, as with other mAbs like dalotuzumab and ganitumab. The authors concluded that neutropenia did not seem to be significantly worsened when combined with chemotherapy 25,26. However, in our analysis, when figitumumab was combined with carboplatin and paclitaxel, the incidence of neutropenia was significantly higher than in the single figitumumab group (29.5% vs. 0%, P = 0.00) as is shown in Table 7. Besides, the suspended study of a phase III trial of carboplatin and paclitaxel with or without figitumumab also revealed that there were more life-threatening AEs in patients who received figitumumab combined with chemotherapy. So whether and how figitumumab should be combined with chemotherapy is somehow worth discussing. More information about inherent side effects of combined chemotherapies, clinical efficiency of mAbs and biomarkers should be included in this discussion, too.
Cardiotoxicity
Interestingly, despite of the expression of IGF-1R in vascular smooth muscle and endothelial cells, and potential cardiotoxicity associated with anti-IGF-1R mAbs, no cardiac toxicities were reported in any of the enrolled studies. Even in the case of sarcoma patients treated with figitumumab in study 6, three quarters of the patients were pretreated with anthracyclines but none developed cardiotoxicity 27. However, cardiotoxicities were reported to be more frequent when figitumumab was in combination with carboplatin and paclitaxel compared with carboplatin and paclitaxel in patients with non-small cell lung cancer (NSCLC) 1,28, although the trial was suspended as mentioned above.
Conclusion
Differences in AE incidences exist between individual anti-IGF-1R mAbs, as well as between the two age groups studied, and combination with chemotherapies seem to cause more AEs. These data suggest that when using this class of drugs, some more frequently observed AEs for specific mAbs should be paid adequate attention, and it is essential to choose the proper drug and combination with chemotherapies to reduce the occurrence of AEs. Furthermore, prepubertal patients who receive these mAbs possibly need more prudent medical care.
The potential insufficiencies of this systematic review
The number of the enrolled studies was not quite sufficient and we only searched one database, namely Pubmed for our analysis. In some enrolled studies, specific mAbs were combined with chemotherapies, and some of these studies selected the mAb-related toxicities while the others did not. Some toxicities not related to mAbs might thus be extracted from the latter studies.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and we declare this work was supported by Natural Science Foundation of China (81141100), Provincial Science and Technology Development Planning of Shandong (2011GGH21819) and the Natural Science Foundation of Shandong Province of China (ZR2011HM077). There are no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and there are also no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Gombos A, Metzger-Filho O, Dal Lago L, Awada-Hussein A. Clinical development of insulin-like growth factor receptor—1 (IGF-1R) inhibitors: at the crossroad? Invest New Drugs. 2012;30:2433–2442. doi: 10.1007/s10637-012-9811-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKian KP, Haluska P. Cixutumumab. Expert Opin Investig Drugs. 2009;18:1025–1033. doi: 10.1517/13543780903055049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jassem J, Langer CM, Karp DD, Mok T, Benner RJ, Green SJ. Randomized, open label, phase III trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with non-small cell lung cancer. J Clin Oncol. 2010;28:7500. doi: 10.1200/JCO.2013.54.4932. (Suppl.) abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pappo AS, Patel SR, Crowley J, Reinke DK, Kuenkele KP, Chawla SP, Toner GC, Maki RG, Meyers PA, Chugh R, Ganjoo KN, Schuetze SM, Juergens H, Leahy MG, Geoerger B, Benjamin RS, Helman LJ, Baker LH. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory ewing sarcoma family of tumors: results of a phase II sarcoma alliance for research through collaboration study. J Clin Oncol. 2011;29:4541–4547. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yee D. Insulin-like growth factor receptor inhibitors: baby or the bathwater? J Natl Cancer Inst. 2012;104:975–981. doi: 10.1093/jnci/djs258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olmos D, Tan DS, Jones RL, Judson IR. Biological rationale and current clinical experience with anti-insulin-like growth factor 1 receptor monoclonal antibodies in treating sarcoma: twenty years from the bench to the bedside. Cancer J. 2010;16:183–194. doi: 10.1097/PPO.0b013e3181dbebf9. [DOI] [PubMed] [Google Scholar]
- 7.Karp DD, Paz-Ares LG, Novello S, Haluska P, Garland L, Cardenal F, Blakely LJ, Eisenberg PD, Langer CJ, Blumenschein G, Jr, Johnson FM, Green S, Gualberto A. Phase II study of the anti-insulin-like growth factor type 1 receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol. 2009;27:2516–2522. doi: 10.1200/JCO.2008.19.9331. [DOI] [PubMed] [Google Scholar]
- 8.Pollak M, Eisenberg P, Karp D, Cohen R, Kreisman H, Adjei A, Langer C, Melvin C, Yin D, Gualberto A. Safety and tolerability of the anti-IGF-I receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in patients with advanced solid tumors. 2007. Proceedings of the American Association for Cancer Research. 2007 (1_Annual_Meeting):LB-343.
- 9.Weickhardt A, Doebele R, Oton A, Lettieri J, Maxson D, Reynolds M, Brown A, Jackson MK, Dy G, Adjei A, Fetterly G, Lu X, Franklin W, Varella-Garcia M, Hirsch FR, Wynes MW, Youssoufian H, Camidge DR. A phase I/II study of erlotinib in combination with the anti-insulin-like growth factor-1 receptor monoclonal antibody IMC-A12 (cixutumumab) in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2012;7:419–426. doi: 10.1097/JTO.0b013e31823c5b11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reidy DL, Vakiani E, Fakih MG, Saif MW, Hecht JR, Goodman-Davis N, Hollywood E, Shia J, Schwartz J, Chandrawansa K, Dontabhaktuni A, Youssoufian H, Solit DB, Saltz LB. Randomized, phase II study of the insulin-like growth factor-1 receptor inhibitor IMC-A12, with or without cetuximab, in patients with cetuximab-or panitumumab-refractory metastatic colorectal cancer. J Clin Oncol. 2010;28:4240–4246. doi: 10.1200/JCO.2010.30.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramalingam SS, Spigel DR, Chen D, Steins MB, Engelman JA, Schneider CP, Novello S, Eberhardt WE, Crino L, Habben K, Liu L, Janne PA, Brownstein CM, Reck M. Randomized phase II study of erlotinib in combination with placebo or R1507, a monoclonal antibody to insulin-like growth factor-1 receptor, for advanced-stage non-small-cell lung cancer. J Clin Oncol. 2011;29:4574–4580. doi: 10.1200/JCO.2011.36.6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodon J, DeSantos V, Ferry RJ, Jr, Kurzrock R. Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: lessons from the first clinical trials. Mol Cancer Ther. 2008;7:2575–2588. doi: 10.1158/1535-7163.MCT-08-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene. 2009;28:3009–3021. doi: 10.1038/onc.2009.172. [DOI] [PubMed] [Google Scholar]
- 14.Kurzrock R, Patnaik A, Aisner J, Warren T, Leong S, Benjamin R, Eckhardt SG, Eid JE, Greig G, Habben K, McCarthy CD, Gore L. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res. 2010;16:2458–2465. doi: 10.1158/1078-0432.CCR-09-3220. [DOI] [PubMed] [Google Scholar]
- 15.Gualberto A, Pollak M. Clinical development of inhibitors of the insulin-like growth factor receptor in oncology. Curr Drug Targets. 2009;10:923–936. doi: 10.2174/138945009789577945. [DOI] [PubMed] [Google Scholar]
- 16.Belfiore A. The role of insulin receptor isoforms and hybrid insulin/IGF-I receptors in human cancer. Curr Pharm Des. 2007;13:671–686. doi: 10.2174/138161207780249173. [DOI] [PubMed] [Google Scholar]
- 17.Benyoucef S, Surinya KH, Hadaschik D, Siddle K. Characterization of insulin/IGF hybrid receptors: contributions of the insulin receptor L2 and Fn1 domains and the alternatively spliced exon 11 sequence to ligand binding and receptor activation. Biochem J. 2007;403(Pt 3):603–613. doi: 10.1042/BJ20061709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.del Rincon JP, Iida K, Gaylinn BD, McCurdy CE, Leitner JW, Barbour LA, Kopchick JJ, Friedman JE, Draznin B, Thorner MO. Growth hormone regulation of p85alpha expression and phosphoinositide 3-kinase activity in adipose tissue: mechanism for growth hormone-mediated insulin resistance. Diabetes. 2007;56:1638–1646. doi: 10.2337/db06-0299. [DOI] [PubMed] [Google Scholar]
- 19.Bagatell R, Herzog CE, Trippett TM, Grippo JF, Cirrincione-Dall G, Fox E, Macy M, Bish J, Whitcomb P, Aikin A, Wright G, Yurasov S, Balis FM, Gore L. Pharmacokinetically guided phase 1 trial of the IGF-1 receptor antagonist RG1507 in children with recurrent or refractory solid tumors. Clin Cancer Res. 2011;17:611–619. doi: 10.1158/1078-0432.CCR-10-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laron Z, Erster B, Klinger B, Anin S. Effect of acute administration of insulin-like growth factor I in patients with Laron-type dwarfism. Lancet. 1988;332:1170–1172. doi: 10.1016/s0140-6736(88)90236-x. [DOI] [PubMed] [Google Scholar]
- 21.Di Cola G, Cool MH, Accili D. Hypoglycemic effect of insulin-like growth factor-1 in mice lacking insulin receptors. J Clin Invest. 1997;99:2538–2544. doi: 10.1172/JCI119438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karp DD, Pollak MN, Cohen RB, Eisenberg PD, Haluska P, Yin D, Lipton A, Demers L, Leitzel K, Hixon ML, Terstappen LW, Garland L, Paz-Ares LG, Cardenal F, Langer CJ, Gualberto A. Safety, pharmacokinetics, and pharmacodynamics of the insulin-like growth factor type 1 receptor inhibitor figitumumab (CP-751,871) in combination with paclitaxel and carboplatin. J Thorac Oncol. 2009;4:1397–1403. doi: 10.1097/JTO.0b013e3181ba2f1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zha J, Lackner MR. Targeting the insulin-like growth factor receptor-1R pathway for cancer therapy. Clin Cancer Res. 2010;16:2512–2517. doi: 10.1158/1078-0432.CCR-09-2232. [DOI] [PubMed] [Google Scholar]
- 24.Gualberto A, Karp DD. Development of the monoclonal antibody figitumumab, targeting the insulin-like growth factor-1 receptor, for the treatment of patients with non–small-cell lung cancer. Clin Lung Cancer. 2009;10:273–280. doi: 10.3816/CLC.2009.n.038. [DOI] [PubMed] [Google Scholar]
- 25.Molife L, Fong P, Paccagnella L, Reid A, Shaw H, Vidal L, Arkenau H, Karavasilis V, Yap T, Olmos D. The insulin-like growth factor-I receptor inhibitor figitumumab (CP-751,871) in combination with docetaxel in patients with advanced solid tumours: results of a phase Ib dose-escalation, open-label study. Br J Cancer. 2010;103:332–339. doi: 10.1038/sj.bjc.6605767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camidge DR, Dziadziuszko R, Hirsch FR. The rationale and development of therapeutic insulin-like growth factor axis inhibition for lung and other cancers. Clin Cancer Rev. 2009;3:19–29. doi: 10.3816/CLC.2009.n.037. [DOI] [PubMed] [Google Scholar]
- 27.Olmos D, Postel-Vinay S, Molife L, Okuno SH, Schuetze SM, Paccagnella ML, Batzel GN, Yin D, Pritchard-Jones K, Judson I. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: a phase 1 expansion cohort study. Lancet Oncol. 2010;11:129–135. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gualberto A. Figitumumab (CP-751,871) for cancer therapy. Expert Opin Biol Ther. 2010;10:575–585. doi: 10.1517/14712591003689980. [DOI] [PubMed] [Google Scholar]
- 29.Tolcher AW, Sarantopoulos J, Patnaik A, Papadopoulos K, Lin CC, Rodon J, Murphy B, Roth B, McCaffery I, Gorski KS. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27:5800–5807. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 30.Naing A, Kurzrock R, Burger A, Gupta S, Lei X, Busaidy N, Hong D, Chen HX, Doyle LA, Heilbrun LK. Phase I trial of cixutumumab combined with temsirolimus in patients with advanced cancer. Clin Cancer Res. 2011;17:6052–6060. doi: 10.1158/1078-0432.CCR-10-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atzori F, Tabernero J, Cervantes A, Prudkin L, Andreu J, Rodriguez-Braun E, Domingo A, Guijarro J, Gamez C, Rodon J. A phase I, pharmacokinetic and pharmacodynamic study of dalotuzumab (MK-0646), an anti-IGF-1R monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2011;17:6304–6312. doi: 10.1158/1078-0432.CCR-10-3336. [DOI] [PubMed] [Google Scholar]
- 32.Quek R, Wang Q, Morgan JA, Shapiro GI, Butrynski JE, Ramaiya N, Huftalen T, Jederlinic N, Manola J, Wagner AJ, Demetri GD, George S. Combination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res. 2011;17:871–879. doi: 10.1158/1078-0432.CCR-10-2621. [DOI] [PubMed] [Google Scholar]
- 33.Goto Y, Sekine I, Tanioka M, Shibata T, Tanai C, Asahina H, Nokihara H, Yamamoto N, Kunitoh H, Ohe Y, Kikkawa H, Ohki E, Tamura T. Figitumumab combined with carboplatin and paclitaxel in treatment-naive Japanese patients with advanced non-small cell lung cancer. Invest New Drugs. 2011;30:1548–1556. doi: 10.1007/s10637-011-9715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malempati S, Weigel B, Ingle AM, Ahern CH, Carroll JM, Roberts CT, Reid JM, Schmechel S, Voss SD, Cho SY, Chen HX, Krailo MD, Adamson PC, Blaney SM. Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2012;30:256–262. doi: 10.1200/JCO.2011.37.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lacy MQ, Alsina M, Fonseca R, Paccagnella ML, Melvin CL, Yin D, Sharma A, Sarano ME, Pollak M, Jagannath S. Phase I, pharmacokinetic and pharmacodynamic study of the anti–insulinlike growth factor type 1 receptor monoclonal antibody CP-751,871 in patients with multiple myeloma. J Clin Oncol. 2008;26:3196–3203. doi: 10.1200/JCO.2007.15.9319. [DOI] [PubMed] [Google Scholar]


