Abstract
Restoration of male fertility associated with use of the cryopreserved testicular tissue would be a significant advance in human and animal assisted reproductive technology. The purpose of this study was to test the effects of four different cryoprotectant agents (CPA) on spermatogenesis and steroidogenesis in cryopreserved and allotransplanted neonatal mouse testicular tissue. Hank's balanced salt solution (HBSS) with 5% fetal bovine serum including either 0.7 M dimethyl sulfoxide (DMSO), 0.7 M propylene glycol (PrOH), 0.7 M ethylene glycol (EG), or glycerol was used as the cryoprotectant solution. Donor testes were collected and dissected from neonatal pups of CD-1 mice (one day old). Freezing and seeding of the testicular whole tissues was performed using an automated controlled-rate freezer. Four fresh (non-frozen) or frozen–thawed pieces of testes were subcutaneously grafted onto the hind flank of each castrated male NCr nude recipient mouse and harvested after 3 months. Fresh neonatal testes grafts recovered from transplant sites had the most advanced rate of spermatogenesis with elongated spermatid and spermatozoa in 46.6% of seminiferous tubules and had higher levels of serum testosterone compared to all other frozen–thawed-graft groups (p < 0.05). Fresh grafts and frozen–thawed grafts in the DMSO group had the highest rate of tissue survival compared to PrOH, EG, and glycerol after harvesting (p > 0.05). The most effective CPA for the freezing and thawing of neonatal mouse testes was DMSO in comparison with EG (p < 0.05) in both pre-grafted and post-grafted tissues based on histopathological evaluation. Likewise, the highest level of serum testosterone was obtained from the DMSO CPA group compared to all other cryoprotectants evaluated (p < 0.05). The typical damage observed in the frozen–thawed grafts included disruption of the interstitial stroma, intercellular connection ruptures, and detachment of spermatogonia from the basement membrane. These findings indicate that neonatal mouse testes were most effectively preserved when frozen with HBSS medium with DMSO and that the type of CPA is a significant factor to obtain the most advanced stages of spermatogenesis and steroidogenesis after cryopreservation, thawing, and transplantation of neonatal mouse testes.
Keywords: Mouse testes, Spermatogenesis, Spermatozoa, Grafting, Cryopreservation, Testosterone
Introduction
The mouse has become a significant research tool in genetic and molecular biology allowing the study of many models of human diseases [5,32]. Cryopreservation of mouse testis is a significant tool in assisted reproductive technology for preservation of genetic resources and strain rescue. This technique would enable the preservation of the cell integrity and the endocrine functions of the testes. The existence of spermatogonial stem cells in the testis of prepubertal males offers clinically relevant options for preservation and restoration of fertility later in life. New approaches based on male germ cell transplantation and grafting of testicular tissue can be applied to generate a limited number of sperm cells and could therefore be considered important new alternatives for restoration of fertility in testicular cancer patients by cryopreserving healthy testicular tissue before chemotherapy. Most studies on cryopreservation of testicular tissue have been proposed to recover spermatozoa by mechanical or enzymatic processing of the testicular tissue or grafting of testicular tissue for future use in ICSI (intracytoplasmic sperm injection) treatment of infertility [1,12,24,29,30].
Grafting of fresh testicular tissue from neonatal mouse, hamster, goat, pig, and marmoset [10,14,31] and cryopreserved testicular tissue after controlled-rate freezing [10,28] onto immune-deficient mice have obtained spermatogenesis and in vivo [28] and in vitro production of testosterone [16]. Transplantation of testicular germ cells in mice [3], domestic animals [11,13,28], primates [21,27,28], and human [22] have also been reported as innovative approaches leading to rapid scientific progress for experimental and clinical studies.
Cryopreservation with automated controlled-rate freezing is the most widely used and well established technique in gamete and somatic tissue banking. Testicular tissue from infertile men has been cryopreserved using glycerol as a CPA, either as cell suspensions [7,8,25] or as pieces of tissue [12,33]. Brook et al. [4] successfully froze human testicular tissue with slow programmed cooling using different CPAs in Leibovitz medium containing 4% fetal bovine serum. Other studies on cryopreservation of mature and immature testicular tissue using slow freezing programs have also been reported for mouse, [28,31], rat [15], and humans [2,6,18,34]. However, there is no published information to our knowledge that compares the cryoprotective efficiency of different CPAs for neonatal mouse testicular tissue. The aim of this study was to test the effects of different CPAs on spermatogenesis and steroidogenesis of cryopreserved and grafted neonatal mouse testicular tissue.
Materials and methods
Animals
Neonatal donor testes were obtained from 1-day old CD-1 pups born to CD-1 pregnant females purchased from Charles River Canada (St.-Constant QC, Canada). Ten week old male NCr nude mice purchased from Taconic (Germantown, NY, USA) were used as graft recipients. During all experiments, mice were housed under controlled light conditions (12 h light:12 h dark) at the Laboratory Animal Facilities of Mount Sinai Hospital and received a standard mouse diet and water ad libitum. Their use and care were reviewed in advance and performed according to standards of the Canadian Council on Animal Care (CACC).
Chemicals
Unless otherwise stated, all chemicals were purchased from Sigma Chemical Company (St. Louis, MO, USA).
Freezing and thawing protocols of neonatal testicular tissue
Standard cryopreservation solutions (CPA; cryoprotective agent) were prepared by using HBSS (Hank's balanced salt solution) with 5% fetal bovine serum including either 0.7 M (Molar) DMSO, 0.7 M propylene glycol, 0.7 M ethylene glycol, or 0.7 M glycerol as previously described for human adult testicular tissue by Keros et al. [16]. The cryopreservation process for whole testicular tissues in the study groups was performed using a computer-controlled automated freezer (Thermo Forma 7452, Marietta, Ohio, USA) according to Keros et al. [16] (Table 1). Neonatal pups were euthanized by carbon dioxide (CO2) and both whole testes were immediately dissected from the abdominal cavity and transferred into HTF (human tubal fluid) media at room temperature [26]. The testicular tissue was washed three times with DPBS (Dulbecco's phosphate buffered saline) and transferred into 1 ml freezing solution in a 1.8 ml cryovial at room temperature. The tissues were equilibrated onto ice for 10 min at 4 °C and loaded into the controlled-rate freezer before starting the cryopreservation process. The seeding process was performed manually after holding for 5 min before inducing ice formation at −8 °C. The cryovials were plunged immediately into liquid nitrogen (LN2) when reaching −80 °C at the end of the freezing program and stored for one month until use. Cryopreserved tissue was thawed in a water bath for 1 min at 37 °C until the ice melted and washed three times in HBSS. All frozen-thawed tissue samples in the study were incubated at 37 °C in HTF for 10 min before grafting onto NCr nude mice or were fixed in Bouin's fixative solution (HT10132, Sigma) for histopathological evaluation. Control samples (fresh testes with no exposure to CPA) were collected from six different mice and immediately fixed for histopathological evaluation.
Table 1.
Computer-controlled automatic freezing protocol used for neonatal mouse testicular tissue.
| Stages | Cooling rates |
|---|---|
| Start | 4 °C |
| Ramp | −1 °C/min to 0 °C |
| Hold | 0 °C for 5 min |
| Ramp | −0.5 °C/min to −8 °C |
| Hold | −8 °C for 15 min |
| Ramp | −0.5 °C/min to −40 °C |
| Hold | −40 °C for 10 min |
| Ramp | −7 °C/min to −80 °C |
| Liquid nitrogen | −196 °C |
Castration of NCr nude graft recipients
The castration process was done according to Schlatt et al. [28]. Briefly, animals were anesthetized with a combination of 100 mg/kg ketamine and 20 mg/kg xylazine and a ventral mid-line incision was made in the skin and underlying body wall. The vasa deferantia were identified and the testes were exteriorized. The spermatic cord was ligatured and divided, and both testes were detached from adhering tissue and from the vasa efferentia. The body wall was closed with sutures.
Transplantation of donor testicular pieces
Four pieces of whole testes from each fresh and cryopreserved groups were implanted subcutaneously using sterile surgical technique onto the hind flank of a castrated NCr nude mouse. In total, sixteen whole testes were grafted from each CPA group. Graft sites were located at the anterior lateral and posterior lateral hind flank, 2–3 mm right and left of the midline. Graft sites were closed using absorbable glycomer 5–0 suture (um-202, Biosyn, MA, USA) to secure their fibrous capsules. The overlying skin incision was closed using the same suture material. Grafting of 16 fresh testicular tissue and 16 sham-operated groups with no testicular tissues were done as a positive and negative control using different castrated NCr nude mice.
Graft collection
The animals were euthanized by cervical dislocation 90 days after grafting. Immediately prior to euthanasia, a blood sample was collected via cardiac puncture and serum was prepared and stored at −20 °C for assay of testosterone. An incision was made in the dorsal skin to expose the grafts which were dissected for immediate fixation in Bouin's fixative solution.
Histological tissue processing and histopathological analysis
Histological tissue processing and analysis were modified according to Milazzo et al. [20]. Briefly, cryopreserved testicular tissues were thawed from liquid nitrogen after 1 month of storage. After thawing, tissue was immediately fixed in Bouin's solution then embedded in paraffin and cut into serial sections of 4 μm in thickness. Sections were stained with hematoxylin–eosin. Histolopathological evaluation was performed using a conventional bright-field microscope (Nikon Eclipse E 400, NY, USA). Digital images of serial sections were captured using a digital camera (Nikon, Coolpix950, NY, USA) at 10×, 20×, 40×, and 100× magnification. Histological features of the frozen–thawed testicular tissue samples were compared to fresh and frozen–thawed non-grafted control tissue. Damaged seminiferous tubules, spermatogonia, pachytene spermatocytes, round spermatids, elongated spermatids, and spermatozoa number were counted from pre- and post-grafting fresh and frozen–thawed testicular samples.
The tissue and cellular integrity and morphological changes in the fresh control and frozen–thawed sections were evaluated semi-quantitatively. Normal structure was scored as 1 and damage as 0. The total number of all sections per piece of testis scored as normal or damaged was divided by the number of sections investigated in the whole testis. This gave the percentage of sections with normal morphology in each testis sample. For each fresh and cryopreservation procedure tested, four independent samples were scored using two glass slides. The slides were evaluated by a single observer blinded to sample identity and counts were performed by two independent observers.
When the tissue and cellular integrity of the seminiferous tubules was scored as normal (scored as 1), the structure was scored as ‘good’. The tubules were scored as 0 when two or more of the following changes in structure were observed: disruption of cell–cell connections in the seminiferous tubules, rupture of the stroma, intercellular connection rupture, swelling of the lamina propria, and detachment of spermatogonia from the basement membrane.
Competitive immunoassay for serum testosterone
Quantification of total testosterone in serum was performed with a commercial total testosterone assay (Elecsys 2010 Testosterone II, product #05200067190 Roche Diagnostics, Mannheim, Germany). The assay was performed according the manufacturer's recommendations. It is based on a competitive principle in which testosterone in the sample and labeled testosterone are competing for binding to a biotinylated antibody. The assay has the following analytical characteristics, according to the manufacturer. Measuring range = 0.09–52.0 nmol/L. The lower limit of detection is 0.09 nmol/L and precision within the measurement range was <20%. The assay is not affected by icterus hemolysis or lipemia and exhibited recovery of 90–110%.
Statistical analyses
Statistical analyses were performed using the Graphpad Prism version 3.0 computer program (Graphpad Software, San Diego, CA, USA). All data from the histological comparison between the experimental groups and the testosterone results were compared statistically using repeated-measures one-way ANOVA, and expressed as the mean ± SEM. The Dunnett multiple-comparison test was used to compare differences among groups studied.
Results
Comparison of fresh and frozen–thaw neonatal mouse testis using different cryoprotectants
The effect of the four different CPAs on the morphological structure of testicular tissue was evaluated according to the criteria described above. Fresh neonatal testes maintain their structural integrity with only minimal damage (0.3 ± 0.5%). The frozen–thaw process resulted in significantly higher damage to seminiferous tubules with all cryoprotectants used (p < 0.05, Table 2). Significant structural changes observed included cell detachment, intercellular connection ruptures, and swelling of the lamina propria (Fig. 2). Cryopreservation using HBSS with 5% fetal bovine serum containing dimethyl sulfoxide (DMSO) provided greater protection compared to other CPA's, propylene glycol (PrOH), ethylene glycol (EG), and glycerol (p < 0.05); while using EG resulted in the highest rate of damage in seminiferous tubule.
Table 2.
Rates of seminiferous tubule damage in pre-grafting fresh and cryopreserved neonatal CD-1 mouse testes (mean ± SEM)
| Type of cryoprotectant | Rate of seminiferous tubule damage (%) |
|---|---|
| Fresh (control) | 0.3 ± 0.5a |
| DMSO | 6.3 ± 1.7b |
| PrOH | 9.9 ± 2.3bc |
| EG | 12.1 ± 1.6c |
| Glycerol | 9.4 ± 0.9bc |
Groups with different letters in the same column are significantly different (p < 0.05).
First column is dimethyl sulfoxide (DMSO), propylene glycol (PrOH), ethylene glycol (EG), glycerol, respectively.
Fig. 2.
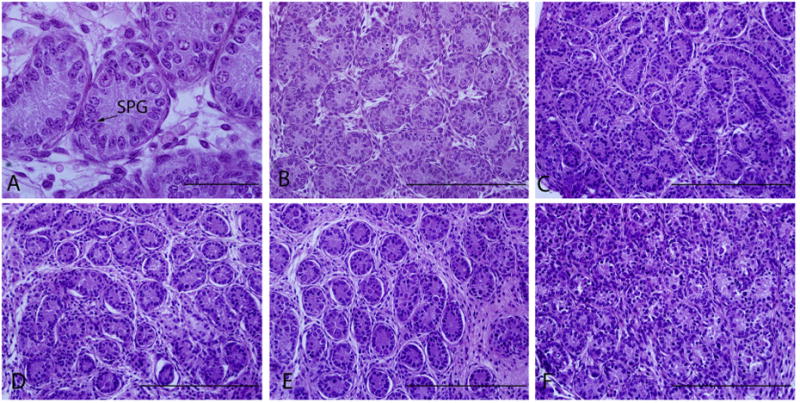
Morphology of fresh and frozen–thawed pre-grafted testicular tissue. Fresh control (A and B), cryopreserved neonatal testes with DMSO (C), PrOH (D), glycerol (E), and EG (F). Original magnification of A is ×100 and scale bar represents 50 μm. Original magnification of B–F are ×40 and scale bars represent 200 μm. Arrow points spermatogonia (SPG).
Functional spermatogenic assay for fresh and frozen–thawed neonatal mouse testes
Grafted testes were harvested after 3 months from the control and study groups. The rate of graft survival and damage to seminiferous tubules found in post-grafted fresh and cryopreserved CD-1 mouse testes are presented in Table 3 and Fig. 3. Fresh grafted and frozen–thawed grafted testes using freezing using DMSO CPA had the highest tissue survival rates compared to the other CPA groups (p < 0.05). The frozen–thawed testes using glycerol had the lowest graft survival rate (58.6 ± 1.9%, p < 0.05). Harvested testes from fresh grafted neonatal testes exhibited the lowest rate of seminiferous tubular damage and EG group had the highest rate of damaged seminiferous tubules (21.9 ± 2.7) among the CPA groups. The most common damage was stromal and intercellular connection rupture, and spermatogonia detachment from the basement membrane (Fig. 3).
Table 3.
Survival and testicular cell distribution rates of fresh and cryopreserved neonatal CD-1 mouse testes post-grafting onto Nude NCr mice (mean ± SEM)
| Type of cryoprotectant | Survival rate (%) | No. of damaged seminiferous tubules (%) | Spermatogonia (%) | Pachytene spermatocyte (%) | Round spermatid (%) | Elongated spermatid and spermatozoa (%) |
|---|---|---|---|---|---|---|
| Fresh (control) | 100 ± 0.0a | 3.2 ± 1.7a | 86.6 ± 2.6a | 84.6 ± 3.1a | 73.3 ± 1.9a | 46.6 ± 2.7a |
| DMSO | 100 ± 0.0a | 14.3 ± 1.2b | 71.4 ± 1.6a | 50.0 ± 0.9b | 39.7 ± 1.4bd | 16.2 ± 1.9b |
| PrOH | 83.3 ± 2.1b | 13.4 ± 1.5b | 87.3 ± 2.3a | 66.6 ± 2.9c | 54.5 ± 3.2bc | 11.1 ± 1.4b |
| EG | 85.6 ± 3.2b | 21.9 ± 2.7c | 71.8 ± 3.1a | 51.1 ±3.1b | 28.5 ± 2.6d | 0.0 ± 0.0c |
| Glycerol | 58.6 ± 1.9c | 10.1 ± 2.0b | 66.6 ± 1.9b | 53.0 ± 1.7b | 33.3 ± 1.6d | 14.6 ± 1.8b |
Groups with different letters in the same column are significantly different (p < 0.05).
First column is dimethyl sulfoxide (DMSO), propylene glycol (PrOH), ethylene glycol (EG), glycerol, respectively.
Fig. 3.
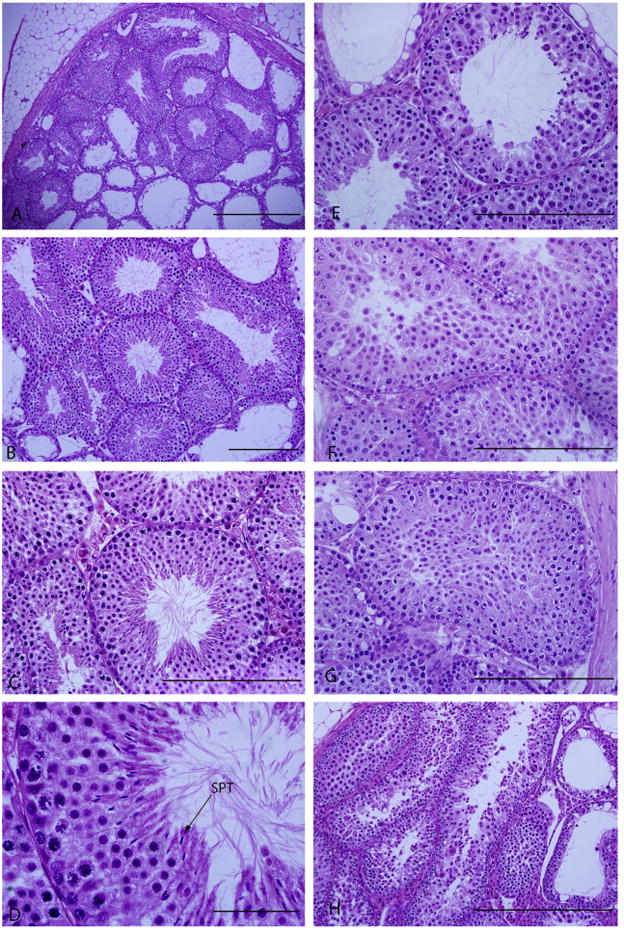
Morphology of fresh and frozen-thawed post-grafted testicular tissue. Magnification and scale bars for post-grafted fresh controls are A (×10–500 μm), B (×20–200 μm), C (×40–200 μm), D (× 100–50 μm), respectively. Magnification of E (DMSO), F (EG), G (PrOH), and H (glycerol) are ×40 and scale bars represent 200 μm, respectively. Arrow points spermatozoa (SPT).
From the functional point of view, the extent of germ cell differentiation in the grafted testes is the most significant endpoint using this model. Our positive control (freshly grafted group) has the most advanced spermatogenesis stage (elongated spermatid and spermatozoa) in 46.6% of the seminiferous tubules examined (Table 3). Although significanly lower rates of spermatozoa compared to control were observed in the DMSO, PrOH, and glycerol CPA groups, with 16.2%, 11.1%, and 14.4% respectively, these results demonstrate that complete spermatogenesis can be achieved in the frozen–thaw testes using these three CPA's. Whereas in the EG group, spermatogenesis arrested at the round spermatids stage after 3 months of grafting.
Comparison of the steroidogenic function in fresh and frozen–thawed testes grafts
Serum testosterone measured in the castrated hosts represents androgen production from the grafted testes (Fig. 1). Testosterone was non-detectable in the negative (castrated) control mice. While there was no statistical difference between fresh grafted and DMSO cryopreserved group, recipients with PrOH, EG, and glycerol groups had significantly lower serum testosterone levels (p < 0.05).
Fig. 1.
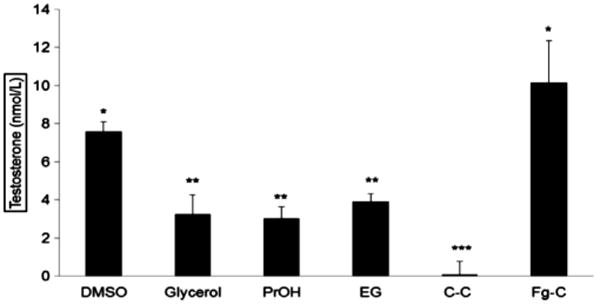
Testosterone rates of post-grafting fresh and cryopreserved CD-1 mouse testes onto Nude NCr mice (mean ± SEM). *p < 0.05; Asterisks indicate significant differences between each groups. Columns are dimethyl sulfoxide (DMSO), glycerol, propylene glycol (PrOH), ethylene glycol (EG), castrated control with no grafted testis (C–C), and fresh-grafted control (Fg-C).
Discussion
In this study, we have demonstrated differential rates of survival, spermatogenesis, and steroidogenesis in neonatal mouse testes grafted onto immunodeficient NCr Nude mice recipients between fresh grafted tissue and frozen–thawed testis. In addition, significant differences were observed among the CPA's used.
An understanding of the response of neonatal testes to the cryopreservation process is critical to generate improved freezing techniques and more efficient CPA protocols for recovery of functional tissue from frozen samples. Cryopreservation and recovery of functional tissue requires better permeation of the cryoprotectant compared with cell suspensions [23]. Different cell types may need different freezing protocols for optimal survival [19]. Automated controlled-rate slow freezing takes advantage of the regulatory properties of extracellular ice formation to dehydrate cells during cooling, thus minimizing the probability of intracellular ice crystal formation and avoiding toxicity to cells by exposing them to lower concentrations of CPAs while slowly decreasing the temperature. Based on our current results, the type of CPA used in cryopreservation of neonatal testicular tissue using the slow freezing program with an automated controlled-rate freezer was critical. Dimethyl sulfoxide (DMSO) provided a greater protection against freezing compared to propylene glycol (PrOH), ethylene glycol (EG), and glycerol with regard to testicular survival rates in the grafted samples (Table 3). This agrees with previously reported data supporting DMSO with its low molecular weight and high tissue penetration offering better results than PrOH [20], glyerol [16], or EG [9,16] in minimizing cryoinjury to tissue components.
Milazzo et al. [20] reported that freezing testes with DMSO maintained not only immature testicular tissue architecture, but also viability of testicular cells. In our study, the best morphology of the basal compartment was found when the CPA contained DMSO. This is in agreement with the findings of Keros et al. [17], and Goossens et al. [9]. Both research groups reported that the most typical damage was rupture of the cell-to-cell connections inside the seminiferous tubules, mainly in the basal compartment. Likewise, in the present study, rupture of the stroma, intercellular connection ruptures, and detachment of spermatogonia from the basement membrane were the most typical damage (Fig. 3).
According to our data presented here, the freeze–thaw process had a negative impact on the capacity for spermatogenesis in the grafted neonatal testes compared to the fresh grafted testes irrespective of the type of CPA used (Table 3, and Fig. 3). Among the different CPA groups, complete spermatogenesis was observed between 11% and 16% using DMSO, PrOH, and glycerol. However, no mature sperm was recovered from the EG group. This is in contrast to the report from Goossens and colleagues [9] who noted that both DMSO and EG CPA groups produced spermatozoa in frozen–thawed and grafted immature mouse testes. The discrepancy with our findings could be dependent on the age of neonatal testes; one day old in our study, and the use of a different freezing program in our experiments.
The endocrine function of Leydig cells from frozen–thawed testes was maintained after long-term grafting onto NCr mice (Fig. 1). Compared to the fresh grafted controls, DMSO group recipients had similar serum testosterone levels indicating good androgenic function from the grafted testes. These results confirmed that a testicular cryopreservation protocol using DMSO maintains the in vivo hormonal activity of Leydig cells, which is in agreement with previous studies using DMSO for in vitro endocrine and partial exocrine functions in both human [16–18] and mouse immature testes [20]. Although serum testosterone was also detectable in each of the PrOH, EG, and glycerol groups, the levels were significantly lower compared to the DMSO group (Fig. 3). This suggests that DMSO provides more effective preservation of androgenic activity compared to the other CPAs evaluated in the current study.
In summary, the results obtained conclude that: (1) neonatal mouse testes are well-preserved when frozen with HBSS medium including 5% fetal bovine serum and 0.7 M DMSO using slowrate automated controlled-rate freezing; and (2) the type of cryoprotectant is a significant factor to preserve the capacity of frozen–thawed neonatal mouse testes for spermatogenesis and steroidogenesis.
Acknowledgments
Statement of funding: This project is funded by the Canadian Urological Association Scholarship Fund.
References
- 1.Al-Hasani S, Demirel LC, Schopper B, Bals-Pratsch M, Nikolettos N, Kupker W, Ugur M, Sturm R, Diedrich K. Pregnancies achieved after frozen–thawed pronuclear oocytes obtained by intracytoplasmic sperm injection with spermatozoa extracted from frozen–thawed testicular tissues from non-obstructive azoospermic men. Hum Reprod. 1999;14:2031–2035. doi: 10.1093/humrep/14.8.2031. [DOI] [PubMed] [Google Scholar]
- 2.Baukloh V. Retrospective multicentre study on mechanical and enzymatic preparation of fresh and cryopreserved testicular biopsies. Hum Reprod. 2002;17:1788–1794. doi: 10.1093/humrep/17.7.1788. [DOI] [PubMed] [Google Scholar]
- 3.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA. 1994;22:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brook PF, Radford JA, Shalet SM, Joyce AD, Gosden RG. Isolation of germ cells from human testicular tissue for low temperature storage and autotransplantation. Fertil Steril. 2001;75:269–274. doi: 10.1016/s0015-0282(00)01721-0. [DOI] [PubMed] [Google Scholar]
- 5.Critser JK, Mobraaten LE. Cryopreservation of murine spermatozoa. ILAR J. 2000;41:197–206. doi: 10.1093/ilar.41.4.197. [DOI] [PubMed] [Google Scholar]
- 6.De Oliveira NM, Vaca Sánchez R, Rodriguez Fiesta S, Lopez Salgado T, Rodríguez R, Bethencourt JC, Blanes Zamora R. Pregnancy with frozen–thawed and fresh testicular biopsy after motile and immotile sperm microinjection, using the mechanical touch technique to assess viability. Hum Reprod. 2004;19:262–265. doi: 10.1093/humrep/deh083. [DOI] [PubMed] [Google Scholar]
- 7.Gianaroli L, Magli MC, Selman HA, Colpi G, Belgrano E, Trombetta C, Vitali G, Ferraretti AP. Diagnostic testicular biopsy and cryopreservation of testicular tissue as an alternative to repeated surgical openings in the treatment of azoospermic men. Hum Reprod. 1999;14:1034–1038. doi: 10.1093/humrep/14.4.1034. [DOI] [PubMed] [Google Scholar]
- 8.Gil-Salom M, Romero J, Minguez Y, Rubio C, De los Santos MJ, Remohí J, Pellicer A. Pregnancies after intracytoplasmic sperm injection with cryopreserved testicular spermatozoa. Hum Reprod. 1996;11:1309–1313. doi: 10.1093/oxfordjournals.humrep.a019377. [DOI] [PubMed] [Google Scholar]
- 9.Goossens E, Frederickx V, Geens M, De Block G, Tournaye H. Cryosurvival and spermatogenesis after allografting prepubertal mouse tissue: comparison of two cryopreservation protocols. Fertil Steril. 2008;89:725–727. doi: 10.1016/j.fertnstert.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 10.Honaramooz A, Snedaker A, Boiani M, Scholer H, Dobrinski I, Schlatt S. Sperm from neonatal mammalian testes grafted in mice. Nature. 2000;15:778–781. doi: 10.1038/nature00918. [DOI] [PubMed] [Google Scholar]
- 11.Honaramooz A, Behboodi E, Megee SO, Overton SA, Galantino-Homer H, Echelard Y, Dobrinski I. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod. 2003;69:1260–1264. doi: 10.1095/biolreprod.103.018788. [DOI] [PubMed] [Google Scholar]
- 12.Hovatta O, Foudila T, Siegberg R, Johansson K, von Smitten K, Reima I. Pregnancy resulting from intracytoplasmic injection of spermatozoa from a frozen–thawed testicular biopsy specimen. Hum Reprod. 1996;11:2472–2476. doi: 10.1093/oxfordjournals.humrep.a019140. [DOI] [PubMed] [Google Scholar]
- 13.Izadyar F, Den Ouden K, Creemers LB, Posthuma G, Parvinen M, De Rooij DG. Proliferation and differentiation of bovine type A spermatogonia during long-term culture. Biol Reprod. 2003;68:272–281. doi: 10.1095/biolreprod.102.004986. [DOI] [PubMed] [Google Scholar]
- 14.Jahnukainen K, Ehmcke J, Hergenrother SD, Schlatt S. Effect of cold storage and cryopreservation of immature non-human primate testicular tissue on spermatogonial stem cell potential in xenografts. Hum Reprod. 2007;22:1060–1067. doi: 10.1093/humrep/del471. [DOI] [PubMed] [Google Scholar]
- 15.Jezek D, Schulze W, Kalanj-Bognar S, Vukelic Z, Milavec-Puretic V, Krhen I. Effects of various cryopreservation media and freezing–thawing on the morphology of rat testicular biopsies. Andrologia. 2001;33:368–378. doi: 10.1046/j.1439-0272.2001.00459.x. [DOI] [PubMed] [Google Scholar]
- 16.Keros V, Rosenlund B, Hultenby K, Aghajanova L, Levkov L, Hovatta O. Optimizing cryopreservation of humantesticular tissue: comparison of protocols with glycerol, propanediol and dimethylsulphoxide as cryoprotectants. Hum Reprod. 2005;20:1676–1687. doi: 10.1093/humrep/deh797. [DOI] [PubMed] [Google Scholar]
- 17.Keros V, Hultenby K, Borgström B, Fridström M, Jahnukainen K, Hovatta O. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod. 2007;22:1384–1395. doi: 10.1093/humrep/del508. [DOI] [PubMed] [Google Scholar]
- 18.Kvist K, Thorup J, Byskov AG, Høyer PE, Møllgård K, Yding Andersen C. Cryopreservation of intact testicular tissue from boys with cryptorchidism. Hum Reprod. 2006;21:484–491. doi: 10.1093/humrep/dei331. [DOI] [PubMed] [Google Scholar]
- 19.Leibo SP, Mazur P. The role of cooling rates in low-temperature preservation. Cryobiology. 1971;8:447–452. doi: 10.1016/0011-2240(71)90035-6. [DOI] [PubMed] [Google Scholar]
- 20.Milazzo JP, Vaudreuil L, Cauliez B, Gruel E, Massé L, Mousset-Siméon N, Macé B, Rives N. Comparison of conditions for cryopreservation of testicular tissue from immature mice. Hum Reprod. 2008;23:17–28. doi: 10.1093/humrep/dem355. [DOI] [PubMed] [Google Scholar]
- 21.Nagano M, McCarrey JR, Brinster RL. Primate spermatogonial stem cells colonize mouse testes. Biol Reprod. 2001;64:1409–1416. doi: 10.1095/biolreprod64.5.1409. [DOI] [PubMed] [Google Scholar]
- 22.Nagano M, Patrizio P, Brinster RL. Long-term survival of human spermatogonial stem cells in mouse testes. Fertil Steril. 2002;78:1225–1233. doi: 10.1016/s0015-0282(02)04345-5. [DOI] [PubMed] [Google Scholar]
- 23.Nugent D, Meirow D, Brook PF, Aubard Y, Gosden RG. Transplantation in reproductive medicine: previous experience, present knowledge and future prospects. Hum Reprod Update. 1997;3:267–280. doi: 10.1093/humupd/3.3.267. [DOI] [PubMed] [Google Scholar]
- 24.Oates RD, Mulhall J, Burgess C, Cunningham D, Carson R. Fertilization and pregnancy using intentionally cryopreserved testicular tissue as the sperm source for intracytoplasmic sperm injection in 10 men with non-obstructive azoospermia. Hum Reprod. 1997;12:734–739. doi: 10.1093/humrep/12.4.734. [DOI] [PubMed] [Google Scholar]
- 25.Podsiadly BT, Woolcott RJ, Stanger JD, Stevenson K. Pregnancy resulting from intracytoplasmic injection of cryopreserved spermatozoa recovered from testicular biopsy. Hum Reprod. 1996;11:1306–1308. doi: 10.1093/oxfordjournals.humrep.a019376. [DOI] [PubMed] [Google Scholar]
- 26.Quinn P, Kerin JF, Warnes GM. Improved pregnancy rate in human in vitro fertilization with the use of a medium based on the composition of human tubal fluid. Fertil Steril. 1985;44:493–498. doi: 10.1016/s0015-0282(16)48918-1. [DOI] [PubMed] [Google Scholar]
- 27.Schlatt S, von Schönfeldt V, Nieschlag E. Germ cell transplantation in the male: animal studies with a human perspective. Hum Fertil (Camb) 1999;2:143–148. doi: 10.1080/1464727992000198531. [DOI] [PubMed] [Google Scholar]
- 28.Schlatt S, Kim SS, Gosden R. Spermatogenesis and steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and heterotopic grafting to castrated hosts. Reproduction. 2002;124:339–346. doi: 10.1530/rep.0.1240339. [DOI] [PubMed] [Google Scholar]
- 29.Scholtes MC, van Hoogstraten DG, Schmoutziguer A, Zeilmaker GH. Extraction of testicular sperm from previously cryopreserved tissue in couples with or without transport of oocytes and testicular tissue. Fertil Steril. 1999;72:785–791. doi: 10.1016/s0015-0282(99)00359-3. [DOI] [PubMed] [Google Scholar]
- 30.Shinohara T, Inoue K, Ogonuki N, Kanatsu-Shinohara M, Miki H, Nakata K, Kurome M, Nagashima H, Toyokuni S, Kogishi K, Honjo T, Ogura A. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Hum Reprod. 2002;17:3039–3045. doi: 10.1093/humrep/17.12.3039. [DOI] [PubMed] [Google Scholar]
- 31.Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Restoration of spermatogenesis in infertile mice by Sertoli cell transplantation. Biol Reprod. 2003;68:1064–1071. doi: 10.1095/biolreprod.102.009977. [DOI] [PubMed] [Google Scholar]
- 32.Thornton CE, Brown SD, Glenister PH. Large numbers of mice established by in vitro fertilization with cryopreserved spermatozoa: implications and applications for genetic resource banks, mutagenesis screens, and mouse backcrosses. Mamm Genome. 1999;10:987–992. doi: 10.1007/s003359901145. [DOI] [PubMed] [Google Scholar]
- 33.Tuuri T, Moilanen J, Kaukoranta S, Makinen S, Kotola S, Hovatta O. Testicular biopsy gun needle biopsy in collecting spermatozoa for intracytoplasmic injection, cryopreservation and histology. Hum Reprod. 1999;14:1274–1278. doi: 10.1093/humrep/14.5.1274. [DOI] [PubMed] [Google Scholar]
- 34.Wood S, Aziz N, Millar A, Schnauffer K, Meacock S, El Ghobashy A, Lewis-Jones I. Morphological and morphometric attributes of epididymal and testicular spermatozoa following surgical sperm retrieval for obstructive and nonobstructive azoospermia. Andrologia. 2003;35:358–367. doi: 10.1046/j.0303-4569.2003.00591.x. [DOI] [PubMed] [Google Scholar]


