Abstract
Objective
To investigate the use of random ultrasensitive (US) luteinizing hormone (LH) levels to monitor children being treated with a histrelin implant for central precocious puberty (CPP).
Study design
This was a prospective, uncontrolled, observational study at a pediatric endocrinology tertiary center. Thirty-three children (26 girls; mean age 7.2 ± 2.5 years) treated with a histrelin implant for CPP were enrolled. A random US LH measurement was obtained at 6 months, and a gonadotropin-releasing hormone analog stimulation test was performed at 12 months. Clinic visits occurred at baseline and at 6-month intervals.
Results
In 59% of the patients (17 of 29), the 6-month random US LH exceeded the prepubertal range of ≤0.3 IU/L. In contrast, gonadotropin-releasing hormone analog stimulation tests revealed complete hypothalamic-pituitary-gonadal axis suppression (peak LH <4 IU/L) in all 31 patients who underwent testing. US LH levels were highly correlated with peak stimulated LH levels. The mean peak stimulated LH level was higher in patients with a pubertal random LH than in those with a prepubertal random LH (1.2 ± 0.5 IU/L vs 0.5 ± 0.1 IU/L; P < .01). No patient had clinical evidence of pubertal progression.
Conclusion
The random US LH level does not revert to a prepubertal range in more than one-half of patients with a histrelin implant and documented hypothalamic-pituitary-gonadal axis suppression. Long-term studies are needed to elucidate the optimal strategy for monitoring treatment in children with CPP.
Histrelin implants provide continuous release of the potent gonadotropin-releasing hormone analog (GnRHa) histrelin, and have been shown to cause profound hypothalamic-pituitary-gonadal (HPG) axis suppression by 1 month in children being treated for central precocious puberty (CPP).1 Currently, no consensus exists regarding routine biochemical monitoring of GnRHa therapy during treatment. However, a 2009 consensus statement on the use of GnRHa in children suggested that a random ultrasensitive (US) luteinizing hormone (LH) level above the prepubertal range may indicate inadequate suppression from GnRHa therapy.2 Whether or not this is the case has not been investigated previously.
Methods
Following Institutional Review Board approval, girls aged 2–10 years and boys aged 2–11 years who received a histrelin implant for treatment of CPP during the first year of an ongoing prospective study were recruited for this study. Inclusion criteria included biochemical confirmation of CPP via peak stimulated or random US LH level (defined as peak LH >4 mIU/L or random US LH >0.3 mIU/L), as well as an advanced bone age and clinical evidence of pubertal development (Tanner breast stage ≥II in girls and testicular volume ≥4 mL in boys). Patients diagnosed with CPP by their primary endocrinologist based on classical radiographic, clinical, and auxologic evidence but without biochemical confirmation were also eligible for inclusion.
The children were evaluated in the pediatric endocrine clinic every 6 months. At every visit, history was obtained and physical examination was performed, including determination of growth velocity and Tanner staging. At 6 months, an US LH level was drawn and sent to Esoterix (Calabasas Hills, California), with the exception of 2 patients whose insurance mandated that the LH analysis be performed at Quest Diagnostics (Auburn Hills, Michigan). LH at Quest and initially at Esoterix was measured by an immunochemiluminometric assay, which has a lower limit of detection of 0.02 IU/L. During the course of the study, Esoterix changed its methodology to an electrochemiluminescence assay owing to greater sensitivity (lower limit of detection, 0.005 IU/L). The pediatric reference ranges were verified and correlated with the previous immunochemiluminometric assay methods and remained the same with the electrochemiluminescence method. At 12 months, a GnRHa stimulation test was performed according to the following protocol: Leuprolide acetate 20 ug/kg was given subcutaneously, and samples for LH and follicle-stimulating hormone analysis were drawn at 0, 30, and 60 minutes and sent to the institution’s laboratory (Clarian Pathology Lab, Indianapolis, Indiana) where they were measured by a chemiluminescence assay, which has a lower limit of detection of 0.2 IU/L. The prepubertal reference range for these tests is <3 IU/L. Estradiol (in girls) and testosterone (in boys) were also measured at time 0 using the same methodology. The lower limit of detection is 20 pg/mL for estradiol and 10 ng/dL for testosterone. Bone age radiographs were obtained at baseline and 12 months. Patients found to have an US LH of >1 IU/L at 6 months underwent a full leuprolide stimulation test shortly thereafter owing to concerns about insufficient suppression. Suppression was defined as peak LH ≤4 IU/L, which is in line with general consensus regarding diagnostic cutoff values for CPP.2
For statistical analysis, continuous variables were summarized using descriptive statistics (eg, sample size, mean, SD, minimum, maximum). Discrete variables were summarized by frequency and percentage. Continuous variables were compared using the 2-sided t test, and categorical variables were compared using the Fisher exact test. Correlation between US LH and peak LH values were described using the Pearson coefficient.
Results
Thirty-three patients (26 girls, mean age 7.2 ± 2.5 years) were included in the study. One patient withdrew because of transfer of care within the first 6 months of participation. A random US LH level measured at 6 months was available for 29 of the remaining 32 patients. Two patients did not attend the 6-month visit, and 1 patient did not have an US LH level measured. All but 1 patient underwent GnRHa stimulation testing at or close to 12 months (mean, 12.5 ± 1.4 months). Three patients (2 boys and 1 girl) underwent GnRHa stimulation testing at the 6-month time point because of a random US LH level >1 IU/L, which was originally interpreted as suggestive of treatment failure.
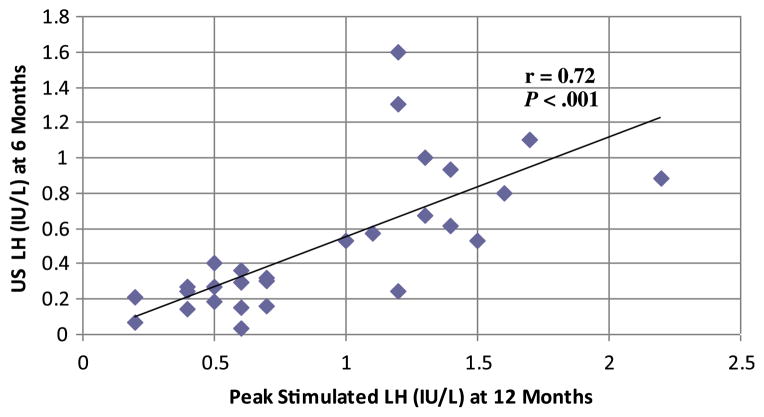
In 17 of 29 patients (59%), the 6-month random US LH was above the prepubertal reference range of ≤0.3 IU/L. In the 3 patients with a US LH >1 IU/L, the values were 1.1, 1.6, and 1.3 IU/L. Testosterone levels in the 2 boys were 17 ng/dL (59 nmol/L) and 15 ng/dL (52 nmol/L), and estradiol level in the girl was 28 pg/mL(103 pmol/L). None of these 3 patients had clinical evidence of pubertal progression, and all 3 exhibited suppression by subsequent GnRHa stimulation testing, with peak LH values of 1.9, 1.7, and 1.2 IU/L, respectively. GnRHa stimulation tests revealed complete HPG axis suppression (peak LH ≤4 IU/L) in all patients (n = 31) at 12 months. As expected, the time 0 LH levels assessed as part of the GnRHa stimulation test at 12 months were also prepubertal (defined as <3 IU/L using our institution’s methodology) in all patients (mean, 0.6 ± 0.3 IU/L; range, <0.2–1.2 IU/L). US LH levels at 6 months were highly correlated with peak stimulated LH levels at 12 months (Figure), and the mean peak stimulated LH level was higher in patients with a pubertal random LH level than in those with a prepubertal random LH level (1.2 ± 0.5 IU/L vs 0.5 ± 0.1 IU/L; P < .01). There was no statistically significant difference in clinical or radiographic characteristics at baseline or during treatment in those with a 6-month prepubertal US LH level and those with a pubertal US LH level (Table). Similarly, no differences were noted between children who had been previously treated with a GnRHa and those who were naïve to therapy. However, the 2 girls who achieved menarche before GnRHa therapy were noted to have pubertal 6-month US LH levels, and the average testicular volume was greater in boys with pubertal 6-month LH levels compared with those with prepubertal levels. Two girls had estradiol values of 23 pg/mL (84 pmol/L) and 28 pg/mL (103 pmol/L) but peak stimulated LH levels of 0.4 IU/L and 1.2 IU/L, respectively, and the remaining 22 (92%) patients had estradiol levels of <20 pg/mL (<73 pmol/L) at 12 months. Testosterone levels in boys were <10–21 ng/dL (<0.35–0.73 nmol/L) at 12 months. None of the patients exhibited growth acceleration or bone age advancement, and 90% of girls demonstrated stabilization or regression of breast Tanner stage. The 4 girls with an increased breast Tanner stage on physical examination at 12 months all had an estradiol level <20 pg/mL (<73 pmol/L) and a suppressed peak stimulated LH (1.6, 1.1, 0.5, and 1.4 IU/L).
Figure.
Correlation of US LH levels at 6 months and peak stimulated LH levels at 12 months. r, Pearson correlation coefficient.
Table.
Comparison of patients with 6-month prepubertal versus pubertal US LH at baseline and 12 months
| All patients at baseline (n = 33) | Prepubertal LH at baseline (n = 12) | Pubertal LH at baseline (n = 17) | All patients at 12 months (n = 32) | Prepubertal LH at 12 months (n = 12) | Pubertal LH at 12 months (n = 17) | |
|---|---|---|---|---|---|---|
| Sex, n (%) | M: 7 (21) F: 26 (79) |
M: 2 (17) F: 10 (83) |
M: 5 (29) F: 12 (71) |
|||
| Age at implantation, years, mean ± SD | 7.2 ± 2.5 | 7.4 ± 2.6 | 7.0 ± 2.4 | |||
| Previous GnRHa therapy, n (%) | 12 (39) | 5 (42) | 5 (29) | |||
| CNS abnormality on imaging, n (%) | 4 (13) | 3 (25) | 1 (6) | |||
| BMI, kg/m2, mean ± SD | 18.3 ± 3.8 | 19.6 ± 4.6 | 17.3 ± 3.0 | 19.7 ± 5.1 | 21.6 ± 6.2 | 18.6 ± 3.8 |
| Growth velocity, cm/year, mean ± SD | 5.8 ± 2.4 | 5.8 ± 2.4 | 5.6 ± 2.3 | |||
| Growth velocity SDS, mean ± SD | −0.41 ± 2.7 | −0.43 ± 3.2 | −0.46 ± 2.4 | |||
| BA/CA, mean ± SD | 1.31 ± 0.24 | 1.35 ± 0.19 | 1.31 ± 0.26 | 1.25 ± 0.22 | 1.22 ± 0.17 | 1.28 ± 0.27 |
| Tanner breast staging, n | II: 1/26 III: 19/26 IV: 6/26 |
III: 9/10 IV: 1/10 |
II: 1/12 III: 7/12 IV: 4/12 |
I: 1/25 II: 1/25 III: 19/25 IV: 1/25 V: 3/25 |
II: 1/10 III: 8/10 V: 1/10 |
I: 1/12 III: 8/12 IV: 1/12 V: 2/12 |
| Testicular volume, mL | 4–15 (n = 7) | 4, 10 (n = 2) | 5, 10, 12, 12, 15 (n = 5) | 5–10 (n = 7) | 5, 7 (n = 2) | 5, 8, 8, 9, 9 (n = 5) |
| Menarche | 2 (8) | 0 (0) | 2 (16) | 0 (0) | 0 (0) | 0 (0) |
BA/CA, bone age to chronological age ratio; BMI, body mass index; CNS, central nervous system.
P value not significant for all comparisons between patients with prepubertal and pubertal US LH values.
Discussion
Although the safety and efficacy of GnRHa treatment for CPP is undisputed,3 the optimal strategy for biochemical monitoring of therapy has not been established.4–7 Gonadotropin-releasing hormone stimulation testing has long been considered the gold standard for evaluating the degree of activation of the HPG axis, and was also used historically to document suppression once treatment was underway. However, the lack of availability of gonadotropin-releasing hormone in the US has led to the use of GnRHa stimulation testing as an alternative approach.8 The US assay for measuring random LH provides a less invasive and less time-intensive option for diagnosing CPP that has been adopted by many centers.9 Despite its ease of use for diagnosis, the utility of random US LH for routine monitoring during GnRHa treatment of CPP has not been rigorously investigated. Regardless, the 2009 consensus statement on the use of GnRHa in children states that “if elevated, random LH levels obtained by using an US assay may indicate lack of suppression.”2 This likely reflects an assumption that the degree of HPG axis suppression during treatment can be extrapolated from random US LH values in the same way as in the untreated state. Anecdotally, clinicians using monthly depot leuprolide injections for treatment of CPP have frequently increased the dose in response to a random US LH in the pubertal range (personal communication), significantly increasing the costs associated with this already expensive therapy. Remarkably, our findings indicate that US LH remains elevated in more than one-half of patients treated with a histrelin implant despite clinical and biochemical evidence of pubertal suppression. To our knowledge, such results have not been reported previously.
Our study has several limitations. Because the study was not specifically designed to address the utility of US LH, we did not obtain baseline or 12-month US LH levels. Rather, the unexpected discovery that US LH does not revert to the prepubertal range was made during the course of an ongoing prospective study in which random US LH was assumed to be a valuable indicator of HPG axis suppression. In addition, because random US LH levels were not obtained at the same time as the GnRHa stimulation testing in the majority of patients, we cannot say with certainty that the observed correlation between random US LH levels and peak LH levels would be seen if 12-month US LH levels were used. However, the time 0 LH levels obtained as part of the GnRHa stimulation test at 12 months were all prepubertal, and the 3 subjects who underwent stimulation tests at 6 months owing to concerns about lack of suppression all had peak values well below the cutoff for suppression (≤4 IU/L). Another flaw is that the LH levels with stimulation testing were obtained using a different assay in a different laboratory than the random US LH levels. Despite these limitations, however, all patients clearly demonstrated biochemical suppression by GnRHa stimulation testing and did not exhibit clinical progression of puberty even with US LH values up to 1.6 IU/L.
Random US LH remained elevated in more than one-half of patients treated for CPP with a histrelin implant. Although there was excellent correlation between random US LH and peak stimulated LH values, our data do not allow for extrapolation to determine a US LH value that indicates lack of pubertal suppression. Until such a value is identified, the finding of a pubertal US LH level in a patient with a good clinical response should not be interpreted as indicative of treatment failure. Whether US LH levels should be measured at all as part of routine clinical care in patients being treated for CPP is open to debate, given that growth velocity, pubertal progression, and bone age advancement are likely the most important indicators of therapeutic efficacy. Anecdotally, an on-treatment pubertal random US LH level may simply reflect that the child was farther along in pubertal development when GnRHa therapy was initiated, as suggested by the pubertal LH values in the girls who were postmenarchal and the boys with larger testicular volumes. Further study is needed to determine the optimal approach to monitoring treatment of CPP with histrelin implants and other modes of delivering GnRHa therapy.
Glossary
- CPP
Central precocious puberty
- GnRHa
Gonadotropin-releasing hormone analog
- HPG
Hypothalamic-pituitary-gonadal
- LH
Luteinizing hormone
- US
Ultrasensitive
Footnotes
E.E. participates in clinical trials sponsored by Endo Pharmaceuticals and Abbott Laboratories. K.L. declares no conflicts of interest.
Portions of this study were presented as a poster at the Pediatric Academic Societies Meeting, Vancouver, Canada, May 1–4 2010.
References
- 1.Eugster EA, Clarke W, Kletter GB, Lee PA, Neely EK, Reiter EO, et al. Efficacy and safety of histrelin subdermal implant in children with central precocious puberty: a multicenter trial. J Clin Endocrinol Metab. 2007;92:1697–704. doi: 10.1210/jc.2006-2479. [DOI] [PubMed] [Google Scholar]
- 2.Carel J, Eugster EA, Rogol A, Ghizzoni L, Palmert MR on behalf of the members of the ESPE-LWPES GnRH Analogs Consensus Conference Group. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. 2009;123:e752–62. doi: 10.1542/peds.2008-1783. [DOI] [PubMed] [Google Scholar]
- 3.Neely EK, Lee PA, Bloch CA, Larsen L, Yang D, Mattia-Goldberg C, et al. Leuprolide acetate 1-month depot for central precocious puberty: hormonal suppression and recovery. Int J Pediatr Endocrinol. 2010;2010:1–9. doi: 10.1155/2010/398639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawson ML, Cohen N. A single-sample subcutaneous luteinizing hormone (LH)-releasing hormone (LHRH) stimulation test for monitoring LH suppression in children with central precocious puberty receiving LHRH agonists. J Clin Endocrinol Metab. 1999;84:4536–40. doi: 10.1210/jcem.84.12.6181. [DOI] [PubMed] [Google Scholar]
- 5.Acharya SV, Gopal RA, George J, Bandgar TR, Menon PS, Shah NS. Utility of single luteinizing hormone determination 3 h after depot leuprolide in monitoring therapy of gonadotropin-dependent precocious puberty. Pituitary. 2009;12:335–8. doi: 10.1007/s11102-009-0184-0. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia S, Neely EK, Wilson DM. Serum luteinizing hormone rises within minutes after depot leuprolide injection: implications for monitoring therapy. Pediatrics. 2002;109:E30. doi: 10.1542/peds.109.2.e30. [DOI] [PubMed] [Google Scholar]
- 7.Salerno M, Di Maio S, Gasparini N, Mariano A, Macchia V, Tenore A. Central precocious puberty: a single blood sample after gonadotropin-releasing hormone agonist administration in monitoring treatment. Horm Res. 1998;50:205–11. doi: 10.1159/000023275. [DOI] [PubMed] [Google Scholar]
- 8.Garibaldi LR, Aceto T, Jr, Weber C, Pang S. The relationship between luteinizing hormone and estradiol secretion in female precocious puberty: evaluation by sensitive gonadotropin assays and the leuprolide stimulation test. J Clin Endocrinol Metab. 1993;76:851–6. doi: 10.1210/jcem.76.4.8473395. [DOI] [PubMed] [Google Scholar]
- 9.Houk CP, Kunselman AR, Lee PA. Adequacy of a single unstimulated lutienizing hormone level to diagnose central precocious puberty in girls. Pediatrics. 2009;123:e1059–63. doi: 10.1542/peds.2008-1180. [DOI] [PubMed] [Google Scholar]