ABSTRACT
We reported previously that stem cells associated with adult rat testis seminiferous tubules are able to give rise to differentiated Leydig cells in vitro. The regulatory mechanisms by which they do so, however, are uncertain. Herein, we hypothesized that the proliferation and differentiation of Leydig cell stem cells (stem Leydig cells, SLCs) depend upon locally produced factors from the seminiferous tubules. Microarray analysis revealed that platelet-derived growth factor receptor alpha (PDGFRalpha) is up-regulated and PDGFRbeta is down-regulated with postnatal differentiation of SLCs. This suggested that their ligands, PDGF-AA and PDGF-BB, respectively, might have important roles in SLC proliferation and differentiation. To test this, we developed a unique in vitro culture system in which SLCs proliferate on the surfaces of cultured seminiferous tubules largely during Week 1 of culture and their progeny subsequently differentiate to testosterone-forming Leydig cells during Weeks 2 through 4. Using this system, seminiferous tubules from adult rat testes were cultured with PDGF-AA or PDGF-BB for up to 4 wk. Both ligands stimulated SLC proliferation during the first week of culture, with PDGF-BB significantly more potent than PDGF-AA. Furthermore, PDGF-AA had a stimulatory effect on SLC differentiation from Weeks 2 through 4 of culture. In contrast, PDGF-BB, which stimulated cell proliferation during Week 1, had a significant inhibitory effect on differentiation during Weeks 2 through 4. These findings, made possible by the development of the seminiferous tubule culture system, reveal distinct roles by locally produced PDGFs in SLC regulation.
Keywords: Leydig cells, PDGF, stem cells, testosterone
INTRODUCTION
Leydig cells produce testosterone via a multistep process that includes luteinizing hormone (LH) stimulation, cholesterol translocation into the mitochondria, and an enzymatic cascade in the mitochondria and smooth endoplasmic reticulum [1]. Adult Leydig cells (ALCs) arise from undifferentiated stem cells (SLCs), first identified in the early postnatal rat testis and shown to have the ability to self-renew indefinitely or to differentiate into testosterone-producing cells [2]. Studies [3–5] have demonstrated that the elimination of Leydig cells from the adult testis by a single i.p. injection of ethane dimethanesulfonate (EDS) results in Leydig cell repopulation within 6 to 10 wk thereafter, suggesting that there also might be stem cells in the adult testis. Indeed, precursor cells lacking the LH receptor and steroidogenic enzymes, as well as having the ability to proliferate indefinitely or to differentiate into testosterone-producing Leydig cells in vitro, have been shown to be present on the surfaces of seminiferous tubules [6]. The regulatory mechanisms by which the stem cells proliferate and/or differentiate remain uncertain.
Members of the platelet-derived growth factor (PDGF) family have been shown to be involved in the development of ALCs [7–9]. PDGF-A and PDGF-B are expressed in the rat testis, homodimerizing to form PDGF-AA and PDGF-BB [9]. The PDGF homodimers bind to their corresponding tyrosine kinase receptors PDGFRα and PDGFRβ, thereby activating unique downstream pathways [10, 11]. Previous investigations have suggested that Leydig cells are among the testicular cells that are potential targets for the local action of testicular PDGFs [9]. During the periods of prenatal and postnatal testis development, the primary PDGF-expressing and PDGF-responsive testicular cells are Sertoli cells and Leydig cells, respectively [7, 8, 12]. The SLCs from neonatal and adult testes also express both PDGFRα and PDGFRβ [2, 13, 14]. Knocking out either PDGFRα or PDGF-A was found to block the formation of ALCs, perhaps a consequence of the inability of precursor cells to differentiate into Leydig cells [9, 15, 16]. Both PDGFRα and PDGFRβ have been shown to be necessary for the development of steroidogenic cells in the major steroid-producing organs, including the ovary, testis, and adrenal gland [9, 17].
Our objective herein was to analyze the possible regulatory effects of PDGF-AA and PDGF-BB on SLC proliferation and/or differentiation. To do so, we utilized a newly developed seminiferous tubule culture system to evaluate the direct effects of PDGF-AA and PDGF-BB on SLC regulation in vitro. We show that, although both PDGF-AA and PDGF-BB stimulated SLC proliferation, the two ligands had opposite effects on the differentiation process, with PDGF-AA being stimulatory and PDGF-BB being inhibitory.
MATERIALS AND METHODS
Chemicals
Rat PDGF-AA was obtained from R&D Systems Inc. (Minneapolis, MN). Rat PDGF-BB and anti-laminin rabbit antibody were from Sigma (St. Louis, MO). PDGFRβ tyrosine kinase inhibitor IV 521233 was purchased from Calbiochem (EMD Biosciences Inc., San Diego, CA). Bovine LH (USDA-bLH-B-6) was provided by the United States Department of Agriculture Animal Hormone Program (Beltsville, MD). The Invitrogen Click-iT EdU (5-ethynyl-2′-deoxyuridine) imaging kit was from Life Technologies (Grand Island, NY). Testosterone was obtained from Steraloids Inc. (Newport, RI), and (1,2,6,7,16,17-3H [N])-testosterone was obtained from PerkinElmer (Boston, MA). Testosterone antibody was purchased from MP Biomedicals (Solon, OH). Ethane dimethanesulfonate was house synthesized according to the method described by Jackson and Jackson [18].
Animals
Young adult male brown Norway rats, 3 to 5 mo of age, were used. The rats were purchased from Harlan Sprague-Dawley Inc. (Indianapolis, IN). They were housed in the animal facilities at the Johns Hopkins Bloomberg School of Public Health under controlled light (14L:10D) and with free access to water and rat chow. All animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals according to protocols approved by the Johns Hopkins Animal Care and Use Committee.
Cell Purification and Microarray Analysis
DNA microarray analyses (Affymetrix Inc., Santa Clara, CA) of SLCs, progenitor Leydig cells (PLCs), immature Leydig cells (ILCs), and ALCs were published previously by our group [13]. The data were deposited in the National Center for Biotechnology Information Gene Expression Omnibus under the accession number GSE26703. For the present study, we conducted secondary analyses of the data. From each array, probe sets were normalized to a mean signal intensity of 100.
Isolation and Culture of Seminiferous Tubules
Rats were injected i.p. with a single dose (75 mg/kg of body weight) of EDS dissolved in a 1:3 solution mixture of dimethyl sulfoxide and PBS. Rats were killed by decapitation 4 days after EDS treatment, by which time all ALCs had been eliminated [3–5]. Testes were placed in Dulbecco modified Eagle medium (DMEM)/F-12 culture medium and decapsulated. Seminiferous tubules were mechanically separated from the interstitium by fine forceps under a transillumination dissection microscope [6, 19]. The tubules were distributed randomly into 24-well or 12-well plates, with each well containing a group of tubule fragments of equivalent total length (1–2 inches). Tubule fragments were cultured at 34°C and 5% carbon dioxide for up to 4 wk in a 1:1 mixture of DMEM/F-12 and medium 199 (pH 7.2–7.4), sodium bicarbonate (2.2 mg/ml), bovine serum albumin (1 mg/ml) and penicillin-streptomycin (100 U/ml and 100 μg/ml, respectively). The medium contained LH (10 ng/ml), LH plus PDGF-AA (10 ng/ml), LH plus PDGF-BB (10 ng/ml), or LH plus PDGFRβ inhibitor 52133 (20 nM). Duplicate wells were used at each time point, and each experiment was repeated at least three times. Media were collected and frozen (−20°C) for testosterone measurement, and the tubules were immediately fixed for morphological study.
SLC Differentiation and Proliferation
Testosterone levels were determined by radioimmunoassay as a measure of SLC differentiation. To measure proliferation, dividing cells on the surface of the tubules were labeled using the Click-iT EdU imaging kit. In brief, the seminiferous tubules were labeled with EdU (10 μM for 24 h) and examined at times thereafter. Click-iT-positive cells on the surface of tubule fragments were visualized under a fluorescence microscope (excitation/emission at 495/519 nm) and quantified. The total positive cells were counted along the surface of the tubules and expressed as the number per square unit with the side length comparable to the diameter of the tubules. More than 100 square areas were counted that came from at least three different experiments. For some studies, the tubules were cultured with LH plus PDGF-AA or LH plus PDGF-BB as described above and then labeled with EdU for 24 hours, fixed in 10% formalin (15 min), embedded in paraffin, and sectioned. After visualization of EdU-labeled nuclei, the tubule sections were counterstained for laminin.
Statistical Analysis
Data were analyzed using SigmaStat software version 2.03 (Systat Software Inc., Richmond, CA). Student t-test was used for two-group comparisons. One-way ANOVA, followed by Tukey test, was used for multigroup comparisons. Data were analyzed from at least three separate experiments. Differences were considered significant at P < 0.05. Data are presented as the mean (SEM).
RESULTS
Effects of PDGF-AA and PDGF-BB on Leydig Cell Development
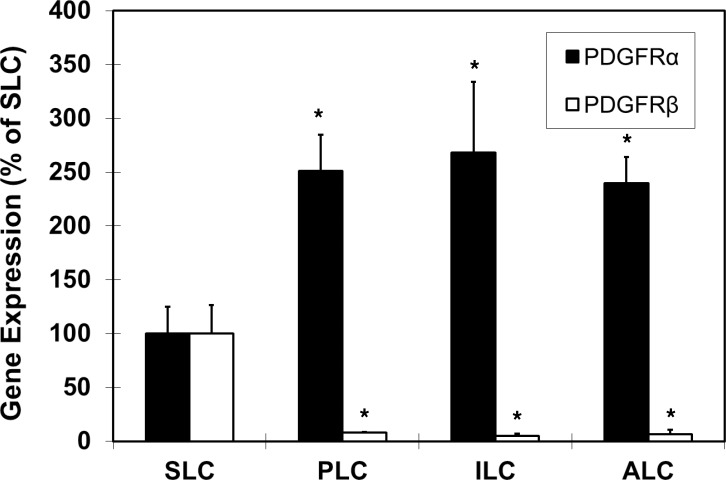
Previous studies [20, 21] demonstrated that ALCs arise from SLCs that are present in the neonatal testis and that undergo phased transitions through progenitor (PLC) and immature (ILC) stages before ALCs are formed. We conducted microarray analysis to identify differentially regulated genes during differentiation of neonatal SLCs. As shown in Figure 1, PDGFRα expression increased in PLCs, ILCs, and ALCs compared with SLCs, while PDGFRβ expression decreased. The significant changes in the expression of these receptors suggested that they and their primary ligands, PDGF-AA and PDGF-BB, respectively, might have important, perhaps opposite, roles in Leydig cell development.
FIG. 1.
Microarray analysis showing gene expression levels of PDGFRα (black bars) and PDGFRβ (white bars) during Leydig cell differentiation from SLC through PLC, ILC, and ALC phases. The levels are expressed as percentages relative to SLC expression, with SLC expression normalized to 100%. Asterisks show significant differences relative to SLC expression of PDGFRα or PDGFRβ. Error bars are the mean (SEM).
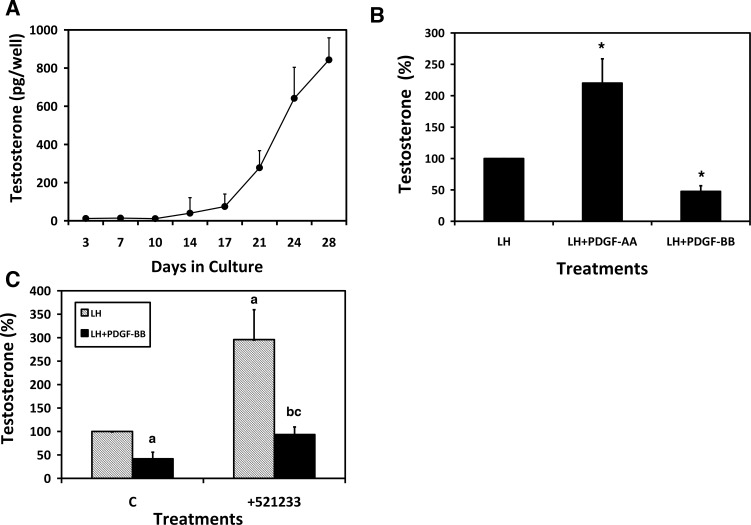
To directly test the potential effects of PDGF-AA and PDGF-BB on the formation of Leydig cells from undifferentiated SLCs, we used a newly developed in vitro system that involves the culture of adult rat seminiferous tubules physically separated from the interstitium of the adult testis after the elimination of the existing Leydig cells from the testis with EDS [6]. In the initial study, isolated seminiferous tubules were cultured for up to 4 wk in the presence of LH, and testosterone was measured in the medium throughout this period. As also reported previously [6], little testosterone was produced during the first 2 wk in culture but increased dramatically in the period from 2 to 4 wk (Fig. 2A). To examine the possible effects of PDGF-AA and PDGF-BB during the 4-wk culture period, seminiferous tubules were cultured in media containing LH, LH plus PDGF-AA, or LH plus PDGF-BB for 4 wk (Fig. 2B). With LH plus PDGF-AA, the testosterone concentration in the culture media was significantly greater than that in the media of the tubules cultured with LH alone. In contrast, with LH plus PDGF-BB there was a significant decrease in the testosterone concentration compared with LH alone. Previous investigations had shown that PDGF-BB preferentially binds to PDGFRβ [9]. To determine whether binding of PDGF-BB to its receptor inhibited testosterone formation, the tubules were cultured with LH, LH plus PDGF-BB, LH plus the specific PDGFRβ inhibitor 521233 (20 nM), or LH plus PDGF-BB plus the inhibitor. Culturing the tubules with the PDGFRβ inhibitor along with LH and PDGF-BB prevented the PDGF-BB-induced reduction in testosterone production (Fig. 2C). Interestingly, culturing the tubules with LH alone plus the inhibitor resulted in significantly higher testosterone formation than with LH alone (Fig. 2C). This suggests that in our culture system PDGF-BB produced locally by Sertoli cells may have a role in suppressing testosterone formation and that its inhibition by the PDGFRβ inhibitor results in enhanced testosterone production in response to LH.
FIG. 2.
Effects of LH, PDGF-AA, and PDGF-BB on SLC differentiation from stem cells. A) Testosterone in the media of isolated seminiferous tubules cultured with LH for 1 to 4 wk. B) Effects of PDGF-AA and PDGF-BB on testosterone formation. Seminiferous tubules were cultured for 4 wk with LH, LH plus PDGF-AA, or LH plus PDGF-BB. Asterisks indicate significant differences from testosterone formation in response to LH alone. C) Mechanism by which PDGF-BB inhibits testosterone formation. The testosterone concentrations resulting from the tubules cultured with LH, LH plus PDGF-BB, or LH plus PDGF-BB plus the PDGFRβ inhibitor 521233 were determined. aSignificantly different from LH-only control. bSignificantly different from LH plus PDGF-BB control. cSignificantly different from LH plus 521233. In B and C, testosterone levels are expressed as percentages relative to LH alone, which is set at 100%. Error bars are the mean (SEM).
Effects of PDGF-AA and PDGF-BB on SLC Proliferation and Differentiation
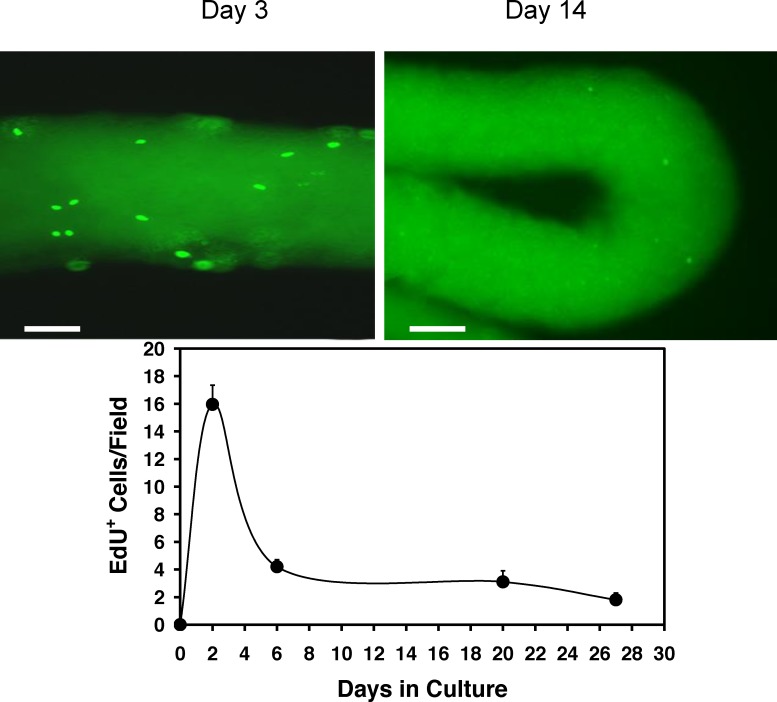
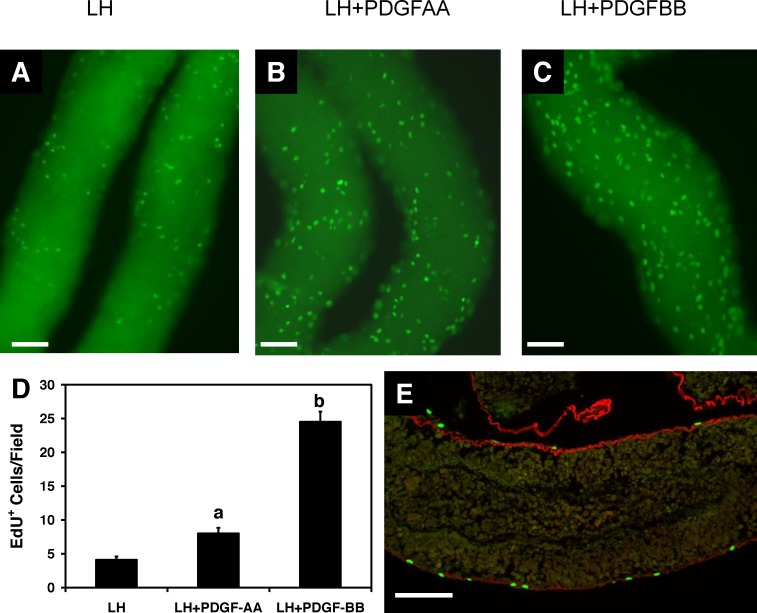
Having shown that during a 4-wk culture period PDGF-AA had a stimulatory effect on testosterone production and PDGF-BB had a suppressive effect, we hypothesized that the two might have significant, perhaps opposite, the effects on SLC cell proliferation and/or differentiation. To address the effects on cell proliferation, seminiferous tubules were first cultured with LH alone, and dividing cells were labeled with EdU at Days 2, 6, 20, and 27. There was a peak in EdU-positive cells on the surfaces of the tubules within the first few days of culture, followed by a decline such that after approximately 1 wk of culture and thereafter relatively few cells were labeled (Fig. 3). We then examined the effects of PDGF-AA and PDGF-BB on SLC proliferation (Fig. 4). To this end, the tubules were cultured with LH alone, LH plus PDGF-AA, or LH plus PDGF-BB, and dividing cells were labeled with EdU on Day 5 of culture, a period of active cell proliferation. As shown in the micrographs (A–C) and bar graph (D) of Figure 4, treatment with PDGF-AA resulted in a 2-fold increase in EdU-positive cells, and PDGF-BB resulted in a 5-fold increase. To determine whether the EdU-positive cells indeed were on the tubule surfaces rather than within the tubules, the tubules were cultured with LH plus PDGF-BB, labeled with EdU, and then fixed, sectioned, and stained for the basement membrane marker laminin (Fig. 4E). The EdU-positive cells (green) were almost exclusively located on the outer surfaces of the tubules, outside of the basement membrane (red). These results indicate that both PDGF-AA and PDGF-BB are able to act as mitogens for SLCs. Thus, although incubation of the tubules with PDGF-BB for 4 wk had been found to suppress testosterone production, as seen in Figure 2B, PDGF-BB was even more effective than PDGF-AA in inducing SLC proliferation.
FIG. 3.
Cell proliferation on the surfaces of seminiferous tubules over time in culture. Seminiferous tubules were cultured with LH, and dividing cells were labeled with EdU at 0 to 27 days. The EdU-stained tubules at Days 3 and 14 (micrographs above) and the numbers of EdU-positive cells per tubule length are shown. Bar = 100 μm. Error bars are the mean (SEM).
FIG. 4.
Cell proliferation in response to PDGF-AA and PDGF-BB during the first week of culture. Micrographs showing EdU-positive cells after the tubules were cultured for 5 days with LH (A), LH plus PDGF-AA (B), or LH plus PDGF-BB (C). D) The numbers of EdU-positive cells per tubule length for groups A through C were determined. Different letters indicate significant differences. Error bars are the mean (SEM). E) Click-iT EdU staining (green) on the surface of the tubules. The basement membrane is immunofluorescently labeled with anti-laminin antibody (red). Bar = 100 μm.
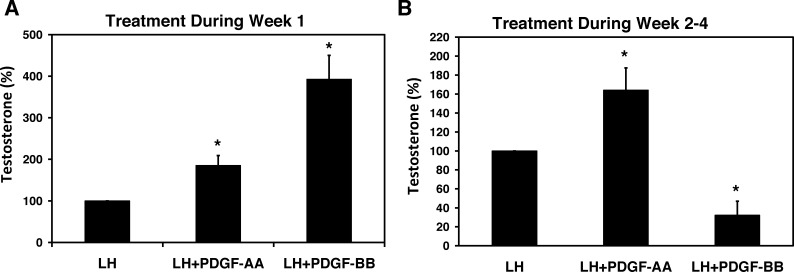
We next asked whether the cells produced by culturing the tubules with PDGF-BB and PDGF-AA during the first week were equally capable of differentiating and ultimately producing testosterone. The tubules were incubated with LH alone, LH plus PDGF-AA, or LH plus PDGF-BB for 1 wk and then switched to LH-only medium for an additional 3 wk to allow SLCs to differentiate. At 4 wk, the testosterone concentration in the media was determined. Treatment with PDGF-AA for the first week of culture, which had resulted in a doubling of the numbers of cells, also resulted in a doubling of testosterone production at the end of the 4-wk period compared with the LH-only controls (Fig. 5A). Similarly, treatment with PDGF-BB, which had resulted in a 5-fold increase in the numbers of cells, resulted in almost a 5-fold increase in testosterone formation (Fig. 5A). These results indicate that cells resulting from the stimulation of SLC proliferation by PDGF-AA or PDGF-BB during Week 1 were equally capable of differentiating into testosterone-producing Leydig cells during the subsequent 3 wk.
FIG. 5.
Testosterone production in response to incubating the tubules with PDGF-AA or PDGF-BB. A) The tubules were cultured with LH alone, LH plus PDGF-AA, or LH plus PDGF-BB during Week 1, and then all three groups were cultured with LH alone for an additional 3 wk (Weeks 2–4). B) The tubules were cultured with LH alone for Week 1 and then with LH, LH plus PDGF-AA, or LH plus PDGF-BB for Weeks 2 through 4. Asterisks indicate significant difference from LH alone. Error bars are the mean (SEM).
Based on these results, we hypothesized that the inhibitory and stimulatory effects of PDGF-BB and PDGF-AA, respectively, on testosterone formation by the tubules that had been cultured for the full 4-wk period (Fig. 2B) must be related to their effects on differentiation of SLCs during Weeks 2 through 4. To test this hypothesis, the tubules were initially cultured with LH alone for 1 wk and then cultured with LH, LH plus PDGF-AA, or LH plus PDGF-BB during Weeks 2 through 4 (Fig. 5B). PDGF-AA treatment resulted in a significant 3-fold increase in testosterone production compared with the LH-only group. In contrast, PDGF-BB had a significant suppressive effect on testosterone production during the same period.
DISCUSSION
It is well established that, after the elimination of Leydig cells from the adult rat testis with EDS, a new population of ALCs develops [3–5]. The cells that serve as precursors to the newly formed Leydig cells were recently isolated and characterized. The isolated cells lacked the LH receptor and steroidogenic enzymes and had the ability to proliferate indefinitely or to differentiate into steroid-producing cells [6]. These characteristics identified the cells as stem cells, with properties similar, if not identical, to those of SLCs identified by Ge and colleagues [2] in Postnatal Day 7 rat testes.
The mechanisms by which SLC proliferation or self-renewal and differentiation are regulated remain uncertain, although previous studies [17, 22–25] suggested the possible involvement of factors produced locally by Sertoli cells. Among these were PDGF-AA and PDGF-BB [2, 7–9, 12, 15, 16, 26–30], the receptors for which PDGFRα and PDGFRβ, respectively, are expressed by SLCs [2, 14, 26]. Using microarray analysis, we found that, with differentiation of SLCs to steroid-producing PLCs during the early neonatal period, there is up-regulation of PDGFRα and down-regulation of PDGFRβ [13]. The changes in the expression of these receptors led us to hypothesize that their ligands, PDGF-AA and PDGF-BB, respectively, might have important, perhaps opposite, roles in the proliferation and/or differentiation of SLCs in Leydig cell-depleted adult testis.
It was possible to test this directly by utilizing an in vitro culture system that we developed [6]. In this system, SLCs (located on the outer surfaces of isolated seminiferous tubules) maintain direct interaction with their niche. When the tubules were cultured for 4 wk with LH-containing media that also contained PDGF-AA, there was increased testosterone production compared with the tubules cultured in the absence of PDGF-AA. With PDGF-BB, however, testosterone production was reduced. These results suggested that PDGF-AA and PDGF-BB, which are produced by Sertoli cells [7, 9, 12], might exert positive and negative effects, respectively, on SLC function but did not address whether the effects were on SLC proliferation, differentiation, or both.
We found that the seminiferous tubule culture method that we used has the fortunate quality that proliferation and differentiation of SLCs occur sequentially rather than simultaneously, with most proliferation occurring during the first week of culture and most differentiation occurring subsequently. This temporal separation of proliferation and differentiation parallels the in vivo restoration of Leydig cells to the EDS-treated testis, wherein precursor cells have been shown to proliferate during the first week after EDS treatment, followed by differentiation [31–33]. Using the in vitro system, it was possible to analyze the effects of PDGF-AA and PDGF-BB on SLC proliferation and differentiation separately. That is, by adding PDGF-AA or PDGF-BB to the cultured tubules only for the first week, the effects on proliferation could be assessed; adding these ligands from Weeks 2 through 4 allowed an assessment of the effects on differentiation. We found that the addition of PDGF-AA to the tubules cultured for the first week resulted in significantly increased numbers of EdU-positive cells compared with control tubules cultured with LH alone. In light of the observation that culturing the tubules with PDGF-BB for 4 wk resulted in a negative effect on testosterone production, we were particularly surprised to find that PDGF-BB had an even more robust effect on SLC proliferation than PDGF-AA, with a 5-fold increase in the number of EdU-labeled cells during the first week compared with the control. These results indicated that the enhanced testosterone production by PDGF-AA treatment for 4 wk might have resulted at least in part from increased SLC proliferation and thus increased cell numbers. However, the inhibition of testosterone production by PDGF-BB treatment during the 4-wk period was not a result of reduced SLC proliferation but rather occurred despite increased SLC proliferation.
These results suggested that, whereas PDGF-AA treatment seemed likely to enhance testosterone formation during Weeks 2 through 4 of culture, PDGF-BB would be expected to reduce testosterone formation despite having stimulated cell proliferation during Week 1. As hypothesized, testosterone formation was higher with LH plus PDGF-AA than with LH alone when added during Weeks 2 through 4, indicating its positive effect on Leydig cell differentiation in addition to its positive effect on SLC proliferation. In contrast, LH plus PDGF-BB significantly reduced testosterone levels when added to the culture during this period. This indicated that, although PDGF-BB increased the number of precursor cells that are capable of differentiating into testosterone-producing cells, its presence during differentiation had a negative effect on the process.
As yet, the mechanisms by which the two closely related factors, PDGF-AA and PDGF-BB, have opposite roles in SLC differentiation are not known. Also, it is unclear how the local concentration of the two ligands may change during Leydig cell development in pubertal or adult rat testis after EDS treatment. A 2008 study [34], however, has indeed found a transient increase in PDGF-AA mRNA levels in the first week of EDS-treated rat testis. This is consistent with the period in which active SLC proliferation takes place in EDS-treated rat testis [31, 32] and in our culture system. Clearly, understanding the underlying mechanisms of PDGF-AA and PDGF-BB and their physiological roles in vivo, as well as other regulatory factors of the SLC niche, will require further investigations. Our newly designed in vitro culture system makes it possible to pursue such studies under highly controlled conditions.
Footnotes
Supported by National Institutes of Health grant R37 AG21092 from the National Institute on Aging to B.R.Z. and by National Natural Science Foundation of China grant NSFC30972840 to H.C. and grants NSFC30871434 and NSFC31171425 to R.G.
REFERENCES
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Ge RS, Dong Q, Sottas CM, Papadopoulos V, Zirkin BR, Hardy MP. In search of rat stem Leydig cells: identification, isolation, and lineage-specific development. Proc Natl Acad Sci U S A. 2006;103:2719–2724. doi: 10.1073/pnas.0507692103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JB, Donachie K, Rommerts FF. Selective destruction and regeneration of rat Leydig cells in vivo: a new method for the study of seminiferous tubular-interstitial tissue interaction. Cell Tissue Res. 1985;242:145–156. doi: 10.1007/BF00225571. [DOI] [PubMed] [Google Scholar]
- Jackson AE, O'Leary PC, Ayers MM, de Kretser DM. The effects of ethylene dimethane sulphonate (EDS) on rat Leydig cells: evidence to support a connective tissue origin of Leydig cells. Biol Reprod. 1986;35:425–437. doi: 10.1095/biolreprod35.2.425. [DOI] [PubMed] [Google Scholar]
- Morris ID, Phillips DM, Bardin CW. Ethylene dimethanesulfonate destroys Leydig cells in the rat testis. Endocrinology. 1986;118:709–719. doi: 10.1210/endo-118-2-709. [DOI] [PubMed] [Google Scholar]
- Stanley E, Lin CY, Jin S, Liu J, Sottas CM, Ge R, Zirkin BR, Identification Chen H. proliferation, and differentiation of adult Leydig stem cells. Endocrinology. 2012;153:5002–5010. doi: 10.1210/en.2012-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnessi L, Emidi A, Jannini EA, Carosa E, Maroder M, Arizzi M. Testicular development involves the spatiotemporal control of PDGFs and PDGF receptors gene expression and action. J Cell Biol. 1995;131:1105–1121. doi: 10.1083/jcb.131.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveland KL, Zlatic K, Stein-Oakley A, Risbridger G, deKretser DM. Platelet-derived growth factor ligand and receptor subunit mRNA in the Sertoli and Leydig cells of the rat testis. Mol Cell Endocrinol. 1995;108:155–159. doi: 10.1016/0303-7207(94)03471-5. [DOI] [PubMed] [Google Scholar]
- Basciani S, Mariani S, Spera G, Gnessi L. Role of platelet-derived growth factors in the testis. Endocr Rev. 2010;31:916–939. doi: 10.1210/er.2010-0004. [DOI] [PubMed] [Google Scholar]
- Matsui T, Heidaran M, Miki T, Popescu N, La Rochelle W, Kraus M, Pierce J, Aaronson S. Isolation of a novel receptor cDNA establishes the existence of two PDGF receptor genes. Science. 1989;243:800–804. doi: 10.1126/science.2536956. [DOI] [PubMed] [Google Scholar]
- Rosenkranz S, Kazlauskas A. Evidence for distinct signaling properties and biological responses induced by the PDGF receptor alpha and beta subtypes. Growth Factors. 1999;16:201–216. doi: 10.3109/08977199909002130. [DOI] [PubMed] [Google Scholar]
- Basciani S, Mariani S, Arizzi M, Ulisse S, Rucci N, Jannini EA, Della Rocca C, Manicone A, Carani C, Spera G, Gnessi L. Expression of platelet-derived growth factor-A (PDGF-A), PDGF-B, and PDGF receptor-alpha and -beta during human testicular development and disease. J Clin Endocrinol Metab. 2002;87:2310–2319. doi: 10.1210/jcem.87.5.8476. [DOI] [PubMed] [Google Scholar]
- Stanley EL, Johnston DS, Fan J, Papadopoulos V, Chen H, Ge RS, Zirkin BR, Jelinsky SA. Stem Leydig cell differentiation: gene expression during development of the adult rat population of Leydig cells. Biol Reprod. 2011;85:1161–1166. doi: 10.1095/biolreprod.111.091850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreh L, Stukenborg JB, Söder O, Svechnikov K. Phenotype and steroidogenic potential of PDGFRα-positive rat neonatal peritubular cells. Mol Cell Endocrinol. 2013;372:96–104. doi: 10.1016/j.mce.2013.03.019. [DOI] [PubMed] [Google Scholar]
- Brennan J, Tilmann C, Capel B. Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003;17:800–810. doi: 10.1101/gad.1052503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnessi L, Basciani S, Mariani S, Arizzi M, Spera G, Wang C, Bondjers C, Karlsson L, Betsholtz C. Leydig cell loss and spermatogenic arrest in platelet-derived growth factor (PDGF)-A-deficient mice. J Cell Biol. 2000;149:1019–1026. doi: 10.1083/jcb.149.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahl J, Rizzolo K, Soriano P. The PDGF signaling pathway controls multiple steroid-producing lineages. Genes Dev. 2008;22:3255–3267. doi: 10.1101/gad.1723908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CM, Jackson H. Comparative protective actions of gonadotrophins and testosterone against the antispermatogenic action of ethane dimethanesulphonate. J Reprod Fertil. 1984;71:393–401. doi: 10.1530/jrf.0.0710393. [DOI] [PubMed] [Google Scholar]
- Vihko KK, Suominen JJ, Parvinen M. Cellular regulation of plasminogen activator secretion during spermatogenesis. Biol Reprod. 1984;31:383–389. doi: 10.1095/biolreprod31.2.383. [DOI] [PubMed] [Google Scholar]
- Chen H, Stanley E, Jin S, Zirkin BR. Stem Leydig cells: from fetal to aged animals. Birth Defects Res C Embryo Today. 2010;90:272–283. doi: 10.1002/bdrc.20192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Ge RS, Zirkin BR. Leydig cells: from stem cells to aging. Mol Cell Endocrinol. 2009;306:9–16. doi: 10.1016/j.mce.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AJ, Capel B. Signaling at the crossroads of gonad development. Trends Endocrinol Metab. 2005;16:19–25. doi: 10.1016/j.tem.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Haider SG. Cell biology of Leydig cells in the testis. Int Rev Cytol. 2004;233:181–241. doi: 10.1016/S0074-7696(04)33005-6. [DOI] [PubMed] [Google Scholar]
- Saez JM. Leydig cells: endocrine, paracrine, and autocrine regulation. Endocr Rev. 1994;15:574–626. doi: 10.1210/edrv-15-5-574. [DOI] [PubMed] [Google Scholar]
- Habert R, Lejeune H, Saez JM. Origin, differentiation and regulation of fetal and adult Leydig cells. Mol Cell Endocrinol. 2001;179:47–74. doi: 10.1016/s0303-7207(01)00461-0. [DOI] [PubMed] [Google Scholar]
- Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF, Muller D. Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol. 2004;167:935–944. doi: 10.1083/jcb.200409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnessi L, Emidi A, Farini D, Scarpa S, Modesti A, Ciampani T, Silvestroni L, Spera G. Rat Leydig cells bind platelet-derived growth factor through specific receptors and produce platelet-derived growth factor-like molecules. Endocrinology. 1992;130:2219–2224. doi: 10.1210/endo.130.4.1312451. [DOI] [PubMed] [Google Scholar]
- Ricci G, Catizone A, Galdieri M. Embryonic mouse testis development: role of platelet derived growth factor (PDGF-BB) J Cell Physiol. 2004;200:458–467. doi: 10.1002/jcp.20035. [DOI] [PubMed] [Google Scholar]
- Risbridger GP. Discrete stimulatory effects of platelet-derived growth factor (PDGF-BB) on Leydig cell steroidogenesis. Mol Cell Endocrinol. 1993;97:125–128. doi: 10.1016/0303-7207(93)90218-9. [DOI] [PubMed] [Google Scholar]
- Puglianiello A, Campagnolo L, Farini D, Cipollone D, Russo MA, Siracusa G. Expression and role of PDGF-BB and PDGFR-beta during testis morphogenesis in the mouse embryo. J Cell Sci. 2004;117:1151–1160. doi: 10.1242/jcs.00981. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, De Rooij DG, Rommerts FF, van den Hurk R, Wensing CJ. Stimulation of the proliferation and differentiation of Leydig cell precursors after the destruction of existing Leydig cells with ethane dimethyl sulphonate (EDS) can take place in the absence of LH. J Androl. 1989;10:472–477. doi: 10.1002/j.1939-4640.1989.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, de Rooij DG, Rommerts FF, van den Hurk R, Wensing CJ. Proliferation and differentiation of possible Leydig cell precursors after destruction of the existing Leydig cells with ethane dimethyl sulphonate: the role of LH/human chorionic gonadotrophin. J Endocrinol. 1989;122:689–696. doi: 10.1677/joe.0.1220689. [DOI] [PubMed] [Google Scholar]
- Yan W, Kero J, Huhtaniemi I, Toppari J. Stem cell factor functions as a survival factor for mature Leydig cells and a growth factor for precursor Leydig cells after ethylene dimethane sulfonate treatment: implication of a role of the stem cell factor/c-Kit system in Leydig cell development. Dev Biol. 2000;227:169–182. doi: 10.1006/dbio.2000.9885. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Morris ID, Baker PJ. Leydig cell re-generation and expression of cell signaling molecules in the germ cell-free testis. Reproduction. 2008;135:851–858. doi: 10.1530/REP-07-0529. [DOI] [PubMed] [Google Scholar]