Abstract
Swine fecal samples collected from seven farms were screened for group C rotaviruses (RVCs) using a reverse transcription-polymerase chain reaction assay. A total of 380 samples were tested and 19.5% were positive. Of the 128 samples collected in 2012, 23.5% from nursing piglets and 8.5% from weaned piglets were RVC positive, with a higher RVC frequency in diarrheic (28.4%) than in non-diarrheic (6.6%) piglets. Two strains (RVC/Pig-wt/USA/RV0104/2011/G3PX and RVC/Pig-wt/USA/RV0143/2012/G6Px) from two different farms were characterized genetically to gain information on virus diversity based on full length sequences of the inner capsid VP6, enterotoxin NSP4 and the outer capsid VP7 and VP4 (partial for RV0104) genes. The VP6 gene of the two strains showed high (99%) nucleotide identity to one another, 84–91% identity to other porcine RVCstrains and 81–82% identity to human and bovine RVC strains. The NSP4 gene analysis revealed that RVC/Pig-wt/USA/RV0104/2011/G3PX and RVC/Pig-wt/USA/RV0143/2012/G6Px strains were not closely related to each other (87% identity), but shared higher identity with prototype RVC/Pig-wt/USA/Cowden/1980/G1Px strain (93% and 89%, respectively) and were more distantly related to human strains (72–76% identity). The VP7 gene analysis indicated that the two strains were distantly related to one another (72% identity). RVC/Pig-wt/USA/RV0143/2012/G6Px was most closely related to porcine RVC G6 strains (82–86% identity), whereas RVC/Pig-wt/USA/RV0104/2011/G3PX was most closely related to porcine HF (G3) strain (94% identity). Analysis of the full length nucleotide sequence of the VP4 gene revealed that RVC/Pig-wt/USA/RV0143/2012/G6Px was distantly related to porcine (75%), bovine (74%) and human (70%) strains. The deduced amino acid identities (69.5–75.6%) of VP4 between RVC/Pig-wt/USA/RV0143/2012/G6Px and other RVCs were low; hence, we propose that this strain comprises a new VP4 genotype. Our results indicate high genetic heterogeneity in RVCs genes and the concurrent co-circulation of different genotypes at the same time. Our findings are useful for the development of more accurate diagnostic tools, for basic research to understand gene function and to provide information for RVC diversity germane to vaccine development.
Keywords: Group C rotavirus, Prevalence, Genetic diversity, Swine
1. Introduction
Neonatal diarrhea is a major endemic health problem for most swine herds worldwide due to its economic impacts. Diarrhea in piglets is a multi-factorial and multi-etiological disease. Rotaviruses (RVs) are a major cause of viral gastroenteritis in children and young animals worldwide, including nursing and weaned piglets (Chang et al., 2012). RVs comprise a genus in the Reoviridae family of dsRNA viruses, with a genome of 11 segments of dsRNA encoding 6 structural viral proteins (VP1–VP4, VP6 and VP7) and 5 nonstructural proteins (NSP1–NSP5/6). RVs are classified into 7 groups (A–G) based on antigenic relationships of their VP6 proteins (Kapikian, 2001). The most common groups that infect humans and animals are groups A, B and C, with the highest prevalence for Group A RV. The outer capsid proteins, VP4 and VP7, induce neutralizing antibodies and form the basis for G and P genotype/serotype assignment (Estes and Kapikian, 2007). A dual typing system based on the genome segments encoding VP4 (P genotypes) and VP7 (G genotypes) is used. To date, 27 different G- and 35 P-genotypes have been described in both humans and animals (Matthijnssens et al., 2011) for group A RVs; however, a formal classification system for RVCs based on VP4 and VP7 genes has not yet been established, although 6 G-types (G1–G6) have been identified and proposed by researchers using sequence analysis of multiple human and animal RVC strains (Rahman et al., 2005). Porcine RVCs exhibit G1, G3, G5 and G6 genotypes, bovine RVCs exhibit G2 genotype, and human RVCs exhibit G4 genotype (Collins et al., 2008; Martella et al., 2007a; Rahman et al., 2005).
Group C RVs were first identified in swine in 1980 (Saif et al., 1980; Bohl et al., 1982). Subsequently, RVCs have been detected in humans, cows, ferrets and dogs (Mawatari et al., 2004; Otto et al., 1999; Saif and Jiang, 1994). In humans, RVC infection has been associated with gastroenteritis worldwide, leading to the recognition that RVCs are important enteric pathogens (Bányai et al., 2006). Previous studies have reported 28–70% antibody prevalence against RVCs in pigs by 8 weeks of age. The antibody prevalence increases with age and reaches 79–100% in adult pigs (Saif and Jiang, 1994; Terrett et al., 1987). Diarrhea outbreaks associated with RVCs have been documented in nursing, weaning and post-weaning pigs (Kim et al., 1999; Saif and Jiang, 1994; Saif et al., 1980) either alone or in mixed infection with other enteric pathogens (Chang et al., 2012). A possible zoonotic role of animal RVCs has also been hypothesized based on increased seroprevalence rates to RVCs in human populations (Iturriza-Gómara et al., 2004). Furthermore, RVC surveillance has revealed transmission of RVCs between animal species: bovine strain WD534tc was shown to be of porcine origin (Chang et al., 1999a). Detection of animal-like RVCs in humans necessitates further detailed studies of the epidemiology and genetic diversity of animal RVCs, especially in regions where humans and animals, or different animal species often live in close contact making mixed infections more common.
The limited information on porcine RVC prevalence and diversity and lack of a commercial diagnostic assay for the detection of RVCs has hindered detailed knowledge of RVCs with regard to their epidemiology, their genetic heterogeneity and their pathogenic potential in pigs of different ages. To date the RV vaccine (ProSystem Rota, Intervet Inc., Merck Animal Health) licensed in the US that has been developed against RVs in swine reportedly contains only G4 and G5 genotypes of group A RV. Since there is no heterologous protection between RVs belonging to different groups or genotypes/serotypes, understanding the genetic heterogeneity of RVCs is critical to design effective prophylactic tools against RV infections, including development, optimization and improvement of RV vaccines. Therefore, the aim of this study was to investigate the prevalence of porcine RVC strains and to genetically characterize them from historical (2004) and recent (2011 and 2012) fecal samples collected from swine herds located in the state of Ohio, USA.
The GenBank accession numbers of RVC/Pig-wt/USA/RV0104/2011 are KC164673 (NSP4), KC164674 (VP6), KC164675 (VP7) and RVC/Pig-wt/USA/RV0143/2012 are KC164676 (NSP4), KC164677 (VP6), KC164678 (VP7), and KC164679 (VP4). The accession numbers of all representative known RVC strains for each gene mentioned in this study are listed in Appendix A.
Appendix A.
GenBank accession numbers for each gene segment of group C RV strains used in this study
| Gene | Strain | Accession |
|---|---|---|
| VP6 nucleotide | RVC/Pig-wt/USA/Cowden/1980/G1Px | M94157 |
| RVC/Pig-wt/KOR/CUK-5/2011/G5Px | HQ833829 | |
| RVC/Pig-wt/KOR/CUK-6/2011/G1Px | HQ323753 | |
| RVC/Pig-wt/KOR/CA-2/2009/GxPx | GQ925781 | |
| RVC/Pig-wt/BRA/BRA61/10/2010/GxPx | JF810453 | |
| RVC/Pig-wt/BRA/BRA905/07/2007/GxPx | JF810449 | |
| RVC/Human-wt/NGA/Jajeri/1999-00/G4Px | AF325802 | |
| RVC/Human-wt/NGA/Moduganari/1999-00/G4Px | AF325806 | |
| RVC/Human-wt/RUS/Nsk09-B43/2009/G4Px | GU592519 | |
| RVC/Human-wt/KOR/CAU-10-312/2010/G4Px | HQ896714 | |
| RVC/Human-wt/JPN/OH567/2003/GxPx | HQ185667 | |
| RVC/Human-wt/GBR/Preston/xxxx/G4Px | M94156 | |
| RVC/Human-wt/BRA/Belem/xxxx/G4Px | M94155 | |
| RVC/Human-wt/GBR/Bristol/xxxx/G4Px | X59843 | |
| RVC/Cow-wt/JPN/Yamagata/2002/G2Px | AB108680 | |
| NSP4 nucleotide | RVC/Human-wt/JPN/Ehime9301/1993/GxPx | D88353 |
| RVC/Human-wt/JPN/BK0830/2008/GxPx | HQ185682 | |
| RVC/Human-wt/CHN/208/xxxx/G4Px | AB008673 | |
| RVC/Human-wt/GBR/Bristol/xxxx/G4Px | X83967 | |
| RVC/Human-wt/KOR/CAU-10-312/2010/G4Px | HQ896719 | |
| RVC/Human-wt/JPN/OH567/2003/GxPx | HQ185671 | |
| RVC/Human-wt/IND/V508/2001/GxPx | AY770976 | |
| RVC/Human-wt/RUS/Nsk09-B43/2009/G4Px RVC/Pig-wt/USA/Cowden/1980/G1Px | JN969080 AF093202/AF093203 | |
| VP4 nucleotide | RVC/Human-wt/KOR/CAU-10-312/2010/G4Px | HQ896713 |
| RVC/Human-wt/BGD/BS347/2005/G4Px | HQ185635 | |
| RVC/Human-wt/CHN/208/xxxx/G4Px | AB008670 | |
| RVC/Human-wt/JPN/Y11-1/2011/G4Px | AB648917 | |
| RVC/Human-wt/NGA/Jajeri/1999-00/G4Px | AF323980 | |
| RVC/Human-wt/NGA/Moduganari/1999-00/G4Px | AF323981 | |
| RVC/Pig-wt/USA/Cowden/1980/G1Px | M74218 | |
| RVC/cow-wt/USA/Shintoku/xxxx/G2Px | U26551 | |
| RVC/Pig-wt/KOR/CUK-5/2011/G5Px | HQ833828 | |
| RVC/Pig-wt/KOR/CUK-6/2011/G1Px | HQ833827 | |
| VP7 nucleotide | RVC/Pig-wt/ITA/118/05-1/2005/G1Px | EF464651 |
| RVC/Pig-wt/IRL/45/06/Cork/2006/G1Px | EU624405 | |
| RVC/Pig-wt/USA/Cowden/1980/G1Px | M61101 | |
| RVC/Pig-wt/KOR/CUK-6/2011/G1Px | HQ323754 | |
| RVC/Pig-wt/USA/WH/xxxx/G1Px | U31749 | |
| RVC/cow-wt/USA/Shintoku/xxxx/G2Px | U31750 | |
| RVC/Cow-wt/JPN/Yamagata/2002/G2Px | AB108681 | |
| RVC/Pig-wt/USA/HF/xxxx/G3Px | U31748 | |
| RVC/Human-wt/BRA/Belem/xxxx/G4Px | X72256 | |
| RVC/Human-wt/KOR/CAU-10-312/2010/G4Px | HQ896715 | |
| RVC/Human-wt/NGA/Jajeri/1999-00/G4Px | AF325805 | |
| RVC/Human-wt/NGA/Moduganari/1999-00/G4Px | AF325806 | |
| RVC/Human-wt/RUS/Nsk09-B43/2009/G4Px | JN934901 | |
| RVC/Human-wt/JPN/OK450/1989/G4Px | D87544 | |
| RVC/Human-wt/GBR/Preston/xxxx/G4Px | X77258 | |
| RVC/Pig-wt/ITA/134/04-18/2004/G5Px | EF464653 | |
| RVC/Pig-wt/KOR/CUK-5/2011/G5Px | HQ833830 | |
| RVC/Pig-wt/ITA/134/04-2/2004/G6Px | EF464655 | |
| RVC/Pig-wt/IRL/1GA/05/Cork/2005/G6Px | EU624403 | |
| RVC/Pig-wt/IRL/281/07/Dublin/2007/G6Px | EU624404 | |
| RVC/Pig-wt/ITA/344-04-7/2004/G6Px | EF464654 | |
| RVC/Pig-wt/ITA/43/06-16/2006/G6Px | EF464656 | |
| RVC/Pig-wt/ITA/43/06-22/2006/G6Px | EF464657 | |
| VP4 amino acid | RVC/Human-wt/KOR/CAU-10-312/2010/G4Px | AEJ21069 |
| RVC/Human-wt/BGD/BS347/2005/G4Px | ADP76599 | |
| RVC/Human-wt/CHN/208/xxxx/G4Px | BAB83827 | |
| RVC/Human-wt/JPN/Y11-1/2011/G4Px | BAK53408 | |
| RVC/Human-wt/NGA/Moduganari/1999-00/G4Px | AAK26531 | |
| RVC/Human-wt/NGA/Jajeri/1999-00/G4Px | AAK26532 | |
| RVC/Pig-wt/USA/Cowden/1980/G1Px | AAB00802 | |
| RVC/cow-wt/USA/Shintoku/xxxx/G2Px | AAB01672 | |
| RVC/Pig-wt/KOR/CUK-5/2011/G5Px | AEK20775 | |
| RVC/Pig-wt/KOR/CUK-6/2011/G1Px | AEK20774 |
2. Materials and methods
2.1. Samples
Two sets of porcine fecal samples collected from diarrheic and non-diarrheic nursing and post weaning piglets from 7 swine herds located in Ohio, USA were screened for RVCs (Table 1). One set included historical fecal samples collected in 2004 (118) and the second set included recent samples collected in 2011 (134) and 2012 (128). Samples collected in 2012 were from both nursing (81) and weaned (47) piglets and they were collected from piglets with diarrhea (67) and without diarrhea (61) (Table 1). All samples were screened by reverse transcription-polymerase chain reaction (RT-PCR), using validated primers (Appendix B) designed from multiple alignment of the VP6 gene of representative human and porcine RVC reference strains.
Table 1.
The year and number of samples collected, pig ages, diarrhea status and RVC infection incidence.
| Year of collection, total # of samples | Farm IDa | Month samples collected in | Number of samples | Diarrhea status | Ageb | RVC positive samples (%) |
|---|---|---|---|---|---|---|
| 2004, n = 118 | Farm 1 (Central OH) | April | n = 32 | N/Ac | <3 weeks | 5 (15.6%) |
| Farm 2 (NW OH) | July | n = 31 | N/A | <3 weeks | 6 (19.4%) | |
| Farm 2 (NW OH) | December | n = 40 | N/A | <3 weeks | 3 (7.5%) | |
| Farm 3 (Central OH) | May | n = 15 | N/A | <3 weeks | 0 (0%) | |
| 2011, n = 134 | Farm 2 (NW OH) | July | n = 39 | N/A | N/A | 14 (36%) |
| Farm 2 (NW OH) | December | n = 20 | N/A | <3 weeks | 11 (55%) | |
| Farm 4 (NE OH) | June | n = 37 | N/A | <3 weeks | 12 (32.4%) | |
| Farm 5 (NE OH) | April | n = 34 | 5 diarrhea; others no diarrhea | <3 weeks | 0 (0%) | |
| Farm 6 (Central OH) | November | n = 4 | Diarrhea | <3 weeks | 0 (0%) | |
| 2012, n = 128 | Farm 2 (NW OH) | January | n = 8 | Diarrhea | <3 weeks (n = 4), gilts (n = 4) | 0 (0%) |
| Farm 2 (NW OH) | March | n = 60 | Most no diarrhea (only 6 with diarrhea) | <3 weeks (n = 17), >3 weeks (n = 43) | 4 (6.8%) | |
| Farm 2 (NW OH) | May | n = 55 | Most diarrhea (only 5 with no diarrhea) | <3 weeks | 14 (25.5%) | |
| Farm 6 (Central OH) | January | n = 1 | Diarrhea | <3 weeks | 1 (100%) | |
| Farm 7 (NE OH) | May | n = 4 | Diarrhea | <3 weeks | 4 (100%) |
Central OH – Central Ohio, NW OH – North-western Ohio, NE OH – North-eastern OH.
Within same year, each farm # appears as many times as it was sampled.
<3 weeks – nursing piglets, >3 weeks – weaned piglets.
N/A – not available.
Appendix B.
Primers used for detection and full length sequencing of RVCs NSP4, VP4 (VP8* and VP5*), VP6 and VP7 genes designed from human and porcine RVC strains
| Gene | Name | Sequence | Segment | Amplicon (bp) |
|---|---|---|---|---|
| Diagnostic (partial VP6) | VP6F | ACAGTATTTCAGCCAGGDTTTC | 1095–1116 | 260 |
| VP6R | AGCCACATAGTTCACATTTCATC | 1332–1354 | ||
| NSP4 | NSP4-F | GGCTTTAAATTTTTCAGATCAC | 1–22 | 613 |
| NSP4-R | AGCCWCATGAATTTTTCAYATC | 592–613 | ||
| VP6 | 5′comVP6-F | GCAWTWAAAATCTCATTCACAATGG | 3–27 | 1352 |
| 3′comVP6-R | AGCCACATAGTTCACATTTCATCC | 1331–1354 | ||
| VP7 | 5′comVP7-F | GCTGTCTGACAAACTGGTC | 20–38 | 1043 |
| 3′comVP7-R | GCCACATGATCTTGTTTACGC | 1042–1062 | ||
| VP4-1 (VP8*) | VP4-17Fdeg | GATCRATGGCGTCYTCAC | 17–34 | 1222 |
| VP4-1238R | CCTGATGAATGTAATCCWGGAT | 1238–1216 | ||
| VP4-2 (VP5*) | VP4-1108F | GATTATTGGGACGATTCAG | 1108–1126 | 1179 |
| VP4-2267F | AGCCACATTTCAAGCTGGTC | 2267–2286 |
2.2. RNA extraction and RT-PCR
The RNA was extracted from 250 μl of 10% (w/v) fecal suspensions of rectal swabs or stool samples in Minimum Essential Media (MEM) using RNeasy mini kit (Qiagen, CA, USA) according to the manufacturer’s instructions. The total RNA recovered was suspended in 40 μl of nuclease free water and stored at −70 °C until used. PCR inhibitors in the samples were determined to be negligible based upon results obtained by making 10-fold and 100-fold dilutions of RNA. Conventional RT-PCR was used for detection of the RVCs with validated primer sets (Appendix B) using Promega reagents according to the manufacturers’ instructions. The amplicons were analyzed in 1.5–3% agarose gel.
2.3. Sequencing and molecular analysis
To confirm the PCR results and to obtain genetic information on virus diversity, the VP6 amplicons of six positive samples were sequenced. The DNA was purified by using a QIAquick gel extraction kit (QIAGEN, Inc.) according to the manufacturer’s protocol. The amplicons were sequenced directly using forward and reverse RT-PCR primers. DNA sequencing was carried out by using BigDye Terminator Cycle chemistry and 3730 DNA Analyzer (Applied Biosystems, Foster, CA). The resulting partial (260 bp) VP6 gene sequences of the RVCs were aligned with corresponding fragments of selected reference strains of human, porcine and bovine RVC strains. Distance analysis and phylogenetic inference were conducted using the Mega 5.0 software package (Tamura et al., 2011).
Based on results of the partial VP6 gene sequences, two of the RVC strains (RVC/Pig-wt/USA/RV0104/2011/G3PX and RVC/Pig-wt/USA/RV0143/2012) were selected from two different clusters and further characterized using full-length sequences of their NSP4, VP6 and VP7 and VP4 genes (Partial VP4 gene for RVC/Pig-wt/USA/RV0104/2011/G3Px strain) using specific primers for each gene (Appendix B). These two samples contained only RVCs (no group A or B RVs or sapoviruses) and were collected from nursing piglets. The RVC/Pig-wt/USA/RV0104/2011/G3PX was from a piglet from Farm 2, 2011, with diarrhea status unknown, and RVC/PIG-WT/USA/RV0143/2012 was from a diarrheic piglet from Farm 6, 2012. cDNA was synthesized using 3 μl of the total RNA using SuperScript III™ first-strand synthesis system for RT-PCR according to manufacturer’s protocol (Invitrogen) using gene specific primers for each gene. Two μl of cDNA was used as a template for the PCR reaction, PrimeSTAR GXL DNA Polymerase (Takara, Japan) was used for high fidelity synthesis and PCR was conducted under the following conditions: 35 cycles at 98 °C for 10 s, 50 °C for 20 s and 68 °C for 2 min. The amplicons were analyzed in 1.5% agarose gel, the DNA was purified using QIAquick gel extraction kit (QIAGEN, Inc.) and sequenced in both directions using the same primers. The nucleotide sequences obtained were compared with those of similar sequences from reference strains available from GenBank using BLAST software (http://www.ncbi.nlm.nih.gov/BLAST/). The DNA sequences were aligned using the Clustal W method and phylogenetic dendrograms were constructed for each gene using the neighbor-joining method supported with a bootstrap test of 1000 replicates in MEGA 5 software (Tamura et al., 2011).
3. Results
3.1. Incidence of RVCs in nursing piglets
A total of 380 samples were tested and 19.5% (74/380) were positive for RVCs. When these data were compiled with surveillance results for groups A and B RVs, we found that 10.8% of RVC positive samples also contained either group A (6.7%) or B (4.1%) RVs (data not shown). The prevalence of RVCs varied among the farms and between different years within the same farm ranging from 15.6% to 100% (Fig. 1). RVC were detected in most farms (except Farms 3 and 5) but not for every sampling: for example, no RVC were detected on Farm 3 in 2004 and Farms 5 and 6 in 2011. It is noteworthy that samples from Farms 6 and 7 were from diarrheic piglets submitted to the laboratory for diagnosis. Yearly differences in the prevalence of RVCs were observed among the 3 years: 2004 (11.9%), 2011 (27.6%) and 2012 (18%). Overall, the RVC incidence was highest in summer (26%) followed by winter (19.5%) and then spring (16.4%). No autumn samples were available. The RVC prevalence differed within each year: in 2004, the highest prevalence occurred in summer, while in 2011, winter had the highest prevalence and in 2012, the detection rate was highest in spring. However, seasonal influences on the prevalence of RVC infections were not evident across the years studied even for Farm 2 that was sampled at least twice a year in different seasons (Fig. 2, Table 1).
Fig. 1.

Prevalence of RVCs between and within surveyed farms and years. In 2004, Farms 1 and 3 were sampled once, Farm 2 was sampled twice; in 2011, Farms 4, 5 and 6 were each sampled once and Farm 2 was sampled twice; in 2012, Farms 6 and 7 were sampled once and Farm 2 was sampled 3 times.
Fig. 2.

Annual and seasonal variability of RVC prevalence within and among the 3 years. Only Farms 2 and 6 were sampled repeatedly in different years: Farm 2 (July and December, 2004; July and December, 2011; January, March and May, 2012); Farm 6 (November, 2011; January, 2012). Farms 1, 3, 4 and 5 were each sampled just once in April, 2004, June, 2011, April, 2011, and May, 2012, respectively.
Based on 128 samples collected in 2012, the RVC prevalence was higher in the nursing (23.5%, 19/81) compared to the weaned (8.5%, 4/47) piglets. Interestingly, all the weaned pigs positive for RVC infection were asymptomatic, whereas all nursing piglets positive for RVC infection were symptomatic. The relationship between RV detection and diarrhea was evaluated in samples collected in 2012. The detection rate of RV was significantly higher in piglets with diarrhea (50.7%, 34/67) than in non-diarrheic piglets (16.4%, 10/61). Three (2.3%) samples had mixed RV infections (2 group A + group B RVs and 1 group B + group C RV) and all were from diarrheic piglets. Regardless of the age of piglets, RVC was detected more frequently (28.4%) in diarrheic piglets than in piglets without diarrhea (6.6%). The relationship between RVC detection and diarrheal and age status of the piglets could not be evaluated for samples collected in 2004 and 2011 because the information about the diarrheal status was not always available.
3.2. Molecular analysis of NSP4, VP6, VP7 and VP4 genes RVC strains
Molecular analysis of the partial (260 bp) VP6 gene segment of six selected positive samples confirmed them as porcine RVCs, although an unexpected heterogeneity in a relatively short fragment was revealed. The partial VP6 gene segment of the six strains had high nucleotide sequence identity with reference sequences of human (82.5–86%) and porcine (86.2–97.2%) RVCs. Most of the field strains shared high nucleotide identity between one another (97.2–99.5%) and clustered (>92% identity) with recent Korean and Brazilian strains (data not shown). To gain more information on genetic heterogeneity of RVC strains circulating in swine in the surveyed farms, two likely divergent (based on preliminary sequencing data) strains (RVC/Pig-wt/USA/RV0104/2011/G3PX and RVC/Pig-wt/USA/RV0143/2012/G6PX) from two different swine herds collected in 2011 and 2012 were selected and their full-length sequences for NSP4, VP6, VP7 and VP4 genes were determined and analyzed.
VP6 gene
Full length sequences of the VP6 gene of the RVC/Pig-wt/USA/RV0104/2011/G3PX and RVC/Pig-wt/USA/RV0143/2012/G6PX strains showed 99% nucleotide identity to each other and 84–91% nucleotide identity to other porcine strains. Phylogenetic analysis revealed that RVC/Pig-wt/USA/RV0104/2011/G3PX was clustered with the RVC/Pig-wt/USA/RV0143/2012/G6PX in a monophyletic branch, closely related to other porcine strains, specifically Brazilian strains (90–91% nt identity); however, they were distantly related to the human and bovine branches (data not shown).
NSP4 gene
The sequence analysis of the full length NSP4 gene showed that the two field strains (RVC/Pig-wt/USA/RV0104/2011/G3PX and RVC/Pig-wt/USA/RV0143/2012/G6PX) were distantly related to each other (87% nucleotide identity). They were closely related to prototype RVC/Pig-wt/USA/Cowden/1980/G1Px strain with sequence identity of 93% and 89% for RVC/Pig-wt/USA/RV0104/2011/G3PX and RVC/Pig-wt/USA/RV0143/2012/G6PX, respectively, while distantly related to human strains (72–76%) (data not shown). NSP4 based phylogenetic analysis revealed that RVC/Pig-wt/USA/RV0104/2011/G3PX and the prototype RVC/Pig-wt/USA/Cowden/1980/G1Px strain belonged to the same cluster while RVC/Pig-wt/USA/RV0143/2012/G6PX formed a different branch indicating increased diversity in porcine RVCs in the NSP4 gene (data not shown).
VP7 gene
The complete coding nucleotide sequence of the VP7 gene of the RVC/Pig-wt/USA/RV0104/2011/G3PX and RVC/Pig-wt/USA/RV0143/2012/G6PX strains was determined and compared with VP7 gene sequences of other RVC strains available in the GenBank database. Sequence comparison indicated that one strain (RVC/Pig-wt/USA/RV0143/2012/G6PX) was most closely related to RVC G6 strains with sequence identities ranging from 82 to 86% while the other strain (RVC/Pig-wt/USA/RV0104/2011/G3PX) was closely related to RVC/Pig-wt/USA/HF/xxxx/G3Px strain (G3) with sequence identity of 94%. The two strains were distantly related to one another with sequence identity of 72% and they were also distantly related to other porcine RVC G-types, human and bovine strains with sequence identities ranging from 72 to 77% (Table 2). Phylogenetic analysis (Fig. 3) that included available VP7 gene sequences of RVCs confirmed that our RVC/Pig-wt/USA/RV0104/2011/G3PX and RVC/Pig-wt/USA/RV0143/2012/G6PX strains belonged to the two distinct porcine RVC genotypes, G3 and G6 strains, respectively and were distantly related to the human (74–77%) and bovine strains (74–77%).
Table 2.
Full length nucleotide sequence identity between the VP7 gene of RVC/Pig-wt/USA/RV0104/2011/G3Px and RVC/Pig-wt/USA/RV0143/2012/G6Px RVC strains and the available published RVC strains in GenBank.
| Strains | Host | G-type | % nucleotide identity
|
|
|---|---|---|---|---|
| RVC/Pig-wt/USA/RV0104/2011/G3Px | RVC/Pig-wt/USA/RV0143/2012/G6Px | |||
| RVC/Pig-wt/ITA/118/05-1/2005/G1Px | Porcine | G1 | 77 | 75 |
| RVC/Pig-wt/IRL/45/06/Cork/2006/G1Px | Porcine | G1 | 71 | 75 |
| RVC/Pig-wt/USA/Cowden/1980/G1Px | Porcine | G1 | 77 | 76 |
| RVC/Pig-wt/KOR/CUK-6/2011/G1Px | Porcine | G1 | 77 | 75 |
| RVC/Pig-wt/USA/WH/xxxx/G1Px | Porcine | G1 | 75 | 76 |
| RVC/cow-wt/USA/Shintoku/xxxx/G2Px | Bovine | G2 | 74 | 77 |
| RVC/Cow-wt/JPN/Yamagata/2002/G2Px | Bovine | G2 | 74 | 76 |
| RVC/Pig-wt/USA/HF/xxxx/G3Px | Porcine | G3 | 94 | 73 |
| RVC/Human-wt/BRA/Belem/xxxx/G4Px | Human | G4 | 75 | 77 |
| RVC/Human-wt/KOR/CAU-10-312/2010/G4Px | Human | G4 | 75 | 76 |
| RVC/Human-wt/NGA/Jajeri/1999-00/G4Px | Human | G4 | 76 | 77 |
| RVC/Human-wt/NGA/Moduganari/1999-00/G4Px | Human | G4 | 76 | 77 |
| RVC/Human-wt/RUS/Nsk09-B43/2009/G4Px | Human | G4 | 74 | 77 |
| RVC/Human-wt/JPN/OK450/1989/G4Px | Human | G4 | 75 | 77 |
| RVC/Human-wt/GBR/Preston/xxxx/G4Px | Human | G4 | 75 | 77 |
| RVC/Pig-wt/ITA/134/04-18/2004/G5Px | Porcine | G5 | 74 | 77 |
| RVC/Pig-wt/KOR/CUK-5/2011/G5Px | Porcine | G5 | 74 | 76 |
| RVC/Pig-wt/ITA/134/04-2/2004/G6Px | Porcine | G6 | 76 | 83 |
| RVC/Pig-wt/IRL/1GA/05/Cork/2005/G6Px | Porcine | G6 | 74 | 81 |
| RVC/Pig-wt/IRL/281/07/Dublin/2007/G6Px | Porcine | G6 | 72 | 81 |
| RVC/Pig-wt/ITA/344-04-7/2004/G6Px | Porcine | G6 | 75 | 85 |
| RVC/Pig-wt/ITA/43/06-16/2006/G6Px | Porcine | G6 | 77 | 86 |
| RVC/Pig-wt/ITA/43/06-22/2006/G6Px | Porcine | G6 | 77 | 84 |
| RVC/Pig-wt/USA/RV0104/2011/G3Px | Porcine | G3? | 72 | |
| RVC/Pig-wt/USA/RV0143/2012/G6Px | Porcine | G6? | 72 | |
For each strain the highest identity to the reference strain is shown in bold.
Fig. 3.
Phylogenetic dendrogram of RVC full-length VP7 gene of selected divergent field strains and Lab adapted RVC/Pig-wt/USA/Cowden/1980/G1Px strain (bold, dots) compared with VP7 gene sequences for human, bovine and porcine RVCs available in GenBank.
VP4 gene
Partial (602nt) sequence of the VP4 gene (VP8* segment) of the RVC/Pig-wt/USA/RV0143/2012/G6PX strain revealed higher diversity of RVCs, with low sequence identity with porcine (76.3%), bovine (76%) and human (77.4–78.4%) RVC strains (Table 3). The human RVC/Human-wt/BGD/BS347/2005/G4Px strain showed higher (78.4%) identity with the RVC/Pig-wt/USA/RV0143/2012/G6PX strain than with bovine and other porcine strains. The partial (311nt) VP4 (VP8*) gene sequence of the RVC/Pig-wt/USA/RV0104/2011/G3PX strain also showed higher diversity, with lower sequence identity to RVC/Pig-wt/USA/RV0143/2012/G6PX strain (71.4%) and bovine RVC/cow-wt/USA/Shintoku/xxxx/G2Px strain (79.1%); however, it was closely related to porcine strain RVC/Pig-wt/USA/Cowden/1980/G1PX (84.2%) and human strains (79.4–83.3%). A phylogenetic tree was constructed of the RVC reference strains (human and porcine) and the two identified strains (RVC/Pig-wt/USA/RV0143/2012/G6PX and RVC/Pig-wt/USA/RV0104/2011/G3PX). Based on the partial VP4 (VP8*) gene all human strains clustered in a monophyletic branch while the RVC/Pig-wt/USA/RV0104/2011/G3PX and RVC/Pig-wt/USA/RV0143/2012/G6PX strains were in separate branches in the phylogenetic tree (Fig. 4). The two strains were distantly related to each other and were in different branches as was also observed for the VP8* segment of the VP4 gene. After sequencing the full length VP4 gene of the RVC/Pig-wt/USA/RV0143/2012/G6PX strain, we observed that it was more closely related to both RVC/Cow-wt/USA/Shintoku/xxxx/G2Px (74.1%) and RVC/Pig-wt/USA/Cow-den/1980/G1PX (74.6%) RVC strains than to human strains (70%) (data not shown). Similarly, the deduced amino acid sequence of VP4 of the RVC/Pig-wt/USA/RV0143/2012/G6PX strain also showed lower identity to known human (69.5–69.9%), bovine RVC/cow-wt/USA/Shintoku/xxxx/G2Px (74.4%) and porcine RVC/Pig-wt/USA/Cowden/1980/G1PX (75.6%) strains (data not shown). Phylogenetic analysis of the full length VP4 gene of the RVC/Pig-wt/USA/RV0143/2012/G6PX strain and reference strains in GenBank confirmed this relationship with the RVC/Pig-wt/USA/RV0143/2012/G6PX, RVC/cow-wt/USA/Shintoku/xxxx/G2Px and RVC/Pig-wt/USA/Cowden/1980/G1PX strains clustering in one branch (Fig. 5). The full length VP4 gene for RVC/Pig-wt/USA/RV0104/2011/G3PX was not obtained presumably due to polymorphic positions in some primer binding sites.
Table 3.
Sequence identity of partial VP4 (VP8*) gene of selected divergent field strains (RVC/Pig-wt/USA/RV0104/2011/G3Px and RVC/Pig-wt/USA/RV0143/2012/G6Px) compared with VP4 gene sequences for known human, bovine and porcine RVCs available in GenBank.
| Name | RVC/Pig-wt/USA/RV0104/2011/G3Px (311nt) | RVC/Pig-wt/USA/RV0143/2012/G6Px (602nt) |
|---|---|---|
| RVC/Human-wt/KOR/CAU-10-312/2010/G4Px | 83.3 | 78.2 |
| RVC/Human-wt/JPN/Y11-1/2011/G4Px | 82.3 | 78.4 |
| RVC/Human-wt/BGD/BS347/2005/G4Px | 79.4 | 78.6 |
| RVC/Human-wt/CHN/208/xxxx/G4Px | 82.3 | 77.4 |
| RVC/Human-wt/NGA/Moduganari/1999-00/G4Px | 82.0 | 77.7 |
| RVC/cow-wt/USA/Shintoku/xxxx/G2Px | 79.1 | 75.9 |
| RVC/Pig-wt/USA/Cowden/1980/G1Px | 84.2 | 76.3 |
| RVC/Pig-wt/USA/RV0104/2011/G3Px | – | 71.4 |
For each strain the highest identity to the reference strain is shown in bold.
Fig. 4.
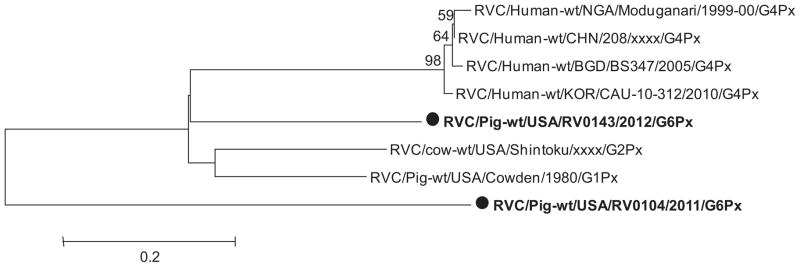
Phylogenetic dendrogram of RVC partial VP4 gene (VP8*) of selected divergent field strains (bold, dots) compared with VP4 gene sequences for human, bovine and porcine RVCs available in GenBank.
Fig. 5.
Phylogenetic dendrogram of RVC full length VP4 gene of RVC/PIG-WT/USA/RV0143/2012 strain compared with VP4 gene sequences for human, bovine and porcine RVCs available in GenBank.
4. Discussion
RVC are a cause of diarrheal disease outbreaks either in nursing or older pigs, leading to mortality (Kim et al., 1999). Seroprevalence studies of RVCs have also revealed high rates of infection and indicated that the virus is widespread (Terrett et al., 1987; Tsunemitsu et al., 1992). However, the information on prevalence and genetic characterization of porcine RVCs is very limited, especially in the US, hampering the implementation of control measures for RV infections. In this study, we report an overall prevalence of RVCs of 19.5% in fecal samples from 7 swine herds in Ohio, which was slightly lower than the 26.3% reported in S. Korea (Jeong et al., 2009) and 28.7% in Italy (Martella et al., 2007b). However, these prior studies analyzed samples from only diarrheic pigs, whereas we analyzed samples from both diarrheic and non-diarrheic pigs. Of the 128 samples collected in 2012, the prevalence of RVC among nursing piglets was 23.5%, but only 8.5% among weaned piglets. Interestingly, all the weaned piglets positive for RVC were non-diarrheic, whereas, all nursing piglets with RVC infection were diarrheic. These results indicate that RVC is an important enteric pathogen causing diarrhea more frequently in nursing piglets than weaned piglets as has also been described in other studies (Chang et al., 2012; Kim et al., 1999; Saif and Jiang, 1994). Overall, the high prevalence and distribution of porcine RVCs suggests that they are widespread in nursing piglets from Ohio swine farms, similar to that documented in S. Korea and Italy (Jeong et al., 2009; Martella et al., 2007b). Further studies are required to ascertain the pathogenic potential of RVC in different age groups in swine to be able to implement effective prophylactic measures for prevention and control of RVC infections in swine.
We detected RVCs in most farms sampled (excluding Farms 3 and 5), and the prevalence varied among the farms and between different years within the same farm, indicating that differences in swine herd management might influence RV prevalence. Overall we detected porcine RVCs more commonly in summer (26%) followed by winter (19.5%) and then spring (16.4%). Seasonal distribution of RVC infections among the years was not evident from our results because in each year the seasonal prevalence of porcine RVCs differed (Fig. 2). To date there is lack of information showing a clear seasonal distribution of porcine RVCs in the US; hence, further epidemiological studies with repetitive sampling throughout the year are required to fully understand the seasonal distribution of this virus.
Porcine RVCs have been detected in diarrheic and non-diarrheic fecal samples from nursing, weaning and post-weaning pigs, either alone or in combination with other enteric pathogens (Collins et al., 2008; Kim et al., 1999; Martella et al., 2007b; Morin et al., 1991; Saif and Jiang, 1994). In this study, 28.4% (19/67) of diarrheic fecal samples collected in 2012 tested positive for RVCs while 6.6% (4/61) of the non-diarrheic fecal samples collected at the same time were positive for RVCs. Consistent with findings by others, we observed that 11% (8/74) of RVC positive samples were mixed infections with groups A and B RVs, suggesting that a number of enteric pathogens, either singly or in combination can augment the clinical presentation of porcine RVC infections (Jeong et al., 2009; Martella et al., 2007b). This argument is supported by the observation that experimental co-infection of calves with group A RVs enhanced fecal shedding of a bovine RVC and the extent of histopathological lesions in the small intestine (Chang et al., 1999a).
We analyzed the full length sequences of NSP4, VP4 (VP8*/VP5* for RVC/Pig-wt/USA/RV0104/2011/G3PX strain), VP6 and VP7 genes of two strains (RVC/Pig-wt/USA/RV0104/2011/G3PX and RVC/Pig-wt/USA/RV0143/2012/G6PX) to evaluate the genetic relatedness of these cotemporary RVC strains. Initial analysis of the partial (260 bp) VP6 gene of six porcine RVCs showed that they share lower nucleotide sequence identity (82.5–86%) with human RVCs which is consistent with previous reports (Collins et al., 2008; Jeong et al., 2009; Martella et al., 2007b).
The intermediate-layer capsid VP6 protein mediates RV group and subgroup specificity. Analysis of the full length VP6 gene of the two RVC/Pig-wt/USA/RV0104/2011/G3PX and RVC/Pig-wt/USA/RV0143/2012/G6PX strains revealed that they were closely related to each other with nucleotide sequence identity of 99%: however, the genetic distance was variable between these and other known RVCs, ranging from 81% to 91% and they were most closely related to recent RVCs identified in Brazil (91%). Similar genetic variability in the same gene of porcine RVC has been reported in Italy (Martella et al., 2007b), Brazil (Medici et al., 2010) and S. Korea (Jeong et al., 2009; Lee et al., 2011).
The full length sequence analysis of the NSP4 gene of the two field strains showed that they were not closely related to each other (87%): however, they were more closely related to the porcine prototype RVC/Pig-wt/USA/Cowden/1980/G1Px strain (89–93%), a further indication of genetic heterogeneity of RVCs. The two strains were distantly related to human strains which is consistent with a previous report by Chang et al. (1999b) who showed a higher divergence between porcine RVC/Pig-wt/USA/Cow-den/1980/G1Px strain and human Bristol strain. The NSP4 protein has an important function as the viral enterotoxin which causes secretory diarrhea in mice (Ball et al., 1996).
Major serotype-specific neutralization sites have been associated with the VP7 protein of RVs. The VP7 gene sequences of different serotypes revealed extensive sequence conservation among serotypes, which was suggested to reflect structural and functional constraints necessary to preserve the architecture of the VP7 protein (Gunn et al., 1985). To date 27 G genotypes have been identified for group A RVs (Matthijnssens et al., 2011); however, a formal classification system of RVCs based on the VP7 gene has not yet been established, although 6 G-type (G1–G6) strains have been identified and proposed by researchers using sequence analysis of multiple human and animal RVC strains (Rahman et al., 2005). For group A RVs, it was observed that strains having −80% nucleotide identity in VP7 gene sequences belongs to the same G-genotype (Matthijnssens et al., 2008a). We studied the identity between two field strains (RVC/Pig-wt/USA/RV0104/2011/G3PX and RVC/Pig-wt/USA/RV0143/2012/G6PX) complete VP7 gene sequences and RVC strains available in GenBank (Table 2). The RVC/Pig-wt/USA/RV0143/2012/G6PX strain was most closely related to porcine RVC G6 strains with nucleotide sequence identities ranging from 82% to 86% while RVC/Pig-wt/USA/RV0104/2011/G3PX was closely related to RVC/Pig-wt/USA/HF/xxxx/G3Px strain with nucleotide sequence identity of 94%. Lineages were confirmed by the VP7 gene phylogenetic tree (Fig. 3). The two strains were distantly related to one another with sequence identity of 72% and also to other porcine G-types, human and bovine strains with sequence identities ranging from 72 to 77%, an indication of greater genetic heterogeneity. Furthermore, the two strains were detected in two different swine herds suggesting high variability among wild-type RVC strains and that divergent strains are widely spread in US swine farms.
The VP4 gene is a multifunctional spike protein with hemagglutinating, neutralizing and fusion activities and antigenic properties (Chang et al., 2012; Estes et al., 1983). To date based on cutoff value of −80% nucleotide identity for the VP4 gene there are 35 P genotypes of group A RVs identitied (Matthijnssens et al., 2011); however, there is no information on formal classification of RVCs based on differences in the VP4 gene. To date only one porcine RVC (RVC/Pig-wt/USA/Cowden/1980/G1Px, accession no. M74218) has full length sequence available in GenBank. In this study we charaterized the RVC/Pig-wt/USA/RV0104/2011/G3PX and RVC/Pig-wt/USA/RV0143/2012/G6PX strains based on on their partial VP4 gene (VP8* and VP5* segments) sequences. The VP8* segment of the VP4 gene of the RVC/Pig-wt/USA/RV0143/2012 strains showed higher identity (77.4–78.4%) to human than to bovine (75.9%) and porcine (76.3%) strains, whereas the VP8* segment of the RVC/Pig-wt/USA/RV0104/2011/G3PX strain showed high identity with both porcine (84.2%) and human strains (79.4–83.3%). Although both RVC/Pig-wt/USA/RV0104/2011/G3PX and RVC/Pig-wt/USA/RV0143/2012/G6PX strains showed a closer relationship to human strains, they were distantly related to one another (71.3%). The analysis of the VP5* segment of the VP4 gene showed similar results with nucleotide sequence identity between the two strains (78%) and the known human (71–72%), bovine (78%) and porcine (76%) strains below the recommended cutoff value (80%) (data not shown). In the phylogenetic tree of the partial VP4 gene (VP8* and VP5* segments), all human RVCs clustered in one branch, an indication that they originate from a common ancestor. These results suggest that there might be interspecies transmission of RVCs; however, this is unclear and requires further studies. Other RVCs are thought to be associated with interspecies transmission events (Chang et al., 1999a; Gabbay et al., 2008; Jeong et al., 2009; Martella et al., 2007a, 2008). For example, Iturriza-Gómara et al. (2004) reported increasing seroprevalence rates to RVCs in human populations living in rural areas.
Analysis of the full length nucleotide sequence of the VP4 gene of the RVC/Pig-wt/USA/RV0143/2012 and other RVC strains revealed that the RVC/Pig-wt/USA/RV0143/2012 VP4 gene is closely related to that of bovine RVC/cow-wt/USA/Shintoku/xxxx/G2Px (74.1%) and porcine RVC/Pig-wt/USA/Cowden/1980/G1PX(74.6%) strains than to human (70–70.4%) strains. Similarly, analysis of the deduced amino acid sequence identity between the full length VP4 of the RVC/Pig-wt/USA/RV0143/2012/G6Px strain and VP4 of the known human, bovine and porcine RVC strains revealed that the RVC/Pig-wt/USA/RV0143/2012/G6Px strain shares less than 76% amino acid sequence identity with VP4 of the known human, bovine and porcine RVC strains (69.5–69.9%, 74.4% and 75.6%, respectively). Based on the cutoff values for group A RVs of −80% nucleotide identity (Matthijnssens et al., 2008b) and −89% deduced amino acid identity (Ciarlet et al., 1997; Estes, 2001) for new VP4 genotypes, the nucleotide and deduced amino acid identity between strain RVC/Pig-wt/USA/RV0143/2012/G6Px and the known RVC strains was below these cutoff values. Therefore, we propose that the RVC/Pig-wt/USA/RV0143/2012/G6Px strain represents a new RVC VP4 genotype.
In summary, this study demonstrates that porcine RVC are widespread and genetically diverse in swine herds in Ohio, USA, which poses challenges for development of diagnostic and prophylactic tools for prevention of RV infections. Therefore, detailed molecular epidemiological surveillance is needed for a better understanding of the diversity of RVCs and their role in diarrhea in swine of various ages. Information acquired from full length sequences of NSP4, VP6 and VP7 and VP4 genes in this study will be useful in the development of accurate diagnostic tools and also in basic research for understanding the functions of these genes. Additionally, the data generated in this study will be helpful in understanding the pathogenic potential and diversity of the current porcine RVCs.
Acknowledgments
We gratefully acknowledge the cooperation and assistance of the Ohio Swine farm Veterinarians & Managers in sample collection. This work was supported be the Ohio Agricultural Research and Development Center (OARDC), Ohio State University SEED Grant – #2011-077. Salaries and research support were provided by state and federal funds provided to the Ohio Agricultural Research and Development Center (OARDC), The Ohio State University. The work was done at the Food Animal Health Research Program, OARDC, Department of Veterinary Preventive Medicine, The Ohio State University, Wooster, Ohio.
Footnotes
Conflict of interest
None.
References
- Ball JM, Tian P, Zeng CQ, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–104. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- Bányai K, Jiang B, Bogdán A, Horváth B, Jakab F, Meleg E, Martella V, Magyari L, Melegh B, Szucs G. Molecular characterization of human group C rotavirus genes 6, 7 and 9. J Gen Virol. 2006;80 (Pt 12):317–322. doi: 10.1016/j.jcv.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Bohl EH, Saif LJ, Theil KW, Agnes AG, Cross RF. Porcine pararotavirus: detection, differentiation from rotavirus, and pathogenesis in gnotobiotic pigs. J Clin Microbiol. 1982;15:312–319. doi: 10.1128/jcm.15.2.312-319.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KO, Nielsen PR, Ward LA, Saif LJ. Dual infection of gnotobiotic calves with bovine strains of group A and porcine-like group C rotaviruses influences pathogenesis of the group C rotavirus. J Virol. 1999a;73:9284–9293. doi: 10.1128/jvi.73.11.9284-9293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KO, Kim YJ, Saif LJ. Comparisons of nucleotide and deduced amino acid sequences of NSP4 genes of virulent and attenuated pairs of group A and C rotaviruses. Virus Genes. 1999b;18:229–233. doi: 10.1023/a:1008068218966. [DOI] [PubMed] [Google Scholar]
- Chang K, Kim Y, Saif LJ. Rotavirus and reovirus. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, editors. Diseases of Swine. 10. Wiley-Blackwell; West Sussex, UK: 2012. pp. 621–634. [Google Scholar]
- Ciarlet M, Estes MK, Conner ME. Comparative amino acid sequence analysis of the outer capsid protein VP4 from four lapine rotavirus strains reveals identity with genotype P[14] human rotaviruses. Arch Virol. 1997;142:1059–1069. doi: 10.1007/s007050050142. [DOI] [PubMed] [Google Scholar]
- Collins PJ, Martella V, O’Shea H. Detection and characterization of group C rotaviruses in asymptomatic piglets in Ireland. J Clin Microbiol. 2008;46:2973–2979. doi: 10.1128/JCM.00809-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes MK. Rotaviruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott-Raven; Philadelphia: 2001. pp. 1747–1785. [Google Scholar]
- Estes MK, Kapikian AZ. Rotaviruses and their replication. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields virology. 5. Lippincott Williams & Wilkins; Philadelphia, PA: 2007. pp. 1917–1974. [Google Scholar]
- Estes MK, Palmer EL, Obijeski JF. Rotaviruses: a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- Gabbay YB, Borges AA, Oliveira DS, Linhares AC, Mascarenhas JDP, Barardi CRM, Simões CMO, Wang Y, Glass RI, Jiang B. Evidence for zoonotic transmission of group C rotaviruses among children in Belém, Brazil. J Med Virol. 2008;80:1666–1674. doi: 10.1002/jmv.21250. [DOI] [PubMed] [Google Scholar]
- Gunn PR, Sato F, Powell KF, Bellamy aR, Napier JR, Harding DR, Hancock WS, Siegman LJ, Both GW. Rotavirus neutralizing protein VP7: antigenic determinants investigated by sequence analysis and peptide synthesis. J Virol. 1985;54:791–797. doi: 10.1128/jvi.54.3.791-797.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriza-Gómara M, Clarke I, Desselberger U, Brown D, Thomas D, Gray J. Seroepidemiology of group C rotavirus infection in England and Wales. Eur J Epidemiol. 2004;19:589–595. doi: 10.1023/b:ejep.0000032381.36658.cb. [DOI] [PubMed] [Google Scholar]
- Jeong YJ, Park SI, Hosmillo M, Shin DJ, Chun YH, Kim HJ, Kwon HJ, Kang SY, Woo SK, et al. Detection and molecular characterization of porcine group C rotaviruses in South Korea. Vet Microbiol. 2009;138:217–224. doi: 10.1016/j.vetmic.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian AZ. A rotavirus vaccine for prevention of severe diarrhoea of infants and young children: development, utilization and withdrawal. Novartis Found Symp. 2001;238:153–171. doi: 10.1002/0470846534.ch10. (discussion 171–179) [DOI] [PubMed] [Google Scholar]
- Kim Y, Chang KOO, Straw B, Saif LJ. Characterization of group C rotaviruses associated with diarrhea outbreaks in feeder pigs. J Clin Microbiol. 1999;37:1484–1488. doi: 10.1128/jcm.37.5.1484-1488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SG, Youn SH, Oh MH, Rhee OJ, Oh S, Paik SY. Molecular characterization of two strains of porcine group C rotavirus. J Microbiol (Seoul, Korea) 2011;49:1058–1062. doi: 10.1007/s12275-011-1088-z. [DOI] [PubMed] [Google Scholar]
- Martella V, Bányai K, Lorusso E, Decaro N, Bellacicco A, Desario C, Corrente M, Greco G, Moschidou P, et al. Genetic heterogeneity in the VP7 of group C rotaviruses. Virology. 2007a;367:358–366. doi: 10.1016/j.virol.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Martella V, Bányai K, Lorusso E, Bellacicco AL, Decaro N, Camero M, Bozzo G, Moschidou P, Arista S, et al. Prevalence of group C rotaviruses in weaning and post-weaning pigs with enteritis. Vet Microbiol. 2007b;123:26–33. doi: 10.1016/j.vetmic.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Martella V, Colombrita D, Lorusso E, Draghin E, Fiorentini S, De Grazia S, Banyai K, Ciarlet M, Caruso A, Buonavoglia C. Detection of a porcine-like rotavirus in a child with enteritis in Italy. J Clin Microbiol. 2008;46:3501–3507. doi: 10.1128/JCM.00983-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J, Ciarlet M, Heiman E, Arijs I, Delbeke T, McDonald SM, Palombo EA, Iturriza-Gomara M, Maes P, et al. Full genome-based classification of rotaviruses reveals a common origin between human Wa-like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J Virol. 2008a;82:3204–3219. doi: 10.1128/JVI.02257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J, Ciarlet M, Rahman M, Attoui H, Banyai K, Estes MK, Gentsch JR, Iturriza-Gomara M, Kirkwood CD, et al. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch Virol. 2008b;153:1621–1629. doi: 10.1007/s00705-008-0155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J, Ciarlet M, McDonald SM, Attoui H, Banyai K, Brister JR, Buesa J, Esona MD, Estes MK, et al. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG) Arch Virol. 2011;156:1397–1413. doi: 10.1007/s00705-011-1006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawatari T, Taneichi A, Kawagoe T, Hosokawa M, Togashi K, Tsunemitsu H. Detection of a bovine group C rotavirus from adult cows with diarrhea and reduced milk production. J Vet Med Sci Jpn Soc Vet Sci. 2004;66:887–890. doi: 10.1292/jvms.66.887. [DOI] [PubMed] [Google Scholar]
- Medici KC, Barry AF, Alfieri AF, Alfieri AA. VP6 gene diversity in Brazilian strains of porcine group C rotavirus. Genet Mol Res. 2010;9:506–513. doi: 10.4238/vol9-1gmr715. [DOI] [PubMed] [Google Scholar]
- Morin M, Magar R, Robinson Y. Identification of atypical rota-viruses in outbreaks of preweaning and postweaning diarrhea in quebec swine herds. Can J Vet Res – Rev Can Rech Vet. 1991;55:385–389. [PMC free article] [PubMed] [Google Scholar]
- Otto P, Schulze P, Herbst W. Demonstration of group C rotaviruses in fecal samples of diarrheic dogs in Germany. Arch Virol. 1999;144:2467–2473. doi: 10.1007/s007050050659. [DOI] [PubMed] [Google Scholar]
- Rahman M, Banik S, Faruque ASG, Taniguchi K, Sack DA, Van Ranst M, Azim T. Detection and characterization of human group C rotaviruses in Bangladesh. J Clin Microbiol. 2005;43:4460–4465. doi: 10.1128/JCM.43.9.4460-4465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif LJ, Jiang B. Nongroup A rotaviruses of humans and animals. Curr Top Microbiol Immunol. 1994;185:339–371. doi: 10.1007/978-3-642-78256-5_11. [DOI] [PubMed] [Google Scholar]
- Saif LJ, Bohl EH, Theil KW, Cross RF, House JA. Rotavirus-like, calicivirus-like, and 23-nm virus-like particles associated with diarrhea in young pigs. J Clin Microbiol. 1980;12:105–111. doi: 10.1128/jcm.12.1.105-111.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;113:1530–1534. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrett LA, Saif LJ, Theil KW, Kohler EM. Physicochemical characterization of porcine pararotavirus and detection of virus and viral antibodies using cell culture immunofluorescence. J Clin Microbiol. 1987;25:268–272. doi: 10.1128/jcm.25.2.268-272.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemitsu H, Jiang B, Yamashita Y, Oseto M, Ushijima H, Saif LJ. Evidence of serologic diversity within group C rotaviruses. J Clin Microbiol. 1992;30:3009–3012. doi: 10.1128/jcm.30.11.3009-3012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]




