ABSTRACT
Bazedoxifene (BZA), a selective estrogen receptor modulator (SERM), inhibits the action of estrogens on endometrial proliferation. Here, we evaluate the effect of a tissue-selective estrogen complex (TSEC) containing BZA and conjugated estrogens (CE) on ectopic endometrial lesions in a mouse model of endometriosis. Experimental endometriosis was created in 60 female CD-1 mice. The mice were randomly divided into 10 groups that received varying doses of either BZA (1, 2, 3, or 5 mg/kg/day), BZA (1, 2, 3, or 5 mg/kg/day) in combination with CE (3 mg/kg/day), CE treatment alone (3 mg/kg/day), or vehicle control for 8 wk. Treatment with BZA alone or the TSEC containing BZA/CE led to a decrease in endometriotic lesion size compared to controls. The mean surface area of the untreated lesions was 19.6 mm2. Treatment with BZA or BZA/CE resulted in reduced lesion size (to 8.8 and 7.8 mm2, respectively). No significant difference was found in lesion size between the BZA and BZA/CE treatment groups or between different doses of either treatment. Ovarian cyst formation was not evident in the treated groups. Treatment with the TSEC containing higher BZA dosages (3 and 5 mg/kg/day) led to significantly lower levels of estrogen receptor (Esr1) mRNA expression compared to the control treatment. No differences were observed in expression of progesterone receptor (Pgr). Immunohistochemical analysis also demonstrated a decrease in ESR protein. The combination of CE and BZA may prove to be a novel treatment option for endometriosis.
Keywords: bazedoxifene (BZA), conjugated estrogen (CE), endometriosis, hormone receptors, tissue-specific estrogen complex (TSEC)
INTRODUCTION
Endometriosis is an estrogen-dependent disorder that affects 5%–10% of the female population with clinical features that include pelvic pain, dysmenorrhea, and infertility [1, 2]. The defining characteristic of endometriosis is the ectopic growth of endometrial tissue in sites outside of the uterine cavity.
While the etiology remains enigmatic, dependence on estrogens for growth and a modified response to estrogens and progesterone affecting the ectopic endometrium are essential to the development of endometriosis [3, 4]. Current medical treatments include hormonal manipulations to achieve pseudo-pregnancy or pseudo-menopause. These therapies have a high failure rate, and side effects are common [5–9]. Treatment with progestins often results in irregular bleeding as well as fluid retention and mood changes. In contrast, treatments with gonadotropin-releasing hormone (GnRH) agonists result in menopausal symptoms [10, 11]. The need for improved therapies is evident.
Selective estrogen receptor modulators (SERMs), used for treatment of breast cancer and osteoporosis, display tissue-specific estrogen agonist or antagonist activities [12, 13]. SERMs bind to the estrogen receptor (ESR1) with high affinity and exert estrogenic effects on several estrogen target tissues, in particular the skeletal system. In the presence of other estrogens, such as estradiol, SERMs display antagonistic activity on the breast and prevent endometrial proliferation [14–17]. Due to their antiproliferative effect on the endometrium, they have been considered for use in the treatment of endometriosis. The SERM raloxifene is approved for the prevention and treatment for postmenopausal osteoporosis and the prevention of breast cancer in postmenopausal women [18–22]. Raloxifene binds to the ESR1 with an affinity similar to that of 17β-estradiol and has been demonstrated not to lead to endometrial proliferation in a rat model [23]. It has also been used to treat endometriosis in a single study using the ovariectomized rat model [24]. In a randomized clinical trial, raloxifene was used to treat women with chronic pain due to endometriosis [25]. The trial was terminated when women using raloxifene experienced greater pain than that of women in the placebo group. Unfortunately, the dose of raloxifene used in this clinical trial was lower than the weight-adjusted effective dose used in the animal model. Additionally, SERMs block feedback inhibition of sex steroids on the hypothalamus and pituitary. The effects of raloxifene on follicle-stimulating hormone (FSH) production and subsequent ovarian stimulation would not have been apparent in the ovariectomized rat model. Ovarian stimulation may have contributed to the failure of raloxifene in the single clinical trial designed to treat endometriosis with this drug.
A new-generation SERM, bazedoxifene (BZA), has been shown to effectively maintain bone mass in postmenopausal women [26–28]. BZA has also been combined with conjugated estrogens (CE) to create a tissue-specific estrogen complex (TSEC). Unlike a SERM alone, this TSEC provides effective relief of vasomotor symptoms in menopausal women [29, 30]. Further, BZA/CE did not affect the endometrium of menopausal women any differently than placebo [27]. In other TSECs, the SERM has not been shown to be capable of countering the effects of estrogens on the endometrium. The combination of raloxifene and estradiol resulted in an unacceptable rate of endometrial hyperplasia, suggesting that combination would not be an effective or safe treatment for endometriosis [31]. The effects of BZA/CE on the endometrium suggest that this TSEC may be a more effective agent in the treatment of endometriosis. The addition of estrogens to the SERM may result in improved feedback inhibition and prevent ovarian stimulation when used in a premenopausal woman.
The objective of the present study was to evaluate the effect of a TSEC (BZA/CE) on ectopic endometrial lesions in a murine endometriosis model. Using clinically relevant doses in the mouse model, we examined the effects of several doses of BZA with or without CE (3 mg/kg/day) on experimental endometriosis in animals with intact ovarian function.
MATERIALS AND METHODS
Animal Care and Treatment
Eight-week old CD1 female mice were purchased from Charles River Laboratories. Animals were kept under a regulated photoperiod of 12L:12D. Laparotomy was performed following i.p. anesthesia with xylazine (Lloyd Laboratories) and ketamine (Vedco); the whole uterus was removed, washed in PBS, and divided into two horns. The uterine horns were then split longitudinally, exposing the lumen, and sutured to the parietal peritoneum of the recipient mice to create experimental endometriosis, after which the abdominal wall was sutured closed. Experimental endometriosis was created in 60 mice with intact ovaries. Eight weeks after establishment of disease, the mice were divided into 10 groups. Groups 1–4 (n = 5 mice/group) received i.p. injections of varying doses of BZA (1, 2, 3, or 5 mg/kg/day in dimethyl sulfoxide [DMSO; 10%] plus sesame oil [90%]) for 8 wk. Groups 5–8 (n = 5 mice/group) received the same i.p. of BZA (1, 2, 3, or 5 mg/kg/day) in combination with CE (3 mg/kg/day; administered orally by gavage) for 8 wk. Group 9 (n = 10 mice) received CE (3 mg/kg/day) alone. Group 10 (controls; n = 10 mice) received i.p. injections of DMSO (10%) plus sesame oil (90%) simultaneously for 8 wk. Following the completion of treatments, mice were euthanized, and the uteri, ovaries, and ectopic endometrial lesions were measured and collected. One uterine horn was snap-frozen in TRIzol Reagent (Invitrogen) for RNA extraction, and the other horn was formalin-fixed and paraffin-embedded for hematoxylin-and-eosin staining and immunohistochemical analysis.
Ethical guidelines for the use of animals as established by Institutional Animal Care and Use Committee, Yale University, and the U.S. Government Principles for Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training were followed.
Quantitative Real-Time RT-PCR
Total RNA was isolated from eutopic endometrium using TRIzol Reagent and then purified with the RNeasy MinElute Cleanup Kit (Qiagen) according to the manufacturer's instructions. Purified RNA (50 ng) was reverse-transcribed using iScript cDNA synthesis kit (Bio-Rad Laboratories). Quantitative real-time PCR was performed using SYBR Green (Bio-Rad Laboratories) and optimized in the MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad Laboratories). The specificity of the amplified transcript was confirmed by a melting-curve analysis. The reactions for each gene were performed in duplicate and repeated three times. Expression of Esr1 and progesterone receptor (Pgr) mRNA was quantified and standardized to that of a reference gene (β-actin). The relative amount of transcript generated for each primer was analyzed on the basis of the cycle threshold (Ct) value. The relative gene expression ratio was calculated using 2−ΔΔCt. Statistical significance was evaluated using a two-tailed t-test; a P-value of 0.05 or less was considered to be significant.
Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded uterine tissue cut into 5-μm sections. Slides were deparaffinized and hydrated through series of 15-min xylene and 10-min ethanol washes. After being rinsed for 5 min in fresh distilled water, slides were steamed in 0.01 M citric acid for 15 min to promote antigen presentation and cooled for 15 min in the citrate buffer, followed by three 5-min washes in Tris-buffered saline (TBS). Endogenous peroxidase activity was blocked using a 3% hydrogen peroxide solution for 10 min before a wash in TBS-Tween 20 (TBST). To block nonspecific antibody binding, the slides were incubated for 30 min at room temperature in a solution of 1.5% blocking serum in TBS. The slides were then incubated overnight at 4°C with the primary antibody against ESR1 (ERα) (1:250 dilution) or against PGR H-190 (sc-7208; Santa Cruz Biotechnology). After three 5-min rinses in TBS, slides were incubated with either a horse anti-goat biotinylated secondary antibody (ESR1) or goat α-rabbit antibody (PGR; Vector Laboratories) for 30 min at room temperature. Slides were washed three times in TBS and incubated for 30 min in ABC Elite solution (Vector Laboratories), and then the slides were incubated for 2.5 min in diaminobenzidine (Vector Laboratories). Slides were exposed to hematoxylin for 16 sec and then rehydrated through multiple 3-min ethanol and xylene washes. All slides were mounted with Permount (Fisher Scientific). The H-score was used to quantify the glandular and stromal expression of steroid receptors. In all, 100 positively stained cell nuclei for each microscopic field were examined from each animal to quantify the expression of ESR1 and PGR in the tissue. The H-score was calculated with the following equation: HSCORE = Σπ(i + 1), where i is the intensity of staining with a value 0–3 (none, weak, moderate, or strong staining, respectively) and π is the percentage (0%–100%) of stained cells for each intensity [32]. The average score was calculated, and a statistical analysis was performed using the Mann-Whitney rank-sum test to compare differences in the eutopic endometrium of the control and treated groups. A P-value of 0.05 or less was considered to be significant.
RESULTS
Endometrial lesions were established in controls and in animals treated with CE alone. Groups treated with BZA alone or any dose of BZA/CE combination (TSEC) for 8 wk displayed a similar decrease in endometriotic lesion size compared to controls (Table 1). The mean surface area of the untreated lesions was 19.6 mm2. Treatment with BZA (all doses combined) or BZA/CE resulted in lesion reduction to 8.8 and 7.8 mm2, respectively (both P < 0.05). No significant difference in lesion size was found between the BZA and BZA/CE treatment groups or between different doses of either treatment. Further, all doses of BZA/CE treatment reduced many lesions of endometriosis to fibrosis or scar, with little endometrial tissue remaining. All mice continued to cycle on treatment as assessed by vaginal histology. Ovarian weight was not significantly different between groups (Table 1). Ovarian cyst formation was not evident in the treated groups. Endometrial histological evaluation revealed no evidence of hyperplasia in any of the treated animals. Similarly, histological analysis of the ovaries did not reveal hyperstimulation or changes in follicle number.
TABLE 1.
Mean endometrial lesion size and ovarian weights.
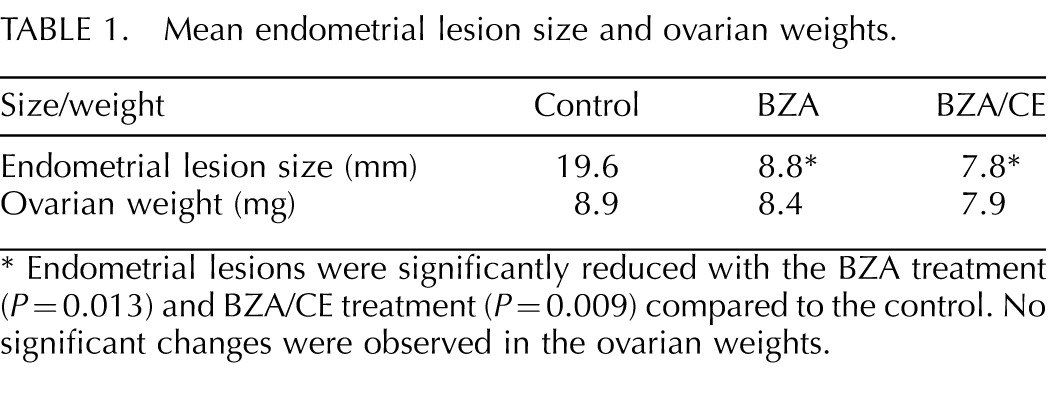
Endometrial lesions were significantly reduced with the BZA treatment (P = 0.013) and BZA/CE treatment (P = 0.009) compared to the control. No significant changes were observed in the ovarian weights.
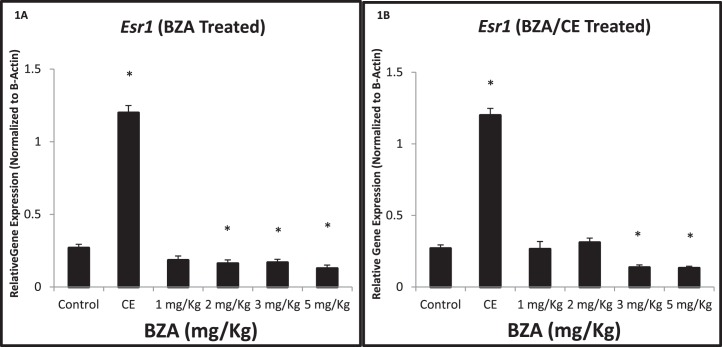
Expression of Esr1, an essential mediator of endometrial proliferation, was altered in a dose-dependent manner (Fig. 1A). Treatment with CE (3 mg/kg/day) increased Esr1 expression 446% (4.46-fold) compared to controls (P = 0.009). Esr1 mRNA expression was reduced to 60% of control levels after treatment with 2 mg/kg/day of BZA (P = 0.029). After treatment with 3 or 5 mg/kg/day of BZA, Esr1 expression was reduced to 63% and 48%, respectively, of the control value (P = 0.037 and 0.006, respectively).
FIG. 1.
Quantitative real-time RT-PCR demonstrates Esr1 gene expression in the uterus of CE, BZA-treated, BZA/CE-treated, and control mice. Esr1 expression was increased by CE treatment, as expected (*P = 0.009). A) Esr1 expression was significantly reduced after treatment with 2, 3, and 5 mg/kg/day of BZA when compared to the control (*P = 0.0295, 0.0374, and 0.0066, respectively). B) Esr1 expression was significantly reduced after BZA/CE treatment consisting of 3 and 5 mg/kg/day of BZA (*P = 0.004 and 0.006, respectively).
Treatment with either 3 or 5 mg/kg/day of BZA (Fig. 1B) used in combination with CE (BZA/CE) resulted in reductions of Esr1 expression when compared to the control. Treatment with the TSEC containing 3 mg/kg/day of BZA and CE reduced Esr1 expression to 51% of control (P = 0.004), and treatment with the TSEC containing 5 mg/kg/day of BZA and CE reduced Esr1 expression to 49% of control (P = 0.006). In addition, treatments with 3 and 5 mg/kg/day of BZA used in combination with CE demonstrated reduced expression of Esr1 compared to treatments with 1 and 2 mg/kg/day of BZA and CE (P = 0.040 and 0.002, respectively).
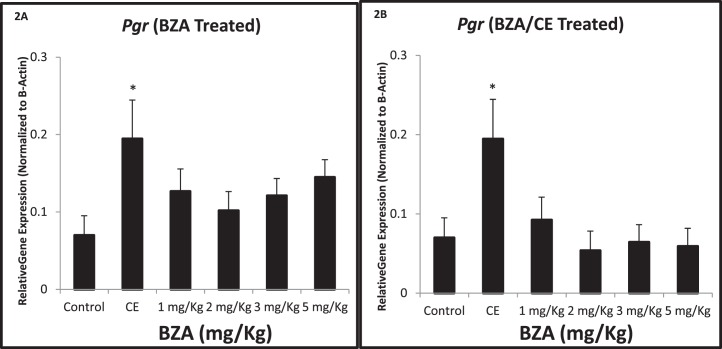
Expression of Pgr mRNA was used as a marker of endometrial differentiation. Pgr expression was increased 278% (2.78-fold) compared to controls after treatment with CE (3 mg/kg/day; P = 0.02). Pgr expression remained unchanged after either BZA or BZA/CE treatment compared to the vehicle-treated controls and did not vary throughout the range of treatment doses (Fig. 2, A and B).
FIG. 2.
Quantitative real-time RT-PCR demonstrates Pgr gene expression in the uterus of BZA-treated, BZA/CE-treated, and control mice. A and B) Pgr expression was increased by CE (*P = 0.02), as expected, but no significant treatment effects related to BZA or BZA/CE were found.
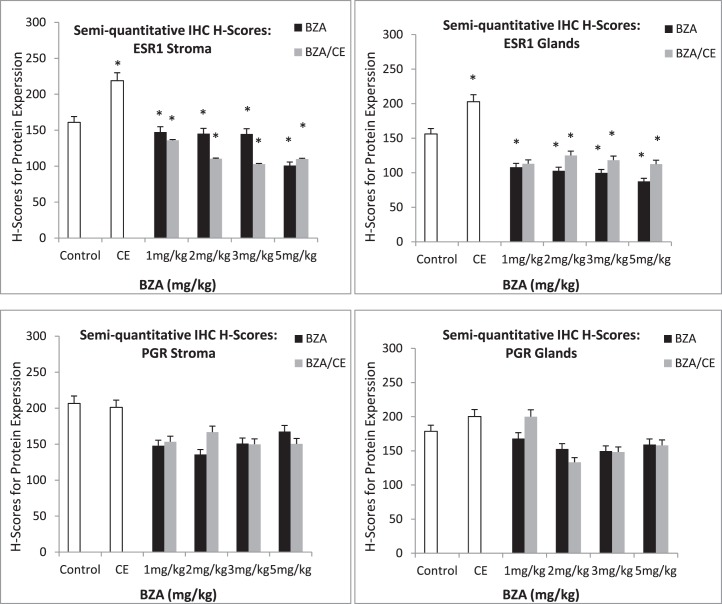
Immunohistochemistry was used to identify ESR1 and PGR protein expression in the eutopic endometrium (Fig. 3). Consistent with the quantitative real-time RT-PCR results, ESR1 expression was decreased in the endometrium of the BZA- and BZA/CE-treated groups. A decrease in ESR1 expression was also evident in the stromal and glandular cells of both treatment groups. On a continuous scale of 0–300, the mean ESR1 H-score for the control group was 161 and 156.2 in the stromal cells and endometrial glands, respectively (Fig. 4). The mean ESR1 H-score for the CE-treated group was 219 and 202 in the stromal cells and endometrial glands, respectively (P = 0.002 and 0.03, respectively). In the stromal cells of the BZA treatment groups, the mean ESR1 H-score was 147, 145, 144, and 100 for doses of 1, 2, 3, and 5 mg/kg/day, respectively (P = 0.02, 0.017, 0.013, and 6.81 × 10−7, respectively). The mean ESR1 H-score in the endometrial glands for each corresponding dose of BZA was 108, 102, 100, and 88, (P = 2.69 × 10−5, 8.71 × 10−6, 5.46 × 10−6, and 1.19 × 10−6, respectively). The mean ESR1 H-score in the stromal cells for the BZA/CE (1, 2, 3, or 5 mg/kg/day of BZA plus 3 mg/kg/day of CE) treatment group was 136, 110, 103, and 110 (P = 0.0008, 5.74 × 10−6, 8.39 × 10−7, and 3.02 × 10−6, respectively). The mean ESR1 H-score of the endometrial glands for each corresponding dose in the BZA/CE-treated group was 113, 125, 118, and 112 (P = 0.19, 0.0014, 0.0002, and 0.0001, respectively).
FIG. 3.
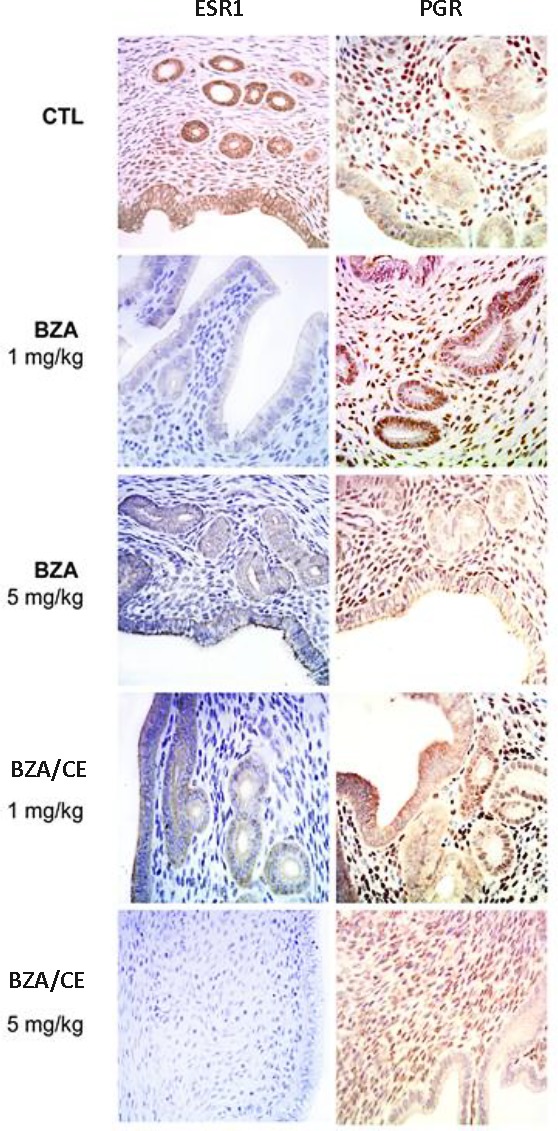
Immunohistochemistry (IHC) demonstrating ESR1 and PGR protein expression in the endometrial stroma and glands of the control, BZA-treated, and BZA/CE-treated endometrium. A greatly reduced ESR1 expression was evident in the stromal and glandular cells of the BZA-treated endometrium at all doses used. BZA treatment led to reduction of both glandular and stromal expression of ESR1. Similarly, BZA/CE treatment led to decreased ESR1 expression at all doses. PGR expression was not significantly altered by treatment. Representative images are shown at the lowest and highest dose. Original magnification ×400.
FIG. 4.
H-scores corresponding to glandular and stromal expression of ESR1 and PGR protein in controls as well as CE-, BZA-, and BZA/CE-treated animals. Increased ESR1 expression was noted in the glands and stroma of animals treated with CE. Significantly decreased ESR1 expression was seen in animals treated with either BZA or BZA/CE (*P < 0.05) compared to the control.
Expression of PGR protein did not appear to be significantly altered by the BZA or BZA/CE treatment and did not vary by dose (Fig. 3). The mean PGR H-score for the control group was 207 and 179 in the stromal cells and endometrial glands, respectively (Fig. 4). The mean PGR H-score for the CE treated group was 201 and 200 in the stromal cells and endometrial glands, respectively. The mean PGR H-score for the BZA treatment groups was 168 in the stromal cells and 168 in the endometrial glands (P = 0.2). The mean PGR H-score for the BZA/CE-treated group was 155 in the stromal cells and 160 in the endometrial glands (P = 0.1).
DISCUSSION
The treatment of endometriosis continues to be a dilemma, hampered by our lack of treatment options. However, endometriosis is clearly an estrogen-dependent disease, and all therapies rely on alteration of sex steroid levels. Common therapies used to suppress the progression of endometriosis include GnRH agonists, progestins, aromatase inhibitors, androgens, and oral contraceptives [1, 2, 33–35]. The use of many of these treatments has been associated with numerous and, in some cases, serious side effects. Oral contraceptive therapy has a high long-term failure rate. While the GnRH agonist therapy improves pain, its use leads to estrogen deficiency, which can lead to bone loss, vaginal dryness, and vasomotor symptoms if not administered with “add back” hormone therapy [36, 37]. Progestin therapy has been associated with weight gain, breast tenderness and mood alterations [38–40]. Clearly, alternative medical regiments for the treatment of endometriosis are needed [41].
The ideal endometriosis treatment would effectively treat the lesions and block the undesirable stimulation of the breast while retaining an estrogenic effect on the skeletal and central nervous systems. Elimination of a progestin would improve the side effect profile that severely limits patient compliance. SERMs display estrogen receptor (ESR) agonist and antagonistic effects in a tissue-specific profile. BZA blocks the estrogen-dependent growth of endometrium and endometriosis [27, 42–47]. To prevent hypothalamic and pituitary stimulation, which increase FSH production and ovarian cyst formation, a novel approach combining BZA and CE in a TSEC was used to treat endometriosis. TSECs block endometrial stimulation, and the addition of the estrogens in CE to the SERM would hypothetically contribute to the alleviation of symptoms. The addition of CE would also be expected to enhance the beneficial effects of estrogenic feedback on the central nervous system (CNS), preventing the increase in FSH that would otherwise be seen with the estrogenic inhibition of a SERM alone. Further, the CE may prevent the hot flashes typically associated with SERMs.
We have previously demonstrated that BZA is an effective treatment of endometriosis as evaluated by the decrease in endometrial implant size [42]. In the present study, we demonstrate that the use of BZA/CE decreased endometrial lesion size. The addition of estrogens did not reduce the efficacy of the SERM. Further, the estrogens did not promote endometrial hyperplasia. BZA/CE is an effective treatment for murine experimental endometriosis and is expected to yield a superior side effect profile in humans due to estrogenic action on the CNS. In addition, no significant effect of BZA dose on lesion size was found, suggesting that doses below the equivalent to be used in humans may still be fully efficacious.
In the treatment of endometriosis, this TSEC (BZA/CE) likely functions through decreased Esr1 expression. While estrogens, including CE, as demonstrated here, increase Esr1 expression, the TSEC did not induce ESR1 expression, clearly demonstrating that BZA antagonizes the effect of CE. In fact, both BZA alone and the TSEC reduce Esr1 mRNA and ESR1 protein expression. A mechanism of action may be posttranscriptional; BZA affects Esr1 receptor stability based on a recent report describing a BZA-induced conformational change in ESR1 that resulted in its proteosomal degradation [48] . As previously demonstrated in endometrial cells, Pgr expression was not significantly altered by BZA or BZA/CE treatment [49]. In women participating in phase II clinical trials (for postmenopausal vasomotor symptom treatment and prevention of osteoporosis), BZA/CE treatment did not induce endometrial growth or endometrial hyperplasia [21, 47]. The effects of BZA and TSEC treatment on Esr1 suggest a mechanism by which BZA inhibits endometrial proliferation. BZA induced decreases in ESR1 may inhibit endometrial cell growth in both the endometrium and endometriosis.
The TSECs have been developed as a treatment for menopausal vasomotor symptoms, vaginal atrophy, and bone loss; the advantage of at least one TSEC (BZA/CE) includes the ability to selectively antagonize estrogen action in the endometrium and breast while maintaining estrogen action in the CNS, all without the need for a progestin. Similarly, the ability to inhibit endometrial growth without a progestin makes BZA/CE an ideal agent for the treatment of endometriosis.
In summary, BZA/CE is a potential novel therapy for endometriosis that is predicted to have a high level of efficacy without the side effects of currently available treatments.
Footnotes
Supported by NICHD and Pfizer, Inc.
REFERENCES
- Giudice LC, Kao LC. Endometriosis Lancet 2004. 364 9447: 1789 1799 [DOI] [PubMed] [Google Scholar]
- Bulun SE. Endometriosis N Engl J Med 2009. 360 3: 268 279 [DOI] [PubMed] [Google Scholar]
- Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer Endocr Rev 2013. 34 1: 130 162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho L, Podgaec S, Bellodi-Privato M, Falcone T, Abrão MS. Role of eutopic endometrium in pelvic endometriosis J Minim Invasive Gynecol 2011. 18 4: 419 427 [DOI] [PubMed] [Google Scholar]
- Allen C, Hopewell S, Prentice A, Gregory D. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis Cochrane Database Syst Rev 2009. CD004753. [DOI] [PubMed] [Google Scholar]
- Al Kadri H, Hassan S, Al-Fozan HM, Hajeer A. Hormone therapy for endometriosis and surgical menopause Cochrane Database Syst Rev 2009. CD005997. [DOI] [PubMed] [Google Scholar]
- Nawathe A, Patwardhan S, Yates D, Harrison GR, Khan KS. Systematic review of the effects of aromatase inhibitors on pain associated with endometriosis. BJOG. 2008;115:818–822. doi: 10.1111/j.1471-0528.2008.01740.x. [DOI] [PubMed] [Google Scholar]
- Rodgers AK, Falcone T. Treatment strategies for endometriosis. Expert Opin Pharmacother. 2008;9:243–255. doi: 10.1517/14656566.9.2.243. [DOI] [PubMed] [Google Scholar]
- Davis L, Kennedy SS, Moore J, Prentice A. Modern combined oral contraceptives for pain associated with endometriosis Cochrane Database Syst Rev 2007. CD001019. [DOI] [PubMed] [Google Scholar]
- Surrey ES. Gonadotropin-releasing hormone agonist and add-back therapy: what do the data show? Curr Opin Obstet Gynecol 2010. 22 4: 283 288 [DOI] [PubMed] [Google Scholar]
- Divasta AD, Laufer MR. The use of gonadotropin releasing hormone analogues in adolescent and young patients with endometriosis Curr Opin Obstet Gynecol 2013. 25 4: 287 292 [DOI] [PubMed] [Google Scholar]
- Taylor HS. Designing the ideal selective estrogen receptor modulator—an achievable goal? Menopause 2009. 16 3: 609 615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs BL, Hartmann LC. Selective estrogen receptor modulators—mechanisms of action and application to clinical practice. N Engl J Med. 2003;348:618–629. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Sestak I, Bonanni B, Costantino JP, Cummings S, DeCensi A, Dowsett M, Forbes JF, Ford L, LaCroix AZ, Mershon J, Mitlak BH, et al. Chemoprevention of Breast Cancer Overview Group. Selective estrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data Lancet 2013. 381 9880: 1827 1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton JV, Goldstein SR. Endometrial safety: a key hurdle for selective estrogen receptor modulators in development Menopause 2010. 17 3: 642 653 [DOI] [PubMed] [Google Scholar]
- Ethun KF, Wood CE, Cline JM, Register TC, Appt SE, Clarkson TB. Endometrial profile of bazedoxifene acetate alone and in combination with conjugated equine estrogens in a primate model Menopause 2013. 20 7: 777 784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer DF, Pinkerton JV, Utian WH, Menegoci JC, de Villiers TJ, Yuen CK, Levine AB, Chines AA, Constantine GD. Bazedoxifene, a selective estrogen receptor modulator: effects on the endometrium, ovaries, and breast from a randomized controlled trial in osteoporotic postmenopausal women Menopause 2009. 16 6: 1109 1115 [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Grady D, Sashegyi A, Anderson PW, Cox DA, Hoszowski K, Rautaharju P, Harper KD. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial JAMA 2002. 287 7: 847 857 [DOI] [PubMed] [Google Scholar]
- Delmas PD, Ensrud KE, Adachi JD, Harper KD, Sarkar S, Gennari C, Reginster JY, Pols HA, Recker RR, Harris ST, Wu W, Genant HK, et al. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial J Clin Endocrinol Metab 2002. 87 8: 3609 3617 [DOI] [PubMed] [Google Scholar]
- Fuchs-Young R, Glasebrook AL, Short LL, Draper MW, Rippy MK, Cole HW, Magee DE, Termine JD, Bryant HU. Raloxifene is a tissue-selective agonist/antagonist that functions through the estrogen receptor. Ann N Y Acad Sci. 1995;761:355–360. doi: 10.1111/j.1749-6632.1995.tb31392.x. [DOI] [PubMed] [Google Scholar]
- Bryant HU, Dere WH. Selective estrogen receptor modulators: an alternative to hormone replacement therapy Proc Soc Exp Biol Med 1998. 217 1: 45 52 [DOI] [PubMed] [Google Scholar]
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER, Jr, , Wade JL , Robidoux A, Margolese RG, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial JAMA 2006. 295 23: 2727 2741 [DOI] [PubMed] [Google Scholar]
- Black LJ, Sato M, Rowley ER, Magee DE, Bekele A, Williams DC, Cullinan GJ, Bendele R, Kauffman RF, Bensch WR. Raloxifene (LY139481 HCI) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats. J Clin Invest. 1994;93:63–69. doi: 10.1172/JCI116985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Shen X, Capodanno I, Donnelly M, Fenyk-Melody J, Hausamann J, Nunes C, Strauss J, Vakerich K. Validation of rat endometriosis model by using raloxifene as a positive control for the evaluation of novel SERM compounds. J Invest Surg. 2005;18:177–183. doi: 10.1080/08941930591004412. [DOI] [PubMed] [Google Scholar]
- Stratton P, Sinaii N, Segars J, Koziol D, Wesley R, Zimmer C, Winkel C, Nieman LK. Return of chronic pelvic pain from endometriosis after raloxifene treatment: a randomized controlled trial. Obstet Gynecol. 2008;111:88–96. doi: 10.1097/01.AOG.0000297307.35024.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genant HK. Bazedoxifene: a new selective estrogen receptor modulator for postmenopausal osteoporosis Menopause Int 2011. 17 2: 44 49 [DOI] [PubMed] [Google Scholar]
- Pickar JH, Yeh I-T, Bachmann G, Speroff L. Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril. 2009;92:1018–1024. doi: 10.1016/j.fertnstert.2009.05.094. [DOI] [PubMed] [Google Scholar]
- Pinkerton JV, Archer DF, Utian WH, Menegoci JC, Levine AB, Chines AA, Constantine GD. Bazedoxifene effects on the reproductive tract in postmenopausal women at risk for osteoporosis. Menopause. 2009;16:1102–1108. doi: 10.1097/gme.0b013e3181a816be. [DOI] [PubMed] [Google Scholar]
- Utian W, Yu H, Bobula J, Mirkin S, Olivier S, Pickar JH. Bazedoxifene/conjugated estrogens and quality of life in postmenopausal women Maturitas 2009. 20; 63 4: 329 335 [DOI] [PubMed] [Google Scholar]
- Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial Menopause 2009. 16 6: 1116 1124 [DOI] [PubMed] [Google Scholar]
- Stovall DW, Utian WH, Gass ML, Qu Y, Muram D, Wong M, Plouffe L., Jr. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety Menopause 2007. 14 (3 pt 1): 510 517 [DOI] [PubMed] [Google Scholar]
- McCarty KS, Szabo E, Flowers JL, Cox EB, Leight GS, Miller L, Konrath J, Soper JT, Budwit DA, Creasman WT, Seigler HF, McCarty KS., Sr. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res. 1986;46:4244S–4248S. [PubMed] [Google Scholar]
- Olive DL, Pritts EA. Treatment of endometriosis N Engl J Med 2001. 345 4: 266 275 [DOI] [PubMed] [Google Scholar]
- Taylor HS, Osteen KG, Bruner-Tran KL, Lockwood CJ, Krikun G, Sokalska A, Duleba AJ. Novel therapies targeting endometriosis Reprod Sci 2011. 18 9: 814 823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility Obstet Gynecol Clin North Am 2012. 39 4: 535 549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwalisz K, Surrey E, Stanczyk FZ. The hormonal profile of norethindrone acetate: rationale for add-back therapy with gonadotropin-releasing hormone agonists in women with endometriosis Reprod Sci 2012. 19 6: 563 571 [DOI] [PubMed] [Google Scholar]
- Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study Obstet Gynecol 1998. 91 1: 16 24 [DOI] [PubMed] [Google Scholar]
- Mahutte NG, Arici A. Medical management of endometriosis-associated pain. Obstet Gynecol Clin North Am. 2003;30:133–150. doi: 10.1016/s0889-8545(02)00057-8. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Hornung D, Seitz C, Faustmann T, Gerlinger C, Luisi S, Lazzeri L, Strowitzki T. Reduced pelvic pain in women with endometriosis: efficacy of long-term dienogest treatment Arch Gynecol Obstet 2012. 285 1: 167 173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellini P, Somigliana E, Viganò P, Abbiati A, Barbara G, Crosignani PG. Endometriosis Drugs 2009. 69 6: 649 675 [DOI] [PubMed] [Google Scholar]
- Rogers PA, D'Hooghe TM, Fazleabas A, Giudice LC, Montgomery GW, Petraglia F, Taylor RN. On etiology or lack of understanding: defining future directions for endometriosis research: workshop report from the 2011 World Congress of Endometriosis in Montpellier, France Reprod Sci 2013. 20 5: 483 499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak J, Jr, , Fischer C, Komm B, Taylor HS. Treatment with bazedoxifene, a selective estrogen receptor modulator, causes regression of endometriosis in a mouse model Endocrinology 2011. 152 8: 3226 3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkin S, Komm BS, Pan K, Chines AA. Effects of bazedoxifene/conjugated estrogens on endometrial safety and bone in postmenopausal women Climacteric 2013. 16 3: 338 346 [DOI] [PubMed] [Google Scholar]
- Taylor HS, Ohleth K. Using bazedoxifene plus conjugated estrogens for treating postmenopausal women: a comprehensive review Menopause 2012. 19 4: 479 485 [DOI] [PubMed] [Google Scholar]
- Pinkerton JV, Pickar JH, Racketa J, Mirkin S. Bazedoxifene/conjugated estrogens for menopausal symptom treatment and osteoporosis prevention. Climacteric. 2012;15:411–418. doi: 10.3109/13697137.2012.696289. [DOI] [PubMed] [Google Scholar]
- Komm BS, Mirkin S. Incorporating bazedoxifene/conjugated estrogens into the current paradigm of menopausal therapy. Int J Womens Health. 2012;4:129–140. doi: 10.2147/IJWH.S29346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrodin TJ, Chang KCN, Komm BS, Freedman LP, Nagpal S. Differential biochemical and cellular actions of Premarin estrogens: distinct pharmacology of bazedoxifene-conjugated estrogens combination Mol Endocrinol 2009. 23 1: 74 85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardell SE, Nelson ER, Chao CA, McDonnell DP. Bazedoxifene exhibits antiestrogenic activity in animal models of tamoxifen-resistant breast cancer: implications for treatment of advanced disease Clin Cancer Res 2013. 19 9: 2420 2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak J, Jr, , Ferriani RA, Komm BS, Taylor HS. Tissue selective estrogen complexes (TSECs) differentially modulate markers of proliferation and differentiation in endometrial cells Reprod Sci 2013. 20 2: 129 137 [DOI] [PMC free article] [PubMed] [Google Scholar]