Abstract
STUDY QUESTION
Are the transmembrane mucins, MUC1, MUC4 and MUC16, differentially expressed in endometriosis compared with normal endometrium?
SUMMARY ANSWER
This study revealed that transmembrane mucin expression does not vary significantly in normal endometrium during the menstrual cycle and is not altered in endometriosis relative to the epithelial marker, cytokeratin-18 (KRT18).
WHAT IS KNOWN ALREADY
Increased serum levels of the transmembrane mucin fragments MUC1, MUC4 and MUC16 that normally dominate the apical surface of simple epithelia are found in several pathological conditions, including endometriosis. Altered mucin expression in gynecologic diseases may promote infertility or endometrial pathologies.
STUDY DESIGN, SIZE, DURATION
This was a laboratory-based study of samples from 12 endometriosis patients as well as non-endometriosis control samples obtained from 31 patients.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Total RNA was isolated from endometrial biopsies of ectopic and eutopic endometrium from women with endometriosis and control patients from different stages of the menstrual cycle. Quantitative (q)-RT–PCR analyses were performed for the mucins, MUC1, MUC4 and MUC16, relative to the epithelial marker, cytokeratin-18 (KRT18), or β-actin (ACTB). Frozen sections from endometrial biopsies of proliferative and mid-secretory stage women with endometriosis were immunostained for MUC1, MUC4 and MUC16.
MAIN RESULTS AND THE ROLE OF CHANCE
qRT–PCR analyses of MUC1 and MUC16 mRNA revealed that these mucins do not vary significantly during the menstrual cycle nor are they altered in women with endometriosis relative to the epithelial marker, KRT18. MUC4 mRNA is expressed at very low levels relative to MUC1 and MUC16 under all conditions. There was little difference in MUC1 and MUC16 expression between eutopic endometrial and ectopic endometriotic tissues. MUC4 expression also was not significantly higher in the ectopic endometriotic tissues. Immunostaining for all three mucins reveals robust expression of MUC1 and MUC16 at the apical surfaces of endometrial epithelia, but little to no staining for MUC4.
LIMITATIONS, REASONS FOR CAUTION
qRT–PCR analysis was the main method used for mucin detection. Additional studies with stage III–IV endometriotic tissue would be useful to determine if changes in MUC1 and MUC16 expression occur, or if MUC4 expression increases, at later stages of endometriosis.
WIDER IMPLICATIONS OF THE FINDINGS
We report a comprehensive comparative profile of the major transmembrane mucins, MUC1, MUC4 and MUC16, relative to the epithelial marker, KRT18, in normal cycling endometrium and in endometriosis, and indicate constitutive expression. Previous studies have profiled the expression of individual mucins relative to β-actin and indicate accumulation in the luteal phase. Thus, these differences in interpretation appear to reflect the increased epithelial content of endometrium during the luteal phase.
STUDY FUNDING
This study was supported by: NIH R01HD29963 to D.D.C.; NIH U54HD007495 to S.M.H.; and NIH R01HD067721 to S.L.Y. and B.A.L. The authors have no competing interests to declare.
Keywords: mucins, endometrium, endometriosis, cytokeratin-18
Introduction
The transmembrane mucins, MUC1, MUC4 and MUC16, belong to a family of very large, heavily glycosylated proteins that are characterized by a variable number of tandem repeat (VNTR) motifs. These mucins are expressed on the apical surface of nearly all simple epithelial tissues as well as various tumor cells and serve to keep epithelial surfaces lubricated and hydrated, protecting them from pathogens and environmental challenges (Hattrup and Gendler, 2008). Uterine membrane-tethered mucins play a key role in embryo implantation, a process facilitated by the loss or reduction of the mucin barrier (Braga and Gendler, 1993; Brayman et al., 2004; Dharmaraj et al., 2009). Dysregulated mucin expression is associated with various disease states (Hollingsworth and Swanson, 2004; Rose and Voynow, 2006; Vlad et al., 2006; Margarit et al., 2010; Chang et al., 2011; He et al., 2011) and high levels of transmembrane mucin expression reduce drug efficacy and protect cells from apoptosis (Hollingsworth and Swanson, 2004; Ren et al., 2004). Transmembrane mucin expression is hormonally regulated during the estrous/menstrual cycle in many species (Hey et al., 1994; Surveyor et al., 1995; Hild-Petito et al., 1996; Hoffman et al., 1998; Julian et al., 2005; Gipson et al., 2008) and altered expression of membrane-tethered mucins is associated with infertility (Koscinski et al., 2006; Margarit et al., 2010). In addition, increased serum levels of transmembrane mucin fragments are found in gynecological pathologies such as endometrial cancer and endometriosis (He et al., 2011; Szubert et al., 2012). Endometriosis is a benign chronic inflammatory disorder associated with an increased risk of progression to ovarian cancer and impaired fecundity (Lessey et al., 2013). The Center for Disease Control reports that 11.8% of all women (∼17 million in the USA alone) between the ages of 15 and 44 have endometriosis. Identifying approaches to control transmembrane mucin expression provides novel opportunities to enhance embryo implantation success, improve protective functions of the female reproductive tract and potentially reduce endometriotic spread and/or enhance the efficacy of existing therapies.
MUC1 expression has been extensively studied in human endometrium (Aplin et al., 1994, 1998; DeLoia et al., 1998; Horne et al., 2005; Wang et al., 2008); however, there is limited information available on MUC4 and MUC16 expression in human uterine tissues. Studies from Gipson and colleagues demonstrate MUC16 (CA-125) expression at the apical surface of luminal epithelia with little change during the menstrual cycle (Gipson et al., 2008), in contrast to changing patterns observed for MUC1 (Hey et al., 1994). Recently, serological studies indicated that CA 125 levels are useful markers of endometriosis (Patrelli et al., 2011; Socolov et al., 2011; Szubert et al., 2012). Genetic polymorphisms in MUC4 are associated with endometriosis in certain populations (Chang et al., 2011), but little information on protein and mRNA expression is available. An isolated report has shown MUC4 expression by immunostaining of secretory stage samples from women in the secretory stage of the cycle and undergoing in vitro fertilization (IVF) (Koscinski et al., 2006). However, careful comparative studies of the expression of these three major membrane-tethered mucins have not been performed in the cycling endometrium and endometriosis.
In the current study, we report a comprehensive expression profile of MUC1, MUC4 and MUC16 in normal endometrium during the menstrual cycle and in eutopic and ectopic endometrium of women with and without endometriosis. Mucin expression profile studies so far have been performed relative to the housekeeping gene, β-actin (ACTB). Here, we use the epithelial-specific markers, cytokeratin-18 mRNA (KRT18) or pan-cytokeratin antibodies, as controls for changes in the content of the uterine epithelial population that occur during the menstrual cycle (Moll, 1983).
Materials and Methods
Sample collection
All patient samples were collected according to the Institutional Review Board for Baylor College of Medicine and affiliated hospitals, Greenville Hospital System and University of North Carolina School of Medicine and processed at Rice University with approval from the Institutional Review Board at Rice University. Sample collection was performed as described previously (Hawkins et al., 2011; Plante et al., 2012). All tissue samples were collected with patient consent and approvals from the Institutional Review Board for Baylor College of Medicine and affiliated hospitals, Greenville Hospital System and University of North Carolina School of Medicine and processed at Rice University with approval from the Institutional Review Board at Rice University. Tissue collection was performed as described previously (Hawkins et al., 2011; Plante et al., 2012). Normal endometrium tissue samples from S.L.Y. (Plante et al., 2012) were obtained from a tissue library, with participants’ cycles ranging from 25 to 35 days. Samples were randomized to a specific proliferative cycle day or a specific day post-luteinizing hormone (LH) surge. Endometrial histology and dating was performed according to Noyes criteria (Noyes et al., 1950). Matched samples of endometrium and endometriosis obtained were not LH timed but confirmed histologically according to Noyes criteria (Noyes et al., 1950). Samples were categorized as proliferative (n = 6), early secretory (n = 4), mid-secretory (n = 6) and late secretory (n = 4), and stage-matched eutopic and ectopic endometrial samples (total n = 6; early secretory, stages I-II, n = 1; secretory, stage II, n = 3; proliferative, stage II, n = 1; mid-secretory, stage III, n = 1).
For the purpose of immunohistochemistry, samples from normal secretory endometrium, and proliferative and mid-secretory endometriosis grade stage II were used. These samples obtained from S.M.H. (Hawkins et al., 2011) were from women with regular menstrual cycles, who were free from hormones for at least 30 days before surgery. Menstrual cycle phase was determined by patient-provided information and confirmed by pathology according to standard criteria (Noyes et al., 1950). Samples were categorized as non-endometriosis proliferative (n = 5), non-endometriosis secretory (n = 6) and endometriotic samples (total n = 6; proliferative, n = 2; secretory, n = 2; interval phase, n = 1; inactive phase, n = 1).
RNA isolation, DNAse treatment and qRT–PCR
Total RNA was isolated as described (Hawkins et al., 2011; Plante et al., 2012). RNA was diluted with nuclease free water to a volume of 40 µl and DNAse treated (Ambion, Austin, TX, USA; AM1906) according to the manufacturer's instructions. Reverse transcriptase reactions were performed using 1 µg of total RNA in a 20 µl reaction using qScript cDNA Super mix (Quanta; 95048) incubated for 5 min at 25°C, 30 min at 42°C and 5 min at 85°C. Real-time PCR was performed using the primer sequences shown in Table I and SYBR Green Super mix (Quanta Bioscience). Samples were cycled for 30 s at 95°C, 30 s at 59°C and 30 s for 72°C for 40 cycles for MUC1, MUC16, KRT18 and ACTB. For MUC4, and for accompanying KRT18, samples were cycled for 30 s at 95°C, 15 s at 58°C and 15 s for 72°C for 40 cycles. The relative amounts of mucin mRNA were identified using the comparative threshold cycle method and normalized to that of either KRT18 or ACTB. Primer sets not previously described were designed using Primer-BLAST (NCBI) and were validated according to protocols in the BioRad CFX96 manual.
Table I.
Oligonucleotide primer sequences used in this study.
| Gene | Forward | Reverse | Amplicon | Reference |
|---|---|---|---|---|
| MUC1 | 5′ AGAGAAGTTCAGTGCCCAGC | 5′ TGACATCCTGTCCCTGAGTG | 114 bp | Dharmaraj et al. (2010) |
| MUC4 | 5′ GCCCAAGCTACAGTGTGACTC | 5′ ATGGTGCCGTTGTAATTTGTT | 102 bp | Argueso et al. (2002) |
| MUC4-CT | 5′ CCATGGAGGCCAGTGCCAGC | 5′ GTGCTCACAGTGCTCGCCCC | 94 bp | aThis Paper |
| MUC16 | 5′ GCCTCTACCTTAACGGTTACAATGAA | 5′ GGTACCCCATGGCTGTTGTG | 114 bp | Argueso et al. (2003) |
| ACTB | 5′ GATGAGATTGGCATGGCTTT | 5′ CACCTTCACCGGTCCAGTTT | 100 bp | Dharmaraj et al. (2010) |
| KRT18 | 5′ GACACCAATATCACACGACTG | 5′ GGCTTGTAGGCCTTTTACTTC | 108 bp | aThis Paper |
aRefer to materials and methods for primer validation.
Immunofluorescence analysis
Frozen sections from mid-secretory stage of cycling endometrium were thawed at room temperature for 20 min, rehydrated with 1× PBS for 10 min and fixed with 4% (wt/vol) paraformaldehyde for 10 min. Slides were washed three times for 10 min each with 1× PBS, blocked for 1 h with [1% (wt/vol) BSA in PBS] and washed three times with 1× PBS. Primary antibody was added and incubated overnight at the indicated dilutions: rabbit polyclonal antibody to wide spectrum cytokeratin, 1:50 (Abcam; ab9377); mouse monoclonal anti-MUC4, 1:250 (kindly provided by Dr S.K. Batra, University of Nebraska); rabbit anti-MUC1, 1:50 (CT-1, ref.); mouse monoclonal anti-MUC16 (OC125), 1:200 (kindly provided by Dr Robert Bast, MD Anderson Cancer Center). Mouse and rabbit IgG controls (Jackson ImmunoResearch Laboratories) at 250 ng each as well as a secondary antibody only were used as controls. Slides were washed three times for 10 min each with 1× PBS, then incubated with 1:400 dilution of secondary antibody, Alexa-fluor 647 goat anti-mouse (Invitrogen; A21235) or Alexa-fluor 488 goat anti-rabbit (Invitrogen; A11034) in 1% (wt/vol) BSA in PBS for 1 h in the dark, and washed three times for 10 min with 1× PBS. Samples were mounted with Prolong-Antifade with DAPI as per manufacturer's instructions (Invitrogen) and subjected to analyses by confocal microscopy (LSM710).
Tissue samples from proliferative stage endometriosis were embedded in O.C.T. and stored at −80°C. Tissue blocks were incubated at −20°C overnight prior to sectioning in a Leica cryotome. Ten micron tissue sections were then processed as described above. Tissue sections were imaged using a Nikon A1-Rsi confocal microscope.
The pancreatic adenocarcinoma cell line, HPAF was plated in an 8-well chamber slide in RPMI + 10% (vol/vol) FBS until confluent. Cells were fixed in 4% (vol/vol) paraformaldehyde for 10 min, then in 0.02% (vol/vol) Triton X-100 for 2 min. Cells were blocked in filtered 3% (wt/vol) BSA for 1 h, followed by overnight incubation at 4°C with MUC4 antibody (8G7 from Dr Batra) at a 1:100 dilution in filtered 3% (wt/vol) BSA. Cells were washed three times for 5 min each in PBS, and then incubated with goat anti-mouse AlexaFluor 647 at a dilution of 1:400 in filtered 3% (wt/vol) BSA for 1 h at room temperature, followed by two 5 min washes in PBS. Cells were then washed in PBS + DAPI for 5 min. Coverslips were mounted using ProLong Gold anti-fade agent and cells were imaged on a Zeiss LSM 710 confocal microscope (Supplementary data, Fig. S1).
Statistical analysis
Statistical analyses were performed using a two-tailed student's t-test (GraphPad InStat program; GraphPad Software, Inc., San Diego, CA, USA).
Results
Mucin mRNA profiles in normal endometrium across the menstrual cycle
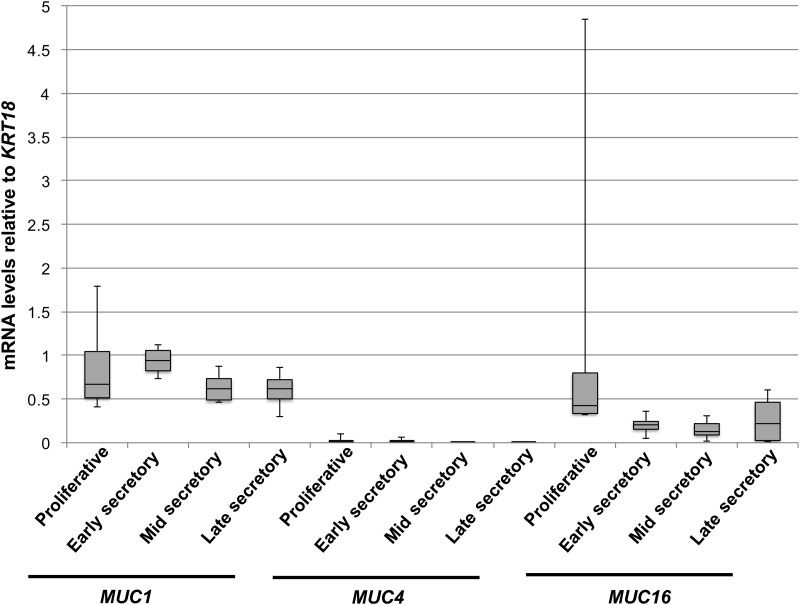
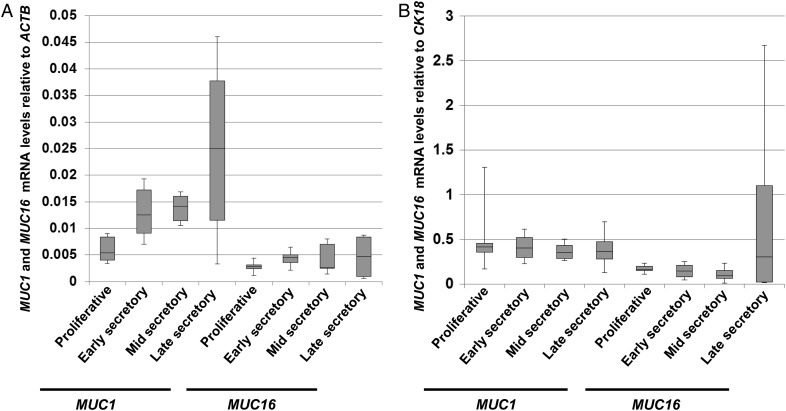
Levels of MUC1 and MUC16 mRNAs have been previously profiled in human endometrial tissues (Hey et al., 1994; Gipson et al., 1997, 2008; Hebbar et al., 2005). These studies did not account for variations in uterine epithelial cell populations during the menstrual cycle. We therefore examined mucin mRNA expression levels by qRT–PCR in the different stages of the cycling endometrium relative to the epithelial marker, cytokeratin-18, KRT18 (Fig. 1). The mucins, MUC1, MUC4 and MUC16, were expressed in all stages of the cycle with very low expression of MUC4 detected in all cases. There was no significant change in MUC1 and MUC16 mRNA expression across the cycle when compared with KRT18. Since this was in apparent conflict with previous reports, we also compared MUC1 and MUC16 expression relative to ACTB used by others as a load control in similar studies. In agreement with previous reports (Hey et al., 1994; Gipson et al., 2008), we observed a rise in MUC1 and MUC16 mRNA expression relative to ACTB during the secretory phase; however, no significant changes were observed in the expression of these mucin mRNAs relative to KRT18 (Fig. 2A and B, respectively). Thus, the apparent rise in MUC1 and MUC16 reported previously parallels the increased epithelial content of the endometrium during the secretory phase.
Figure 1.
Mucin mRNA profile in normal endometrium. Box plots were used to express levels of transmembrane mucin mRNA levels in the cycling human endometrium. Mucin mRNA expression was determined relative to that of epithelial marker, cytokeratin-18 (KRT18) by qRT–PCR as described in Materials and Methods. Mucins were expressed at similar levels at all stages of the cycle with very low expression of MUC4. The box plot shades the second and third quartiles of the data. The black line in the box is drawn at the median value. The whiskers above and below show the maximum and minimum values, respectively.
Figure 2.
Mucin mRNA levels relative to those of KRT18 (A) and ACTB (B). MUC1, MUC16, KRT18 and ACTB mRNA levels were determined by qRT–PCR as described in Materials and Methods. Box plots were used to express these data as described in the legend to Fig. 1. No significant differences in mucin expression were observed across the cycle when compared with KRT18 mRNA levels.
Mucin mRNA profiles in normal endometrium and endometrium of women with endometriosis
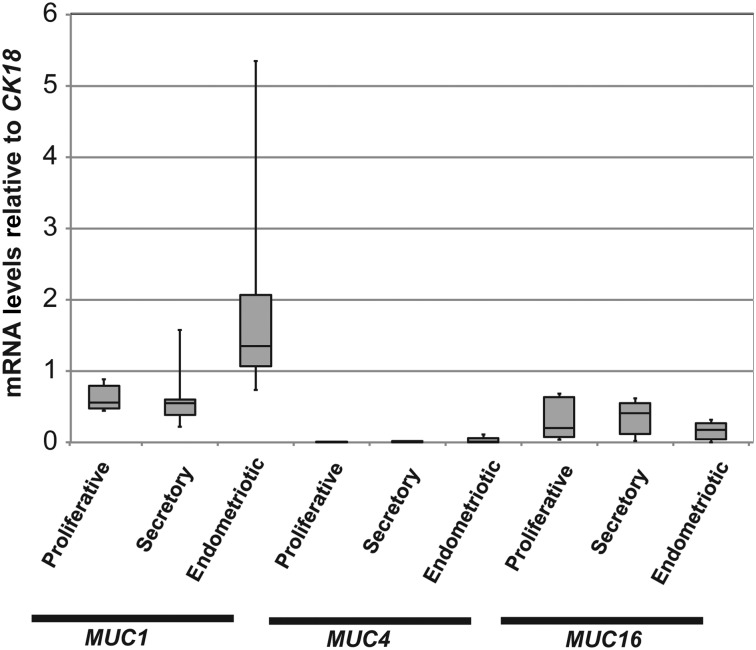
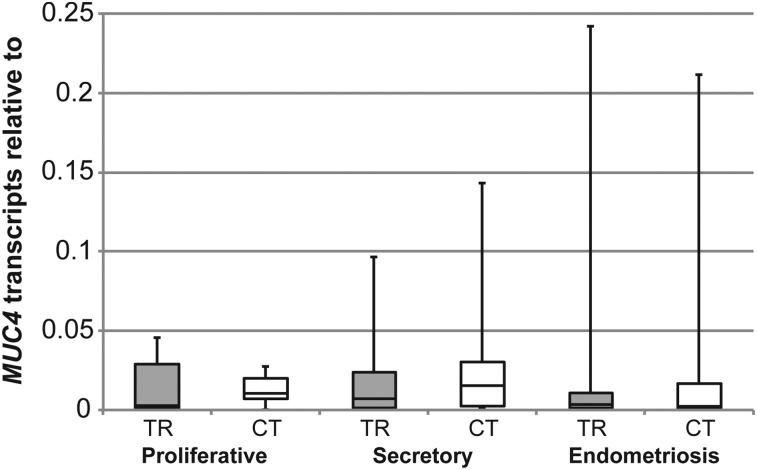
Mucin glycoproteins have been assessed as potential biomarkers or diagnostic tools in endometriosis because of their utility as markers of malignant diseases (May et al., 2010). We sought to profile MUC1, MUC4 and MUC16 mRNA expression in normal endometrium and in endometrium of women with endometriosis again relative to the epithelial marker, KRT18 mRNA. MUC1 and MUC16 mRNA expression did not vary significantly between these conditions (Fig. 3). MUC4 mRNA was much more difficult to detect than MUC1 and MUC16 in normal endometrium and in endometrium from endometriosis patients (Fig. 3). Primers to the cytoplasmic tail (CT) region of the MUC4 gene were therefore designed, in addition to the primers to the tandem repeat (TR) region, to detect MUC4 mRNA expression to account for any variability in MUC4 mRNA expression (Table I). We confirmed minimal levels of MUC4 mRNA expression in both normal endometrium and that of women with endometriosis (Fig. 4). Collectively, these data indicated little variation in MUC1, MUC4 and MUC16 mRNA expression in the eutopic endometrium of women with endometriosis when compared with normal endometrium.
Figure 3.
Relative abundance of mucin mRNAs in normal endometrium and in endometrium of women with endometriosis. MUC1, MUC4 and MUC16 mRNA in normal endometria at different stages of the menstrual cycle and in endometria of women with endometriosis relative to KRT18 mRNA were determined as described in Materials and Methods. Box plots were used to express the data as described in the legend to Fig. 1. No significant differences were observed between these conditions.
Figure 4.
A comparison of MUC4 mRNA detection by primers against tandem repeat (TR) and cytoplasmic tail (CT) regions of the MUC4 gene. MUC4 mRNA in normal endometria at different stages of the menstrual cycle and in endometria of women with endometriosis relative to KRT18 mRNA using primers to the TR and CT regions. Box plots were used to express these data as described in the legend to Fig. 1. No significant differences were observed using either set of primers.
Mucin mRNA profiles in stage-matched endometriotic tissue
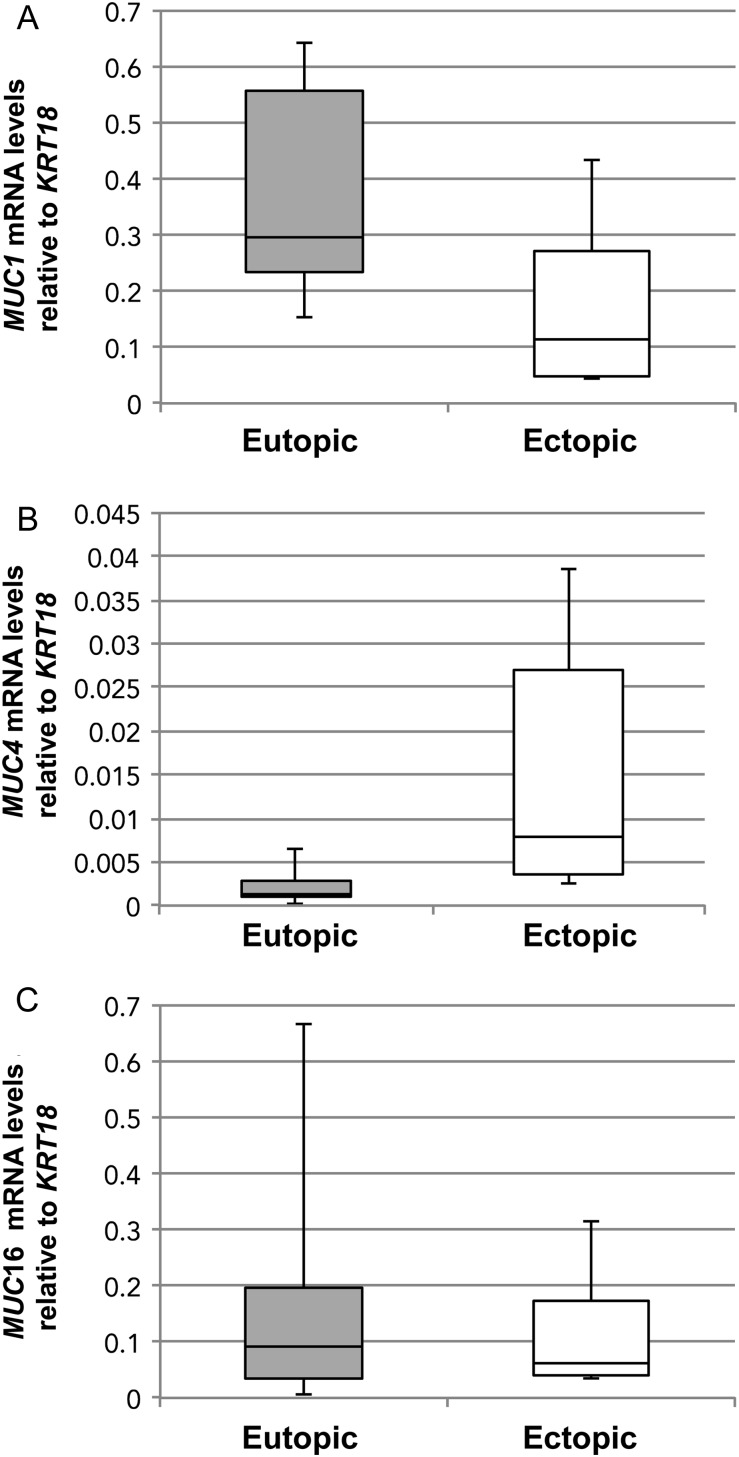
We then sought to compare mucin expression in stage-matched eutopic and ectopic samples from endometriosis patients relative to KRT18 mRNA (Fig. 5). qRT–PCR analyses revealed that MUC1 (Fig. 5A), MUC4 (Fig. 5B) and MUC16 (Fig. 5C) mRNA expression did not vary between eutopic and ectopic stage-matched endometriotic tissues. MUC4 mRNA expression was not significantly higher in ectopic endometriotic tissue (Fig. 5B) and was still very low relative to that of MUC1 and MUC16. Collectively, these results were in accordance with the above experiment and showed little variation in mucin expression in stage-matched eutopic and ectopic endometriotic tissue.
Figure 5.
(A) MUC1, (B) MUC4 and (C) MUC16 mRNA expression in stage-matched eutopic and ectopic endometria of endometriosis patients. Six menstrual stage-matched samples, either from secretory or proliferative stage of the menstrual cycle and stage II or III (one sample) endometriosis were analyzed by qRT–PCR for the different mucins as described in Materials and Methods. Box plots were used to express these data as described in the legend to Fig. 1. Changes in levels of MUC4 expression was observed in endometriotic tissue when compared with eutopic endometriotic tissue.
Transmembrane mucin protein detection by immunohistochemistry
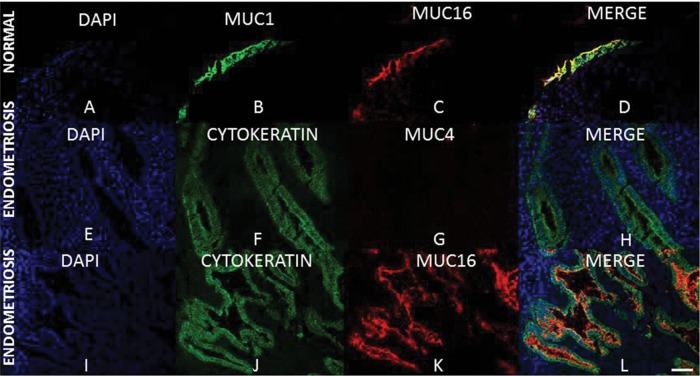
To determine the localization of each mucin, immunohistochemistry was performed on frozen sections from normal and mid-secretory endometriosis grade Stage II endometrium (Fig. 6A–L). Robust MUC1 and MUC16 localization was confirmed on the apical surface of epithelia (Fig. 6A–D). Localization of MUC16 protein expression to epithelia was confirmed using the pan-cytokeratin antibody (Fig. 6I–L). We performed immunohistochemistry for MUC4 only in endometrium from endometriosis patients since MUC4 mRNA expression was barely detectable in normal cycling endometrium. Confirming the results from MUC4 qRT–PCR analyses, a lack of reactivity with the MUC4-specific antibody was observed in sections of mid-secretory endometriotic tissue (Fig. 6E–H). Pan-cytokeratin staining was performed to identify epithelial cells in each case (Fig. 6F and J). MUC4 immunohistochemistry on frozen sections from proliferative stage endometrium from endometriosis patients was also performed (Supplementary data, Fig. S1). Similar to the results obtained in mid-secretory endometriosis grade tissues, MUC4 protein expression was undetectable (Supplementary data, Fig. S1B) while MUC1 and MUC16 were expressed (Supplementary data, Fig. S1A). Recognition of MUC4 by the 8G7 antibody was confirmed by positive immunostaining for MUC4 in the pancreatic adenocarcinoma cell line, HPAF (Supplementary data, Fig. S1C and D). Collectively, these data confirm that MUC1 and MUC16 expression are restricted to endometrial epithelial cells and that MUC4 protein is not present at appreciable levels in endometriosis.
Figure 6.
MUC1, MUC4 and MUC16 expression in normal endometrium and in endometriosis. Frozen sections were stained with antibodies to the indicated mucins as described in Materials and Methods. Pan-cytokeratin staining was used to detect epithelia in each case (F, H, J and L). DAPI staining was used to detect nuclei as described in Materials and Methods. Robust staining for both MUC1 (B and D) and MUC16 (C, D, K and L) were observed in all cytokeratin positive (epithelial) cells. No MUC4 staining was detected (G and H). DAPI: blue; pan-cytokeratin or MUC1: green; MUC4 or MUC16: red. Magnification for all fields is as indicated in (L), 50 μm.
Discussion
Mucin expression and distribution changes in several gynecological pathologies, including endometrial cancer and endometriosis (He et al., 2011). Endometriosis is a prevalent endocrine-driven disease occurring in almost 45% of all women receiving laparoscopic examination and in ∼10% of all women presenting with clinical symptoms (Lessey, 2000; Winkel, 2003; de Ziegler et al., 2010). Symptoms range from mild to severe pelvic pain and infertility (Winkel 2003; de Ziegler et al., 2010) and are cyclical following the rise and fall of ovarian steroid hormones through the menstrual cycle that act on endometriotic tissue as well as the endometrium (Winkel, 2003). The etiology of endometriosis remains unclear, but a common theory is that retrograde menstruation leads to the deposition of endometrial tissue on the ovaries and within the peritoneal cavity. Retrograde menstruation is common in menstruating women, but the reasons that some women get endometriosis and others do not remain an area of active investigation.
The current study focused on quantitative analyses of membrane-tethered mucin (MUC1, MUC4 and MUC16) expression in normal endometrium and eutopic and ectopic endometrium of endometriosis patients. This is the first comprehensive study of these three key membrane-associated mucins in these contexts. Only three reports have examined MUC4 expression by immunohistochemistry in human endometrium (Gipson et al., 1997; Koscinski et al., 2006; Alameda et al., 2007) with no agreement on the expression pattern of MUC4. Gipson et al. (1997) reported a lack of MUC4 expression in normal endometrium while Koscinski et al. (2006) report isolated patches of MUC4 expressed in luminal and glandular epithelium using the same 8G7 antibody utilized in the present studies in infertile and women undergoing IVF. A lack of information on MUC4-specific antibody used by Alameda et al. (2007) hinders interpretation of their results which indicate endometrial MUC4 protein expression in a subset of women. There also is limited information on MUC16 expression in the cycling endometrium, although qRT–PCR has been used to assess levels of MUC16 mRNA levels during the cycle (Gipson et al., 2008). Previous reports have indicated that MUC1 and MUC16 vary in the cycling endometrium with maximal expression in the secretory phase (Hey et al., 1994; Gipson et al., 2008). These studies relied on the use of ACTB or 28S RNA as RNA load controls, i.e. an RNA species found in all endometrial cells. When MUC1 and MUC16 mRNA levels were compared relative to ACTB mRNA, our results were in agreement with previous reports (Hey et al., 1994; Gipson et al., 2008).
We used KRT18 mRNA as a control for potential changes in the epithelial populations in endometrial samples (Moll, 1983). Our data indicate that endometrial MUC1 and MUC16 mRNA expression does not change significantly relative to KRT18 and rather seems to vary in parallel with the changes in the epithelial populations in the endometrium. While it is possible that KRT18 is hormonally regulated, this has not been described. A number of agents including progesterone and proinflammatory cytokines modulate transmembrane mucin expression in uterine cell types and endometrium of various animal models (Hoffman et al., 1998; Brayman et al., 2006, 2007; Dharmaraj et al., 2010). Nonetheless, the current studies indicate that changes in mucin expression are not observed during the normal menstrual cycle nor in endometriotic tissues. Several reports also indicate that serum MUC1 levels are not changed in endometriosis (reviewed in May et al. 2010). One report describes a reduction of MUC1 in endometriosis (Margarit et al. 2010). Endometriosis has been suggested as a precursor for ovarian cancer (Worley et al., 2013) and the serum MUC16 levels test (detected as CA-125) is a widely used test for ovarian cancer. Elevated serum MUC16/CA-125 has also been used as a marker for endometriosis, particularly for Stage III–IV disease (Mol et al., 1998; Vodolazkaia et al., 2012). Our data indicate that MUC16 mRNA expression does not vary in endometriotic tissue through the cycle relative to the levels observed in normal endometrium. Nonetheless, our samples were primarily obtained from patients presenting with stage I–II endometriosis and thus we can only support the notion that MUC16/CA 125 is not a useful marker for early-stage disease.
Previous reports have examined MUC4 expression in the human endometrium (Gipson et al., 1997; Koscinski et al., 2006; Alameda et al., 2007). Using an established qRT–PCR protocol that detects a region of MUC4 mRNA encoding a portion of the ectodomain, we found that MUC4 mRNA expression is at very low levels under all conditions examined. Consistent with this, MUC4 protein was undetectable by immunohistochemistry in proliferative and mid-secretory stage endometriosis. Thus, we conclude that full-length MUC4 is not expressed at appreciable levels in normal endometrium and endometriosis. Although we found a trend toward higher MUC4 mRNA levels in ectopic versus eutopic endometrium of stage-matched endometriosis patients, this change was not significant. It would be interesting to determine whether a significant accumulation of MUC4 expression occurs in advanced stages of endometriosis.
Progesterone resistance is now considered a key player in the etiology of endometriosis (Brosens et al., 2012). Nuclear receptors and nuclear receptor coregulator dysregulation are associated with the progression of endometriosis either due to an increase in estrogen or due to signaling by inflammatory molecules (Han and O'Malley, 2014). Several of these factors, such as PR-A and PR-B, PPARγ and TNFα regulate the expression of transmembrane mucins in various systems (Brayman et al., 2006; Dharmaraj et al., 2010; Wang et al., 2010; Morgado and Carson, unpublished observations). Thus, an understanding of mucins in endometriosis is important. There is great need for endometriosis biomarkers to aid clinical diagnosis and also to monitor the disease. Changes in mucin glycoforms have been suggested to characterize endometrial disease (Sivridis et al., 2002; Seeber et al., 2008). Although we have found no alterations in mucin expression in endometriosis, a comprehensive profile of transmembrane mucin expression, including changes in the oligosaccharide moieties carried by the mucin core proteins, in normal and endometriotic tissues may yet allow for development of meaningful biomarkers. In addition, changes in the oligosaccharide structures carried by mucins may have important biological consequences such as binding of selectins (Carson et al., 2006) or suppression of the immune system (Daniels et al., 2002; Hauselmann and Borsig, 2014). Future studies should focus on how glycoforms of MUC1 and MUC16 change in endometrial disease states in ways that might promote disease progression.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors’ roles
N.D., P.J.C. and M.M. performed all the qRT–PCR and immunostaining experiments, participated in the experimental design and co-wrote the manuscript. S.M.H., S.L.Y. and B.A.L. secured the patient samples and participated in the experimental design, critical discussions and manuscript editing. D.D.C. participated in the experimental design and manuscript preparation.
Funding
This study was supported by: NIH R01HD29963 to D.D.C.; NIH U54HD007495 to S.M.H.; and NIH R01HD067721 to S.L.Y. and B.A.L.
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
The authors appreciate the excellent secretarial and graphics assistance of Ms Sharron Kingston. We also greatly appreciate the routine critical input of Drs Pamela Constantinou-Papadopoulos and Cindy Farach-Carson as well as Mr Brian Engel during the course of these studies.
References
- Alameda F, Mejias-Luque R, Garrido M, de Bolos C. Mucin genes (MUC2, MUC4, MUC5AC, and MUC6) detection in normal and pathological endometrial tissues. Int J Gynecol Pathol. 2007;26:61–65. doi: 10.1097/01.pgp.0000225837.32719.c1. [DOI] [PubMed] [Google Scholar]
- Aplin JD, Seif MW, Graham RA, Hey NA, Behzad F, Campbell S. The endometrial cell surface and implantation. Expression of the polymorphic mucin MUC-1 and adhesion molecules during the endometrial cycle. Ann N Y Acad Sci. 1994;734:103–121. doi: 10.1111/j.1749-6632.1994.tb21739.x. [DOI] [PubMed] [Google Scholar]
- Aplin JD, Hey NA, Graham RA. Human endometrial MUC1 carries keratan sulfate: characteristic glycoforms in the luminal epithelium at receptivity. Glycobiology. 1998;8:269–276. doi: 10.1093/glycob/8.3.269. [DOI] [PubMed] [Google Scholar]
- Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Investigative ophthalmology & visual science. 2002;43:1004–1011. [PubMed] [Google Scholar]
- Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Investigative ophthalmology & visual science. 2003;44:2487–2495. doi: 10.1167/iovs.02-0862. [DOI] [PubMed] [Google Scholar]
- Braga VM, Gendler SJ. Modulation of Muc-1 mucin expression in the mouse uterus during the estrus cycle, early pregnancy and placentation. J Cell Sci. 1993;105(Pt 2):397–405. doi: 10.1242/jcs.105.2.397. [DOI] [PubMed] [Google Scholar]
- Brayman M, Thathiah A, Carson DD. MUC1: a multifunctional cell surface component of reproductive tissue epithelia. Reprod Biol Endocrinol. 2004;2:4. doi: 10.1186/1477-7827-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayman MJ, Julian J, Mulac-Jericevic B, Conneely OM, Edwards DP, Carson DD. Progesterone receptor isoforms A and B differentially regulate MUC1 expression in uterine epithelial cells. Mol Endocrinol. 2006;20:2278–2291. doi: 10.1210/me.2005-0343. [DOI] [PubMed] [Google Scholar]
- Brayman MJ, Dharmaraj N, Lagow E, Carson DD. MUC1 expression is repressed by protein inhibitor of activated signal transducer and activator of transcription-y. Mol Endocrinol. 2007;21:2725–2737. doi: 10.1210/me.2006-0539. [DOI] [PubMed] [Google Scholar]
- Brosens I, Brosens JJ, Benagiano G. The eutopic endometrium in endometriosis: are the changes of clinical significance? Reprod Biomed Online. 2012;24:496–502. doi: 10.1016/j.rbmo.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Carson DD, Julian J, Lessey BA, Prakobphol A, Fisher SJ. MUC1 is a scaffold for selectin ligands in the human uterus. Front Biosci. 2006;11:2903–2908. doi: 10.2741/2018. [DOI] [PubMed] [Google Scholar]
- Chang CY, Chang HW, Chen CM, Lin CY, Chen CP, Lai CH, Lin WY, Liu HP, Sheu JJ, Tsai FJ. MUC4 gene polymorphisms associate with endometriosis development and endometriosis-related infertility. BMC Med. 2011;9:19. doi: 10.1186/1741-7015-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels MA, Hogquist KA, Jameson SC. Sweet ‘n’ sour: the impact of differential glycosylation on T cell responses. Nat Immunol. 2002;3:903–910. doi: 10.1038/ni1002-903. [DOI] [PubMed] [Google Scholar]
- DeLoia JA, Krasnow JS, Brekosky J, Babaknia A, Julian J, Carson DD. Regional specialization of the cell membrane-associated, polymorphic mucin (MUC1) in human uterine epithelia. Hum Reprod. 1998;13:2902–2909. doi: 10.1093/humrep/13.10.2902. [DOI] [PubMed] [Google Scholar]
- de Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet. 2010;376:730–738. doi: 10.1016/S0140-6736(10)60490-4. [DOI] [PubMed] [Google Scholar]
- Dharmaraj N, Gendler SJ, Carson DD. Expression of human MUC1 during early pregnancy in the human MUC1 transgenic mouse model. Biol Reprod. 2009;81:1182–1188. doi: 10.1095/biolreprod.109.079418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmaraj N, Wang P, Carson DD. Cytokine and progesterone receptor interplay in the regulation of MUC1 gene expression. Mol Endocrinol. 2010;24:2253–2266. doi: 10.1210/me.2009-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Ho SB, Spurr-Michaud SJ, Tisdale AS, Zhan Q, Torlakovic E, Pudney J, Anderson DJ, Toribara NW, Hill JA., 3rd Mucin genes expressed by human female reproductive tract epithelia. Biol Reprod. 1997;56:999–1011. doi: 10.1095/biolreprod56.4.999. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Blalock T, Tisdale A, Spurr-Michaud S, Allcorn S, Stavreus-Evers A, Gemzell K. MUC16 is lost from the uterodome (pinopode) surface of the receptive human endometrium: in vitro evidence that MUC16 is a barrier to trophoblast adherence. Biol Reprod. 2008;78:134–142. doi: 10.1095/biolreprod.106.058347. [DOI] [PubMed] [Google Scholar]
- Han SJ, O'Malley BW. The dynamics of nuclear receptors and nuclear receptor coregulators in the pathogenesis of endometriosis. Hum Reprod Update. 2014;20:467–484. doi: 10.1093/humupd/dmu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–457. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- Hauselmann I, Borsig L. Altered tumor-cell glycosylation promotes metastasis. Front Oncol. 2014;4:28. doi: 10.3389/fonc.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins SM, Creighton CJ, Han DY, Zariff A, Anderson ML, Gunaratne PH, Matzuk MM. Functional microRNA involved in endometriosis. Mol Endocrinol. 2011;25:821–832. doi: 10.1210/me.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He RH, Yao WM, Wu LY, Mao YY. Highly elevated serum CA-125 levels in patients with non-malignant gynecological diseases. Arch Gynecol Obstet. 2011;283(Suppl 1):107–110. doi: 10.1007/s00404-010-1717-5. [DOI] [PubMed] [Google Scholar]
- Hebbar V, Damera G, Sachdev GP. Differential expression of MUC genes in endometrial and cervical tissues and tumors. BMC Cancer. 2005;5:124. doi: 10.1186/1471-2407-5-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hey NA, Graham RA, Seif MW, Aplin JD. The polymorphic epithelial mucin MUC1 in human endometrium is regulated with maximal expression in the implantation phase. J Clin Endocrinol Metab. 1994;78:337–342. doi: 10.1210/jcem.78.2.8106621. [DOI] [PubMed] [Google Scholar]
- Hild-Petito S, Fazleabas AT, Julian J, Carson DD. Mucin (Muc-1) expression is differentially regulated in uterine luminal and glandular epithelia of the baboon (Papio anubis) Biol Reprod. 1996;54:939–947. doi: 10.1095/biolreprod54.5.939. [DOI] [PubMed] [Google Scholar]
- Hoffman LH, Olson GE, Carson DD, Chilton BS. Progesterone and implanting blastocysts regulate Muc1 expression in rabbit uterine epithelium. Endocrinology. 1998;139:266–271. doi: 10.1210/endo.139.1.5750. [DOI] [PubMed] [Google Scholar]
- Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- Horne AW, Lalani EN, Margara RA, Ryder TA, Mobberley MA, White JO. The expression pattern of MUC1 glycoforms and other biomarkers of endometrial receptivity in fertile and infertile women. Mol Reprod Dev. 2005;72:216–229. doi: 10.1002/mrd.20307. [DOI] [PubMed] [Google Scholar]
- Julian J, Enders AC, Fazleabas AT, Carson DD. Compartmental distinctions in uterine Muc-1 expression during early pregnancy in cynomolgous macaque (Macaca fascicularis) and baboon (Papio anubis) Hum Reprod. 2005;20:1493–1503. doi: 10.1093/humrep/deh801. [DOI] [PubMed] [Google Scholar]
- Koscinski I, Viville S, Porchet N, Bernigaud A, Escande F, Defossez A, Buisine MP. MUC4 gene polymorphism and expression in women with implantation failure. Hum Reprod. 2006;21:2238–2245. doi: 10.1093/humrep/del189. [DOI] [PubMed] [Google Scholar]
- Lessey BA. Medical management of endometriosis and infertility. Fertil Steril. 2000;73:1089–1096. doi: 10.1016/s0015-0282(00)00519-7. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Lebovic DI, Taylor RN. Eutopic endometrium in women with endometriosis: ground zero for the study of implantation defects. Semin Reprod Med. 2013;31:109–124. doi: 10.1055/s-0032-1333476. [DOI] [PubMed] [Google Scholar]
- Margarit L, Taylor A, Roberts MH, Hopkins L, Davies C, Brenton AG, Conlan RS, Bunkheila A, Joels L, White JO, et al. MUC1 as a discriminator between endometrium from fertile and infertile patients with PCOS and endometriosis. J Clin Endocrinol Metab. 2010;95:5320–5329. doi: 10.1210/jc.2010-0603. [DOI] [PubMed] [Google Scholar]
- May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010;16:651–674. doi: 10.1093/humupd/dmq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol BW, Bayram N, Lijmer JG, Wiegerinck MA, Bongers MY, van der Veen F, Bossuyt PM. The performance of CA-125 measurement in the detection of endometriosis: a meta-analysis. Fertil Steril. 1998;70:1101–1108. doi: 10.1016/s0015-0282(98)00355-0. [DOI] [PubMed] [Google Scholar]
- Moll R, Levy R, Czernobilsky B, Hohlweg-Majert P, Dallenbach-Hellweg G, Franke WW. Cytokeratins of normal and some neoplasms of the female genital tract. Lab Invest. 1983;49:599–610. [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the Endometrial Biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- Patrelli TS, Berretta R, Gizzo S, Pezzuto A, Franchi L, Lukanovic A, Nardelli GB, Modena AB. CA 125 serum values in surgically treated endometriosis patients and its relationships with anatomic sites of endometriosis and pregnancy rate. Fertil Steril. 2011;95:393–396. doi: 10.1016/j.fertnstert.2010.08.043. [DOI] [PubMed] [Google Scholar]
- Plante BJ, Lessey BA, Taylor RN, Wang W, Bagchi MK, Yuan L, Scotchie J, Fritz MA, Young SL. G protein-coupled estrogen receptor (GPER) expression in normal and abnormal endometrium. Reprod Sci. 2012;19:684–693. doi: 10.1177/1933719111431000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Agata N, Chen D, Li Y, Yu WH, Huang L, Raina D, Chen W, Kharbanda S, Kufe D. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell. 2004;5:163–175. doi: 10.1016/s1535-6108(04)00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- Seeber B, Sammel MD, Fan X, Gerton GL, Shaunik A, Chittams J, Barnhart KT. Panel of markers can accurately predict endometriosis in a subset of patients. Fertil Steril. 2008;89:1073–1081. doi: 10.1016/j.fertnstert.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Sivridis E, Giatromanolaki A, Koukourakis MI, Georgiou L, Anastasiadis P. Patterns of episialin/MUC1 expression in endometrial carcinomas and prognostic relevance. Histopathology. 2002;40:92–100. doi: 10.1046/j.1365-2559.2002.01316.x. [DOI] [PubMed] [Google Scholar]
- Socolov R, Butureanu S, Angioni S, Sindilar A, Boiculese L, Cozma L, Socolov D. The value of serological markers in the diagnosis and prognosis of endometriosis: a prospective case-control study. Eur J Obstet Gynecol Reprod Biol. 2011;154:215–217. doi: 10.1016/j.ejogrb.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, Julian J, Pimental RA, Wegner CC, Dey SK, Carson DD. Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology. 1995;136:3639–3647. doi: 10.1210/endo.136.8.7628404. [DOI] [PubMed] [Google Scholar]
- Szubert M, Suzin J, Wierzbowski T, Kowalczyk-Amico K. CA-125 concentration in serum and peritoneal fluid in patients with endometriosis—preliminary results. Arch Med Sci. 2012;8:504–508. doi: 10.5114/aoms.2012.29407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad AM, Diaconu I, Gantt KR. MUC1 in endometriosis and ovarian cancer. Immunol Res. 2006;36:229–236. doi: 10.1385/IR:36:1:229. [DOI] [PubMed] [Google Scholar]
- Vodolazkaia A, El-Aalamat Y, Popovic D, Mihalyi A, Bossuyt X, Kyama CM, Fassbender A, Bokor A, Schols D, Huskens D, et al. Evaluation of a panel of 28 biomarkers for the non-invasive diagnosis of endometriosis. Hum Reprod. 2012;27:2698–2711. doi: 10.1093/humrep/des234. [DOI] [PubMed] [Google Scholar]
- Wang P, Julian JA, Carson DD. The MUC1 HMFG1 glycoform is a precursor to the 214D4 glycoform in the human uterine epithelial cell line, HES. Biol Reprod. 2008;78:290–298. doi: 10.1095/biolreprod.107.064584. [DOI] [PubMed] [Google Scholar]
- Wang P, Dharmaraj N, Brayman MJ, Carson DD. Peroxisome proliferator-activated receptor gamma activation inhibits progesterone-stimulated human MUC1 expression. Mol Endocrinol. 2010;24:1368–1379. doi: 10.1210/me.2009-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel CA. Evaluation and management of women with endometriosis. Obstet Gynecol. 2003;102:397–408. doi: 10.1016/s0029-7844(03)00474-5. [DOI] [PubMed] [Google Scholar]
- Worley MJ, Welch WR, Berkowitz RS, Ng SW. Endometriosis-associated ovarian cancer: a review of pathogenesis. Int J Mol Sci. 2013;14:5367–5379. doi: 10.3390/ijms14035367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.