Abstract
Aims
Voclosporin is a novel calcineurin inhibitor intended for prevention of organ graft rejection and treatment of lupus nephritis. Pharmacokinetic drug interactions between voclosporin and a CYP3A inhibitor, inducer and substrate and a P-glycoprotein inhibitor and substrate were evaluated.
Methods
Voclosporin 0.4 mg kg−1 was administered to 24 subjects in each of five studies, as follows: every 12 h (Q12H) alone and concomitantly with ketoconazole 400 mg once daily (QD); single dose before and single dose after rifampin 600 mg QD; Q12H where midazolam 7.5 mg was administered as a single dose alone before voclosporin and with last the dose of voclosporin; Q12H alone and concomitantly with verapamil 80 mg every 8 h; and Q12H with digoxin 0.25 mg QD. The noncompartmental pharmacokinetic parameters maximal concentration (Cmax) and area under the concentration–time curve (AUC) were obtained, and geometric least squares mean ratios and 90% confidence intervals were evaluated.
Results
Ketoconazole increased voclosporin Cmax (6.4-fold) and AUC (18-fold); rifampin reduced voclosporin AUC (0.9-fold); voclosporin did not change exposure of midazolam or α-hydroxy-midazolam; verapamil increased voclosporin Cmax (2.1-fold) and AUC (2.7-fold); and voclosporin increased digoxin Cmax (0.5-fold), AUC (0.25-fold) and urinary excretion (0.2-fold).
Conclusions
Administration of voclosporin concomitantly with strong inhibitors and inducers of CYP3A resulted in increased and decreased exposures, respectively, and should be considered contraindicated. Drug–drug interactions involving voclosporin and CYP3A substrates are not expected. Administration of voclosporin concomitantly with inhibitors and substrates of P-glycoprotein resulted in increased voclosporin and substrate exposures, respectively. Appropriate concentration and safety monitoring is recommended with co-administration of voclosporin and P-glycoprotein substrates and inhibitors.
Keywords: calcineurin inhibitor, cytochrome P450, drug interaction, P-glycoprotein, pharmacokinetics, voclosporin
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Calcineurin inhibitors are the gold standard for immunosuppression in transplantation.
Drug–drug interactions with cytochrome P450 inducers, inhibitors and substrates and with P-glycoprotein inhibitors and substrates in these drugs with narrow therapeutic windows may impact both safety and efficacy.
Knowledge of patient and drug factors that influence drug disposition enhances the optimal dosing and successful therapy with the drug.
WHAT THIS STUDY ADDS
These results provide a rationale for avoiding the co-administration of voclosporin with strong CYP3A inhibitors and CYP3A inducers.
These results suggest that co-administration of voclosporin with substrates of CYP3A would not result in increased exposure to the substrate.
These results provide a rationale for conducting appropriate concentration and safety monitoring when voclosporin is co-administered with P-glycoprotein substrates and inhibitors.
Introduction
Voclosporin is a novel calcineurin inhibitor intended for use in the prevention of organ graft rejection and the treatment of autoimmune diseases, such as lupus nephritis. Voclosporin was created by adding a single carbon extension to the amino acid-1 region of ciclosporin A (CsA). X-Ray crystallography studies have shown that this increase in carbon length alters the way in which the cyclophilin–voclosporin complex binds to a composite surface of catalytic and regulatory subunits in calcineurin (latch region). This change in binding has increased the potency of voclosporin in comparison to CsA 1, which has been confirmed in vivo using a 32P-labelled calcineurin activity assay 2. Calcineurin activity has been studied as a potential biomarker throughout the development of voclosporin. As amino acid-1 is the primary site of metabolism of CsA, modification of this site has shifted the primary site for voclosporin metabolism to the amino acid-9 position. In vitro studies suggest that the primary metabolites for CsA and voclosporin occur at amino acids 1 and 9, respectively, which are equipotent in their ability to prevent T-cell activation and possess ∼10% of the parent compound activity (personal communication from R. Huizinga, Isotechnika Pharma Inc.). Critically, amino acid 9 is produced in significantly smaller amounts than amino acid 1, resulting in less competitive antagonism of their respective parent molecules. The combination of increased potency and a change in metabolite profile for voclosporin allows for administration of lower doses, less pharmacokinetic–pharmacodynamic variability and a potentially improved safety profile compared with CsA.
Voclosporin is a large molecular weight, highly lipophilic molecule, which suggests that it would favour biliary excretion and hepatic metabolism as the primary elimination routes. It is estimated that over 99% of the drug is eliminated as metabolite, mainly via the cytochrome P450 3A (CYP3A) isoform (personal communication from R. Huizinga, Isotechnika Pharma Inc.). In vitro studies suggest that voclosporin is a direct, competitive inhibitor of CYP3A, with the potential for clinically relevant drug–drug interactions (personal communication from R. Huizinga, Isotechnika Pharma Inc.). As with CsA, drug–drug interactions are expected when voclosporin is administered concomitantly with medications that interact with CYP3A. In vitro studies suggest that voclosporin could be a substrate for P-glycoprotein and a potential inhibitor of P-glycoprotein (personal communication from R. Huizinga, Isotechnika Pharma Inc.). Given that immunosuppressants are frequently co-administered with P-glycoprotein-modulated drugs, it was necessary to evaluate the potential for drug–drug interactions between voclosporin and a model P-glycoprotein inhibitor and a model P-glycoprotein substrate. Based on the US Food and Drug Administration (FDA) Draft Guidance, ketoconazole, rifampin and midazolam are considered to be a suitable model CYP3A inhibitor, inducer and substrate, respectively, and verapamil and digoxin are considered a suitable model P-glycoprotein inhibitor and substrate, respectively, because these drugs would be most sensitive for identifying a pharmacokinetic interaction 3,4.
Five drug–drug interaction studies were conducted to determine the pharmacokinetic drug interaction between voclosporin and a model CYP3A inhibitor (ketoconazole), inducer (rifampin) and substrate (midazolam) and between voclosporin and a model P-glycoprotein inhibitor (verapamil) and substrate (digoxin) in healthy adult volunteers.
Methods
These open-label, multi-arm, sequential studies were conducted at two clinical sites in Canada, following the FDA drug interaction guidance. The protocol and all modifications and appropriate consent procedures were reviewed and approved by a Research Ethics Board or Institutional Review Board (REB/IRB) at each site in accordance with the current regulations. All subjects signed an informed consent form at the screening visit. The principal investigators ensured that each study adhered fully to the principles outlined in the Good Clinical Practice (GCP) International Conference on Harmonization (ICH) Tripartite Guideline (January 1997), which is based on the principles of the Declaration of Helsinki (1996).
Subjects
Nonsmoking, male or female subjects aged 18–45 years, with a body mass index between 19 and 30 kg m−2, were included.
Main exclusion criteria included the following: any clinically significant abnormality (including clinically significant ECG or vital sign abnormalities) or abnormal laboratory test results, clinically significant illness or surgery within 4 weeks prior to dosing, clinically significant history or presence of any condition known to interfere with the absorption, distribution, metabolism or excretion of the drug, use of any tobacco products within 3 months, history of latent or active tuberculosis or exposure to endemic areas within 8 weeks prior to PPD tuberculin testing, history of positive PPD testing or positive PPD testing result (≥5 mm) indicating possible tuberculosis infection, history of opportunistic infection or serious local or systemic infection within 3 months, or symptoms of fever, viral or bacterial infection.
Medication exclusion included the following: history of significant alcohol or drug abuse within 1 year prior to the screening visit, regular use of alcohol (within 6 months), use of soft drugs, such as marijuana (within 3 months), or hard drugs, such as cocaine, phencyclidine and crack (within 1 year), use of any drugs known to induce or inhibit hepatic drug metabolism within 30 days, or use of prescription medication within 14 days.
Main laboratory exclusions included the following: positive test for hepatitis B, hepatitis C or human immunodeficiency virus, haemoglobin <128 g l−1 (males) and <115 g l−1 (females), haematocrit <0.37 l l−1 (males) and <0.32 l l−1 (females), positive pregnancy test at screening, positive urine cotinine test, positive alcohol breath test, or a positive urine drug screen.
Study design
Voclosporin was administered orally every 12 h (Q12H) at approximately the same time every day, with subjects in a fasting condition for at least 8 h prior to the morning dose and 2 h prior to the evening dose. The voclosporin oral dose of 0.4 mg kg−1 was selected as the anticipated therapeutic dose based on phase 1 and 2 clinical studies.
The duration of blood sampling differed between studies and between drugs as described individually for each study. Blood samples were collected to characterize the pharmacokinetic profile of the substrate drug over approximately five half-lives following single doses and during an interval between doses after multiple dosing.
Ketoconazole study
The objective of this study was to determine the effects of ketoconazole on the pharmacokinetics of voclosporin when both drugs are at steady state. Twenty-four subjects were given voclosporin 0.4 mg kg−1 orally Q12H for 20 consecutive days (days 1–20). Subjects were to be given oral ketoconazole 400 mg once daily (QD) for 10 consecutive days (days 11–20) concomitantly with the morning dose of voclosporin. For voclosporin and ketoconazole, blood samples were drawn from each subject for pharmacokinetic analyses. Voclosporin samples were drawn at 0, 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2, 3, 4, 5, 6, 8, 10 and 12 h after the morning dose on days 10 and 20. Ketoconazole samples were drawn at 0, 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2, 3, 4, 5, 6, 8, 10, 12 and 24 h after the morning dose on day 20.
Rifampin study
The objective of this study was to determine the effects of rifampin on the pharmacokinetics of voclosporin after single doses when rifampin is at steady state. Twenty-four subjects received a single 0.4 mg kg−1 dose of voclosporin orally on days 1 and 16. Subjects received oral rifampin 600 mg QD for 10 consecutive days (days 6–15). For voclosporin and rifampin, blood samples were drawn from each subject for pharmacokinetic analyses. Voclosporin samples were drawn at 0, 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2, 3, 4, 5, 6, 8, 10, 12, 24, 48, 72, 96 and 120 h after the morning dose on days 1 and 16. Rifampin samples were drawn prior to rifampin administration on days 14 and 15, and at 24 h after the day 15 dose. The predose sample obtained on day 1 for quantification of voclosporin was also analysed for rifampin.
Midazolam study
The objective of this study was to determine the effects of voclosporin on the pharmacokinetics of midazolam when voclosporin is at steady state and midazolam is given as a single dose. Twenty-four male and female subjects received midazolam 7.5 mg as a single oral dose on days 1 and 12, and voclosporin 0.4 mg kg−1 orally Q12H on days 2 through to the evening dose on day 12. For voclosporin and midazolam, blood samples were drawn from each subject for pharmacokinetic analyses. Voclosporin samples were drawn at 0, 0.25, 0.5, 1.0, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 10 and 12 h after the morning dose on day 12. Midazolam samples were drawn at 0, 0.25, 0.5, 1.0, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 10, 12, 16 and 24 h after the morning dose on days 1 and 12.
Verapamil study
The objective of the study was to determine the effects of verapamil on the pharmacokinetics of voclosporin with both drugs at steady state. Twenty-four subjects were given voclosporin 0.4 mg kg−1 orally Q12H for 20 consecutive days (days 1–20). Subjects were given verapamil 80 mg every 8 h (Q8H) for 10 consecutive days (days 11–20) concomitantly with the morning dose of voclosporin. Blood samples for pharmacokinetic analyses were drawn from each subject at 0, 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2, 3, 4, 5, 6, 8, 10 and 12 h after the morning dose on days 10 (voclosporin) and 20 (voclosporin and verapamil).
Digoxin study
The objective of this study was to determine the effects of voclosporin on the pharmacokinetics of digoxin when both drugs are at steady state. Twenty-four subjects received oral digoxin 0.5 mg on day 1 followed by digoxin 0.25 mg every day for 17 consecutive days (days 2–18). Subjects were administered voclosporin 0.4 mg kg−1 Q12H orally for 11 consecutive days (days 8–18), with the final dose on the evening of day 18. The morning dose of voclosporin was given concomitantly with the dose of digoxin. Blood samples for pharmacokinetic analyses were drawn from each subject at 0, 0.25, 0.5, 1.0, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 10, 12, 16 and 24 h after the morning dose on day 7 (digoxin) and day 18 (digoxin and voclosporin). Pooled urine samples were collected for the measurement of digoxin concentration over the 24 h dosing interval on days 7 and 18.
Sample analysis
Whole blood concentrations of voclosporin were measured using a validated liquid chromatography–tandem mass spectrometry (LC/MS/MS) method by Isotechnika Pharma Inc. 5. Plasma concentrations of ketoconazole, rifampin, midazolam, α-hydroxy-midazolam, verapamil and digoxin were measured using a validated LC/MS/MS method by Anapharm (Québec City, Québec, Canada). The limit of quantification for voclosporin, ketoconazole, rifampin, midazolam, α-hydroxy-midazolam, verapamil, digoxin and urinary digoxin was 2 ng ml−1, 40.1 ng ml−1, 0.5 ng ml−1, 100 pg ml−1, 100 pg ml−1, 50.1 pg ml−1 and 2 ng ml−1, respectively. Assays were evaluated for possible interference before study samples were analysed.
Pharmacokinetic analysis
Pharmacokinetic parameters for voclosporin, ketoconazole, midazolam, α-hydroxy-midazolam, verapamil and digoxin were calculated using noncompartmental analysis. Only whole blood or plasma concentrations greater than or equal to the limit of quantification for the respective assays were used in the pharmacokinetic analyses. Actual blood sampling times were used in all pharmacokinetic analyses. Nominal times were used to calculate mean concentrations for graphical displays. The maximal whole blood or plasma concentrations (Cmax) and time to Cmax (Tmax) were taken directly from the data without interpolation. For single-dose data, the elimination rate constant (Kel) was estimated by linear regression of at least three points in the elimination phase. Elimination half-life (t1/2) was calculated as 0.693/Kel. Area under the curve over the 8 h [AUC(0–8)], 12 h [AUC(0–12)] or 24 h [AUC(0–24)] dosing intervals was calculated using the linear trapezoidal method. Area under the curve from zero to infinity [AUC(0–inf)] was calculated as the sum of AUC(0–t) and the last observed concentration divided by Kel. The 24 h urinary excretion (Ue) of digoxin was calculated from the urinary concentration of drug and volume of urine and renal clearance as the quotient of Ue(0–24)/AUC(0–24). Given that only predose plasma rifampin concentrations were measured, no formal pharmacokinetic analysis was conducted on these samples.
All pharmacokinetic calculations were done using SAS® for Windows® version 9.1.3.
Statistical analysis
Comparisons were made for the pharmacokinetic parameters: Cmax and AUC(0–12) on days 10 and 20 for voclosporin in the ketoconazole study; Cmax, AUC(0–t) and AUC(0–inf) on days 1 and 16 for voclosporin in the rifampin study; Cmax, AUC(0–t) and AUC(0–inf) on days 1 and 12 for midazolam and α-hydroxy-midazolam in the midazolam study; Cmax and AUC(0–12) on days 10 and 20 for voclosporin in the verapamil study; and Cmax, AUC(0–24) and Ue(0–24) on days 7 and 18 for digoxin in the digoxin study. The comparisons were made with an analysis of variance (ANOVA) model using the natural logarithms of the data. The 90% confidence intervals (CIs) were constructed for the treatment ratios (substrate + inhibitor/inducer-to-substrate alone) of both parameters using the natural log-transformed data. The point estimates and confidence limits were exponentiated back to the original scale.
Following the FDA Draft Guidance, if the 90% CIs for the geometric mean ratios for Cmax and AUC fell within the default no effect boundary of 80–125%, then the conclusion that no clinically significant differences are present was to be made.
Statistical analyses were performed using SAS® for Windows® version 9.1.3. Graphs were produced using ggplot2 in R version 2.15.0 (Vienna, Austria).
The sample sizes chosen for these studies were based on feasibility and on a typical screening panel that was judged to be sufficient to detect a clinically important effect. The sample sizes were not based on formal power calculations.
Safety evaluation
Safety assessments were conducted according to the principles outlined in the GCP ICH Tripartite Guideline (January 1997) and included physical examinations, adverse events (AEs), vital signs (heart rate and blood pressure), ECG and clinical laboratory tests. Vital signs were assessed at the time of blood work on screening, from day −1 to the end of the study and follow-up using clinically acceptable methods and devices. The measurements were taken only after the subject had remained in a supine position for at least 5 min and prior to any blood sampling. The total bodyweight was measured, and this value was used to calculate the exact dose of medication required on a milligram per kilogram basis rounded to 10 mg. A 12-lead safety ECG was taken before dosing at screening and on day −1 to ensure the subject's eligibility, and throughout the study for subject safety.
Results
Analysis populations
A total of 24 subjects were enrolled into each study. Subjects' baseline characteristics are summarized in Table 1. A total of 11 subjects completed the ketoconazole study. Nine subjects were withdrawn on day 18 due to increased serum creatinine. Two subjects withdrew consent due to AEs and two subjects were withdrawn due to AEs. The analysis population was therefore composed of the 11 subjects who completed the study. A total of 24 subjects completed the rifampin study. Voclosporin data for two subjects on day 16 were too sparse for pharmacokinetic analysis. The analysis population was therefore composed of 22 subjects with voclosporin data for both days 1 and 16. A total of 22 subjects completed the midazolam study. Two subjects withdrew due to high white blood cell count and due to flu-like symptoms and were not included in the pharmacokinetic and statistical analyses. The analysis population was therefore composed of the 22 subjects who completed the study. A total of 20 subjects completed the verapamil study. Three subjects were withdrawn due to AEs and one subject withdrew consent. The analysis population was therefore composed of the 20 subjects who completed the study. A total of 23 subjects completed the digoxin study. One subject was withdrawn due to missed sampling time points and not included in the statistical analyses. The analysis population was therefore composed of the 23 subjects who completed the study.
Table 1.
Summary of demographic data
| Demographics | Ketoconazole study | Rifampin study | Midazolam study | Verapamil study | Digoxin study |
|---|---|---|---|---|---|
| Subjects completing the study (n) | 11 | 24 | 22 | 20 | 23 |
| Male/female (n) | 7/4 | 17/7 | 21/1 | 15/5 | 15/8 |
| Age (years) | 30 (20–42) | 31 (18–45) | 33 (19–44) | 29 (19–43) | 32 (18–45) |
| Race | |||||
| Black | 0 | 4 | 0 | 1 | 1 |
| Caucasian | 11 | 20 | 22 | 18 | 22 |
| Hispanic | 0 | 0 | 0 | 0 | 0 |
| Other | 0 | 0 | 0 | 1 | 0 |
| Height (cm) | 167.0 | 172.0 | 172.1 | 174.2 | 168.5 |
| (149.0–180.5) | (152.5–190.0) | (155.5–189.0) | (160.0–193.5) | (153.0–183.5) | |
| Weight (kg) | 66.3 | 74.3 | 75.3 | 76.9 | 68.7 |
| (45.3–92.1) | (56.8–98.8) | (58.2–102.0) | (56.9–103.5) | (55.4–89.1) | |
| Body mass index (kg m−2) | 23.5 | 25.0 | 25.4 | 25.2 | 24.2 |
| (19.8–29.6) | (20.7–29.3) | (20.6–29.3) | (19.1–28.8) | (20.6–27.4) |
Values are shown as means (range).
Pharmacokinetics
Ketoconazole study
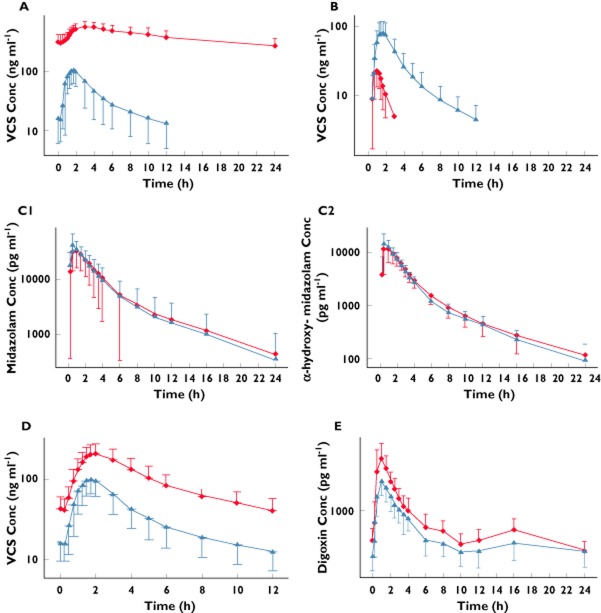
There was an increase in the mean whole blood concentrations of voclosporin when concomitantly administered with ketoconazole (Figure 1A). There were corresponding increases in both Cmax and AUC(0–12) with geometric least squares mean ratios for voclosporin + ketoconazole to voclosporin alone of 645 and 1855%, respectively, demonstrating a significant drug–drug interaction (Table 2). Median Tmax increased from 1.5 to 3.0 h when voclosporin was administered with ketoconazole.
Figure 1.
Whole blood or plasma concentrations (means ± SD) of voclosporin (VCS) 0.4 mg kg−1 every 12 h (Q12H) alone and with ketoconazole 400 mg once daily (QD; A); VCS 0.4 mg kg−1 alone and following 10 days of rifampin 600 mg QD (B); midazolam 7.5 mg alone and with VCS 0.4 mg kg−1 Q12H (C1); α-hydroxy-midazolam after administration of midazolam 7.5 mg alone and with VCS 0.4 mg kg−1 Q12H (C2); VCS 0.4 mg kg−1 Q12H alone and with verapamil 80 mg every 8 h (D); and digoxin 0.25 mg QD alone and with VCS 0.4 mg kg−1 Q12H (E). (A)  , VCS alone;
, VCS alone;  , VCS + ketoconazole. (B)
, VCS + ketoconazole. (B)  , VCS alone;
, VCS alone;  , VCS + rifampin. (C1)
, VCS + rifampin. (C1)  , midazolam alone;
, midazolam alone;  , midazolam + VCS. (C2)
, midazolam + VCS. (C2)  , midazolam alone;
, midazolam alone;  , midazolam + VCS. (D)
, midazolam + VCS. (D)  , VCS alone;
, VCS alone;  , VCS + verapamil. (E)
, VCS + verapamil. (E)  , digoxin alone;
, digoxin alone;  , digoxin + VCS
, digoxin + VCS
Table 2.
Pharmacokinetic parameters for voclosporin and ketoconazole after oral administration of voclosporin 0.4 mg kg−1 every 12 h alone and with ketoconazole 400 mg once daily
| Parameter | Voclosporin alone (n = 11) | Voclosporin + ketoconazole (n = 11) | |
|---|---|---|---|
| Voclosporin | Voclosporin | Ketoconazole | |
| Tmax (h) | 1.5 | 3.0 | 2.0 |
| Cmax (ng ml−1) | 97.0 ± 62.3 | 562 ± 125 | 12 258 ± 2928 |
| GMR % (90% CI) | – | 645 (502–829) | – |
| AUC(0–12) (h ng ml−1) | 304 ± 87 | 5513 ± 1358 | 108 428 ± 30 791 |
| GMR % (90% CI) | – | 1855 (1589–2165) | – |
Values are shown as means ± SD except for Tmax, for which the median is reported. Abbreviations are as follows: AUC, area under the curve; CI, confidence interval; Cmax, maximal concentration; GMR, geometric least squares mean ratio (comparison to voclosporin alone) based on analysis of natural log-transformed data; Tmax, time to reach maximal concentration.
Nine subjects were withdrawn from the study on the morning of day 18 due to an elevated serum creatinine and were not included in the pharmacokinetic analyses. The nine withdrawn subjects had a mean creatinine increase from baseline of 58 μmol l−1 (88% mean increase from baseline), in comparison to 21 μmol l−1 (32% mean increase from baseline) for the other 11 subjects remaining in the study. The highest recorded serum creatinine was 163 μmol l−1, with a mean maximal serum creatinine of 132 μmol l−1 (laboratory normal ranges: female, 44–80 μmol l−1; and male, 53–106 μmol l−1). All serum creatinine concentrations in these subjects returned to the normal range by follow-up. In these withdrawn subjects, higher predose voclosporin concentrations were observed on day 10 when voclosporin was administered alone, suggesting that increased voclosporin exposure may be associated with the development of an elevated serum creatinine (Figure 2).
Figure 2.
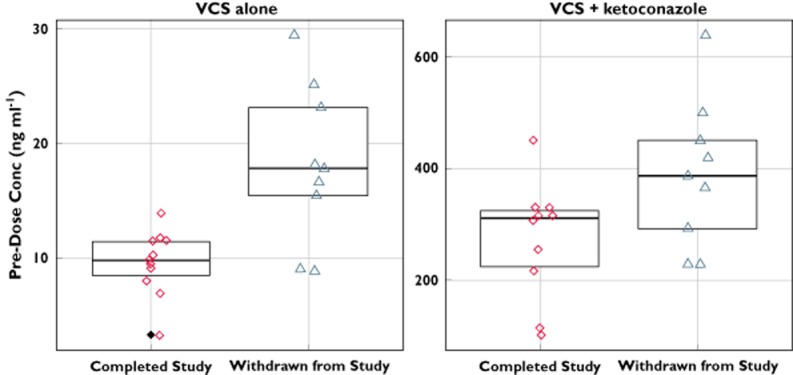
Individual predose concentrations of voclosporin on day 10 [voclosporin (VCS) alone] and day 18 (VCS + ketoconazole) for subjects completing the study and for subjects withdrawn due to elevated serum creatinine levels
Rifampin study
There was a decrease in the mean whole blood concentrations of voclosporin when administered after 10 days of rifampin dosing (Figure 1B). There were corresponding decreases in Cmax, AUC(0–t) and AUC(inf), with geometric least squares mean ratios for voclosporin + rifampin to voclosporin alone of 32, 13 and 13%, respectively, and 90% CIs well below the 80–125% no-effect boundary, demonstrating a significant drug–drug interaction (Table 3). Median Tmax decreased from 1.75 to 1.0 h when voclosporin was administered with rifampin, while t1/2 decreased from ∼5 to <1 h.
Table 3.
Pharmacokinetic parameters for voclosporin after oral administration of voclosporin 0.4 mg kg−1 alone and after 10 days of rifampin 600 mg once daily
| Parameter | Voclosporin alone | Voclosporin + rifampin |
|---|---|---|
| (n = 24) | (n = 22) | |
| Tmax (h) | 1.75 | 1.0 |
| Cmax (ng ml−1) | 90.0 ± 39.6 | 27.3 ± 12.7 |
| GMR % (90% CI) | – | 31.8 (27.5–36.8) |
| AUC(0–t) (h ng ml−1) | 306 ± 151 | 37.1 ± 14.8 |
| GMR % (90% CI) | – | 13.2 (11.5–15.2) |
| AUC(inf) (h ng ml−1) | 331 ± 166 | 40.5 ± 17.4 |
| GMR % (90% CI) | – | 13.1 (11.4–15.1) |
| Λz (h−1) | 0.155 ± 0.072 | 0.998 ± 0.380 |
| t1/2 (h) | 4.96 ± 3.05 | 0.82 ± 0.45 |
Values are shown as means ± SD except Tmax, for which the median is reported. Abbreviations are as follows: AUC, area under the curve; CI, confidence interval; Cmax, maximal concentration; GMR, geometric least squares mean ratio (comparison to voclosporin alone) based on analysis of natural log-transformed data; Λz, elimination rate constant; Tmax, time to reach maximal concentration.
Based on the decreases in whole blood voclosporin concentrations, Cmax, AUC(0–t) and AUC(inf), concomitant administration of voclosporin with rifampin, a CYP3A inducer, results in a decrease in exposure.
Midazolam study
Mean plasma concentrations of midazolam and α-hydroxy-midazolam were comparable when administered alone on day 1 and concomitantly with voclosporin on day 12 (Figure 1C1 and 1C2). There were no significant differences in either AUC(0–24) or AUC(0–inf) of either drug or metabolite, with geometric least squares mean ratios ranging from 102 to 104% and 90% CIs for both parameters within the 80–125% no-effect boundary (Table 4).
Table 4.
Pharmacokinetic parameters for midazolam, α-hydroxy-midazolam and voclosporin after oral administration of midazolam 7.5 mg alone and with voclosporin 0.4 mg kg−1 every 12 h
| Parameter | Midazolam | α-Hydroxy-midazolam | Voclosporin | ||
|---|---|---|---|---|---|
| Midazolam alone (n = 22) | Midazolam + voclosporin (n = 22) | Midazolam alone (n = 22) | Midazolam + voclosporin (n = 22) | Midazolam + voclosporin (n = 22) | |
| Tmax (h) | 0.5 (0.5–1) | 1 (0.25–1.5) | 0.5 (0.5–1.5) | 1 (0.5–2) | 2 (1–4) |
| Cmax (ng ml−1) | 43 469 ± 26 534 | 37 641 ± 18 014 | 16 121 ± 6500 | 13 661 ± 6983 | 134.6 ± 35.8 |
| GMR % (90% CI) | – | 88.86 | – | 81.93 | – |
| (79.92–98.79) | (69.32–96.85) | ||||
| AUC(0–24)* (h ng ml−1) | 124 038 ± 92 674 | 125 904 ± 90 724 | 38 690 ± 12 185 | 39 199 ± 10 230 | 569.3 ± 229.2 |
| GMR % (90% CI) | – | 101.72 | – | 102.73 | – |
| (92.83–111.47) | (93.79–112.51) | ||||
| AUC(inf) (h ng ml−1) | 127 682 ± 99 577 | 130 604 ± 99 542 | 40 518 ± 13 016 | 41 472 ± 11 561 | – |
| GMR % (90% CI) | – | 102.11 | – | 103.64 | – |
| (94.60–113.54) | |||||
| (93.41–111.63) | |||||
| t1/2 (h) | 4.8 ± 1.2 | 5.0 ± 1.3 | 6.9 ± 5.5 | 6.8 ± 5.4 | – |
Values are shown as means ± SD except Tmax, for which the median (range) is reported. Abbreviations are as follows: AUC, area under the curve; CI, confidence interval; Cmax, maximal concentration; GMR, geometric least squares mean ratio (comparison to midazolam alone) based on analysis of natural log-transformed data; Tmax, time to reach maximal concentration.
AUC(0–12) for voclosporin data.
Mean concentrations of midazolam and α-hydroxy-midazolam at 0.5 h appeared lower on day 12 than on day 1. There was a corresponding decrease in Cmax, with geometric least squares mean ratios of 89 and 82%, and the lower limit of the 90% confidence interval was 79.9 and 69.3%, respectively. However, plots of individual Cmax values for midazolam and α-hydroxy-midazolam when midazolam was administered alone and with voclosporin demonstrated almost complete overlap of Cmax values on both days with the exception of one subject, who had a midazolam Cmax value 3.5-fold higher than the mean for the group (Figure 3). These data suggest that the concomitant administration of voclosporin and midazolam did not affect the rate or extent of exposure to midazolam or α-hydroxy-midazolam.
Figure 3.
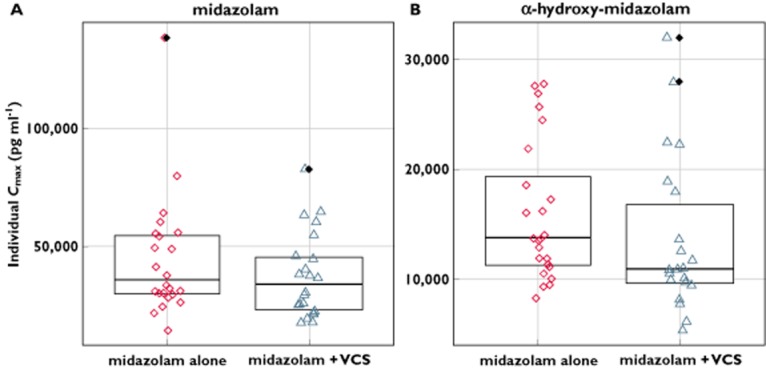
Individual subject maximal concentration (Cmax) values of midazolam and α-hydroxy-midazolam after oral administration of midazolam 7.5 mg alone and with voclosporin (VCS) 0.4 mg kg−1 every 12 h
Verapamil study
There was an increase in the mean whole blood concentrations of voclosporin, including a 2.8-fold increase in mean predose concentrations, when voclosporin was administered concomitantly with verapamil (Figure 1D). There were corresponding increases in both Cmax and AUC(0–12), with geometric least squares mean ratios for voclosporin + verapamil to voclosporin alone of 208 and 271%, respectively, and 90% CIs well above the 80–125% equivalence window, demonstrating a significant drug–drug interaction (Table 5).
Table 5.
Pharmacokinetic parameters for voclosporin after oral administration of voclosporin 0.4 mg kg−1 every 12 h alone and with verapamil 80 mg every 8 h
| Parameter | Voclosporin alone | Voclosporin + verapamil | |
|---|---|---|---|
| (n = 20) | (n = 20) | ||
| Voclosporin | Voclosporin | Verapamil | |
| Tmax (h) | 1.63 | 2.00 | 1.00 |
| Cmax (ng ml−1) | 105 ± 30.9 | 219 ± 64.1 | 268 ± 95.4 |
| GMR % (90% CI) | – | 207.76 (189.21–228.14) | – |
| AUC(0–12)* (h ng ml−1) | 434 ± 158 | 1165 ± 390 | 1164 ± 358 |
| GMR % (90% CI) | – | 271.15 (255.97–287.24) | – |
Values are shown as means ± SD except Tmax, for which the median is reported. Abbreviations are as follows: AUC, area under the curve; CI, confidence interval; Cmax, maximal concentration; GMR, geometric least squares mean ratio (comparison to voclosporin alone) based on analysis of natural log-transformed data; Tmax, time to reach maximal concentration.
AUC(0–8) for verapamil data.
Digoxin study
There was an increase in the mean plasma concentrations of digoxin when it was administered concomitantly with voclosporin (Figure 1E). There were corresponding increases in both Cmax and AUC(0–24), with geometric least squares mean ratios for digoxin + voclosporin to digoxin alone of 151 and 125%, respectively (Table 6). As the upper limits of the associated 90% CIs were >125%, this demonstrates a statistically significant drug–drug interaction and confirms that voclosporin is an inhibitor of P-glycoprotein.
Table 6.
Pharmacokinetic parameters for digoxin after oral administration of digoxin 0.25 mg once daily alone and with voclosporin 0.4 mg kg−1 every 12 h
| Parameter | Digoxin alone | Digoxin + voclosporin | |
|---|---|---|---|
| (n = 23) | (n = 23) | ||
| Digoxin | Digoxin | Voclosporin | |
| Tmax (h) | 1.00 | 1.00 | 1.55 |
| Cmax (pg ml−1)* | 1689 ± 317 | 2566 ± 624 | 124 ± 38.7 |
| GMR % (90% CI) | – | 150.93 (139.67–163.10) | – |
| AUC(0–24)† (h pg ml−1) | 15 651 ± 3012 | 19 514 ± 3575 | 497 ± 188 |
| GMR % (90% CI) | – | 124.88 (119.39–130.62) | – |
| Ue(0–24) (mg) | 0.12 ± 0.03 | 0.14 ± 0.04 | – |
| GMR % (90% CI) | – | 120.54 (107.94–134.60) | – |
| Renal clearance (ml min−1) | 127 ± 36.6 | 121 ± 32.8 | – |
Values are shown as means ± SD except Tmax, for which the median is reported. Abbreviations are as follows: AUC, area under the curve; CI, confidence interval; Cmax, maximal concentration; GMR, geometric least squares mean ratio (comparison to digoxin alone) based on analysis of natural log-transformed data; Tmax, time to reach maximal concentration, Ue, urinary excretion.
Values are in nanograms per millilitre for voclosporin data.
AUC(0–12) for voclosporin data.
An increase in renal excretion of digoxin was also observed, with a geometric least squares mean ratio of 120% and a 90% CI of 108–135%, providing further evidence of a significant drug–drug interaction. There was no apparent change in the renal clearance of digoxin, as urinary excretion and AUC changed correspondingly.
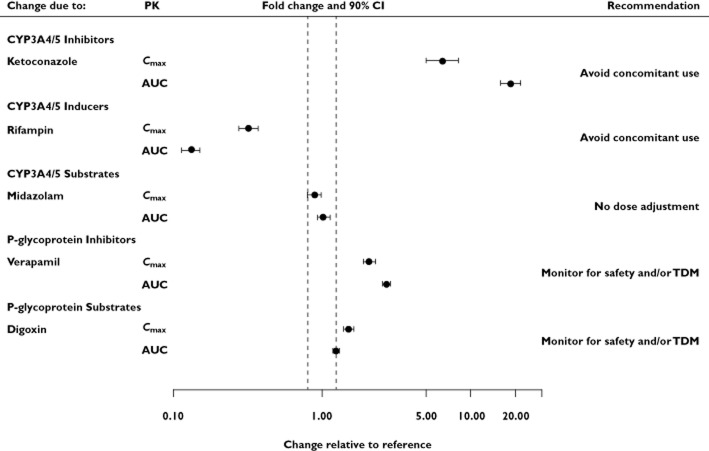
A clear visual presentation of the pharmacokinetic information has been proposed to facilitate efficient and accurate clinical decision-making 4,6. A summary of the five drug–drug interaction studies presented as forest plots, including the mean fold change and 90% CIs in Cmax and AUC and dose adjustment recommendations, is displayed in Figure 4.
Figure 4.
Forest plot presentation of voclosporin drug–drug interaction studies. Point estimate and 90% confidence interval is shown for change in substrate exposure following co-administration of voclosporin with ketoconazole, rifampin and verapamil, where voclosporin is the substrate; and co-administration of voclosporin with midazolam and digoxin, where midazolam and digoxin are the substrates. Abbreviations are as follows: AUC, area under the concentration–time curve; CI, confidence interval; Cmax, maximal whole blood or plasma concentration; PK, pharmacokinetics; TDM, therapeutic drug monitoring
Discussion
The five studies described in this paper investigated the potential for drug–drug interactions when voclosporin, a CYP3A and P-glycoprotein substrate and inhibitor in vitro, was co-administered with a model CYP3A inhibitor, inducer and substrate and a model P-glycoprotein inhibitor and substrate. The choice of interacting drugs was based on the FDA Draft Guidance for Industry (i.e. the 2006 Draft Guidance) at the time the studies were conducted to maximize the potential to detect a drug interaction. The choice of interacting drugs is unchanged in the 2012 Draft Guidance.
The ketoconazole interaction study examined the effect that this strong CYP3A and P-glycoprotein inhibitor would have on the pharmacokinetics of voclosporin when both drugs were at steady state, following co-administration. Large increases in mean voclosporin concentrations were observed, with corresponding increases in Cmax and AUC(0–12) of 6.4- and 18.5-fold, respectively. Although the results of this study do not allow for a determination of the specific mechanism for the drug–drug interaction (i.e. inhibition of CYP3A and/or P-glycoprotein), due to the magnitude of the increase in voclosporin exposure and results from the verapamil and digoxin studies, it is likely to be a combination of both CYP3A and P-glycoprotein. The presence and magnitude of the interaction was not unexpected, because voclosporin has been shown in vitro to be both a CYP3A and a P-glycoprotein substrate, with CYP3A being the predominant isoform involved in voclosporin metabolism and elimination. Inhibition of CYP3A4 by ketoconazole 400 mg QD for 4 days has been shown to reduce midazolam AUC by 16-fold 7. Increased exposure of other calcineurin inhibitors, co-administered with ketoconazole, has been reported 8,9, while the concomitant use of ketoconazole with calcineurin inhibitors has been evaluated for its potential to reduce the dosage requirements for CsA 10,11. Based upon the nine withdrawn subjects, it is clear that at supratherapeutic concentrations of voclosporin, renal function may be adversely, but reversibly, affected.
The rifampin study was designed to determine the effect of rifampin, a potent inducer of CYP3A, on the pharmacokinetics of voclosporin after single doses when rifampin is at steady state. Voclosporin was administered 1 day following the last dose of rifampin rather than concomitantly on the same day to avoid the paradoxical inhibitory effect of rifampin on organic anion transporter-mediated hepatic uptake, of which voclosporin may be a substrate when the two drugs are administered concomitantly 12–14. Following administration of rifampin, mean voclosporin concentrations decreased, with corresponding decreases in Cmax, AUC and t1/2 of approximately 70, 90 and 85%, respectively. The geometric least squares mean ratios point estimates and the lower limit of the 90% CIs for Cmax and AUC were well below the 80% no-effect boundary, demonstrating a significant drug–drug interaction. These results are consistent with increased clearance of voclosporin by rifampin due to induction of CYP3A metabolism. Drug interactions between rifampin and CsA have been observed in renal transplant patients (Cmax and AUC reduced by 30 and 40%, respectively) 15, and decreased CsA trough concentrations have been reported following addition of rifampin to stable CsA therapy 16,17.
In vitro, voclosporin has been shown to be a direct, competitive inhibitor of CYP3A with an IC50 of 1.2 μm and a Ki of 1.1 μm (personal communication from R. Huizinga, Isotechnika Pharma Inc.). Based on voclosporin Cmax values obtained from clinical studies in psoriasis patients 18,19 and de novo renal transplant patients 20, the expected [I]/Ki (where [I] is the whole blood concentration of inhibitor for which Cmax is considered an estimate) for voclosporin ranges from 0.125 to 0.216. This suggests that the potential for a clinically relevant drug–drug interaction is between remote ([I]/Ki < 0.1) and possible ([I]/Ki > 1) 3. Therefore, the midazolam study was designed to determine the effect of voclosporin on the pharmacokinetics of midazolam when voclosporin is at steady state and midazolam is given as a single dose. Concomitant administration of voclosporin and midazolam did not result in statistically significant changes in the rate or extent of exposure to midazolam or α-hydroxy-midazolam. The geometric least squares mean ratios point estimates for AUC ranged from 102 to 104%, with 90% CIs between the 80–125% no-effect boundaries. Mean peak concentrations were reduced slightly at the 0.5 h time point, with the lower 90% CIs for the mean ratios just below the 80% no-effect boundary. Examination of the individual Cmax plots, however, demonstrates almost complete overlap of values for both midazolam and α-hydroxy-midazolam when midazolam was administered alone or with voclosporin. This suggests that co-administration of voclosporin with other substrates of CYP3A would not lead to clinically significant drug–drug interactions.
The verapamil study was designed to determine the effect of verapamil on the pharmacokinetics of voclosporin when both drugs were at steady state. A large increase in mean whole blood concentrations, including a 3-fold increase in predose concentrations and AUC, as well as a 2-fold increase in Cmax, were observed when voclosporin was co-administered with verapamil. These results are consistent with the inhibition of P-glycoprotein by verapamil leading to increased bioavailability of voclosporin, perhaps via reduced P-glycoprotein-mediated efflux of voclosporin in the gastrointestinal tract or reduced P-glycoprotein-mediated biliary excretion in the liver 21. Other immunosuppressants, including CsA, sirolimus and tacrolimus, are known P-glycoprotein substrates, requiring appropriate caution when co-administered with inhibitors of P-glycoprotein 22–24.
The data demonstrate an augmented CYP3A effect in comparison to P-glycoprotein. Interestingly, voclosporin predose concentrations, AUC and Cmax increased 32.5-, 18.5- and 6.5-fold, respectively, when voclosporin was co-administered with ketoconazole, a CYP3A and P-glycoprotein inhibitor. The disproportionate elevation of predose concentrations and AUC relative to the increase in Cmax suggests profound inhibition of systemic clearance of voclosporin and increased elimination half-life in addition to increased bioavailability due to suppressed first-pass metabolism. In contrast, these parameters were increased by 2.8-, 2.7- and 2.1-fold when voclosporin was co-administered with verapamil, a P-glycoprotein inhibitor. The similar magnitude of effect of verapamil on all three parameters is consistent with inhibition of gut P-glycoprotein, resulting in enhanced oral bioavailability with no effect on systemic clearance or half-life.
The digoxin study was designed to determine the effect of voclosporin on the pharmacokinetics of digoxin when both drugs were at steady state. A 50% increase in Cmax, 25% increase in AUC and 20% increase in urinary excretion of digoxin were observed when digoxin was co-administered with voclosporin. Although P-glycoprotein-mediated renal secretion of digoxin has been reported 25, in the present study digoxin renal clearance was unchanged, indicating that voclosporin had a negligible effect on renal elimination of digoxin. The increase in Cmax by 50% with only a 25% increase in AUC suggests a greater effect on the rate rather than the extent of absorption. This could suggest a greater influence on first-pass bioavailability rather than on renal tubular secretion. This could be due a higher drug exposure in the gastrointestinal tract than the bloodstream. Therefore, renal P-glycoprotein exposure is less, as demonstrated by no apparent change in renal clearance. Ciclosporin is a well-known P-glycoprotein inhibitor, requiring appropriate monitoring and dosage adjustments of the interacting drugs when co-administered with CsA 26. These results demonstrate a modest inhibitory effect of voclosporin on P-glycoprotein in vivo and are consistent with in vitro studies demonstrating that voclosporin is an inhibitor of P-glycoprotein.
Conclusion
Co-administration of voclosporin with ketoconazole, a strong CYP3A inhibitor, increases voclosporin peak concentration and extent of exposure by 6.4- and 18-fold, respectively. Co-administration of voclosporin with rifampin, a potent inducer of CYP3A, reduced voclosporin exposure by 90%. Administration of voclosporin concomitantly with strong inhibitors and inducers of CYP3A should be considered contraindicated. Co-administration of voclosporin with midazolam, a known CYP3A substrate, did not result in significant changes to exposure of midazolam or its metabolite α-hydroxy-midazolam after a single dose. Drug–drug interactions involving voclosporin and other CYP3A substrates are not expected.
Co-administration of voclosporin with verapamil increased voclosporin Cmax, AUC and predose concentrations by 2.1-, 2.7- and 2.8-fold, respectively. Co-administration of voclosporin with digoxin resulted in a significant increase in digoxin Cmax, AUC and urinary excretion by 50, 25 and 20%, respectively, while digoxin renal clearance was unchanged. Administration of voclosporin concomitantly with inhibitors of P-glycoprotein would be expected to result in increased voclosporin exposures. Administration of voclosporin concomitantly with substrates of P-glycoprotein would be expected to result in increased P-glycoprotein substrate exposures. Therefore, appropriate concentration and safety monitoring is recommended with co-administration of voclosporin and P-glycoprotein substrates and inhibitors.
Acknowledgments
The authors thank Drs Denis Audet, Benoit J. Deschamps and Francois St-Maurice for participating in this study.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: SYL, RBH, PRM, RL, DGF, LJA and RTF had support from Isotechnika Pharma Inc. for the submitted work; RBH and RTF are employees of Isotechnika Pharma Inc., and there are no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Kuglstatter A, Mueller F, Kusznir E, Gsell B, Stihle M, Thoma R, Benz J, Aspeslet L, Freitag D, Hennig M. Structural basis for the cyclophilin A binding affinity and immunosuppressive potency of E-ISA247 (voclosporin) Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 2):119–123. doi: 10.1107/S0907444910051905. doi: 10.1107/S0907444910051905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stalder M, Bîrsan T, Hubble RW, Paniagua RT, Morris RE. In vivo evaluation of the novel calcineurin inhibitor ISATX247 in non-human primates. J Heart Lung Transplant. 2003;22:1343–1352. doi: 10.1016/s1053-2498(03)00033-0. [DOI] [PubMed] [Google Scholar]
- 3.Draft Guidance for Industry. Drug Interaction Studies – Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), September 2006.
- 4.Draft Guidance for Industry. Drug Interaction Studies – Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), February 2012.
- 5.Handy R, Trepanier D, Scott G, Foster R, Freitag D. Development and validation of a LC/MS/MS method for quantifying the next generation calcineurin inhibitor, voclosporin, in human whole blood. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;874:57–63. doi: 10.1016/j.jchromb.2008.08.023. doi: 10.1016/j.jchromb.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Menon-Andersen D, Yu B, Madabushi R, Bhattaram V, Hao W, Uppoor RS, Mehta M, Lesko L, Temple R, Stockbridge N, Laughren T, Gobburu JV. Essential pharmacokinetic information for drug dosage decisions: a consice visual presentation in the drug label. Clin Pharmacol Ther. 2011;90:471–474. doi: 10.1038/clpt.2011.149. [DOI] [PubMed] [Google Scholar]
- 7.Olkkola KT, Backman JT, Neuvonen PJ. Midazolam should be avoided in patients receiving the systemic antimycotics ketoconazole or itraconazole. Clin Pharmacol Ther. 1994;55:481–485. doi: 10.1038/clpt.1994.60. [DOI] [PubMed] [Google Scholar]
- 8.Gomez DY, Wacher VJ, Tomlanovich SJ, Hebert MF, Benet LZ. The effects of ketoconazole on the intestinal metabolism and bioavailability of cyclosporine. Pharmacokinet Drug Dispos. 1995;58:15–19. doi: 10.1016/0009-9236(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 9.Floren LC, Bekersky I, Benet LZ, Mekki Q, Dressler D, Lee JW, Roberts JP, Hebert MF. Tacrolimus oral bioavailability doubles with coadministration of ketoconazole. Clin Pharmacol Ther. 1997;62:41–49. doi: 10.1016/S0009-9236(97)90150-8. [DOI] [PubMed] [Google Scholar]
- 10.First MR, Schroeder TJ, Michael A, Hariharan S, Weiskittel P, Alexander JW. Cyclosporine-ketoconazole interaction. Transplantation. 1993;55:1000–1004. [PubMed] [Google Scholar]
- 11.Abraham MA, Thomas PP, John GT, Job V, Shankar V, Jacob CK. Efficacy and safety of low-dose ketoconazole (50 mg) to reduce the cost of cyclosporine in renal allograft recipients. Transplant Proc. 2003;35:215–216. doi: 10.1016/s0041-1345(02)03839-3. [DOI] [PubMed] [Google Scholar]
- 12.Bidstrup TB, Stilling N, Damkier P, Scharling B, Thomsen MS, Brøsen K. Rifampin seems to act as both an inducer and an inhibitor of the metabolism of repaglinide. Eur J Clin Pharmacol. 2004;60:109–114. doi: 10.1007/s00228-004-0746-z. [DOI] [PubMed] [Google Scholar]
- 13.Xiong H, Carr RA, Locke CS, Katz DA, Achari R, Doan TT, Wang P, Jankowski JR, Sleep DJ. Dual effects of rifampin on the pharmacokinetics of atrasentan. J Clin Pharmacol. 2007;47:423–429. doi: 10.1177/0091270007299928. [DOI] [PubMed] [Google Scholar]
- 14.Srinivas NR. Unsuspected and paradoxical potential for drug interaction by rifampin: things to ponder with antiretroviral therapy. J Infect Dis. 2009;199:766–767. doi: 10.1086/596746. author reply 767. [DOI] [PubMed] [Google Scholar]
- 15.Kim YH, Yoon YR, Kim YW, Shin JG, Cha IJ. Effects of rifampin on cyclosporine disposition in kidney recipients with tuberculosis. Transplant Proc. 1998;30:3570–3572. doi: 10.1016/s0041-1345(98)01139-7. [DOI] [PubMed] [Google Scholar]
- 16.Freitag VL, Skifton RD, Lake KD. Effect of short-term rifampin on stable cyclosporine concentrations. Ann Pharmacother. 1999;33:871–872. doi: 10.1345/aph.19044. [DOI] [PubMed] [Google Scholar]
- 17.Zelunka EJ. Intravenous cyclosporine-rifampin interaction in a pediatric bone marrow transplant recipient. Pharmacotherapy. 2002;22:387–390. doi: 10.1592/phco.22.5.387.33190. [DOI] [PubMed] [Google Scholar]
- 18.Bissonnette R, Papp K, Poulin Y, Lauzon G, Aspeslet L, Huizinga R, Mayo P, Foster RT, Yatscoff RW, Maksymowych WP. ISA247 Psoriasis Study Group. A randomized, multicenter, double-blind, placebo-controlled phase 2 trial of ISA247 in patients with chronic plaque psoriasis. J Am Acad Dermatol. 2006;54:472–478. doi: 10.1016/j.jaad.2005.10.061. [DOI] [PubMed] [Google Scholar]
- 19.Papp K, Bissonnette R, Rosoph L, Wasel N, Lynde CW, Searles G, Shear NH, Huizinga RB, Maksymowych WP. Efficacy of ISA247 in plaque psoriasis: a randomised, multicentre, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1337–1342. doi: 10.1016/S0140-6736(08)60593-0. doi: 10.1016/S0140-6736(08)60593-0. [DOI] [PubMed] [Google Scholar]
- 20.Busque S, Cantarovich M, Mulgaonkar S, Gaston R, Gaber AO, Mayo PR, Ling S, Huizinga RB, Meier-Kriesche H-U PROMISE Investigators. The PROMISE study: a phase 2b multicenter study of voclosporin (ISA247) versus tacrolimus in de novo kidney transplantation. Am J Transplant. 2011;11:2675–2684. doi: 10.1111/j.1600-6143.2011.03763.x. doi: 10.1111/j.1600-6143.2011.03763.x. [DOI] [PubMed] [Google Scholar]
- 21.Müller F, Fromm MF. Transporter-mediated drug-drug interactions. Pharmacogenomics. 2011;12:1017–1037. doi: 10.2217/pgs.11.44. [DOI] [PubMed] [Google Scholar]
- 22.Hesselink DA, van Gelder T, van Schaik RH. The pharmacogenetics of calcineurin inhibitors: one step closer toward individualized immunosuppression? Pharmacogenomics. 2005;6:323–337. doi: 10.1517/14622416.6.4.323. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman JJ. Exposure-response relationships and drug interactions of sirolimus. AAPS J. 2004;6:1–12. doi: 10.1208/aapsj060428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawwa AF, McKiernan PJ, Shields M, Millership JS, Collier PS, McElnay JC. Influence of ABCB1 polymorphisms and haplotypes on tacrolimus nephrotoxicity and dosage requirements in children with liver transplant. Br J Clin Pharmacol. 2009;68:413–421. doi: 10.1111/j.1365-2125.2009.03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koren G, Woodland C, Ito S. Toxic digoxin-drug interactions: the major role of renal P-glycoprotein. Vet Hum Toxicol. 1998;40:45–46. [PubMed] [Google Scholar]
- 26.Novartis. 2009. Neoral Product Monograph.