Abstract
The objective of the Biomarkers of Nutrition for Development (BOND) project is to provide state-of-the-art information and service with regard to selection, use, and interpretation of biomarkers of nutrient exposure, status, function, and effect. Specifically, the BOND project seeks to develop consensus on accurate assessment methodologies that are applicable to researchers (laboratory/clinical/surveillance), clinicians, programmers, and policy makers (data consumers). The BOND project is also intended to develop targeted research agendas to support the discovery and development of biomarkers through improved understanding of nutrient biology within relevant biologic systems. In phase I of the BOND project, 6 nutrients (iodine, vitamin A, iron, zinc, folate, and vitamin B-12) were selected for their high public health importance because they typify the challenges faced by users in the selection, use, and interpretation of biomarkers. For each nutrient, an expert panel was constituted and charged with the development of a comprehensive review covering the respective nutrient’s biology, existing biomarkers, and specific issues of use with particular reference to the needs of the individual user groups. In addition to the publication of these reviews, materials from each will be extracted to support the BOND interactive Web site (http://www.nichd.nih.gov/global_nutrition/programs/bond/pages/index.aspx). This review represents the first in the series of reviews and covers all relevant aspects of iodine biology and biomarkers. The article is organized to provide the reader with a full appreciation of iodine’s background history as a public health issue, its biology, and an overview of available biomarkers and specific considerations for the use and interpretation of iodine biomarkers across a range of clinical and population-based uses. The review also includes a detailed research agenda to address priority gaps in our understanding of iodine biology and assessment.
Background: Overview of Iodine Nutrition
Introduction
Iodine is an essential nutrient due to its role as a component of thyroid hormones and because it must be obtained via exogenous/dietary sources. Unlike most other essential dietary nutrients, iodine status is not linked to socioeconomic development but rather to geography. The iodine content of local foods is dependent on soil iodine content and therefore low iodine concentrations in soil and water will result in iodine-deficient plants and animals.
Although iodine (as iodide) is present in soils, the content may fluctuate widely within and across regions as a result of a number of factors (e.g., differences that occurred during geological formation, impact of glaciation, flooding, and soil erosion). In the ocean, iodide is converted into elemental iodine, a volatile form that is carried into the atmosphere as part of an ecological cycle, returning it to the land through rain and snow. However, this cycle is slow and inconsistent in its impact/redistribution and is incapable of adequately replenishing soils and freshwater otherwise poor in iodine (1). Consequently, humans dependent on food sources grown in iodine-deplete or -deficient areas become iodine deficient in the absence of any other source of the nutrient. In such areas, iodine can only enter the food supply through deliberate efforts such as the iodization of salt (2). A summary of available sources of iodine is found in Table 1.
TABLE 1.
Dietary sources of iodine
| • Iodine is naturally low in most foods and beverages. Generally, common food sources provide 3–80 μg/serving (3, 4), but the content largely depends on the food’s origin and is usually insufficient to meet daily requirements. |
| • Iodine content is relatively high in most saltwater fish and seafood because of their ability to concentrate iodine from seawater. However, they do not contribute substantially to dietary iodine intake unless consumed regularly (5–7). Iodine content in some seaweed is relatively high (8), and the population consuming seaweed may obtain high iodine concentrations through their diet (9–11). Sometimes, iodine can also be obtained through drinking water drawn from certain aquifers or water disinfected with iodine (9, 12, 13). |
| • Iodine from household salt and milk consumption: Iodized salt used for cooking and at the table in households continues to be the major source of iodine in many countries around the world (5, 8, 9, 14). According to label declarations, the iodine content in commercially available salt ranges from 15–80 mg iodine/kg salt (5, 9, 14–16). In addition to iodized salt, milk and other dairy products are good sources of iodine (9), especially in children, in countries such as the United States, Canada, and Switzerland (3, 5, 7, 17, 18). Although naturally low, the iodine content in fortified winter cattle fodder and perhaps the iodine residues in milk from the disinfecting agents (iodophors) used in dairying contribute to dairy iodine (3, 19–22). Some countries such as the United Kingdom and Norway do not have regulation of iodized salt, and in those countries adventitious iodine in the milk is the main source (7, 9, 23). |
| • Iodine in processed foods: The salt used in processed foods contributes to ∼60–80% of total salt intake in industrialized countries (9, 24–26). In a typical Western diet, processed foods such as bread, dairy products, and processed meats provide the main salt source, which may or may not be iodized (9, 27–29). Although processing would entail boiling, baking, and canning of foods manufactured with iodated salt, it results in minimal loss (generally ≤10%) in iodine content (8, 30). |
| • Iodine from dietary supplements: Based on data from the NHANES 1996–2006, 18.5% of nonpregnant and 22% of pregnant women in the United States used supplements that contain iodine; for adults, the median iodine intake from supplements was ∼144 μg/d (8, 31). |
Role in health and disease
Iodine deficiency and related disorders.
Among the roles of thyroid hormones in human health are the regulation of numerous physiologic processes, including growth, key aspects of neurologic development, and reproductive function. During the first trimester of pregnancy, maternal thyroid hormone crosses the placenta to serve as the primary source of this essential hormone to the fetus prior to the development of its own functional thyroid and may account for up to 20–40% of cord blood thyroid hormone at birth (32).
Known and potential roles of thyroid hormones and iodine are listed in Table 2. The numerous effects of iodine deficiency on growth and development are known collectively known as iodine deficiency disorders (IDDs)16 (Table 3). Resulting fundamentally from a deficiency of thyroid hormone, the constellation of adverse effects associated with IDD represents some of the most important and common human diseases (35).
TABLE 2.
Thyroid hormone functions
| • Integral for brain development and influences cell growth and migration (33) |
| • Important for growth and maturation |
| • Elevates energy metabolism and increases the basal metabolic rate |
| Other suggested functions for iodine include the following: |
| • Potential link to fibrocystic breast disease |
| • May be important for the immune response |
| • May alter gastric cancer risk (31, 34) |
TABLE 3.
Iodine deficiency disorders by age group
| Age group | Health consequences of iodine deficiency |
| All ages | Goiter |
| Increased susceptibility of the thyroid gland to nuclear radiation | |
| Fetuses | Abortion |
| Stillbirth | |
| Congenital anomalies | |
| Perinatal mortality | |
| Neonates | Infant mortality |
| Endemic cretinism | |
| Children and adolescents | Impaired mental function |
| Delayed physical development | |
| Adults | Impaired mental function |
| Reduced work productivity | |
| Toxic nodular goiter; iodine-induced hyperthyroidism | |
| Increased occurrence of hypothyroidism in moderate-to-severe iodine deficiency; increased occurrence of hypothyroidism in mild-to-moderate iodine deficiency |
Many regions most affected by iodine-depleted soils are also among the most heavily populated (Table 4), resulting in a large at-risk population for deficiency. Before the introduction of salt iodization programs or other measures to correct iodine deficiency, cretinism was frequent and goiter affected up to 80% of children in severely deficient areas (38) and likely affected other population groups. The classical manifestation of iodine deficiency is an enlargement of the thyroid referred to as goiter. The sequence of events and clinical manifestations associated with goiter include
TABLE 4.
Regions with low soil iodine1
| • Asia, including parts of China, India, Bangladesh, the Himalayan hillsides, and Indonesia |
| • Africa, including the mountains of Morocco and Algeria (e.g., Atlas Mountains); much of west and central Africa (e.g., Nigeria, Cameroon, the Central African Republic, Democratic Republic of Congo), and some areas of East Africa (e.g., Uganda, Ethiopia) |
| • Europe, including the European Alps and the Pyrenees, inland areas of England and Wales, Greece, and The Netherlands |
| • South America, including the Andes and inland Brazil |
| • Midwestern United States |
| • Southern Australia |
| • Highlands of New Guinea |
increased secretion of thyroid-stimulating hormone (TSH), representing an effort to maximize thyroid uptake of diminishing sources of iodine;
development of diffuse, homogeneous thyroid enlargement in early stages and fusion of thyroid follicles, which become encapsulated, leading to a condition termed nodular goiter during later stages (35); and
physical problems created by large goiters, including obstruction of the trachea and esophagus and damage to the recurrent laryngeal nerves, which can lead to hoarseness; surgery to reduce goiter has substantial risks, including bleeding, nerve damage, and hypothyroidism consequent to removal of thyroid tissue (32).
In adults with mild-moderate iodine deficiency, an increased risk exists for diffuse goiter, nodular goiter, and accompanying hyperthyroidism, as well as the potential for secondary neurologic impairment (e.g., decreased work capacity, physical endurance, and cognitive ability) (40). In iodine-deficient individuals, particularly children, thyroidal radioactive iodine uptake after exposures from nuclear accidents is high, resulting in an increased risk of thyroid cancer, compared with iodine-sufficient individuals.
Iodine and child growth and development.
Although goiter remains the cardinal sign of iodine deficiency, IDD in children has many other manifestations including subclinical hypothyroidism associated with a more atherogenic lipid profile that may be associated with increased cardiovascular disease risk (41, 42). But the most serious consequences of iodine deficiency are associated with its impact on infant/child growth and development. Iodine status may influence growth through its effects on the thyroid axis. Putative mechanisms for the impact of iodine deficiency on growth have been suggested and may be associated with a decrease in insulin-like growth factor (IGF) 1 and IGF binding protein (IGFBP) 3 concentrations (43). The role of iodine in growth and the prophylactic effect of iodine interventions have been well documented (44).
Iodine deficiency during pregnancy has substantial consequences, with the most severe outcome being cretinism and short stature. The characteristics of the 2 types of cretinism are listed in Table 5. Additional adverse outcomes of severe iodine deficiency during pregnancy include increased risk of stillbirths, abortions, and congenital abnormalities (35).
TABLE 5.
Types and characteristics of cretinism1
| Neurologic cretinism is characterized by the following: |
| • Growth retardation |
| • Severe mental retardation with squint |
| • Deaf-mutism |
| • Motor spasticity |
| Myxedematous cretinism is characterized by the following: |
| • Growth retardation |
| • Less severe mental retardation when compared with neurologic cretinism |
| • Other clinical manifestations of hypothyroidism and delayed sexual maturation |
Globally, perhaps the most profound impact of iodine deficiency is its impact on neurologic development and related outcomes. Iodine deficiency is one of the most common causes of preventable mental retardation worldwide. Even in areas of mild-to-moderate iodine deficiency, cognitive impairment in school-age children is at least partially reversible by administration of iodine (49, 50). A meta-analysis of 18 studies concluded that moderate-to-severe iodine deficiency is associated with reductions in mean intelligence quotient (IQ) scores of 13.5 points (51). A more recent systematic review focusing on young children estimated that iodine deficiency in utero or during early childhood may reduce IQ scores by ∼8 IQ points (N. Aburto, personal communication, 2014).
Among the functions of thyroid hormones for the developing nervous system are normal neuronal migration and myelination of the brain. Low concentrations of thyroid hormone during the fetal stage and early infancy are associated with irreversible brain damage, including mental retardation and neurologic abnormalities (33). The key factors influencing the extent and magnitude of the neurologic complications are the timing and severity of the iodine deficiency and consequent thyroid hormone deficits. Subtle impairment of cognitive function is likely to occur even among offspring of pregnant women with mild or asymptomatic hypothyroidism (52, 53).
Consequences of iodine excess.
Iodine malnutrition includes not only insufficiency/deficiency but also excess. The putative mechanisms and causes by which iodine excess can affect thyroid function and effects have been described (54–58) and are highlighted in Table 6.
TABLE 6.
Mechanisms and causes of iodine excess1
| • Wolff and Chaikoff effect (59): high iodide exposure in rats has been shown to result in a transient inhibition of thyroid hormone synthesis lasting ∼24 h |
| ◦Mechanism: possibly via the generation of intrathyroidal iodolactones or iodolipids, which inhibit TPO activity |
| ◦Continued administration of iodide results in normal thyroid hormone synthesis referred to as “escape from the acute Wolff-Chaikoff effect” and may be caused by an inhibition of NIS synthesis, a reduction in intrathyroidal iodine, and a decrease in the iodine-induced inhibitors of hormone synthesis (57) |
| • The Jöd-Basedow phenomenon or iodine-induced hyperthyroidism represents a failure of the acute Wolff-Chaikoff effect, may occur in individuals with a history of nodular goiters caused by iodine deficiency |
| • Failure to “escape” from the acute Wolff-Chaikoff effect may also result in iodine-induced hypothyroidism |
| • Risk factors for iodine-induced hypothyroidism include underlying thyroid autoimmunity such as Hashimoto thyroiditis or a history of partial thyroidectomy |
| • Causes of iodine excess and related consequences might include the following: |
| ◦Exposure to amiodarone, an antiarrhythmic medication that is 37% iodine by weight, or after exposure to iodinated radiographic contrast agents (34) |
| ◦Ingestion of foods or supplements with very high iodine content, such as kelp, or where there is high iodine content in drinking water |
| ◦Transient increases in rates of hyperthyroidism have been reported in historically iodine-deficient regions with the initiation of salt iodization |
NIS, sodium/iodide symporter; TPO, thyroperoxidase.
Global efforts to address iodine malnutrition.
In 1952, a WHO technical group recommended iodization of all “food salt” in iodine-deficient areas (60). In the first global estimate of the prevalence of goiter, WHO estimated that 20–60% of the world’s population was at risk of IDD, with the highest risk in low- to middle-income countries (61). Despite the recognition that many countries were affected by goiter, the serious health implications of this problem were not widely accepted. Consequently, little attention and limited resources were devoted to addressing iodine deficiency in public health programs or policies. During the period of 1970–1990, as a result of emerging evidence from controlled trials in areas with endemic iodine insufficiency, this trend began to change. Aside from the predicted outcomes of these intervention studies, several other important functional consequences were highlighted. One of the important emerging areas was the recognition that iodine interventions improved cognitive function and thus had a clear benefit from an economic/development perspective (40). Iodine fortification also had ancillary benefits to humans because iodine fortification of salt for animal consumption improved the viability and quality of livestock. Iodine deficiency was thus shown to have social and economic consequences beyond the immediate benefits to human health. Table 7 provides an overview of some of the seminal events in recognition of iodine as an important medical and public health issue.
TABLE 7.
Seminal events in the history of iodine
| • Greeks (Galen) used sponge and seaweed as cures for swollen neck |
| • Italian physicians of the School of Salerno were the first to report the specific use of the sponge and dried seaweed to treat goiter |
| • 13th century: de Villanova cautioned that the effect of the sponge on goiter was limited; it could cure goiter of recent origin in young people but had only a modest effect on large, chronic goiters |
| • 1811: Courtois discovered iodine when he added sulfuric acid to burnt seaweed ash and produced an intense violet vapor that crystallized on cold surfaces |
| • Gay-Lussac subsequently identified this substance as a new element, and named it iodine from the Greek for “violet” |
| • 1813: Coindet, a physician in Geneva, Switzerland, hypothesized that the effectiveness of seaweed or sponges in treating goiter was due to their iodine content (62). He began giving oral iodine tinctures to goitrous patients and claimed his treatment was effective and safe |
| • Boussingault, working in the Andes, was the first to advocate prophylaxis with naturally iodine-rich salt to prevent goiter |
| • 1851: Chatin became the first to publish the hypothesis that iodine deficiency was the cause of goiter and subsequently proposed distributing iodized salt in the goitrous zones of France |
| • 1915–1918: Marine and Kimball introduced iodine supplements in the Midwest region of the United States to treat endemic goiter |
| • 1918: the Swiss physician Bayard conducted the first dose-response trial of iodine to treat goiter (63). Bayard established that as little as 30 μg of iodine daily had a clear beneficial effect on goiter |
| • 1922: the surgeon Eggenberger introduced iodized salt to prevent goiter and cretinism in northeastern Switzerland |
This changing view allowed iodine deficiency to be repositioned from a purely medical concern to one with a broader development perspective. The actualization of a global strategy was further aided by the coining of the more expansive term “iodine deficiency disorder,” rather than the traditional and limited reference to “goiter.” It was also noted that due to their relatively high biologic needs, pregnant women and young children are particularly susceptible. Consequently, the term “iodine deficiency disorder” more comprehensively covers those iodine-related conditions that affect almost 2 billion people worldwide (40, 64).
The potential of low-cost interventions to affect the health and development of billions of individuals made programs to address IDD politically and morally attractive. The fact that a program that costs between $0.02 and $0.05 per capita can result in such huge benefits both in humanitarian and economic terms continues to be a compelling impetus for the global health community (65).
By 1990, a handful of countries (United States, Australia, Canada, several Scandinavian countries, The Netherlands, and Switzerland) were considered to be iodine sufficient. Generally, salt is considered adequately iodized if iodine amounts are 15–40 mg/kg, which is based on the assumption that daily per capita intake of salt is in the range of 10 g. The key policy developments in a global effort to eliminate IDD are highlighted in Table 8. The number of countries with effective salt iodization as a safe, cost-effective, and sustainable strategy to ensure sufficient intake of iodine has increased (35) to the point that ∼70% of the world has access to adequately iodized salt (71, 72).
TABLE 8.
IDD Policy Development and Milestones1
| • 1990: the United Nations World Summit for Children and the World Health Assembly codified efforts to eliminate IDD |
| • 1991: the Conference on Ending Hidden Hunger adopted the ambitious goal of virtually eliminating IDD as a public health problem by the turn of the century (66–69) |
| • 1993: the WHO reaffirmed the utility of salt iodization and proclaimed this as the key strategy to achieving this goal (9, 69) |
| • 2007: the International Child Development Steering Group identified iodine deficiency as 1 of the 4 key global risk factors for impaired child development where the need for intervention remains urgent (70) |
IDD, iodine deficiency disorder.
Despite the local, regional, and global efforts to eradicate IDD, the prevalence remains high in many settings (Supplemental Fig. 1, Table 9), which provides an underpinning for why this continued monitoring is important and will set the stage for discussions on biomarkers. The following is an overview of the relevant features of iodine absorption, metabolism, and utilization.
TABLE 9.
Countries in 2013 that were mildly or moderately iodine deficient or had excessive iodine intakes based on median UICs in school-aged children1
| Moderately iodine deficient (median UIC: 20–49 μg/L) | Mildly iodine deficient (median UIC: 50–99 μg/L) | Excessive iodine intake (median UIC: >300 μg/L) |
| Afghanistan | Albania | Armenia |
| Algeria | Burundi | Benin |
| Angola | Democratic People‘s Republic of Korea | Brazil |
| Central African Republic | Estonia | Colombia |
| Ethiopia | Finland | Georgia |
| Gambia | Guatemala | Honduras |
| Ghana | Haiti | Paraguay |
| Papua New Guinea | Hungary | Somalia |
| Vanuatu | Ireland | Uganda |
| Italy | Uruguay | |
| Lebanon | ||
| Lithuania | ||
| Mali | ||
| Morocco | ||
| Mozambique | ||
| New Zealand | ||
| Russian Federation | ||
| Sudan | ||
| Ukraine | ||
| United Kingdom |
Countries are listed alphabetically in each category (34). School-aged children are defined as children aged 6–12 y. UIC, urinary iodine concentration.
Iodine Biology: Factors Affecting Iodine Digestion, Absorption, Metabolism, and Utilization
Absorption and metabolism
The absorption and metabolism of iodine has been well characterized and consists of the following key elements:
- To maintain the normal concentration of body iodine (10–20 mg of iodine, of which 70–80% is in the thyroid), humans require an exogenous source that may be provided in any of several chemical forms, each with unique absorptive or metabolic features:
- ◦ Iodate: the primary source of iodine for salt iodization, iodate is reduced to iodide and absorbed in the gut
- ◦ Organically bound iodine: most of organically bound iodine is digested to release the iodide for absorption; however, some forms, e.g., thyroxine (T4), can be absorbed intact (∼70% of an oral T4 dose) (74)
- Absorbed iodine is cleared from the circulation primarily by the thyroid and kidney:
- ◦ Renal iodine clearance is fairly constant
- Circulating iodine is rapidly turned over
- ◦ Under normal circumstances, plasma iodine has a half-life of ∼10 h
- ◦ During lactation, the mammary gland concentrates iodine and secretes it into breast milk to provide iodine for the newborn (78)
Iodine metabolism and homeostatic control
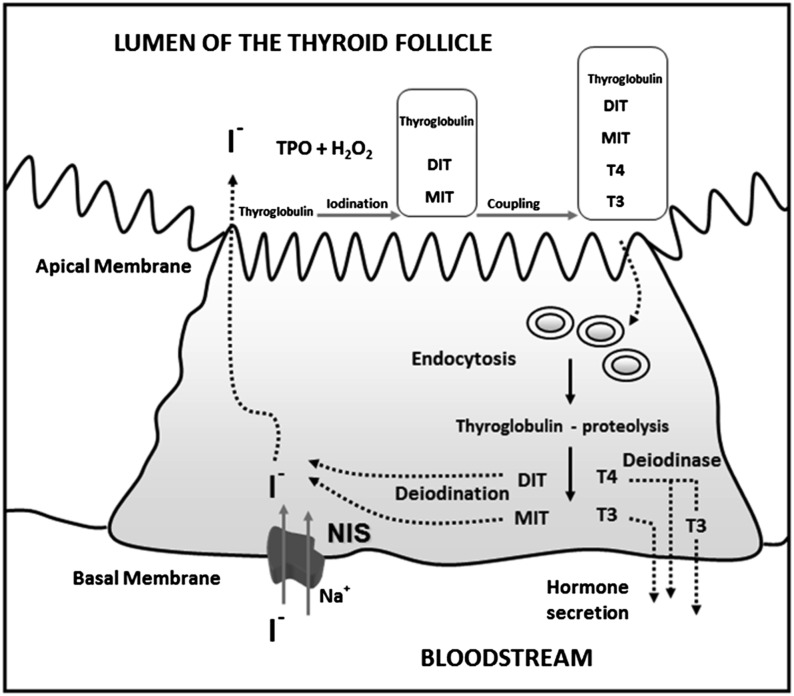
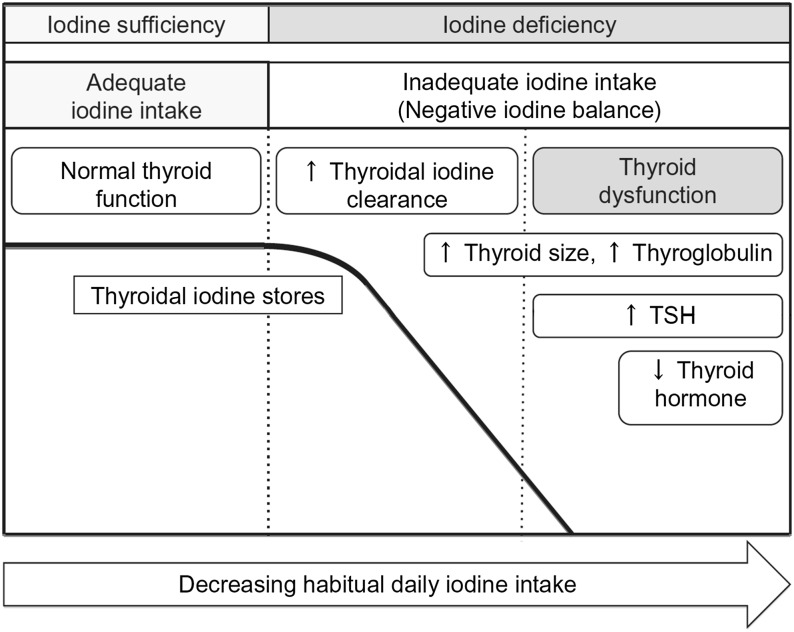
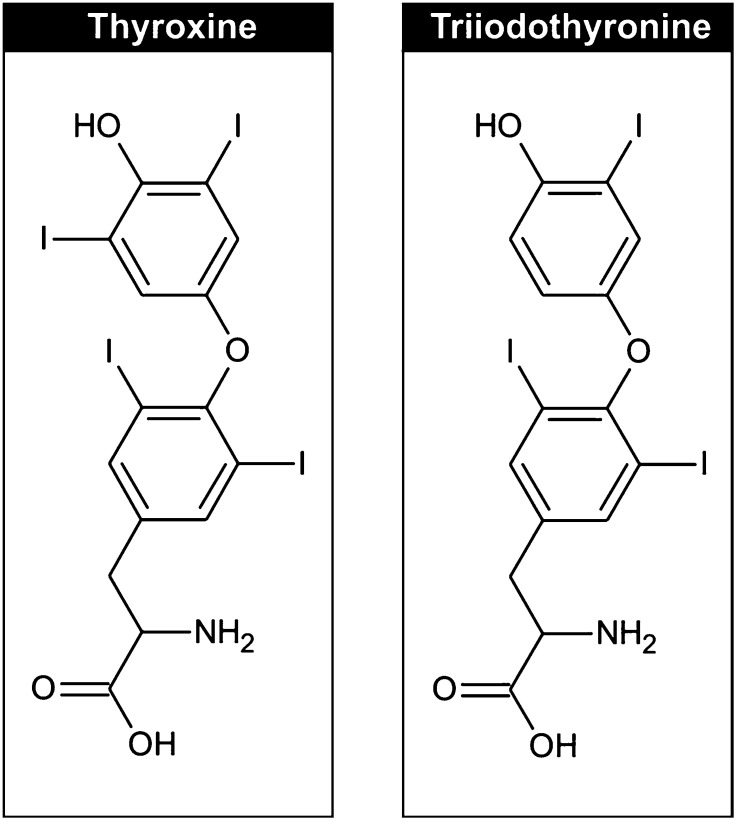
Understanding of iodine absorption, metabolism, homeostasis, and thyroid function was previously reviewed (62). For the purposes of this review, some essential components are highlighted in Table 10 and in Figures 1–3.
TABLE 10.
Thyroid hormone production and metabolism during iodine insufficiency and sufficiency1
| Mechanisms of normal thyroid hormone production and metabolism |
| • Under conditions of iodine adequacy, adults accumulate ∼60 μg iodine/d in the thyroid gland to account for losses and thyroid hormone production |
| • A transmembrane protein in the basolateral membrane of the thyrocyte, referred to as the NIS, is responsible for transferring iodide into the thyroid gland (79) (see Fig. 1) |
| • Production of thyroid hormone is a multistep process that includes: |
| ◦ Oxidation of iodide via the action of the enzymes TPO and hydrogen peroxide at the apical surface of the thyrocyte |
| ◦ The product is attached to tyrosyl residues on thyroglobulin to produce MIT and DIT |
| ◦ TPO then catalyzes the linkage of the phenyl groups of the iodotyrosines to form T4 (via linkage of 2 DIT molecules) and T3 (via linkage of an MIT and a DIT); T3 and T4 are therefore structurally identical except for an extra iodine |
| • Relevant features of T3/T4: |
| ◦ 65% and 59% of the weights of T4 and T3 is iodine (see Fig. 2) (62) |
| ◦ Thyroglobulin contains 0.1–1.0% of its weight as iodine and is stored extracellularly in the luminal colloid of the thyroid follicle |
| • Thyroglobulin that is digested undergoes endocytosis and subsequent digestion by endosomal and lysosomal proteases to release T4 and T3 into the circulation |
| • T4 and T3 are broken down in the periphery (the half-life of circulating T4 is 5–8 d; that of T3 is 1.5–3 d) |
| • The fate of the liberated iodine is to enter the plasma iodine pool for uptake by the thyroid or be excreted by the kidney |
| • More than 90% of ingested iodine is ultimately excreted in the urine (35), with only a small amount appearing in the feces |
| Mechanisms of response to dietary iodine insufficiency |
| • In non-pregnant, non-lactating adults, maintenance of intake above a threshold of ∼60 μg/d, even in the face of a decrease in circulating plasma inorganic iodine, allows the iodine content of the thyroid to remain within normal limits (∼10–20 mg) |
| • Below this threshold, despite high fractional clearance of plasma inorganic iodine, the iodine content of the thyroid is depleted, and the potential for development of goiter ensues |
| • Responses to low intake include: |
| ◦ Marked modification of thyroid activity, triggered by increased secretion of TSH by the pituitary (see Fig. 3) |
| ◦ Increased plasma iodine clearance by the thyroid; in otherwise healthy individuals, iodine intake below ∼100 μg/d results in increased TSH secretion resulting in increasing plasma inorganic iodide clearance by the thyroid through stimulation of NIS expression (8) |
| ◦ The increased iodine clearance by the thyroid leads to a progressive reduction in renal iodide excretion |
| ◦ TSH also stimulates breakdown of thyroglobulin and preferential synthesis and release of T3 into the blood |
| • The profile of thyroid hormones in iodine deficiency is: |
| ◦ In areas of moderate-to-severe iodine deficiency, children will have a variably elevated TSH, a low serum T4, and a normal or high-normal T3 |
| ◦ A similar pattern is seen in adults, but less predictably, and it may not be present |
| ◦ In conditions of low iodine intake, serum thyroglobulin concentration is typically elevated |
| ◦ Thyroid failure and cretinism usually develop only in regions of chronic, severe iodine deficiency accompanied by low circulating T4 and T3 and dramatically elevated TSH (80) |
DIT, diiodotyrosine; MIT, monoiodotyrosine; NIS, sodium/iodide symporter; T3, triiodothyronine; T4, thyroxine; TPO, thyroperoxidase; TSH, thyroid-stimulating hormone.
FIGURE 1.
Iodine pathway in the thyroid cell. Iodide (I−) is transported into the thyrocyte by NIS at the basal membrane and migrates to the apical membrane. I− is oxidized by the enzymes TPO and H2O2 and attached to tyrosyl residues in thyroglobulin to produce the hormone precursors MIT and DIT. The residues then couple to form T4 and T3 within the thyroglobulin molecule in the follicular lumen. Thyroglobulin enters the cell by endocytosis and is digested. T4 and T3 are released into the circulation, and nonhormonal iodine on MIT and DIT is recycled within the thyrocyte. Adapted from reference 79 with permission. DIT, diiodotyrosine; H2O2, hydrogen peroxidase; MIT, monoiodotyrosine; NIS, sodium/iodide symporter; TPO, thyroperoxidase; T3, triiodothyronine; T4, thyroxine.
FIGURE 3.
The physiologic stages of iodine status. The graph shows a simplified model of human iodine and thyroid status at different stages (left to right) of iodine intake: sufficient iodine intake, low iodine intake without thyroid dysfunction, and finally, low iodine intake with hypothyroidism. The 3 stages are separated by vertical dashed bars. The scientific evidence is limited with regard to the absolute levels of habitual daily iodine intake at which thyroid stores decrease and thyroid dysfunction occurs; this likely varies strongly among individuals. Reproduced from reference 9 with permission. TSH, thyroid-stimulating hormone.
FIGURE 2.
Iodine is an essential component of the thyroid hormones triiodothyronine and thyroxine. Reproduced from reference 62 with permission.
Even though we have learned much about the mechanisms and impact of iodine deficiency it should be noted that the presentation and severity of IDD (e.g., goiter, thyroid dysfunction) are extremely variable between and within populations even in endemic areas. Our ability to understand why this might be the case is limited by an incomplete appreciation of the dietary, environmental, and/or genetic factors that might contribute to this variability. The following sections describe some factors that might contribute to this variability.
Dietary considerations
Dietary inhibitors of iodine metabolism: goitrogens.
A number of dietary factors have been identified that negatively affect thyroid metabolism. These substances are collectively referred to as “goitrogens” and seem to exert their deleterious effects primarily via an exacerbation of iodine deficiency through several mechanisms (81–83) (Table 11). More than 100 additional naturally occurring and synthetic substances were reported to affect thyroid function or thyroid hormone metabolism, but most of these have not yet been well characterized in human studies (84).
TABLE 11.
Goitrogens and micronutrient deficiencies that affect iodine metabolism and thyroid function1
| Mechanism | |
| Cassava, lima beans, linseed, sorghum, sweet potato | Contain cyanogenic glucosides that are metabolized to thiocyanates and act by competing with iodine for uptake by thyroid gland |
| Cruciferous vegetables: cabbage, kale, cauliflower, broccoli, turnips, rapeseed | Contain glucosinolates; these metabolites act by competing with iodine for thyroidal uptake (36) |
| Soy, millet | Flavonoids impair thyroid peroxidase activity |
| Industrial pollutants (that enter food and water) | |
| Perchlorate, nitrate | Competitive inhibitors of the sodium/iodide symporter; they act by decreasing transportation of iodine to the thyroid |
| Others (e.g., disulfides from coal processes) | Reduce thyroidal iodine uptake |
| Smoking | One of the important goitrogens; during breastfeeding, smoking is known to be linked to reduced iodine content in the breast milk; the mechanism behind this is that in smoking women, the higher serum concentrations of thiocyanate compete with iodine for active transport into the secretory epithelium of the lactating breast (35) |
| Nutrients | |
| Selenium deficiency | Accumulated peroxides due to selenium deficiency may damage the thyroid, and deiodinase deficiency may impair the synthesis of thyroid hormone |
| Iron deficiency | The activity of heme-dependent TPO is reduced and the efficacy of iodine prophylaxis may be compromised |
| Vitamin A deficiency | Due to the deficiency there is a decrease in vitamin A–mediated suppression of the pituitary TSHβ gene and an associated increase in TSH stimulation and goiter |
TPO, thyroperoxidase; TSH, thyroid-stimulating hormone.
Population groups that may be particularly vulnerable to exposure to goitrogens are infants and young children. Examples of substances of concern include perchlorate, which was shown to interfere with iodine transport into the thyroid (85, 86). Another group of suspected substances are flavonoids commonly found in soy and millet. Although the consumption of soy-based foods does not appear to present a problem for adults, without the addition of iodine it can pose a risk to infants consuming soy-based formulas (87).
Nutrient-nutrient interactions.
Although iodine clearly plays a unique role in human health, like most nutrients it is also the case that interactions can occur that affect absorption, metabolism, and function. In the case of iodine, examples of these interactions are outlined in Table 11. The following provides some additional detail with regard to specific nutrients:
Selenium: the selenium-dependent enzymes glutathione peroxidase and the deiodinases are impaired in selenium deficiency. The accumulated peroxidases may damage the thyroid, whereas deiodinase deficiency impairs thyroid hormone synthesis. These effects have been implicated in the etiology of myxedematous cretinism (88).
- Iron deficiency
- ◦ reduces heme-dependent thyroperoxidase activity in the thyroid, resulting in impaired production of thyroid hormone;
- ◦ in goitrous children, iron deficiency anemia negatively affects the efficacy of interventions to prevent iodine deficiency.
- ◦ Iron supplementation improves the efficacy of iodized oil and iodized salt (89).
- ◦ Iron deficiency anemia during pregnancy can result in both higher TSH and lower T4 concentrations (90).
Requirements and Tolerable Upper Intake Levels
Iodine requirements by age and life stage are shown in Table 12. The definitions of key categories and their use, according to the U.S. Institute of Medicine (IOM) in the derivation of the current dietary recommendations, are discussed below. These definitions, and in particular their use, will set the stage for the following sections on specific biomarkers and their relative utility for clinical and population assessments.
TABLE 12.
Recommendations for iodine intake by age or population group1
| Institute of Medi cine |
WHO |
|||
| Life-stage group | EAR | AI or RDA | Life-stage group | RNI |
| μg/d | μg/d | μg/d | ||
| Infants 0–12 mo | — | 110–130 | Children 0–5 y | 90 |
| Children 1–8 y | 65 | 90 | Children 6–12 y | 120 |
| Children 9–13 y | 73 | 120 | Adults >12 y | 150 |
| Adults ≥14 y | 95 | 150 | ||
| Pregnancy | 160 | 220 | Pregnancy | 250 |
| Lactation | 200 | 290 | Lactation | 250 |
Estimated Average Requirement (EAR): The daily nutrient intake that meets the requirement of half of the healthy individuals in a particular life-stage (age/gender) group. Use: This category is not used for individual assessment but can be used for population/group analyses.
RDA: The average daily intake sufficient to meet the nutrient requirement of 97–98% of healthy individuals in a life-stage (age/gender) group. Use: It can be used as a reference point or goal for the daily nutrient intake of individuals.
Adequate Intake (AI): A recommended average daily intake amount based on observed or experimentally determined estimates of nutrient intake by a (gender/age) group (or groups) of apparently healthy people presumed to have adequate intakes. Use: The AI is derived when there are insufficient data to generate an RDA. Because the AI is intended to define the amount of a nutrient needed in “essentially all” individuals in a target group, it can be used as a goal for individual intake. For iodine, there is an AI for infants based on observed mean intakes by healthy full-term breast-fed infants in iodine-sufficient areas.
Tolerable Upper Intake Level (UL): The highest average daily nutrient intake that poses no risk of adverse health effects to almost all individuals in an otherwise healthy population (88). The UL for iodine is listed in Table 13. Use: The UL is used as a reference for safety. For iodine, metabolic adaptations to chronic deficiency may result in adverse responses to intakes lower than the UL.
TABLE 13.
Tolerable Upper Intake Level for iodine1
| Life-stage group | European Commission/Scientific Committee on Food | Institute of Medicine |
| μg/d | μg/d | |
| 1–3 y | 200 | 200 |
| 4–6 y | 250 | 300 |
| 7–10 y | 300 | 600 |
| 11–14 y | 450 | 900 |
| 15–17 y | 500 | |
| Adult | 600 | 1100 |
| Pregnant and lactating women | 600 | 1100 |
In addition to the IOM terminology, the joint FAO/WHO committee published several definitions derived in a consultation conducted in 2001 (92). Several of the terms used are similar to those used by the IOM (e.g., UL), but some, although essentially representing the same concepts, are different in name [e.g., Recommended Nutrient Intake (RNI)]. FAO/WHO defines the RNI as “the intake estimated to cover the needs of ‘nearly all’ healthy individuals in a specific age/gender group.” The RNI for iodine is included in Table 12.
Currently Available Biomarkers
Overview
Table 14 summarizes the currently available biomarkers of iodine and provides an overview of the utility for the different purposes and user groups (i.e., research, clinical, program, policy) and includes a rating system for the usefulness of each indicator for a specific use.
TABLE 14.
Overview of currently available biomarkers for the assessment of iodine nutrition1
| Usefulness assessment2 |
||||||||||||
| Exposure |
Status |
Function |
Effect |
|||||||||
| Biomarker | Research | Clinical | Program | Research | Clinical | Program | Research | Clinical | Program | Research | Clinical | Program |
| Salt iodine content (table or cooking) | ++ | 0 | +++ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dietary assessment | ++ | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Urinary iodine | +++ | + | +++ | +++ | + | +++ | + | 0 | + | + | 0 | + |
| TSH | + | + | 0 | ++ | ++ | 0 | ++ | ++ | 0 | 0 | 0 | 0 |
| Thyroglobulin | ++ | ++ | 0 | +++ | ++ | ++ | +++ | ++ | ++ | 0 | 0 | 0 |
| T3/T4 | 0 | 0 | 0 | 0 | 0 | 0 | + | + | 0 | 0 | 0 | 0 |
| Goiter | 0 | 0 | 0 | 0 | 0 | 0 | ++ | ++ | ++ | ++ | ++ | ++ |
T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone.
The usefulness assessment uses the following grading system: 0, not useful for the specific purpose; +, useful to some extent and in certain population groups but either not commonly used or has important disadvantages (e.g., no reference values); ++, useful in certain population groups, often used with some limitations (e.g., lack of specificity or sensitivity); +++, useful, often used in relevant population groups; no or only minor limitations.
Biomarker-specific issues
This section is an overview of the conclusions of the Iodine Expert Panel (I-EP) with regard to those iodine biomarkers that are ready for wide application across different user groups targeted by the Biomarkers of Nutrition for Development (BOND) program. For each of the priority biomarkers selected, the I-EP provided responses to a specific set of issues derived from an outline developed by the BOND Secretariat. This approach was used by each of the nutrient-specific BOND expert panels. The outline can be found in Supplemental Table 1 and on Tier 1 of the BOND Web site (93). The aim of this approach was to
provide a common format for the work of all BOND nutrient expert panels, and
address core issues that might be important to the range of user groups to be served by the BOND.
On the basis of the evaluation system presented in Table 14, the I-EP selected the following priority biomarkers for in-depth evaluation in this review:
urinary iodine concentration (UIC);
TSH;
thyroglobulin;
T4 and triiodothyronine (T3); and
goiter.
The characteristics and technical considerations for each of these biomarkers are listed in Supplemental Table 2. The following sections provide some general information about these biomarkers as well as some additional considerations with regard to specific exposure-related aspects of iodine assessment.
In addition to the consideration of the priority biomarkers, the I-EP concluded that a summary of issues pertaining to iodine exposure methodologies was an essential precursor to the discussion of iodine nutrition. Consequently, the following sections provide overviews of several issues pertaining to iodine exposure including household coverage of (adequately) iodized salt and dietary assessment of iodine intake. As opposed to the presentation of the priority biomarkers found in Supplemental Table 2, the discussion of exposure is presented in text because neither household surveillance nor dietary assessment are biomarkers per se but rather proxies and thus answer a slightly different set of questions.
Household coverage of (adequately) iodized salt
Iodine in salt used in households.
The importance of salt iodization was codified in 1994 by the Joint UNICEF/WHO Committee on Health Policy (94), which outlined policy for salt iodization intended for all types of salt destined for human and animal consumption, including salt used in the food industry. Momentum for universal salt iodization programs grew substantially throughout the 1990s, and by 2007 salt iodization strategies had been enacted in at least 120 countries (95). Rapid and inexpensive field kits that visibly detect iodine in salt have been widely used to measure the presence of iodized salt in households throughout the world. This has helped to document the progress of universal salt iodization strategies.
Measuring the iodine concentration in household salt.
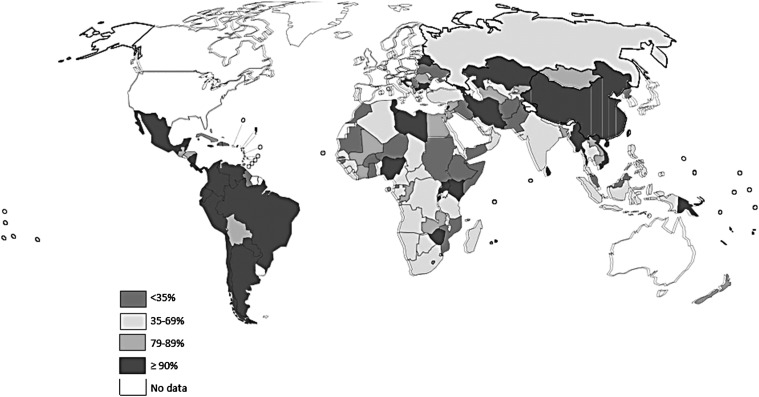
Household iodized salt coverage is the most feasible process indicator for assessing the reach of salt iodization strategies across countries and is commonly used as the key indicator of progress of salt iodization strategies over time. Figure 4 shows the global map with percentages of household consuming iodized salt. The success of delivering iodized salt to households is assessed either by the proportion of households using salt with any iodine concentration or, preferably, those using adequately iodized salt, i.e., salt containing at least 15 mg/kg of iodine. According to the WHO, the salt iodine content should be in the range of 15 to 40 mg/kg; however, in large national surveys the upper end of this range is rarely applied for practical reasons. The accepted standard threshold defining a successful iodization program is coverage of ≥90% of households using adequately iodized salt (64).
FIGURE 4.
Percentage of households consuming iodized salt. With exception of New Zealand, this indicator is not formally measured in industrialized nations. However, examples of effective iodized salt regulation in the Western world include Austria, Czech Republic, Belgium, Canada, Denmark, The Netherlands, and Switzerland. Adapted from reference 72 with permission.
To generate data on the adequacy of iodine in salt, it is necessary to use quantitative tools. An additional benefit derived from quantitative data on the iodine content in household salt is the ability to generate a distribution curve of the iodine content in household salt to compare the relative value of different iodine cutoff points (66). Moreover, because neither the coverage nor the median iodine value gives an indication of the existence or extent of excessively iodized salt in the households, the distribution curve provides important additional information in this regard.
Quantitative vs. qualitative measurements at the household level.
Ideally, national iodine strategy planning, surveillance, and decision making rely on quantitative data on salt iodine contents. Quantitative data also facilitate accurate tracking of the progress of the salt iodine content as well as coverage of adequately iodized household salt over time. Currently, rapid test kits are the most prominent tool used in national surveys to estimate the proportion of households using iodized salt. By implication, this estimate also provides the proportion of households not using iodized salt, which can provide useful information for planning corrective action. There are, however, certain limitations to the use of rapid test kits (F. van der Haar, R. Houston, A. Timmer, K. Codling, J. Gorstein, unpublished results, 2009) (66). Paramount among these is an inability to accurately identify salt iodized at <15 mg/kg of iodine, and in particular salt containing <10 mg/kg of iodine. In addition, the rapid test kits cannot identify overiodized salt at concentrations that may exceed 80–100 mg/kg (see “Household coverage of iodized salt” section). In light of these limitations, the use of the rapid test kits should be viewed as more of a qualitative test rather than a semiquantitative or even quantitative test.
Sampling salt at the household level.
The analytical method used for measuring salt iodine content determines the amount of salt sample required. For quantitative analysis by means of the titration or equivalent method, at least 10 g (2 teaspoons) are required. Using <10 g of salt for the titration analysis may lead to dubious results, particularly in the case of coarse salt and salt containing impurities. For repeat or duplicate analyses, at least 20 g of salt is required.
In many demographic and health surveys, multiple indicator cluster surveys, or similar population surveys, household salt iodine content is analyzed by using rapid test kits. However, in some recent large-scale surveys [e.g., the 2010 Tanzania Demographic and Health Survey (96)], household salt was sampled and brought to a central laboratory for quantitative measurements of iodine content. In terms of statistical sampling methods, the probability proportional to population size, a cluster sampling method, has been reliably applied to household salt sampling as part of national or subnational surveys in communities or in schools. Other sampling methods, such as a representative sample weighted for population density in different geographical areas, will also work (97).
Analytical capacity.
As outlined in the “Quantitative vs. qualitative measurements at the household level” section, accurate and reliable quantitative analysis of salt iodine content (i.e., by means of the titration method) requires suitably equipped public or private laboratories. Many countries have central as well as regional analytical laboratories for this purpose. As is further elaborated in the “Household coverage of iodized salt” section, it is critical that the laboratory or the network of laboratories provides reliable data from continuous quality assessments of the results obtained with their salt iodine measurements.
Relation between coverage of iodized salt and iodine deficiency and IDD.
The coverage of households with (adequately) iodized salt is a population indicator reflecting the iodine intake above the customary iodine consumption from common foods and water. In countries where the salt iodization regulation also mandates the iodization of salt used in the food industry, any foods manufactured with iodized salt also add to the dietary iodine supply. This situation is especially relevant for assessments in wealthier households and in urban areas in developing countries.
UIC is the prime indicator of iodine status in a population and is used in conjunction with analyses reflecting salt iodization to assess the nature, timeliness, and extent of the relation of household coverage with the population’s iodine status. It has been well documented that when iodized salt is introduced in the markets, the distribution of UIC levels in initially deficient populations rapidly shifts to the right (98). Irrespective of whether the coverage of households is expressed as any iodized salt or as adequately iodized salt, the nature of the response in a population’s UIC distribution to increases in household coverage is consistent over time and with the salt iodine intake. Several factors influence the pace by which salt iodization programs influence household/population iodine status (Table 15).
TABLE 15.
Factors affecting implementation of salt iodization programs1
Dietary assessment of iodine intake
Variability of iodine intake.
Although assessment of salt iodization (104–106) can serve as a useful proxy for iodine exposure under defined circumstances and despite its relative ease of implementation [see “Household coverage of (adequately) iodized salt” section], the quantification of iodine content in table salt may not be sufficient in assessing iodine exposure in certain contexts. In part, this challenge is due to settings where there is a shift to consumption of fewer home-prepared foods. Such shifts will necessitate a greater need to assess overall dietary iodine intake.
As summarized in Table 1, the natural iodine content of food sources differs considerably, which contributes to the fluctuations in daily iodine intake of individuals (107, 108). The iodine exposure scenarios vary greatly due to not only differences in inherent content of food (3, 19, 23, 109, 110) but also to factors such as seasonality of food intake patterns (20, 110), sources, and iodine content of milk (23, 110). Food processing is another critical factor affecting iodine exposure (e.g., choice of processed food made with iodized salt, discretionary use of iodized salt in cooking or at the table).
Methods used to assess dietary iodine intake.
The goal of dietary iodine assessment is to quantify the relative contribution of habitual intake from iodine-containing foods in terms of amounts and frequency (111, 112). The primary tools to accomplish this include FFQs (105, 113, 114), food diaries or 24-h recalls (115), or weighted food records (5). The relative strengths and weaknesses of the dietary assessment methods and other biomarkers identified by the I-EP are listed in Table 16. As is true across the field of dietary assessment, the assessment tools used to measure iodine exposure are imprecise (104–106).
TABLE 16.
Relative strengths and weaknesses of iodine biomarkers1
| Biomarker | Usefulness for the purpose | Advantages | Disadvantages | Analytical considerations |
| Salt iodine content | Is a good proxy for exposure in contexts of low iodine intakes from other sources. | Has clear advantages in dietary iodine monitoring in that it is noninvasive, inexpensive, and provides immediate visible results (RTK). If a quantitative method is used, results provide information on the various magnitudes of exposure. | RTKs are qualitative not quantitative; they can detect the presence of iodine in a salt sample but cannot accurately quantify the amount. Thus, salt that is not adequately iodized will test positive but will not deliver the appropriate amount of iodine to populations. In some areas, other sources of iodine (e.g., iodine-rich groundwater) can contribute large amounts of ingested iodine, and iodine intakes may be excessive in a local population, but household iodized salt coverage data do not reflect this. | The qualitative testing of household salt samples is usually done with simple RTKs. |
| Assesses the penetration and coverage of a population by iodized salt. In low-resource settings, household coverage with iodized salt may be the only affordable indicator of iodine nutrition in a population. | Although titration is accurate and requires only basic equipment, it is time-consuming and requires some training. Simpler alternatives, such as the WYD checker, the I-Reader, or the iCheck, are accurate and less demanding. | Titration is recommended for determination of iodine in salt at points of the salt distribution system (import points, manufacturers) at which accurate results are required. | ||
| An alternative to titration is to use a portable spectrophotometer, such as the WYD checker, I-Reader, or iCheck device, to back up RTK results, but they are a little more expensive than titration. | ||||
| An adequate iodine amount in household salt is defined as salt containing 15–40 mg/kg of iodine, although often >15 mg/kg is used. | ||||
| Dietary assessment | For dietary assessment of iodine intake, the 3 major instruments are the FFQ, food diaries, or 24-h food intake recall and weighed food records. FFQs assess the frequency and portion sizes of iodine-containing foods and/or food groups consumed over a predefined time frame, usually 1 y. The FFQ method captures iodine-rich sources that are irregularly consumed and accounts, to some extent, for day-to-day variation in the overall consumption patterns. Food diaries or 24-h recalls measure short-term intakes. To capture the day-to-day variation in dietary iodine intake, at least 10 repeated assessment days and/or a large sample size is needed, as shown for 24-h UIC collections. Research in small groups should use duplicate portion approaches. | Provides a broader picture beyond the household salt iodine content. In particular, intake of iodine from other sources such as processed foods is captured through this. Dietary assessment methods do not accurately quantify the “usual” iodine intake, but dietary data can be used to identify the most important food sources of iodine. This information is useful to design or adapt iodine intervention strategies. | Time-consuming to collect data, because it is difficult to identify all other potential sources of iodine. There are only a few food composition databases containing information on iodine-rich foods; additionally, due to wide variations in iodine content in the same food grown in different areas, iodine content of specific foods may need to be reanalyzed. Although an FFQ may include questions on discretionary salt added at the table or in cooking, it is difficult to accurately quantify the total amount of iodized salt consumed. Therefore, the FFQ method is not a reliable quantitative method for assessing the total iodine intake. Dietary recall is often inaccurate with regard to salt intake and generally underestimates salt consumption. The lack of accuracy in measuring iodine intake from iodized salt is thus a major limitation of dietary assessment. Food iodine content can be accurately measured only by using advanced methods, such as ICP-MS. The quality of iodine data in food composition tables is often poor and depends on whether the food iodine analysis is up-to-date and to what extent natural variability in iodine content is taken into account. Food composition databases generally contain information on the salt content of foods, but they rarely specify if the salt used in processed foods is iodized or not. | In most cases, no comprehensive and locally adapted food composition databases are available; analysis of iodine content in food matrices may require sophisticated methods, such as ICP-MS. |
| UIC | Because >90% of dietary iodine eventually appears in the urine, UI is an excellent indicator of recent iodine intake. UI can be expressed as a 24-h excretion (μg/d), as a concentration (UIC; μg/L), or in relation to creatinine excretion (μg iodine/g creatinine). But these are not interchangeable. | Relatively easy to collect in most population groups (except neonates and infants). For populations, because it is impractical to collect 24-h samples in field studies, UI can be measured in spot urine specimens from a representative sample of the target group. Variations in hydration between individuals generally even out in a large number of samples, so that the median UI in spot samples correlates well with that from 24-h samples. | Needs sufficiently large sample size to even out inter- and intraindividual variations. Thresholds not established for all population groups: widely accepted cutoffs exist for school-aged children, pregnant and lactating women, and infants (not neonates). Even with apparently similar exposure, the results of UI assessment in 1 subgroup may not necessarily be representative of other groups in the same population. The median UI does not provide direct information on thyroid function, but a low value suggests a population is at higher risk of developing thyroid disorders. UI in schoolchildren may not adequately characterize the risk of iodine deficiency among pregnant women, the main target group for iodine interventions. | Relatively simple, important to avoid contamination. Requires laboratory equipment and trained technicians. Spot urine samples are most widely used; requires relatively large populations due to inter- and intraindividual variation. |
| TSH | Mainly for newborn screening to detect congenital hypothyroidism; for other population groups, mean TSH values do not reliably discriminate between iodine-deficient and iodine-sufficient populations. Because TSH is determined mainly by the concentrations of circulating thyroid hormone, which in turn reflect iodine intake, TSH can be used as an indicator of iodine nutrition. | TSH is a sensitive indicator of iodine status in the newborn period. Neonatal TSH screening is an existing universal practice in most industrialized countries. The results have been used to assess the risk of iodine deficiency in the mothers when they were pregnant. | In older children and adults, although serum TSH may be slightly increased by iodine deficiency, the values often remain within the normal range. TSH is therefore a relatively insensitive indicator of iodine nutrition in school-aged children and adults. | Relatively sophisticated equipment required to quantitatively measure TSH. Can be measured from a DBS. |
| Thyroglobulin | In areas of iodine deficiency and endemic goiter, serum thyroglobulin increases due to greater thyroid cell mass and TSH stimulation. | Serum thyroglobulin is well correlated with the severity of iodine deficiency as measured by UI. Intervention studies examining the potential of thyroglobulin as an indicator of response to iodized oil and potassium iodide have shown that thyroglobulin falls rapidly with iodine repletion and that thyroglobulin is a more sensitive indicator of iodine repletion than TSH or T4. | The need for concurrent measurement of antithyroglobulin antibodies to avoid potential underestimation of thyroglobulin is uncertain; it is unclear how prevalent antithyroglobulin antibodies are in iodine deficiency, or whether they are precipitated by iodine prophylaxis. A limitation is large interassay variability and poor reproducibility, even with the use of standardization. There are no cutoffs for thyroglobulin to distinguish severity of iodine deficiency. | A new assay for thyroglobulin has been developed for DBSs taken by a finger prick, simplifying collection and transport. An international reference range and a reference standard for DBS-thyroglobulin in iodine- sufficient schoolchildren (4–40 μg/L) is available. |
| T3/T4 | In iodine-deficient populations, serum T3 increases or remains unchanged, and serum T4 usually decreases. | T3/T4 concentrations are a direct reflection of thyroid function. | Except in areas of severe iodine deficiency, thyroid hormone concentrations are poor indicators of iodine status. Changes in T3/T4 are often within the normal range, and the overlap with iodine-sufficient populations is large enough to make thyroid hormone concentrations an insensitive measure of iodine nutrition. | Widely accepted population cutoffs are established. |
| Goiter | Two methods are available for measuring goiter: neck inspection and palpation and thyroid ultrasonography. Inspection and palpation for goiter is simple and quick. To assess goiter in field studies, children and adults are examined with the examiner sitting or standing directly in front of the individual, palpating the base of the neck with both thumbs simultaneously. The normal thyroid should not be visible with the neck in the normal position. | Thyroid ultrasound is noninvasive, quickly done (2–3 min per individual), and feasible even in remote areas using portable equipment. Reference ranges for thyroid volume by ultrasound are available for school-aged children. | Palpation of goiter in areas of mild iodine deficiency has poor sensitivity and specificity; in such areas, measurement of Tvol by ultrasound is preferable. Thyroid ultrasound is somewhat subjective and requires judgment and experience. Differences in technique can produce large interobserver errors in Tvol. | The WHO recommends that the total goiter rate be used to define severity of iodine deficiency in populations by using the following criteria: <5%, iodine sufficiency; 5.0–19.9%, mild deficiency; 20.0– 29.9%, moderate deficiency; and >30%, severe deficiency. In the classification system of the WHO, grade 0 is defined as a thyroid that is not palpable or visible, grade 1 is a goiter that is palpable but not visible when the neck is in the normal position (i.e., the thyroid is not visibly enlarged), and grade 2 goiter is a thyroid that is clearly visible when the neck is in a normal position. |
DBS, dried blood spot; ICP-MS, inductively coupled plasma MS; RTK, rapid test kit; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone; Tvol, thyroid volume; UI, urinary iodine; UIC, urinary iodine concentration.
Food composition tables.
All dietary assessment methods require accurate information on the iodine content of foods. A number of constraints exist that impinge on the accurate analysis and documentation of iodine content of foods, including the following:
Methodologies: The most reliable method for measuring the iodine content of food is inductively coupled plasma MS (3). The major limitation to this method is that it is only available to a relatively limited number of laboratories worldwide.
- The quality of food iodine content data found in food composition tables is contingent on
- ◦ the method used to assess the iodine content (i.e., is it up-to-date and valid and reliable?);
- ◦ whether the natural variability in iodine content was taken into account;
- ◦ whether the source and nature of salt used in processed foods included in composition tables were accounted for in the analyses (i.e., tables generally contain information on the salt content of foods but rarely specify if the salt is iodized or not; commercial producers often fail to declare this information); and
- ◦ the generalizability of certain databases (processed food even from the same manufacturer or the same brand often contains different amounts/types of salt in different settings/countries, limiting the generalizability of certain databases).
In summary, dietary assessment of iodine intake to determine “usual” intakes is challenged by all of the factors above, as well as the large day-to-day variation in individual intake. That said, assessment of dietary data can be used to determine the primary sources of iodine, which can then be used to design or adapt iodine intervention strategies. In addition, simulation models can be used to anticipate the effects of changes in the food supply, precipitated by events such as iodine fortification, the impact of reduced salt intake as part of a public health intervention to prevent hypertension, and changes in food vehicles used for iodine fortification (14, 116–118).
Estimating iodine intake in populations using UIC.
The assessment of iodine intake at a population level was recently reviewed (9). The current consensus is that UIC is a reliable biomarker of recent iodine intake in populations at all amounts of chronic iodine intake. Some key characteristics of UIC include the following:
For individuals in positive iodine balance, >90% of ingested iodine is excreted in the urine (35).
Compared with dietary assessment or assessment of household salt consumption, UIC more accurately represents the total iodine intake from all dietary sources.
Because the concentration of iodine in urine depends on the urine volume, daily iodine intake can be estimated from spot UIC by calculating the daily urinary iodine excretion from the creatinine concentration.
In an attempt to simplify the calculation of daily iodine intake, the IOM proposed the following equation (31):
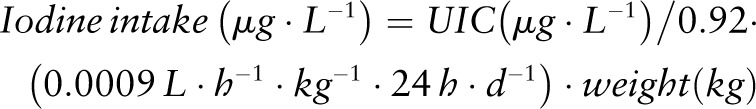 |
With regard to this equation, it should be noted that
the value 0.92 refers to 92% bioavailability and 0.0009 L ⋅ h−1 ⋅ kg−1 refers to excreted urine volume based on studies in (pre-) adolescent girls;
although body weight is poorly correlated with urine volume in adults, the factor of 0.0009 L ⋅ h−1 ⋅ kg−1 is accepted as a good approximation considering an average 24-h urine volume of 1.5 L/d in adults (119); and
by using this equation, the estimated dietary intake for a population of women with a mean UIC of 120 μg/L and an average body weight of 64 kg would be 180 μg/d (120).
The following sections briefly describe the iodine biomarkers identified by the I-EP as most suitable for broad use. Additional details on each may be found in Supplemental Table 2.
UIC
As discussed above, UIC has been extensively used to assess iodine status of populations, most often through measurement in school-aged children. Because of high diurnal and interday variation in individuals, the use of UIC as a marker for assessing individual status is limited, particularly in the absence of accurate assessment of intake; these variations level out when used in large population samples (27).
UIC can be expressed in several different (and not interchangeable) formats, such as
24-h excretion (μg/d);
concentration (μg/L); or
in relation to creatinine excretion (μg iodine/g creatinine).
UIC is usually measured in spot urine collections, which is advantageous in that it overcomes the challenges of 24-h urine collections particularly in field settings. In otherwise healthy, well-nourished adults, daily creatinine excretion is fairly constant at ∼1 g, so expressing the UIC from spot samples in adults as μg iodine/g creatinine represents a reasonable approximation of a 24-h collection. One important caveat is that it may not work in individuals with protein/calorie malnutrition because the daily creatinine would be more variable and often lower than normal ranges, thereby resulting in increased variability and decreased precision of UIC assessments (64). Overall, the current practice is to report UIC as a concentration (μg/L), expressed as the median, from spot samples in the target population.
TSH
TSH is secreted by the anterior pituitary gland and regulates thyroid hormone synthesis and secretion. TSH is the principal screening test for thyroid dysfunction. Serum TSH concentrations are increased when thyroid hormone concentrations are low (hypothyroidism) and decreased when thyroid hormone concentrations are high (hyperthyroidism or thyrotoxicosis).
T4 and T3
T4 and T3 are the hormones secreted by the thyroid gland. T3 is the active hormone; T4 is converted to T3 in peripheral tissues by deiodinases, which remove an outer ring iodine from the T4 molecule. More than 99% of the T4 and T3 circulating in the blood is bound to proteins. However, it is only the small amount of free (unbound) thyroid hormone that is bioactive. Total thyroid hormone concentrations can be easily measured. Free T4 and T3 concentrations are present in the blood at such low amounts that they cannot be readily measured. However, the amount of free T4 and T3 in the blood can be estimated on the basis of total thyroid hormone concentrations and measures of thyroid hormone binding. As thyroid function diminishes, serum TSH concentrations begin to increase. The presentation with elevated TSH concentrations and normal T4 and T3 concentrations is termed subclinical hypothyroidism. As hypothyroidism progresses, serum T4 concentrations decrease, and the combination of elevated TSH and low T4 concentrations is termed overt hypothyroidism. Serum total and free T3 concentrations may not decrease until disease is far advanced, because increased TSH concentrations stimulate T3 release from the thyroid.
Thyroglobulin
Thyroglobulin is the scaffold protein within which T3 and T4 are synthesized, and small amounts may be secreted into the blood along with T4 and T3 secretion. Clinically, serum thyroglobulin is used most frequently as a tumor marker for differentiated thyroid carcinoma. A recent development in the measurement of thyroglobulin is the application of dried blood spot (DBS) technology to categorize iodine status of populations of school-aged children (64, 121)
Goiter
Traditionally, the diagnosis of goiter is made when palpation-revealed thyroid lobes are bigger than the terminal phalanx of the patient’s thumbs. A grading system (grades 0, 1, and 2) is now recommended (64). Thyroid palpation in areas of mild IDD lacks specificity and sensitivity, necessitating the use of ultrasound to improve diagnostic precision. The advent of improved (both in terms of portability and resolution) instrumentation has facilitated the use of ultrasound even in remote and resource-limited areas. In experienced hands, this new technology allows a rapid and accurate analysis. The major drawback of this approach is expense, the need for experienced/well-trained technicians, and a high-level of inter-rater variability in its application and interpretation (9).
Although iodine deficiency is the primary cause of goiter, sometimes it is the result of a combination of low intake with high goitrogen intake (Table 11). As outlined in Figure 3, when dietary iodine intake is inadequate for thyroid hormone synthesis, serum T4 decreases and the pituitary gland reacts by releasing more TSH. The latter stimulates the growth and metabolic activity of thyroid follicular cells, leading to enlargement of the thyroid gland. Thus, goiter begins as an adaptive process to low iodine intake. Initially, goiter is diffuse, but in many individuals it eventually becomes nodular. Although most children with diffuse goiter will respond well to iodine treatment, nodular goiter may be more intractable in its response to iodine interventions (9).
Assay-Specific Queries
This section contains a more detailed discussion of analytical aspects of the priority biomarkers outlined in the “Biomarker-specific issues” section and is organized in a similar fashion. Specific technical issues pertaining to the priority biomarkers selected by the I-EP may be found in Supplemental Table 2.
Household coverage of iodized salt
The details and motivation for regulation of salt iodization programs were outlined in the “Biomarker-specific issues” section. The analytic requirements for monitoring and evaluating salt iodization programs and related policies might include information on the iodine content of retail and household salt content (122). Because the mandated iodization levels are generally stated in quantitative terms, the quality assurance and quality control protocols for safeguarding the iodized salt supplies should be based on a quantitative analytical method, the most frequently used and recommended being iodometric titration (64, 122).
Quantitative determination of iodine helps to identify successful elements of an iodization strategy, as well as factors that may weaken the supply of adequately iodized salt, some of which are mentioned in Table 17. Another advantage of quantitative measurement of the iodine in salt is the availability of a complete distribution curve along with enhanced ability to calculate the coverage of percentage of households using iodized salt (>0 mg/kg iodine), adequately iodized salt (>15 mg/kg iodine), and the percentage of households with excessively iodized salt (66).
TABLE 17.
Factors contributing to poor salt iodization
| • Inconsistent production or limitations in quality-controlled iodization technology at the factory |
| • Poor packaging and ineffective transport channels |
| • Noniodized salt entering the market in areas with large numbers of small-scale salt producers |
| • Absence of an effective monitoring system |
As reviewed in the “Household coverage of (adequately) iodized salt” section, rapid test kits are often used to estimate the percentage of households using iodized salt. Although these rapid test kits perform with sufficient accuracy in distinguishing between iodized and noniodized salt they lack the capacity to quantify iodine concentrations and lack precision to detect concentrations below or above 15 mg/kg (123–125). Consequently, the value of these kits is limited to their ability to determine whether or not salt is iodized, which is useful for education and advocacy but not for surveillance of salt iodine content/exposure.
Analytical methods for iodine in salt
Titration method.
The titration method for potassium iodate was first reported in 1979 and was subsequently modified (122, 126–129). Although well accepted, standardized, and relatively easy to implement, this method does require specific training to ensure consistent results. The method includes the following features:
- The process begins with the preparation of reagents followed by 2 analytical steps:
- ◦ liberation of free iodine from the salt, and
- ◦ titration of free iodine with thiosulfate using starch as an indicator.
The results are expressed as milligrams of iodine per kilogram of salt (mg/kg) or the equivalent parts of iodine per million parts of salt (mg/kg).
- For salt iodized with potassium iodide, in addition to the steps described above, before the analytical titration step, the analysis must be
- ◦ initiated with a step using bromine water to oxidize the iodide anions to free iodine, followed by
Quality assurance of the titration method is critical to ensure consistent results, especially when the method is performed by inexperienced technicians
WYD iodine checker.
Using a colorimetric methodology, the WYD iodine checker (Salt Research Institute of China National Salt Industry Corporation) is a single-wavelength spectrophotometer designed to measure the iodine concentration in salt (130). The method is based on the concept that iodine can be liberated from iodized salt under acidic conditions. The resultant color reaction that occurs in the presence of excess potassium iodide and starch, as an external color indicator, is directly proportional to the iodine content of the salt. The method detects iodine at a range of 10 to 90 mg/kg.
Some of the core characteristics of the WYD iodine checker include the following: 1) the assay is specific for salt fortified with iodate, 2) sample extraction should start with 10 g of well-mixed salt to ensure homogeneity of the salt particle size, 3) the sample used for the analysis should be 1 g, and 4) as with the titration methods and with the exception of assessment of total iodine (129), salt fortified with potassium iodide needs to be oxidized by bromine water and neutralized with sodium formate before the colorimetric assay.
The WYD iodine checker offers the following advantages:
portability;
rapid testing, allowing an “on the spot” result;
the results compare well with the titration method (131); and
unlike the titration methods, this method does not require a laboratory.
The WYD device can be used by anyone after minimal training unlike the titration method for which trained laboratory personnel are required to conduct the analysis. The device performs well in the concentration range of 10 to 90 mg/kg, which encompasses the usual range of salt iodine standards. Characteristics of newer methods similar to the WYD device that have emerged on the market are described in Table 18.
TABLE 18.
Newer methods to quantitatively assess salt iodine content
| iCheck |
| • Used for simplified quantitative testing of iodine |
| • Has been validated against the titration method in salt samples from several countries (132) |
| I-Reader |
| • Developed by Mahidol University in Bangkok, Thailand |
| • Has been independently validated to produce good results against the titration method |
| • Has found wide practical acceptance in Thailand (133) |
Potentiometric method.
The details of this method were previously described (134, 135). In brief, the efficiency of measuring iodine in salt fortified with iodate was documented as 99.7% with a CV of 1.56 at an iodine concentration of 20 mg/kg and 0.31 at a concentration of 50 mg/kg (136). As with the previous methods described above, this method can also be adapted for salt using iodide. Although offering many advantages, due to its high cost and the need for technical capacity particularly with regard to maintenance of the equipment, this method has not been widely used in public health laboratories.
Rapid test kits.
While commonly used, the available rapid test kits, although fairly effective at distinguishing between iodized and noniodized salt, are unreliable for the assessment of the iodine content above the zero point (123–125). Using a colorimetric method, these kits are used as a qualitative reflection of household iodized salt coverage. The primary advantages of these kits are their simplicity and the rapid results generated, making them particularly attractive for use in large-scale household surveys. However, it has been suggested that the results be corroborated whenever possible with a titration assay, minimally in a subsample of households with the survey (64, 125).
Quality control and quality assurance.
Currently, no international quality assurance support programs exist for methods to assess the iodine content of salt. One suggested approach is the application of certification process used by the International Organization for Standardization (137). Some examples exist for laboratory networks within countries (e.g., Bulgaria and Romania) where salt iodine assessments occur but within the larger context of nationally certified laboratories that function as a component of public safety and hygiene more broadly defined for the performance of a range of public health–related assays. In some other countries (e.g., Tanzania and India), a reputable central public health laboratory performs assessment of iodine content of salt as a validation/corroboration of data provided by salt manufacturers.
The absence of external quality assurance service places a greater onus on existing salt iodine laboratory managers and analysts to ensure adherence to the principles of good laboratory practice. Moreover, as is the case with laboratories measuring UIC, laboratories measuring iodine content of salt must ensure the accuracy, reliability, recovery, and minimum detection limits of their methods (127, 138). In addition, they must ensure that some sort of external reference material be analyzed on a regular basis as outlined in Supplemental Table 3. Ideally, laboratories engaged in the measurement of iodine content should meet certain minimal specifications as outlined in Table 19.
TABLE 19.
Laboratory instrumentation requirements for quantitative analysis of salt iodine content
| Titration method |
| • Requires a balance accurate to at least 2, preferably 3, decimal points (0.001 g) |
| • Burette |
| • Hotplate and appropriate glassware |
| Analytical method |
| • Single and multiposition magnetic stirrers |
| • Bottle-top dispensers |
| • A self-zeroing burette |
| • An analog or digital bottle-top burette for rapid titration |
| • A fume hood for the preoxidation step |
| Potentiometric method |
| • Potentiometer instrument, including dedicated ion selective electrodes for iodine |
| Measurement |
| WYD iodine checker method |
| • Instrument procured from China; chemicals can be obtained locally |
Interpreting iodine content in salt.
The need for effective quality control and assurance programs are obvious both in terms of the public health and economic implications. In an attempt to determine the success or failures of salt iodination programs, comparisons are made on the data generated from standardized reliable quantitative tests (e.g., titration method) and accepted national cutoffs set by appropriate regulatory bodies. Such comparisons allow for determinations of both the effectiveness of the iodization process and the percentage of salt that meets the standard. Obviously, the consequences for salt that does not meet specifications loom large both in terms of public health (implications to the consuming population) and to the manufacturers who wish to avoid the additional costs that would accrue from the need to rectify an “out-of-spec” product batch.
In the context of quality control, 2 critical cutoffs exist at the household level, as follows:
1. Salt containing no iodine, which may include salt with iodine measured at levels just above zero due to measurement variability; for purposes such as surveys designed to determine the percentage of households using iodized salt, there may be a variation in the classification of salt as not containing iodine. In some circumstances/laboratories, salt containing <2 mg/kg is classified as salt without iodine, whereas in other laboratories salt with <5 mg/kg is classified as salt without iodine.
2. Salt defined as containing adequate levels of iodine (i.e., ≥15 mg/kg of iodine).
Conclusions on coverage of (adequately) iodized salt
Specific technical considerations about the priority biomarkers identified by the I-EP (UIC, TSH, thyroglobulin, T4/T3, and goiter) can be found in Supplemental Table 2. The following reflects the I-EP’s conclusions about population-based methodologies assessing coverage of (adequately) iodized salt.
Because of its accuracy, relative ease of operation, and low cost, the titration method is recognized as the reference method recommended for assessing the iodine content of salt (122). The I-EP endorsed the following:
Every country with an iodized salt regulation should have ≥1 laboratories equipped to perform the titration method on a fairly large (>1000) number of samples.
Where salt iodization is mandatory, all salt producers should be required to routinely operate and maintain a titration laboratory or use the WYD iodine checker, iCheck IODINE (BioAnalyt), I-Reader (Mahidol University), or a similar device (132) at production sites (128).
For small production sites, an effort should be made to have access to such a facility, provided the result can be made available within a short time.
While emphasizing that titration remains the method of choice for establishing the percentage of households using salt containing at least 15 mg/kg, the I-EP recognized that the rapid test kit is also used in surveys to estimate the percentage of households using iodized salt. In such cases the I-EP suggested that a subsample of at least 10%, preferably 20%, of salt samples be sent to a laboratory for confirmatory analyses via the titration method to ensure the accuracy of coverage estimates and to validate the utility of rapid test kits.
New Directions and Technologies
Use of the EAR cut-point method.
As highlighted in section the above “Dietary assessment of iodine intake” section of this report, the ability to assess short- and long-term iodine intake is challenged by a number of factors, including the following: variability of intake between individuals and populations, adequacy of food composition tables, and limitations of available tools to assess intake of individuals and populations. Currently, the consensus approach to establish dietary adequacy is the EAR cut-point method (139–144). On the basis of the population distribution of intake as opposed to single mean or median intakes, the EAR cut-point method was also recommended as the preferred method for defining optimal levels of fortification of foods (145). With this method, the nutrient inadequacy of a group is defined as the proportion of people in a target group with usual intakes below the EAR (139–141, 146). Adequacy is defined as 97–98% of the target group meeting the EAR.
The I-EP suggests that, in light of acceptance of UIC as the most reliable biomarker of recent iodine intake, it could be used in conjunction with the EAR cut-point method to assess the prevalence of iodine deficiency, thereby improving iodine monitoring in populations. The I-EP cautioned that this approach would need to be validated before wide application, and it identified several points of concern that would need to be addressed to establish the viability of this approach, including the following:
Recognizing that the EAR has been used to estimate the prevalence of inadequate iodine intakes based on 24-h urine collections (5), the I-EP cautioned that the EAR cut-point method has not yet been evaluated for spot UIC collections.
Different methods need to be tested to adjust UIC distributions.
The sample size and number of repeated samples required for adjustment need to be established.
There is a need to validate the UIC distribution against 24-h urine collections, urinary iodine excretion, urine volume, body weight, and dietary intake data in populations with different iodine status.
The UIC cutoff corresponding to the EAR for different age and population groups should be defined and validated against thyroid function (technical and statistical considerations with regard to this application are highlighted in Table 20).
TABLE 20.
Justification and considerations for the use of UIC in application of the EAR method1
| • The population distribution of UICs is typically skewed toward lower intakes with a scattered tail of high intakes due not only to variations in iodine intake between individuals but also to high day-to-day variability of iodine intakes for individuals in the target population |
| • The sample size of the group takes interindividual variation into account (when properly powered) but does not account for individual day-to-day variations (147, 148) |
| • Therefore, before UIC data from a target group can be used for this application, the UIC distribution in populations with large variations in iodine intakes must be adjusted for intraindividual variation (144, 146) |
| • This can be done by collecting ≥2 repeated samples from the same individual in a subset of the study population |
| • The information on variability obtained can then be used to correct for within-person variation in the group (149) |
| • This approach adjusts the observed intake distribution closer to the mean (i.e., to more closely resemble a normal intake distribution of habitual intakes) (146, 150) |
| Advantages and additional considerations: |
| • The normalization of the distribution generates a prevalence estimate that is closer to the true prevalence of inadequacy |
| • Adjustment will generally reduce the prevalence compared with noncorrected distributions, except in populations with low or homogeneous iodine intakes (140) |
| • In populations with low iodine intake (i.e., mean intakes below the EAR), the distribution is typically skewed to lower intakes and adjustment may underestimate the prevalence |
| • In countries with a well-functioning salt iodization program, the iodine intake is less variable and the population distribution is close to normal without correction, as observed in Swiss schoolchildren (151) |
| • A further advantage of collecting a repeat urine sample and accounting for intraindividual variation is that the required sample size can likely be smaller than the 500 samples now recommended for population assessment (147, 148) |
EAR, Estimated Average Requirement; UIC, urinary iodine concentration.
Once these considerations are addressed, the I-EP concluded that this approach can be successfully implemented to define the prevalence of iodine deficiency as the proportion of the population below the EAR, from the adjusted distribution. The I-EP acknowledged the existence of several statistical methods that might be applied to adjust the population distribution of iodine intake data. These approaches were previously reviewed (152, 153) and include those proposed by the following organizations: Iowa State University (154–158), the IOM (141), and the National Cancer Institute of the NIH (159–161; the National Cancer Institute approach is currently used by the CDC/National Center for Health Statistics as part of the survey in the United States) (162). For those interested in learning more about these approaches, tutorials are available (162–164).
Development of “field-friendly” and “point-of-care” biomarker methods.
Despite the tools available, a great need still exists for functional biomarkers of thyroid status to complement the current markers of exposure and further improve clinical and population efforts to monitor iodine deficiency. An additional need is for tools that are easy to use, available, and accessible in resource-limited settings in the field.
An example of a “field-friendly” functional biomarker is DBS-thyroglobulin technology, which has gained a measure of acceptance with the WHO, which recommends DBS-thyroglobulin for monitoring iodine status (64). A reference range and cutoffs for the use of DBS-thyroglobulin in monitoring iodine status (both deficiency and excess) in school-aged children are available (121). But several questions need to be resolved before thyroglobulin can be widely adopted as a functional indicator of iodine status (see Table 21).
TABLE 21.
Unresolved issues regarding DBS-thyroglobulin1
| • It is uncertain whether a need exists for concurrent measurement of antithyroglobulin antibodies to avoid potential underestimation of elevated thyroglobulin: |
| it is unclear how prevalent antithyroglobulin antibodies are in iodine deficiency, or |
| whether they are precipitated by iodine prophylaxis |
| • Large interassay variability exists, even with the use of standardization |
DBS, dried blood spot.
Other “point-of-care” methods are being developed that may prove useful in assessment of iodine status in field studies. These include rapid semiquantitative assessment of TSH on a drop of fresh blood and simplified rapid methods and/or “dipstick” methods for semiquantitative measurement of iodine concentrations in urine samples.
Iodine biomarker development through “-omics” technologies.
It is likely that rapidly developing “-omics” technologies will contribute a new generation of biomarkers that may be applied to iodine assessment. Micronutrient research is focusing on metabolic, genomic, metabolomics, and transcriptomic changes that indicate micronutrient malnutrition (over-/undernutrition) or responses to micronutrient interventions in individuals and populations. Three major transitions that may further define the iodine-health relation include change in emphasis
from status biomarkers to mechanism-driven health biomarkers;
from single parameters to a biomarker profile or fingerprint; and
from a single micronutrient to function-oriented micronutrient networks/systems biology (165).
Iodine is 1 of the nutrients included in the Micronutrient Genomics Project (MGP). The MGP, 1 of the collaborating partners of the BOND project, is a community-driven collaboration intended to facilitate the development, collection, analysis, and dissemination of new data generated from studies applying the range of -omics approaches to enhance our understanding of micronutrients within relevant biologic systems. The MGP has created a public portal and open-source bioinformatics toolbox for all -omics information and evaluation of micronutrient and health studies. The core of the project focuses on access to, and visualization of, genetic/genomic, transcriptomic, proteomic, and metabolomics information related to micronutrients (166).
Sequencing technology is developing rapidly and offers the capacity to look at large numbers of genes to analyze for new sequence variants. The MGP has developed a list of genes for each micronutrient either in the pathway used for metabolizing the micronutrient or in regulating those pathway genes (i.e., networks of genes involved with each micronutrient) that could be used to screen for micronutrient-related genetic variations. This would allow the MGP to analyze the genetic variation in different ancestral populations by using targeted resequencing. The list of genes included for iodine is shown in Supplemental Table 4.
Research Needs
Despite much success in the global efforts to control and eliminate iodine deficiency, including the elimination of severe deficiency and endemic goiter in most countries, mild to moderate iodine deficiency continues to affect 32 countries, more than half of them in the industrialized world. Where efforts have succeeded, ensuring sustainability of those efforts becomes a high priority requiring regular surveillance and vigilance to ensure the adequacy of salt iodine content. Conversely, as salt iodization efforts become more commonplace and effective, attention must be paid to the potential for excess iodine intakes and consequent health effects.
Each of these trends highlights the need for greater efforts to develop and implement accurate and reliable biomarkers to monitor iodine status. Yet, as highlighted in this report, current assessment methods are limited. To address this limitation, the I-EP has offered the following suggested research priorities:
Development of biomarkers of exposure and function at the individual level, likely through new “-omics” technologies
Personalization of iodine requirements based on age, gender, ethnicity, environment, and health status
Development of robust, inexpensive, and field-friendly functional biomarkers of thyroid status that respond to varying amounts of iodine exposure
Validation of extrapolation of iodine intakes from UICs by using concurrent dietary assessment
Validation/optimization of current UIC cutoffs for vulnerable groups, such as pregnant women and infants, by using functional biomarkers
Assessment and validation of the EAR cut-point model as outlined in the “Use of the EAR cut-point method” section
Clarification of the potential role of perchlorate and other environmental contaminants on iodine biomarkers and thyroid status
Further development and assessment of the DBS-thyroglobulin assay as a functional biomarker
Evaluation of the combination of DBS with UIC data to assess efforts to ensure normalization of thyroid function in children in response to improved iodine intake
Expansion of DBS use to other subgroups such as pregnant women and infants
In light of efforts to reduce salt intake as a strategy to prevent cardiovascular disease risk, developing sensitive and specific biomarkers needs to be continued and iodized salt interventions recalibrated
Iodine biomarkers and the role of varying iodine intake on the incidence of autoimmune thyroid disease and papillary thyroid cancer
Iodine biomarkers and the role of varying iodine intake on antioxidant and immune response
Better understanding of the relation between household salt coverage and UIC and its interpretation
Effects of nutrients that interact with iodine (e.g., vitamin A, iron, zinc, and selenium) on iodine biomarkers
Acknowledgments
The authors thank John Egbuta, Roland Kupka, Stephen Long, Elizabeth N. Pearce, Christine Swanson, Paula Trumbo, and Frits van der Haar for their contributions. They also thank Alexandra Porter for her help with manuscript preparation. This review is the result of the deliberations and contributions of the Iodine Expert Panel: M.Z., F.R., P.J., C.P., and K.C.; D.J.R. and R.R. were responsible for synthesizing and summarizing the results of the I-EP deliberations. All authors read and approved the final version of the manuscript.
Footnotes
Abbreviations used: AI, Adequate Intake; DBS, dried blood spot; BOND, Biomarker of Nutrition for Development; EAR, Estimated Average Requirement; IDD, iodine deficiency disorder; I-EP, Iodine Expert Panel; IGF, insulin-like growth factor; IGFBP, insulin-like growth factor binding protein; IOM, Institute of Medicine; IQ, intelligence quotient; MGP, Micronutrient Genomics Project; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone; UIC, urinary iodine concentration; UL, Tolerable Upper Intake Level.
References
- 1.Zimmermann MB. Symposium on 'Geographical and geological influences on nutrition’: iodine deficiency in industrialised countries. Proc Nutr Soc 2010;69:133–43. [DOI] [PubMed] [Google Scholar]
- 2.Hetzel BS, Dunn JT. The iodine deficiency disorders: their nature and prevention. Annu Rev Nutr 1989;9:21–38. [DOI] [PubMed] [Google Scholar]
- 3.Haldimann M, Alt A, Blanc A, Blondeau K. Iodine content of food groups. J Food Compos Anal 2005;18:461–71. [Google Scholar]
- 4.Pennington JAT, Schoen SA, Salmon GD, Young B, Johnson RD, Marts RW. Composition of core foods in the U.S. food supply. J Food Compos Anal 1995;8:171–217. [Google Scholar]
- 5.Johner SA, Gunther AL, Remer T. Current trends of 24-h urinary iodine excretion in German schoolchildren and the importance of iodised salt in processed foods. Br J Nutr 2011;106:1749–56. [DOI] [PubMed] [Google Scholar]
- 6.Julshamn K, Dahl L, Eckhoff K. Determination of iodine in seafood by inductively coupled plasma/mass spectrometry. J AOAC Int 2001;84:1976–83. [PubMed] [Google Scholar]
- 7.Dahl L, Johansson L, Julshamn K, Meltzer HM. The iodine content of Norwegian foods and diets. Public Health Nutr 2004;7:569–76. [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann MB. Iodine deficiency. Endocr Rev 2009;30:376–408. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann MB, Andersson M. Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev 2012;70:553–70. [DOI] [PubMed] [Google Scholar]
- 10.Teas J, Pino S, Critchley A, Braverman LE. Variability of iodine content in common commercially available edible seaweeds. Thyroid 2004;14:836–41. [DOI] [PubMed] [Google Scholar]
- 11.Nagataki S. The average of dietary iodine intake due to the ingestion of seaweeds is 1.2 mg/day in Japan. Thyroid 2008;18:667–8. [DOI] [PubMed] [Google Scholar]
- 12.Andersen S, Guan H, Teng W, Laurberg P. Speciation of iodine in high iodine groundwater in China associated with goitre and hypothyroidism. Biol Trace Elem Res 2009;128:95–103. [DOI] [PubMed] [Google Scholar]
- 13.Henjum S, Barikmo I, Gjerlaug AK, Mohamed-Lehabib A, Oshaug A, Strand TA, Torheim LE. Endemic goitre and excessive iodine in urine and drinking water among Saharawi refugee children. Public Health Nutr 2010;13:1472–7. [DOI] [PubMed] [Google Scholar]
- 14.Charlton K, Skeaff S. Iodine fortification: why, when, what, how, and who? Curr Opin Clin Nutr Metab Care 2011;14:618–24. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization; United Nations Children’s Fund; International Council for Control of Iodine Deficiency Disorders. Recommended iodine levels in salt and guidelines for monitoring their adequacy and effectiveness. Geneva (Switzerland): WHO; 1996.
- 16.Andersson AM, de Benoist B, Darnton-Hill I, Delange F. Iodine deficiency in Europe: a continuing public health problem. Geneva: World Health Organization; 2007. [Google Scholar]
- 17.Murray CW, Egan SK, Kim H, Beru N, Bolger PM. US Food and Drug Administration's Total Diet Study: dietary intake of perchlorate and iodine. J Expo Sci Environ Epidemiol 2008;18:571–80. [DOI] [PubMed] [Google Scholar]
- 18.Caldwell KL, Makhmudov A, Ely E, Jones RL, Wang RY. Iodine status of the U.S. population, National Health and Nutrition Examination Survey, 2005–2006 and 2007–2008. Thyroid 2011;21:419–27. [DOI] [PubMed] [Google Scholar]
- 19.Pearce EN, Pino S, He X, Bazrafshan HR, Lee SL, Braverman LE. Sources of dietary iodine: bread, cows’ milk, and infant formula in the Boston area. J Clin Endocrinol Metab 2004;89:3421–4. [DOI] [PubMed] [Google Scholar]
- 20.Dahl L, Opsahl JA, Meltzer HM, Julshamn K. Iodine concentration in Norwegian milk and dairy products. Br J Nutr 2003;90:679–85. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Waite KV, Ma G, Eastman CJ. Declining iodine content of milk and re-emergence of iodine deficiency in Australia. Med J Aust 2006;184:307. [DOI] [PubMed] [Google Scholar]
- 22.Schöne F, Leiterer M, Lebzien P, Bemmann D, Spolders M, Flachowsky G. Iodine concentration of milk in a dose-response study with dairy cows and implications for consumer iodine intake. J Trace Elem Med Biol 2009;23:84–92. [DOI] [PubMed] [Google Scholar]
- 23.Bath SC, Button S, Rayman MP. Iodine concentration of organic and conventional milk: implications for iodine intake. Br J Nutr 2012;107:935–40. [DOI] [PubMed] [Google Scholar]
- 24.Mattes RD, Donnelly D. Relative contributions of dietary sodium sources. J Am Coll Nutr 1991;10:383–93. [DOI] [PubMed] [Google Scholar]
- 25.Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol 2009;38:791–813. [DOI] [PubMed] [Google Scholar]
- 26.Institute of Medicine. Strategies to reduce sodium intake in the United States. Washington; 2010.
- 27.Thomson BM. Nutritional modelling: distributions of salt intake from processed foods in New Zealand. Br J Nutr 2009;102:757–65. [DOI] [PubMed] [Google Scholar]
- 28.Ni Mhurchu C, Capelin C, Dunford EK, Webster JL, Neal BC, Jebb SA. Sodium content of processed foods in the United Kingdom: analysis of 44,000 foods purchased by 21,000 households. Am J Clin Nutr 2011;93:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Vital signs: food categories contributing the most to sodium consumption—United States, 2007–2008. MMWR Morb Mortal Wkly Rep 2012;61:92–8. [PubMed] [Google Scholar]
- 30.Chavasit V, Malaivongse P, Judprasong K. Study on stability of iodine in iodated salt by use of different cooking model conditions. J Food Compos Anal 2002;15:265–76. [Google Scholar]
- 31.Institute of Medicine, Academy of Sciences. Iodine. In: Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington: National Academies Press; 2001. [PubMed]
- 32.Smith P, Williams ED, Wynford-Thomas D. In vitro demonstration of a TSH-specific growth desensitising mechanism in rat thyroid epithelium. Mol Cell Endocrinol 1987;51:51–8. [DOI] [PubMed] [Google Scholar]
- 33.Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol 2004;151: Suppl 3:U25–37. [DOI] [PubMed] [Google Scholar]
- 34.International Council for the Control of Iodine Deficiency Disorders. Consequences of iodine deficiency [cited 2014 Jan 31]. Available from: http://www.iccidd.org/p142000352.html.
- 35.Zimmermann MB, Jooste PL, Pandav CS. Iodine-deficiency disorders. Lancet 2008;372:1251–62. [DOI] [PubMed] [Google Scholar]
- 36.Eastman CJ, Zimmermann MB. The iodine deficiency disorders. In: Thyroid disease manager. 2009 [cited 2009 Sep 1]. Available from: http://www.thyroidmanager.org/chapter/the-iodine-deficiency-disorders/.
- 37.World Health Organization; United Nations Children’s Fund, International Council for Control of Iodine Deficiency Disorders. Progress towards the elimination of iodine deficiency disorders (IDD). Geneva (Switzerland): WHO; 1999. [Google Scholar]
- 38.Kelly FC, Snedden WW. Prevalence and geographical distribution of endemic goitre. Bull World Health Organ 1958;18:5–173. [PMC free article] [PubMed] [Google Scholar]
- 39.Stübner D, Gartner R, Greil W, Gropper K, Brabant G, Permanetter W, Horn K, Pickardt CR. Hypertrophy and hyperplasia during goitre growth and involution in rats—separate bioeffects of TSH and iodine. Acta Endocrinol (Copenh) 1987;116:537–48. [DOI] [PubMed] [Google Scholar]
- 40.Hetzel BS. Iodine deficiency disorders (IDD) and their eradication. Lancet 1983;2:1126–9. [DOI] [PubMed] [Google Scholar]
- 41.Paoli-Valeri M, Guzman M, Jimenez-Lopez V, Arias-Ferreira A, Briceno-Fernandez M, Arata-Bellabarba G. [Atherogenic lipid profile in children with subclinical hypothyroidism.] An Pediatr (Barc) 2005;62:128–34. [DOI] [PubMed] [Google Scholar]
- 42.Biondi B, Cooper D. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008;29:76–131. [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann MB, Jooste PL, Mabapa NS, Mbhenyane X, Schoeman S, Biebinger R, Chaouki N, Bozo M, Grimci L, Bridson J. Treatment of iodine deficiency in school-age children increases insulin-like growth factor (IGF)-I and IGF binding protein-3 concentrations and improves somatic growth. J Clin Endocrinol Metab 2007;92:437–42. [DOI] [PubMed] [Google Scholar]
- 44.Koutras DA, Christakis G, Trichopoulos D, Dakou-Voutetaki A, Kyriakopoulos V, Fontanares P, Livadas DP, Gatsios D, Malamos B. Endemic goiter in Greece: nutritional status, growth, and skeletal development of goitrous and non goitrous populations. Am J Clin Nutr 1973;26:1360–8. [DOI] [PubMed] [Google Scholar]
- 45.Delange F. Endemic goitre and thyroid function in Central Africa. Monogr Paediatr 1974;2:1–171. [PubMed] [Google Scholar]
- 46.Halpern JP, Boyages SC, Maberly GF, Collins JK, Eastman CJ, Morris JG. The neurology of endemic cretinism: a study of two endemias. Brain 1991;114:825–41. [DOI] [PubMed] [Google Scholar]
- 47.McCarrison R. Observations on endemic cretinism in the Chitral and Gilgit valleys. Lancet 1908;2:1275–80. [PMC free article] [PubMed] [Google Scholar]
- 48.Nordenberg D, Sullivan K, Maberly G, Wiley V, Wilcken B, Bamforth F. Congenital hypothyroid screening programs and the sensitive thyrotropin assay: strategies for the surveillance of iodine deficiency disorders. In: Delange F, Dunn JT, Glinoer D, editors. Iodine deficiency in Europe: a continuing concern. New York: Plenum Publishing; 1993. p. 211–7. [Google Scholar]
- 49.Zimmermann MB, Connolly K, Bozo M, Bridson J, Rohner F, Grimci L. Iodine supplementation improves cognition in iodine-deficient schoolchildren in Albania: a randomized, controlled, double-blind study. Am J Clin Nutr 2006;83:108–14. [DOI] [PubMed] [Google Scholar]
- 50.Gordon RC, Rose MC, Skeaff SA, Gray AR, Morgan KM, Ruffman T. Iodine supplementation improves cognition in mildly iodine-deficient children. Am J Clin Nutr 2009;90:1264–71. [DOI] [PubMed] [Google Scholar]
- 51.Bleichrodt N, Shrestha RM, West CE, Hautvast JG, van de Vijver FJ, Born MP. The benefits of adequate iodine intake. Nutr Rev 1996;54:S72–8. [DOI] [PubMed] [Google Scholar]
- 52.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O'Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 1999;341:549–55. [DOI] [PubMed] [Google Scholar]
- 53.Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, Vulsma T, Wiersinga WM, Drexhage HA, Vader HL. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50:149–55. [DOI] [PubMed] [Google Scholar]
- 54.Todd CH, Allain T, Gomo ZA, Hasler JA, Ndiweni M, Oken E. Increase in thyrotoxicosis associated with iodine supplements in Zimbabwe. Lancet 1995;346:1563–4. [DOI] [PubMed] [Google Scholar]
- 55.Bourdoux PP, Ermans AM, Mukalay wa Mukalay A, Filetti S, Vigneri R. Iodine-induced thyrotoxicosis in Kivu, Zaire. Lancet 1996;347:552–3. [DOI] [PubMed] [Google Scholar]
- 56.Delange F, de Benoist B, Alnwick D. Risks of iodine-induced hyperthyroidism after correction of iodine deficiency by iodized salt. Thyroid 1999;9:545–56. [DOI] [PubMed] [Google Scholar]
- 57.Eng PH, Cardona GR, Fang SL, Previti M, Alex S, Carrasco N, Chin WW, Braverman LE. Escape from the acute Wolff-Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein. Endocrinology 1999;140:3404–10. [DOI] [PubMed] [Google Scholar]
- 58.Bürgi H. Iodine excess. Best Pract Res Clin Endocrinol Metab 2010;24:107–15. [DOI] [PubMed] [Google Scholar]
- 59.Wolff J, Chaikoff I. Plasma inorganic iodide as a homeostatic regulator of thyroid function. J Biol Chem 1948;174:555–64. [PubMed] [Google Scholar]
- 60.Study Group on Endemic Goitre. Final report. Bull World Health Organ 1953;9:293–301. [PMC free article] [PubMed] [Google Scholar]
- 61.Hetzel B. The nature and magnitude of the iodine deficiency disorders: towards the global elimination of brain damage due to iodine deficiency. Delhi (India): Oxford University Press; 2004.
- 62.Zimmermann MB. Iodine and iodine deficiency disorders. In: Erdman JW, Macdonald IA, Zeisel SH, editors. Present knowledge in nutrition. Oxford (United Kingdom): Wiley-Blackwell; 2012. p. 554–67.
- 63.Bayard O. Über das Kropfproblem. Schweiz Med Wochenschr 1923;53:732–7. [Google Scholar]
- 64.World Health Organization; United Nations Children’s Fund; International Council for Control of Iodine Deficiency Disorders. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. Geneva (Switzerland): World Health Organization; 2007.
- 65.Caulfield LE, Richard SA, Rivera JA, Musgrove P, Stunting REB. Wasting and micronutrient deficiency disorders. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. Disease control priorities in developing countries. 2nd ed. Washington: World Bank; 2006, p. 551–67. [PubMed] [Google Scholar]
- 66.Jooste PL, Weight MJ, Lombard CJ. Iodine concentration in household salt in South Africa. Bull World Health Organ 2001;79:534–40. [PMC free article] [PubMed] [Google Scholar]
- 67.WHO. Overcoming iodine deficiency disorders. Geneva (Switzerland): WHO; 1990.
- 68.UNICEF. World declaration on the survival, protection and development of children the World Summit for Children. New York: UNICEF; 1990. [DOI] [PubMed]
- 69.World Health Organization; United Nations Children’s Fund; International Council for the Control of Iodine Deficiency Disorders. Indicators for assessment of iodine deficiency disorders and their control program. Geneva: World Health Organization, United Nations Children’s Fund, International Council for the Control of Iodine Deficiency Disorders; 1993.
- 70.Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, Carter JA. Child development: risk factors for adverse outcomes in developing countries. Lancet 2007;369:145–57. [DOI] [PubMed] [Google Scholar]
- 71.Pearce EN, Andersson M, Zimmermann MB. Global iodine nutrition: where do we stand in 2013? Thyroid 2013;23:523–8. [DOI] [PubMed] [Google Scholar]
- 72.UNICEF. Children in an urban world. The state of the world's children 2012. New York: UNICEF; 2012.
- 73.Nath SK, Moinier B, Thuillier F, Rongier M, Desjeux JF. Urinary excretion of iodide and fluoride from supplemented food grade salt. Int J Vitam Nutr Res 1992;62:66–72. [PubMed] [Google Scholar]
- 74.Alexander WD, Harden RM, Harrison MT, Shimmins J. Some aspects of the absorption and concentration of iodide by the alimentary tract in man. Proc Nutr Soc 1967;26:62–6. [DOI] [PubMed] [Google Scholar]
- 75.Stanbury JB, Brownell GL, Riggs DS, Itioz J, Perinetti H, Del Castillo EB. Endemic goiter: the adaptation of man to iodine deficiency. Cambridge, MA: Harvard University Press; 1954. [Google Scholar]
- 76.Wayne EJ, Koutras DA, Alexander WD. Clinical aspects of iodine metabolism. Oxford (United Kingdom): Blackwell Scientific; 1964. [Google Scholar]
- 77.DeGroot LJ. Kinetic analysis of iodine metabolism. J Clin Endocrinol Metab 1966;26:149–73. [DOI] [PubMed] [Google Scholar]
- 78.Weaver J. Excretion of radioiodine in human milk. JAMA 1960;173(8):872–5. [DOI] [PubMed] [Google Scholar]
- 79.Eskandari S, Loo DD, Dai G, Levy O, Wright EM, Carrasco N. Thyroid Na+/I- symporter: mechanism, stoichiometry, and specificity. J Biol Chem 1997;272:27230–8. [DOI] [PubMed] [Google Scholar]
- 80.Andersson M, Zimmermann MB. Influence of iodine deficiency and excess on thyroid function tests. In: Brent GA, editor. Thyroid function testing. New York: Springer; 2010. p. 45–69.
- 81.Ermans AM, Delange F, Van der Velden M, Kinthaert J. Possible role of cyanide and thiocyanate in the etiology of endemic cretinism. Adv Exp Med Biol 1972;30:455–86. [PubMed] [Google Scholar]
- 82.Laurberg P, Nohr SB, Pedersen KM, Fuglsang E. Iodine nutrition in breast-fed infants is impaired by maternal smoking. J Clin Endocrinol Metab 2004;89:181–7. [DOI] [PubMed] [Google Scholar]
- 83.Gaitan E. Environmental goitrogenesis. Boca Raton (FL): CRC Press; 1989. [Google Scholar]
- 84.Pearce EN, Braverman LE. Environmental pollutants and the thyroid. Best Pract Res Clin Endocrinol Metab 2009;23:801–13. [DOI] [PubMed] [Google Scholar]
- 85.Urbansky ET. Perchlorate as an environmental contaminant. Environ Sci Pollut Res Int 2002;9:187–92. [DOI] [PubMed] [Google Scholar]
- 86.Kirk AB. Environmental perchlorate: why it matters. Anal Chim Acta 2006;567:4–12. [DOI] [PubMed] [Google Scholar]
- 87.Messina M, Redmond G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: a review of the relevant literature. Thyroid 2006;16:249–58. [DOI] [PubMed] [Google Scholar]
- 88.Zimmermann MB, Kohrle J. The impact of iron and selenium deficiencies on iodine and thyroid metabolism: biochemistry and relevance to public health. Thyroid 2002;12:867–78. [DOI] [PubMed] [Google Scholar]
- 89.Zimmermann MB. The influence of iron status on iodine utilization and thyroid function. Annu Rev Nutr 2006;26:367–89. [DOI] [PubMed] [Google Scholar]
- 90.Zimmermann MB, Burgi H, Hurrell RF. Iron deficiency predicts poor maternal thyroid status during pregnancy. J Clin Endocrinol Metab 2007;92:3436–40. [DOI] [PubMed] [Google Scholar]
- 91.European Commission. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of iodine. Brussels (Belgium): Scientific Committee on Food, European Commission; 2002. [Google Scholar]
- 92.World Health Organization. Human vitamin and mineral requirements. Rome: World Health Organization; 2002. [Google Scholar]
- 93.National Institute of Child Health and Human Development. Biomarkers of Nutrition for Development (BOND) program. 2011 [cited 2013 Jun 24]. Available from: http://www.nichd.nih.gov/global_nutrition/programs/bond/pages/index.aspx.
- 94.UNICEF, WHO. World summit for children–mid-decade goal: Iodine deficiency disorders. Geneva (Switzerland): UNICEF; 1994. [Google Scholar]
- 95.UNICEF. Sustainable elimination of iodine deficiency. New York: UNICEF; 2008. [Google Scholar]
- 96.National Bureau of Statistics. The 2010 Tanzania Demographic and Health Survey. Calverton (MD): ICF Macro; 2011. [Google Scholar]
- 97.Myatt M, Feleke T, Sadler K, Collins S. A field trial of a survey method for estimating the coverage of selective feeding programmes. Bull World Health Organ 2005;83:20–6. [PMC free article] [PubMed] [Google Scholar]
- 98.Andersson M, Karumbunathan V, Zimmermann MB. Global iodine status in 2011 and trends over the past decade. J Nutr 2012;142:744–50. [DOI] [PubMed] [Google Scholar]
- 99.Wheeler S, van der Haar F. Household wealth and iodised salt consumption: iodised salt at any price? Atlanta (GA): Emory University Rollins School of Public Health; 2004. [Google Scholar]
- 100.van der Haar F, Gerasimov G, Tyler VQ, Timmer A. Universal salt iodization in the Central and Eastern Europe, Commonwealth of Independent States (CEE/CIS) region during the decade 2000–09: experiences, achievements, and lessons learned. Food Nutr Bull 2011;32:S258–68. [DOI] [PubMed] [Google Scholar]
- 101.Ategbo EA, Sankar R, Schultink W, van der Haar F, Pandav CS. An assessment of progress toward universal salt iodization in Rajasthan, India, using iodine nutrition indicators in school-aged children and pregnant women from the same households. Asia Pac J Clin Nutr 2008;17:56–62. [PubMed] [Google Scholar]
- 102.Shamim AA, Christian P, Schulze KJ, Ali H, Kabir A, Rashid M, Labrique A, Salamatullah Q, West KP., Jr Iodine status in pregnancy and household salt iodine content in rural Bangladesh. Matern Child Nutr 2012;8:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sultanalieva RB, Mamutova S, van der Haar F. The current salt iodization strategy in Kyrgyzstan ensures sufficient iodine nutrition among school-age children but not pregnant women. Public Health Nutr 2010;13:623–30. [DOI] [PubMed] [Google Scholar]
- 104.Bentley B. A review of methods to measure dietary sodium intake. J Cardiovasc Nurs 2006;21:63–7. [DOI] [PubMed] [Google Scholar]
- 105.Leung A, Pearce EN. Iodine nutrition in North America. Hot Thyroidology 2007;5:1–12. [Google Scholar]
- 106.World Health Organization; Pan American Health Organization. A review of methods to determine the main sources of salt in the diet. 2010 [cited 9 Jun 2014]. Available from: http://new.paho.org/hq/index/php?option=com_content#x0026task=view#x0026id=3072#x0026ltemid=2376.
- 107.Gowans EM, Fraser CG. Biological variation in analyte concentrations in urine of apparently healthy men and women. Clin Chem 1987;33:847–50. [PubMed] [Google Scholar]
- 108.Ricós C, Jimenez CV, Hernandez A, Simon M, Perich C, Alvarez V, Minchinela J, Macia M. Biological variation in urine samples used for analyte measurements. Clin Chem 1994;40:472–7. [PubMed] [Google Scholar]
- 109.Fordyce FM. Database of the iodine content of food and diets populated with data from published literature. Keyworth (United Kingdom): British Geological Survey: Department for International Development; 2003. [Google Scholar]
- 110.Soriguer F, Gutierrez-Repiso C, Gonzalez-Romero S, Olveira G, Garriga MJ, Velasco I, Santiago P, de Escobar GM, Garcia-Fuentes E. Iodine concentration in cow's milk and its relation with urinary iodine concentrations in the population. Clin Nutr 2011;30:44–8. [DOI] [PubMed] [Google Scholar]
- 111.Gibson RS. Principles of nutritional assessment. New York: Oxford University Press; 2005. [Google Scholar]
- 112.Thompson FE, Subar AF. Dietary assessment methodology. In: Coulston AM, Boushey CJ, editors. Nutrition in the prevention and treatment of disease. 2nd ed. London: Elsevier Academic Press; 2008. p. 3–39.
- 113.Rasmussen LB, Carle A, Jorgensen T, Knudsen N, Laurberg P, Pedersen IB, Perrild H, Vejbjerg P, Ovesen L. Iodine intake before and after mandatory iodization in Denmark: results from the Danish Investigation of Iodine Intake and Thyroid Diseases (DanThyr) study. Br J Nutr 2008;100:166–73. [DOI] [PubMed] [Google Scholar]
- 114.Brantsaeter AL, Haugen M, Julshamn K, Alexander J, Meltzer HM. Evaluation of urinary iodine excretion as a biomarker for intake of milk and dairy products in pregnant women in the Norwegian Mother and Child Cohort Study (MoBa). Eur J Clin Nutr 2009;63:347–54. [DOI] [PubMed] [Google Scholar]
- 115.Flynn A, Hirvonen T, Mensink GB, Ocke MC, Serra-Majem L, Stos K, Szponar L, Tetens I, Turrini A, Fletcher R, et al. Intake of selected nutrients from foods, from fortification and from supplements in various European countries. Food Nutr Res 2009;53:1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Verkaik-Kloosterman J, van't Veer P, Ocke MC. Simulation model accurately estimates total dietary iodine intake. J Nutr 2009;139:1419–25. [DOI] [PubMed] [Google Scholar]
- 117.Verkaik-Kloosterman J, van't Veer P, Ocke MC. Reduction of salt: will iodine intake remain adequate in The Netherlands? Br J Nutr 2010;104:1712–8. [DOI] [PubMed] [Google Scholar]
- 118.Schiess S, Cressey PJ, Thomson BM. Predictive modelling of interventions to improve iodine intake in New Zealand. Public Health Nutr 2012;15:1932–40. [DOI] [PubMed] [Google Scholar]
- 119.Manz F, Johner SA, Wentz A, Boeing H, Remer T. Water balance throughout the adult life span in a German population. Br J Nutr 2012;107:1673–81. [DOI] [PubMed] [Google Scholar]
- 120.Ekelund U, Besson H, Luan J, May AM, Sharp SJ, Brage S, Travier N, Agudo A, Slimani N, Rinaldi S, et al. Physical activity and gain in abdominal adiposity and body weight: prospective cohort study in 288,498 men and women. Am J Clin Nutr 2011;93:826–35. [DOI] [PubMed] [Google Scholar]
- 121.Zimmermann MB, Aeberli I, Andersson M, Assey V, Yorg JA, Jooste P, Jukic T, Kartono D, Kusic Z, Pretell E, et al. Thyroglobulin is a sensitive measure of both deficient and excess iodine intakes in children and indicates no adverse effects on thyroid function in the UIC range of 100–299 mug/L: a UNICEF/ICCIDD study group report. J Clin Endocrinol Metab 2013;98:1271–80. [DOI] [PubMed] [Google Scholar]
- 122.Jooste PL, Strydom E. Methods for determination of iodine in urine and salt. Best Pract Res Clin Endocrinol Metab 2010;24:77–88. [DOI] [PubMed] [Google Scholar]
- 123.Pandav CS, Arora NK, Krishnan A, Sankar R, Pandav S, Karmarkar MG. Validation of spot-testing kits to determine iodine content in salt. Bull World Health Organ 2000;78:975–80. [PMC free article] [PubMed] [Google Scholar]
- 124.Jooste PL, Strydom EE. Performance of rapid test kits designed to monitor salt iodine content. New York: WHO/UNICEF; 2004. [Google Scholar]
- 125.van der Haar F, Gorstein J, Houston R, Codling K, Jooste P, Strydom E, Tripp K, Ivanova L, Ategbo E, Timmer A. Toward the development of a practical manual for estimating household coverage of adequately iodized salt. New York: UNICEF; 2008. [Google Scholar]
- 126.Demaeyer EM, Lowenstein FW, Thilly CH. The control of endemic goitre. Geneva (Switzerland): World Health Organization; 1979.
- 127.Sullivan K, Houston R, Cervinskas J, Gorstein J, editors. Monitoring universal salt iodization programs. Ottowa (Canada): The Programme Against Micronutrient Malnutrition, The Micronutrient Initiative, The International Council for Control of Iodine Deficiency Disorders; 1995.
- 128.Codex Alimentarius Commission. Standard for food grade salt. Geneva (Switzerland): Food and Agriculture Organization of the United Nations, WHO; 2006.
- 129.European Salt Producersapos Association. Determination of total iodine: titrimetric method with sodium thiosulphate. 2005. Available from: http://eusalt.com/sites/www.eusalt.com/files/page-documents/EuSalt%20AS002-2005%20Total%20Iodine%20-%20Titrimetric%20Method%20with%20Sodium%20Thiosulphate.pdf.
- 130.Salt Research Institute of China National Salt Industry Corp. WYD iodine checker instruction manual [cited 2013 Feb 22]. Available from: http://ebookbrowse.com/salt-iodine-wyd-iodine-checker-instruction-manual-doc-d403972037.
- 131.Dearth-Wesley T, Makhmudov A, Pfeiffer CM, Caldwell K. Fast and reliable salt iodine measurement: evaluation of the WYD iodine checker in comparison with iodometric titration. Food Nutr Bull 2004;25:130–6. [DOI] [PubMed] [Google Scholar]
- 132.Rohner F, Garrett GS, Laillou A, Frey SK, Mothes R, Schweigert FJ, Locatelli-Rossi L. Validation of a user-friendly and rapid method for quantifying iodine content of salt. Food Nutr Bull 2012;33:S330–5. [DOI] [PubMed] [Google Scholar]
- 133.Ma G, Maberly K, Waite K. Evidence based evaluation of iodine checkers. Report to UNICEF Regional Office for East Asia and the Pacific. Sydney (Australia): UNICEF; 2006. [Google Scholar]
- 134.Zakharova EA, Slepchenko GB, Kolpakova E. [Electrochemical methods of control of iodine contents in drinks.] Vopr Pitan 2001;70:32–6. [PubMed] [Google Scholar]
- 135.Shah MH, Jaffar M, Shaheen N, Rasool N. Estimation of iodine in fortified salts by an improved electrometric method. Nutr Food Sci 2007;37:115–22. [Google Scholar]
- 136.Harris MJ, Jooste PL, Charlton KE. The use of iodised salt in the manufacturing of processed foods in South Africa: bread and bread premixes, margarine, and flavourants of salty snacks. Int J Food Sci Nutr 2003;54:13–9. [DOI] [PubMed] [Google Scholar]
- 137.International Organization for Standardization. ISO/IEC 17025 resource center. 2013 [cited 2014 Feb 10]. Available from: http://www.isoiec17025.com/.
- 138.Sullivan KM, May S, Maberly G. Urinary iodine assessment: a manual on survey and laboratory methods. Atlanta (GA): Rollins School of Public Health at Emory University; 2000. [Google Scholar]
- 139.Carriquiry AL. Assessing the prevalence of nutrient inadequacy. Public Health Nutr 1999;2:23–33. [DOI] [PubMed] [Google Scholar]
- 140.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes: applications in dietary assessment. Washington: National Academies Press; 2000. [PubMed] [Google Scholar]
- 141.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes: applications in dietary planning. Washington: National Academies Press; 2003. [PubMed] [Google Scholar]
- 142.de Lauzon B, Volatier JL, Martin A. A Monte Carlo simulation to validate the EAR cut-point method for assessing the prevalence of nutrient inadequacy at the population level. Public Health Nutr 2004;7:893–900. [DOI] [PubMed] [Google Scholar]
- 143.Ribas-Barba L, Serra-Majem L, Roman-Vinas B, Ngo J, Garcia-Alvarez A. Effects of dietary assessment methods on assessing risk of nutrient intake adequacy at the population level: from theory to practice. Br J Nutr 2009;101 Suppl 2:S64–72. [DOI] [PubMed] [Google Scholar]
- 144.Murphy SP, Barr SI. Practice paper of the American Dietetic Association: using the Dietary Reference Intakes. J Am Diet Assoc 2011;111:762–70. [DOI] [PubMed] [Google Scholar]
- 145.Allen L, de Benoist B, Dary O, Hurrell R. Guidelines on food fortification with micronutrients. Geneva: WHO/FAO; 2006. [Google Scholar]
- 146.Murphy SP, Barr SI, Poos MI. Using the new dietary reference intakes to assess diets: a map to the maze. Nutr Rev 2002;60:267–75. [DOI] [PubMed] [Google Scholar]
- 147.Andersen S, Karmisholt J, Pedersen KM, Laurberg P. Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br J Nutr 2008;99:813–8. [DOI] [PubMed] [Google Scholar]
- 148.König F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr 2011;141:2049–54. [DOI] [PubMed] [Google Scholar]
- 149.Murphy SP. How consideration of population variance and individuality affects our understanding of nutritional requirements in human health and disease. J Nutr 2001;131 Suppl:361S–5S. [DOI] [PubMed] [Google Scholar]
- 150.Murphy SP, Guenther PM, Kretsch MJ. Using the dietary reference intakes to assess intakes of groups: pitfalls to avoid. J Am Diet Assoc 2006;106:1550–3. [DOI] [PubMed] [Google Scholar]
- 151.Andersson M, Aeberli I, Wüst N, Piacenza AM, Bucher T, Henschen I, Haldimann M, Zimmermann MB. The Swiss iodized salt program provides adequate iodine for school children and pregnant women, but weaning infants not receiving iodine-containing complementary foods as well as their mothers are iodine deficient. J Clin Endocrinol Metab 2010;95:5217–24. [DOI] [PubMed] [Google Scholar]
- 152.Hoffmann K, Boeing H, Dufour A, Volatier JL, Telman J, Virtanen M, Becker W, De Henauw S. Estimating the distribution of usual dietary intake by short-term measurements. Eur J Clin Nutr 2002;56 Suppl 2:S53–62. [DOI] [PubMed] [Google Scholar]
- 153.Dodd KW, Guenther PM, Freedman LS, Subar AF, Kipnis V, Midthune D, Tooze JA, Krebs-Smith SM. Statistical methods for estimating usual intake of nutrients and foods: a review of the theory. J Am Diet Assoc 2006;106:1640–50. [DOI] [PubMed] [Google Scholar]
- 154.Nusser SM, Carriquiry AL, Dodd KW, Fuller WA. A semiparametric transformation approach to estimating usual daily intake distributions. J Am Stat Assoc 1996;91:1440–9. [Google Scholar]
- 155.Guenther PM, Kott PS, Carriquiry AL. Development of an approach for estimating usual nutrient intake distributions at the population level. J Nutr 1997;127:1106–12. [DOI] [PubMed] [Google Scholar]
- 156.Carriquiry AL. Estimation of usual intake distributions of nutrients and foods. J Nutr 2003;133 Suppl:601S–8S. [DOI] [PubMed] [Google Scholar]
- 157.Jahns L, Carriquiry A, Arab L, Mroz TA, Popkin BM. Within- and between-person variation in nutrient intakes of Russian and US children differs by sex and age. J Nutr 2004;134:3114–20. [DOI] [PubMed] [Google Scholar]
- 158.Jahns L, Arab L, Carriquiry A, Popkin BM. The use of external within-person variance estimates to adjust nutrient intake distributions over time and across populations. Public Health Nutr 2005;8:69–76. [DOI] [PubMed] [Google Scholar]
- 159.Tooze JA, Midthune D, Dodd KW, Freedman LS, Krebs-Smith SM, Subar AF, Guenther PM, Carroll RJ, Kipnis V. A new statistical method for estimating the usual intake of episodically consumed foods with application to their distribution. J Am Diet Assoc 2006;106:1575–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tooze JA, Kipnis V, Buckman DW, Carroll RJ, Freedman LS, Guenther PM, Krebs-Smith SM, Subar AF, Dodd KW. A mixed-effects model approach for estimating the distribution of usual intake of nutrients: the NCI method. Stat Med 2010;29:2857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang S, Midthune D, Guenther PM, Krebs-Smith SM, Kipnis V, Dodd KW, Buckman DW, Tooze JA, Freedman L, Carroll RJ. A new multivariate measurement error model with zero-inflated dietary data, and its application to dietary assessment. Ann Appl Stat 2011;5(2B):1456–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Centers for Disease Control and Prevention. NHANES dietary web tutorial. 2013 [cited 2014 Feb 5]. Available from: http://www.cdc.gov/nchs/tutorials/Dietary/.
- 163.Center for Survey Statistics and Methodology. Estimation of usual intake distributions. 2013 [cited 2014 Feb 5]. Available from: http://streaming.stat.iastate.edu/cssm/.
- 164.National Cancer Institute. Usual dietary intakes: SAS macros for analysis of a single dietary component. 2012 [cited 2014 Feb 5]. Available from: http://riskfactor.cancer.gov/diet/usualintakes/macros_single.html.
- 165.van Ommen B, El-Sohemy A, Hesketh J, Kaput J, Fenech M, Evelo CT, McArdle HJ, Bouwman J, Lietz G, Mathers JC, et al. The Micronutrient Genomics Project: a community-driven knowledge base for micronutrient research. Genes Nutr 2010;5:285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Micronutrient Genomics Project [homepage on the Internet] [cited 2013 Oct 18]. Available from: http://www.micronutrientgenomics.org./index.php/Main_Page.