Abstract
This paper reviews the medical use of helium oxygen mixture in obstructive airway disease in patients with croup, narrow endotracheal tubes (ETTs), respiratory distress syndrome, asthma, bronchiolitis, as well as patients with acute exacerbation of chronic obstructive pulmonary disease (COPD) and acute lung injury. In addition, some other indications of heliox use and some innovative methods of ventilation applied in pediatrics and adults are presented through review of the literature of current decade. Yet, to recommend heliox use seems to require more research based on clinical practice and observation through vaster and more robust investigations.
Keywords: Administration, airway obstruction, dyspnea, heliox, mechanical ventilation
INTRODUCTION
The atmosphere comprises various distinct gases, the most abundant being nitrogen (~78%), followed by oxygen (~21%). Helium is only present in five parts per million (0.0005%) in the lower atmosphere. Nitrogen and helium have comparable viscosity, but helium has higher thermal conductivity compared to nitrogen. As a result, when a heliox gas mixture (79% helium and 21% oxygen) is produced, it has a viscosity similar to, but a density nearly six times lowers than atmospheric air. Due to these properties, heliox has potential applications in respiratory medicine.[1,2] Heliox gas mixtures are known to be nontoxic, noncarcinogenic, and have no lasting effects on any human organs.[3]
Due to its lower density, inhalation of heliox results in significantly lower turbulence, particularly in the more distal portions of the lung. This effect translates to a greater proportion of laminar flow and lower overall airway resistance. The decreased turbulence effect results in increased flow rates by up to 50% during heliox inhalation. This decreased turbulence remained evident even when airflow was restricted, as in the case of obstructive lung disease.[2,4]
PHYSICAL PROPERTIES OF HELIUM TO APPLY FOR RESPIRATORY CARE
Heliox is a low density gas mixture of helium and oxygen commonly used in deep diving, and also for clinical purposes, particularly in the critical care setting. Heliox breathing reduces air flow resistance within the bronchial tree in patients with obstructive lung disease, and has beneficial effects in severe asthma attacks. Heliox may also reduce the work of breathing and improve pulmonary gas exchange efficiency. Despite the encouraging results, heliox use in routine practice remains controversial because of technical implications and high costs.[5] Lower density of heliox compared with the air or oxygen regardless of the concentration of helium in the mixture; improves air flow through constricted ways by transforming turbulent flow into laminar flow. Benefits are seen quickly, usually within an hour of initiation of treatment.[6]
Clinical applications of heliox
What follows are some clinical applications of heliox in respiratory medicine [Table 1].
Table 1.
Clinical applications of Heliox-driven respiratory support
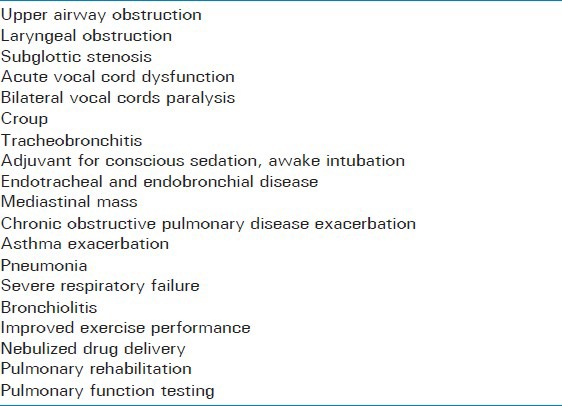
Heliox in airway obstruction
The potential benefits of replacing nitrogen in the inspired air with helium were initially recognized in the 1930s when Barach administered helium to asthmatic patients and patients with laryngeal obstruction for relief of dyspnea.[7]
Conscious sedation, awake intubation
In 1997, Milner and colleagues reported the use of heliox with a laryngeal mask airway (LMA) to allow for tracheostomy under conscious sedation in an extremely anxious patient. The patient was given heliox (80:20 ratio) by means of a non-rebreathing face mask and reported subjective improvement in dyspnea. Anesthesia was induced with midazolam and small doses of propofol while maintaining spontaneous respirations. A size 3 LMA was inserted and manual ventilation was started.[8] Administration of heliox is usually achieved through the use of a non-rebreathing face mask with gas flows in the range of 10 L/min. We should note that while the viscosity values for helium and oxygen are similar, the density of helium is much less than that of oxygen. The low density of helium allows it to play a significant clinical role in the temporary management of some forms of airway obstruction associated with gas turbulence.[3,9]
Acute vocal cord dysfunction, bilateral vocal cords paralysis
Administering a helium and oxygen mixture (heliox) reduces airway resistance and may result in rapid improvement in patients with acute vocal cord dysfunction. A trial of heliox may be appropriate because of its relatively low cost and minimal adverse effects, and this has been reported in one case series.[10,11,12]
Heliox has also been used effectively in cases of bilateral vocal cords paralysis after radiation therapy, post-extubation stridor, and as a temporizing measure in cases of external tracheal compression due to tumor.[13,14,15,16,17]
Intrinsic endotracheal and endobronchial, mediastinal masses
Intrinsic endotracheal or endobronchial disease can pose significant airway difficulties due to airway obstruction and loss of available lung for oxygenation and ventilation. Mediastinal masses also may present extreme hazards to the airway, leading to complete airway collapse. Much of the literature on the use of heliox is limited to case descriptions.[18,19,20,21]
Croup
Duncan treated seven children with severe croup refractory to epinephrine with heliox 70:30. They found that all patients showed a significant reduction in their croup scores and none required intubation.[22] Weber, et al., measured croup scores in 29 children treated either with heliox via a non-rebreather mask or nebulized racemic adrenaline, and found similar improvements with both.[23]
Tracheobronchitis, subglottic stenosis
Connolly and McGuirt, detailed 14 pediatric patients (five with viral tracheobronchitis, five with inflammatory exacerbations of subglottic stenosis, and four with acute iatrogenic subglottic injury) in whom heliox was used, it would avoid intubation in 10.[24]
Exacerbations of chronic obstructive pulmonary disease
The lower density and the higher viscosity of helium, compared with nitrogen, increase the probability that flow in a rigid tube will be laminar instead of turbulent at any given flow rate. As the pressure differential that must be generated by the patient or ventilator to produce laminar flow is substantially less than that associated with turbulent flow, a helium oxygen mixture (heliox) might be expected to reduce the work of breathing in patients with air flow obstruction.[25] The administration of heliox has been shown in numerous case reports and case series to reduce the work of breathing in patients with obstruction in the extrathoracic and central intrathoracic airways.[6] In patients with small airway disease, including asthma or COPD, heliox is predicted to be much less effective because the cross-sectional area of the smaller airways is quite large.[26] Several groups of investigators have reported that the administration of heliox to patients with moderate or severe COPD is associated with improved exercise performance. These improvements are primarily attributable to reductions in end-expiratory lung volume or dynamic hyperinflation, which contributes to the increased work of breathing in COPD patients. Furthermore, the resulting reduced intrathoracic pressure improves hemodynamic and peripheral oxygen delivery.[25,26,27,28,29]
In 10 patients with pulmonary arterial catheters, heliox decreased mean pulmonary arterial pressure, right atrial pressure, and pulmonary arterial occlusion pressure and increased cardiac index. Heliox may be a useful adjunct therapy in patients with severe COPD and acute respiratory failure with persistent intrinsic positive end-expiratory pressure (PEEP)-induced hemodynamic changes despite ventilator management.[28]
Asthma
There have been four previous, randomized, placebo-controlled studies examining
heliox for the treatment of acute asthma in children. There will still be a role for a trial of heliox in selected children with refractory status asthmaticus.[30]
In the first two studies by Carter et al., and Kudukis et al., 15 min of heliox therapy was provided to small groups of children.[31,32] In Kudukis et al., findings; there was improvement in clinical asthma score and pulsus paradoxus, whereas Carter et al., found no improvement in pulmonary function testing or clinical asthma score.
The next two randomized placebo-controlled studies were conducted in children with acute asthma in the emergency department setting: The study by Kim et al., with the longer duration of heliox therapy showed improvement in clinical asthma score, whereas the study by Rivera et al., did not.[33,34]
Pneumonia and other conditions involving lower airway obstruction
Lower respiratory tract disease caused by respiratory syncytial virus (RSV) is characterized by narrowing of the airways resulting in increased airway resistance, air-trapping, and respiratory acidosis. These problems might be overcome using helium-oxygen gas mixture. In a study of electrical impedance tomography (EIT) measurements in nine subjects showed, mechanical ventilation (MV) with heliox significantly decreased respiratory system resistance. This was not accompanied by an improved CO2 elimination, decreased peak expiratory flow rate or decreased end expiratory lung volume. Importantly, oxygenation remained unaltered throughout the experimental protocol.[35] Heliox has been used in a number of other clinical situations in which lower airway obstruction plays a significant role, particularly in children with bronchiolitis and cystic fibrosis.[13] Again, the common theme is that heliox is a temporizing measure, but is not a treatment in itself.[13] Similar low-level evidence suggests that heliox is effective in reducing airway pressure and improving ventilation in various forms of lower airway obstruction. These therapies generally are supportive and may facilitate patient management.[36]
Other research on the use of heliox
Nebulized drug delivery
Helium-driven albuterol would be expected to increase nebulized drug delivery, and improve gas exchange to the distal airways. Bigham et al., investigated the effect of heliox-powered albuterol therapy on hospital length of stay and clinical status in children with moderate to severe status asthmaticus. According to that study, heliox-powered nebulized albuterol therapy for children admitted to the hospital with moderate to severe status asthmaticus does not shorten hospital length of stay or hasten rates of clinical improvement when compared with air/oxygen-powered nebulized albuterol.[37] A study performed to compare the bronchodilator effects of albuterol and ipratropium bromide through nebulization is driven by heliox with that if driven by compressed room air (AIR) during the treatment of acute exacerbation of COPD. The change in percentage of predicted forced expiratory flow after 25-75% of vital capacity that had been expelled (FEF25-75), and forced expiratory volume in 1 s (FEV1) were measured. According to this study, use of heliox as a driving gas for the updraft nebulization of bronchodilators during the first 2 h of treatment of an acute COPD exacerbation failed to improve FEV1 faster than the use of AIR. The faster improvement in FEF25-75 during the first 2 h of treatment was small and of uncertain clinical significance.[38]
Exercise tolerance
The maximum flow in the airways is determined not only by their size and compliance at the choke point, but also by physical properties of the inhaled gases. During heliox breathing, since the density of it is approximately one-third of air, flow is expected to increase as a result of decreased turbulence within the large airways. Heliox breathing increases exercise endurance tolerance in severe COPD patients.[39]
Pulmonary rehabilitation
The most promising use of Heliox mixtures would be as an adjunct to pulmonary rehabilitation in patients with severe COPD, who are still disabled by dyspnea and are unable to achieve full benefits of training, despite pharmacologic treatment and ambulatory oxygen therapy. Use of Heliox with rehabilitation needs to be tested in large controlled studies with appropriate outcome measures.[40]
Hunt et al., in a systematic review, explored whether symptom modification (perceived levels of dyspnea) and exercise performance in COPD (either intensity or duration of work) are modified by inhalation of heliox. They summarized that eight studies supported the effectiveness of heliox in improving the intensity and endurance of exercise when compared to room air for people with COPD.[2] Further studies are also needed to verify the utility of heliox as an ergogenic aid to training in pulmonary rehabilitation.[2,39]
Severe respiratory failure
Winters et al., reported five intubated patients with acute hypoxemic respiratory failure who were treated with high-frequency oscillatory ventilation (HFOV) combined with heliox. This bimodal treatment resulted in a fast reduction of PaCO2 in all patients.[41]
HELIOX AND MODE OF MECHANICAL VENTILATION
The bulk of evidence for heliox-assisted therapy is in the areas of controlled MV (CMV) and synchronized intermittent mandatory ventilation (SIMV) modes of ventilation. Heliox driven volume-controlled ventilation is currently not recommended in patients needing tidal volume (VT) <40 mL (i.e. approximately <6 kg). Heliox-driven pressure-controlled ventilation may be used in patients down to premature neonates with endotracheal tube (ETT) size 2.0 mm internal diameter and a weight of at least 500 g and above 30. It is important to maximize the helium content to gain the greatest potential benefit of heliox MV. This means that inspired fraction of oxygen (FiO2) should be kept to the minimum required for adequate oxygenation, in order to optimize FiHe. This necessitates greater flexibility and tolerance of oxygen saturations. The aim is to achieve FiHe ≥0.6, that is, FiO2≤0.4 where possible. If a patient is hypoxic, it is preferable to use volume recruitment strategies; increase PEEP, VT, or peak inspiratory pressure (PIP) in the case of pressure-controlled ventilation before increasing FiO2.[42]
The addition of helium has a significant effect on fraction of inspired oxygen (FiO2) delivery, displayed inspiratory VT, and actual delivered VT during both volume- and pressure-controlled ventilation in four ventilators commonly used in pediatric critical care. These effects are both ventilator specific and ventilation mode specific, mandating vigilance during helium ventilation in clinical practice.[43]
Noninvasive ventilation
Noninvasive high-frequency percussive ventilation (NIHFPV) differs from HFOV in several aspects. The most evident differences are the mode of delivery of NIHFPV through a facial mask, the applied frequency, and the pressure waveform. It stresses the potential benefits of heliox combined with high-frequency ventilation for improving severe respiratory failure, particularly in the setting of uncontrollable hypercapnia.[44] The use of heliox in combination with noninvasive positive pressure ventilation (NIPPV) in patients presenting with exacerbations of COPD will have to be added to the growing list of promising therapies for critically ill patients that could not be treated through other methods.[25] Helium is an inert gas with a very low density (0.18 g/L), which allows it to pass through narrowed passages with less turbulence than nitrogen or oxygen. Most studies agree that heliox is extremely safe; no adverse effects have been reported. However, heliox must be administered with vigilance and continuous monitoring to avoid technical complications.[45] The improved flow properties and higher CO2 diffusion coefficient of heliox make it an interesting adjunct in the treatment of severe airway obstruction. It is imperative to keep in mind that heliox has no direct treatment effects and is only a temporizing measure until definitive therapies take effect or the disease process resolves.[45]
In a study to assess whether noninvasive ventilation with heliox may decrease the incidence of extubation failure in preterm infants with respiratory distress syndrome (RDS): Infants <29 weeks of gestation were treated immediately after extubation with heliox combined with nasal continuous airway pressure (Hx-NCPAP) or bilevel NCPAP (Hx-BiPAP) for 24 h, while infants in the control groups were treated with conventional NCPAP or BiPAP. The primary endpoint was the comparison of the extubation failure rate in the two groups, where failure was defined as the need for MV during the 24 h following extubation. According to this study, noninvasive ventilation with heliox was not effective in decreasing extubation failure in preterm infants with RDS, but did improve their respiratory function. The findings might support the planning of large randomized controlled studies to evaluate the effectiveness of heliox noninvasive ventilation for decreasing extubation failure in premature infants.[46]
CONCLUSION
Reliable physical and physiological theories support the assertion that helium can improve ventilator function, especially by reducing the resistance of the airways, which is considered the main physiopathology element of obstructive syndromes. However, the level of evidence does not permit a formal recommendation to be made regarding the use of He/O2 in the intensive care unit (ICU). Numerous questions remain unanswered concerning the use of He/O2, for instance, which patients may benefit from He/O2 use, in the setting of COPD or asthma? Is He/O2 useful in combination with noninvasive MV or with aerosol delivery? And, what is the best delivery system for He/O2?[47] Evidence continues to evolve that heliox can effectively reduce airway resistance and work of breathing in patients with severe airway obstruction and can improve delivery of aerosol by reducing turbulence and aerosol particle impact en route to the lungs. As the practice of heliox administration continues to evolve, it is very important for clinicians to understand how heliox works, and how it will affect devices and patients. No heliox administration device should be used clinically without ample training and bench testing.[48] To recommend, heliox use seems to require more research based on clinical practice and observation through vaster investigations.
ACKNOWLEDGMENT
The authors would like to thank Dr. Mohsen Azimi for the accompanying illustrations and for his technical assistance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Oliver BM, Farrar H, Bradley JG. Helium concentration in the earth's lower atmosphere. Geochim Cosmochima Acta. 1984;48:1759–67. [Google Scholar]
- 2.Hunt T, Williams MT, Frith P, Schembri D. Heliox, dyspnoea and exercise in COPD. Eur Respir Rev. 2010;19:30–8. doi: 10.1183/09059180.00006009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris PD, Barnes R. The uses of helium and xenon in current clinical practice. Anaesthesia. 2008;63:284–93. doi: 10.1111/j.1365-2044.2007.05253.x. [DOI] [PubMed] [Google Scholar]
- 4.Eves ND, Ford GT. Helium-oxygen: A versatile therapy to “lighten the load” of chronic obstructive pulmonary disease. Respir Med COPD Update. 2007;3:87–94. [Google Scholar]
- 5.Valli G, Paoletti P, Savi D, Martolini D, Palange P. Clinical use of Heliox in asthma and COPD. Monaldi Arch Chest Dis. 2007;67:159–64. doi: 10.4081/monaldi.2007.488. [DOI] [PubMed] [Google Scholar]
- 6.McGarvey JM, Pollack CV. Heliox in airway management. Emerg Med Clin North Am. 2008;26:905–20. doi: 10.1016/j.emc.2008.07.007. viii. [DOI] [PubMed] [Google Scholar]
- 7.Barach AL. The use of helium in the treatment of asthma and obstructive lesions in the larynx and trachea. Ann Intern Med. 1935;9:739–65. [Google Scholar]
- 8.Milner QJ, Abdy S, Allen JG. Management of severe tracheal obstruction with helium/oxygen and a laryngeal mask airway. Anaesthesia. 1997;52:1087–9. doi: 10.1111/j.1365-2044.1997.252-az0386.x. [DOI] [PubMed] [Google Scholar]
- 9.Galway U, Doyle DJ, Gildea T. Anesthesia for endoscopic palliative management of a patient with a large anterior mediastinal mass. J Clin Anesth. 2009;21:150–1. doi: 10.1016/j.jclinane.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Weir M. Vocal cord dysfunction mimics asthma and may respond to heliox. Clin Pediatr (Phila) 2002;41:37–41. doi: 10.1177/000992280204100108. [DOI] [PubMed] [Google Scholar]
- 11.Berkenbosch JW, Grueber RE, Graff GR, Tobias JD. Patterns of helium-oxygen (heliox) usage in the critical care environment. J Intensive Care Med. 2004;19:335–44. doi: 10.1177/0885066604269670. [DOI] [PubMed] [Google Scholar]
- 12.Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81:156–9. [PubMed] [Google Scholar]
- 13.Wigmore T, Stachowski E. A review of the use of heliox in the critically ill. Crit Care Resusc. 2006;8:64–72. [PubMed] [Google Scholar]
- 14.Khanlou H, Eiger G. Safety and efficacy of heliox as a treatment for upper airway obstruction due to radiation-induced laryngeal dysfunction. Heart Lung. 2001;30:146–7. doi: 10.1067/mhl.2001.112026. [DOI] [PubMed] [Google Scholar]
- 15.Kemper KJ, Ritz RH, Benson MS, Bishop MS. Helium-oxygen mixture in the treatment of postextubation stridor in pediatric trauma patients. Crit Care Med. 1991;19:356–9. doi: 10.1097/00003246-199103000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Polaner DM. The use of heliox and the laryngeal mask airway in a child with an anterior mediastinal mass. Anesth Analg. 1996;82:208–10. doi: 10.1097/00000539-199601000-00037. [DOI] [PubMed] [Google Scholar]
- 17.Balkissoon R, Kenn K. Asthma: Vocal cord dysfunction (VCD) and other dysfunctional breathing disorders. Semin Respir Crit Care Med. 2012;33:595–605. doi: 10.1055/s-0032-1326959. [DOI] [PubMed] [Google Scholar]
- 18.Pullerits J, Holzman R. Anaesthesia for patients with mediastinal masses. Can J Anaesth. 1989;36:681–8. doi: 10.1007/BF03005421. [DOI] [PubMed] [Google Scholar]
- 19.Frawley G, Low J, Brown TC. Anesthesia for an anterior mediastinal mass with ketamine and midazolam infusion. Anesth Intensive Care. 1995;23:610–2. doi: 10.1177/0310057X9502300515. [DOI] [PubMed] [Google Scholar]
- 20.Dasan J, Littleford J, McRae K, Farine D, Winton T. Mediastinal tumour in a pregnant patient presenting as acute cardiorespiratory compromise. Int J Obstet Anesth. 2002;11:52–6. doi: 10.1054/ijoa.2001.0915. [DOI] [PubMed] [Google Scholar]
- 21.Slinger P, Karsli C. Management of the patient with a large anterior mediastinal mass: Recurring myths. Curr Opin Anaesthesiol. 2007;20:1–3. doi: 10.1097/ACO.0b013e328011390b. [DOI] [PubMed] [Google Scholar]
- 22.Duncan PG. Efficacy of helium--oxygen mixtures in the management of severe viral and post-intubation croup. Can Anaesth Soc J. 1979;26:206–12. doi: 10.1007/BF03006983. [DOI] [PubMed] [Google Scholar]
- 23.Weber JE, Chudnofsky CR, Younger JG, Larkin GL, Boczar M, Wilkerson MD, et al. A randomized comparison of helium-oxygen mixture (Heliox) and racemic epinephrine for the treatment of moderate to severe croup. Pediatrics. 2001;107:E96. doi: 10.1542/peds.107.6.e96. [DOI] [PubMed] [Google Scholar]
- 24.Connolly KM, McGuirt WF., Jr Avoiding intubation in the injured subglottis: The role of heliox therapy. Ann Otol Rhinol Laryngol. 2001;110:713–7. doi: 10.1177/000348940111000803. [DOI] [PubMed] [Google Scholar]
- 25.Mutlu GM, Budinger GR. Not much turbulence: Addition of heliox to noninvasive ventilation fails to improve outcomes in patients with exacerbations of chronic obstructive pulmonary disease. Crit Care Med. 2010;38:319–20. doi: 10.1097/CCM.0b013e3181bc7cce. [DOI] [PubMed] [Google Scholar]
- 26.Colebourn CL, Barber V, Young JD. Use of helium-oxygen mixture in adult patients presenting with exacerbations of asthma and chronic obstructive pulmonary disease: A systematic review. Anaesthesia. 2007;62:34–42. doi: 10.1111/j.1365-2044.2006.04897.x. [DOI] [PubMed] [Google Scholar]
- 27.Chiappa GR, Queiroga F, Jr, Meda E, Ferreira LF, Diefenthaeler F, Nunes M, et al. Heliox improves oxygen delivery and utilization during dynamic exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:1004–10. doi: 10.1164/rccm.200811-1793OC. [DOI] [PubMed] [Google Scholar]
- 28.Lee DL, Lee H, Chang HW, Chang AY, Lin SL, Huang YC. Heliox improves hemodynamics in mechanically ventilated patients with chronic obstructive pulmonary disease with systolic pressure variations. Crit Care Med. 2005;33:968–73. doi: 10.1097/01.ccm.0000163403.42842.fe. [DOI] [PubMed] [Google Scholar]
- 29.Eves ND, Petersen SR, Haykowsky MJ, Wong EY, Jones RL. Helium hyperoxia, exercise, and respiratory mechanics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174:763–71. doi: 10.1164/rccm.200509-1533OC. [DOI] [PubMed] [Google Scholar]
- 30.Carroll CL. Heliox for children with acute asthma: Has the sun set on this therapy? Pediatr Crit Care Med. 2010;11:428–9. doi: 10.1097/PCC.0b013e3181ce6d19. [DOI] [PubMed] [Google Scholar]
- 31.Carter ER, Webb CR, Moffitt DR. Evaluation of heliox in children hospitalized with acute severe asthma. A randomized crossover trial. Chest. 1996;109:1256–61. doi: 10.1378/chest.109.5.1256. [DOI] [PubMed] [Google Scholar]
- 32.Kudukis TM, Manthous CA, Schmidt GA, Hall JB, Wylam ME. Inhaled helium-oxygen revisited: Effect of inhaled helium-oxygen during the treatment of status asthmaticus in children. J Pediatr. 1997;130:217–24. doi: 10.1016/s0022-3476(97)70346-9. [DOI] [PubMed] [Google Scholar]
- 33.Kim IK, Phrampus E, Venkataraman S, Pitetti R, Saville A, Corcoran T, et al. Helium/oxygen-driven albuterol nebulization in the treatment of children with moderate to severe asthma exacerbations: A randomized, controlled trial. Pediatrics. 2005;116:1127–33. doi: 10.1542/peds.2004-2136. [DOI] [PubMed] [Google Scholar]
- 34.Rivera ML, Kim TY, Stewart GM, Minasyan L, Brown L. Albuterol nebulized in heliox in the initial ED treatment of pediatric asthma: A blinded, randomized controlled trial. Am J Emerg Med. 2006;24:38–42. doi: 10.1016/j.ajem.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Kneyber MC, van Heerde M, Twisk JW, Plötz FB, Markhors DG. Heliox reduces respiratory system resistance in respiratory syncytial virus induced respiratory failure. Crit Care. 2009;13:R71. doi: 10.1186/cc7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bigham MT, Jacobs BR, Monaco MA, Brilli RJ, Wells D, Conway EM, et al. Helium/oxygen-driven albuterol nebulization in the management of children with status asthmaticus: A randomized, placebo-controlled trial. Pediatr Crit Care Med. 2010;11:356–61. [PubMed] [Google Scholar]
- 37.Kallet RH. Adjunct therapies during mechanical ventilation: Airway clearance techniques, therapeutic aerosols, and gases. Respir Care. 2013;58:1053–73. doi: 10.4187/respcare.02217. [DOI] [PubMed] [Google Scholar]
- 38.deBoisblanc BP, DeBleiux P, Resweber S, Fusco EE, Summer WR. Randomized trial of the use of heliox as a driving gas for updraft nebulization of bronchodilators in the emergent treatment of acute exacerbations of chronic obstructive pulmonary disease. Crit Care Med. 2000;28:3177–80. doi: 10.1097/00003246-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Palange P. Lighter than air: Heliox breathing improves exercise tolerance in COPD. Eur Respir Rev. 2010;19:1–3. doi: 10.1183/09059180.00000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wedzicha JA. Heliox in chronic obstructive pulmonary disease: Lightening the airflow. Am J Respir Crit Care Med. 2006;173:825–6. doi: 10.1164/rccm.2512007. [DOI] [PubMed] [Google Scholar]
- 41.Winters JW, Willing MA, Sanfilippo D. Heliox improves ventilation during high-frequency oscillatory ventilation in pediatric patients. Pediatr Crit Care Med. 2000;1:33–7. doi: 10.1097/00130478-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Chowdhury MM, Brown MK, Habibi P. Heliox and ventilatory support: What does it mean for the future of infant care? Infant. 2006;2:194–203. [Google Scholar]
- 43.Berkenbosch JW, Grueber RE, Dabbagh O, McKibben AW. Effect of helium oxygen (heliox) gas mixtures on the function of four pediatric ventilators. Crit Care Med. 2003;31:2052–8. doi: 10.1097/01.ccm.0000084804.15352.48. [DOI] [PubMed] [Google Scholar]
- 44.Stucki P, Scalfaro P, de Halleux Q, Vermeulen F, Rappaz I, Cotting J. Successful management of severe respiratory failure combining heliox with noninvasive high-frequency percussive ventilation. Crit Care Med. 2002;30:692–4. doi: 10.1097/00003246-200203000-00032. [DOI] [PubMed] [Google Scholar]
- 45.Hurford WE, Cheifetz IM. Respiratory controversies in the critical care setting. Should heliox be used for mechanically ventilated patients? Respir Care. 2007;52:582–91. [PubMed] [Google Scholar]
- 46.Dani C, Fontanelli G, Lori I, Favelli F, Poggi C. Heliox non-invasive ventilation for preventing extubation failure in preterm infants. J Matern Fetal Neonatal Med. 2013;26:603–7. doi: 10.3109/14767058.2012.745501. [DOI] [PubMed] [Google Scholar]
- 47.Gainnier M, Forel JM. Clinical review: Use of helium-oxygen in critically ill patients. Crit Care. 2006;10:241. doi: 10.1186/cc5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fink JB. Opportunities and risks of using heliox in your clinical practice. Respir Care. 2006;51:651–60. [PubMed] [Google Scholar]


