Abstract
Aims
The use of topiramate, which is prescribed for the management of epilepsy, for migraine headache prophylaxis and as a weight-loss agent, has been associated with the development of metabolic acidosis, hypokalaemia and renal stone disease. We systematically reviewed all the literature.
Methods
The systematic review of the literature was realized using the principles underlying the UK Economic and Social Research Council guidance on the conduct of narrative synthesis and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.
Results
Fourty-seven reports published between 1996 and 2013 were retained for the final analysis. Five case–control studies and six longitudinal studies addressed the effect of topiramate on acid–base and potassium balance. A significant tendency towards mild-to-moderate hyperchloraemic metabolic acidosis (with bicarbonate ≤21.0 mmol l−1 in approximately every third case) and mild hypokalaemia (with potassium ≤3.5 mmol l−1 in 10% of the cases) was noted on treatment with topiramate, which was similar in children and adults. A single study observed that topiramate causes mild hyperuricaemia in male adults. A tendency towards hypocitraturia, a recognized promoter of renal stone formation, was noted in all patients on topiramate.
Conclusions
Increasing evidence supports the use of topiramate. Topiramate is generally well tolerated, and serious adverse events are rare. Nonetheless, the present systematic review of the literature indicates that its use is linked with the development of acidosis, hypokalaemia, hyperuricaemia and hypocitraturia.
Keywords: Acidosis, citraturia, hyperuricaemia, hypokalaemia, renal stone promotion, topiramate
Introduction
Topiramate is widely prescribed for the management of epilepsy, for migraine headache prophylaxis and as a weight-loss agent 1,2. The use of this rather new agent has been associated with the development of metabolic disturbances, such as acidosis, hypokalaemia, hyperuricaemia and renal stone disease 3,4. This tendency, addressed so far in various reports published since 1996, has never been addressed analytically. We therefore reviewed and analysed all the available literature. Our purposes in conducting this study were to describe in detail these disturbances, to highlight the causal pathophysiological mechanisms, to suggest preventive and therapeutic tools and to alert physicians about the possible occurrence of these disturbances as complications of management with topiramate.
Materials and methods
Data source and types of data
Between December 2012 and May 2013, we conducted a thorough computer-based search of the terms ‘acidosis topiramate’, ‘hypokal[a]emia topiramate’, ‘potassium topiramate’, ‘dyselectrolyt[a]emia topiramate’, ‘hyperuric[a]emia topiramate’, ‘uric acid topiramate’, ‘renal stone topiramate’, ‘kidney stone topiramate’, ‘urolithiasis topiramate’, ‘nephrolithiasis topiramate’, ‘citrate topiramate’ and ‘hypocitraturia topiramate’ in the US National Library of Medicine database and in the Web-based search engine Google Scholar. For this purpose, we used the principles established by the UK Economic and Social Research Council guidance on the conduct of narrative synthesis and on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement 5. For the final analysis, we selected reports available as a full-length article or as a letter, which included topiramate-associated cases of acidosis, hypokalaemia, hyperuricaemia or renal stone disease. Studies addressing the biochemical parameters that contribute to urinary stone promotion were also selected. Secondary references found in the articles that were relevant to the subject were also reviewed. Cases with dyselectrolytaemias induced by a topiramate poisoning and reports published in languages other than English, French, German, Italian, Portuguese or Spanish were excluded. If the same case was present in different publications, we considered exclusively the most complete description.
Data extraction, nomenclature and statistical analysis
Two persons independently extracted data from the retained publications. The Fifth Edition of the ‘Guide to Receptors and Channels’ was used as the nomenclature standard 6. To pool the effect of topiramate on acid–base, chloride, potassium or uric acid balance and parameters that contribute to urinary stone promotion, the weighted average effect was calculated using standard equations. Data are presented as means and standard deviations 7. Comparisons of weighted means were made using analysis of variance with correction for multiple comparisons.
Results
Search results
The flowchart of the literature search process (Figure 1) indicates that the initial search revealed 252 publications, of which 131 remained after exclusion of duplicates (i.e. publications found with both search terms). A total of 72 of them were reviewed in detail, and 47 articles 8–54 published between 1996 and 2013 were retained for the final analysis, following inclusion of reports found in references (41 in English 8–48, five in Spanish 49–53 and one in German 54). They had been reported from following countries: USA (n = 17), Spain (n = 5), Great Britain (n = 4), France (n = 3), Italy (n = 3), Brazil (n = 2), Switzerland (n = 2), Turkey (n = 2), Argentina (n = 1), Australia (n = 1), Belgium (n = 1), China (n = 1), Germany (n = 1), Hong Kong (n = 1), Mexico (n = 1), The Netherlands (n = 1) and Saudi Arabia (n = 1).
Figure 1.
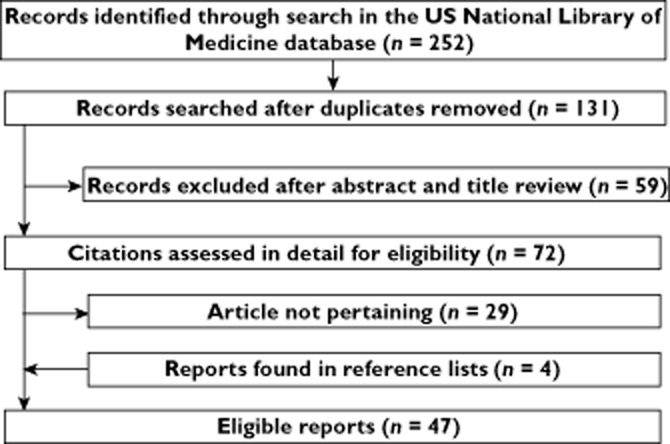
Flowchart of the literature search process
Acid–base, chloride, potassium and uric acid balance
Five articles addressed the effect of topiramate on acid–base and potassium balance by means of a case–control study in a total of 150 (female:male ratio = 1.62) epileptic patients on treatment with this agent for ≥3 months 9,17,30,45,47. Three studies were performed in adults, one in children and one in both children ad adults. A significant tendency towards metabolic acidosis was noted in the five reports; circulating bicarbonate was significantly (P < 0.0001) lower in patients (22.3 ± 2.8 mmHg) than in control subjects (25.4 ± 2.5 mmHg), with bicarbonate levels ≤21.0 mmol l−1 in 31% of the cases. In these reports, no significant correlation was observed between the dosage of topiramate and circulating bicarbonate or between topiramate blood level and circulating bicarbonate 9,17. Finally, no significant correlation was noted between the tendency to acidosis and the polymorphisms in the gene for carbonic anhydrase type XII in a study including 65 patients on topiramate 30. The effect of topiramate on chloride 9,17,47 and potassium levels 9,47 was addressed in some of the aforementioned reports; a significant tendency towards hyperchloraemia (108 ± 4 vs. 104 ± 4 mmol l−1; P < 0.001) and hypokalaemia (3.9 ± 0.4 vs. 4.2 ± 0.3 mmol l−1; P < 0.001) was noted. The frequency of hypokalaemia (≤ 3.5 mmol l−1), reported exclusively in one report, was 8.3% 9.
The effect of topiramate on acid–base balance was also addressed in six longitudinal studies (prospective, n = 4; retrospective, n = 2) including a total of 132 patients, comprising 68 children and 64 adults with a female:male ratio of 1.53 [16, 32, 34, 43, 44, 47]. In these studies, bicarbonataemia was 25.3 ± 3.2 mmmol l−1 before treatment and 20.6 ± 3.9 mmol l−1 on topiramate for ≥3 months (with a significant reduction of bicarbonate level of 4.7 ± 2.2 mmol l−1; P < 0.0001). The relationship between circulating bicarbonate level and daily topiramate dose or topiramate level, analysed exclusively in one report, was not significant 43. The decrease in plasma bicarbonate 44 level was significantly (P < 0.01) higher in topiramate patients concurrently on a ketogenic diet (6.0 ± 3.2 mmol l−1) than in those without a ketogenic diet (4.6 ± 1.9 mmol l−1). Interestingly, Takeoka et al. [44] observed that in children on a ketogenic diet the supplemental prescription of topiramate was associated with a significantly (P < 0.05) less profound decrease (2.9 ± 1.8 mmol l−1) in bicarbonate levels than when a ketogenic diet was added to prior topiramate therapy (7.6 ± 2.4 mmol l−1). None of the longitudinal studies investigated the influence of topiramate on circulating potassium and chloride levels, with the exception of Mirza et al. 30, who observed a tendency to hyperchloraemia (>105 mmol l−1) in patients with acidosis. The weighted levels of bicarbonate, chloride and potassium in patients treated with topiramate for ≥3 months (both case–control and longitudinal studies) are given in Table 1.
Table 1.
Circulating bicarbonate, chloride and potassium levels in patients on treatment with topiramate for ≥3 months
| Ion | n | Concentration (mmol l−1) |
|---|---|---|
| Bicarbonate | 289 | 21.5 ± 3.48 |
| Chloride | 142 | 108 ± 3.7 |
| Potassium | 110 | 3.9 ± 0.4 |
Both case–control and longitudinal studies are included. Results are given as weighted means ± SD.
In a cross-sectional study addressing 320 consecutive adult patients undergoing a neurosurgical procedure, a tendency towards intra-operative metabolic acidosis (bicarbonate level ≤21.0 mmol l−1) was found exclusively in four patients on long-term medication with topiramate. In that series, there were four further topiramate patients with normal acid–base balance despite similar doses and duration of treatment 36.
In most cases, metabolic acidosis was asymptomatic. Symptoms likely to be related to metabolic acidosis were noted in two (1.3%) of the patients included in case–control studies and in 28 (22%) of those included in longitudinal studies. Symptoms of acidosis were also reported in 10 patients included in anecdotal reports containing two or fewer cases 11,12,15,21,29,31,38,41,50. The reported symptoms included deep and laboured breathing with increased frequency (in one case associated with chest pain), nausea with or without vomiting and central nervous system disturbances, such as lethargy or irritability and hallucinations. At least 11 further cases of asymptomatic metabolic acidosis (bicarbonate level ≤21.0 mmol l−1) were included in anecdotal reports 18,19,28,33,37,42,48,52. In these subjects, circulating bicarbonate ranged from 12.0 to 20.0 mmol l−1. In 14 case reports 12,19,21,23–25,29,31,33,38,41,48–50, acidosis was associated with a tendency towards hyperchloraemia (level ranging between 111 and 120 mmol l−1).
In a case–control study 22, the circulating uric acid level was significantly (P < 0.01) higher in 53 patients (female:male ratio = 3.8) with migraine on topiramate (231 ± 79 μmol l−1) than in 44 healthy control subjects (184 ± 111 μmol l−1). Furthermore, the uric acid level increased significantly (P < 0.01) from 215 ± 53 to 262 ± 96 μmol l−1 in 23 patients (female:male ratio = 3.6) following initiation of treatment with topiramate 22. In this study, the tendency towards hyperuricaemia was significantly (P < 0.05) more prominent in males. Not surprisingly, therefore, in a case–control study including 32 patients on topiramate with a very high female:male ratio of 15, the level of uric acid in blood was similar (268 ± 59 vs. 274 ± 65 μmol l−1) in patients and control subjects 47. In a small longitudinal study with seven patients (female:male ratio = 6.0), 3 months of topiramate did not change the circulating uric acid level 47. No further reports addressed the influence of topiramate on circulating uric acid.
Kidney stones
Epidemiology
At least 10 longitudinal reports (prospective, n = 9; retrospective, n = 1), including a total of 1264 subjects, addressed the frequency of kidney stones on drug management with topiramate. The overall annual incidence of symptomatic kidney stones was found to be 2.1% 8,10,13,14,26,35,39,40,45,51. The studies were uncontrolled, with the exception of a small study performed in Mexico 51, a country with a very high prevalence of renal stones. In this study, the annual incidence of kidney stones was 19% on treatment with topiramate and 14% in control subjects (difference not significant). The tendency towards kidney stone formation was significantly higher in adults treated with topiramate at ≥300 mg day−1 51.
Kidney stones are sometimes found by imaging studies in people without symptoms. In a study among 75 topiramate-treated adult patients with a median daily dose of 300 mg and median treatment duration of 48 months, the prevalence of symptomatic nephrolithiasis was 11% and that of asymptomatic nepohrolithiasis 20% 26. In a further study performed among infants and toddlers 17, stone fragments were evident in the nappies of 13 of 24 individuals (54%) on topiramate (radiological confirmation with imaging was achieved in seven of 13 patients). Finally 27, among 96 children on topiramate, imaging studies disclosed asymptomatic kidney stones in five children (5.2%; four girls and one boy).
Mechanisms of stone promotion
Metabolic factors that promote renal stone formation consist of either solute excess or decreased levels of inhibitors of stone formation. Eleven reports 17,20,23,24,30,45–47,49,53,54, including a total of 84 patients (female:male ratio = 2.9) on medication with topiramate, addressed at least one promoter of stone formation, as shown in Table 2. Rather discrepant findings were noted with respect to a possible solute excess of calcium and oxalate. On the contrary, the urinary uric acid level was almost always normal and the urinary citrate excretion always low. Stone composition was analysed exclusively in two reports for a total of five patients 17,26. In addition to calcium, disclosed in all cases, phosphate and oxalate were present in three cases and struvite and phosphate in two cases.
Table 2.
Biochemical promoters of renal stone formation assessed in patients on drug management with topiramate
Differences between children and adults
The tendency towards hypobicarbonataemia, hyperchloraemia, hypokalaemia and hypocitraturia on treatment with topiramate was similar in children and adults. No study addressed the influence of topiramate on uric acid levels in childhood.
Discussion
Topiramate is structurally distinct from other antiepileptics 1,2. Its rather complex mechanism of action includes the blockade of voltage-gated sodium channels (similar to many traditional antiepileptics), enhancement of γ-aminobutyric acid receptor activity, inhibition of glutamate receptors, inhibition of L-type voltage-gated calcium ion channels and inhibition of carbonic anhydrases 1,2. The relative importance of these mechanisms in the functioning of topiramate is not clearly known, but it does not appear that any other single antiepileptic drug shares these five properties 1,2.
The present systematic review of the literature indicates that management with this broad-spectrum agent is associated with mild-to-moderate hyperchloraemic metabolic acidosis (bicarbonate level generally decreases by ∼3–5 mmol l−1), mild hypokalaemia (potassium level generally decreases by ∼0.3 mmol l−1), hyperuricaemia (the latter almost exclusively in male subjects) and a propensity for renal stone formation associated with hypocitraturia. These abnormalities result from tubular dysfunction caused by inhibition of renal carbonic anhydrases 3,4. The elevation of uricaemia observed on topiramate in a single study 22 is not surprising. Such an elevation is a side-effect of all diuretics, except those agents that retain potassium. The main underlying mechanism is a tubular reabsorption of uric acid that results from extracellular fluid volume depletion 55.
The severity of acidosis is increased if there are other factors that predispose to metabolic acidosis, such as concurrent use of a ketogenic diet. The failure to demonstrate a relationship between the degree of acidosis and either topiramate dose or topiramate level supports the notion that genetic factors contribute to the risk of developing acidosis. It is true, however, that such a link was not found in a preliminary study. Chronic metabolic acidosis is mostly asymptomatic. Rarely, it presents with Kussmaul breathing or even with alterations in mental status. Potential complications of long-lasting metabolic acidosis, which include impaired growth in children and low bone mass in both children and adults, have not yet been addressed in the literature.
This review of the literature indicates that management with topiramate is associated with a considerable tendency towards renal stone promotion because of a low urinary citrate excretion 55. Urinary citrate enables humans to excrete finite amounts of calcium (and phosphate) in the urine while elaborating an extremely low urine volume, because it inhibits the crystallization of several stone-forming salts 56. The excretion of citrate is impaired by any factor that increases the systemic acid load or produces a tendency to acidosis and by any factor that induces potassium depletion, two recognized consequences of medication with topiramate and other drugs that inhibit renal carbonic anhydrases 57. The potential of carbonic anhydrase inhibition to reduce citraturia was first demonstrated ∼60 years ago 57.
Monitoring drug treatment is one way of seeing that a treatment works, while protecting from adverse drug effects 58. Monitoring treatment with topiramate to anticipate metabolic acidosis, hypokalaemia and especially renal stone promotion before these complications become clinically relevant is undoubtedly essential. Unfortunately, so far no study has addressed the effects of alkali therapy on acid–base and potassium status, urinary citrate excretion and especially the tendency to renal stone formation in patients treated with topiramate. In glaucoma patients without kidney stone disease treated with the carbonic anhydrase inhibitor acetazolamide, low-dose alkali treatment did not correct hypocitraturia 59. Consequently, whether alkali therapy would ultimately alter the urinary stone risk remains unclear. It is, therefore, imperative to initiate well-designed studies focusing on the influence of alkali therapy on acid–base and potassium status and urinary stone risk profile in subjects managed with topiramate. For the moment, clinicians prescribing topiramate have to remain watchful of these possible adverse effects. Nearly all patients experiencing these adverse effects withdraw from topiramate treatment because alternatives are mostly available. However, if withdrawal of treatment is not possible, remedial therapy in the form of alkali replacement should be considered.
Conclusion
The aim of all drug medication is to improve the patient's condition by achieving the maximal therapeutic benefit and concurrently minimizing unwanted adverse drug reactions. Increasing evidence, based on controlled studies, supports the use of topiramate for epilepsy, for migraine headache prophylaxis and as a weight-loss drug. Topiramate is generally well tolerated, and serious adverse events are rare. Nonetheless, the present review indicates that its use is linked with metabolic acidosis, hypokalaemia, hyperuricaemia and renal stone promotion.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Ben-Menachem E, Sander JW, Stefan H, Schwalen S, Schäuble B. Topiramate monotherapy in the treatment of newly or recently diagnosed epilepsy. Clin Ther. 2008;30:1180–1195. doi: 10.1016/s0149-2918(08)80045-8. [DOI] [PubMed] [Google Scholar]
- 2.Silberstein SD, Ben-Menachem E, Shank RP, Wiegand F. Topiramate monotherapy in epilepsy and migraine prevention. Clin Ther. 2005;27:154–165. doi: 10.1016/j.clinthera.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Vega D, Maalouf NM, Sakhaee K. Increased propensity for calcium phosphate kidney stones with topiramate use. Expert Opin Drug Saf. 2007;6:547–557. doi: 10.1517/14740338.6.5.547. [DOI] [PubMed] [Google Scholar]
- 4.Mirza NS, Marson AG, Pirmohamed M. Effect of topiramate on acid-base balance: extent, mechanism and effects. Br J Clin Pharmacol. 2009;68:655–661. doi: 10.1111/j.1365-2125.2009.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 6.Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl 1):S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abou-Khalil B. Topiramate in the long-term management of refractory epilepsy. Topiramate YOL Study Group. Epilepsia. 2000;41(Suppl 1):S72–76. doi: 10.1111/j.1528-1157.2000.tb02176.x. [DOI] [PubMed] [Google Scholar]
- 9.Belotti EA, Taddeo I, Ragazzi M, Pifferini R, Simonetti GD, Bianchetti MG, Ramelli GP. Chronic impact of topiramate on acid-base balance and potassium in childhood. Eur J Paediatr Neurol. 2010;14:445–448. doi: 10.1016/j.ejpn.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Bootsma HP, Coolen F, Aldenkamp AP, Arends J, Diepman L, Hulsman J, Lambrechts D, Leenen L, Majoie M, Schellekens A, de Krom M. Topiramate in clinical practice: long-term experience in patients with refractory epilepsy referred to a tertiary epilepsy center. Epilepsy Behav. 2004;5:380–387. doi: 10.1016/j.yebeh.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Burmeister JE, Pereira RR, Hartke EM, Kreuz M. Topiramate and severe metabolic acidosis: case report. Arq Neuropsiquiatr. 2005;63:532–534. doi: 10.1590/s0004-282x2005000300032. [DOI] [PubMed] [Google Scholar]
- 12.Cheng M, Wen S, Tang X, Zhong Z. Hallucinations and comorbid renal tubular acidosis caused by topiramate in a patient with psychiatric history. Gen Hosp Psychiatry. 2013;35:213. doi: 10.1016/j.genhosppsych.2012.04.008. e1–3. [DOI] [PubMed] [Google Scholar]
- 13.Coppola G, Caliendo G, Veggiotti P, Romeo A, Tortorella G, De Marco P, Pascotto A. Topiramate as add-on drug in children, adolescents and young adults with Lennox-Gastaut syndrome: an Italian multicentric study. Epilepsy Res. 2002;51:147–153. doi: 10.1016/s0920-1211(02)00103-1. [DOI] [PubMed] [Google Scholar]
- 14.Coppola G, Capovilla G, Montagnini A, Romeo A, Spanò M, Tortorella G, Veggiotti P, Viri M, Pascotto A. Topiramate as add-on drug in severe myoclonic epilepsy in infancy: an Italian multicenter open trial. Epilepsy Res. 2002;49:45–48. doi: 10.1016/s0920-1211(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 15.Delpirou-Nouh C, Gelisse P, Chanez P, Carlander B. Migraine and topiramate induced dyspnea. Headache. 2007;47:1453–1455. doi: 10.1111/j.1526-4610.2007.00921.x. [DOI] [PubMed] [Google Scholar]
- 16.Garris SS, Oles KS. Impact of topiramate on serum bicarbonate concentrations in adults. Ann Pharmacother. 2005;39:424–426. doi: 10.1345/aph.1E437. [DOI] [PubMed] [Google Scholar]
- 17.Goyal M, Grossberg RI, O'Riordan MA, Davis ID. Urolithiasis with topiramate in nonambulatory children and young adults. Pediatr Neurol. 2009;40:289–294. doi: 10.1016/j.pediatrneurol.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Groeper K, McCann ME. Topiramate and metabolic acidosis: a case series and review of the literature. Paediatr Anaesth. 2005;15:167–170. doi: 10.1111/j.1460-9592.2005.01415.x. [DOI] [PubMed] [Google Scholar]
- 19.Izzedine H, Launay-Vacher V, Deray G. Topiramate-induced renal tubular acidosis. Am J Med. 2004;116:281–282. doi: 10.1016/j.amjmed.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Kaplon DM, Penniston KL, Nakada SY. Patients with and without prior urolithiasis have hypocitraturia and incident kidney stones while on topiramate. Urology. 2011;77:295–298. doi: 10.1016/j.urology.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 21.Ko CH, Kong CK. Topiramate-induced metabolic acidosis: report of two cases. Dev Med Child Neurol. 2001;43:701–704. doi: 10.1017/s0012162201001268. [DOI] [PubMed] [Google Scholar]
- 22.Koçer A, Dikici S, Atakay S, Okuyucu S. Serum uric acid and lipid levels while taking topiramate for migraine. Headache. 2008;48:1056–1060. doi: 10.1111/j.1526-4610.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- 23.Kuo RL, Moran ME, Kim DH, Abrahams HM, White MD, Lingeman JE. Topiramate-induced nephrolithiasis. J Endourol. 2002;16:229–231. doi: 10.1089/089277902753752188. [DOI] [PubMed] [Google Scholar]
- 24.Lamb EJ, Stevens PE, Nashef L. Topiramate increases biochemical risk of nephrolithiasis. Ann Clin Biochem. 2004;41:166–169. doi: 10.1258/000456304322880104. [DOI] [PubMed] [Google Scholar]
- 25.Laskey AL, Korn DE, Moorjani BI, Patel NC, Tobias JD. Central hyperventilation related to administration of topiramate. Pediatr Neurol. 2000;22:305–308. doi: 10.1016/s0887-8994(99)00143-5. [DOI] [PubMed] [Google Scholar]
- 26.Maalouf NM, Langston JP, Van Ness PC, Moe OW, Sakhaee K. Nephrolithiasis in topiramate users. Urol Res. 2011;39:303–307. doi: 10.1007/s00240-010-0347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahmoud AA, Rizk T, El-Bakri NK, Riaz M, Dannawi S, Al Tannir M. Incidence of kidney stones with topiramate treatment in pediatric patients. Epilepsia. 2011;52:1890–1893. doi: 10.1111/j.1528-1167.2011.03245.x. [DOI] [PubMed] [Google Scholar]
- 28.Malik R, Iacoune J. Metabolic acidosis in a pediatric patient receiving topiramate. J Pediatr. Pharmacol Ther. 2003;8:287–292. doi: 10.5863/1551-6776-8.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathews KD, Stark JE. Hyperchloremic, normal anion-gap, metabolic acidosis due to topiramate. Am J Health Syst Pharm. 2008;65:1430–1434. doi: 10.2146/ajhp070395. [DOI] [PubMed] [Google Scholar]
- 30.Mirza NS, Alfirevic A, Jorgensen A, Marson AG, Pirmohamed M. Metabolic acidosis with topiramate and zonisamide: an assessment of its severity and predictors. Pharmacogenet Genomics. 2011;21:297–302. doi: 10.1097/FPC.0b013e3283441b95. [DOI] [PubMed] [Google Scholar]
- 31.Montcriol A, Meaudre E, Kenane N, Asencio Y, Bordes J, Palmier B. Hyperventilation and cerebrospinal fluid acidosis caused by topiramate. Ann Pharmacother. 2008;42:584–587. doi: 10.1345/aph.1K508. [DOI] [PubMed] [Google Scholar]
- 32.Montenegro MA, Guerreiro MM, Scotoni AE, Guerreiro CA. Predisposition to metabolic acidosis induced by topiramate. Arq Neuropsiquiatr. 2000;58:1021–1024. doi: 10.1590/s0004-282x2000000600007. [DOI] [PubMed] [Google Scholar]
- 33.Ozer Y, Altunkaya H. Topiramate induced metabolic acidosis. Anaesthesia. 2004;59:830. doi: 10.1111/j.1365-2044.2004.03884.x. [DOI] [PubMed] [Google Scholar]
- 34.Philippi H, Boor R, Reitter B. Topiramate and metabolic acidosis in infants and toddlers. Epilepsia. 2002;43:744–747. doi: 10.1046/j.1528-1157.2002.37201.x. [DOI] [PubMed] [Google Scholar]
- 35.Reith D, Burke C, Appleton DB, Wallace G, Pelekanos J. Tolerability of topiramate in children and adolescents. J Paediatr Child Health. 2003;39:416–419. doi: 10.1046/j.1440-1754.2003.00180.x. [DOI] [PubMed] [Google Scholar]
- 36.Rodríguez L, Valero R, Fàbregas N. Intraoperative metabolic acidosis induced by chronic topiramate intake in neurosurgical patients. J Neurosurg Anesthesiol. 2008;20:67–68. doi: 10.1097/ANA.0b013e31815613ad. [DOI] [PubMed] [Google Scholar]
- 37.Sacré A, Jouret F, Manicourt D, Devuyst O. Topiramate induces type 3 renal tubular acidosis by inhibiting renal carbonic anhydrase. Nephrol Dial Transplant. 2006;21:2995–2996. doi: 10.1093/ndt/gfl251. [DOI] [PubMed] [Google Scholar]
- 38.Shiber JR. Severe non-anion gap metabolic acidosis induced by topiramate: a case report. J Emerg Med. 2010;38:494–496. doi: 10.1016/j.jemermed.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 39.Shorvon SD. Safety of topiramate: adverse events and relationships to dosing. Epilepsia. 1996;37(Suppl 2):S18–22. doi: 10.1111/j.1528-1157.1996.tb06029.x. [DOI] [PubMed] [Google Scholar]
- 40.Stephen LJ, Sills GJ, Brodie MJ. Topiramate in refractory epilepsy: a prospective observational study. Epilepsia. 2000;41:977–980. doi: 10.1111/j.1528-1157.2000.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 41.Stowe CD, Bollinger T, James LP, Haley TM, Griebel ML, Farrar HC., 3rd Acute mental status changes and hyperchloremic metabolic acidosis with long-term topiramate therapy. Pharmacotherapy. 2000;20:105–109. doi: 10.1592/phco.20.1.105.34662. [DOI] [PubMed] [Google Scholar]
- 42.Sujan S, Torres de Rueda A, Montero L, Toledo R, Ros S, Martín Reyes G. Topiramate-induced renal tubular acidosis. A case report. Nefrologia. 2008;28:656–657. [PubMed] [Google Scholar]
- 43.Takeoka M, Holmes GL, Thiele E, Bourgeois BF, Helmers SL, Duffy FH, Riviello JJ. Topiramate and metabolic acidosis in pediatric epilepsy. Epilepsia. 2001;42:387–392. doi: 10.1046/j.1528-1157.2001.04500.x. [DOI] [PubMed] [Google Scholar]
- 44.Takeoka M, Riviello JJ, Jr, Pfeifer H, Thiele EA. Concomitant treatment with topiramate and ketogenic diet in pediatric epilepsy. Epilepsia. 2002;43:1072–1075. doi: 10.1046/j.1528-1157.2002.00602.x. [DOI] [PubMed] [Google Scholar]
- 45.Tartara A, Sartori I, Manni R, Galimberti CA, Di Fazio M, Perucca E. Efficacy and safety of topiramate in refractory epilepsy: a long-term prospective trial. Ital J Neurol Sci. 1996;17:429–432. doi: 10.1007/BF01997718. [DOI] [PubMed] [Google Scholar]
- 46.Warner BW, LaGrange CA, Tucker T, Bensalem-Owen M, Pais VM., Jr Induction of progressive profound hypocitraturia with increasing doses of topiramate. Urology. 2008;72:29–32. doi: 10.1016/j.urology.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 47.Welch BJ, Graybeal D, Moe OW, Maalouf NM, Sakhaee K. Biochemical and stone-risk profiles with topiramate treatment. Am J Kidney Dis. 2006;48:555–563. doi: 10.1053/j.ajkd.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Wilner A, Raymond K, Pollard R. Topiramate and metabolic acidosis. Epilepsia. 1999;40:792–795. doi: 10.1111/j.1528-1157.1999.tb00781.x. [DOI] [PubMed] [Google Scholar]
- 49.Alarcón-Martínez H, Casas-Fernández C, Escudero-Rodríguez N, Cao-Avellaneda E, Domingo-Jiménez R, Puche-Mira A, Rodríguez-Costa T. Nefrolitiasis y topiramato. Rev Neurol. 2006;42:91–94. [PubMed] [Google Scholar]
- 50.Fernández-de Orueta L, Esteban-Fernández J, Aichner HF, Casillas-Villamor A, Rodríguez-Álvarez S. Acidosis metabólica inducida por topiramato: a propósito de un caso. Nefrologia. 2012;32:403–404. doi: 10.3265/Nefrologia.pre2011.Dec.11308. [DOI] [PubMed] [Google Scholar]
- 51.Gutiérrez López C, Plascencia-Álvarez N, Quiñones Aguilar S, Toriz-Ortiz A, Núñez-Orozco L. Relación entre topiramato y nefrolitiasis en una muestra de pacientes mexicanos con epilepsia refractaria. Rev Mex Neuroci. 2008;9:438–444. [Google Scholar]
- 52.López Gastón O, Pastorino ML, Alfonso A, Varela J, Giannaula R. Topiramato e inhibición de la anhidrasa carbónica. Nephrol Dial Transplant. 2009;29:69–73. [Google Scholar]
- 53.López-Real AM, García-Antelo MJ, Fernández-Couto D, López-Facal S, Rubio-Nazabal E. Cálculo coraliforme secundario a topiramato. Farm Hosp. 2008;32:299–301. doi: 10.1016/s1130-6343(08)75952-x. [DOI] [PubMed] [Google Scholar]
- 54.Gerber L, Wüthrich RP, Mohebbi N. Nierenstein durch Migräne. Praxis (Bern 1994) 2012;101:665–668. doi: 10.1024/1661-8157/a000936. [DOI] [PubMed] [Google Scholar]
- 55.Wile D. Diuretics: a review. Ann Clin Biochem. 2012;49:419–431. doi: 10.1258/acb.2011.011281. [DOI] [PubMed] [Google Scholar]
- 56.Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest. 2005;115:2598–2608. doi: 10.1172/JCI26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanley T, Platts MM. Observations on the metabolic effects of the carbonic anhydrase inhibitor acetazolamide: mode and rate of recovery from the drug's action. J Clin Invest. 1956;35:20–30. doi: 10.1172/JCI103248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pirmohamed M, Ferner RE. Monitoring drug treatment. BMJ. 2003;327:1179–1181. doi: 10.1136/bmj.327.7425.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Higashihara E, Nutahara K, Takeuchi T, Shoji N, Araie M, Aso Y. Calcium metabolism in acidotic patients induced by carbonic anhydrase inhibitors: responses to citrate. J Urol. 1991;145:942–948. doi: 10.1016/s0022-5347(17)38496-3. [DOI] [PubMed] [Google Scholar]


