Abstract
Hemodynamic monitoring in the form of invasive arterial, central venous pressure and pulmonary capillary wedge pressure monitoring may be required in seriously ill Intensive care unit patients, in patients undergoing surgeries involving gross hemodynamic changes and in patients undergoing cardiac surgeries. These techniques are considered the gold standards of hemodynamic monitoring but are associated with their inherent risks. A number of non-invasive techniques based on various physical principles are under investigation at present. The goal is to not only avoid the risk of invasive intervention, but also to match the gold standard set by them as far as possible. Techniques based on photoplethysmography, arterial tonometry and pulse transit time analysis have come up for continuous arterial pressure monitoring. Of these the first has been studied most extensively and validated, however it has been shown to be substandard in patients with gross hemodynamic instability. The other two still need further evaluation. While the non-invasive methods for arterial blood pressure monitoring are based on diverse technologies, those for measurement of central venous and pulmonary pressures are mostly based on imaging techniques such as echocardiography, Doppler ultrasound, computed tomography scan and chest X ray. Most of these techniques are based on measurement of the dimensions of the great veins. This makes them operator and observer dependent. However, studies done till now have revealed adequate inter-observer agreement. These techniques are still in their incipience and although initial studies are encouraging, further research is needed on this front.
Keywords: Non-invasive central venous pressure measurement, Non-invasive monitoring in intensive care unit, Non-invasive pulmonary capillary wedge pressure
INTRODUCTION
Hemodynamic monitoring in the form of invasive arterial, central venous pressure and pulmonary capillary wedge pressure monitoring may be required in seriously ill Intensive care unit (ICU) patients, in patients undergoing surgeries involving gross hemodynamic changes and in patients undergoing cardiac surgeries. These techniques are considered the gold standards of hemodynamic monitoring but are associated with their inherent risks. A number of non-invasive techniques based on various physical principles are under investigation at present. We present the view point along with available evidence for these non-invasive monitoring techniques.
ARTERIAL BLOOD PRESSURE
Blood pressure is probably one of the commonest monitored hemodynamic parameters. The conventional measurement using a sphygmomanometer with cuff/Doppler method although convenient and reliable fails to show continuous changes and is fraught with inaccuracies in patients with alterations in vascular compliance (atherosclerosis/arteriosclerosis) or systemic vascular resistance.[1] All these limitations are often seen in intensive care units with patients on inotropes and invasive blood pressure monitoring replaces the non-invasive methods. The invasive blood pressure monitoring does not come without its own complications. Distal ischemia and limb gangrene is one dreaded complication. Although the reported incidence of ischemia post arterial cannulation is very low (up to 0.1%), it is a disastrous complication. Interestingly the size of cannula seems to be unrelated to incidence of ischemia. Allen's test, which was traditionally used to test the patency of collaterals, has been proven to not be reliable.[2,3] Infection, thrombosis, embolism and nerve injury are other rare complications reported with invasive arterial cannula.[4] Ringing and damping may lead to erroneous results. The main advantage of invasive arterial monitoring is that a continuous beat-to-beat idea of blood pressure can be got.
There has been a surge of interest in inventing methods of non-invasive continuous blood pressure monitoring over the last decade so as to avoid these complications while retaining the benefits of continuous blood pressure measurements.
Non-invasive Continuous measuring blood pressure
Penaz method
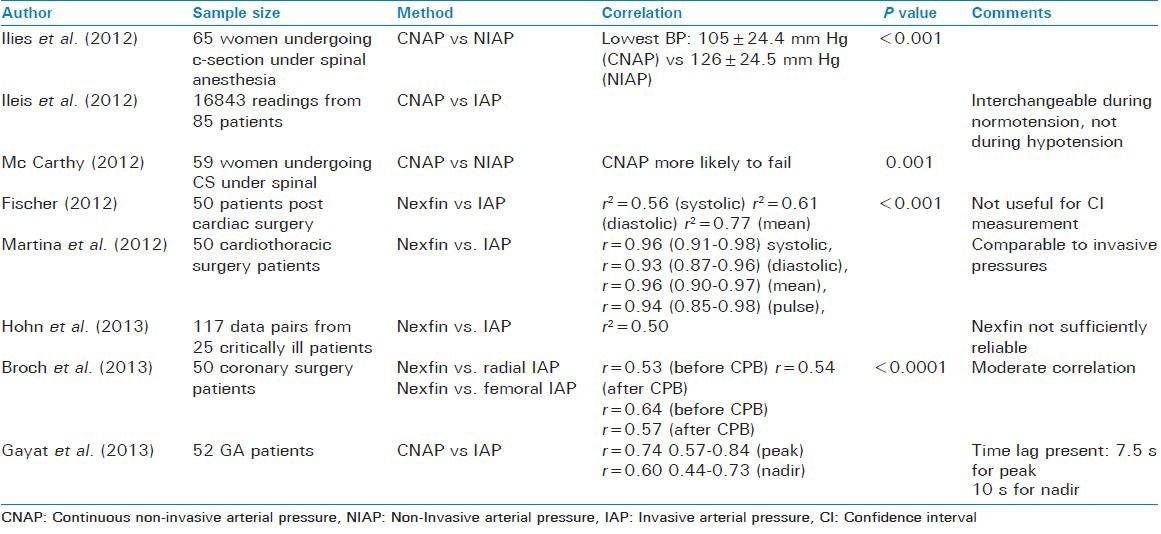
Described first by Penaz in 1973 this method uses the principle of vascular unloading. The cuff with a light source on one side and a receiver on the other side measures the absorption of light as it passes across the finger and thus estimates the volume of blood flowing in the finger. The cuff then adjusts in order to keep the volume of the blood in the finger constant. The cuff pressure, which is required to do so in the finger, is extrapolated to brachial pressure in the Finapres system (Nexfin™). This pressure is correlated to the non-invasive pressure obtained by using an upper arm cuff in Continuous non-invasive arterial pressure (CNAP) system. Both these techniques have been validated.[5] The accuracy and stability of blood pressure recording is maintained by using several concentrically interlocking control loops.[6] Although CNAP has been found to be reliable in patients at risk of hypotensive episodes such as those undergoing caesarean sections under subarachnoid blocks,[7] it is not without its disadvantages which include a lag time of 7.5-10 s,[8] decreased accuracy as compared to invasive techniques[9] and missed blood pressure measurements.[10] Although there are studies supporting the reliability of Nexfin™[11,12,13] some other studies indicate that it is not a reliable replacement for invasive blood pressure where the latter is indicated.[14,15] This method is vulnerable to inaccuracies resulting from peripheral vasoconstriction due to vasopressors, cold or systemic and collagen vascular disorders [Table 1].
Table 1.
Techniques based on Penaz method
Tonometry
The principle of applanation tonometry has also been explored for non-invasive measurement of blood pressure. The vessel is compressed enough to flatten it but not occlude it. The pressure is then measured against a bony surface. The pressure required to flatten the artery is then used to derive the systolic, diastolic and mean pressures using an algorithm.[5,16] T-line TL-200 and TL-200pro are the devices currently available that work on this principle. The studies on tonometry have produced controversial results. Positive results have been found for its use both in ICU settings and during anesthesia.[17,18] However, other studies have precluded its use in hemodynamically unstable patients and spine surgery patients due to wide agreement limits and lack of reliability.[19,20] Since this method uses a larger artery such as the radial, it is devoid of the limitations related to peripheral vasoconstriction [Table 2].
Table 2.
Techniques based on applanation tonometry
Pulse transit time
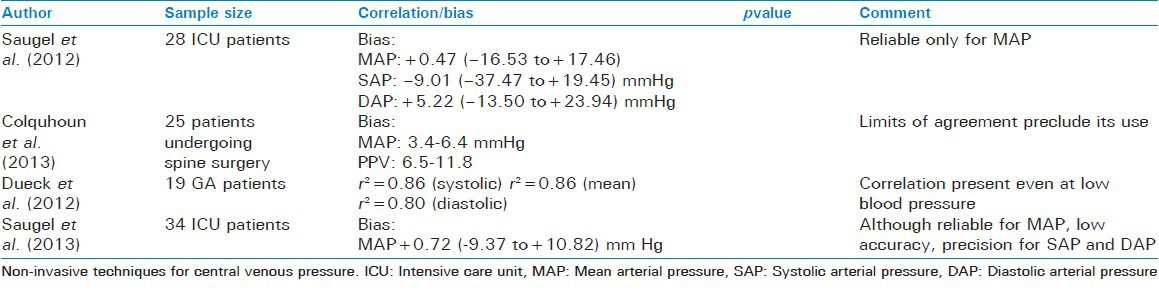
This method is based on the concept of pulse wave velocity first described by Bramwell and Hill. This method measures the time taken by systolic pressure wave to travel between two points to measure the blood pressure. The systolic blood pressure is inversely proportional to this time.[5] Alternatively, time from the R-wave on the ECG to the time arterial pulse reaches a peripheral site may be measured. Photometry may be used for this purpose. A recent study used electrical impedance tomography to measure the time of arrival of pulse at the descending aorta from the time of closure of aortic valve (detected from arterial line) in mechanically ventilated anesthetized pigs.[21] A strong correlation between the pulse transit time and invasively measured central blood pressure was found (r = -0.97, P < 0.00001). MRI has also been used and validated for the measurement of pulse wave velocity.[22,23] More recently, radio frequency transmission has been used in the form of nanosecond pulse near field sensing to measure the pulse wave velocity.[24]
Pulse wave analysis
Pulse wave obtained from pulse oximeter was subjected to complex analysis to obtain arterial blood pressure. The blood pressure thus obtained was compared to that measured invasively. Although a correlation was found between the two, due to its intrinsic variability and wide limits of agreement, the technology was considered not be ready yet for clinical implementation.[25]
CENTRAL VENOUS PRESSURE
Central venous pressure (CVP) has been invasively measured to get an idea of the volume status of the body. However a word of caution is needed here. The absolute values of the central venous pressure carry little significance. It's the trend of central venous pressure that is of more importance. The absolute values of CVP measured in reference to the atmosphere are affected by the intrathoracic pressure.[26] Since this pressure varies with respiration and thus affects the CVP, a time point where this pressure is zero relative to the atmosphere should be chosen to take the reading. Such a point is end-expiration. Hence the CVP should always be taken at the end-expiration.
Estimation of central venous pressure by non-invasive method
The inferior vena cava (IVC) and deep veins of the neck [internal jugular (IJV) and subclavian] form a direct valveless route to the superior vena cava and hence they were the first ones to be explored for this purpose.
Inferior vena cava-based techniques
Inferior vena cava diameter
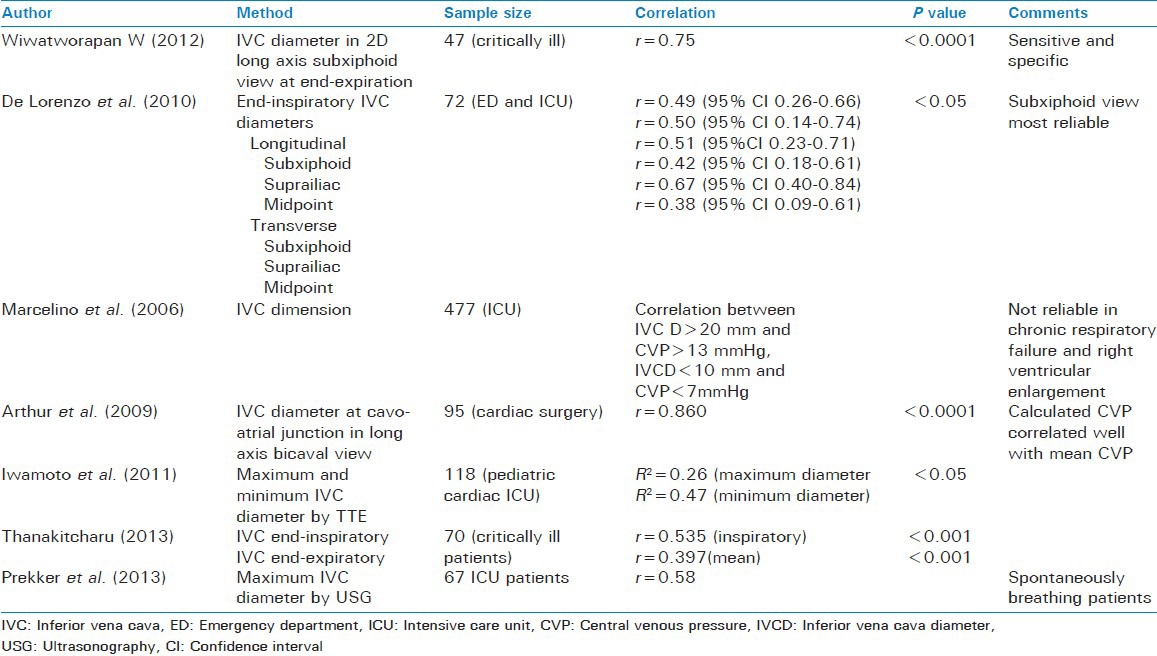
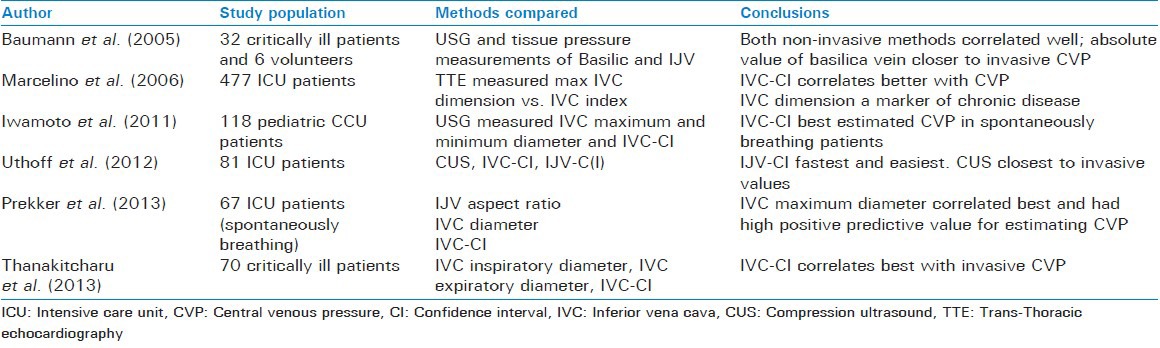
Marcelino et al. studied the relationship of IVC diameter to CVP in 477 ICU patients. They found that a maximum IVC diameter of >20 mm correlated with a CVP of >13 mmHg, while a maximum IVC diameter of <10 mm correlated with a CVP <7 mmHg in patients with normal right ventricular and pulmonary function.[27]
De Lorenzo et al. correlated the maximum transverse and longitudinal diameter of the IVC at the subxiphoid, suprailiac and mid abdomen levels with1 the invasively measured CVP in ICU and ED patients. The subxiphoid view was the most reliable, since the adequate measurement was possible in 89% of the cases. Weak correlation was found between the IVC diameter and CVP in all the six views. The correlation was found to be highest for the suprailiac view. The measurements correlated best in the end-inspiratory state.[28]
Thanakitcharu et al. also found a weak, though significant, correlation of CVP with end-inspiratory and mean IVC diameters.[29]
Wiwatworapan W, however found a strong correlation between subxiphoid end-expiratory IVC diameters and the CVP. It was found that an IVC diameter of 10 mm or less predicted a CVP of ≤10 cm H2O with a sensitivity and specificity of 70% and 90%, respectively, while an IVC diameter of 15 mm or more predicted a CVP of ≥15 cmH2O.[30]
Arthur et al. used TEE in 95 patients undergoing cardiac surgery to measure the IVC diameter and predict the central venous pressure. They found a good correlation (r = 0.860) between the two. They even formulated an equation to predict the CVP from IVC diameter at the cavo-atrial junction in long axis bicaval view[31] i.e., CVP = (IVCD – 4.004)/0.751.
Iwamoto et al. studied the relation of IVC diameter to CVP in children in CCU and found a weak but statistically significant correlation between the two[32] [Table 3].
Table 3.
Techniques based on IVC diameter
Variation in inferior vena cava diameter with respiration
The IVC diameter changes depending on the phase of respiration. In spontaneously breathing patients, during inspiration the negative pressure develops in the thorax, causing the IVC to drain into the right atrium and decreasing its diameter. The measure of this is called the IVC collapsibility index (IVC-CI) given by (IVC diameter in expiration-IVC diameter in inspiration)/IVC diameter in expiration. For patients who are being mechanically ventilated, the dynamics of respiration are different and this parameter is given by (IVCDmax – IVCDmin)/IVCDmax. The IVC diameters are measured regardless of the phases of the respiratory cycle.[33]
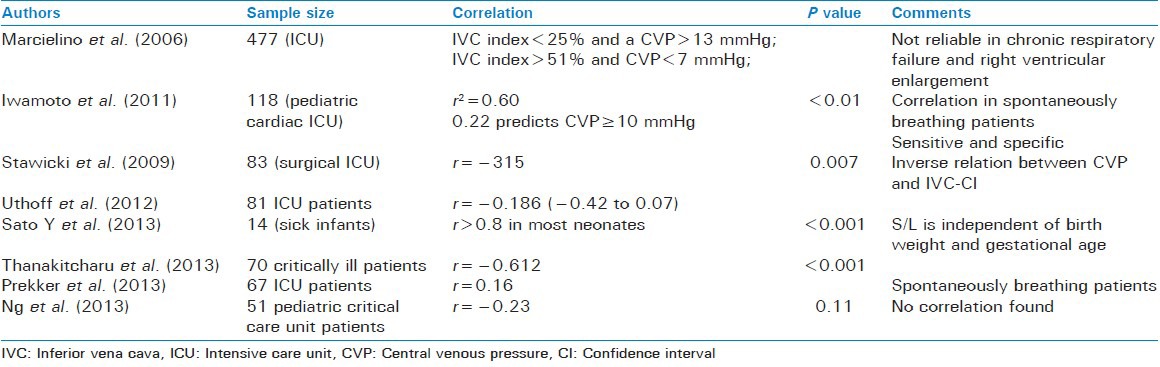
In 1993, Minutiello suggested that this parameter could be used to estimate the CVP. After studying the IVC-CI in 65 patients, he concluded that it varied inversely with the CVP. An IVC index of <20% predicted an elevated level of CVP.[34]
Marcelino et al. found that in critically ill patients an IVC-CI of <25% correlated with a CVP of >13 mmHg, while that of >51% corresponded to a CVP of <7 mmHg.[27]
Iwamoto et al. used this parameter to study the CVP in pediatric CCU patients. They found that IVC-CI correlated with CVP only in spontaneously breathing patients. A high sensitivity and specificity of 1.0 and 0.98 of an ICV-CI of less than or equal to 22% was found in predicting a high CVP Of ≥10 mmHg.[32] Stawicki et al. conducted a study in 83 surgical ICU patients and found that as the IVC-CI increased from <0.2 to 0.2-0.6 to >0.6 the CVP fell (P = 0.023). The measurements were taken by intensivists 1-2 cm below the level of hepatic veins using the subxiphoid or subcostal views. They concluded that this was a quick, easy and repeatable parameter that did not require any expert skill.[33] Thankitcharu also found a strong correlation between the two.[29]
Sato et al. used a slight modification of the above parameter. They studied the ratio of minimum IVC diameter to the maximum IVC diameter (S/L) in neonates. They found that this ratio had a strong correlation (>0.8) to the CVP in most of the neonates.[35] Ng et al., however, could find no such correlation.[36]
Studies by Uthoff et al. and Prekker et al., however, found a weak correlation between the two.[37,38]
Ratio of inferior vena cava diameter to aortic diameter
Ng et al. studied this parameter and its relation to CVP in pediatric critical care patients. They could not find any significant correlation.
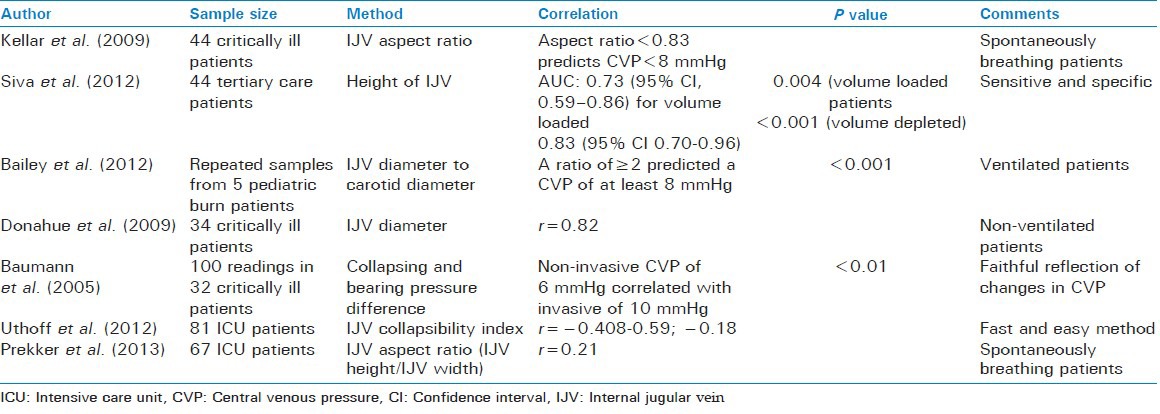
Internal jugular vein based methods
Height of internal jugular vein
Traditionally, the CVP has been estimated by measuring the distance above the strenal angle where the collapse of IJV is seen. Bloch et al. in 1991 used inductive plethysmography to detect this point and correlated it to the invasively measured CVP. They found that this method was able to predict the CVP with an error of 20% when the CVP ranged from 0 to 19 cm of H2O.[39] Lipton et al. in 2000 used ultrasound imaging in a similar manner to detect the point of collapse of IJV to predict the CVP.[40] Siva et al. in a recent study have found a good correlation between the two (“top” of IJV and CVP) and reported that this method had a positive predictive value of 85.7% and a negative predictive value of 96.4% for detecting volume overload in patients admitted to tertiary care.[41]
Internal jugular vein diameter
Donahue et al. studied the IJV diameter in 34 spontaneously breathing acutely ill patients and found that it had a significant strong correlation with CVP.[42] Bailey et al. studied the relationship of the ratio of IJV and carotid diameters to CVP in mechanically ventilated pediatric burn patients. They reported that an IJV diameter that was twice that of carotid predicted a CVP of at least 8 mmHg.[43]
Collapsing pressure of Internal jugular vein
This method was pioneered by Aggarwal et al. They attached a pressure measuring device to the ultrasound probe and measured the pressure needed to collapse the IJV. This pressure was correlated to the CVP. They opined that this was a cheap and easy method of estimating CVP, which even an untrained person could use.[44]
Internal jugular vein collapsing pressure and bearing pressure difference
Bearing pressure is defined as the pressure required to deform the IJV, while the collapsing pressure is defined as the pressure required to completely occlude it. The difference between the two has been found to reflect the CVP, although the absolute values differed significantly from the invasive CVP i.e., a non-invasive CVP of 6 mmHg corresponded to an invasive CVP of 10 mmHg.[45] This method however faithfully represents the changes in CVP [Table 4].
Table 4.
Techniques based on IVC collapsibility index
Internal jugular vein collapsibility index
Uthoff et al. found IJV collapsibility index to be a fast and easy method to measure the CVP taking a median time of 60 s (IQR 50-109). They found a significant correlation between the two and opined that this might be valuable method in the emergency department.[37]
Internal jugular vein aspect ratio
The internal jugular vein aspect ratio is defined as the ratio of the height of the IJV to its width on ultrasound. Kellar et al. studied the utility of this entity in predicting the CVP in spontaneously breathing critically ill patients. They found that an aspect ratio of <0.83 accurately estimated a CVP of less than 8 mmHg.[46] Prekker et al. however found a poor correlation between the two[38] [Table 5].
Table 5.
Techniques based on IJV measurements
Peripheral veins
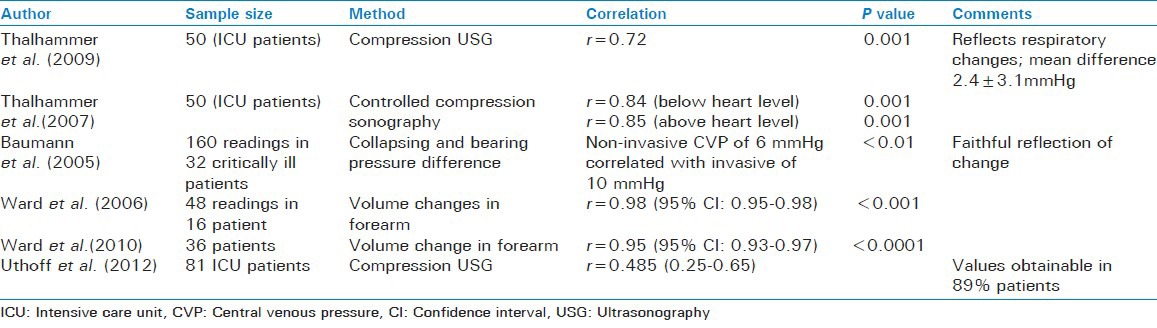
Compression ultrasound
Estimation of CVP using compression ultrasound uses the pressure required to collapse the basilica vein to estimate the CVP. Thalhammer found that noninvasive CVP measured in this way correlated very well with that measured invasively in ICU patients irrespective of the level of arm.[47] In another study Thalhammer et al. reported that it also reflected faithfully the change in CVP with respiration.[48] Uthoff et al. also found similar results and opined that this method could predict the CVP with an error of 3 mmHg.[37]
Basilic vein collapsing pressure and bearing pressure difference
As described for the IJV, the above-mentioned parameters were taken for basilica vein and it was found that again there was a difference in the absolute values although a correlation was present between the invasive and non-invasive CVP measurements. A pressure measurement of 8 mmHg corresponded to a CVP of 10 mmHg.[45]
Forearm volume changes
In this method, plethysmography was used to determine the maximum derivative of the forearm volume decrease during release of external pressure and this was correlated to the invasively measured CVP. A good correlation has been found between the two.[49,50]
Vascular pedicel width
It is a parameter measured on the chest X ray. It is defined as the horizontal distance between the point where the left subclavian artery exits the aorta to the point where the superior vena cava crosses the right main-stem bronchus. This parameter has been found to correlate with CVP with a small correlation coefficient of 0.21[51] [Table 6].
Table 6.
Techniques based on forearm measurements
Doppler echocardiography
The right atrial pressures can be estimated by Doppler echocardiography as well. The right atrial pressures measured this way have been found to correlate with the invasive pressures (r = 0.39, P < 0.001). However, the authors concluded that this method was not reliable to estimate the right atrial pressures.[52]
Marcelino et al. have come up with a formula using echocardiographic parameter for the calculation of CVP:[53]
(tricuspid E deceleration) ×0.11+ (RU/RA gradient) ×0.16 – (IVC variation)
Prekker et al. compared IVC collapsibility index, IVC aspect ratio and maximum IVC diameter in 65 spontaneously breathing patients and found that only IVC diameter correlated moderately with the CVP. Also as a diagnostic test this outperformed the other two.[38] Uthoff et al. compared compression ultrasound of forearm veins (CUS), IVC and IJV collapsibility index to see which correlated best to invasive CVP. They found that while all three correlated only moderately with CVP, CUS gave absolute values closest to the invasive CVP, IVC collapsibility index was easiest and fastest (median time of measurement 60 s).[37] Further comparison of various non-invasive techniques is summarized in Table 7. As is clear from this table superiority of any technique over the other is controversial. Although a number of studies support the IVC-CI to be the best among all IVC based techniques,[29,32,37,53] there is at least one which shows contradictory results.[38]
Table 7.
Comparison of various methods of CVP measurement
PULMONARY CAPILLARY WEDGE PRESSURE
It is the pressure obtained by inserting a catheter in the superior vena cava and then guiding it through the right ventricle into the pulmonary artery with the help of an inflated balloon and then wedging it in pulmonary capillaries.
The position in the lungs where the balloon gets wedged is of importance. It should ideally get wedged in the West zone 3 (i.e., where both pulmonary arterial and venous pressures exceed the alveolar pressure). If wedged higher up (i.e., in zones 1 or 2) where the alveolar pressures are higher than pulmonary venous or both venous and arterial pressures, the catheter will read the alveolar rather than the wedge pressure. The supine position has been found to favor wedging I zone 3.[54]
Complications of a PA catheter
Pulmonary artery rupture is one of the most devastating complications of this procedure, which frequently results in death. Interestingly, in a study by Kaczmarek et al. women were found to be at a higher risk of rupture as compared to men[54] (OR = 5.84, CI: 2.97-11.46, P < 0.001). Less severe injury may lead to the formation of pulmonary artery aneurysm. Other complications include tachyarrhythmias and heart blocks, thromboembolic complications, pulmonary infarctions and valvular and endocardial damage. In order to avoid such complications, there has been a recent interest in developing non-invasive techniques for estimation of the pulmonary capillary wedge pressure.
Non-invasive measurement of pulmonary capillary wedge pressure
ECG-gated computed tomography
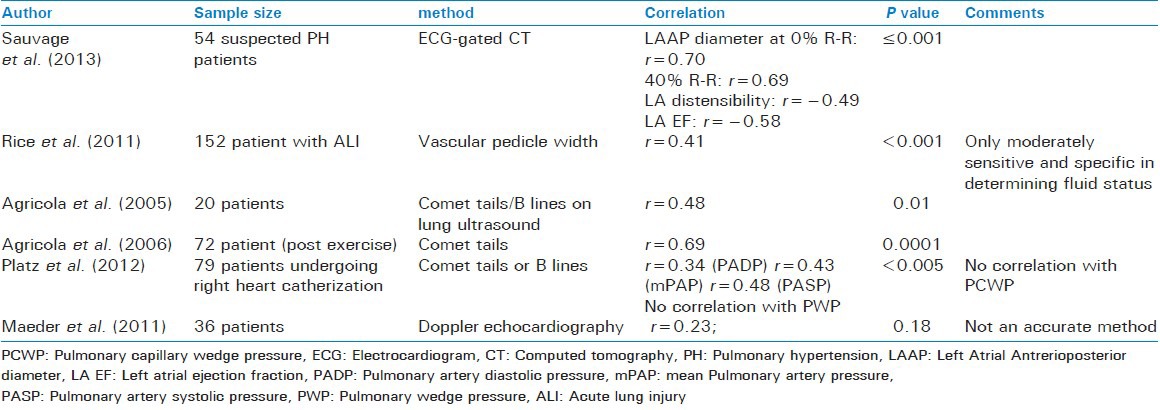
Sauvage et al. hypothesized that morphological and functional characteristics of the left atrium on Electrocardiogram (ECG)-gated CT scan correlated with the PCWP. In a retrospective study on 54 patients they found that antero-posterior diameter at 0% and 50% of R-R interval correlated with PCWP (r = 0.70, P ≤ 0.001 and r = 0.69, P ≤ 0.001). Functional characteristics of left atrium such as distensibility (r = 0.49, P ≤ 0.001) and ejection fraction (r = 0.58, P ≤ 0.001) also correlated well with the PCWP.[55] It also helps distinguish between pre- and post-capillary pulmonary hypertension.
Vascular pedicle width
As mentioned above it is a horizontal distance measured on the chest x ray. Although its correlation with the CVP has been found to be low, the correlation with PCWP is better i.e., r = 0.41 (P < 0.001). A VPW of ≤67 mm detects a PCWP of <8 mmHg with 71% sensitivity and 68% specificity, while a VPW of ≥72 mm detects a PCWP of ≥18 mmHg with a sensitivity of 61.4% and a specificity of 61%.[51]
B lines on the lung ultrasound
B lines are the hyperechoic vertical lines arising from the pleural line and extending to the edge of the ultrasound screen in the ultrasound of the chest. They are thought to arise from interlobular septa thickened by fluid. Platz et al. studied the relationship of the number of these lines to the PCWP to see if these could be used as its surrogate for estimation of pulmonary congestion. The maximum number of B lines in any intercostal space were recorded and the sum of the number of B-lines in a total of four zones of lung (four on each side) were recorded. It was found that, although the number of B lines correlated with the systolic, diastolic and mean pulmonary arterial pressures in a graded manner, the same was not true for PCWP.[56] However a number of previous studies have shown otherwise.[57,58]
Doppler echocardiography
Doppler echocardiography can be used to estimate the PCWP. Transmitral Doppler variables and pulmonary venous flow have been studied for this purpose. Although both of them have been found to be useful in estimating PCWP,[59,60] Poelzl et al. found a combination of the two to be most useful.[61] However in a more recent study, Maeder et al. found that although, transmitral variables could reliably predict diastolic and mean pulmonary artery pressures, this was not true for PCWP. The authors considered this technique unreliable for estimating pulmonary pressures[52] [Table 8].
Table 8.
Noninvasive techniques for PCWP measurement
CONCLUSION
Invasive arterial, central venous pressure and pulmonary capillary wedge pressure have formed the corner stone of hemodynamic monitoring in ICU patients and in patients undergoing major surgeries. Due to their many complications, which arise because of the invasive nature of the technique, and due to the residence of catheters in the bloodstream for prolonged period, efforts have been made to look for non-invasive methods of monitoring the hemodynamics. An ideal noninvasive method of measurement of these important hemodynamic parameters should be easily available, cheap, reproducible, easy to learn and use and simple to interpret. Although a number of these methods have come up, some of them rather innovative, none of them fulfill the above criteria. Further exploration and validation is needed. Although methods for non-invasive continuous blood pressure measurement have been validated, the hemodynamically unstable patient, requiring inotrope support who is the primary candidate for invasive monitoring, does not seem to benefit much from these as of date. This calls for further research into these methods.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Tholl U, Forstner K, Anlauf M. Measuring blood pressure: Pitfalls and recommendations. Nephrol Dial Transplant. 2004;19:766–70. doi: 10.1093/ndt/gfg602. [DOI] [PubMed] [Google Scholar]
- 2.Slogoff S, Keats AS, Arlund C. On the safety of radial artery cannulation. Anesthesiology. 1983;59:42–7. doi: 10.1097/00000542-198307000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Mangano DT, Hickey RF. Ischemic injury following uncomplicated radial artery catheterization. Anesth Analg. 1979;58:55–7. [PubMed] [Google Scholar]
- 4.Brzezinski M, Luisetti T, London MJ. Radial artery cannulation: A comprehensive review of recent anatomic and physiologic investigations. Anesth Analg. 2009;109:1763–81. doi: 10.1213/ANE.0b013e3181bbd416. [DOI] [PubMed] [Google Scholar]
- 5.Chung E, Chen G, Alexander B, Cannesson M. Non-invasive continuous blood pressure monitoring: A review of current applications. Front Med. 2013;7:91–101. doi: 10.1007/s11684-013-0239-5. [DOI] [PubMed] [Google Scholar]
- 6.Fortin J, Marte W, Grüllenberger R, Hacker A, Habenbacher W, Heller A, et al. Continuous non-invasive blood pressure monitoring using concentrically interlocking control loops. Comput Biol Med. 2006;36:941–57. doi: 10.1016/j.compbiomed.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Ilies C, Kiskalt H, Siedenhans D, Meybohm P, Steinfath M, Bein B, et al. Detection of hypotension during Caesarean section with continuous non-invasive arterial pressure device or intermittent oscillometric arterial pressure measurement. Br J Anaesth. 2012;109:413–9. doi: 10.1093/bja/aes224. [DOI] [PubMed] [Google Scholar]
- 8.Gayat E, Mongardon N, Tuil O, Sievert K, Chazot T, Liu N, et al. CNAP(®) does not reliably detect minimal or maximal arterial blood pressures during induction of anaesthesia and tracheal intubation. Acta Anaesthesiol Scand. 2013;57:468–73. doi: 10.1111/aas.12028. [DOI] [PubMed] [Google Scholar]
- 9.Ilies C, Bauer M, Berg P, Rosenberg J, Hedderich J, Bein B, et al. Investigation of the agreement of a continuous non-invasive arterial pressure device in comparison with invasive radial artery measurement. Br J Anaesth. 2012;108:202–10. doi: 10.1093/bja/aer394. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy T, Telec N, Dennis A, Griffiths J, Buettner A. Ability of non-invasive intermittent blood pressure monitoring and a continuous non-invasive arterial pressure monitor (CNAPTM) to provide new readings in each 1-min interval during elective caesarean section under spinal anaesthesia. Anaesthesia. 2012;67:274–9. doi: 10.1111/j.1365-2044.2011.06996.x. [DOI] [PubMed] [Google Scholar]
- 11.Fischer MO, Avram R, Cârjaliu I, Massetti M, Gérard JL, Hanouz JL, et al. Non-invasive continuous arterial pressure and cardiac index monitoring with Nexfin after cardiac surgery. Br J Anaesth. 2012;109:514–21. doi: 10.1093/bja/aes215. [DOI] [PubMed] [Google Scholar]
- 12.Martina JR, Westerhof BE, van Goudoever J, de Beaumont EM, Truijen J, Kim YS, et al. Noninvasive continuous arterial blood pressure monitoring with Nexfin®. Anesthesiology. 2012;116:1092–103. doi: 10.1097/ALN.0b013e31824f94ed. [DOI] [PubMed] [Google Scholar]
- 13.Nowak RM, Sen A, Garcia AJ, Wilkie H, Yang JJ, Nowak MR, et al. Noninvasive continuous or intermittent blood pressure and heart rate patient monitoring in the ED. Am J Emerg Med. 2011;29:782–9. doi: 10.1016/j.ajem.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Hohn A, Defosse JM, Becker S, Steffen C, Wappler F, Sakka SG. Non-invasive continuous arterial pressure monitoring with Nexfin (R) does not sufficiently replace invasive measurements in critically ill patients. Br J Anaesth. 2013;111:178–84. doi: 10.1093/bja/aet023. [DOI] [PubMed] [Google Scholar]
- 15.Broch O, Bein B, Gruenewald M, Carstens A, Illies C, Schöneich F, et al. A comparison of continuous non-invasive arterial pressure with invasive radial and femoral pressure in patients undergoing cardiac surgery. Minerva Anestesiol. 2013;79:248–56. [PubMed] [Google Scholar]
- 16.Kemmotsu O, Ueda M, Otsuka H, Yamamura T, Winter DC, Eckerle JS. Arterial tonometry for noninvasive, continuous blood pressure monitoring during anesthesia. Anesthesiology. 1991;75:333–40. doi: 10.1097/00000542-199108000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Dueck R, Goedje O, Clopton P. Noninvasive continuous beat-to-beat radial artery pressure via TL-200 applanation tonometry. J Clin Monit Comput. 2012;26:75–83. doi: 10.1007/s10877-012-9336-2. [DOI] [PubMed] [Google Scholar]
- 18.Saugel B, Meidert AS, Hapfelmeier A, Eyer F, Schmid RM, Huber W. Non-invasive continuous arterial pressure measurement based on radial artery tonometry in the intensive care unit: A method comparison study using the T-Line TL-200pro device. Br J Anaesth. 2013;111:185–90. doi: 10.1093/bja/aet025. [DOI] [PubMed] [Google Scholar]
- 19.Saugel B, Fassio F, Hapfelmeier A, Meidert AS, Schmid RM, Huber W. The T-Line TL-200 system for continuous non-invasive blood pressure measurement in medical intensive care unit patients. Intensive Care Med. 2012;38:1471–7. doi: 10.1007/s00134-012-2617-x. [DOI] [PubMed] [Google Scholar]
- 20.Colquhoun DA, Forkin KT, Dunn LK, Bogdonoff DL, Durieux ME, Thiele RH. Non-invasive, minute-to-minute estimates of systemic arterial pressure and pulse pressure variation using radial artery tonometry. J Med Eng Technol. 2013;37:197–202. doi: 10.3109/03091902.2013.774443. [DOI] [PubMed] [Google Scholar]
- 21.Solà J, Adler A, Santos A, Tusman G, Sipmann FS, Bohm SH. Non-invasive monitoring of central blood pressure by electrical impedance tomography: First experimental evidence. Med Biol Eng Comput. 2011;49:409–15. doi: 10.1007/s11517-011-0753-z. [DOI] [PubMed] [Google Scholar]
- 22.Laffon E, Marthan R, Montaudon M, Latrabe V, Laurent F, Ducassou D. Feasibility of aortic pulse pressure and pressure wave velocity MRI measurement in young adults. J Magn Reson Imaging. 2005;21:53–8. doi: 10.1002/jmri.20227. [DOI] [PubMed] [Google Scholar]
- 23.Grotenhuis HB, Westenberg JJ, Steendijk P, van der Geest RJ, Ottenkamp J, Bax JJ, et al. Validation and reproducibility of aortic pulse wave velocity as assessed with velocity-encoded MRI. J Magn Reson Imaging. 2009;30:521–6. doi: 10.1002/jmri.21886. [DOI] [PubMed] [Google Scholar]
- 24.Lin HD, Lee YS, Chuang BN. Using dual-antenna nanosecond pulse near-field sensing technology for non-contact and continuous blood pressure measurement. Conf Proc IEEE Eng Med Biol Soc 2012. 2012:219–22. doi: 10.1109/EMBC.2012.6345909. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz-Rodríguez JC, Ruiz-Sanmartín A, Ribas V, Caballero J, García-Roche A, Riera J, et al. Innovative continuous non-invasive cuffless blood pressure monitoring based on photoplethysmography technology. Intensive Care Med. 2013;39:1618–25. doi: 10.1007/s00134-013-2964-2. [DOI] [PubMed] [Google Scholar]
- 26.Barbeito A, Mark JB. Arterial and central venous pressure monitoring. Anesthesiol Clin. 2006;24:717–35. doi: 10.1016/j.atc.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Marcelino P, Borba A, Fernandes AP, Marum S, Germano N, Lopes MR. Non invasive evaluation of central venous pressure using echocardiography in the intensive care--specific features of patients with right ventricular enlargement and chronic exacerbated pulmonary disease. Rev Port Pneumol. 2006;12:637–58. [PubMed] [Google Scholar]
- 28.De Lorenzo RA, Morris MJ, Williams JB, Haley TF, Straight TM, Holbrook-Emmons VL, et al. Does a simple bedside sonographic measurement of the inferior vena cava correlate to central venous pressure? J Emerg Med. 2012;42:429–36. doi: 10.1016/j.jemermed.2011.05.082. [DOI] [PubMed] [Google Scholar]
- 29.Thanakitcharu P, Charoenwut M, Siriwiwatanakul N. Inferior vena cava diameter and collapsibility index: A practical non-invasive evaluation of intravascular fluid volume in critically-ill patients. J Med Assoc Thai. 2013;96(Suppl 3):S14–22. [PubMed] [Google Scholar]
- 30.Wiwatworapan W, Ratanajaratroj N, Sookananchai B. Correlation between inferior vena cava diameter and central venous pressure in critically ill patients. J Med Assoc Thai. 2012;95:320–4. [PubMed] [Google Scholar]
- 31.Arthur ME, Landolfo C, Wade M, Castresana MR. Inferior vena cava diameter (IVCD) measured with transesophageal echocardiography (TEE) can be used to derive the central venous pressure (CVP) in anesthetized mechanically ventilated patients. Echocardiography. 2009;26:140–9. doi: 10.1111/j.1540-8175.2008.00772.x. [DOI] [PubMed] [Google Scholar]
- 32.Iwamoto Y, Tamai A, Kohno K, Masutani S, Okada N, Senzaki H. Usefulness of respiratory variation of inferior vena cava diameter for estimation of elevated central venous pressure in children with cardiovascular disease. Circ J. 2011;75:1209–14. doi: 10.1253/circj.cj-10-0690. [DOI] [PubMed] [Google Scholar]
- 33.Stawicki SP, Braslow BM, Panebianco NL, Kirkpatrick JN, Gracias VH, Hayden GE, et al. Intensivist use of hand-carried ultrasonography to measure IVC collapsibility in estimating intravascular volume status: correlations with CVP. J Am Coll Surg. 2009;209:55–61. doi: 10.1016/j.jamcollsurg.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 34.Minutiello L. [Non-invasive evaluation of central venous pressure derived from respiratory variations in the diameter of the inferior vena cava] Minerva Cardioangiol. 1993;41:433–7. [PubMed] [Google Scholar]
- 35.Sato Y, Kawataki M, Hirakawa A, Toyoshima K, Kato T, Itani Y, et al. The diameter of the inferior vena cava provides a noninvasive way of calculating central venous pressure in neonates. Acta Paediatr. 2013;102:e241–6. doi: 10.1111/apa.12247. [DOI] [PubMed] [Google Scholar]
- 36.Ng L, Khine H, Taragin BH, Avner JR, Ushay M, Nunez D. Does bedside sonographic measurement of the inferior vena cava diameter correlate with central venous pressure in the assessment of intravascular volume in children? Pediatr Emerg Care. 2013;29:337–41. doi: 10.1097/PEC.0b013e31828512a5. [DOI] [PubMed] [Google Scholar]
- 37.Uthoff H, Siegemund M, Aschwanden M, Hunziker L, Fabbro T, Baumann U, et al. Prospective comparison of noninvasive, bedside ultrasound methods for assessing central venous pressure. Ultraschall Med. 2012;33:E256–62. doi: 10.1055/s-0031-1299506. [DOI] [PubMed] [Google Scholar]
- 38.Prekker ME, Scott NL, Hart D, Sprenkle MD, Leatherman JW. Point-of-care ultrasound to estimate central venous pressure: A comparison of three techniques. Crit Care Med. 2013;41:833–41. doi: 10.1097/CCM.0b013e31827466b7. [DOI] [PubMed] [Google Scholar]
- 39.Bloch KE, Krieger BP, Sackner MA. Noninvasive measurement of central venous pressure by neck inductive plethysmography. Chest. 1991;100:371–5. doi: 10.1378/chest.100.2.371. [DOI] [PubMed] [Google Scholar]
- 40.Lipton B. Estimation of central venous pressure by ultrasound of the internal jugular vein. Am J Emerg Med. 2000;18:432–4. doi: 10.1053/ajem.2000.7335. [DOI] [PubMed] [Google Scholar]
- 41.Siva B, Hunt A, Boudville N. The sensitivity and specificity of ultrasound estimation of central venous pressure using the internal jugular vein. J Crit Care. 2012;27:315.e7–11. doi: 10.1016/j.jcrc.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Donahue SP, Wood JP, Patel BM, Quinn JV. Correlation of sonographic measurements of the internal jugular vein with central venous pressure. Am J Emerg Med. 2009;27:851–5. doi: 10.1016/j.ajem.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Bailey JK, McCall J, Smith S, Kagan RJ. Correlation of internal jugular vein/common carotid artery ratio to central venous pressure: A pilot study in pediatric burn patients. J Burn Care Res. 2012;33:89–92. doi: 10.1097/BCR.0b013e318234d965. [DOI] [PubMed] [Google Scholar]
- 44.Aggarwal V, Chatterjee A, Cho Y, Cheung D. Ultrasound-guided noninvasive measurement of a patient's central venous pressure. Conf Proc IEEE Eng Med Biol Soc. 2006;1:3843–9. doi: 10.1109/IEMBS.2006.260703. [DOI] [PubMed] [Google Scholar]
- 45.Baumann UA, Marquis C, Stoupis C, Willenberg TA, Takala J, Jakob SM. Estimation of central venous pressure by ultrasound. Resuscitation. 2005;64:193–9. doi: 10.1016/j.resuscitation.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 46.Keller AS, Melamed R, Malinchoc M, John R, Tierney DM, Gajic O. Diagnostic accuracy of a simple ultrasound measurement to estimate central venous pressure in spontaneously breathing, critically ill patients. J Hosp Med. 2009;4:350–5. doi: 10.1002/jhm.503. [DOI] [PubMed] [Google Scholar]
- 47.Thalhammer C, Aschwanden M, Odermatt A, Baumann UA, Imfeld S, Bilecen D, et al. Noninvasive central venous pressure measurement by controlled compression sonography at the forearm. J Am Coll Cardiol. 2007;50:1584–9. doi: 10.1016/j.jacc.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 48.Thalhammer C, Siegemund M, Aschwanden M, Gassmann M, Baumann UA, Jaeger KA, et al. Non-invasive central venous pressure measurement by compression ultrasound--A step into real life. Resuscitation. 2009;80:1130–6. doi: 10.1016/j.resuscitation.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 49.Ward KR, Tiba MH, Barbee RW, Ivatury RR, Arrowood JA, Spiess BD, et al. A new noninvasive method to determine central venous pressure. Resuscitation. 2006;70:238–46. doi: 10.1016/j.resuscitation.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 50.Ward KR, Tiba MH, Draucker GT, Proffitt EK, Barbee RW, Gunnerson KJ, et al. A novel noninvasive impedance-based technique for central venous pressure measurement. Shock. 2010;33:269–73. doi: 10.1097/SHK.0b013e3181ab9b9b. [DOI] [PubMed] [Google Scholar]
- 51.Rice TW, Ware LB, Haponik EF, Chiles C, Wheeler AP, Bernard GR, et al. Vascular pedicle width in acute lung injury: Correlation with intravascular pressures and ability to discriminate fluid status. Crit Care. 2011;15:R86. doi: 10.1186/cc10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maeder MT, Karapanagiotidis S, Dewar EM, Gamboni SE, Htun N, Kaye DM. Accuracy of Doppler echocardiography to estimate key hemodynamic variables in subjects with normal left ventricular ejection fraction. J Card Fail. 2011;17:405–12. doi: 10.1016/j.cardfail.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Marcelino P, Fernandes AP, Marum S, Ribeiro JP. Non-invasive evaluation of central venous pressure by echocardiography. Rev Port Cardiol. 2002;21:125–33. [PubMed] [Google Scholar]
- 54.Kronberg GM, Quan SF, Schlobohm RM, Lindauer JM, Goodman PC. Anatomic locations of the tips of pulmonary-artery catheters in supine patients. Anesthesiology. 1979;51:467–9. doi: 10.1097/00000542-197911000-00021. [DOI] [PubMed] [Google Scholar]
- 55.Sauvage N, Reymond E, Jankowski A, Prieur M, Pison C, Bouvaist H, et al. ECG-gated computed tomography to assess pulmonary capillary wedge pressure in pulmonary hypertension. Eur Radiol. 2013;23:2658–65. doi: 10.1007/s00330-013-2911-1. [DOI] [PubMed] [Google Scholar]
- 56.Platz E, Lattanzi A, Agbo C, Takeuchi M, Resnic FS, Solomon SD, et al. Utility of lung ultrasound in predicting pulmonary and cardiac pressures. Eur J Heart Fail. 2012;14:1276–84. doi: 10.1093/eurjhf/hfs144. [DOI] [PubMed] [Google Scholar]
- 57.Agricola E, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A, et al. “Ultrasound comet-tail images”: A marker of pulmonary edema: A comparative study with wedge pressure and extravascular lung water. Chest. 2005;127:1690–5. doi: 10.1378/chest.127.5.1690. [DOI] [PubMed] [Google Scholar]
- 58.Agricola E, Picano E, Oppizzi M, Pisani M, Meris A, Fragasso G, et al. Assessment of stress-induced pulmonary interstitial edema by chest ultrasound during exercise echocardiography and its correlation with left ventricular function. J Am Soc Echocardiogr. 2006;19:457–63. doi: 10.1016/j.echo.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 59.Temporelli PL, Scapellato F, Corrà U, Eleuteri E, Imparato A, Giannuzzi P. Estimation of pulmonary wedge pressure by transmitral Doppler in patients with chronic heart failure and atrial fibrillation. Am J Cardiol. 1999;83:724–7. doi: 10.1016/s0002-9149(98)00978-3. [DOI] [PubMed] [Google Scholar]
- 60.Capomolla S, Pozzoli M, Gola A, Maestri R, Sisti M, Cobelli F, et al. [Pulmonary venous flow in patients with chronic heart failure: Feasibility and additional value compared to transmitral flow for non-invasive estimation of pulmonary wedge pressure] G Ital Cardiol. 1996;26:1123–37. [PubMed] [Google Scholar]
- 61.Poelzl G, Gattermeier M, Kratzer H, Zeindlhofer E, Kuehn P. Feasibility and accuracy of transthoracic Doppler echocardiographic estimation of pulmonary capillary wedge pressure applying different methods. Eur J Heart Fail. 2001;3:553–60. doi: 10.1016/s1388-9842(01)00166-0. [DOI] [PubMed] [Google Scholar]


