Abstract
Tube thoracostomy (TT) placement belongs among the most commonly performed procedures. Despite many benefits of TT drainage, potential for significant morbidity and mortality exists. Abdominal or thoracic injury, fistula formation and vascular trauma are among the most serious, but more common complications such as recurrent pneumothorax, insertion site infection and nonfunctioning or malpositioned TT also represent a significant source of morbidity and treatment cost. Awareness of potential complications and familiarity with associated preventive, diagnostic and treatment strategies are fundamental to satisfactory patient outcomes. This review focuses on chest tube complications and related topics, with emphasis on prevention and problem-oriented approaches to diagnosis and treatment. The authors hope that this manuscript will serve as a valuable foundation for those who wish to become adept at the management of chest tubes.
Keywords: Chest tube, complications, diagnosis, prevention, review, thoracostomy tube, treatment
INTRODUCTION
Descriptions of procedural drainage of the pleural space date back to the times of Hippocrates.[1] Despite different techniques and devices used throughout the history, the basic principles did not change.[1] Today, tube thoracostomy (TT) placement remains among the most commonly performed procedures, from bedside to operating room, from life-threatening emergencies to postoperative chest drainage in elective surgery.[2] In the injured patient, TT may be lifesaving and facilitates evacuation (and monitoring) of hemothorax, prevents the development of tension pneumothorax [Figure 1] while promoting lung reexpansion, tamponades low pressure pulmonary bleeding, and improves respiratory function.[2] In the surgical patient, chest tubes facilitate postoperative recovery. Patients with malignancies may benefit from symptomatic relief brought about by drainage of persistent, large pleural effusions.[3] Despite potential benefits of TT drainage, many possible complications exist. This manuscript reviews chest tube complications and provides an overview of topics related to TT-related morbidity.
Figure 1.
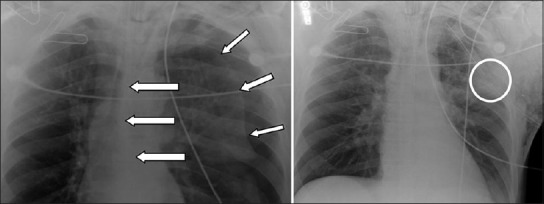
Tension pneumothorax (short arrows, left) with contralateral mediastinal shift (long arrows, left). The pneumothorax was managed with emergently placed tube thoracostomy (right). Note the position of the most peripheral intake portal (circled, right). This “sentinel hole” should be located inside the thoracic cavity, and the distance markers on the chest tube indicate the distance between the “sentinel hole” and the numbered marker located at the skin level. The distance between the “sentinel hole” and the skin varies based on various factors (i.e., the position of the TT entry site on the chest wall, patient body habitus, obesity, etc)
Chest tubes: Indications and contraindications
Indications for chest tube placement include: (a) pneumothorax; (b) penetrating chest trauma; (c) severe blunt chest trauma; (d) hemothorax; (e) chylothorax; (f) symptomatic pleural effusion; (g) bronchopleural fistula; (h) chemical pleurodesis for benign and malignant conditions; (i) postoperative use in thoracic/cardiac surgery; and (j) complicated parapneumonic effusion or empyema.[4,5,6] In addition, a number of relative indications for TT placement exist. Due to the limited scope of this article, the reader is referred elsewhere for a complete list.[1,4,5,6] Contraindications to thoracostomy placement have to be considered in the context of the overall risk-benefit assessment. For example, there are no contraindications to TT placement for tension pneumothorax. However, previous history of pleurodesis or lung transplantation may preclude “blind” chest tube placement and trigger the performance of image-guided TT procedure. Patients with extensive pulmonary blebs also have a relative contraindication to TT placement because of the risk of bronchial fistula formation.[4,7] Elective or semi-elective TT placement should be avoided in patients with clinically significant coagulopathy and consideration should be given to normalization of coagulation parameters prior to commencing with the procedure.
Overview of chest tube related complications
Cumulative rates of early (<24hrs post-placement) and late (>24hrs post-placement period) chest tube complications are approximately 3 and 8-10%, respectively.[8,9] Thoracostomy procedures carry multiple, often underestimated risks and the list of published complications of TT is extensive. The number of anatomic structures potentially affected during TT placement includes primary and secondary injuries of the lung, intercostal/intrathoracic vasculature, esophagus, stomach, liver, spleen, diaphragm, major blood vessels, and even cardiac structures.[8,9,10,11,12,13,14,15,16] While TT complications can be categorized as acute or chronic, procedural and non-procedural, provider- and patient-related, we have chosen to categorize them into organ-specific complications and thoracic cage complications/injuries (extrapulmonary anatomic, physiological, technical, and miscellaneous). We will briefly address procedural technical/safety considerations and follow with a review of individual complications.
Procedural and technical considerations
Before proceeding with TT placement, one must carefully consider pertinent indications and contraindications. Different chest tube types and their characteristics have been described elsewhere,[1] and this section will focus on general peri-procedural, operative, and safety-oriented aspects. A chest tube's length and internal diameter are important determinants of effluent flow rates and risk of blockage/occlusion. Drainage of more viscous fluid requires a larger diameter tube (i.e., for hemothorax use 36-French diameter or larger). On the other hand, for iatrogenic pneumothorax, a smaller (i.e., 20-French) or even a “pigtail” or “dart” thoracostomy may be sufficient. The patient's anatomy and body habitus direct the extent of the skin incision, soft tissue dissection, and intra-thoracic excursion of the TT. Commonly utilized approaches to TT placement have been described elsewhere.[1,17,18,19]
The chest tube needs to be advanced far enough into the thorax so that its most peripheral intake portal (i.e. the “sentinel hole”, [Figure 1] rests within the pleural space. Chest tubes directed medially (i.e., towards the midline) rather than superiorly (i.e., towards lung apex) are more likely to abut the mediastinum. Creating a tunneled tract helps optimize tube positioning. Post-procedure chest roentgenogram is used to confirm proper placement and identify tube misplacement.
Procedural safety considerations
Although TT insertion is ubiquitous in everyday clinical practice, the procedure may be complicated by patient misidentification or wrong side/site placement. Errors during patient safety checks and communication breakdowns are among causes of “wrong-procedure”, “wrong-site/side”, and “wrong-patient” adverse events. Additionally, natural symmetry of the body may contribute to confusion regarding procedural laterality, especially if surgical drapes, patient positioning, and/or operative table rotation are involved.[20] In one study of intercostal chest drain procedures, the authors proposed that a pre-insertion checklist could improve procedural safety, including reduction in wrong-site placements.[21]
To maximize procedural safety, certain critical steps must be followed during TT placement. The Joint Commission has implemented the Universal Protocol for invasive procedures. Although physicians performing procedures in the non-surgical setting do not always adhere to this protocol,[22,23] the Universal Protocol should not be limited to the classic procedural or operative settings,[22,24] and established guidelines should be followed prior to performing a TT insertion to ensure proper safety.[24] One of the most important considerations is the utilization of a safety checklist, which may help reduce the incidence of wrong-site procedures, eliminate omissions of important adjuncts such as procedural adherence to sterile techniques, and minimize other critical errors associated with lack of familiarity with equipment, etc.[23] Nearly all modern chest tubes contain an important landmark termed “the sentinel hole”-the most peripheral intra-thoracic hole that contains a radiographic marker and should always be positioned within the markings of the ribcage [Figure 1]. Another critical safety step is the review of both pre- and post-procedure radiographs by the physician performing the TT, with emphasis on education regarding critical findings such as retained surgical items, residual pneumothorax, new or incompletely drained hemothorax, and pleural/mediastinal abnormalities that suggest iatrogenic injury.
The urgent conditions of the emergency department (ED) often present challenges to the performance of peri-procedural patient safety checks. One ED survey indicated lack of awareness of the “time out” requirement for procedures among ~ 10% physicians, although 4% mentioned that such protocols may help prevent errors.[25] Only three-fourths of physicians who routinely place chest tubes responded that they would perform a “time out” before the procedure, allowing for the possibility of laterality errors.[25] For chest tube procedures across all areas of medicine, pre-procedure verification, specific site marking, and a “time out” should be universally performed.
Organ-specific complications
Esophageal injury
Esophageal injury associated with TT is rare. Direct injury to the esophagus may occur during or after tube placement. Drainage of enteric or salivary contents through the tube should raise suspicion and contrast studies confirm the diagnosis. Esophageal perforations have been reported for TT placed in the setting of normal esophagus,[26] various pathologic states, and post-esophageal surgery.[16] Esophageal injuries are best avoided by repositioning chest tubes that appear to terminate close to the mediastinum. Management of esophageal injuries related to TT follows general principles of esophageal injury management. Non-operative approaches have been reported, but surgical repair is the mainstay of treatment. Options include primary repair (with or without tissue flap), esophageal exclusion/diversion, drainage, and/or resection.[27]
Gastric injury
The stomach is rarely injured during TT placement, and usually occurs with intra-abdominal placement (may also be associated with concurrent diaphragm injury).[5] Proper technique and avoiding trocar use during chest tube placement reduces the risk of this complication. Gastric trauma during TT insertion has also been reported in patients with intrathoracic gastric herniation.[15,28] The presentation of gastrothorax may mimic a tension pneumothorax,[29] increasing the risk of gastric TT insertion. While chest radiographs may help prevent this complication, <40% of patients have radiographs suggestive of diaphragm rupture. Clinical suspicion of gastrothorax is necessary as shock and respiratory insufficiency, along with the nonspecific radiographic findings of pleural effusion, basilar opacity and a non-visualized diaphragm may mask the diagnosis.[28,30] Injured stomach/diaphragm must be promptly repaired.
Bowel injury
Chest tube misplacement into the abdominal cavity [Figures 2 and 3], although rare, can lead to injury of any intra-abdominal organ and significant morbidity.[5] The incidence of intra-abdominal TT placement is <1% but approximately one third of cases result in bowel injury.[5] Clinical presentation may include enteric content drainage from the chest tube, peritonitis, or sub-diaphragmatic tube location on follow-up chest roentgenogram. Similar to penetrating abdominal trauma with enteric injury, TT-related bowel injury mandates an exploratory laparotomy. The extent of bowel injury will determine the type of repair performed. Intraoperatively, care should be taken to identify and repair any other associated injuries. This may include but is not limited to liver, splenic, and diaphragmatic injuries. Patients with gross enteric spillage may require post-operative antibiotics.
Figure 2.
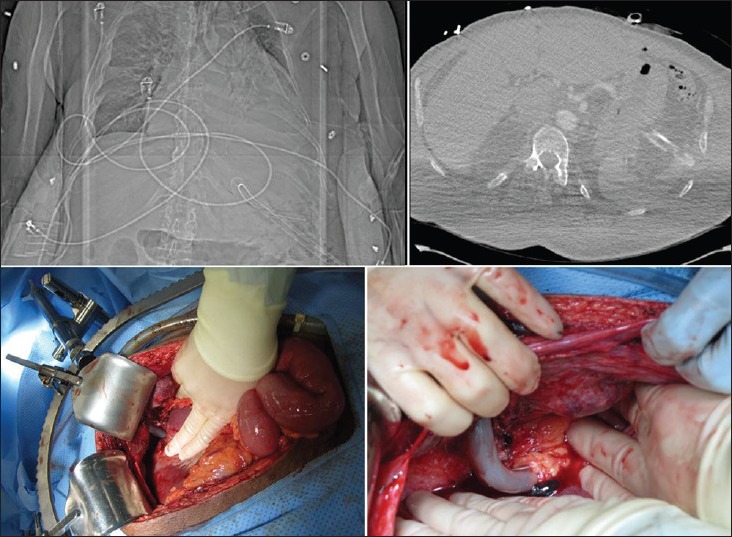
Sub-diaphragmatic tube thoracostomy (TT) mis-placement. Plain radiograph showing the TT in the left upper quadrant (left upper image). Computed tomography confirms intra-abdominal location of the chest tube, along with free air (right upper). The patient underwent laparotomy due to the combination of both radiographic (free air) and clinical (abdominal pain) findings. Intraoperative photographs show the TT causing a superficial injury to the left hepatic lobe (left lower) and then proceeding deeper in to the left upper quadrant (peri-splenic) wrapped by omental tissue (right lower)
Figure 3.
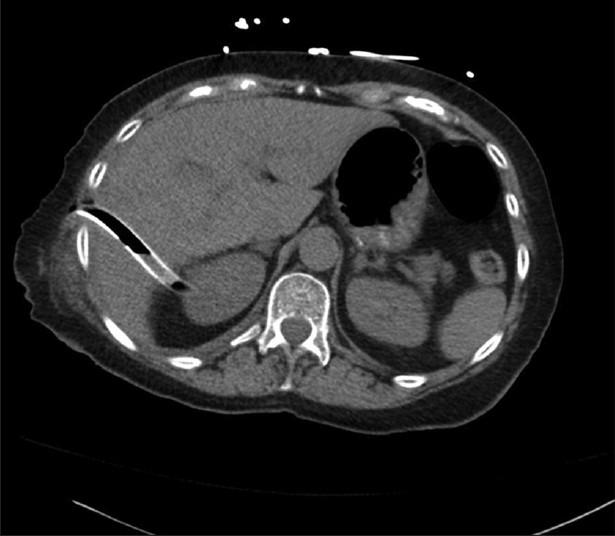
An example of hepatic injury secondary to thoracostomy tube. This elderly patient underwent a thoracoscopic-assisted right lower lobectomy and developed a symptomatic right-sided loculated pleural effusion several weeks later. An attempt to place right-sided chest tube at the bedside resulted in an iatrogenic injury to the hepatic parenchyma. The tube was removed in a monitored setting, with interventional radiology and surgical teams standing-by for possible hepatic parenchymal bleeding. Following tube removal, the patient remained asymptomatic and showed no signs or symptoms of bleeding
During expiration the diaphragm can rise to the level of the 5th intercostal space. Placement of the chest tube no lower than the 5th intercostal space (approximately the level of nipple in men or infra-mammary fold in women) in the mid axillary line helps prevent subdiaphragmatic insertion. Important landmarks to consider during TT placement include the “triangle of safety” (i.e., anterior border of the latissimus dorsi, lateral border of the pectoralis major, and a line superior to the horizontal level of the nipple).[31] Confirmation of intrathoracic placement by carefully inserting a sterile gloved finger into the thoracostomy tract prior to introducing the TT can identify misplacement before injury happens.
Hepatic injury
Liver injury [Figure 3] associated with TT placement is uncommon.[8] For patients with resultant major hepatic hemorrhage, vascular injury, and/or hemodynamic instability, surgery is indicated. A nonoperative approach to this complication has been described, provided that the patient is hemodynamically stable, there is no evidence of major vascular injury on advanced imaging, and there are no contraindications to percutaneous intervention (i.e., hepatic embolization).[8] Prevention of this injury follows the same rules as prevention of other complications related to ectopic TT placement.
Splenic injury
The close proximity of the spleen to the left hemidiaphragm places the spleen at risk for injury if a TT is placed through or below the diaphragm [Figure 2]. Although rare,[9] splenic placement may result in hemoperitoneum and/or shock. At times, it may be discovered incidentally on follow-up imaging. While splenic injuries in general can be managed non-operatively, patients with splenic injury from TT warrant an exploratory laparotomy to assess the extent of the injury and to rule out any potential associated injuries. The extent of injury will determine whether splenectomy is required. Embolization has been successful in cases of liver injury following TT placement and could be considered for splenic injuries.[8] Prevention includes attention to anatomic landmarks, including the “triangle of safety”.[31]
Diaphragm injury
Most diaphragmatic injuries during TT placement result in either laceration, perforation, or muscle dysfunction. Diaphragmatic laceration or perforation occurs when the tip of the trocar or the distal portion of the tube comes in contact with the diaphragm. Several conditions may increase the risk of this complication. During full expiration, the diaphragm rises as high as the 4th intercostal space.[1] Other conditions predisposing the diaphragm to TT-related injury include hemidiaphragm paralysis, late pregnancy, obesity, massive ascites and intraabdominal tumors.[32,33] Proper procedure and technique reduce the likelihood of this complication. Additionally, real-time scanning or ultrasonography guided insertion can help ensure TT placement is safe despite movement or elevation of the diaphragm.[34]
Malpositioning of the chest tube can also cause diaphragmatic dysfunction. Most common in neonates, this complication occurs secondary to phrenic neuropraxia from TT causing nerve compression.[35] The timing of diaphragm dysfunction is unknown; However, eventration was observed within hours of tube insertion.[36] Patients may be asymptomatic or show signs of respiratory insufficiency. Continuous positive airway pressure may depress the affected diaphragm and obscure the diagnosis.[36] The incidence of phrenic nerve paralysis can be reduced by maintaining the tip of the tube at least 2 cm from the vertebral line.[10] Prompt recognition and tube repositioning result in improvement.[35]
Lung injury
The lung is the most commonly injured organ during TT placement. Patients with decreased lung compliance, consolidation of the underlying parenchyma or significant pleural adhesions are at increased risk for laceration. These conditions prevent normal displacement of the lung when confronted by the chest tube. The use of a trocar and an inability to sufficiently explore the pleural space prior to tube placement also increase the risk of lung laceration.[14,1,37] Diagnosis of this complication is often delayed or missed because radiographic evidence of laceration is absent and many patients do not exhibit signs or symptoms, especially patients with preexisting pulmonary disease.[37]
Infarction is another rare form of chest tube-related lung injury. When excessive pleural suction is applied, aspiration of a lung segment into the TT leads to pulmonary infarction. The use of a low pressure, high volume pump can avoid this complication.[1,5,38] Pulmonary artery canalization is a rare complication of TT placement. Rapid drainage of pulsatile, dark red blood, shortness of breath, tachycardia, and hypotension are signs/symptoms of this serious complication. Volume replacement, clamping the TT and surgical repair of the injured artery are required.[39]
Delayed lung perforation, although rare, is well recognized as a complication of TT use.[40] The mechanism for such occurrence is multifactorial, including the duration of TT dwelling as well as tube repositioning maneuvers and prolonged use of suction. Chest tube removal has been advocated as the initial therapy in such occurrence.[40]
Cardiac/vascular injury
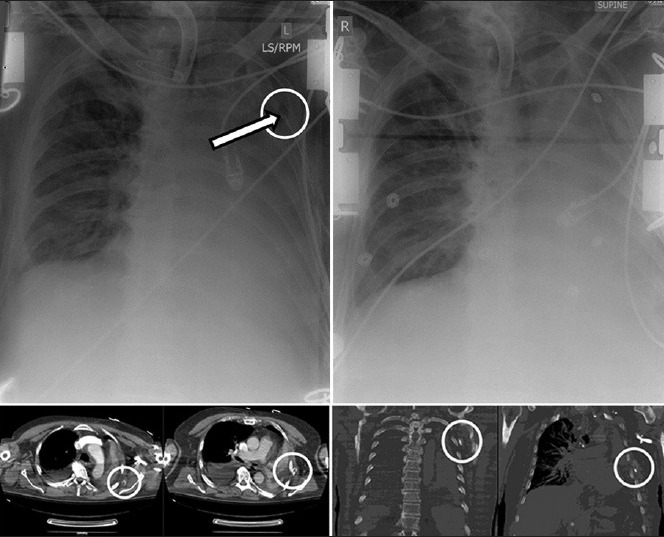
Cardiovascular complications following tube thoracostomy are rare. However, these rare complications can lead to mortality if not recognized. Cardiovascular complications can occur from tube compression of critical structures causing vascular compromise [Figure 4], or from penetrating cardiac injury during tube insertion.[41,42,43]
Figure 4.
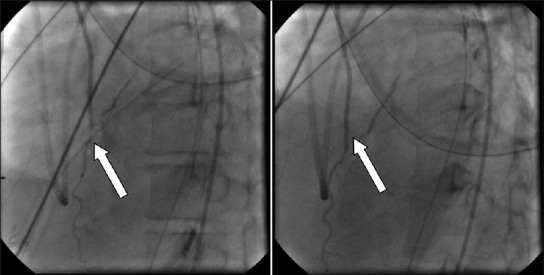
An example of postoperative chest tube compression of a coronary bypass graft (CBG).”. The chest tube is seen crossing the CBG and producing pressure-related flow-limiting lesion (arrow, left); Withdrawal of the chest tube results in improved distal flow (right)
When advanced too far into the pleural cavity, TT can abut the mediastinum and in rare instances mechanically compress cardiovascular structures. Chest tubes have been described compressing the right ventricle or the descending aorta.[41,42] In severe cases this can lead to hemodynamic instability. More commonly a TT will be seen crossing the midline of the thorax or bending on itself on chest roentgenogram. When a TT is found abutting the mediastinum it should be appropriately withdrawn and secured at its new position, with radiographic re-verification of position. Even in some cases of cardiogenic shock, withdrawing the tube has resolved the symptoms.
Penetrating cardiac injury has been described following TT placement.[44] Clinical presentation includes rapid blood drainage from the tube following insertion and hemodynamic collapse. These patients require an emergent thoracotomy or sternotomy to repair the cardiac injury. Penetrating cardiac injuries usually result from inappropriate use of the trocar technique, where the trocar tip lacerates/penetrates the heart and provides a route of intracardiac TT passage. While this technique allows for rapid insertion and a smaller incision, great force is required to insert the trocar through the chest wall and into the pleural space. Loss of control of the sharp trocar tip can have devastating consequences. An open TT placement with blunt dissection allows for controlled entry into the pleural space and limits the use of sharp instruments. Digital palpation of the pleural cavity prior to inserting the tube can verify the presence of adhesions, misplacement, or proximity of organs that may interfere with tube insertion.
At this juncture mediastinal tubes deserve special mention. Mediastinal tubes are inserted as routine post-operative practice following cardiac surgery to assist the clearance of blood and fluid from the pericardial and pleural spaces and to prevent cardiac tamponade and pleural effusion. Literature on mediastinal TT morbidity is limited. Complications occur mainly when the drain is malpositioned or malfunctioning; manipulation techniques including milking, stripping, fanfolding and tapping may be applied to keep tubes from blocking. Common complications include pain from nerve impingement, subcutaneous emphysema, pneumothorax, direct injuries to the lung, injury of the diaphragm and intraabdominal organs, chylothorax, and neurologic complications (Horner's syndrome, winging of the scapula, acute diaphragmatic paralysis).[13] Tube entrapment under sternal sutures is uncommon; Berkow and Salo reported 5 cases among 759 median sternotomies performed.[45]
Mediastinitis is a devastating complication of cardiac surgical procedures. The reported incidence ranges from 0.15 to 8% but is 1 to 2% in most series.[46] Massive hemorrhage caused by rupture of a vein graft varicosity that was suctioned into the tube and life-threatening complication of aortic perforation caused by the friction of the chest tube have also been reported.[47,48] In such an event, reexploration is necessary because of the significant mortality associated with nonoperative management. Use of the pericardial membrane between the heart and the chest tube and the choice of smaller and more flexible silastic chest tubes may reduce the incidence of these complications. Postoperative myocardial ischemia manifested by reduced ventricular contraction, caused by TT compressing a vein graft has also been reported.[49] Tube repositioning is required in these situations.
Extrapulmonary anatomic complications
Bronchopleural fistula
Bronchopleural fistula (BPF) is the abnormal connection between the pleural space and the bronchial tree. It is both an indication for and a complication of chest tube placement. Although rare, acute BPF is difficult to manage and is associated with high morbidity, prolonged hospital stay, high resource utilization, and mortality.[50,51] While a TT may be beneficial in the management of existing BPF, the tube itself may also contribute to or worsen a BPF (especially when malpositioned).
Patients with TT often require concurrent mechanical ventilation, which may contribute to BPF. Loss of tidal volume, gas exchange abnormalities, and the appearance of ventilator cycling are initial diagnostic clues related to this form of BPF.[50] Furthermore, negative pressure applied to TT can increase flow through the fistulous tract and delay BPF closure and healing.[52] Acute signs and symptoms of BPF include dyspnea, hypotension, subcutaneous emphysema, cough, and persistent air leak. Diagnosis can be confirmed via bronchoscopy, bronchography, or computed tomography (CT). Broncho-mediastinal fistulae have also been reported.[7]
BPF resulting from TT insertion is synonymous with a failure of the chest drainage. Therefore, alternative methods to treat the condition must be used. Initial treatment should target life-threatening conditions, such as massive air leaks or tension pneumothorax. A number of clinical approaches to BPF include application of sealants via bronchoscopy, sclerosing and occluding methodologies, up to and including balloons, stents, as well as biologic and non-biologic adhesives.[50] However, BPF as a direct complication of TT likely will require more aggressive intervention, such as direct open repair (with or without tissue flap), partial pneumonectomy, or video assisted thoracoscopy.[51] These risky procedures are generally used in cases of advanced BPF, often in poor (high-risk) surgical candidates.
Proper TT insertion technique, precise tube placement, appropriate patient positioning, and utilization of sealants may all play a role in prevention and management of BPF.[1] Timely chest tube removal limits the duration of time exposure to possible tube erosion etc.[53] For patients with chest tubes who require mechanical ventilation, the goal should be to reduce airway pressure and minimize tube suction, therefore reducing fistula flow and loss of tidal volume.[50]
Recurrent pneumothorax
The dreaded complication following chest tube removal is the recurrence of a previously resolved pneumothorax. A recurrent pneumothorax can be associated with premature TT removal (i.e., before full lung re-expansion), an occult air leak, or air entering the pleural space during removal. The latter is especially common in patients with relatively thin, muscular, chest wall anatomy. Patients may be asymptomatic, develop shortness of breath, or tension pneumothorax in extreme cases. The pneumothorax can be detected on chest roentgenogram following TT removal, highlighting the need for confirmatory imaging.
Small pneumothoraces in patients that are clinically stable can be managed with clinical observation and follow-up chest radiography. Patients should be placed on supplemental oxygen (to maximize the “nitrogen washout” effect) and have follow-up chest roentgenogram to assess for resolution. In symptomatic patients or patients with a large pneumothorax, a new TT is inserted to allow lung reexpansion. In a study of 33 patients with a recurrent pneumothorax following TT removal, 20 required insertion of a new chest tube.[13] Of note, patients who require insertion of a new TT have been show to have twice the length of hospital stay.[54]
Proper technique for TT removal is critical for preventing recurrent pneumothorax. The thoracostomy site needs to be kept occluded during the removal and an occlusion dressing needs to be placed over the site immediately. Chest tube removal will be discussed in detail in a later section.
Intercostal arterial hemorrhage
The intimate association of the intercostal arteries with the inferior border of their respective rib makes them potentially vulnerable to injury during TT placement. Given this anatomic consideration, tissue dissection during chest tube placement should be done on the superior border of the rib to avoid arterial trauma (i.e., “above to avoid”). Failure to do so may result in hemorrhage into a “negative pressure” space. Cases of massive hemothorax and sudden death after injuring intercostal arteries during TT placement have been reported.[55] Proximal surgical artery ligation and transcatheter arterial embolization have both been utilized to control intercostal artery hemorrhage.[56]
Arteriovenous fistula, including chest wall (intercostals)
Although intercostal vessel laceration during TT placement is usually associated with profuse bleeding during or persistent bleeding after tube placement, the chest tube itself may tamponade such an injury and encourage the development of an arteriovenous fistula. Systemic fistulas between an intercostal artery and a subcutaneous vein may present immediately or after several years. The most common physical signs include machinery-like murmur, pulsatile mass and palpable thrill. Selective angiography or contrast-enhanced MRI establishes the diagnosis. Sonography may be useful and, on occasion, plain radiographs may show an abnormal density, or bone erosion. Surgical excision of the fistula has been the mainstay of treatment. In addition, transcatheter embolization has been successful.[57] Prevention of this complication relies on proper TT placement technique, and is best accomplished by dissecting and placing the tube above the superior border of the rib to avoid the neurovascular bundle (see previous section).
Chylothorax
Disruption of the thoracic duct may result in the drainage of lymphatic fluid into the pleural space, known as a chylothorax. Chylothorax following TT is very rare with only a handful of reported cases.[58] It is characterized by a milky appearing fluid drainage due to high fat content of the lymph. The diagnosis is supported by pleural fluid triglyceride levels >110 mg/dL. The thoracic duct runs cephalad along the right side of the aorta, crosses to the left side at the level of the fifth thoracic vertebra, and runs along the left side of the aorta until entering the venous system at the junction of the left internal jugular and subclavian veins. An injury below the fifth vertebrae will cause right chylothorax and an injury above the fifth vertebrae will cause left chylothorax.
Initial management of chylothorax is conservative, with continued TT drainage. A second thoracostomy may be considered if the original tube does not provide adequate drainage. Patients are placed on a strict low fat diet, supplemented by medium chain fatty acids. This management course is usually attempted for 1-2 weeks. Therapeutic failures are taken to the operating room for ligation of the thoracic duct through either a thoracotomy or thoracoscopy. Patients may be given a cream meal immediately prior to the procedure to help identify the site of the leak. More recently, techniques have evolved wherein lympangiographic “embolization” of the leaking duct is accomplished percutaneously.[59] To avoid thoracic duct injury the tube needs to be directed toward the pulmonary apex rather than the mediastinum.
Fibrothorax
Failure to fully evacuate a hemothorax can lead to development of fibrothorax. Retained blood within the pleural space causes inflammation and fibrous changes involving the parietal and visceral pleurae. Fibrothorax “traps” the lung, preventing it from fully expanding, and can begin as early as 1-2 weeks after the initial injury. Patients with fibrothorax report initial dyspnea. For mechanically ventilated patients, progressive difficulty with adequate ventilation due to restrictive lung mechanics can be seen. Radiographic imaging may show the retained hemothorax, loculated collections, and/or collapsed lung. Once fibrothorax develops, primary management is operative and consists of “freeing” the trapped lung. Any retained fluid is evacuated surgically and the fibrous adhesions removed to facilitate lung re-expansion. A formal decortication may be required.
Fibrothorax can be prevented by adequately draining the hemothorax early in the course of the process. A retained hemothorax should be evacuated before it causes fibrosis. Over 95% of hemothoraces are effectively treated by TT alone.[60] An additional TT may be necessary to fully drain the “difficult” (i.e., dense or loculated) hemothorax. However, this is usually only effective during early management, before the hemothorax begins to organize and clot. Fibrinolytics (i.e., streptokinase or urokinase) used to dissolve and then evacuate clot contents and/or loculated hemothorax can be administered through the TT as an “intermediate measure” in select cases.[61] When performed early, thoracoscopy allows for complete evacuation of the retained clot with minimal morbidity, decreased hospital stays and lower hospital costs compared to placement of a second thoracostomy.[62]
Technical complications
Non-functioning tube
Chest tube non-function may be associated with disconnection of the tube from the suction mechanism, mechanical obstruction (i.e., blood clot) or tube kinking, or from a dysfunctional closed system apparatus. If a closed suction system becomes disconnected, the tube should be cleaned with an antiseptic and the tubing reconnected. The chest tube should not be clamped because doing so in a patient who has active air leak can result in tension pneumothorax.[34] A malfunctioning suction apparatus needs to be replaced promptly. Likewise, whenever significant contamination is noted, the tube itself should be replaced.
Tube kinking and clotting, especially in smaller tubes, are other common causes of nonfunctioning drainage.[38] The cardinal sign of blocked TT drain is failure of the fluid column within the tube to fluctuate with coughing or respiration. Several mechanical procedures including milking, stripping, and fan folding have been described to promote/maintain chest tube patency. Although, commonly utilized, the usefulness of these procedures is uncertain. In one study, two-thirds of clogged chest tubes were found to clear spontaneously without manipulation.[63] Additionally, several studies have shown that prophylactic tube milking/stripping does not prevent clotting.[64,65] Moreover, due to the potential buildup of negative pressure within the pleural cavity, these procedures have been associated with tissue entrapment, increased bleeding, and even left ventricular dysfunction.[66,67]
Contralateral pneumothorax
Contralateral pneumothorax is an uncommon complication of TT placement.[68] This complication may develop when the tube is advanced across the anterior mediastinum. Chronic obstructive pulmonary disease, due to a widened retrosternal airspace, and trocar use have been identified as risk factors for this complication.[69] Trans-mediastinal positioning of chest tubes is usually identified radiographically, but antero-posterior radiographs alone may be inadequate and lateral views increase detection rate. Computed tomography is most accurate in determining TT position.[70] Management includes repositioning of the culprit thoracostomy tube and inserting additional TT on the side of the newly developed pneumothorax.
Subcutaneous placement
Ectopic TT insertion occurs in up to 10% of placements.[9] Of those, about 15-20% are subcutaneous, wherein the tube is inserted into the chest wall and fails to enter the pleural space (i.e., the distal tip is located outside the parietal pleura).[9] Subcutaneously placed tubes [Figure 5] will fail to function either immediately or shortly after insertion. The fluid chamber in the collection system will not show respiratory variation. Improper TT placement leads to ineffective/failed drainage of pleural fluid or air. A follow-up chest radiograph confirms subcutaneous TT placement.[71]
Figure 5.
Subcutaneous chest tube placement in a patient with a large left-sided hemothorax. Immediately upon tube thoracostomy placement, large amount of sanguineous effusion was liberated. The patient was also noted to have an air leak in the suction apparatus. The initial chest radiograph (top left) was difficult to interpret due to ECG wires interfering with proper visualization, but the tip of the chest tube (arrow) was determined to be intrathoracic. Despite improved clinical picture, both the TT drainage and the air leak quickly stopped. The subsequent CT scan shows the TT to be extrathoracic (bottom images). A new chest tube was promptly inserted and the initial chest tube removed (top right).
Risk factors for this complication include patient obesity, multiple rib fractures, chest wall hematoma, and rushed TT insertion. When inserted too cranially and too medially, subcutaneous chest tubes have been associated with bleeding from the pectoralis major muscle (especially in men) and damage to breast tissue in women.[5] Subcutaneous chest tubes are identified clinically by absence of fluid fluctuation or radiographically by subcutaneous position of the chest tube [Figure 5]. Once identified, subcutaneous tubes must be removed and should not be advanced or repositioned. The TT should be removed and a new TT inserted at a new site under sterile conditions. Proper technique reduces placement-related complications, including subcutaneous TT passage.[2] An adequately sized incision will allow full dissection through the subcutaneous tissue and intercostal muscles prior to entering the pleural space. Passage of sterile gloved finger through the dissected tract confirms that the pleural space has been entered.
Persistent leakage around chest tube
If the thoracostomy site is not properly occluded with the surgical dressing or if the site is left too large relative to the tube, a leak around the TT can develop. The leak allows air back into the pleural space and will result in a residual pneumothorax. An air leak will be evident upon the examination of the collection chamber. At times, air can be heard around the chest tube site itself. Often this problem can be quickly corrected by applying a new occlusive dressing around the thoracostomy site that seals the leak. If the thoracostomy incision is large compared to the size of the tube, placing another heavy suture to reapproximate the incision can solve the problem. Of note, the thoracostomy site needs to be prepped in sterile fashion and local anesthetic should be used prior to placing another suture. The size and location of the initial incision can help decrease the likelihood of a leak around the chest tube. The skin incision should be only slightly larger than the size of the tube to be used in order to decrease the amount of potential space around the tube. Also, the incision can be made one intercostal space below the pleural entry level in order to create a tunnel between the pleura and the atmosphere.
Unintended tube dislodgement
Chest tube dislodgement after insertion is a common occurrence. Chest tube movement is inevitable, especially in critically ill patients who are frequently repositioned for procedures.[71] Patients who purposefully or inadvertently manipulate their chest tubes, increase the chance of dislodgement. When a TT is dislodged the patient should be re-evaluated, the indication for TT insertion re-confirmed, and, if necessary, a new tube reintroduced through a new site. Proper procedure and technique, when used to secure a drain, reduces the likelihood of dislodgement. Two heavy-gauge non-absorbable sutures should be placed, one to secure the drain and the other to close the wound. A simple interrupted or mattress suture is appropriate for a linear incision. A purse string suture should be avoided as it does not offer a better seal, increased risk of skin necrosis and leads to poor cosmetic results.[6,34] A tag of tape is recommended because it maintains the tube close to the chest wall but does not restrict minor fluctuations necessary to prevent kinking and insertion site tension.[1,34] The dressing may offer additional support to reduce movement and dislodgement. However, no study has been conducted to prove this.
Chest tube removal
As previously discussed, proper TT removal is critical to reducing the risk of recurrent pneumothorax. The focus is to prevent air from reentering the pleural space by minimizing the exposure of the thoracostomy site to the atmosphere. Required supplies should be available at the bedside and prepared prior to removal of the drain, including suture removal kit to remove the securing stitch and an occlusive dressing for the tube site. The securing stitch is first cut and removed. With one hand the occlusive dressing is placed at the tube site and the tube is quickly removed with the other hand in one smooth movement. After removal the occlusive dressing must be immediately secured without exposing the site to the atmosphere.
There has been some debate regarding the optimal timing of TT removal in relation to the respiratory cycle. Some propose removing the tube at end of maximal inspiration because the lung has been fully expanded creating the smallest space between the visceral and parietal pleurae.[72] However, when removing the TT during inspiration the patient can potentially suck air into the pleural space through the open thoracostomy site. Others recommend removal at end expiration when the pressure difference between the atmosphere and the thoracic cavity is the least, reducing the potential of air moving through the thoracostomy.[1] When removing the tube during expiration pain may cause the patient to inadvertently inhale and draw air in to the pleural space. One study of TT removal randomized patients to removal at end inspiration versus end expiration, demonstrating that the rate of recurrent pneumothorax between the two groups was similar (8% versus 6%).[73] Given that no method has shown to be superior in preventing pneumothorax, we recommend choosing one method to consistently use when removing the tube. Ultimately, quick removal of the TT while maintaining occlusion of the thoracostomy site helps prevent a recurrent pneumothorax during removal.
There is further disagreement whether chest tubes should be removed while on suction or after a trial on water seal. Placing the tube to water seal allows an occult air leak to become detectable prior to removing the chest tube, but such trials can increase the number of chest x-rays and hospital stays. Two studies have addressed whether removing chest tubes while on suction is safe with neither finding an increased incidence of recurrent pneumothorax; however, one found an increased incidence of pneumothorax requiring reinsertion of a chest tube.[54,74] Because chest tube reinsertion increases hospital stay and subjects patients to another invasive procedure, we recommend a trial on water seal prior to chest tube removal.
Physiologic complications
Reexpansion pulmonary edema
Reexpansion pulmonary edema (RxPE) is a rare complication of chest tube placement.[75] The incidence of RxPE ranges between 1-14%, with mortality rate as high as 20%.[76] It usually occurs on the side ipsilateral to the reexpanded lung, and its presentation can range from an asymptomatic radiographic finding to cardio-respiratory collapse and shock.[77] The exact pathophysiology for this complication has not been fully elucidated, but the mechanism appears to be related to increased endothelial permeability.[78] Oxygen free radicals and multiple inflammatory mediators are important to pathogenesis of RxPE.[78] Risk factors for RxPE include younger age (<40), longer duration of lung collapse (>4 days), and a large pneumothorax (>30% of a single lung).[77] For those at a high risk for RxPE, little or no negative pressure should be applied initially after placing a chest tube. If RxPE develops, the negative pressure applied via thoracostomy tube must be stopped immediately. If clinical deterioration continues, asynchronous differential lung ventilation may be beneficial.[79]
Vagus nerve irritation, including hemodynamic collapse
Sudden death, attributed to vagus nerve irritation following TT placement, has been reported.[80] This exceedingly rare complication may occur if vagal nerve irritation leads to excessive parasympathetic cardiac stimulation and a bradyarrhythmia with a possible fatal escape rhythm. In the reported case, autopsy revealed a hematoma surrounding the vagus nerve. This likely led to continued, direct vagus nerve stimulation following TT placement. The resultant arrhythmia did not respond to pharmacotherapy. Irritation of the nerve due to the chest tube will not resolve without removal or repositioning of the tube. One should consider removing the tube should a profound bradyarrhythmia develop after the placement.
Infectious complications
Insertion site infection, including necrotizing fasciitis
Skin infections, empyema, and even necrotizing infections can occur at the insertion site. In those situations, the site has to be examined for spread of infection to deeper tissues. Tube removal, antibiotics and surgical intervention may be needed. By acting as a foreign body, the TT may provide a “common plane” for infectious exposure between the insertion site, the chest wall, and the pleural space.[1] Necrotizing fasciitis, a rapidly progressive, life-threatening infection of subcutaneous tissue and fascia has been reported in association with TT placement.[81] Risk factors include excessive soft tissue dissection during TT insertion and pre-existing contamination.[82] In the early phases, cellulitis and necrotizing fasciitis may be difficult to clinically differentiate. In the later phases, subcutaneous crepitus may be present. However, in the setting of TT use for pneumothorax with significant pre-existing subcutaneous emphysema even the finding of subcutaneous crepitus may be misinterpreted as “expected”. Prompt recognition of necrotizing fasciitis is paramount due to the fact that urgent surgical chest wall debridement is required in addition to broad-spectrum antibiotic coverage.[81] Antibiotic coverage should include agents effective against mixed aerobic and anaerobic organisms.[82] Another rare but serious infectious complication associated with TT use is the osteomyelitis of the rib.[83,84] The most common presenting signs/symptoms include localized chest wall swelling and actively draining chest wall sinuses.[83] Risk factors for this complication include blunt chest trauma and thoracostomy drainage of empyema.[83] Staphylococcal infection is commonly seen.[84] Therapy is prolonged and includes culture-directed antibiotic coverage as well as surgical resection of the involved rib in cases of therapeutic failure.[83]
Empyema
Placement of a chest tube is considered a “clean contaminated” procedure. Empyema after TT has a reported incidence of 1% to 25%, depending on the study/population characteristics.[85] The presence of a pleural effusion prior to tube placement carries a higher risk of empyema.[86] In trauma patients, factors associated with the development of empyema include prolonged TT dwelling time, length of intensive care stay, the presence of a pulmonary contusion, laparotomy and retained hemothorax. Antibiotic prophylaxis prior to TT placement is controversial. With regards to peri-procedural antibiotic use, a detailed discussion can be found at the end of this manuscript. Staphylococcus aureus is the most frequent organism associated with empyema. Empyema occurs more frequently after penetrating (versus blunt) chest trauma because penetrating injuries allow direct pleural entry of microorganisms. Treatment options for empyema, alone or in combination, include continued TT drainage, new tube placement, image-directed drainage, intrapleural fibrinolytic therapy, thoracoscopy, and open pleural decortication.
Miscellaneous complications and topics
Tube erosion (non-acute)
Erosion of a thoracic structure is a very rare, delayed complication of chest tubes. Direct contact along with continuous pressure between the TT and thoracic structure, in addition to constant motion from respiration/cardiac rhythm, is the most likely mechanism of erosion.[26] Both rigid and soft chest tubes have been reported to cause erosion.[11,26]
Delayed esophageal erosion following TT placement has been reported.[26] A posteriorly placed tube, malnutrition, presence of infection, and an inappropriately sized tube increase the possibility of esophageal erosion.[26,87] An esophageal erosion should be considered whenever a previously functioning TT begins to drain enteric fluid (high amylase, low pH, squamous epithelial cells, multiple pathogens, food particles).[88] Confirmation of erosion is through an esophageal contrast study or computed tomography. Therapy includes continued draining, broad-spectrum antibiotics, nutritional support, and surgical repair or resection.
Erosions of the aorta and subclavian artery are also documented.[11,89,90] Hemodynamic instability and continued bleeding at the chest tube insertion site are signs of arterial erosion and require further investigation. Bleeding at the insertion site is not abnormal. However, it rarely occurs days after placement of the chest tube.[11] Thoracotomy is indicated for arterial erosions. Erosions are preventable with post-insertion chest radiographs allowing for repositioning of the misplaced chest tube.
Horner's syndrome
Horner's syndrome – an injury to the sympathetic pathways characterized by the presence of miosis, ptosis, anhidrosis, enophthalmos, ipsilateral vasodilation – has been reported following TT placement.[12] High insertion in the posterior chest wall has resulted in this complication.[91] The injury likely reflects an interaction of several factors affecting the nerve tissue, including direct pressure of the TT, hematoma formation, inflammation, stretching of the nerve fibers, and scar formation.[12] Variable in onset (hours to days) the clinical course of this TT-associated morbidity is self-limited in one-third to two-thirds of cases.[12,92] Reversibility may be related to how promptly this complication is recognized and treated.[93] Optimal clinical management involves prompt recognition and immediate TT repositioning and/or removal (if indicated).[12] It is important to carefully verify the post-placement position of the TT and to secure the tube adequately in order to prevent primary (direct impingement) or secondary (i.e. impingement related to migration) nerve injury. Any subsequent chest radiographs should be carefully reviewed to ensure continued optimal TT placement.
Determination of tube thoracostomy failure: Indications for surgery
Although the breadth of this section is extensive and beyond the scope of the current review, the authors would like to discuss some of the clinical criteria for declaring the failure of TT management and indications for surgical therapy in such cases of failed therapy. One of such circumstances is the presence of persistent severe air leak and the failure of the lung to re-expand following insertion of one or more thoracostomy tubes.[94] Another indication for escalating therapy is the failure of adequate TT drainage of intrapleural infection or blood (up to and including the use of intrapleural streptokinase) in patients with empyema or retained hemothorax.[95,96] Less frequently, the surgical approach may be utilized in cases of persistent, massive pleural effusion drainage for both benign and malignant conditions.[3,97] Although definitive timelines for determining when the chest tube therapy has failed are difficult to set, there is evidence to suggest that early (3-4 days) versus delayed surgical approach may be more beneficial in cases of empyema[98,99] and retained hemothorax.[99,100]
Miscellaneous topics
Tube thoracostomy in the setting of malignant disease carries a low (<5%) but not negligible rate of tumor seeding of the track.[101,102] Local spread of malignant mesothelioma following minimally invasive thoracic surgical procedures is well documented.[103] Fortunately, it is much less likely for other tumor types to “seed” wounds or tracks associated with pleural biopsies or chest tubes.[104,105]
Among the less common complications is the possibility of pulmonary infarction associated with chest tube placement. Pulmonary infarction has been described in the setting of lung entrapment secondary to TT.[106] Another less common complication is injury to the subclavian vasculature.[107,108] This complication can occur in the setting of perforated/injured parietal pleura and may be more common with trocar thoracostomy devices.[107] Preventive measures include strict adherence to procedural safety, as outlined earlier.[108]
The use of peri-procedural antibiotics during TT placement remains controversial. The practice is widely utilized across different clinical settings, from trauma to drainage of pleural effusion. Although prophylactic antibiotics have been found unnecessary in patients undergoing closed TT for primary spontaneous pneumothorax,[109] at least one prospective randomized trial showed that prophylactic antibiotics significantly reduce the overall rate of infectious complications following chest tube placement.[110] In a meta-analysis of studies on this topic, the use of prophylactic antibiotics was shown to reduce the absolute risk of empyema by approximately 6% and all infectious complications by approximately 12.5%.[111] Of note, most studies utilized cephalosporins or clindamycin for antibiotic coverage.[34] Current British Thoracic Society recommendations advocate the use of aseptic technique for TT insertion (recommendation class C) and prophylactic antibiotics in trauma cases (recommendation class A).[34]
Significant subcutaneous emphysema can be associated with chest tube use.[112] This clinical finding can usually be attributed to subcutaneous side-port migration, suboptimal initial TT placement, poorly secured tube, and thoracostomy blockage.[112] When unanticipated subcutaneous emphysema is clinically or radiographically noted, management includes TT inspection, followed by tube repositioning or replacement, as applicable/indicated. When the subcutaneous air is associated with inadequate drainage despite properly placed chest tube, consideration should be given to placement of additional thoracostomy drainage, investigation of a more serious complication (i.e., bronchopleural fistula) up to and including operative therapy as indicated.
The altered thoracic anatomy of the pregnant patient is worthy of discussion as well. Pregnancy contributes to early changes in the thorax and the thoraco-abdominal interface. These changes persist due to the mass effect of the gravid uterus in later fetal development, but the clinician must be aware that even in the first trimester, the chest is significantly affected. The rib cage increases up to 7 cm in circumference, the diaphragm moves cephalad approximately 4 cm, and its excursion increases by about 2 cm. Also important to TT placement, the subcostal angle increases 50% from 69.5 to 103.5 degrees, the intercostal spaces widen and the cardiac apex moves upward and to the left.[113,114] Consequently, special care should therefore be taken during TT placement in the pregnant patient.
CONCLUSIONS
Chest tubes are life saving instruments. Their use is necessary, and yet operators must be wary of the consequences of such use. Proper placement and evaluation of chest tubes is of paramount importance. An operator's ability to think through a differential diagnosis while trouble-shooting is a matter of knowledge, experience, and vigilance. The authors hope that this chest tube complication primer will serve as a good foundation for those who wish to become adept at the management of chest tubes. It is our belief that good knowledge of potential complications is the fundamental step to improved patient outcomes.
ACKNOWLEDGMENT
The authors would like to acknowledge the assistance of Dr Nikhil P. Jaik with obtaining manuscript figures.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Miller KS, Sahn SA. Chest tubes. Indications, technique, management and complications. Chest. 1987;91:258–64. doi: 10.1378/chest.91.2.258. [DOI] [PubMed] [Google Scholar]
- 2.Ball CG, Lord J, Laupland KB, Gmora S, Mulloy RH, Ng AK, et al. Chest tube complications: How well are we training our residents? Can J Surg. 2007;50:450–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Antunes G, Neville E, Duffy J, Ali N. BTS guidelines for the management of malignant pleural effusions. Thora×. 2003;58(Suppl 2):29–38. doi: 10.1136/thorax.58.suppl_2.ii29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gayer G, Rozenman J, Hoffmann C, Apter S, Simansky DA, Yellin A, et al. CT diagnosis of malpositioned chest tubes. Br J Radiol. 2000;73:786–90. doi: 10.1259/bjr.73.871.11089474. [DOI] [PubMed] [Google Scholar]
- 5.Millikan JS, Moore EE, Steiner E, Aragon GE, Van Way CW., 3rd Complications of tube thoracostomy for acute trauma. Am J Surg. 1980;140:738–41. doi: 10.1016/0002-9610(80)90107-5. [DOI] [PubMed] [Google Scholar]
- 6.Tang AT, Velissaris TJ, Weeden DF. An evidence-based approach to drainage of the pleural cavity: Evaluation of best practice. J Eval Clin Pract. 2002;8:333–40. doi: 10.1046/j.1365-2753.2002.00339.x. [DOI] [PubMed] [Google Scholar]
- 7.Meysman M, Noppen M, Delvaux G, Peters O, Vincken W. Broncho-mediastinal fistula following perforation of the oesophagus. Respirology. 1996;1:217–9. doi: 10.1111/j.1440-1843.1996.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 8.Tait P, Waheed U, Bell S. Successful removal of malpositioned chest drain within the liver by embolization of the transhepatic track. Cardiovasc Intervent Radiol. 2009;32:825–7. doi: 10.1007/s00270-008-9461-y. [DOI] [PubMed] [Google Scholar]
- 9.Osinowo O, Softah AL, Eid Zahrani M. Ectopic chest tube insertions: Diagnosis and strategies for prevention. Afr J Med Med Sci. 2002;31:67–70. [PubMed] [Google Scholar]
- 10.Nahum E, Ben-Ari J, Schonfeld T, Horev G. Acute diaphragmatic paralysis caused by chest-tube trauma to phrenic nerve. Pediatr Radiol. 2001;31:444–6. doi: 10.1007/s002470100428. [DOI] [PubMed] [Google Scholar]
- 11.Taub PJ, Lajam F, Kim U. Erosion into the subclavian artery by a chest tube. J Trauma. 1999;47:972–4. doi: 10.1097/00005373-199911000-00030. [DOI] [PubMed] [Google Scholar]
- 12.Knyazer B, et al. Horner's syndromesecondaryto chest tube insertion for pneumothorax. Asian J Ophthalmol. 2008;10:27–9. [Google Scholar]
- 13.Etoch SW, Bar-Natan MF, Miller FB, Richardson JD. Tube thoracostomy. Factors related to complications. Arch Surg. 1995;130:521–5. doi: 10.1001/archsurg.1995.01430050071012. discussion 525-6. [DOI] [PubMed] [Google Scholar]
- 14.Banagale RC, Outerbridge EW, Aranda JV. Lung perforation: A complication of chest tube insertion in neonatal pneumothorax. J Pediatr. 1979;94:973–5. doi: 10.1016/s0022-3476(79)80241-3. [DOI] [PubMed] [Google Scholar]
- 15.Icoz G, Kara E, Ilkgül O, Yetgin S, Tunçyürek P, Korkut MA. Perforation of the stomach due to chest tube complication in a patient with latrogenic diaphragmatic rupture. Acta Chir Belg. 2003;103:423–4. doi: 10.1080/00015458.2003.11679459. [DOI] [PubMed] [Google Scholar]
- 16.Johnson JF, Wright DR. Chest tube perforation of esophagus following repair of esophageal atresia. J Pediatr Surg. 1990;25:1227–30. doi: 10.1016/0022-3468(90)90511-7. [DOI] [PubMed] [Google Scholar]
- 17.Laub M, Milman N, Müller D, Struve-Christensen E. Role of small calibre chest tube drainage for iatrogenic pneumothorax. Thorax. 1990;45:748–9. doi: 10.1136/thx.45.10.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altman E, Ben-Nun A, Curtis W, Jr, Best LA. Modified Seldinger technique for the insertion of standard chest tubes. Am J Surg. 2001;181:354–5. doi: 10.1016/s0002-9610(01)00579-7. [DOI] [PubMed] [Google Scholar]
- 19.Aziz F, Penupolu S, Flores D. Efficacy of percutaneous pigtail catheters for thoracostomy at bedside. J Thorac Dis. 2012;4:292–5. doi: 10.3978/j.issn.2072-1439.2011.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seiden SC, Barach P. Wrong-side/wrong-site, wrong-procedure, and wrong-patient adverse events: Are they preventable? Arch Surg. 2006;141:931–9. doi: 10.1001/archsurg.141.9.931. [DOI] [PubMed] [Google Scholar]
- 21.Harris A, O’Driscoll BR, Turkington PM. Survey of major complications of intercostal chest drain insertion in the UK. Postgrad Med J. 2010;86:68–72. doi: 10.1136/pgmj.2009.087759. [DOI] [PubMed] [Google Scholar]
- 22.Stahel PF, Sabel AL, Victoroff MS, Varnell J, Lembitz A, Boyle DJ, et al. Wrong-site and wrong-patient procedures in the universal protocol era: Analysis of a prospective database of physician self-reported occurrences. Arch Surg. 2010;145:978–84. doi: 10.1001/archsurg.2010.185. [DOI] [PubMed] [Google Scholar]
- 23.Moffatt-Bruce SD, Ellison EC, Anderson HL, 3rd, Chan L, Balija TM, Bernescu I, et al. Intravascular retained surgical items: A multicenter study of risk factors. J Surg Res. 2012 Mar 15; doi: 10.1016/j.jss.2012.02.053. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Stahel PF, Mehler PS, Clarke TJ, Varnell J. The 5th anniversary of the «Universal Protocol»: Pitfalls and pearls revisited. Patient Saf Surg. 2009;3:14. doi: 10.1186/1754-9493-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly JJ, Farley H, O’Cain C, Broida RI, Klauer K, Fuller DC, et al. A survey of the use of time-out protocols in emergency medicine. Jt Comm J Qual Patient Saf. 2011;37:285–8. doi: 10.1016/s1553-7250(11)37036-5. [DOI] [PubMed] [Google Scholar]
- 26.Shapira OM, Aldea GS, Kupferschmid J, Shemin RJ. Delayed perforation of the esophagus by a closed thoracostomy tube. Chest. 1993;104:1897–8. doi: 10.1378/chest.104.6.1897. [DOI] [PubMed] [Google Scholar]
- 27.Kesieme EB, et al. Tubethoracostomy: Complications and its management. Pulm Med 2012. 2012 doi: 10.1155/2012/256878. 256878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zieren J, Enzweiler C, Muller JM. Tube thoracostomy complicates unrecognized diaphragmatic rupture. Thorac Cardiovasc Surg. 1999;47:199–202. doi: 10.1055/s-2007-1013144. [DOI] [PubMed] [Google Scholar]
- 29.de Jager CP, Trof RJ. Images in clinical medicine. Gastrothorax simulating acute tension pneumothorax. N Engl J Med. 2004;351:e5. doi: 10.1056/NEJMicm030515. [DOI] [PubMed] [Google Scholar]
- 30.Groskin SA. Selected topics in chest trauma. Radiology. 1992;183:605–17. doi: 10.1148/radiology.183.3.1584904. [DOI] [PubMed] [Google Scholar]
- 31.Dev SP, Nascimiento B, Jr, Simone C, Chien V. Videos in clinical medicine. Chest-tube insertion. N Engl J Med. 2007;357:e15. doi: 10.1056/NEJMvcm071974. [DOI] [PubMed] [Google Scholar]
- 32.Ellis H. The applied anatomy of chest drain insertion. Br J Hosp Med (Lond) 2010;71:M52–3. doi: 10.12968/hmed.2010.71.Sup4.47528. [DOI] [PubMed] [Google Scholar]
- 33.Foresti V, Villa A, Casati O, Parisio E, De Filippi G. Abdominal placement of tube thoracostomy due to lack of recognition of paralysis of hemidiaphragm. Chest. 1992;102:292–3. doi: 10.1378/chest.102.1.292. [DOI] [PubMed] [Google Scholar]
- 34.Laws D, Neville E, Duffy J. BTS guidelines for the insertion of a chest drain. Thora×. 2003;58:ii53–9. doi: 10.1136/thorax.58.suppl_2.ii53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salon JE. Reversible diaphragmatic eventration following chest tube thoracostomy. Ann Emerg Med. 1995;25:556–8. doi: 10.1016/s0196-0644(95)70275-x. [DOI] [PubMed] [Google Scholar]
- 36.Marinelli PV, Ortiz A, Alden ER. Acquired eventration of the diaphragm: A complication of chest tube placement in neonatal pneumothorax. Pediatrics. 1981;67:552–4. [PubMed] [Google Scholar]
- 37.Fraser RS. Lung perforation complicating tube thoracostomy: Pathologic description of three cases. Hum Pathol. 1988;19:518–23. doi: 10.1016/s0046-8177(88)80197-7. [DOI] [PubMed] [Google Scholar]
- 38.Bailey RC. Complications of tube thoracostomy in trauma. J Accid Emerg Med. 2000;17:111–4. doi: 10.1136/emj.17.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kao CL, Lu MS, Chang JP. Successful management of pulmonary artery perforation after chest tube insertion. J Trauma. 2007;62:1533. doi: 10.1097/01.ta.0000197918.19306.1c. [DOI] [PubMed] [Google Scholar]
- 40.Resnick DK. Delayed pulmonary perforation. A rare complication of tube thoracostomy. Chest. 1993;103:311–3. doi: 10.1378/chest.103.1.311. [DOI] [PubMed] [Google Scholar]
- 41.Gooding CA, Kerlan RK, Jr, Brasch RC. Partial aortic obstruction produced by a thoracostomy tube. J Pediatr. 1981;98:471–3. doi: 10.1016/s0022-3476(81)80726-3. [DOI] [PubMed] [Google Scholar]
- 42.Kollef MH, Dothager DW. Reversible cardiogenic shock due to chest tube compression of the right ventricle. Chest. 1991;99:976–80. doi: 10.1378/chest.99.4.976. [DOI] [PubMed] [Google Scholar]
- 43.Meisel S, Ram Z, Priel I, Nass D, Lieberman P. Another complication of thoracostomy--perforation of the right atrium. Chest. 1990;98:772–3. doi: 10.1378/chest.98.3.772. [DOI] [PubMed] [Google Scholar]
- 44.Haron H, Rashid NA, Dimon MZ, Azmi MH, Sumin JO, Zabir AF, et al. Chest tube injury to left ventricle: Complication or negligence? Ann Thorac Surg. 2010;90:308–9. doi: 10.1016/j.athoracsur.2010.01.075. [DOI] [PubMed] [Google Scholar]
- 45.Berkow AE, Salo BC. The kinky chest tube: A sign of entrapment following median sternotomy. AJR Am J Roentgenol. 1977;129:883–4. doi: 10.2214/ajr.129.5.883. [DOI] [PubMed] [Google Scholar]
- 46.Baskett RJ, MacDougall CE, Ross DB. Is mediastinitis a preventable complication? A 10-year review. Ann Thorac Surg. 1999;67:462–5. doi: 10.1016/s0003-4975(98)01195-3. [DOI] [PubMed] [Google Scholar]
- 47.Svedjeholm R, Hakanson E. Postoperative myocardial ischemia caused by chest tube compression of vein graft. Ann Thorac Surg. 1997;64:1806–8. doi: 10.1016/s0003-4975(97)01005-9. [DOI] [PubMed] [Google Scholar]
- 48.Yen CC, Yang YS, Liu KY. Aortic perforation caused by friction of a chest tube after coronary artery bypass surgery. Heart Surg Forum. 2010;13:E159–60. doi: 10.1532/HSF98.20091165. [DOI] [PubMed] [Google Scholar]
- 49.Sulimovic S, Noyez L. Postoperative myocardial ischemia caused by compression of a coronary artery by chest tube. J Cardiovasc Surg (Torino) 2006;47:371–2. [PubMed] [Google Scholar]
- 50.Lois M, Noppen M. Bronchopleural fistulas: An overview of the problem with special focus on endoscopic management. Chest. 2005;128:3955–65. doi: 10.1378/chest.128.6.3955. [DOI] [PubMed] [Google Scholar]
- 51.Jain R, Baijal SS, Phadke RV, Pandey CK, Saraswat VA. Endobronchial closure of a bronchopleural cutaneous fistula using angiography catheters. AJR Am J Roentgenol. 2000;175:1646–8. doi: 10.2214/ajr.175.6.1751646. [DOI] [PubMed] [Google Scholar]
- 52.Stawicki SP. Bronchopleural fistula. [Last accessed 2014 Feb 7]. Available online at http://www.surgiwiki.com/surgiwiki/mediawiki-1.11.1/index.php/Bronchopleural_fistula .
- 53.Deschamps C, Bernard A, Nichols FC, 3rd, Allen MS, Miller DL, Trastek VF, et al. Empyema and bronchopleural fistula after pneumonectomy: Factors affecting incidence. Ann Thorac Surg. 2001;72:243–7. doi: 10.1016/s0003-4975(01)02681-9. [DOI] [PubMed] [Google Scholar]
- 54.Martino K, Merrit S, Boyakye K, Sernas T, Koller C, Hauser CJ, et al. Prospective randomized trial of thoracostomy removal algorithms. J Trauma. 1999;46:369–71. doi: 10.1097/00005373-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Heifetz SA, Zeichner MB, Minkowitz S. Sudden death from ruptured intercostal artery aneurysm. A late complication of thoracotomy. Arch Surg. 1975;110:1253–4. doi: 10.1001/archsurg.1975.01360160091019. [DOI] [PubMed] [Google Scholar]
- 56.Chemelli AP, Thauerer M, Wiedermann F, Strasak A, Klocker J, Chemelli-Steingruber IE. Transcatheter arterial embolization for the management of iatrogenic and blunt traumatic intercostal artery injuries. J Vasc Surg. 2009;49:1505–13. doi: 10.1016/j.jvs.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Coulter TD, Maurer JR, Miller MT, Mehta AC. Chest wall arteriovenous fistula: An unusual complication after chest tube placement. Ann Thorac Surg. 1999;67:849–50. doi: 10.1016/s0003-4975(99)00088-0. [DOI] [PubMed] [Google Scholar]
- 58.Limsukon A, Yick D, Kamangar N. Chylothorax: A rare complication of tube thoracostomy. J Emerg Med. 2011;40:280–2. doi: 10.1016/j.jemermed.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 59.Cope C, Kaiser LR. Management of unremitting chylothorax by percutaneous embolization and blockage of retroperitoneal lymphatic vessels in 42 patients. J Vasc Interv Radiol. 2002;13:1139–48. doi: 10.1016/s1051-0443(07)61956-3. [DOI] [PubMed] [Google Scholar]
- 60.Eddy AC, Luna GK, Copass M. Empyema thoracis in patients undergoing emergent closed tube thoracostomy for thoracic trauma. Am J Surg. 1989;157:494–7. doi: 10.1016/0002-9610(89)90643-0. [DOI] [PubMed] [Google Scholar]
- 61.Inci I, Ozçelik C, Ulkü R, Tuna A, Eren N. Intrapleural fibrinolytic treatment of traumatic clotted hemothorax. Chest. 1998;114:160–5. doi: 10.1378/chest.114.1.160. [DOI] [PubMed] [Google Scholar]
- 62.Meyer DM, Jessen ME, Wait MA, Estrera AS. Early evacuation of traumatic retained hemothoraces using thoracoscopy: A prospective, randomized trial. Ann Thorac Surg. 1997;64:1396–400. doi: 10.1016/S0003-4975(97)00899-0. [DOI] [PubMed] [Google Scholar]
- 63.Pierce JD, Piazza D, Naftel DC. Effects of two chest tube clearance protocols on drainage in patients after myocardial revascularization surgery. Heart Lung. 1991;20:125–30. [PubMed] [Google Scholar]
- 64.Dango S, Sienel W, Passlick B, Stremmel C. Impact of chest tube clearance on postoperative morbidity after thoracotomy: Results of a prospective, randomised trial. Eur J Cardiothorac Surg. 2010;37:51–5. doi: 10.1016/j.ejcts.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 65.Lim-Levy F, Babler SA, De Groot-Kosolcharoen J, Kosolcharoen P, Kroncke GM. Is milking and stripping chest tubes really necessary? Ann Thorac Surg. 1986;42:77–80. doi: 10.1016/s0003-4975(10)61841-3. [DOI] [PubMed] [Google Scholar]
- 66.Magder SA, Lichtenstein S, Adelman AG. Effect of negative pleural pressure on left ventricular hemodynamics. Am J Cardiol. 1983;52:588–93. doi: 10.1016/0002-9149(83)90032-2. [DOI] [PubMed] [Google Scholar]
- 67.Duncan C, Erickson R. Pressures associated with chest tube stripping. Heart Lung. 1982;11:166–71. [PubMed] [Google Scholar]
- 68.Gerard PS, Kaldawi E, Litani V, Lenora RA, Tessler S. Right-sided pneumothorax as a result of a left-sided chest tube. Chest. 1993;103:1602–3. doi: 10.1378/chest.103.5.1602. [DOI] [PubMed] [Google Scholar]
- 69.Chen YF, Chen CY, Hsu CL, Yu CJ. Malpositioning of the chest tube across the anterior mediastinum is risky in chronic obstructive pulmonary disease patients with pneumothorax. Interact Cardiovasc Thorac Surg. 2011;13:109–11. doi: 10.1510/icvts.2010.264689. [DOI] [PubMed] [Google Scholar]
- 70.Wechsler RJ, Steiner RM, Kinori I. Monitoring the monitors: The radiology of thoracic catheters, wires, and tubes. Semin Roentgenol. 1988;23:61–84. doi: 10.1016/s0037-198x(88)80018-x. [DOI] [PubMed] [Google Scholar]
- 71.Collop NA, Kim S, Sahn SA. Analysis of tube thoracostomy performed by pulmonologists at a teaching hospital. Chest. 1997;112:709–13. doi: 10.1378/chest.112.3.709. [DOI] [PubMed] [Google Scholar]
- 72.Quigley RL. Thoracentesis and chest tube drainage. Crit Care Clin. 1995;11:111–26. [PubMed] [Google Scholar]
- 73.Bell RL, Ovadia P, Abdullah F, Spector S, Rabinovici R. Chest tube removal: End-inspiration or end-expiration? J Trauma. 2001;50:674–7. doi: 10.1097/00005373-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 74.Davis JW, Mackersie RC, Hoyt DB, Garcia J. Randomized study of algorithms for discontinuing tube thoracostomy drainage. J Am Coll Surg. 1994;179:553–7. [PubMed] [Google Scholar]
- 75.Carlson RI, Classen KL, Gollan F, Gobbel WG, Jr, Sherman DE, Christensen RO. Pulmonary edema following the rapid reexpansion of a totally collapsed lung due to a pneumothorax: A clinical and experimental study. Surg Forum. 1958;9:367–71. [PubMed] [Google Scholar]
- 76.Matsuura Y, Nomimura T, Murakami H, Matsushima T, Kakehashi M, Kajihara H. Clinical analysis of reexpansion pulmonary edema. Chest. 1991;100:1562–6. doi: 10.1378/chest.100.6.1562. [DOI] [PubMed] [Google Scholar]
- 77.Mahfood S, Hix WR, Aaron BL, Blaes P, Watson DC. Reexpansion pulmonary edema. Ann Thorac Surg. 1988;45:340–5. doi: 10.1016/s0003-4975(10)62480-0. [DOI] [PubMed] [Google Scholar]
- 78.Stawicki SP, Sarani B, Braslow BM. Reexpansionpulmonary edema. OPUS 12 Scientist. 2008;2:29–31. [Google Scholar]
- 79.Cho SR, Lee JS, Kim MS. New treatment method for reexpansion pulmonary edema: Differential lung ventilation. Ann Thorac Surg. 2005;80:1933–4. doi: 10.1016/j.athoracsur.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 80.Ward EW, Hughes TE. Sudden death following chest tube insertion: An unusual case of vagus nerve irritation. J Trauma. 1994;36:258–9. [PubMed] [Google Scholar]
- 81.Pingleton SK, Jeter J. Necrotizing fasciitis as a complication of tube thoracostomy. Chest. 1983;83:925–6. doi: 10.1378/chest.83.6.925. [DOI] [PubMed] [Google Scholar]
- 82.Urschel JD, Takita H, Antkowiak JG. Necrotizing soft tissue infections of the chest wall. Ann Thorac Surg. 1997;64:276–9. doi: 10.1016/s0003-4975(97)00514-6. [DOI] [PubMed] [Google Scholar]
- 83.Osinowo O, Adebo OA, Okubanjo AO. Osteomyelitis of the ribs in Ibadan. Thora×. 1986;41:58–60. doi: 10.1136/thx.41.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mayberry JC, Kroeker AD, Ham LB, Mullins RJ, Trunkey DD. Long-term morbidity, pain, and disability after repair of severe chest wall injuries. Am Surg. 2009;75:389–94. [PubMed] [Google Scholar]
- 85.Eren S, Esme H, Sehitogullari A, Durkan A. The risk factors and management of posttraumatic empyema in trauma patients. Injury. 2008;39:44–9. doi: 10.1016/j.injury.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 86.Chan L, Reilly KM, Henderson C, Kahn F, Salluzzo RF. Complication rates of tube thoracostomy. Am J Emerg Med. 1997;15:368–70. doi: 10.1016/s0735-6757(97)90127-3. [DOI] [PubMed] [Google Scholar]
- 87.Johnson AO, Grillo IA, Adebonojo SA. Esophagopleural fistula: A complication of chest intubation for empyema. J Natl Med Assoc. 1980;72:315–8. [PMC free article] [PubMed] [Google Scholar]
- 88.Lakshmanadoss U, Mogili S, Kothari T, Das V. Migration of the chest tube into the esophagus in a case of Boerhaave›s syndrome. Heart Lung. 2011;40:576–9. doi: 10.1016/j.hrtlng.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 89.Symbas PN, Hunter RM, Vlasis SE, Ansley JD. Infected descending aortic fistula. Ann Thorac Surg. 1986;41:647–51. doi: 10.1016/s0003-4975(10)63080-9. [DOI] [PubMed] [Google Scholar]
- 90.Walker FW, Salley AJ. A drainage tube for mediastinal abscesses. Surg Gynecol Obstet. 1981;152:831–2. [PubMed] [Google Scholar]
- 91.Bertino RE, Wesbey GE, Johnson RJ. Horner syndrome occurring as a complication of chest tube placement. Radiology. 1987;164:745. doi: 10.1148/radiology.164.3.3615873. [DOI] [PubMed] [Google Scholar]
- 92.Shen SY, Liang BC. Horner›s syndrome following chest drain migration in the treatment of pneumothorax. Eye (Lond) 2003;17:785–8. doi: 10.1038/sj.eye.6700446. [DOI] [PubMed] [Google Scholar]
- 93.Kaya SO, Liman ST, Bir LS, Yuncu G, Erbay HR, Unsal S. Horner's syndrome as a complication in thoracic surgical practice. Eur J Cardiothorac Surg. 2003;24:1025–8. doi: 10.1016/j.ejcts.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 94.Gabor S, Renner H, Pinter H, Sankin O, Maier A, Tomaselli F, et al. Indications for surgery in tracheobronchial ruptures. Eu J Cardiothorac Surg. 2001;20:399–404. doi: 10.1016/s1010-7940(01)00798-9. [DOI] [PubMed] [Google Scholar]
- 95.Taylor RF, Rubens MB, Pearson MC, Barnes NC. Intrapleural streptokinase in the management of empyema. Thora×. 1994;49:856–9. doi: 10.1136/thx.49.9.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bouros D, Antoniou KM, Chalkiadakis G, Drositis J, Petrakis I, Siafakas N. The role of video-assisted thoracoscopic surgery in the treatment of parapneumonic empyema after the failure of fibrinolytics. Surg Endosc. 2002;16:151–4. doi: 10.1007/s00464-001-9028-3. [DOI] [PubMed] [Google Scholar]
- 97.Jagasia MH, Cole FH, Stegman MH, Deaton P, Kennedy L. Video-assisted talc pleurodesis in the management of pleural effusion secondary to continuous ambulatory peritoneal dialysis: A report of three cases. Am J Kidney Dis. 1996;28:772–4. doi: 10.1016/s0272-6386(96)90264-4. [DOI] [PubMed] [Google Scholar]
- 98.Kalfa N, Allal H, Montes-Tapia F, Lopez M, Forgues D, Guibal MP, et al. Ideal timing of thoracoscopic decortication and drainage for empyema in children. Surg Endosc. 2004;18:472–7. doi: 10.1007/s00464-002-9206-6. [DOI] [PubMed] [Google Scholar]
- 99.Landreneau RJ, Keenan RJ, Hazelrigg SR, Mack MJ, Naunheim KS, et al. Thoracoscopy for empyema and hemothorax. Chest. 1996;109:18–24. doi: 10.1378/chest.109.1.18. [DOI] [PubMed] [Google Scholar]
- 100.Smith JW, Franklin GA, Harbrecht BG, Richardson JD. Early VATS for blunt chest trauma: A management technique underutilized by acute care surgeons. J Trauma. 2011;71:102–5. doi: 10.1097/TA.0b013e3182223080. discussion 105-7. [DOI] [PubMed] [Google Scholar]
- 101.Agarwal PP, Seely JM, Matzinger FR, MacRae RM, Peterson RA, Maziak DE, et al. Pleural mesothelioma: Sensitivity and incidence of needle track seeding after image-guided biopsy versus surgical biopsy. Radiology. 2006;241:589–94. doi: 10.1148/radiol.2412051020. [DOI] [PubMed] [Google Scholar]
- 102.Boutin C, Rey F, Viallat JR. Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. A randomized trial of local radiotherapy. Chest. 1995;108:754–8. doi: 10.1378/chest.108.3.754. [DOI] [PubMed] [Google Scholar]
- 103.Downey RJ, McCormack P, LoCicero J., 3rd Dissemination of malignant tumors after video-assisted thoracic surgery: A report of twenty-one cases. The Video-Assisted Thoracic Surgery Study Group. J Thorac Cardiovasc Surg. 1996;111:954–60. doi: 10.1016/s0022-5223(96)70370-7. [DOI] [PubMed] [Google Scholar]
- 104.Nankhonya JM, Zakhour JD. Malignant seeding of needle aspiration tract: A rare complication. Br J Dermatol. 1991;124:285–6. doi: 10.1111/j.1365-2133.1991.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 105.Lee YCG, De Klerk NH, Henderson D, Musk AW. Malignantmesothelioma. In: Hendrick DJ, Burge PS, Beckett WS, Churg A, editors. Occupational Disorders of the Lung. Recognition, Management and Prevention. London: W B Saunders and Co; 2002. pp. 359–79. [Google Scholar]
- 106.Stahly TL, Tench WD. Lung entrapment and infarction by chest tube suction. Radiology. 1977;122:307–9. doi: 10.1148/122.2.307. [DOI] [PubMed] [Google Scholar]
- 107.Schley M, Rössler M, Konrad CJ, Schüpfer G. [Damage of the subclavian vein with a thorax drainage] Anaesthesist. 2009;58:387–90. doi: 10.1007/s00101-009-1507-2. [DOI] [PubMed] [Google Scholar]
- 108.Tecchio T, Salcuni P, Azzarone M, Soliani P. [A subclavian vein lesion due to the positioning of a chest tube via thoracostomy] G Chir. 1991;12:435–7. [PubMed] [Google Scholar]
- 109.Olgac G, Aydogmus U, Mulazimoglu L, Kutlu CA. Antibiotics are not needed during tube thoracostomy for spontaneous pneumothorax: An observational case study. J Cardiothorac Surg. 2006;13:43. doi: 10.1186/1749-8090-1-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nichols RL, Smith JW, Muzik AC, Love EJ, McSwain NE, Timberlake G, et al. Preventive antibiotic usage in traumatic thoracic injuries requiring closed tube thoracostomy. Chest. 1994;106:1493–8. doi: 10.1378/chest.106.5.1493. [DOI] [PubMed] [Google Scholar]
- 111.Fallon WF, Jr, Wears RL. Prophylactic antibiotics for the prevention of infectious complications including empyema following tube thoracostomy for trauma: Results of meta-analysis. J Trauma. 1992;33:110–6. discussion 116-7. [PubMed] [Google Scholar]
- 112.Jones PM, Hewer RD, Wolfenden HD, Thomas PS. Subcutaneous emphysema associated with chest tube drainage. Respirology. 2001;6:87–9. doi: 10.1046/j.1440-1843.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 113.Gilroy RJ, Mangura BT, Lavietes MH. Rib cage and abdominal volume displacements during breathing in pregnancy. Am Rev Respir Dis. 1988;137:668–72. doi: 10.1164/ajrccm/137.3.668. [DOI] [PubMed] [Google Scholar]
- 114.Crapo RO. Normal cardiopulmonary physiology during pregnancy. Clin Obstet Gynecol. 1996;39:3–16. doi: 10.1097/00003081-199603000-00004. [DOI] [PubMed] [Google Scholar]


