Abstract
Aims
Nefopam is a nonmorphinic central analgesic, for which no recommendation exists concerning adaptation of regimen in aged patients with or without renal impairment. The objective was to describe the pharmacology of nefopam in aged patients to obtain guidelines for practical use.
Methods
Elderly patients (n = 48), 65–99 years old, with severe or moderate renal impairment or with normal renal function, were recruited. Nefopam (20 mg) was administered as a 30 min infusion postoperatively. Simultaneously, a 1 min intravenous infusion of iohexol was performed, in order to calculate the glomerular filtration rate. Blood samples were drawn to determine nefopam, desmethyl-nefopam and iohexol plasma concentrations. Nefopam and desmethyl-nefopam concentrations were analysed using a nonlinear mixed-effects modelling approach with Monolix version 4.1.3. The association between pharmacokinetic parameters and treatment response was assessed using logistic regression.
Results
A two-compartment open model was selected to describe the pharmacokinetics of nefopam. The typical population estimates (between-subject variability) for clearance, volume of distribution, intercompartmental clearance and peripheral volume were, respectively, 17.3 l h−1 (53.2%), 114 l (121%), 80.7 l h−1 (79%) and 208 l (63.6%). Morphine requirement was related to exposure of nefopam. Tachycardia and postoperative nausea and vomiting were best associated with maximal concentration and the rate of increase in nefopam plasma concentration.
Conclusions
We identified the nefopam pharmacokinetic predictors for morphine requirement and side-effects, such as tachycardia and postoperative nausea and vomiting. In order to maintain morphine sparing and decrease side-effects following a single dose of nefopam (20 mg), simulations suggest an infusion time of >45 min in elderly patients with or without renal impairment.
Keywords: analgesia, elderly, logistic regression, nefopam, population pharmacokinetics, renal impairment
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Nefopam is a centrally acting non-opioid analgesic indicated for treatment of postoperative pain after abdominal, urological or orthopaedic surgery, either alone or in combination with other drugs.
There is limited knowledge about nefopam pharmacology in the elderly. This is a challenging patient group to study.
WHAT THIS STUDY ADDS
This study describes nefopam pharmacology in the elderly to propose guidelines for practical use. Nefopam pharmacokinetic predictors of morphine requirement and side effects were determined.
A single dose of nefopam (20 mg) should be infused over a period >45 min in aged patients with or without renal impairment.
Introduction
Elderly people undergo more surgical procedures than younger individuals 1,2, and pain in the elderly is poorly controlled 3–5; therefore, providing adequate postoperative analgesia is critical and essential. Several pharmacological agents combined according to different strategies, as in multimodal analgesia, can be used for pain management 4,6,7. Nefopam (nef) is a centrally acting non-opioid analgesic indicated for treatment of postoperative pain after abdominal, urological or orthopaedic surgery, either alone or in combination with other drugs 8–10. In contrast to opioid analgesics, nefopam has no respiratory-depressive effect 11,12 and provides a better quality of analgesia 10,13–15. After administration, nefopam is mainly metabolized by the liver; seven metabolites have been described, with only one, desmethyl-nefopam (dnef), denoting pharmacological activity and found at very low concentrations 16. The main route of elimination of the metabolites is renal 17. Nevertheless, only one study focused on nefopam pharmacokinetics in patients with end-stage renal disease 18. Furthermore, interpatient variability in pharmacokinetics and pharmacodynamics consequent to physiological age-related impairments and pathological changes in cardiac, renal and hepatic function must be taken into account in order to adjust the analgesic treatment regimen 4–7. Aged patients more frequently experience cardiovascular and respiratory side-effects from drugs 5. However, due to the lack of studies focusing on the link between pharmacokinetics and treatment response, no scientific rationale is available to date for the optimal administration schedule of nefopam in the aged patient. Indeed, no therapeutic monitoring guidelines for nefopam are available. In the present work, the objective was to explore the relationships between nefopam and desmethyl-nefopam pharmacokinetic parameters in aged patients, with or without renal impairment, and morphine requirement and side-effects (nausea and tachycardia).
Patients and Methods
After approval by the Reims Ethics Committee (protocol: PHRC-2004-R11-07; registered at agence française de sécurité sanitaire des produits de santé AFSSAPS: 060493), this single-centre, open prospective study was conducted in the University Hospital of Reims (France). After obtaining written informed consent, patients between 65 and 99 years of age scheduled for repair of a fractured hip were enrolled in this study between June 2006 and August 2011. Renal function was characterized as normal [Cockroft–Gault glomerular filtration rate (GFR) >60 ml min−1 (1.73 m)−2], moderate impairment [Cockroft–Gault GFR <60 ml min−1 (1.73 m)−2] or severe impairment [Cockroft–Gault GFR ≤30 ml min−1 (1.73 m)−2]. Exclusion criteria were nonsigned consent form, nefopam contraindication (hepatic insufficiency defined as a prothrombin level below 70% or haemostasis impairments, epilepsy, glaucoma and prostate adenoma), hypersensitivity to nefopam or to iodinated contrast media, congestive heart failure, unstable angina, sequelae of cardiac infarction, uncontrolled arrhythmia and haemodialysis.
Before surgery, patients received sedative premedication with hydroxyzine. The surgical procedure was performed under general or regional anaesthesia at the discretion of the anaesthesiologist in charge of the patient. In the postanaesthetic care unit, when patients had fully recovered from general anaesthesia (tracheal tube removed) or when the anaesthetic level of spinal anaesthesia was below T10, 20 mg of nefopam hydrochloride (Acupan® injectable; Biocodex, Montrouge, France; IUPAC name, 5-methyl-1-phenyl-1,3,4,6-tetrahydro-2,5-benzoxazocine) diluted with 0.9% of saline solution was infused over a 30 min period using an infusion pump. Twenty-nine minutes after beginning nefopam infusion, a 1 min intravenous infusion of 5 ml iohexol (Omnipaque 180®, GE HEALTHCARE SAS, Vélizy-Villacoublay, France) was performed, in order to estimate the GFR. Iohexol clearance has been described as a gold standard that reflects the GFR in the elderly (GFRio) 19. Blood samples for the determination of nefopam, desmethyl-nefopam and iohexol were drawn 0, 5, 10, 15, 30, 60, 120, 180, 240, 480 and 1440 min after nefopam and iohexol administration. Plasma nefopam, desmethyl-nefopam and iohexol concentrations were determined as previously described 19,20.
Population pharmacokinetics
A population approach, with the nonlinear mixed-effect modelling implemented in Monolix (version 4.1; http://www.lixoft.eu), was used to study the pharmacokinetic profile of nefopam and desmethyl-nefopam 21. Parameters were estimated by computing the maximum likelihood estimator without any approximation of the model, using the stochastic approximation expectation maximization (SAEM) algorithm combined with an MCMC (Markov Chain Monte Carlo: 2 for the number of chain) procedure 21–25. All runs were carried out more than six times to ensure that estimated parameters and likelihood remained stable.
Separate structural model nefopam and desmethyl-nefopam concentrations were described using compartmental pharmacokinetic modelling (Figure 1). For nefopam concentrations, one, two and three mammillary compartment models, included in the Monolix (version 4.1) software library, with first-order distribution constants were tested (see Supporting Information). For desmethyl-nefopam, the MLXTRAN language included in Monolix (version 4.1) was used. One, two and three mammillary compartment models, with zero- or first-order input, metabolic clearance of nefopam with a lag time or a lag time before enterohepatic circulation, and first-order elimination were tested to describe observed desmethyl-nefopam concentrations (see Supporting Information). All individual parameters are defined as log-normally distributed.
Figure 1.
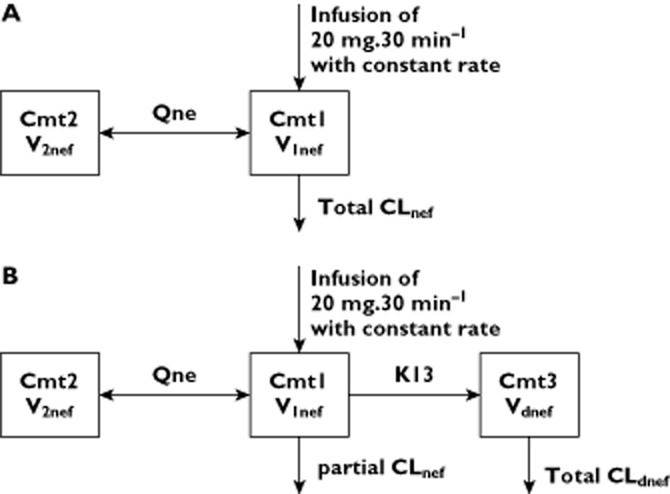
Schematic representation of structural pharmacokinetic models for nefopam (A) and desmethyl-nefopam (B). Abbreviations are as follows: CL, clearance; Cmt, compartment; dnef, desmethyl-nefopam; K13, apparent clearance of metabolization of nefopam to desmethyl-nefopam; Q, intercompartmental clearance; nef, nefopam; V, volume of compartment
Several error models (constant, proportional, additive or mixed, exponential and logit error model) were studied to describe the residual variability (ε). The between-subject variability (BSV) of pharmacokinetic parameters was described using an exponential model, as follows: θi = θTV × exp(ηi),where θi is the estimated individual parameter, θTV the typical value of the parameter and ηi the random effect for the ith patient. The values of θi were assumed to be normally distributed, with mean 0 and variance (ω2) which were parameterized as a diagonal matrix. Correlations between random effects were tested.
The model best describing individual data was selected and evaluated based on the usual diagnostic plot, precision and information criteria with a minimum of pharmacokinetic parameters (to prevent overfitting) 21–27. The likelihood ratio test (LRT), including the −2 log-likelihood, the Akaike information criterion (AIC) and the Bayesian information criterion (BIC), was used to test different hypotheses regarding the final model 23,25,26: covariate effect on pharmacokinetic parameter(s), residual variability model (proportional vs. proportional plus additive error model), and the structure of the variance–covariance matrix for the interindividual variability parameters. As covariate search method, stepwise covariate modelling was used 26. We evaluated the following covariates: age, sex (1, male; 2, female), weight, size, body mass index 26, lean body weight 26, fat free mass 26, ideal body weight 26, body surface area 26, glomerular filtration rate using iohexol clearance (GFRio) 19 or the four-variable Modification of Diet in Renal Disease (MDRD) 26 or the Cockroft–Gault glomerular filtration rate. Covariates were selected in the model if their effect was biologically suspected, using a forward selection (LRT, P = 0.05) followed by backward elimination process (LRT, P = 0.01), they reduced the between-subject variability of the corresponding pharmacokinetic parameter, and the relative standard error of the covariate parameter was lower than 20%.
The goodness of fit was evaluated with the following graphs produced for the final model: observed and predicted concentrations vs. time, observed concentrations vs. population predictions, weighted residuals vs. time and weighted residuals vs. predictions.
Relative standard errors (RSEs) of all estimations of <20% were accepted. To evaluate accuracy and robustness of the model appropriateness across time, prediction-corrected visual checks with 1000 simulated data sets was used 28. The observed concentrations were overlaid on the prediction intervals and compared visually. The normal distribution of normalized prediction distribution errors (NPDE) metrics was tested 29. As for NPDE, population or individual weighted residuals (PWRES or IWRES) vs. time and PWRES or IWRES vs. predictions should be centred on zero, without systematic bias.
Individual pharmacokinetic parameters, such as total clearance, intercompartmental clearance, volume of distribution, predicted nefopam (nef) and desmethyl-nefopam (dnef) plasma concentration as a function of time, area under the curve of plasma concentration as a function of time (in hours) for nefopam (AUCnef0→∞, in micrograms hours per litre) and desmethyl-nefopam (AUCdnef0→∞, in micrograms hours per litre) were derived for each patient by using the Empirical-Bayes-Estimates (EBE) of the individual parameters determined by final model (see Supporting Information).
Assessment of relationship between pharmacokinetics and outcome
The association between predictors [maximal concentration (Cmax), rate of increase in concentration (RCmax), from time of the start the infusion (t = 0 h) to the time corresponding to Cmax, AUC0→∞ for nefopam or desmethyl-nefopam] and outcomes, such as developing the postoperative nausea and vomiting (PONV) as described 30, postoperative tachycardia with heart rate over than 100 beats min−1 and requirement for morphine sulfate (10 mg), were assessed by logistic regression analysis using IBM® SPSS® (version 20.0). To ensure that any observed association between a predictor and a given outcome was not confounded by the presence of reported risk factors, a multivariate model of logistic regression was used. Therefore, we provided an adjusted odds ratio (OR), by modelling the association between predictors and outcomes, controlling the other covariates for confounding effects. We used a binary scale or score to describe PONV and tachycardia (1, presence; 0, absence) and morphine requirement (score 1, administration of at least 10 mg of morphine sulfate as requested by the patient to control the pain; score 0, for patient not requesting morphine sulfate). The assessments of the scores were done during a postoperative period of 24 h.
The omnibus test of model coefficients (OTMC) as LRT for logistic regression in SPSS® software is associated with a P value, which can be used to accept or reject the model. The goodness of fit and appropriateness of the logistic regression model were evaluated using the Nagelkerke r2 values and Hosmer–Lemeshow value and, finally, by the overall correct percentage of prediction.
The covariate factors were chosen by fitting a logistic regression model using a forward selection procedure (P < 0.05). The covariate was retained if it did not reduce the overall correct percentage of prediction and the Hosmer–Lemeshow value of the model. For tachycardia, we assessed as covariate factors body mass index, GFR using iohexol clearance, nefopam clearance (CLnef), desmethy-nefopam clearance (CLdnef) and age, and as binary covariate factors, sex, administration of drug enhancing the probability of tachycardia (drug–tachycardia) as reported in the full prescribing information 17 and the morphine requirement, reflecting the pain, and type of anaesthesia (score 1, general anaesthesia; score 0, local–regional anaesthesia). For PONV, we assessed the impact of body mass index, GFR using iohexol clearance, CLnef, CLdnef, age, and as binary covariate factors, sex, administration of drug enhancing the probability of PONV (drug–PONV) as reported in the full prescribing information 17, a simplified Apfel score 30 and type of anaesthesia (score 1, general anaesthesia; score 0, local–regional anaesthesia). For morphine requirement, we assessed as covariates body mass index, GFR using iohexol clearance, CLnef, CLdnef, age, and as dummy covariate factors, sex, the type of anaesthesia and the use of sufentanil or intravenous acetaminophen during the surgical intervention. To assess the discriminatory performance of a binary logistic model, a receiver operating curve (ROC) was created, and the area under the ROC curve (AUC) was calculated. Values of AUC0→∞, Cmax, RCmax of nefopam and/or desmethyl-nefopam corresponding to a predicted probability higher than 0.5 (i.e. cut-values) for development of side-effects (PONV and tachycardia) were determined using logistic regression analysis. Finally, values of AUC0→∞, Cmax and RCmax of nefopam and/or desmethyl-nefopam corresponding to a predicted probability lower than 0.5 for morphine requirement were also determined using logistic regression analysis. The regimens needed to achieve these targets were studied by Monte Carlo simulations using Monolix (version 4.1) software.
Results
Subject characteristics
Forty-eight patients were included in this study. Their demographic characteristics are described in Table 1. The demographic characteristics were not significantly different between groups based on their expected renal capacity.
Table 1.
Characteristics of patients with severe renal impairment, moderate renal impairment and normal renal function
| Characteristic | Global population (n = 48) | Subdivision group as function of glomerular rate filtration | |||
|---|---|---|---|---|---|
| Severe renal impairment (n = 10) | Moderate renal impairment (n = 28) | Normal renal function (n = 10) | Statistical test | ||
| GFRa (ml.min−1) | 48.36 ± 24.19 [10.77–131.50] | 21.83 ± 5.79†‡ [10.77–29.91] | 43.89 ± 8.26*‡ [30.21–59.84] | 85.20 ± 23.19*† [64.34–131.50] | c |
| Age (years)b | 83 ± 8 [65–99] | 86 ± 8 [76–99] | 83 ± 7 [72–97] | 78 ± 7 [65–89] | c |
| Weight (kg)b | 63 ± 13 [40–100] | 59 ± 9 [45–75] | 61 ± 13 [40–90] | 71 ± 13 [60–100] | c |
| Height (cm)b | 161 ± 7 [144–183] | 164 ± 3 [160–169] | 160 ± 9 [144–183] | 164 ± 7 [155–177] | c |
| Body mass index (kg m−2)b | 23.9 ± 4.7 [15.6–39.1] | 21.9 ± 2.6 [17.6–26.3] | 23.8 ± 4.8 [15.6–36.3] | 26.3 ± 5.3 [20.5–39.1] | c |
| Haemoglobin (g (100 ml)−1)b | 125.3 ± 18.7 [90.0–157.0] | 116.6 ± 23.4 [94.0–156.0] | 127.5 ± 16.8 [90.0–154.0] | 128.2 ± 19.4 [106.0–157.0] | c |
| Fibrinogen (g l−1)b | 4.2 ± 1.2 [2.0–6.8] | 3.8 ± 1.5 [2.0–5.9] | 3.9 ± 1.1 [2.3–6.8] | 4.9 ± 0.7 [4.7–6.4] | c |
| Sex ratio (male to female) | 0.45 | 0.66 | 0.40 | 0.43 | d |
Glomerular filtration rate (GFR) estimated with Cockroft–Gault formula;
mean ± SD [range];
comparison between groups using one-way ANOVA followed with Tukey's multiple comparison test (P < 0.05); and
comparison between groups using χ2 test.
P < 0.05, compared with severe renal impairment;
P < 0.05, compared with moderate renal impairment;
P < 0.05, compared with normal renal function.
Nefopam pharmacokinetic model
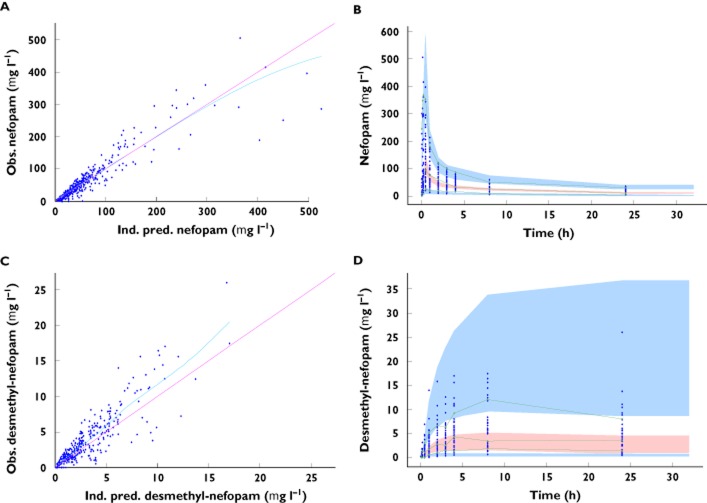
A two-compartment open model, parameterized with clearances (total CLnef and intercompartmental Qnef) and volumes (central V1nef and peripheral V2nef), with zero-order input, best described the pharmacokinetics of nefopam (Figure 1). The BSV was described by exponential terms and residual variability by a combined additive (a = 1.33 μg l−1) and proportional (coefficient = 0.228) error model. Values (estimated mean ± SEM) of CLnef, V1nef, Qnef and V2nef were, respectively, 17.3 ± 1.9 l h−1 (BSV 53 ± 9%), 114 ± 21 l (BSV 121 ± 21%), 80.7 ± 15.0 l h−1 (BSV 79 ± 17%) and 208 ± 31 l (BSV 64 ± 15%). The correlation between observed and predicted concentrations (derived from EBE) of nefopam was evaluated (P < 0.0001, Spearman r = 0.95; Figure 2).
Figure 2.
Goodness-of-fit plots. (A) Observed (Obs) nefopam concentrations vs. individual-predicted (Ind.pred) nefopam concentrations. (B) Observed (Obs) desmethyl-nefopam concentrations vs. individual-predicted (Ind.pred) desmethyl-nefopam concentrations. Prediction-corrected visual predictive check (PC-VPC) for nefopam concentrations (C) and desmethyl-nefopam concentrations (D). The green lines show the 5th, 50th and 95th percentiles of observed data; the areas represent the 90% confidence interval around the simulated percentiles
None of the covariates met the predefined inclusion criteria; however, GFRio with values ≤30 ml min−1 was correlated with CLnef (P < 0.05, Spearman test). Patients with GFRio >30 ml min−1 had higher CLnef and lower area under the curve for nefopam (AUCnef0→∞) than patients with GFRio <30 ml min−1 (P < 0.05; Table 2). The values of Cmax and RCmax for nefopam did not differ between patients with GFRio >30 ml min−1 and patients with GFRio <30 ml min−1 (Table 2).
Table 2.
Individual pharmacokinetic parameters of patients with severe renal impairment (GFRio ≤ 30 ml min−1) or without severe renal impairment
| Severe renal impairment (n = 10) | Without severe renal impairment (n = 38) | |
|---|---|---|
| Nefopam | ||
| Clearance (l h−1) | 13.04 ± 1.45 [8.92–13.50] | 24.61 ± 13.43* [10.44–66.91] |
| Cmax (μg l−1) | 156.2 ± 122.4 [52.9–477.4] | 148.2 ± 131.8 [19.2–512.8] |
| RCmax (μg l−1) h−1) | 315.10 ± 264.40 [103.90–1037.00] | 290.70 ± 268.30 [37.73–1037.00] |
| AUC0→∞ (μg l−1 h) | 1617.0 ± 367.3 [1087.0–2585.0] | 1184 ± 540.6* [382.5–3620] |
| Desmethyl-nefopam | ||
| Clearance (l h−1) | 16.82 ± 8.82 [2.92–28.93] | 17.92 ± 14.88 [3.34–90.46] |
| Cmax (μg l−1) | 7.0 ± 6.1 [1.2–23.0] | 4.9 ± 3.2 [1.3–12.6] |
| RCmax (μg l−1) h−1) | 0.67 ± 0.83 [0.05–2.95] | 1.15 ± 1.16 [0.09–4.87] |
| AUC0→∞ (μg l−1 h) | 141.7 ± 127.0 [25.8–470.5] | 95.1 ± 67.1 [12.0–281.5] |
Abbreviation is as follows: AUC0→∞, Area Under Curve of time course of plasma concentration; Cmax, plasma maximum concentration; GFRio, glomerular filtration rate estimated by iohexol administration; RCmax, rate of increase of plasma concentration.
P < 0.05, compared with severe renal impairment (Mann–Whitney U test). Data are means ± SD [range].
Desmethyl-nefopam pharmacokinetic model
A one-compartment open model, parameterized with total clearances CLdnef and volumes of distribution Vdnef and with first-order input (K13; apparent clearance of metabolization of nefopam to desmethyl-nefopam) best described the pharmacokinetics of desmethyl-nefopam (Figure 1 and Supporting Information). Between-subject variability was described by exponential terms and residual variability by a combined additive (a = 0.01 μg l−1) and proportional (coefficient = 0.68) error model. Typical values for CLdnef, Vdnef and K13 were, respectively, 17.81 ± 0.6 l h−1 (BSV 144 ± 19%), 12.5 ± 0.01 l (BSV 1 ± 0.9%) and 0.35 ± 0.022 l h−1 (BSV 120 ± 67%). The correlation between observed and predicted concentrations (derived from EBE) of desmethyl-nefopam was evaluated (P < 0.0001, Spearman r = 0.94; Figure 2).
As for nefopam, none of the covariates met the predefined inclusion criteria. Clearance, Cmax, RCmax and AUC0→∞ of desmethyl-nefopam were not different between patients with GFRio >30 ml min−1 and patients with GFRio <30 ml min−1 (Table 2).
Evaluation and validation of pharmacokinetic models
The mean and variance of the normalized prediction distribution error (NPDE) metrics 29 for the nefopam model (Figure 3) were not significantly different from zero (P = 0.2, Student's unpaired t test; P = 0.3953, Wilcoxon signed rank test) and one (P = 0.83, Fisher variance test), respectively, and their distribution was not different from a normal distribution (P = 0.21, Shapiro–Wilk test of normality; P = 0.35, D'Agostino and Pearson omnibus normality test).
Figure 3.
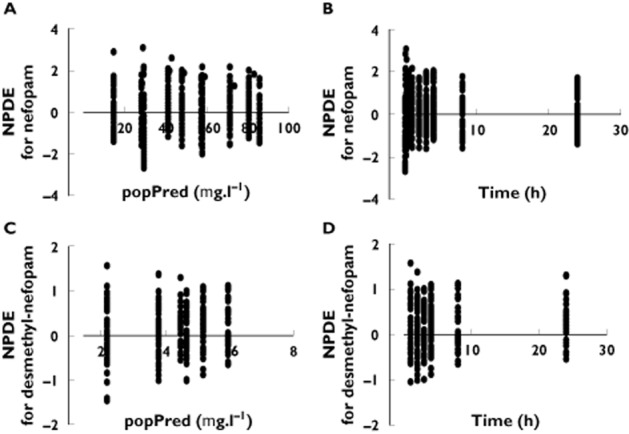
Diagnostic plots. Normalized prediction distribution error (NPDE) as a function of population-predicted (popPred) concentrations of nefopam (A) and desmethyl-nefopam (C), and as a function of time for nefopam (B) and for desmethyl-nefopam (D)
The mean and variance of the NPDE metrics for the desmethyl-nefopam model (Figure 3) were not significantly different from zero (P = 0.10, Student's unpaired t test; P = 0.12, Wilcoxon signed rank test) and one (P = 0.95, Fisher variance test), respectively, and their distribution was not different from a normal distribution (P = 0.11, Shapiro–Wilk test of normality; P = 0.62, D'Agostino and Pearson omnibus normality test).
As shown in Figure 4, PWRES or IWRES vs. time and PWRES or IWRES vs. predictions were centred around zero, without systematic bias, and most values were within ±2 SD (about 5th and 95th percentiles of a normal distribution).
Figure 4.
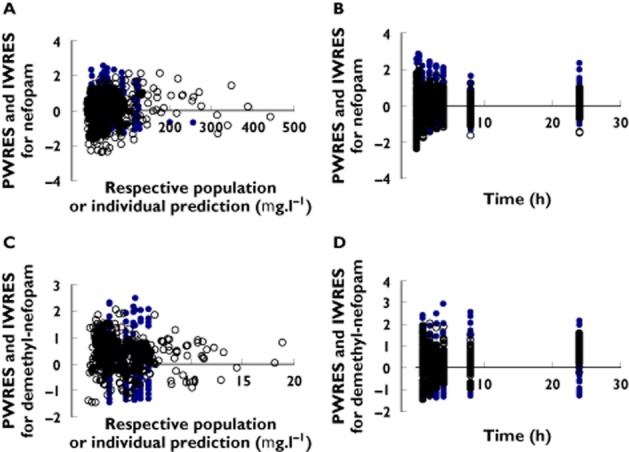
Diagnostic plots. Population (blue dot) or individual (black circle) weighted residuals (PWRES or IWRES) vs. time for nefopam (A) and desmethyl-nefopam (C), and PWRES or IWRES vs. respective predictions for nefopam (B) and desmethyl-nefopam (D)
The prediction-corrected visual predictive check, for nefopam and desmethyl-nefopam, showed that the 5th, 50th and 95th percentiles of observed data are within the 90% confidence interval of 5th, 50th and 95th of simulated percentiles (Figure 2). The observations were contained within prediction intervals, and the models appear adequate to describe the observed data.
Considering these above evaluations, the performance of the model would be considered appropriate and could be used for pharmacokinetic simulations of nefopam in aged patients.
Assessment of relationship between pharmacokinetics and outcome
Tachycardia and PONV were reported in ∼20% of the patients but none showed signs of sweating or sedation. A logistic regression analysis of the data was carried out, considering the presence of tachycardia or PONV (binary outcome measure) and Cmax, RCmax and AUC0→∞ for nefopam or desmethyl-nefopam as the continuous predictors. The logistic model was found to be appropriate for prediction of tachycardia or PONV score using Cmaxnef or RCmaxnef as predictors (Table 3), and inappropriate with predictors such as AUC0→∞ of nefopam even when AUC0→∞ of desmethyl-nefopam was added (P > 0.3, OTMC, Nagelkerke r2 and Hosmer–Lemeshow Test).
Table 3.
Association between pharmacokinetic parameters and pharmacological effect of nefopam analysed by logistic regression
| Logistic regression | Overall correct percentage of prediction* | Receiver operating curve analysis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model acceptance | Goodness of fit | OR and adjusted OR‡ | |||||||||||||
| Predictors | Unit increment | Final −2 log likelihood | P value of OTMC | Nagelkerke r2 | Hosmer–Lemeshow test | Wald test | Exp (B) | Lower 95% CI | Upper 95% CI | Area under curves | P value† | Cut value* | Final model status | ||
| Tachycardia | AUCnef0→∞ | 1 μg l−1 h | 26.390 | 0.128 | 0.250 | 0.867 | 0.049 | 1.001‡ | 1.000 | 1.002 | 80% | 0.788 | 0.019 | 3280 μg l−1 h | |
| Cmaxnef | 1 μg l−1 | 24.338 | 0.05 | 0.330 | 0.770 | 0.025 | 1.010‡ | 1.003 | 1.018 | 83% | 0.887 | 0.002 | 389 μg l−1 | Appropriate | |
| 50 μg l−1 | 0.025 | 1.580‡ | 1.060 | 2.355 | Appropriate | ||||||||||
| RCmaxnef | 1 μg l−1 h−1 | 24.058 | 0.046 | 0.341 | 0.755 | 0.023 | 1.005‡ | 1.001 | 1.009 | 83% | 0.892 | 0.001 | 764 μg l−1 h−1 | Appropriate | |
| 50 μg l−1 h−1 | 0.023 | 1.261‡ | 1.033 | 1.538 | Appropriate | ||||||||||
| PONV | AUCnef0→∞ | 1 μg l−1 h | 28.742 | 0.079 | 0.328 | 0.550 | 0.021 | 1.002‡ | 1.000 | 1.003 | 82.4% | 0.726 | 0.056 | 3280 μg l−1 h | |
| Cmaxnef | 1 μg l−1 | 23.335 | 0.008 | 0.501 | 0.829 | 0.012 | 1.014‡ | 1.003 | 1.026 | 85.3% | 0.774 | 0.021 | 437.44 μg l−1 | Appropriate | |
| 50 μg l−1 | 0.012 | 2.053‡ | 1.169 | 3.605 | Appropriate | ||||||||||
| RCmaxnef | 1 μg l−1 h−1 | 23.482 | 0.009 | 0.497 | 0.882 | 0.012 | 1.007‡ | 1.002 | 1.012 | 85.3% | 0.744 | 0.21 | 859 μg l−1 h−1 | Appropriate | |
| 50 μg l−1 h−1 | 0.012 | 1.416‡ | 1.078 | 1.859 | Appropriate | ||||||||||
| Morphine requirement | AUCnef0→∞ | 1 μg l−1 h | 41.468 | 0.006 | 0.253 | 0.350 | 0.023 | 0.988 | 0.987 | 1.000 | 68% | 0.745 | 0.013 | 947 μg l−1 h | Appropriate |
| Cmaxnef | 1 μg l−1 | 41.797 | 0.007 | 0.243 | 0.349 | 0.048 | 0.988 | 0.977 | 1.000 | 68% | 0.724 | 0.024 | 79 μg l−1 | Appropriate | |
| RCmaxnef | 1 μg l−1 h−1 | 41.567 | 0.006 | 0.250 | 0.353 | 0.047 | 0.994 | 0.988 | 1.000 | 68% | 0.730 | 0.020 | 154 μg l−1 h−1 | Appropriate | |
Abbreviations are as follows: AUCnef0→∞, Area Under Curve of time course of nefopam plasma concentration; Cmaxnef, nefopam plasma maximum concentration; CI, confidence interval; OR, odds ratio; OTMC, omnibus test of model coefficients; PONV, postoperative nausea and vomiting; RCmax, rate of increase of plasma concentration.
The cut value corresponds to the predicted probability of the model higher than 0.60 for tachycardia, 0.80 for PONV and 0.50 for morphine requirement.
Null hypothesis/true area = 0.5.
Adjusted for confounding covariates (see the results of the pharmacodynamics assessment); AUCnef0→∞, Cmaxnef and RCmaxnef were ranged, respectively, within 382.5–3620 μg l−1 h, 19.2–512.8 μg l−1 and 37.7–1037 μg l−1 h−1.
The morphine requirement score was best predicted using AUCnef0→∞ as the predictor (Table 3). Addition of AUCdnef0→∞ to AUCnef0→∞ as a predictor did not improve the model acceptance and goodness-of-fit criteria. Therefore, desmethyl-nefopam pharmacokinetic parameters were excluded from further logistic regression analysis.
The final model of logistic regression for tachycardia included the age and score of drug inducing tachycardia (1, if levobupivacaine at a dose of >75 mg or bupivacaine at a dose of >75 mg was administered; 0, if the same drugs were not administered) as covariates. For logistic regression analysis of PONV, the included covariates were the simplified Apfel score 31, age and drug inducing PONV 17 (score 1, if ropivacaine at a dose >100 mg or levobupivacaine at a dose >125 mg or bupivacaine at a dose >125 mg was administered; score 0, when the same drugs were not administered). However, the final logistic regression model for morphine requirement did not include any covariates.
The odds of developing tachycardia were increased by 58% for every extra 50 μg l−1 of Cmaxnef and by 26.1% for every extra 50 μg l−1 h of RCmaxnef (Table 3). Odds of developing PONV were increased by 205.3% for every extra 50 μg l−1 of Cmaxnef and by 141.6% for every extra 50 μg l−1 h of RCmaxnef (Table 3). There was a decrease of 1.2% in odds of morphine requirement score for every 1 μg l−1 h of AUCnef0→∞ (Table 3). A Cmaxnef higher than 389 μg l−1 and a RCmaxnef higher than 764 μg l−1 h−1 suggested a probability of developing tachycardia higher than 0.6 (60%). A Cmaxnef higher than 437.44 μg l−1 and a RCmaxnef higher than 859 μg l−1 h−1 suggested a probability of developing PONV higher than 0.8 (80%). An AUCnef0→∞ of at least 947 μg l−1 h suggested a probability of morphine sulfate requirement lower than 0.5 (50%).
Simulations of dosage regimens
A dosage regimen was derived from the simulated Cmaxnef, RCmaxnef and AUCnef0→∞. The individual values of Cmaxnef, RCmaxnef and AUCnef0→∞ following different durations of infusion with the same single dose of nefopam (20 mg) were studied by 1000 Monte Carlo simulations using the final population pharmacokinetic model and performed by Monolix (version 4.1) software. As the effect of nefopam on morphine requirement seemed to be linked to AUCnef0→∞ and therefore to dose, we maintained the same dose of nefopam (20 mg) in order to obtain an equivalent morphine-sparing effect. Tachycardia and PONV were associated with Cmaxnef and RCmaxnef (Table 3). Therefore, we decided to optimize infusion time in order to reduce the level of Cmaxnef and RCmaxnef below the determined cut values (Table 3). The worst regimen was a 15 min infusion of 20 mg of nefopam, resulting in a higher probability (>0.2) of simulated Cmaxnef and RCmaxnef higher than their cut values and a probability equal to 0.28 of simulated AUCnef0→∞ below its cut value (Table 3 and Figure 5). Infusions of 20 mg of nefopam over a period of 60, 45 and 30 min were associated with a probability of simulated Cmaxnef and RCmaxnef below their cut values equal to 0.999, 0.94 and 0.88, respectively, and a probability of simulated AUCnef0→∞ below its cut value equal to 0.28, 0.30 and 0.28, respectively (Table 3 and Figure 5).
Figure 5.
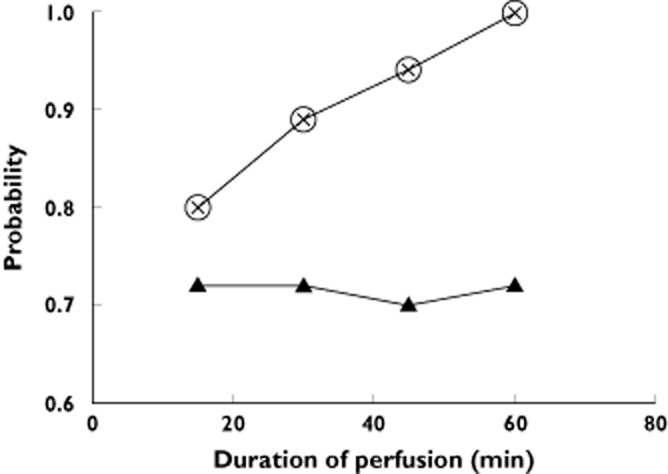
Monte Carlo simulations of different durations in nefopam infusion. The figure shows the probabilities, as a function of duration of infusion, to observe nefopam plasma maximum concentration (Cmaxnef) and rate of increase of nefopam plasma concentration (RCmaxnef) lower than values linked to develop tachycardia or postoperative nausea and vomiting as determined by logistic regression (Table 3) and the probabilities as a function of duration of infusion to observe Area Under Curve of time course of nefopam plasma concentration (AUCnef0→∞) above 950 μg h l−1. The probabilities to observe Cmaxnef and RCmaxnef were the same for all durations of infusion.  , Cmaxnef <389 μg l−1;
, Cmaxnef <389 μg l−1;  , RCmaxnef <764 μg l−1;
, RCmaxnef <764 μg l−1;  , AUCnef0→∞ >950 μg h l−1
, AUCnef0→∞ >950 μg h l−1
Discussion
We report the first study to evaluate the pharmacokinetics of nefopam and its link to treatment response in aged patients with or without renal impairment. First, models able to describe nefopam and desmethyl-nefopam concentrations in the aged patient with or without renal impairment were determined. Then, using a logistic regression approach, the association between pharmacokinetic parameters and morphine requirement, score of tachycardia and score of PONV was assessed. We report that morphine requirement seems to be related to AUC0→∞ of nefopam and that nefopam side-effects seem mainly linked to the speed of administration.
Physiological and homeostatic impairments in the elderly may affect the response to drugs 4,5. A decrease in hepatic extraction 5 as a result of reduced hepatic flow and reduced liver mass 32,33, as well as reduced renal function, with a decrease of 10% per decade in the adult years 34, have been reported. These age-related reductions in renal and hepatic function might partly explain the three times lower total clearance of nefopam reported in our study in comparison to previous studies 18,35,36. A decrease in lean body mass associated with an increase in adipose tissue might explain the differences in intercompartmental clearance of nefopam in our series compared with previous studies performed in younger subjects 18,36. The central volume of distribution for nefopam was two times lower compared with previously reported values 18. For the same infusion duration of 20 mg of nefopam, the decrease in total clearance of nefopam associated with the decrease in central volume of distribution might explain the three times higher values of Cmaxnef in older patients (150 ± 128 μg l−1, range 19.21–512.8 μg l−1) compared with younger subjects 18,35. This higher value of Cmaxnef could also be due to an age-related decrease in cardiac output, as previously described 5. The mean AUC0→∞ of nefopam (1411 ± 724 μg l−1 h, range 382.5–3620 μg l−1 h) in our older population was 1.5 times higher than the value reported for younger subjects 35.
A relationship between AUC0→∞, Cmax and RCmax of nefopam and morphine requirement was reported (Table 3). Moreover, both Cmaxnef and RCmaxnef were linked to tachycardia and PONV, but the association between AUCnef0→∞ and PONV and tachycardia was significant (Table 3). Tachycardia and, to a lesser extent, PONV are probably related to the antimuscarinic and sympathomimetic activity of nefopam 17, to inappropriate physiological responses of aged patients and to a rapid rate of administration of nefopam.
Renal impairment had an impact on AUC0→∞ but not on Cmax and RCmax of nefopam (Table 2). This is explained by the fact that dose and clearance directly influence the AUC, and that the dose and speed of administration, but not clearance, act significantly on Cmax and RCmax of nefopam. The impact of severe renal impairment (GFR <30 ml min−1) on pharmacokinetic parameters of nefopam has been previously reported 18. In our series, we confirmed the impact of severe renal impairment (Table 2), while moderate renal impairment did not reduce total clearance in aged patients, probably because this effect is confounded by the effect of age on the GFR. Furthermore, renal impairment was not associated with development of tachycardia and PONV, as confirmed by the logistic regression analysis (covariate analysis). The values of AUCnef0→∞ of patients with severe renal impairment (Table 2) were above the cut value for morphine requirement (950 μg l−1 h); consequently, patients with severe renal impairment did not require morphine supplementation.
As shown by Monte Carlo simulations for aged patients (with or without renal impairment), a better-tolerated infusion duration for 20 mg of nefopam would be between 45 and 60 min, with an equal morphine requirement compared with the standard protocol (infusion over 30 min of 20 mg nefopam). By choosing a 45 min infusion, the probability of Cmax and RCmax of nefopam being higher than their cut value is about 5%, whereas it is only 0.1% when the duration of infusion is 60 min.
This study has several limitations. As we studied the pharmacokinetics of a single dose of nefopam in aged patients, we could not extrapolate the impact of renal impairment on outcomes after multiple administrations of nefopam and, in particular, on accumulation of nefopam. The analgesic effect of nefopam in the elderly was not evaluated in our study. Nevertheless, the association between various predictors and morphine requirement was assessed. Further studies are therefore required to clarify these limitations.
In conclusion, we validated the first pharmacokinetic model able to describe nefopam and desmethyl-nefopam concentrations in aged patients. By using clearance of iohexol to evaluate renal function, we found that renal impairment is associated with a decrease in the nefopam clearance of aged patients when GFRio is <30 ml min−1, but not associated with development of adverse effects. Using a logistic regression analysis, pharmacokinetic predictors of morphine requirement and side-effects, such as tachycardia and PONV, were identified. In order to maintain morphine sparing and decrease the side-effects of nefopam, Monte Carlo simulations suggest, for a single dose of nefopam (20 mg), an administration period >45 min in elderly patients (>65 years) with or without renal impairment.
Acknowledgments
We thank Dr M.-E. Le Guern for kindly supplying the nefopam hydrochloride and desmethyl-nefopam hydrochloride (Biocodex, Montrouge, France). We thank medical staff who collected the data of the study. We thank laboratory staff of the Department of Pharmacology who performed plasma determinations.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: this study was funded by the Regional Clinical Research Program of Reims University Hospital, in 2004 (PHRC 2004-R11-07); no financial relationships with any organizations that might have an interest in the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Two compartment models for nefopam population pharmacokinetic
Demethylnefopam population pharmacokinetic
References
- 1.Aubrun F, Marmion F. The elderly patient and postoperative pain treatment. Best Pract Res Clin Anaesthesiol. 2007;21:109–127. doi: 10.1016/j.bpa.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Kaye AD, Baluch A, Scott JT. Pain management in the elderly population: a review. Ochsner J. 2010;10:179–187. [PMC free article] [PubMed] [Google Scholar]
- 3.Zyczkowska J, Szczerbińska K, Jantzi MR, Hirdes JP. Pain among the oldest old in community and institutional settings. Pain. 2007;129:167–176. doi: 10.1016/j.pain.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Abdulla A, Bone M, Adams N, Elliott AM, Jones D, Knaggs R, Martin D, Sampson EL, Schofield P. Evidence-based clinical practice guidelines on management of pain in older people. Age Ageing. 2013;42:151–153. doi: 10.1093/ageing/afs199. [DOI] [PubMed] [Google Scholar]
- 5.Aubrun F. Management of postoperative analgesia in elderly patients. Reg Anesth Pain Med. 2005;30:363–379. doi: 10.1016/j.rapm.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Coldrey JC, Upton RN, Macintyre PE. Advances in analgesia in the older patient. Best Pract Res Clin Anaesthesiol. 2011;25:367–378. doi: 10.1016/j.bpa.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Falzone E, Hoffmann C, Keita H. Postoperative analgesia in elderly patients. Drugs Aging. 2013;30:81–90. doi: 10.1007/s40266-012-0047-7. [DOI] [PubMed] [Google Scholar]
- 8.Evans MS, Lysakowski C, Tramèr MR. Nefopam for the prevention of postoperative pain: quantitative systematic review. Br J Anaesth. 2008;101:610–617. doi: 10.1093/bja/aen267. [DOI] [PubMed] [Google Scholar]
- 9.Chanques G, Sebbane M, Constantin JM, Ramillon N, Jung B, Cissé M, Lefrant JY, Jaber S. Analgesic efficacy and haemodynamic effects of nefopam in critically ill patients. Br J Anaesth. 2011;106:336–343. doi: 10.1093/bja/aeq375. [DOI] [PubMed] [Google Scholar]
- 10.Du Manoir B, Aubrun F, Langlois M, Le Guern ME, Alquier C, Chauvin M, Fletcher D. Randomized prospective study of the analgesic effect of nefopam after orthopaedic surgery. Br J Anaesth. 2003;91:836–841. doi: 10.1093/bja/aeg264. [DOI] [PubMed] [Google Scholar]
- 11.Gasser JC, Bellville JW. Respiratory effects of nefopam. Clin Pharmacol Ther. 1975;18:175–179. doi: 10.1002/cpt1975182175. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt AM, Pleuvry BJ, Maddison SE. Respiratory and metabolic effects of oral nefopam in human volunteers. Br J Clin Pharmacol. 1981;11:209–211. doi: 10.1111/j.1365-2125.1981.tb01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mimoz O, Incagnoli P, Josse C, Gillon MC, Kuhlman L, Mirand A, Soilleux H, Fletcher D. Analgesic efficacy and safety of nefopam vs propacetamol following hepatic resection. Anaesthesia. 2001;56:520–525. doi: 10.1046/j.1365-2044.2001.01980.x. [DOI] [PubMed] [Google Scholar]
- 14.McLintock TT, Kenny GN, Howie JC, McArdle CS, Lawrie S, Aitken H. Assessment of the analgesic efficacy of nefopam hydrochloride after upper abdominal surgery: a study using patient controlled analgesia. Br J Surg. 1988;75:779–781. doi: 10.1002/bjs.1800750818. [DOI] [PubMed] [Google Scholar]
- 15.Kapfer B, Alfonsi P, Guignard B, Sessler DI, Chauvin M. Nefopam and ketamine comparably enhance postoperative analgesia. Anesth Analg. 2005;100:169–174. doi: 10.1213/01.ANE.0000138037.19757.ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heel RC, Brogden RN, Pakes GE, Speight TM, Avery GS. Nefopam: a review of its pharmacological properties and therapeutic efficacy. Drugs. 1980;19:249–267. doi: 10.2165/00003495-198019040-00001. [DOI] [PubMed] [Google Scholar]
- 17.Sweetman SC. Martindale: The Complete Drug Reference. 36th edn. London: Pharmaceutical Press; 2009. 36th Revised edn. [Google Scholar]
- 18.Mimoz O, Chauvet S, Grégoire N, Marchand S, Le Guern M-E, Saleh A, Couet W, Debaene B, Levy RH. Nefopam pharmacokinetics in patients with end-stage renal disease. Anesth Analg. 2010;111:1146–1153. doi: 10.1213/ANE.0b013e3181f33488. [DOI] [PubMed] [Google Scholar]
- 19.Stolz A, Hoizey G, Toupance O, Lavaud S, Vitry F, Chanard J, Rieu P. Evaluation of sample bias for measuring plasma iohexol clearance in kidney transplantation. Transplantation. 2010;89:440–445. doi: 10.1097/TP.0b013e3181ca7d1b. [DOI] [PubMed] [Google Scholar]
- 20.Hoizey G, Goglin A, Malinovsky J-M, Robinet A, Binet L, Kaltenbach ML, Millart H, Lamiable D. Specific and sensitive analysis of nefopam and its main metabolite desmethyl-nefopam in human plasma by liquid chromatography-ion trap tandem mass spectrometry. J Pharm Biomed Anal. 2006;42:593–600. doi: 10.1016/j.jpba.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Lavielle M, Mentré F. Estimation of population pharmacokinetic parameters of saquinavir in HIV patients with the MONOLIX software. J Pharmacokinet Pharmacodyn. 2007;34:229–249. doi: 10.1007/s10928-006-9043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertrand J, Treluyer J-M, Panhard X, Tran A, Auleley S, Rey E, Salmon-Céron D, Duval X, Mentré F. Influence of pharmacogenetics on indinavir disposition and short-term response in HIV patients initiating HAART. Eur J Clin Pharmacol. 2009;65:667–678. doi: 10.1007/s00228-009-0660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertrand J, Laffont CM, Mentré F, Chenel M, Comets E. Development of a complex parent-metabolite joint population pharmacokinetic model. AAPS J. 2011;13:390–404. doi: 10.1208/s12248-011-9282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urien S, Firtion G, Anderson ST, Hirt D, Solas C, Peytavin G, Faye A, Thuret I, Leprevost M, Giraud C, Lyall H, Khoo S, Blanche S, Tréluyer J-M. Lopinavir/ritonavir population pharmacokinetics in neonates and infants. Br J Clin Pharmacol. 2011;71:956–960. doi: 10.1111/j.1365-2125.2011.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol. 2013;2:e38. doi: 10.1038/psp.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonate PL. Pharmacokinetic-pharmacodynamic Modeling and Simulation. 2nd edn. New York: Springer-Verlag New York Inc; 2011. [Google Scholar]
- 27.Saint-Marcoux F, Royer B, Debord J, Larosa F, Legrand F, Deconinck E, Kantelip J-P, Marquet P. Pharmacokinetic modelling and development of Bayesian estimators for therapeutic drug monitoring of mycophenolate mofetil in reduced-intensity haematopoietic stem cell transplantation. Clin Pharmacokinet. 2009;48:667–675. doi: 10.2165/11317140-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comets E, Brendel K, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed. 2008;90:154–166. doi: 10.1016/j.cmpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 31.Apfel CC, Kranke P, Eberhart LHJ, Roos A, Roewer N. Comparison of predictive models for postoperative nausea and vomiting. Br J Anaesth. 2002;88:234–240. doi: 10.1093/bja/88.2.234. [DOI] [PubMed] [Google Scholar]
- 32.Wood AJ, Vestal RE, Wilkinson GR, Branch RA, Shand DG. Effect of aging and cigarette smoking on antipyrine and indocyanine green elimination. Clin Pharmacol Ther. 1979;26:16–20. doi: 10.1002/cpt197926116. [DOI] [PubMed] [Google Scholar]
- 33.Sato T, Cespedes RF, Goyenaga PH, Tauchi H. Age changes in the livers of Costa Ricans. Mech Ageing Dev. 1979;11:171–178. doi: 10.1016/0047-6374(79)90052-6. [DOI] [PubMed] [Google Scholar]
- 34.Papper S. The effects of age in reducing renal function. Geriatrics. 1973;28:83–87. [PubMed] [Google Scholar]
- 35.Aymard G, Warot D, Démolis P, Giudicelli JF, Lechat P, Le Guern ME, Alquier C, Diquet B. Comparative pharmacokinetics and pharmacodynamics of intravenous and oral nefopam in healthy volunteers. Pharmacol Toxicol. 2003;92:279–286. doi: 10.1034/j.1600-0773.2003.920605.x. [DOI] [PubMed] [Google Scholar]
- 36.Podranski T, Bouillon TW, Riva T, Kurz AM, Oehmke MJ. Compartmental pharmacokinetics of nefopam during mild hypothermia. Br J Anaesth. 2012;108:784–791. doi: 10.1093/bja/aer517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two compartment models for nefopam population pharmacokinetic
Demethylnefopam population pharmacokinetic