Abstract
Abdominal adiposity, particularly visceral adipose tissue (VAT), is independently linked to the pathogenesis of diabetes and cardiovascular diseases. Emerging evidence suggests that greater intake of sugar-sweetened beverages (SSBs) may be associated with abnormal fat accumulation in VAT. We examined whether habitual SSB consumption and diet soda intakes are differentially associated with deposition of body fat. We conducted a cross-sectional analysis using previously collected data in 2596 middle-aged adults (1306 men and 1290 women) from the Framingham Heart Study Offspring and Third Generation cohorts. VAT and abdominal subcutaneous adipose tissue (SAT) were measured using multidetector computed tomography. Habitual intake of SSBs and diet soda was assessed by a validated food frequency questionnaire. We observed that SSB consumption was positively associated with VAT after adjustment for SAT and other potential confounders (P-trend < 0.001). We observed an inverse association between SSB consumption and SAT (P-trend = 0.04) that persisted after additional adjustment for VAT (P-trend < 0.001). Higher SSB consumption was positively associated with the VAT-to-SAT ratio (P-trend < 0.001). No significant association was found between diet soda consumption and either VAT or the VAT-to-SAT ratio, but diet soda was positively associated with SAT (P-trend < 0.001). Daily consumers of SSBs had a 10% higher absolute VAT volume and a 15% greater VAT-to-SAT ratio compared with nonconsumers, whereas consumption of diet soda was not associated with either volume or distribution of VAT.
Introduction
Obesity is a risk factor for type 2 diabetes and cardiovascular diseases (CVDs);12 however, not all obese individuals display equivalent cardiometabolic risk (1). The location of fat storage, rather than overall fat quantity, may be more predictive of cardiometabolic risk (2, 3). Abdominal adiposity, particularly fat accumulation in visceral adipose tissue (VAT), was independently linked to the pathogenesis of CVD and type 2 diabetes (4). The pathogenic associations of abdominal subcutaneous adipose tissue (SAT) are less understood, but some evidence suggests that SAT may have protective effects (5, 6). Findings from recent observational studies also suggest that a greater propensity of fat stored in VAT relative to SAT, represented by a ratio between the 2 fat depots, is associated with greater risk factors of type 2 diabetes and CVD (7, 8).
Sugar-sweetened beverages (SSBs), sweetened with either sucrose or high-fructose corn syrup, are the leading source of added sugars in the diets of U.S. adults (9). Excess SSB consumption was associated with weight gain, as reported in a recent systematic review and meta-analysis (10). Emerging evidence suggests that greater consumption of SSBs may be preferentially associated with fat accumulation in VAT; that is, more fat may be accumulated in VAT, and less fat may be accumulated in SAT (11, 12). Only 1 observational study examined the association between habitual intake of SSBs and abdominal fat depots in 791 healthy adults (12). This study observed that those who regularly consumed SSBs, rather than diet soda, had a higher proportion of VAT in total abdominal adiposity, although intake of neither type of beverage was associated with absolute VAT or SAT volume. The objective of this study was to examine the cross-sectional associations between consumption of SSBs and diet soda and both the absolute volume of the fat depots and relative distribution of VAT to SAT in participants of the Framingham Heart Study. Our hypothesis was that a higher habitual SSB consumption is associated with a greater fat accumulation in VAT and lesser fat accumulation in SAT, as reflected by a higher VAT-to-SAT ratio, whereas diet soda consumption is associated with none of the 3 variables.
Participants and Methods
Study population.
Study participants were from the Framingham Heart Study Offspring cohort and Third Generation Cohort (Gen3) and were described previously (13, 14). Participants in the 2 cohorts were evaluated approximately every 3–4 y. Each examination included a detailed medical history, physical examination by a physician, and standard laboratory tests for cardiovascular and metabolic risk factors. Data were from Offspring examination cycle 7 (1998–2001) and Gen3 examination cycle 1 (2002–2005). The study sample for the present analysis is a subcohort of 3529 participants whose abdominal adipose tissues were assessed by multidetector computed tomography (MDCT) between June 2002 and April 2005, as described previously (15). The following criteria were applied for study participants to be eligible for the MDCT substudy: 1) body weight < 160 kg; 2) men aged ≥35 y; and 3) nonpregnant women aged ≥40 y. Restriction on body weight was to ensure obtaining MDCT measurement. Of the 3529 participants included in the MDCT study, 3017 had valid FFQs and interpretable measurements for VAT and SAT. We excluded an additional 421 participants with the following: 1) previously diagnosed diabetes as defined by fasting plasma glucose concentrations ≥7.0 mmol/L or treatment with glucose-lowering medication (n = 169); or 2) missing covariates, such as physical activity and smoking status (n = 252). The final sample included 2596 participants. All participants provided written informed consent before study participation. The Framingham Heart Study protocols and procedures were approved by the Institutional Review Board for Human Research at Boston University Medical Center, and the current analyses were approved by the Tufts Medical Center and Tufts University Health Sciences Institutional Review Board.
Abdominal adipose tissue.
Measurement protocols for abdominal visceral and subcutaneous adiposity were described in detail previously (16). In brief, participants underwent an abdominal scanning with an 8-slice MDCT scanner (LightSpeed Ultra; GE Healthcare). This abdominal scanning obtained 25 contiguous slices covering 125 mm superiorly from the upper edge of the S1 vertebrae. The abdominal muscular wall that separates the VAT and SAT was manually traced. Abdominal images were converted to volumes (cubic centimeters) of VAT and SAT using protocol provided by Aquarius 3D Workstation (TeraRecon). Reproducibility between 2 readers was assessed in 100 randomly selected participants. The intraclass correlations were high (>0.99) for both VAT and SAT readings between the 2 readers (17).
Beverage consumption.
Consumption of SSBs and diet soda was assessed using a semiquantitative 126-item FFQ that was designed to capture the habitual dietary intake for the year preceding the physical and medical examinations (18). The FFQ was mailed to participants to be completed at home, and the completed version was returned during the study appointment. The FFQ consisted of a list of foods with standard serving sizes and a selection of 9 frequency categories ranging from 0 or <1 serving/mo to ≥6 servings/d. Nutrient intake was calculated by multiplying the frequency of consumption of a food item by the nutrient content per standard serving size for the given food item. Dietary information was considered valid only if reported energy intake was ≥2.5 MJ/d (600 kcal/d) for both men and women, <16.7 MJ/d (4000 kcal/d) for women, <17.5 MJ/d (4200 kcal/d) for men, and if <13 food items were left blank on the FFQ.
Participants were asked to report their frequency of SSB consumption during the previous year. The SSB assessment included the following: 1) caffeinated colas with sugar; 2) caffeine-free colas with sugar; 3) other carbonated beverages with sugar; and 4) fruit punches, lemonade, or other noncarbonated fruit drinks. Diet soda was captured using 3 FFQ items including the following: 1) low-calorie cola; 2) low-calorie, caffeine-free cola; and 3) other low-calorie carbonated beverage. One serving of an SSB or diet soda was equivalent to 360 mL (12 fl oz). Participants were categorized according to the frequency of SSB consumption: 0 to <1 serving/mo (nonconsumers), ≥1 serving/mo to <1 serving/wk (occasional consumers), ≥1 serving/wk to <1 serving/d (frequent consumers), and ≥1 serving/d (daily consumers). Similarly, we grouped study participants into 4 categories according to their frequency of drinking diet soda. The relative validity of the FFQ was examined for both nutrients and foods in men and women in other cohorts (18–21). The deattenuated correlation coefficients between the FFQ and multiple diet records for women (20) and men (19), respectively, were 0.84 and 0.84 for cola-type soft drinks, 0.36 and 0.55 for other carbonated non-cola beverages, and 0.56 and 0.79 for noncarbonated fruit drinks. The deattenuated correlation coefficients were 0.73 and 0.74 for low-calorie cola and other low-calorie carbonated non-cola beverages for women and men, respectively (19).
Anthropometry and covariates assessment.
At each visit, participants underwent a physical examination using standard protocols and completed a medical history questionnaire. Waist circumference was measured at the level of the umbilicus from a standing position. BMI was calculated as weight (kilograms) divided by height (square meters). Participants who reported that they smoked regularly in the past year were classified as current smokers. Age (years), sex, and education level (college level or above vs. high school or less) were captured from questionnaires. Physical activity level was calculated based on questionnaire-derived time spent performing the activity in a typical day and the intensity of the activity (22). Dietary factors derived from the FFQ included intake of total energy (kilocalories per day), fats, carbohydrates and protein (as percentage of energy intake), multivitamin use (yes/no), dietary fiber (grams per day), fruits and vegetables (grams per day), red meat (grams per day), nuts (grams per day), whole grains (grams per day), and alcohol (grams per day). The 2005 Dietary Guidelines Adherence Index (DGAI) was used to capture overall dietary quality as described previously (23). Because added sugar is a component of the DGAI, we created a modified DGAI in the present study by leaving out the added sugar component. Fasting plasma glucose and serum lipids were measured after an overnight fast. Impaired fasting glucose was defined as fasting glucose concentration ≥ 5.6 mmol/L (24). Dyslipidemia was defined as use of lipid-lowering medications, TG concentration ≥ 1.7 mmol/L, or HDL cholesterol concentration < 1.04 mmol/L in men and <1.29 mmol/L in women (25). Hypertension was defined as systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or use of antihypertensive drugs (25).
Statistical analysis.
Participant characteristics across the categories of SSB consumption were age and sex adjusted and evaluated through use of least-squares means. Dietary characteristics were additionally adjusted for energy intake. A test for linear trend across categories of SSB intake was performed by treating the median value of SSB intake for each category as a continuous independent variable in linear regression models for continuous response variables or in logistic regression models for dichotomous response variables. Participant characteristics are presented as means (95% CI), with the exception of waist circumference, VAT-to-SAT ratio, and dietary fiber intake for which log transformations were applied to correct the positive skewness, and geometric means are presented. We calculated Spearman’s correlation coefficients between all adiposity markers.
In our primary analyses, the relations between SSB intake and BMI, waist circumference, VAT, SAT, and VAT-to-SAT ratio were examined through use of least-squares means determined within categories of SSB intake, with adjustment for the following covariates: 1) age; 2) sex; 3) energy intake; 4) alcohol intake; 5) modified DGAI; 6) physical activity level; 7) educational level; 8) current smoking status (yes/no); and 9) cohort (Offspring/Gen3). We mutually adjusted SSB and diet soda intake, i.e., we adjusted for diet soda intake in the analysis of SSBs and vice versa. We additionally adjusted for SAT in the model for VAT and controlled for VAT in the analysis for SAT. Linear trends for each outcome were tested across categories of SSB using the median value approach with adjustment for the same covariates described above. Because of previously reported sex interactions for the association between abdominal fat and cardiometabolic risk factors (15), we examined whether sex may modify the association between SSB intake and abdominal fat–related measures (waist circumference, SAT, VAT, and VAT-to-SAT ratio). We included the product term of SSB intake and sex in the statistical model to test for a potential interaction. Bonferroni’s correction was applied to account for the multiple comparisons (waist circumference, SAT, VAT, and VAT-to-SAT ratio), and the statistical significance for interaction was set at P < 0.0125 (0.05/4).
In the secondary analyses, we tested for effect modification between SSB and age categorized using the median as an arbitrary cut point (< and ≥ the median age of 50 y) and BMI (<25, ≥25 and <30, and ≥30 kg/m2). Bonferroni’s correction was applied for the tests for interaction, and the significance level was set at P < 0.0125. In a sensitivity analysis, instead of modified DGAI, we controlled for intakes of individual food groups (intakes of fruits, vegetables, whole grains, red meats, and nuts), multivitamin use, and other covariates (age, sex, energy intake, alcohol intake, dietary fat, physical activity level, educational level, smoking status, Framingham cohort, and diet soda). We also examined whether energy intake and glycemic load may mediate the observed associations. For all outcomes, the same models and covariates were used to examine diet soda intake. All statistical analyses were conducted using SAS statistical software (version 9.3; SAS Institute). A 2-tailed P < 0.05 was considered statistically significant, unless specified otherwise.
Results
Approximately 33% of study participants were nonconsumers of SSBs, and 13% were daily consumers. The prevalence of nonconsumers and daily consumers of diet soda was 58% and 8%, respectively. Also, 17% reported consuming neither SSBs nor diet soda, 1% were daily consumers of both types of beverages, and the remaining participants (82%) reported consuming a mixture of SSBs and diet soda. Among daily consumers, the greatest SSB contributor was cola (39.9%), followed by noncarbonated fruit drinks (28.7%), carbonated non-cola beverages (21.5%), and caffeine-free cola (10.7%). The age-, sex-, and/or energy-adjusted characteristics of participants across categories of SSB consumption are shown in Table 1. Compared with nonconsumers, daily SSB consumers were more likely to be men, younger, current smokers, have a lower educational level, and have a higher physical activity level. Daily SSB consumers drank less alcohol, were less likely to take multivitamin supplements, and had an overall less healthy diet as captured by the modified DGAI. In addition, daily SSB consumers had a higher prevalence of dyslipidemia. Compared with nonconsumers, daily diet soda consumers were older and had greater BMI (Table 2). Daily diet soda consumers also demonstrated a higher prevalence of impaired fasting glucose, dyslipidemia, and hypertension.
TABLE 1.
Characteristics according to sugar-sweetened beverage consumption in 2596 adults1
| Non-consumers |
Consumers |
||||
| 0 to <1/mo | ≥1/mo to <1/wk | ≥1/wk to <1/d | ≥1/d | P-trend | |
| Median consumption, servings/wk | 0 | 1 | 4 | 10 | |
| Participants, n | 851 | 910 | 482 | 353 | |
| Age,2 y | 51.8 (51.1, 52.5) | 49.5 (48.9, 50.1) | 49.1 (48.2, 50.0) | 47.4 (46.4, 48.4) | <0.001 |
| Women,3 % | 66.7 (63.5, 69.9) | 50.1 (47.0, 53.1) | 34.1 (29.8, 38.3) | 29.0 (24.0, 34.0) | <0.001 |
| Overweight and obese,4 % | 67.0 (63.8, 70.1) | 64.0 (61.0, 67.0) | 67.4 (63.2, 71.5) | 64.6 (59.8, 69.5) | 0.77 |
| Current smoker,4 % | 11.0 (8.7, 13.2) | 10.8 (8.6, 12.9) | 11.5 (8.6, 14.5) | 21.5 (18.1, 25.0) | <0.001 |
| Alcohol intake,5 g/d | 11.9 (10.9, 12.9) | 11.1 (10.2, 12.1) | 10.0 (8.7, 11.3) | 10.8 (9.2, 12.3) | <0.001 |
| Multivitamin user,4 % | 52.5 (49.1, 55.9) | 46.8 (43.6, 50.0) | 47.0 (42.6, 51.5) | 42.1 (36.9, 47.3) | 0.006 |
| Physical activity score4 | 36.9 (36.4, 37.4) | 37.5 (37.1, 38.0) | 37.2 (36.6, 37.9) | 38.8 (38.1, 39.6) | <0.001 |
| Education level,4,6 % | 48.6 (45.2, 52.0) | 51.1 (47.9, 54.3) | 49.3 (44.9, 53.7) | 37.7 (32.5, 42.9) | <0.001 |
| Antihypertensive drugs,4 % | 17.8 (15.3, 20.2) | 16.4 (14.1, 18.6) | 13.7 (10.5, 16.8) | 13.4 (9.7, 17.1) | 0.03 |
| Hypertension,4 % | 27.4 (24.5, 30.2) | 25.5 (22.8, 28.2) | 23.9 (20.2, 27.6) | 23.8 (19.4, 28.2) | 0.20 |
| Lipid-lowering drugs,4 % | 9.3 (7.5, 11.1) | 7.5 (5.8, 9.2) | 5.4 (3.0, 7.7) | 5.4 (2.7, 8.2) | 0.02 |
| Dyslipidemia,4 % | 40.4 (37.0, 43.7) | 41.5 (38.3, 44.7) | 48.9 (44.5, 53.3) | 49.3 (44.1, 54.4) | 0.001 |
| Fasting plasma glucose,4 mmol/L | 5.3 (5.3, 5.3) | 5.3 (5.3, 5.3) | 5.3 (5.2, 5.3) | 5.3 (5.3, 5.4) | 0.23 |
| Impaired fasting glucose,4 % | 29.0 (26.1, 31.9) | 24.1 (21.4, 26.8) | 23.4 (19.6, 27.2) | 28.1 (23.6, 32.5) | 0.84 |
| Energy intake,4 kcal/d | 1810 (1770, 1850) | 1920 (1880, 1950) | 2090 (2040, 2150) | 2440 (2380, 2510) | <0.001 |
| Fat,4 %EI | 32.2 (31.8, 32.7) | 31.6 (31.2, 32.1) | 31.7 (31.1, 32.3) | 30.6 (29.9, 31.3) | <0.001 |
| Carbohydrate,4 %EI | 45.4 (44.8, 46.0) | 47.7 (47.1, 48.2) | 49.3 (48.6, 50.1) | 52.6 (51.7, 53.5) | <0.001 |
| Total sugar,4 %EI | 19.2 (18.8, 19.7) | 21.1 (20.7, 21.5) | 23.8 (23.2, 24.4) | 29.3 (28.6, 30.0) | <0.001 |
| Dietary fiber,5,7 g/d | 18.7 (18.3, 19.1) | 18.0 (17.7, 18.4) | 16.6 (16.1, 17.1) | 13.6 (13.2, 14.1) | <0.001 |
| Whole grains,5,7 g/d | 20.0 (18.9, 21.1) | 20.3 (19.2, 21.4) | 17.4 (16.1, 18.7) | 12.9 (11.8, 14.1) | <0.001 |
| Fruits,5,7 g/d | 213 (200, 227) | 234 (221, 248) | 215 (198, 233) | 142 (129, 157) | <0.001 |
| Vegetables,5,7 g/d | 212 (204, 220) | 199 (192, 206) | 182 (173, 192) | 150 (141, 160) | <0.001 |
| Red meat,5,7 g/d | 41.8 (39.1, 44.7) | 44.7 (42.1, 47.6) | 49.5 (45.4, 53.9) | 49.7 (44.7, 55.1) | 0.007 |
| Nuts,5 g/d | 6.9 (6.2, 7.6) | 4.7 (4.0, 5.3) | 3.7 (2.8, 4.6) | 1.9 (0.8, 3.0) | <0.001 |
| Glycemic index5 | 52.1 (51.8, 52.3) | 53.4 (53.2, 53.6) | 54.7 (54.3, 55.0) | 55.9 (55.5, 56.3) | <0.001 |
| Glycemic load5 | 119 (117, 120) | 126 (124, 128) | 133 (131, 136) | 148 (145, 151) | <0.001 |
| Diet soda,5 servings/wk | 11.9 (10.3, 13.4) | 6.7 (5.2, 8.1) | 4.7 (2.6, 6.7) | 4.0 (1.5, 6.5) | <0.001 |
| DGAI5 | 9.0 (8.9, 9.2) | 8.9 (8.7, 9.1) | 8.5 (8.3, 8.7) | 7.9 (7.6, 8.2) | <0.001 |
Data are presented as means or geometric means and 95% CIs. The DGAI was modified by leaving out the added sugar component. DGAI, 2005 Dietary Guideline Adherence Index; %EI, percentage of energy intake.
Adjusted for sex.
Adjusted for age.
Values were adjusted for sex and age.
Values were adjusted for sex, age, and energy intake.
College or above.
Values are geometric means.
TABLE 2.
Characteristics according to diet soda consumption in 2596 adults1
| Non-consumers |
Consumers |
||||
| 0 to <1/mo | ≥1/mo to <1/wk | ≥1/wk to <1/d | ≥1/d | P-trend | |
| Median consumption, servings/wk | 0 | 1 | 4 | 11 | |
| Participants, n | 1520 | 599 | 262 | 215 | |
| Age,2 y | 49.8 (49.3, 50.3) | 49.4 (48.6, 50.1) | 50.4 (49.2, 51.6) | 51.6 (50.3, 52.9) | 0.006 |
| Women,3 % | 47.9 (45.4, 50.4) | 52.2 (48.3, 56.2) | 53.0 (47.0, 59.0) | 51.4 (44.7, 58.0) | 0.27 |
| Overweight and obese,4 % | 60.9 (58.7, 63.2) | 70.7 (67.1, 74.4) | 70.5 (65.0, 76.0) | 79.4 (73.3, 85.5) | <0.001 |
| Current smoker,4 % | 13.4 (11.8, 15.1) | 10.0 (7.4, 12.7) | 10.9 (6.9, 14.9) | 14.1 (9.7, 18.5) | 0.85 |
| Alcohol intake,5 g/d | 11.5 (10.7, 12.2) | 10.9 (9.8, 12.1) | 10.6 (8.9, 12.4) | 10.1 (8.1, 12.0) | 0.13 |
| Multivitamin user,4 % | 47.8 (45.3, 50.3) | 49.8 (45.8, 53.7) | 49.2 (43.3, 55.2) | 43.6 (37.0, 50.2) | 0.27 |
| Physical activity score4 | 37.6 (37.2, 38.0) | 37.2 (36.6, 37.8) | 37.2 (36.3, 38.0) | 37.3 (36.3, 38.2) | 0.48 |
| Education level,4,6 % | 48.8 (46.3, 51.3) | 47.9 (44.0, 51.9) | 47.2 (41.3, 53.2) | 44.7 (38.1, 51.3) | 0.24 |
| Antihypertensive drugs,4 % | 14.2 (12.4, 15.9) | 15.3 (12.5, 18.1) | 19.4 (15.2, 23.6) | 25.6 (21.0, 30.3) | <0.001 |
| Hypertension,4 % | 24.3 (22.2, 26.4) | 25.4 (22.1, 28.7) | 27.4 (22.4, 32.4) | 32.7 (27.2, 38.3) | 0.005 |
| Lipid-lowering drugs,4 % | 6.8 (5.5, 8.1) | 7.4 (5.3, 9.5) | 9.5 (6.3, 12.6) | 9.3 (5.8, 12.8) | 0.11 |
| Dyslipidemia,4 % | 42.0 (39.6, 44.5) | 45.0 (41.1, 48.9) | 43.6 (37.7, 49.5) | 50.6 (44.1, 57.1) | 0.02 |
| Fasting plasma glucose,4 mmol/L | 5.3 (5.3, 5.3) | 5.3 (5.3, 5.3) | 5.3 (5.2, 5.3) | 5.4 (5.3, 5.5) | 0.01 |
| Impaired fasting glucose,4 % | 26.0 (23.9, 28.1) | 24.9 (21.5, 28.3) | 25.0 (19.9, 30.1) | 31.5 (25.9, 37.2) | 0.11 |
| Energy intake,4 kcal/d | 1980 (1950, 2020) | 1970 (1920, 2020) | 1990 (1920, 2070) | 2010 (1920, 2090) | 0.57 |
| Fat,4 %EI | 31.5 (31.2, 31.8) | 31.6 (31.1, 32.1) | 32.3 (31.5, 33.0) | 33.0 (32.1, 33.8) | <0.001 |
| Carbohydrate,4 %EI | 48.2 (47.8, 48.7) | 47.9 (47.2, 48.6) | 47.0 (46.0, 48.1) | 46.5 (45.3, 47.6) | 0.002 |
| Total sugar,4 %EI | 22.5 (22.2, 22.9) | 21.8 (21.2, 22.4) | 21.5 (20.6, 22.4) | 20.7 (19.7, 21.7) | <0.001 |
| Dietary fiber,5,7 g/d | 17.1 (16.9, 17.4) | 17.5 (17.0, 17.9) | 17.7 (17.1, 18.4) | 17.4 (16.7, 18.2) | 0.37 |
| Whole grains,5,7 g/d | 18.3 (17.6, 19.1) | 19.5 (18.2, 20.8) | 18.4 (16.6, 20.3) | 16.7 (14.9, 18.6) | 0.10 |
| Fruits,5,7 g/d | 207 (198, 217) | 218 (203, 235) | 211 (189, 236) | 193 (171, 218) | 0.30 |
| Vegetables,5,7 g/d | 190 (185, 196) | 192 (183, 201) | 203 (190, 218) | 197 (182, 212) | 0.25 |
| Red meat,5,7 g/d | 43.4 (41.3, 45.5) | 45.8 (42.4, 49.4) | 49.7 (44.3, 55.7) | 52.6 (46.3, 59.7) | 0.002 |
| Nuts,5 g/d | 5.0 (4.5, 5.5) | 4.3 (3.5, 5.1) | 4.8 (3.6, 6.0) | 5.5 (4.2, 6.9) | 0.44 |
| Glycemic index5 | 53.6 (53.5, 53.8) | 53.6 (53.3, 53.9) | 53.2 (52.8, 53.7) | 53.0 (52.5, 53.5) | 0.01 |
| Glycemic load5 | 129 (128, 131) | 127 (125, 130) | 125 (122, 128) | 123 (119, 126) | <0.001 |
| Diet soda,5 servings/wk | 13.6 (12.7, 14.6) | 9.9 (8.4, 11.4) | 9.9 (7.6, 12.3) | 7.6 (5.0, 10.1) | <0.001 |
| DGAI5 | 8.8 (8.6, 8.9) | 8.7 (8.5, 8.9) | 8.8 (8.5, 9.1) | 8.5 (8.1, 8.8) | 0.14 |
Data are presented as means or geometric means and 95% CIs. The DGAI was modified by leaving out the added sugar component. DGAI: 2005 Dietary Guideline Adherence Index; %EI, percentage of energy intake.
Adjusted for sex.
Adjusted for age.
Values were adjusted for sex and age.
Values were adjusted for sex, age, and energy intake.
College or above.
Values are geometric means.
Spearman’s correlation coefficients between adiposity markers are presented in Table 3. In our primary analysis (Table 4), SSB consumption was marginally associated with lower BMI (P-trend = 0.05) but not associated with waist circumference (P-trend = 0.32). No association was observed between SSB intake and VAT (P-trend = 0.11), but the association became significant (P-trend < 0.001) after additional adjustment for SAT. Adjusted mean volume of VAT was 1640 and 1800 cm3 in SSB nonconsumers and daily consumers, respectively. In contrast to our hypothesis, SSB intake was inversely associated with SAT after additional adjustment for VAT (P-trend < 0.001). In the VAT-adjusted model, SAT was 3010 and 2650 cm3 in SSB nonconsumers and daily consumers, respectively. A significant positive association was observed between SSBs and the VAT-to-SAT ratio (P-trend < 0.001). The geometric mean VAT-to-SAT ratio was 0.54 and 0.62 in SSB nonconsumers and daily consumers. The sensitivity analysis adjusting for individual foods yielded similar results as the primary analyses with the exception that the association between SSB intake and BMI became nonsignificant (P-tend = 0.22). Furthermore, excluding energy intake from the models or including the glycemic load or previously diagnosed CVDs did not substantially change the observed associations.
TABLE 3.
Spearman’s correlation coefficients between markers of adiposity in 2596 adults1
| BMI (kg/m2) | Waist (cm) | VAT (cm3) | SAT (cm3) | |
| Waist | 0.89 | |||
| VAT | 0.73 | 0.81 | ||
| SAT | 0.78 | 0.74 | 0.50 | |
| VAT:SAT ratio | 0.16 | 0.28 | 0.66 | −0.25 |
All P values < 0.001. SAT, abdominal subcutaneous adipose tissue; VAT, visceral adipose tissue.
TABLE 4.
Association between SSB intake and abdominal adiposity in 2596 adults1
| Non-consumers |
Consumers |
P-interaction |
||||||
| 0 to <1/mo | ≥1/mo to <1/wk | ≥1/wk to <1/d | ≥1/d | P-trend | SSB × Sex | SSB × BMI | SSB × Age | |
| Median consumption, servings/wk | 0 | 1 | 4 | 10 | ||||
| Participants, n | 851 | 910 | 482 | 353 | ||||
| BMI, kg/m2 | ||||||||
| Model 1 | 27.8 (27.4, 28.1) | 27.2 (26.9, 27.5) | 27.5 (27.1, 27.9) | 26.9 (26.3, 27.4) | 0.05 | |||
| Waist circumference,2 cm | ||||||||
| Model 1 | 95.9 (95.0, 96.8) | 94.2 (93.4, 95.0) | 95.7 (94.5, 96.8) | 94.2 (92.8, 95.5) | 0.32 | 0.36 | 0.05 | 0.67 |
| VAT, cm3 | ||||||||
| Model 1 | 1700 (1640, 1750) | 1660 (1610, 1720) | 1830 (1760, 1910) | 1740 (1650, 1820) | 0.11 | |||
| Model 1 + SAT | 1640 (1600, 1690) | 1700 (1660, 1740) | 1830 (1770, 1880) | 1800 (1730, 1870) | <0.001 | 0.53 | 0.08 | 0.78 |
| SAT, cm3 | ||||||||
| Model 1 | 2990 (2900, 3090) | 2750 (2660, 2830) | 2850 (2730, 2970) | 2670 (2520, 2820) | 0.02 | |||
| Model 1 + VAT | 3010 (2940, 3080) | 2800 (2730, 2870) | 2730 (2640, 2820) | 2650 (2540, 2770) | <0.001 | 0.94 | <0.001 | 0.65 |
| VAT:SAT ratio2 | ||||||||
| Model 1 | 0.54 (0.52, 0.55) | 0.56 (0.55, 0.58) | 0.60 (0.58, 0.62) | 0.62 (0.59, 0.64) | <0.001 | 0.83 | 0.15 | 0.28 |
Data are presented as means or geometric means and 95% CIs. Model 1 was adjusted for age, sex, energy intake, alcohol intake, diet soda intake, modified DGAI, educational level, physical activity level, smoking status, and Framingham cohort. DGAI, 2005 Dietary Guideline Adherence Index; SAT, abdominal subcutaneous adipose tissue; SSB, sugar-sweetened beverage; VAT, visceral adipose tissue.
Geometric means.
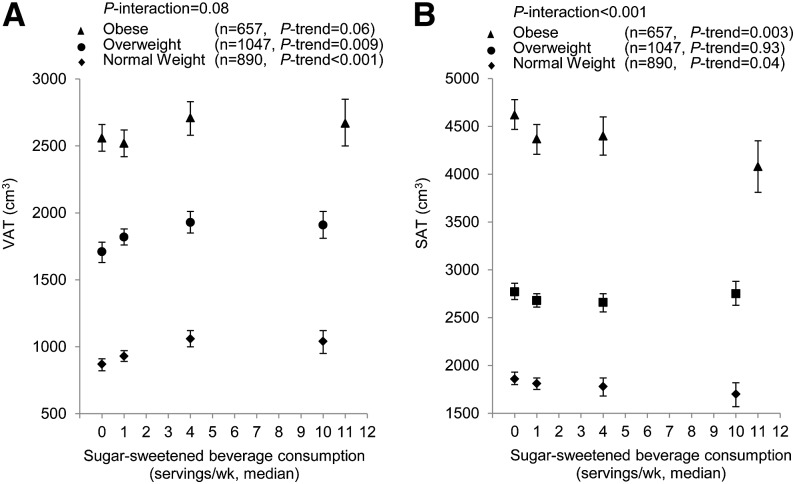
We observed no significant statistical interaction between SSB consumption and sex for all adiposity markers (Table 4). Sex-stratified results for associations between SSB intake and waist circumference, VAT, SAT, and the VAT-to-SAT ratio are displayed in Supplemental Table 1. The associations between SSB intake and VAT, SAT, and the VAT-to-SAT ratio in fully adjusted models were similar in men and women. The interaction term between SSB intake and BMI was significant for SAT (P-interaction < 0.001) and nonsignificant for VAT (P-interaction = 0.08). Nevertheless, BMI-stratified data for both VAT and SAT were presented in Figure 1. The BMI-stratified analysis showed a significant positive association between SSB intake and VAT both with and without adjustment for SAT in normal-weight and overweight participants. In obese individuals, we observed that SSB consumers tended to have larger VAT in both models, but the associations were not statistically significant. In all strata, SSB consumers had lower SAT compared with nonconsumers; however, significant associations were observed only in normal-weight (BMI < 25 kg/m2) and obese (BMI ≥ 30 kg/m2) participants. We observed no significant interaction between SSB intake and BMI for the VAT-to-SAT ratio. The association between SSB intake and the VAT-to-SAT ratio was significant in all BMI strata. There was no significant interaction observed between SSB intake and age for markers of abdominal adiposity (Table 4).
FIGURE 1.
BMI-stratified association between sugar-sweetened beverage intake and VAT and SAT. Symbols are means and 95% CIs. BMI-stratified association for VAT (A). Models were adjusted for age, sex, energy intake, alcohol intake, diet soda intake, modified DGAI, educational level, physical activity level, smoking status, Framingham cohort, and SAT. BMI-stratified association for SAT (B). Same multiple regression model as for VAT was used, except for adjustment for VAT instead of SAT. DGAI, Dietary Guidelines Adherence Index; SAT, abdominal subcutaneous adipose tissue; VAT, visceral adipose tissue.
Finally, a positive association was observed between diet soda consumption and BMI, waist circumference, VAT, and SAT (Table 5). After adjusting for SAT, the association between diet soda intake and VAT was attenuated and no longer statistically significant (P-trend = 0.06). The significant direct association between diet soda and SAT was maintained after additional adjustment for VAT. No significant association was observed between diet soda intake and the VAT-to-SAT ratio. We observed a significant interaction between diet soda intake and sex for VAT and SAT (P-interaction = 0.01 and 0.002, respectively). In a sex-stratified analysis (Supplemental Table 2), the association between diet soda intake and VAT was marginally significant in men (P-trend = 0.05) but not in women (P-trend = 0.74). In contrast, the association between diet soda and SAT was stronger in women compared with that in men; however, the positive associations between diet soda intake and SAT were significant in both sexes. We observed no significant interaction between diet soda intake and age or BMI for abdominal adiposity markers.
TABLE 5.
Association between diet soda intake and abdominal adiposity in 2596 adults1
| Non-consumers |
Consumers |
P-interaction |
||||||
| 0 to <1/mo | ≥1/mo to <1/wk | ≥1/wk to <1/d | ≥1/d | P-trend | Diet soda × Sex | Diet soda × BMI | Diet soda × Age | |
| Median consumption, servings/wk | 0 | 1 | 4 | 11 | ||||
| Participants, n | 1520 | 599 | 262 | 215 | ||||
| BMI, kg/m2 | ||||||||
| Model 1 | 26.8 (26.5, 27.0) | 28.1 (27.7, 28.4) | 27.7 (27.1, 28.3) | 29.7 (29.1, 30.4) | <0.001 | |||
| Waist circumference,2 cm | ||||||||
| Model 1 | 93.7 (93.1, 94.3) | 96.2 (95.2, 97.2) | 96.3 (94.8, 97.8) | 100.0 (98.3, 101.7) | <0.001 | 0.20 | 0.27 | 0.52 |
| VAT, cm3 | ||||||||
| Model 1 | 1640 (1610, 1680) | 1750 (1690, 1820) | 1820 (1730, 1920) | 2000 (1890, 2100) | <0.001 | |||
| Model 1 + SAT | 1700 (1670, 1730) | 1710 (1660, 1760) | 1800 (1730, 1880) | 1760 (1680, 1850) | 0.06 | 0.01 | 0.99 | 0.04 |
| SAT, cm3 | ||||||||
| Model 1 | 2690 (2620, 2750) | 2960 (2860, 3070) | 2890 (2740, 3050) | 3470 (3300, 3640) | <0.001 | |||
| Model 1 + VAT | 2760 (2710, 2810) | 2930 (2840, 3010) | 2780 (2660, 2910) | 3190 (3050, 3330) | <0.001 | 0.002 | 0.07 | 0.10 |
| VAT-to-SAT ratio2 | ||||||||
| Model 1 | 0.57 (0.56, 0.59) | 0.56 (0.54, 0.57) | 0.58 (0.55, 0.60) | 0.55 (0.52, 0.58) | 0.18 | 0.93 | 0.72 | 0.18 |
Data are presented as means or geometric means and 95% CIs. Model 1 was adjusted for age, sex, energy intake, alcohol intake, SSB intake, modified DGAI, educational level, physical activity level, smoking status, and Framingham cohort. DGAI, Dietary Guidelines Adherence Index; SAT, abdominal subcutaneous adipose tissue; SSB, sugar-sweetened beverage; VAT, visceral adipose tissue.
Geometric means.
Discussion
In this cross-sectional analysis examining a large sample of middle-aged adults, we observed that VAT was greater in adults who consumed SSBs daily after accounting for SAT, whereas SAT was lower compared with nonconsumers. Furthermore, daily SSB consumption was positively associated with the VAT-to-SAT ratio. In contrast, diet soda consumption was positively associated with BMI, waist circumference, and SAT. Diet soda intake was also positively associated with VAT in men but not in women. No significant association was observed between diet soda intake and the VAT-to-SAT ratio.
In a previous cross-sectional analysis, Odegaard et al. (12) observed that absolute VAT volume tended to be greater in adult SSB consumers compared with those who rarely consumed SSBs. However, the association did not reach significance, perhaps as a result of insufficient statistical power. In the present study, when holding SAT constant, we observed that daily habitual SSB intake of ≥1 servings of SSB was associated with a greater absolute volume of VAT.
Our cross-sectional observation is supported by results from some recent intervention trials (11, 26). In a 10-wk intervention study, 25% of daily energy required was consumed as liquid fructose or liquid glucose (26). In this study, fructose intake substantially increased the volume of VAT, whereas intake of glucose had the effect of increasing SAT (26). A recent randomized intervention study showed that fat accumulation was greater in VAT (23% or 25 cm3 increase) relative to SAT (5% or 14 cm3 increase) after daily consumption of 1 L of sucrose-sweetened cola for 6 mo and that the VAT-to-SAT ratio increased 18% over this time period (11). In contrast, isocaloric consumption of milk was associated with increased SAT but decreased VAT over the 6 mo intervention period.
Several mechanisms may explain the possible relation between SSB intake and abdominal fat partitioning. However, given the cross-sectional nature of this study, the interpretation of the mechanism behind these observed findings in this study is speculative. Fructose was linked to increased postprandial circulating TGs (27, 28). In addition, fructose consumption may enhance deposition of TGs in VAT. Accumulating fat in adipose tissue is mediated by the activation of lipoprotein lipase (LPL), the rate-limiting enzyme involved in the uptake of TG from the circulation to the storage in the adipose tissue, which is regulated by insulin (29). In normal circumstances, TG deposition in SAT is more efficient than VAT because LPL in SAT is more sensitive to insulin than LPL in VAT (30). In the case of insulin resistance, a greater proportion of circulating TG may be deposited in VAT (4). It was proposed that increased fructose consumption from SSBs results in hepatic fat accumulation, leading to hepatic and peripheral insulin resistance (26). An alternative explanation is that fructose may have a direct effect on adipocytes by promoting intracellular activation of glucocorticoids (31), which activate LPL activity. Supporting this mechanism is the higher concentration of glucocorticoid receptors in VAT compared with SAT (30). Furthermore, as reviewed by Tchernof and Despres (32), both animal and human studies suggest that increased activity of the type 1 11β-hydroxysteroid dehydrogenase, a key enzyme catalyzing the local conversion of inert cortisone to active cortisol, is associated with increased VAT because VAT is rich in glucocorticoid receptors.
The greater VAT-to-SAT ratio in SSB consumers may also represent a potential pathologic alteration in SAT. The lower absolute volume in SAT observed among SSB consumers in the present study may suggest that high SSB consumption alters the development of SAT. It is possible that less fat accumulation in SAT may be due to fat being directly channeled to VAT, as described above. It is also possibly due to the dysfunctional SAT, i.e., the inability of SAT to accommodate more fat derived from excess SSB consumption (4). In this case, lipid flux is channeled to VAT due to decreased capacity in SAT. Although the underlying pathways are not fully understood, the dysfunctional SAT may be due to the inability of normal proliferation and differentiation of adipocytes (33). Multiple factors are involved in abdominal fat distribution (3), including a genetic predisposition (34). Recent genome-wide association studies identified several genetic loci that are associated with greater VAT (35, 36). However, how genetic variation in enzymes, such as LPL or those involved in glucocorticoid action, may trigger dysfunctional SAT or interact with SSB intake to alter regional fat distribution is unknown.
Although we observed a direct association between diet soda intake and BMI, waist circumference, and SAT, such associations are likely confounded by the greater use of diet beverages by overweight and obese individuals as a consequence of their increased adiposity. As reviewed by Malik et al. (37), prospective studies with longer follow-up periods indicate that diet soda consumption is unlikely to be associated with body weight. Diet soda provides no calories, and whether artificial sweeteners in diet soda stimulate appetite remains inconclusive (38). Thus, the biologic plausibility linking diet soda and body weight remains to be determined.
The strengths of our study include the use of comprehensive dietary, lifestyle, and clinical data collected in a well-powered subgroup of the Framingham Heart Study, as well as adipose tissue data that was measured using a highly precise technique. MDCT-derived quantitative data of abdominal fat are both highly reproducible and highly specific. With respect to limitations, the cross-sectional and observational design of this study limits our ability to infer temporality or causality between beverage consumption and adiposity. It is possible that both associations between SSB intake and VAT and the VAT-to-SAT ratio and between diet soda intake and BMI, waist circumference, and SAT could be due to confounding, therefore tempering the conclusions that can be formed. Therefore, our observations are more hypothesis-generating rather than etiologic. Future prospective studies measuring the long-term change of abdominal fat distribution may help to establish the temporal relation that excess SSB intake is preferentially associated with fat accumulation in VAT rather than SAT. Gluteofemoral adipose tissue may affect body fat partitioning (39); however, lower-body adipose tissue was not measured in this study. Misclassification of our dietary assessments including SSBs and diet soda intake may attenuate our results. The consumption of artificially sweetened noncarbonated beverages was not captured using the FFQ. However, diet soda is likely to be the major beverage consumed containing artificial sweeteners and is estimated to account for ∼90% of aspartame used in all foods (40). In addition, although we adjusted for a variety of dietary and lifestyle factors, residual confounding cannot be ruled out. In addition, the majority of our study population is middle-aged and Caucasian, which may minimize confounding from race/ethnicity and socioeconomic factors but limit the generalizability of results to other populations.
In conclusion, regular consumption of SSBs is associated with greater visceral fat in absolute volume and distribution relative to SAT. Although these observational data provide additional evidence to support the association between daily SSB consumption and increased cardiometabolic risk (37), well-powered prospective cohort studies and metabolically controlled intervention trials are required to examine how SSB intake may influence body fat distribution and its underlying mechanisms. Moreover, although diet soda intake was not associated with abdominal fat partitioning in this study, additional studies on the role of these beverages in body weight and cardiometabolic health are warranted.
Supplementary Material
Acknowledgments
The authors thank Adela Hruby and Kara Livingston (Nutrition Epidemiology Department, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University) for editing the manuscript. N.M.M. designed the analysis; J.M., M.S., and G.T.R. analyzed the data; J.M. wrote the manuscript; C.S.F. and U.H. provided the measures of adiposity; N.M.M., C.S.F., P.F.J., C.E.S., and E.S. provided critical editorial comments; N.M.M. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CVD, cardiovascular disease; DGAI, Dietary Guidelines Adherence Index; Gen3, Third Generation Cohort; LPL, lipoprotein lipase; MDCT, multidetector computed tomography; SAT, abdominal subcutaneous adipose tissue; SSB, sugar-sweetened beverage; VAT, visceral adipose tissue.
References
- 1.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Haring HU. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 2008;168:1609–16. [DOI] [PubMed] [Google Scholar]
- 2.Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation 2011;124:e837–41. [DOI] [PubMed] [Google Scholar]
- 3.Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation 2012;126:1301–13. [DOI] [PubMed] [Google Scholar]
- 4.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–7. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab 2011;96:E1756–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care 2009;32:1068–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaess BM, Pedley A, Massaro JM, Murabito J, Hoffmann U, Fox CS. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia 2012;55:2622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S, Cho B, Lee H, Choi K, Hwang SS, Kim D, Kim K, Kwon H. Distribution of abdominal visceral and subcutaneous adipose tissue and metabolic syndrome in a Korean population. Diabetes Care 2011;34:504–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welsh JA, Sharma AJ, Grellinger L, Vos MB. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr 2011;94:726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr 2013;98:1084–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maersk M, Belza A, Stodkilde-Jorgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen SB, Astrup A, Richelsen B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr 2012;95:283–9. [DOI] [PubMed] [Google Scholar]
- 12.Odegaard AO, Choh AC, Czerwinski SA, Towne B, Demerath EW. Sugar-sweetened and diet beverages in relation to visceral adipose tissue. Obesity (Silver Spring) 2012;20:689–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979;110:281–90. [DOI] [PubMed] [Google Scholar]
- 14.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB, Sr, Fox CS, Larson MG, Murabito JM, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol 2007;165:1328–35. [DOI] [PubMed] [Google Scholar]
- 15.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007;116:39–48. [DOI] [PubMed] [Google Scholar]
- 16.Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O'Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond) 2007;31:500–6. [DOI] [PubMed] [Google Scholar]
- 17.Speliotes EK, Massaro JM, Hoffmann U, Foster MC, Sahani DV, Hirschhorn JN, O’Donnell CJ, Fox CS. Liver fat is reproducibly measured using computed tomography in the Framingham Heart Study. J Gastroenterol Hepatol 2008;23:894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26, discussion 1127–36. [DOI] [PubMed] [Google Scholar]
- 19.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6. [DOI] [PubMed] [Google Scholar]
- 20.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 21.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Belanger A, D’Agostino R, Israel I. Physical activity and physical demand on the job and risk of cardiovascular disease and death: the Framingham Study. Am Heart J 1986;112:820–5. [DOI] [PubMed] [Google Scholar]
- 23.Fogli-Cawley JJ, Dwyer JT, Saltzman E, McCullough ML, Troy LM, Jacques PF. The 2005 Dietary Guidelines for Americans Adherence Index: development and application. J Nutr 2006;136:2908–15. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2012;35(Suppl 1):S64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 2013;127:e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanhope KL, Havel PJ. Fructose consumption: potential mechanisms for its effects to increase visceral adiposity and induce dyslipidemia and insulin resistance. Curr Opin Lipidol 2008;19:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallfrisch J. Metabolic effects of dietary fructose. FASEB J 1990;4:2652–60. [DOI] [PubMed] [Google Scholar]
- 29.Mead JR, Irvine SA, Ramji DP. Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med (Berl) 2002;80:753–69. [DOI] [PubMed] [Google Scholar]
- 30.Fried SK, Russell CD, Grauso NL, Brolin RE. Lipoprotein lipase regulation by insulin and glucocorticoid in subcutaneous and omental adipose tissues of obese women and men. J Clin Invest 1993;92:2191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senesi S, Legeza B, Balazs Z, Csala M, Marcolongo P, Kereszturi E, Szelenyi P, Egger C, Fulceri R, Mandl J, et al. Contribution of fructose-6-phosphate to glucocorticoid activation in the endoplasmic reticulum: possible implication in the metabolic syndrome. Endocrinology 2010;151:4830–9. [DOI] [PubMed] [Google Scholar]
- 32.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013;93:359–404. [DOI] [PubMed] [Google Scholar]
- 33.Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord 2004;28(Suppl 4):S12–21. [DOI] [PubMed] [Google Scholar]
- 34.Bouchard C, Tremblay A, Despres JP, Nadeau A, Lupien PJ, Theriault G, Dussault J, Moorjani S, Pinault S, Fournier G. The response to long-term overfeeding in identical twins. N Engl J Med 1990;322:1477–82. [DOI] [PubMed] [Google Scholar]
- 35.Fox CS, Liu Y, White CC, Feitosa M, Smith AV, Heard-Costa N, Lohman K, Consortium G, Consortium M, Consortium G, et al. Genome-wide association for abdominal subcutaneous and visceral adipose reveals a novel locus for visceral fat in women. PLoS Genet 2012;8:e1002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norris JM, Langefeld CD, Talbert ME, Wing MR, Haritunians T, Fingerlin TE, Hanley AJ, Ziegler JT, Taylor KD, Haffner SM, et al. Genome-wide association study and follow-up analysis of adiposity traits in Hispanic Americans: the IRAS Family Study. Obesity (Silver Spring) 2009;17:1932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010;121:1356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr 2009;89:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010;34:949–59. [DOI] [PubMed] [Google Scholar]
- 40.Schernhammer ES, Bertrand KA, Birmann BM, Sampson L, Willett WC, Feskanich D. Consumption of artificial sweetener- and sugar-containing soda and risk of lymphoma and leukemia in men and women. Am J Clin Nutr 2012;96:1419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.