Abstract
STUDY QUESTION
Is there an association between alcohol intake and semen quality and serum reproductive hormones among healthy men from the USA and Europe?
SUMMARY ANSWER
Moderate alcohol intake is not adversely associated with semen quality in healthy men, whereas it was associated with higher serum testosterone levels.
WHAT IS KNOWN ALREADY
High alcohol intake has been associated with a wide range of diseases. However, few studies have examined the correlation between alcohol and reproductive function and most have been conducted in selected populations of infertile men or have a small sample size and the results have been contradictory.
STUDY DESIGN, SIZE, DURATION
A coordinated international cross-sectional study among 8344 healthy men. A total of 1872 fertile men aged 18–45 years (with pregnant partners) from four European cities and four US states, and 6472 young men (most with unknown fertility) aged 18–28 years from the general population in six European countries were recruited.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The men were recruited using standardized protocols. A semen analysis was performed and men completed a questionnaire on health and lifestyle, including their intake of beer, wine and liquor during the week prior to their visit. Semen quality (semen volume, sperm concentration, percentage motile and morphologically normal sperm) and serum reproductive hormones (FSH, LH, testosterone, sex hormone-binding globulin, and inhibin B and free testosterone) were examined.
MAIN RESULTS AND THE ROLE OF CHANCE
The participation rate for our populations was 20–30%. We found no consistent association between any semen variable and alcohol consumption, which was low/moderate in this group (median weekly intake 8 units), either for total consumption or consumption by type of alcohol. However, we found a linear association between total alcohol consumption and total or free testosterone in both groups of men. Young and fertile men who consumed >20 units of alcohol per week had, respectively, 24.6 pmol/l (95% confidence interval 16.3–32.9) and 19.7 pmol/l (7.1–32.2) higher free testosterone than men with a weekly intake between 1 and 10 units. Alcohol intake was not significantly associated with serum inhibin B, FSH or LH levels in either group of men. The study is the largest of its kind and has sufficient power to detect changes in semen quality and reproductive hormones.
LIMITATIONS, REASONS FOR CAUTION
The participation rate was low, but higher than in most previous semen quality studies. In addition, the study was cross-sectional and the men were asked to recall their alcohol intake in the previous week, which was used as a marker of intake up to 3 months before. If consumption in that week differed from the typical weekly intake and the intake 3 months earlier, misclassification of exposure may have occurred. However, the men were unaware of their semen quality when they responded to the questions about alcohol intake. Furthermore, we cannot exclude that our findings are due to unmeasured confounders, including diet, exercise, stress, occupation and risk-taking behavior.
WIDER IMPLICATIONS OF THE FINDINGS
Our study suggests that moderate alcohol intake is not adversely associated with semen quality in healthy men, whereas it was associated with higher serum testosterone levels which may be due to a changed metabolism of testosterone in the liver. Healthy men may therefore be advised that occasional moderate alcohol intake may not harm their reproductive health; we cannot address the risk of high alcohol consumption of longer duration or binge drinking on semen quality and male reproductive hormones.
STUDY FUNDING/COMPETING INTEREST(S)
All funding sources were non-profitable and sponsors of this study played no role in the study design, in data collection, analysis, or interpretation, or in the writing of the article. The authors have no conflicts of interest.
Keywords: semen quality, alcohol, reproductive hormones, male fertility
Introduction
High alcohol intake has been associated with a wide range of diseases, such as liver cirrhosis, as well as cancers of the oral cavity, esophagus, intestine and breast (Parry et al., 2011). Interestingly, a U-shaped relationship between alcohol intake and mortality and cardiovascular disease has been found, suggesting that moderate alcohol intake may be beneficial (Saleem and Basha, 2010).
A Chinese study among 1346 men did not find an association between semen quality and alcohol intake in high doses (>120 units per months) (Li et al., 2009), which has been confirmed by other studies (Marshburn et al., 1989; Dunphy et al., 1991; Oldereid et al., 1992; Chia et al., 1998; Kunzle et al., 2003; Lopez Teijon et al., 2007; Hansen et al., 2012; Povey et al., 2012). However, some studies found association between alcohol intake and semen quality (Martini et al., 2004; Stutz et al., 2004; Muthusami and Chinnaswamy, 2005; Gaur et al., 2010), but included alcoholic men or could not separate the effects of alcohol and smoking. In addition, most previous studies suffered from small sample size or included only selected groups of infertile men. Also, studies of associations between alcohol consumption and sex steroid levels have been inconsistent, with some studies suggesting no association and others supporting lower sex hormone-binding globulin (SHBG) and higher free androgen index among men with higher alcohol consumption (Handa et al., 1997; Tamimi et al., 2001; Allen et al., 2002; Muller et al., 2003; Svartberg et al., 2003; Shiels et al., 2009; Hansen et al., 2012).
To our knowledge no previous studies have investigated associations between semen quality, reproductive hormones and alcohol intake in a large multicenter study. Therefore, we investigated these associations in 8344 healthy men by pooling data from several standardized cross-sectional studies from the USA and Europe.
Materials and Methods
Populations
Informed consent was obtained from all participants.
Group A: young men from the general population (EU)
For the European Union (EU), in Finland, Estonia, Lithuania, Germany, Norway and Denmark all young men, except those suffering from chronic severe diseases (<15%), were required to attend a compulsory medical examination to screen for eligibility for military service. Included men who responded to the questions on alcohol intake had an age range between 18 and 28 years. Men from Turku in Finland (N = 665), Tartu in Estonia (N = 187), Kaunas in Lithuania (N = 170), Hamburg and Leipzig in Germany (N = 966), Oslo in Norway (N = 229) and Copenhagen and Aalborg in Denmark (N = 4255) were recruited into the study population from 1996 to 2007 irrespective of whether they were found to be fit for military service. For a detailed description of these studies see Jorgensen et al. (2002, 2012) and Paasch et al. (2008). The local ethical committees approved the studies.
Group B: fertile men (USA and EU)
Pregnant women were recruited at prenatal clinics affiliated with university hospitals in five US and four European cities between 1996 and 2005 and their partners were invited to participate in the present study. Couples were eligible if both partners were over 18 (USA) or 20 (EU) to 45 years of age at the time of invitation, residing in the local referral area of the recruiting hospital, and in the EU study born in the country in which the participant was currently living, regardless of ethnicity. Further, the current pregnancy of the female partner should not be a result of any infertility treatment or assisted reproductive technologies. A total of 798 US men from four centers participated (men from NY were excluded due to problems with standardization): 188 from Los Angeles, CA; 229 from Minneapolis, MN; 228 from Columbia, MO; and 153 from Iowa City, IA. Altogether, 1074 EU men participated in the study: 347 from Copenhagen, 203 from Paris, 275 from Turku and 249 from Edinburgh. A detailed description has previously been published (Jorgensen et al., 2001; Swan et al., 2003). The local ethical committees approved the studies.
Questionnaire and physical examination
A physical examination, including a genital exam, was performed at the study visit. BMI was calculated as weight in kilograms divided by squared height in meters. In connection with the physical examination the men also delivered a semen sample and a blood sample was taken.
All men completed a questionnaire, which was identical across all studies. Questionnaires were completed before the laboratory test results of semen and blood samples were available and the men therefore did not at the time have any knowledge of their semen quality and reproductive hormone levels. Questionnaires included questions on alcohol consumption and relevant confounders (e.g. center, age, season, period of abstinence adjusted to 96 h, BMI, current smoking, genital surgery, urogenital infections and cryptorchidism). All men were asked to specify the number of bottles of beer (33 cl), glasses of wine (one unit) and units of liquor (2 cl) they had consumed during the week prior to the visit. The total alcohol intake was estimated simply by adding the intake of beer, wine and liquor, assuming that one unit of alcohol contained 12 g of alcohol. The questionnaire included information on current age, cryptorchidism with or without spontaneous descent, genital surgery (operation for hernia, varicocele, torsion or cancer of the testis), and urogenital infections (chlamydia, gonorrhea, cystitis, prostatitis and epididymitis). In addition, information on exposure to smoking in utero and current smoking was obtained (Supplementary data, Table SI).
Semen analysis
All men provided a semen sample, most collected at the laboratory associated room and some collected at home. The ejaculation abstinence time and time between sample collection and analysis were recorded. All semen samples were analyzed according to World Health Organization (WHO) guidelines (Organisation, 1992, 1999), as described in detail (Jorgensen et al., 1997; Swan et al., 2003). Motility assessment was performed, classifying the spermatozoa as either motile (WHO motility classes A, B or C) or immotile (class D). Smears for the morphology analysis for the European study were air dried, fixed in 96% ethanol and sent to Department of Physiology, University of Turku, Finland or The Department of Growth and Reproduction, University Department of Rigshospitalet, Copenhagen, Denmark for staining, and were assessed according to strict criteria (Menkveld et al., 1990). Morphology was assessed for 5089 young men from the general population. The smears from all US sites were sent to UC Davis for fixing, staining and assessment according to strict criteria. The EU study centers participated in an external quality control program of sperm concentration coordinated by the University Department of Growth and Reproduction, Copenhagen, Denmark (Jorgensen et al., 1997). For the US study, a quality control program was maintained by the University of California (Brazil et al., 2004a,b). For the EU program, each month, five quality control samples were sent to the European centers, as well as to the US quality control center at UC Davis and analyzed blind, as described above. For the US study, 12–16 quality control samples for concentration and a videotape of 7–10 samples were sent to each US site approximately every 3 months. Across the study period, inter-laboratory differences remained largely unchanged.
Serum samples
Hormone analyses were performed by Department of Growth and Reproduction, Rigshospitalet. Serum levels of FSH, LH and SHBG were determined using a time-resolved immunofluorometric assay (Delfia, Wallac, Turku, Finland). Testosterone levels were determined using a time-resolved fluoroimmunoassay (Delfia, Wallac, Turku, Finland) and inhibin-B by a specific two-sided enzyme immunometric assay (Serotec, UK). Intra- and inter-assay coefficients of variation (CV) for measurements of both FSH and LH were 3 and 4.5%, respectively. The CV for testosterone and SHBG were <8 and <5%, respectively. The intra- and inter-assay CV for inhibin-B were 15 and 18%, respectively. Free testosterone was calculated (cFT) based on the measured serum concentrations of total testosterone and SHBG using the method of Vermeulen et al. (1999) and a fixed albumin concentration of 0.62 mmol/l (Vermeulen et al., 1999). Analyses for different cohorts were conducted at one time to minimize between-cohort variability.
Statistics
Outcome variables were: semen quality (semen volume, sperm concentration, total sperm count, and percentages of motile and morphologically normal spermatozoa) and reproductive hormones (LH, FSH, testosterone, SBHG, cFT and inhibin B). All analyses were performed separately for young (group A) and fertile men (group B) as these groups of men differed considerably in semen variables, hormone concentrations, covariates and alcohol consumption. Exposure variables were: alcohol intake during the week prior to filling out the questionnaire categorized as 0; 1–10 units (reference); 11–20 units and >20 units (finer strata were also considered, but this did not change the findings). All analyses were initially performed for total alcohol intake during the prior week and then separately by type (beer, wine or liquor) to determine the independent associations with each type of alcohol. In the type-specific analyses the exposure categories were: 0; 1–10 and >10 units due to the small number of men with high intake of only one alcohol type.
First we compared semen quality and reproductive hormones for men in relation to alcohol intake by Kruskal–Wallis test. Then we compared the distributions of the variables from the questionnaires and physical examinations among men with different alcohol intake (0; 1–20; >20 units/week) by chi-square test in order to identify potential confounders.
Finally, data were analyzed using multiple linear regressions. Normally distributed outcome variables were entered directly as continuous variables in the model, whereas sperm concentration, total sperm count, FSH and LH were transformed by use of the natural logarithm (zero set to one) to obtain normality and back-transformed to obtain the percentage change in these variables. Covariates initially included factors possibly associated with semen parameters, reproductive hormone levels, or alcohol intake and were then excluded stepwise if they did not change the estimate by >10%. The same set of confounders was used for all semen outcomes: center, age, season, period of abstinence, BMI (categorized, see Supplementary data, Table SI), current smoking, genital surgery, urogenital infections and cryptorchidism (with or without spontaneous descent) and the time from ejaculation to sample analysis for sperm motility. We adjusted for all confounders since there were large variations in distributions across country, populations and alcohol intake (Supplementary data, Table SI). The results for fertile men (group B) were not adjusted for in utero exposure to smoking as this information was missing for 30% of the US men. The odds of having a sperm concentration <15 or 20 mill/ml, which is the reference for normal concentration (Organisation, 1999, 2010), were calculated by multiple logistic regression analyses taking into account the above confounders. For serum reproductive hormones, confounders were the time of blood sampling (adjusted to 8 AM), center, BMI, age and smoking. We evaluated the fit of the regression models by testing the residuals for normality and by inspecting the residual plots. Analyses were performed using PASW GradPack V.18.0 (SPSS, Inc.) and a P-value of <0.05 was considered statistically significant. Results were presented as regression coefficients with 95% confidence intervals (CI). The analyses were repeated among men from the different centers separately, for all European and all US men, and for all men.
Results
Study population
A total of 8826 men participated. Of these 8344 responded to all questions about alcohol intake the week prior to the examination. Men who did not provide complete information about alcohol intake (5.5%) did not differ significantly from the others with respect to other lifestyle factors and semen quality (data not shown). The mean alcohol intake during the week prior to study visit varied among the different groups of men; the young men (group A) consumed a mean of 12.3 units/week (SD 13.3), EU fertile men 9.1 (9.8) and the US fertile men 4.6 units/week (9.0). More than 60% of the total alcohol intake originated from beer, the young men (group A) consumed more liquor, whereas the fertile men (group B) consumed more wine. Only ∼8 and 2% of the men from group A and B, respectively, consumed >30 units of alcohol per week. As there were differences between group A and B in semen quality and reproductive hormones (Table I) as well as lifestyle (Supplementary data, Table SI), analyses were performed separately for the two groups. Men who drank more alcohol also smoked more and had been exposed to smoking in utero more often. They tended to be from higher social class and have a shorter period of abstinence and a later time of blood sampling, although not consistently in all groups (Supplementary data, Table SI). Age, genital surgery, urogenital infections or cryptorchidism were not related to alcohol intake.
Table I.
Median (25–75 percentiles) of unadjusted semen variables and serum reproductive hormones among young men (group A) and fertile men (group B) from the European Union and USA (total n = 8344) according to alcohol intake in the week prior to the visit.
| Alcohol intake the week prior to the visit (units/week) | Semen variables |
Reproductive hormones |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Semen volume (ml) | Sperm concentration (million/ml) | Total sperm count (million) | Motile sperm (%) | Morphologically normal forms (%)a | FSH (IU/l) | LH (IU/l) | Testosterone (nmol/l) | SHBG (nmol/l) | Free testosterone (pmol/l) | Inhibin B (pg/ml) | |
| Group A (N = 6472) | |||||||||||
| 0 (N = 1133) | 3.0 (2.2–4.1) | 48 (23–84) | 143 (63–263) | 68 (59–76) | 7.0 (4.0–11.0) | 2.9b (2.0–4.2) | 3.6 (2.7–4.6) | 21.2b (17.2–26.1) | 30b (23–39) | 273b (223–331) | 190 (148–244) |
| 1–10 (N = 2467) | 3.0 (2.2–4.1) | 47 (23–83) | 141 (62–256) | 69 (61–76) | 7.0 (4.0–11.0) | 2.8b (1.9–4.1) | 3.6 (2.8–4.7) | 22.2b (17.9–26.8) | 30b (23–38) | 286b (235–346) | 199 (154–256) |
| 11–20 (N = 1635) | 3.2 (2.3–4.3) | 46 (23–80) | 141 (65–252) | 69 (59–76) | 6.5 (3.5–10.0) | 2.7b (1.9–4.0) | 3.6 (2.8–4.7) | 22.7b (18.4–28.0) | 29b (22–37) | 298b (248–359) | 197 (150–251) |
| >20 (N = 1237) | 3.2 (2.3–4.3) | 46 (23–83) | 146 (65–264) | 68 (59–75) | 7.0 (3.5–10.5) | 2.7b (1.8–3.8) | 3.6 (2.8–4.7) | 23.1b (19.1–27.8) | 28b (22–35) | 311b (255–373) | 200 (151–252) |
| Group B (N = 1872) | |||||||||||
| 0 (N = 560) | 3.5 (2.5–4.8) | 64 (36–101) | 220 (118–365) | 59b (52–66) | 9.7 (6.1–14.0) | 3.3 (2.3–4.5) | 3.5 (2.7–4.5) | 18.7b (14.3–23.9) | 28 (20–37) | 239b (191–295) | 195b (148–248) |
| 1–10 (N = 883) | 3.7 (2.6–4.8) | 69 (41–112) | 248 (136–421) | 60b (53–69) | 9.7 (6.5–14.0) | 3.1 (2.2–4.3) | 3.4 (2.7–4.4) | 20.1b (15.9–24.1) | 30 (23–39) | 247b (205–295) | 210b (164–268) |
| 11–20 (N = 261) | 3.6 (2.6–5.0) | 75 (42–116) | 250 (139–400) | 63b (55–71) | 9.7 (5.8–12.9) | 3.1 (2.3–4.5) | 3.6 (2.7–4.7) | 20.3b (16.5–24.4) | 29 (22–38) | 259b (212–312) | 214b (155–271) |
| >20 (N = 168) | 3.5 (2.5–4.7) | 70 (44–113) | 262 (143–408) | 64b (55–73) | 9.7 (6.1–14.3) | 3.2 (2.2–4.4) | 3.6 (2.9–4.7) | 20.3b (16.4–25.4) | 30 (22–37) | 264b (226–314) | 200b (148–258) |
aMorphology counted by Strict criteria among 1872 fertile men and 5089 young men from the general population.
bSignificant difference between the four groups with Kruskal–Wallis test.
SHBG: sex hormone-binding globulin.
Semen quality
Alcohol consumption was not associated with a consistent change in any semen variables in any of the groups in the unadjusted analyses (Table I), although alcohol intake was significantly associated with increased motility in fertile men. Even after adjusting for confounders, we saw no association between alcohol and semen quality (Table II, Fig. 1), although fertile men with an alcohol intake above 20 units the week prior to the visit had more motile sperm (2.8% (95% CI, 0.9–4.8%)) than men with an intake of 1–10 units. The analyses were repeated for men from the different countries, from the USA and Europe separately, and for all men together, and no consistent pattern between alcohol intake and semen quality was found in any population. In addition, the odds of having a sperm concentration <20 mill/ml, which was the WHO cut off level at that time (Organisation, 1999), and the current cut off of 15 mill/ml (Organisation, 2010) was not significantly associated with alcohol intake (data not shown).
Table II.
Adjusteda results from the regression analyses (β coefficients) with 95% confidence intervals of semen quality by total alcohol intake among young men (group A) and fertile men (group B) according to alcohol intake the week prior to the visit.
| Alcohol intake the week prior to the visit (units) | Semen volume ml |
Sperm concentrationb mill/ml |
Total sperm countb mill |
% motile spermatozoac | % morphologically normal spermatozoad | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Young men (group A, N = 6472) Adjusteda difference from reference group in semen variables (sperm concentration and total sperm count as percentage) | ||||||||||
| 0 | −0.1 | −0.2; 0.1 | −0.7 | −8.2; 7.6 | −0.9 | −9.5; 8.4 | −0.7 | −1.7; 0.4 | 0.2 | −0.2; 0.6 |
| 1–10 | Reference | Reference | Reference | Reference | Reference | |||||
| 11–20 | 0.0 | −0.1; 0.1 | 3.6 | −3.4; 11.2 | 6.8 | −1.4; 15.7 | 0.0 | −1.0; 0.9 | −0.5 | −0.9; −0.2 |
| >20 | 0.1 | 0.0; 0.2 | 2.7 | −5.0; 11.0 | 5.8 | −3.1; 15.6 | −0.4 | −1.5; 0.6 | −0.2 | −0.5; 0.2 |
| Fertile men (group B, N = 1872) Adjusteda difference from reference group in semen variables (sperm concentration and total sperm count as percentage) | ||||||||||
| 0 | 0.0 | −0.2; 0.2 | −11.4 | −19.3; −2.8 | −11.2 | −19.9; −1.7 | −0.4 | −1.7; 0.9 | −0.4 | −1.0; 0.3 |
| 1–10 | Reference | Reference | Reference | Reference | Reference | |||||
| 11–20 | −0.1 | −0.3; 0.2 | 3.2 | −8.0; 15.5 | −0.3 | −12.0; 13.0 | 1.5 | −0.1; 3.1 | −0.3 | −1.1; 0.5 |
| >20 | 0.0 | −0.3; 0.3 | 2.7 | −10.5; 17.8 | 1.1 | −13.2; 17.6 | 2.8 | 0.9; 4.8 | 0.1 | −0.9; 1.0 |
aAdjusted for center, age, season, period of abstinence adjusted to 96 h, BMI, current smoking, genital surgery, urogenital infections and cryptorchidism (with or without spontaneous descent), for sperm motility time from delivery until evaluation of the sample and, for young men, in utero exposure to smoking.
bSperm concentration and total sperm count transformed by the use of natural logarithm and back transformed giving the percentage change.
cMotile sperm was defined as the sum of class A, B or C.
dMorphology counted by Strict criteria among 5089 young men from the general population.
Figure 1.
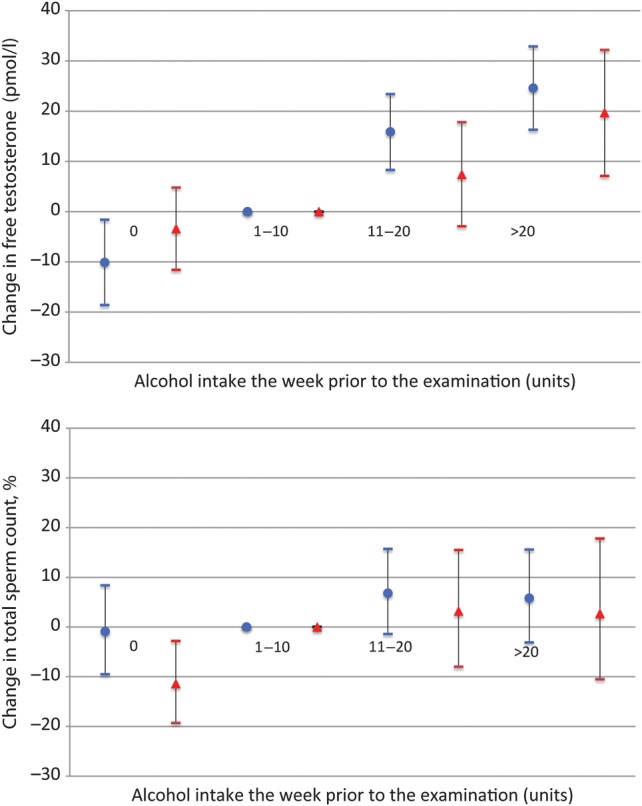
Adjusted changes in total sperm count [adjusted for center, age, season, period of abstinence adjusted to 96 h, BMI, current smoking, genital surgery, urogenital infections and cryptorchidism (with or without spontaneous descent) and, for young men, in utero exposure to smoking] free serum testosterone (pmol/l) [adjusted for current smoking, BMI, age and center and time of blood sampling (8.00 AM reference)] and 95% confidence intervals according to alcohol intake in the week prior to the visit (1–10 units reference) among 8344 young men (blue, group A, n = 6472) and fertile men (red, group B, n = 1872).
Serum hormones
Serum testosterone and cFT were higher among men with higher alcohol intake, whereas SHBG was only related to alcohol intake in group A (Table I), where SHBG was lower among men with higher alcohol intake (Table I; Supplementary data, Table SII). Young (group A) and fertile men (group B) with an alcohol intake >20 units had, respectively, 24.6 pmol/l (16.3–32.9) and 19.7 pmol/l (7.1–32.2) higher cFT than men with a weekly intake between 1 and 10 units. Those with an alcohol intake of 10–20 units had, respectively, 15.9 pmol/l (8.3–23.4) and 7.4 pmol/l (−2.9 to 17.8) higher cFT than men with an intake of 1–10 units. Total testosterone levels were 1.0 nmol/l (0.5–1.6) and 0.9 nmol/l (−0.2 to 1.9) higher among group A and B, respectively, with an alcohol intake >20 units compared with men with an intake of 1–10 units, and the same pattern was found when analyses were repeated among all men and among men from Europe and the USA separately (data not shown). No consistent pattern in FSH, LH or inhibin B was found in relation to units of alcohol intake (Supplementary data, Table SII). Also, no association with semen quality and reproductive hormones was found for men with an alcohol intake the week prior to the visit >30 units (data not shown).
We repeated the analyses for beer, wine or liquor intake separately and found no consistent association with semen quality or serum reproductive hormones (data not shown) except for serum testosterone and cFT, which increased in the same magnitude with increase in beer, wine or liquor intake as with increase in total alcohol intake (data not shown).
Discussion
In this large cross-sectional study among >8000 European and American healthy men we found no consistent association between total alcohol consumption in the week prior to the visit and semen quality either in moderate consumption (up to 20 units per week) or at higher levels of intake (>20 units per week). However, few of our men had a high alcohol intake (6% drank >30 units the week prior to the visit). Alcohol consumption the week prior to the visit was consistently associated with higher serum testosterone levels and cFT and lower SHBG, whereas it was not associated with serum inhibin B or FSH and LH. This pattern was consistent among men from both the USA and Europe.
Previous studies on alcohol intake and semen quality have shown inconsistent results (Marshburn et al., 1989; Dunphy et al., 1991; Oldereid et al., 1992; Chia et al., 1998; Kunzle et al., 2003; Martini et al., 2004; Stutz et al., 2004; Muthusami and Chinnaswamy, 2005; Lopez Teijon et al., 2007; Gaur et al., 2010; Hansen et al., 2012; Povey et al., 2012), as discussed in a review (La Vignera et al., 2013), but most have been conducted among patients attending andrology or infertility clinics (Marshburn et al., 1989; Dunphy et al., 1991; Oldereid et al., 1992; Kunzle et al., 2003; Martini et al., 2004; Povey et al., 2012) or alcoholic men (Muthusami and Chinnaswamy, 2005; Gaur et al., 2010). Infertile men may have changed their drinking habits as a consequence of the infertility. Only four studies were conducted among unselected men similar to our study and they also found no association between semen quality and moderate or high alcohol intake (Chia et al., 1998; Lopez Teijon et al., 2007; Li et al., 2009; Hansen et al., 2012). One study examined the association between different sources of alcohol and semen quality and found no effect of intake of up to 1/2 l of wine daily (Martini et al., 2004). We found no association between beer, wine or liquor consumption and semen quality.
Our observed association between alcohol intake, and testosterone and cFT is in accordance with previous studies showing increased total testosterone and cFT or increased cFT in combination with decreased SHBG (Muller et al., 2003; Shiels et al., 2009; Hansen et al., 2012), whereas other studies found no association with cFT (Handa et al., 1997; Tamimi et al., 2001; Allen et al., 2002; Svartberg et al., 2003). In contrast, decreased testosterone levels have been reported in male alcoholics suggesting that habitual alcohol abuse may damage Leydig cells or impair the hypothalamic–pituitary–gonadal axis (Muthusami and Chinnaswamy, 2005; Maneesh et al., 2006). It has been speculated that chronic/excessive alcohol intake may reduce testosterone synthesis, either through effects on the hypothalamic–pituitary–gonadal axis or through direct toxic effects on the testis (Anderson et al., 1980, 1983; Van Thiel et al., 1983). At high dosages alcohol may also reduce SHBG synthesis by the liver, which secondarily may lead to reduced levels of testosterone. SHBG levels may also be affected by more moderate alcohol consumption as observed in our young men as well as in other studies (Muller et al., 2003; Shiels et al., 2009), which could explain the observed increase in cFT. For men with moderate alcohol consumption, the positive association between alcohol consumption and serum testosterone levels may be explained by alcohol detoxification leading to a changed metabolism of steroids in the liver. Alcohol oxidation increases the NADH/NAD+ ratio and thereby changes the redox state in favor of conversion of androstenedione into the reduced steroids testosterone and estradiol (Sarkola and Eriksson, 2003).
The participation rates for our populations were between 20 and 30%, which is expected when the participation involves semen sampling. Previously among young Danish men we found no differences in serum reproductive hormones between men delivering a semen sample and not (Andersen et al., 2000). In addition, we included both men with unknown fertility (group A) and fertile men (group B) and the men were unaware of the purpose of the study. The young men (group A) had only consumed alcohol for a few years and therefore an adverse effect may not have been detectable. The fertile men (group B) may have consumed alcohol for many years, but were fertile, so if long-term alcohol consumption reduces fertility it cannot be addressed by our study.
The men in our study reported alcohol consumption the week preceding completion of the questionnaire, as we assumed that to be more accurate to recall than the average weekly intake. Ideally, we should have recorded the alcohol intake 3 months earlier, as sperm is developing over 3 months. If the last weeks consumption differed from the typical weekly intake and the intake 3 months earlier, misclassification of exposure may have occurred. We estimated that a glass of wine, a beer or a ‘drink’ contains 1 unit of alcohol (12 g), but this will vary depending on glass and bottle size and the percentage of alcohol in the drink. Likewise, the men from the different cohorts were asked identical questions, but there may be differences across countries in interpretation of the questions. These potential sources of exposure misclassification are all likely to be unrelated to semen quality and reproductive hormones, since the men responded to the questionnaire before they knew the results of the laboratory tests. Therefore, misclassification is likely to be non-differential and would underestimate the associations between alcohol consumption and semen quality and reproductive hormones. In addition, it is noteworthy that we found no consistent association between alcohol consumption and semen quality in any of the populations, whereas the association to testosterone and cFT was consistent across countries and populations. It is well known that inter-observer variability in semen analysis exists. However, all participating laboratories participated in an external quality control program.
As our study was cross-sectional, we cannot totally rule out reverse causation. Men with high sex hormone levels may be ‘risk-takers’ (Stanton et al., 2011) and therefore more often smokers and have a higher alcohol intake. Alcohol consumption was associated with lifestyle, BMI and social class factors, which are known to affect both semen quality and reproductive hormones (Li et al., 2011; Sermondade et al., 2013). To the extent possible we took these into account in the analyses and they did not appear to explain the associations. We can, however, not exclude that our findings are due to unmeasured confounders including diet, exercise, stress, occupation and risk-taking behavior. It is noteworthy that our finding of higher cFT and testosterone with higher alcohol intake was consistent across populations and countries suggesting that the associations are robust.
In conclusion, in a group of healthy men we found no association between moderate alcohol consumption of any type the week prior to the visit and semen quality. However, we found that higher alcohol intake was associated with higher serum testosterone, possibly associated with a changed metabolism of steroids in the liver. Because few men in our study population drank more than moderately and we only obtained information on the intake for the week prior to the visit, our study cannot address the risk of high alcohol consumption of longer duration or binge drinking on semen quality and male reproductive hormones.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
All authors contributed substantially to conception, design and coordination of the study in their respective countries. Analysis of data: T.K.J., N.E.S., A.-M.A. and S.H.W. Interpretation of data: all authors. Drafting the article: T.K.J., S.H.W., N.J. and N.E.S. Revising the article critically for important intellectual content: all authors. Final approval of the version to be published: all authors.
Funding
This work was supported by the following grants from the National Institutes of Health: R01-ES09916 to the University of Missouri; M01-RR00400 to the University of Minnesota; M01-RR0425 and UCLA CTSI Grant UL1TR000124 to the Los Angeles Biomedical Research Institute, Harbor-UCLA Medical Center and the Cedars-Sinai Research Institute. The Danish Ministry of Health, the Danish Environmental Protection Agency, and the Kirsten and Freddy Johansens Foundation grant 95-103-72087: the European Union (contract numbers BMH4-CT96-0314, QLK4-CT-1999-01422, QLK4-CT-2002-00603 and most recently FP7/2007–2013, DEER Grant agreement no. 212844), the Danish Research Council (grants numbers 9700833 2107-05-0006), the Danish Agency for Science, Technology and Innovation (Grant number 271070678), Rigshospitalet (Grant number 961506336), the University of Copenhagen (Grant number 211-0357/07-3012), the Danish Ministry of Health and the Danish Environmental Protection Agency, A.P. Møller and wife Chastine McKinney Møllers foundation and Svend Andersens Foundation. Sigrid Juselius Foundation, Academy of Finland and Turku University Hospital. The sponsors of this study played no role in the study design, in data collection, analysis, or interpretation, or in the writing of the article.
Conflict of interest
None declared.
Supplementary Material
References
- Allen NE, Appleby PN, Davey GK, Key TJ. Lifestyle and nutritional determinants of bioavailable androgens and related hormones in British men. Cancer Causes Control. 2002;13:353–363. doi: 10.1023/a:1015238102830. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Jr, Willis BR, Oswald C, Reddy JM, Beyler SA, Zaneveld LJ. Hormonal imbalance and alterations in testicular morphology induced by chronic ingestion of ethanol. Biochem Pharmacol. 1980;29:1409–1419. doi: 10.1016/0006-2952(80)90437-2. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Jr, Willis BR, Oswald C, Zaneveld LJ. Ethanol-induced male infertility: impairment of spermatozoa. J Pharmacol Exp Ther. 1983;225:479–486. [PubMed] [Google Scholar]
- Andersen AG, Jensen TK, Carlsen E, Jorgensen N, Andersson AM, Krarup T, et al. High frequency of sub-optimal semen quality in an unselected population of young men. Hum Reprod. 2000;15:366–372. doi: 10.1093/humrep/15.2.366. [DOI] [PubMed] [Google Scholar]
- Brazil C, Swan SH, Drobnis EZ, Liu F, Wang C, Redmon JB, et al. Standardized methods for semen evaluation in a multicenter research study. J Androl. 2004a;25:635–644. doi: 10.1002/j.1939-4640.2004.tb02835.x. [DOI] [PubMed] [Google Scholar]
- Brazil C, Swan SH, Tollner CR, Treece C, Drobnis EZ, Wang C, et al. Quality control of laboratory methods for semen evaluation in a multicenter research study. J Androl. 2004b;25:645–656. doi: 10.1002/j.1939-4640.2004.tb02836.x. [DOI] [PubMed] [Google Scholar]
- Chia SE, Tay SK, Lim ST. What constitutes a normal seminal analysis? Semen parameters of 243 fertile men. Hum Reprod. 1998;13:3394–3398. doi: 10.1093/humrep/13.12.3394. [DOI] [PubMed] [Google Scholar]
- Dunphy BC, Barratt CL, Cooke ID. Male alcohol consumption and fecundity in couples attending an infertility clinic. Andrologia. 1991;23:219–221. doi: 10.1111/j.1439-0272.1991.tb02541.x. [DOI] [PubMed] [Google Scholar]
- Gaur DS, Talekar MS, Pathak VP. Alcohol intake and cigarette smoking: impact of two major lifestyle factors on male fertility. Indian J Pathol Microbiol. 2010;53:35–40. doi: 10.4103/0377-4929.59180. [DOI] [PubMed] [Google Scholar]
- Handa K, Ishii H, Kono S, Shinchi K, Imanishi K, Mihara H, et al. Behavioral correlates of plasma sex hormones and their relationships with plasma lipids and lipoproteins in Japanese men. Atherosclerosis. 1997;130:37–44. doi: 10.1016/s0021-9150(96)06041-8. [DOI] [PubMed] [Google Scholar]
- Hansen ML, Thulstrup AM, Bonde JP, Olsen J, Hakonsen LB, Ramlau-Hansen CH. Does last week's alcohol intake affect semen quality or reproductive hormones? A cross-sectional study among healthy young Danish men. Reprod Toxicol. 2012;34:457–462. doi: 10.1016/j.reprotox.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Jorgensen N, Auger J, Giwercman A, Irvine DS, Jensen TK, Jouannet P, et al. Semen analysis performed by different laboratory teams: an intervariation study. Int J Androl. 1997;20:201–208. doi: 10.1046/j.1365-2605.1997.00052.x. [DOI] [PubMed] [Google Scholar]
- Jorgensen N, Andersen AG, Eustache F, Irvine DS, Suominen J, Petersen JH, et al. Regional differences in semen quality in Europe. Hum Reprod. 2001;16:1012–1019. doi: 10.1093/humrep/16.5.1012. [DOI] [PubMed] [Google Scholar]
- Jorgensen N, Carlsen E, Nermoen I, Punab M, Suominen J, Andersen AG, et al. East-West gradient in semen quality in the Nordic-Baltic area: a study of men from the general population in Denmark, Norway, Estonia and Finland. Hum Reprod. 2002;17:2199–2208. doi: 10.1093/humrep/17.8.2199. [DOI] [PubMed] [Google Scholar]
- Jorgensen N, Joensen UN, Jensen TK, Jensen MB, Almstrup K, Olesen IA, et al. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open. 2012;2:e00099. doi: 10.1136/bmjopen-2012-000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzle R, Mueller MD, Hanggi W, Birkhauser MH, Drescher H, Bersinger NA. Semen quality of male smokers and nonsmokers in infertile couples. Fertil Steril. 2003;79:287–291. doi: 10.1016/s0015-0282(02)04664-2. [DOI] [PubMed] [Google Scholar]
- La Vignera S, Condorelli RA, Balercia G, Vicari E, Calogero AE. Does alcohol have any effect on male reproductive function? A review of literature. Asian J Androl. 2013;15:221–225. doi: 10.1038/aja.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lin H, Ma M, Li L, Cai M, Zhou N, et al. Semen quality of 1346 healthy men, results from the Chongqing area of southwest China. Hum Reprod. 2009;24:459–469. doi: 10.1093/humrep/den399. [DOI] [PubMed] [Google Scholar]
- Li Y, Lin H, Li Y, Cao J. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril. 2011;95:116–123. doi: 10.1016/j.fertnstert.2010.06.031. [DOI] [PubMed] [Google Scholar]
- Lopez Teijon M, Garcia F, Serra O, Moragas M, Rabanal A, Olivares R, et al. Semen quality in a population of volunteers from the province of Barcelona. Reprod Biomed Online. 2007;15:434–444. doi: 10.1016/s1472-6483(10)60370-7. [DOI] [PubMed] [Google Scholar]
- Maneesh M, Dutta S, Chakrabarti A, Vasudevan DM. Alcohol abuse-duration dependent decrease in plasma testosterone and antioxidants in males. Indian J Physiol Pharmacol. 2006;50:291–296. [PubMed] [Google Scholar]
- Marshburn PB, Sloan CS, Hammond MG. Semen quality and association with coffee drinking, cigarette smoking, and ethanol consumption. Fertil Steril. 1989;52:162–165. doi: 10.1016/s0015-0282(16)60809-9. [DOI] [PubMed] [Google Scholar]
- Martini AC, Molina RI, Estofan D, Senestrari D, Fiol de Cuneo M, Ruiz RD. Effects of alcohol and cigarette consumption on human seminal quality. Fertil Steril. 2004;82:374–377. doi: 10.1016/j.fertnstert.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Menkveld R, Stander FS, Kotze TJ, Kruger TF, van Zyl JA. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod. 1990;5:586–592. doi: 10.1093/oxfordjournals.humrep.a137150. [DOI] [PubMed] [Google Scholar]
- Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol. 2003;149:583–589. doi: 10.1530/eje.0.1490583. [DOI] [PubMed] [Google Scholar]
- Muthusami KR, Chinnaswamy P. Effect of chronic alcoholism on male fertility hormones and semen quality. Fertil Steril. 2005;84:919–924. doi: 10.1016/j.fertnstert.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Oldereid NB, Rui H, Purvis K. Life styles of men in barren couples and their relationship to sperm quality. Int J Fertil. 1992;37:343–349. [PubMed] [Google Scholar]
- Organisation WH. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 4th edn. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- Organisation WH. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 4th edn. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Organisation WH. WHO Laboratory Manual for the Examination and Processing of Human Semen. Cambridge University Press; 2010. [Google Scholar]
- Paasch U, Salzbrunn A, Glander HJ, Plambeck K, Salzbrunn H, Grunewald S, et al. Semen quality in sub-fertile range for a significant proportion of young men from the general German population: a co-ordinated, controlled study of 791 men from Hamburg and Leipzig. Int J Androl. 2008;31:93–102. doi: 10.1111/j.1365-2605.2007.00860.x. [DOI] [PubMed] [Google Scholar]
- Parry CD, Patra J, Rehm J. Alcohol consumption and non-communicable diseases: epidemiology and policy implications. Addiction. 2011;106:1718–1724. doi: 10.1111/j.1360-0443.2011.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povey AC, Clyma JA, McNamee R, Moore HD, Baillie H, Pacey AA, et al. Modifiable and non-modifiable risk factors for poor semen quality: a case-referent study. Hum Reprod. 2012;27:2799–2806. doi: 10.1093/humrep/des183. [DOI] [PubMed] [Google Scholar]
- Saleem TS, Basha SD. Red wine: a drink to your heart. J Cardiovasc Dis Res. 2010;1:171–176. doi: 10.4103/0975-3583.74259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkola T, Eriksson CJ. Testosterone increases in men after a low dose of alcohol. Alcohol Clin Exp Res. 2003;27:682–685. doi: 10.1097/01.ALC.0000060526.43976.68. [DOI] [PubMed] [Google Scholar]
- Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, Jensen TK, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013;19:221–231. doi: 10.1093/humupd/dms050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels MS, Rohrmann S, Menke A, Selvin E, Crespo CJ, Rifai N, et al. Association of cigarette smoking, alcohol consumption, and physical activity with sex steroid hormone levels in us men. Cancer Causes Control. 2009;20:877–886. doi: 10.1007/s10552-009-9318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton SJ, Liening SH, Schultheiss OC. Testosterone is positively associated with risk taking in the Iowa Gambling Task. Horm Behav. 2011;59:252–256. doi: 10.1016/j.yhbeh.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Stutz G, Zamudio J, Santillan ME, Vincenti L, de Cuneo MF, Ruiz RD. The effect of alcohol, tobacco, and aspirin consumption on seminal quality among healthy young men. Arch Environ Health. 2004;59:548–552. doi: 10.1080/00039890409603432. [DOI] [PubMed] [Google Scholar]
- Svartberg J, Midtby M, Bonaa KH, Sundsfjord J, Joakimsen RM, Jorde R. The associations of age, lifestyle factors and chronic disease with testosterone in men: the Tromso Study. Eur J Endocrinol. 2003;149:145–152. doi: 10.1530/eje.0.1490145. [DOI] [PubMed] [Google Scholar]
- Swan SH, Brazil C, Drobnis EZ, Liu F, Kruse RL, Hatch M, et al. Geographic differences in semen quality of fertile U.S. males. Environ Health Perspect. 2003;111:414–420. doi: 10.1289/ehp.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamimi R, Mucci LA, Spanos E, Lagiou A, Benetou V, Trichopoulos D. Testosterone and oestradiol in relation to tobacco smoking, body mass index, energy consumption and nutrient intake among adult men. Eur J Cancer Prev. 2001;10:275–280. doi: 10.1097/00008469-200106000-00012. [DOI] [PubMed] [Google Scholar]
- Van Thiel DH, Gavaler JS, Cobb CF, Santucci L, Graham TO. Ethanol, a Leydig cell toxin: evidence obtained in vivo and in vitro. Pharmacol Biochem Behav. 1983;18(Suppl 1):317–323. doi: 10.1016/0091-3057(83)90193-4. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


