Abstract
The aims of this study were to evaluate the evolution of the neurogenic bladder after spinal cord contusion and to correlate changes in bladder function with locomotor function and levels of neurotrophic factors. The MASCIS impactor was used to cause a mild contusion injury of the lower thoracic spinal cord of Sprague-Dawley rats. Rats were divided into four groups according to the length of time from injury to sacrifice, at 4, 14, 28, and 56 days after injury. Gait analysis was performed each week, and urodynamic study was performed just before sacrifice. Basso, Beattie, and Bresnahan (BBB) and coupling scores showed gradual recovery, as did the urinary voiding pattern and bladder volume; some parameters of micturition reached normal ranges. Brain-derived neurotrophic factor (BDNF) levels in the spinal cord, as detected by enzyme-linked immunosorbent assay, decreased with time, whereas neurotrophin-3 (NT-3) levels remained unchanged. The micturition pattern, bladder volume, and locomotor function continued to recover during the time of observation; BDNF levels in the spinal cord and bladder were inversely correlated with BBB scores and the restoration of bladder volume. We conclude that urodynamic changes in the bladder correlate with locomotion recovery but not with the levels of BDNF or NT-3 after modified mild contusion injury in rats.
Key words: bladder volume, brain-derived neurotrophic factor, locomotor function, neurogenic bladder, neurotrophin-3, spinal cord injury, urodynamic study
Introduction
Following spinal cord injury, many patients suffer from autonomic dysfunctions such as bladder and bowel problems, as well as from somatic dysfunctions due to motor and sensory impairments. Neurogenic bladder can increase the risk of urinary tract infection, a major cause of morbidity and mortality (Garcia Leoni and Esclarin De Ruz, 2003), and may decrease quality of life (Oh et al., 2005). Because urinary function changes greatly over time after injury, accurate evaluation not only of neurological level, but also of bladder dysfunction is essential for clinical management.
Previous studies of the neurogenic bladder after complete spinal cord transection in rats found no contraction of the detrusor within the first week, irregular contraction with low pressure at 1 week, and high amplitude and long duration following low amplitude at 4 weeks (Takahara et al., 2007). Two weeks after moderate contusion of the rat spinal cord, bladders showed detrusor hyperreflexia (Smith et al., 2002); rats with a mild contusion injury showed complete recovery of coordination between the detrusor and external urethral sphincter. However, in more severely injured rats, detrusor-sphincter-dyssynergia continued at 8 weeks (Pikov and Wrathall, 2001). Few experiments have investigated changes in the neurogenic bladder over time.
The relationship between somatic and autonomic dysfunctions such as neurogenic bladder has been investigated in humans (Weiss et al., 1996; Wyndaele, 1997). The usefulness of somatic sensation as a predictor of bladder recovery and the correlation between somatic sensation and the status of the bladder, however, remain to be clarified (Watanabe et al., 1998; Schurch et al., 2003).
Levels of endogenous neurotrophic factors such as brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and nerve growth factor (NGF) change in rats after spinal cord injury (Vizzard, 2000; Nakamura and Bregman, 2001; Zvarova et al., 2004; Li et al., 2007). These changes are considered to be important because of their impact on axonal regeneration and recovery, but the results of the numerous studies have been inconsistent. For example, BDNF and NGF expression increased in injured spinal cords at 6 weeks after complete spinal cord transection (Zvarova et al., 2004), and the numbers of BDNF- and NT-3-immunostained cells reached peaks at 7 and 14 days, respectively (Li et al., 2007). However, other investigators reported that, within 3 days after ischemic injury to the rat spinal cord, the level of NT-3 protein remained unchanged (Tokumine et al., 2003), and that the expression of BDNF and NT-3 mRNA decreased gradually over 2 weeks after spinal cord hemisection in neonate rats, but did not change after hemisection in adult rats (Nakamura and Bregman, 2001). Vizzard found that levels of NT-3, NGF, and BDNF mRNA in the bladder increased in rats with acute and chronic spinal cord injuries (Vizzard, 2000). Levels of BDNF and NT-3 in the spinal cord also varied according to the distance from the injury site or the type of injury (Uchida et al., 2003) and could be influenced by exercise or electrical muscle stimulation (Gomez-Pinilla et al., 2007; Hutchinson et al., 2004).
Administration of exogenous neurotrophic factors has also been reported to have important effects after experimental spinal cord injury in rats. For example, NT-3 promoted axonal sprouting and reduced glial scar formation (Chen et al., 2006; Taylor et al., 2006) and BDNF increased early functional recovery, axonal growth, proliferation of Schwann cells, and formation of peripheral myelin (Bregman et al., 1997; Namiki et al., 2000). Administration of BDNF and NT-3 also promoted the recovery of bladder function (Mitsui et al., 2005).
Before exogenous neurotrophic factors such as BDNF and NT-3 can be used therapeutically, changes in levels of endogenous BDNF and NT-3 in the spinal cord and bladder over time must be accurately determined. No study has examined the relationship between changes in neurotrophic factors and spontaneous recovery of bladder function after spinal cord injury in rats.
The aims of our study were to evaluate changes in bladder function and in levels of neurotrophic factors over time and to correlate changes in levels of NT-3 and BDNF with changes in urodynamic and locomotor function.
Materials and Methods
Animals
Adult female Sprague-Dawley rats (12 weeks old, 230–250 g) were used in this experiment. All rats were housed individually in a thermo-hygrostat (23°C, 50% humidity) with food and water available ad libitum. All procedures were approved by the Institutional Review Board of Dankook University College of Medicine.
Surgical procedures
Thirty rats were given mild contusion injuries to the thoracic spinal cord, and five rats received a laminectomy, acting as sham-operated controls. Animals were anesthetized with isoflurane (Forane, Choongwae Pharma, Korea). The skin, subcutaneous, and muscle layers were incised, and a laminectomy was performed to expose the T9-10 level of the spinal cord without damaging or compressing the dura mater.
Using a Multicenter Animal Spinal Cord Injury Study (MASCIS) impactor (Rutgers University, New Brunswick, NJ), a 10-g rod was dropped from a vertical distance of 12.5 mm onto the T9 level of the exposed spinal cords, and allowed to rest for 5 s before it was lifted. This caused a mild contusion injury that was modified from Mitsui's method (Mitsui et al., 2005). This model results in more motor deficits than found in a standard mild contusion model. After contusion, muscle, subcutaneous, and skin were closed in layers.
All animals received intramuscular injection of 40 mg/kg cefotiam hydrochloride (Fontiam, Hanmi Pharma, Korea) for 3 days and intraperitoneal injection of normal saline (3 mL) just after surgery. Animals also received oral administration of 10 mg/kg acetaminophen syrup (Tylenol, Janssen Pharmaceutica, Cape Town, South Africa) for 3 days in order to reduce neuropathic and postoperative pain. Bladder expression was performed two times per day, and was continued daily until the amount of expressed urine was less than 0.5 mL/day.
Experimental rats were divided into four groups according to the amount of time since the operation: POD4 (postoperative day), very acute stage (4 days after operation, n = 6); POD14, acute stage (n = 7); POD28, subacute stage (n = 7); and POD56, chronic stage (n = 7). Three rats recovered a normal gait pattern before 14 postoperative days and were excluded from further analysis. Sham-operated rats (n = 5) were used as controls.
Urodynamic study
For the evaluation of bladder function, urodynamic studies (UDS) were performed just before sacrifice. UDS were not performed at multiple time points in individual animals because these procedures are invasive and have the potential to damage the bladder. Rats were anesthetized with isoflurane and the bladder was exposed. A double lumen polyethylene catheter (PE-160 and PE-50, Clay-Adams, Parsippany, NJ) was inserted into the bladder dome and fixed with sutures. Most, if not all, residual urine in the bladder was excreted during the insertion of the double-lumen catheter and little, if any, remained when the urodynamic study began. One lumen (PE50) was connected to the pressure transducer and amplified and recorded by polygraph (Grass polygraph model 7E, Quincy, MA), and another lumen (PE160) was connected to a syringe filled with normal saline and loaded in a Harvard infusion pump. The room temperature normal saline was initially infused into the bladder at a rate of 10 mL/h; the speed was modified to 5 mL/h after the first void and then stopped for 30 min to stabilize micturition cycles. We measured the detrusor pressure just after saline filling, and recorded the timing and numbers of drops of urine voided to reveal the coordination between the detrusor and external urethral sphincters. During the recording procedure, the anesthetic vapor level was gradually reduced to 0.5% in all controls and experimental groups in order to minimize the effect of anesthesia on micturition.
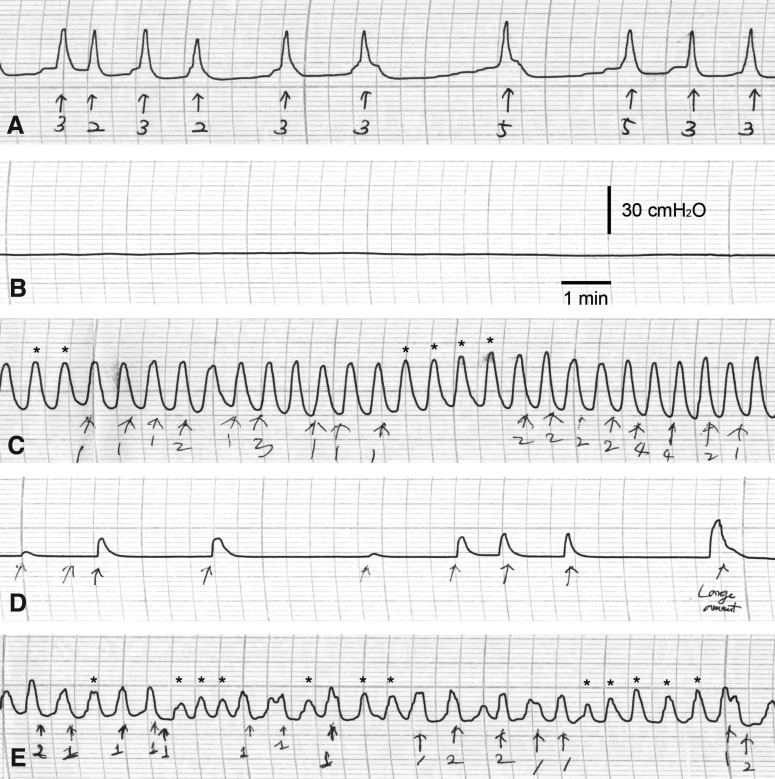
Throughout the urodynamic study, we recorded the maximal micturition pressure (cmH2O) and frequency (time/minute), gross voiding patterns, and regularity of micturition frequency and pressure (Fig. 1). Gross voiding patterns were described as normal, flaccid, or hyperreflexic according to the micturition frequency. A flaccid pattern was defined as no visible detrusor contraction (Fig. 1B), and a hyperreflexic pattern was defined as a higher frequency of detrusor contraction than the mean of the controls plus two standard deviations (Fig. 1C). An irregular micturition pattern was defined as a pattern of frequency or maximal pressure that was too variable to count or calculate (Fig. 1D and E).
FIG. 1.
Representative urodynamic studies for normal (A), flaccid (B), and hyperreflexic (C) bladders. (D and E) Irregular patterns of voiding frequency and pressure. (C and E) Asterisks indicate increased bladder pressure without micturition. Arrows indicate micturition events. Numbers below the arrows represent number of voiding drops.
Calculation of bladder volume
Just before UDS, the diameter of the exposed bladder dome was measured as an indirect indication of bladder hypertrophy. The bladder volume was calculated as an imaginary sphere as follows:
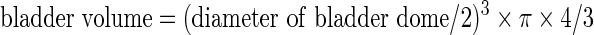 |
We did not use the bladder weight to assess bladder hypertrophy or capacity (Vizzard, 2000; Pikov and Wrathall, 2001; Mitsui et al., 2005) because it could vary depending on the amount of residual urine, the water content of the bladder, or the point at which the bladder was sectioned.
Evaluation of locomotor function
The open-field locomotor scale, known as the Basso, Beattie, and Bresnahan (BBB) scale, was used for the evaluation of hindlimb locomotor recovery (Basso et al., 1995). The BBB score for no hindlimb movement is 0, and for normal hindlimb movement, 21. In the case of rats with incomplete coordination of the forelimb and hindlimb (BBB score between 10 and 14), we recorded the open-field activity with a digital camcorder for the calculation of forelimb/hindlimb coupling score to add more detailed information about gait coordination (Fouad et al., 2005). The coupling score was calculated as the percent of the number of corrected couplings divided by the total number of couplings in the context of a continuous gait for at least six steps from five video captures per rat. A coupling score below 10 was regarded as 0% for the BBB score, and a coupling score above 14 was regarded as a BBB score of 100%.
The BBB and coupling scores of each group of animals were determined every 7 days until sacrifice in a transparent, cylindrical-shaped (90 cm diameter, 15 cm high) acrylic box with a smooth floor. All locomotor tests were recorded for at least 4 min with a digital camcorder and were interpreted by two observers.
Histology
Cresyl violet staining was used to detect morphological changes in neurons after spinal cord injury. One rat in the control group, one rat in each of the POD14 and 56 experimental groups, and two rats in each of the POD4 and POD28 experimental groups were studied immediately after urodynamic study. Control and injured spinal cords were fixed with 4% paraformaldehyde solution, and were cryosectioned at a thickness of 20 μm. After staining for 5 min in 0.1% cresyl violet solution (C5042, Sigma, St. Louis, MO) containing glacial acetic acid, spinal cord tissues were differentiated in ethyl alcohol series (70–95%) and checked under a light microscope. Finally, cresyl violet-stained tissues were cleared in xylene and mounted with resinous medium.
Enzyme-linked immunosorbent assay (ELISA)
Changes in BDNF and NT-3 levels were measured in transverse segments of spinal cord and bladder tissue using BDNF- and NT-3-specific ELISA. After the urodynamic study had been completed, control (n = 4) and spinal cord-injured rats (POD4, n = 4; POD14, n = 6; POD28, n = 4; POD56, n = 6) were sacrificed with an overdose of anesthesia. The bladder was removed and immediately frozen with liquid nitrogen. An equivalent region of spinal cord and bladder was dissected from each sham-operated control rat. Frozen tissues were stored in a deep freezer (−70°C) until just before the ELISA experiment.
The spinal cord segments and bladder tissue were lysed by sonication in lysis buffer (50 μL/mg tissue) containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride) and a protease inhibitor cocktail (P8340, Sigma). Protein was quantified with Bradford solution (500-0006, Bio-Rad, Hercules, CA); 20 pg of total protein was loaded per well. ELISA procedures were performed according to the instructions of the manufacturer (ChemiKine Sandwich ELISA Kit, Chemicon International, Temecula, CA), and the results were obtained using a multiwall plate reader (model 680, Bio-Rad) at 450 nm.
Statistical analysis
SPSS 15.0 (SPSS Inc., Chicago, IL) was used for data analysis. To compare the results of the urodynamic studies from controls and each experimental group, one-way analysis of variance (ANOVA) with a Bonferroni post-hoc test was performed for the numeric data, and Fisher's exact test was performed for the nominal data. The diameter of the bladder dome, calculated bladder volume, BDNF, and NT-3 levels were also compared using one-way ANOVA with a Bonferroni post-hoc test. To delineate the changes of bladder volume and levels of neurotrophic factors over time after spinal cord injury, linear regression analysis was used with the time after injury as an independent factor after confirmation of a linear tendency by curve estimation. Nonlinear regression analysis was used if the curve estimation did not fit the linear model. Repeated-measures ANOVA was used to confirm any difference indicated by gait analysis (BBB scores and coupling scores) among experimental groups (POD14, POD28, and POD56 groups). Correlations among bladder and functional evaluations and ELISA results were evaluated using Pearson's partial correlation analysis with the control of experimental groups for numeric data, one-way ANOVA for trinomial data, and independent t-tests for binomial data.
Results
Urodynamic study and bladder volume
Controls were tested at 4 days after operation (POD4). The micturition frequency of the control group was 0.60 ±0.21 times/min, and the maximal pressure of the control group was 26.86 ± 5.81 cmH2O. In the experimental group, all rats showed a flaccid pattern of micturition at POD4. A hyperreflexic pattern appeared at POD14, peaked at POD28, and returned to normal at POD56 (Table 1). The micturition frequency and maximal pressure did not statistically significantly differ from those of the control group at any time, except for POD4.
Table 1.
Urodynamic Parameters of Control and Spinal Cord Injured Rats
| Control (n = 5) | POD4 (n = 6) | POD14 (n = 6) | POD28 (n = 6) | POD56 (n = 7) | P value | Post-hoc | |
|---|---|---|---|---|---|---|---|
| Micturition frequency (time/min) | 0.60 ± 0.21 | 0 | 0.48 ± 0.68 | 1.13 ± 0.61 | 1.02 ± 0.47 | <0.01* | POD4<>POD28, POD56 |
| Maximal pressure (cmH2O) | 26.86 ± 5.81 | 0 | 12.75 ± 17.68 | 22.75 ± 12.74 | 27.27 ± 7.88 | <0.01* | POD4<>control, POD28, POD56 |
| Pattern of regularity (%)a | 60 | – | 100 | 80 | 57 | 0.06** | ns |
| Gross voiding pattern (%)a | <0.01** | ||||||
| Flaccid | 0 | 100 | 50 | 17 | 0 | ||
| Hyperreflexia | 0 | 0 | 50 | 83 | 29 | ||
| Normal | 100 | 0 | 0 | 0 | 71 |
By one-way ANOVA with the Bonferroni post-hoc test; **By Fisher's exact test.
No. of detectable cases (%).
ns, not significant.
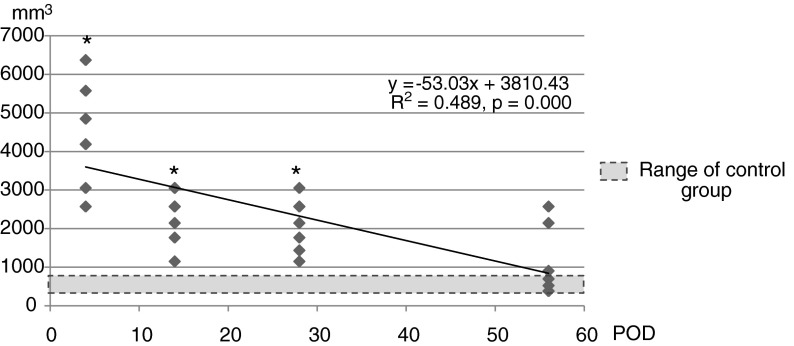
The bladder volume of controls was 564.54 ± 134.01 mm3 at 4 days after sham operation. The bladder volume was highest at POD4 (4434.97 ± 1459.89 mm3), and then decreased (Fig. 2). At POD56, it was 1086.54 ± 896.55 mm3, and was not different from that of the control group (564.54 ± 134.01 mm3).
FIG. 2.
Changes in bladder volume. The large bladder volume detected at 4 days after spinal cord injury gradually decreased throughout the 56 days after injury and ended within the normal range. Groups with asterisks were different from the control group (p < 0.05) by one-way ANOVA with the Bonferroni post-hoc test.
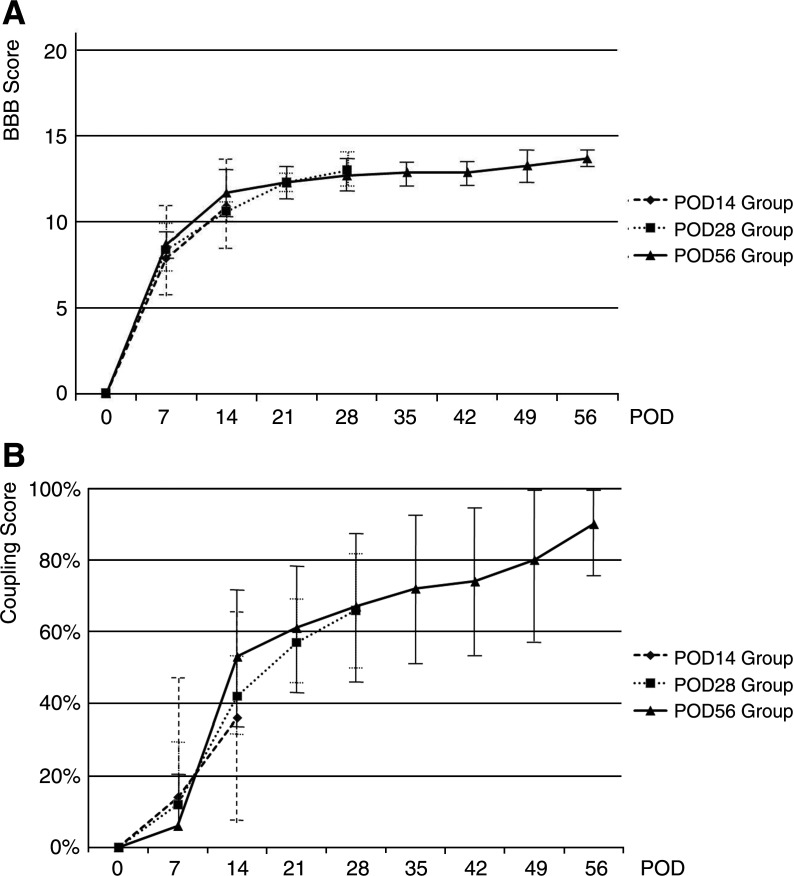
Locomotion: BBB and coupling scores
Figure 3 shows BBB and forelimb and hindlimb coupling scores of groups sacrificed at POD14, POD28, and POD56. At POD21, BBB scores reached a plateau, which indicated incomplete recovery, whereas the coupling scores of all of the experimental groups increased gradually throughout the 56 days following spinal cord injury. The coupling score showed a greater standard deviation than the BBB score because each group included rats scored below 10 or above 14, with a BBB score that equaled 0 and 100% of the coupling score, respectively.
FIG. 3.
Locomotor recovery. There were no differences statistically by repeated-measures ANOVA among the POD14, POD28, and POD56 groups during the 14 days after spinal cord injury or between the POD28 and POD56 groups during the 28 days after spinal cord injury. (A) The BBB score showed a gradual increase until 21 days after spinal cord injury. (B) The coupling score increased throughout the 56 days after injury.
Histology and neurotrophic factors
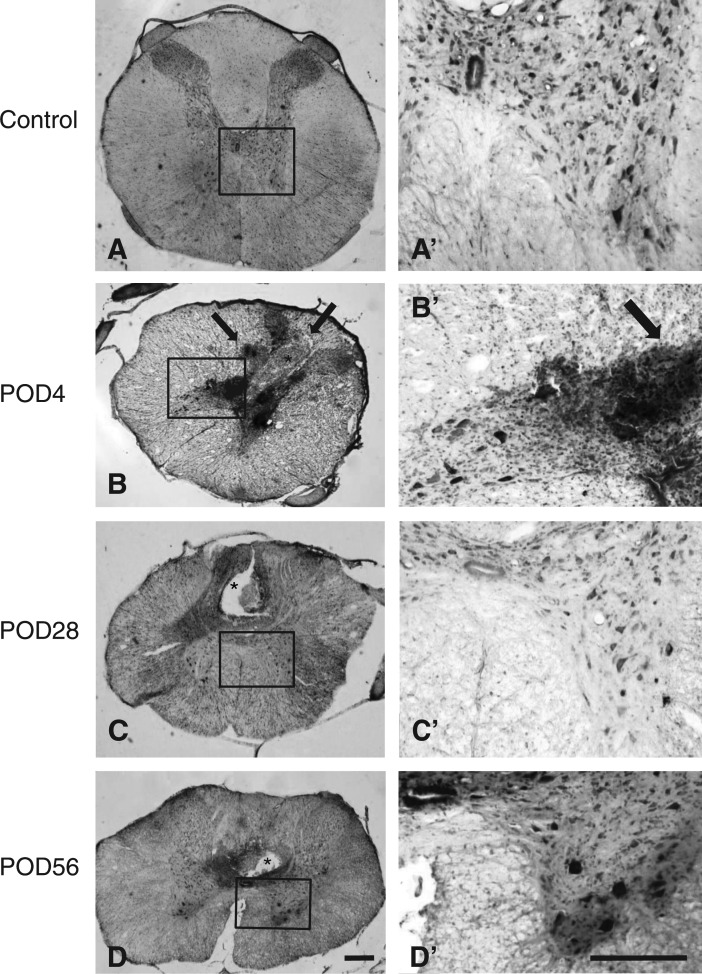
Figure 4 shows spinal cord after sham surgery (A, A’), and from the lesion center 4 days (B, B’), 28 days (C, C’), and 56 days (D, D’) after injury. Damage primarily affects the dorsal horn and includes dorsal cystic cavities (asterisks) and hemorrhage (arrows) (Fig. 4B–D). The numerous neurons that remain in the ventral horn (Fig. 4B’–D’) and the normal appearance of the white matter indicate that the ventral region of the cord was largely spared.
FIG. 4.
Representative photomicrographs show spinal cord from rats with sham surgery (A, A’) and from the lesion center 4 days (B, B’), 28 days (C, C’), and 56 days (D, D’) after mild contusion injury. Coronal sections were stained with cresyl violet. The lesion epicenter is characterized by dorsal cystic cavities (asterisks) and hemorrhage (arrows) (B–D). The ventral region of the cord is largely spared, as indicated by the numerous neurons labeled in the ventral horn (B’–D’) and by the normal appearance of the white matter. Scale bars, 250 μm.
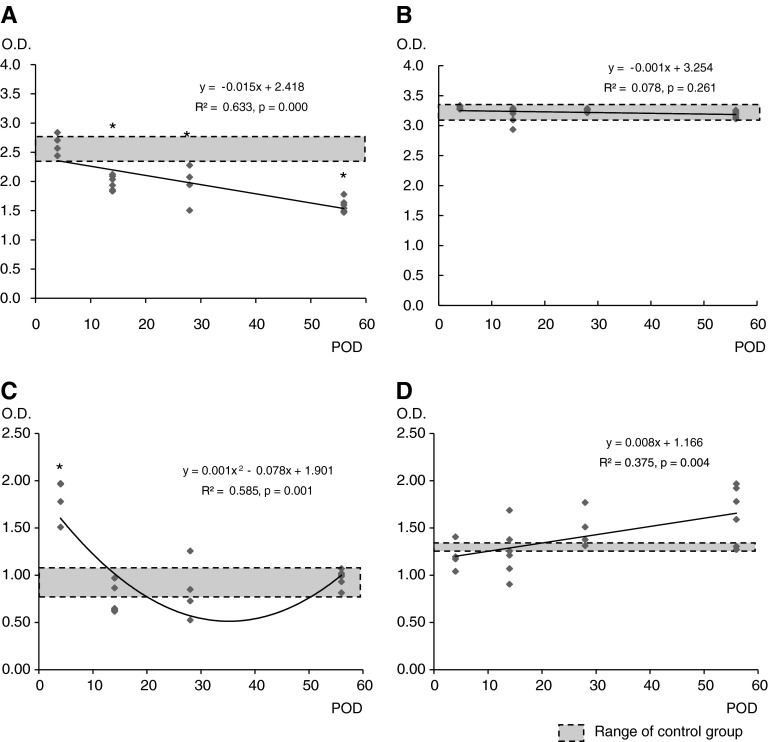
BDNF and NT-3 levels in the spinal cord and bladder of the control and experimental groups are shown in Figure 5. BDNF and NT-3 levels obtained from the spinal cords of the control group at POD4 after sham operation were 2.62 ± 0.25 and 3.34 ± 0.10, respectively, and the BDNF and NT-3 levels in the bladders of the control group were 0.91 ± 0.12 and 1.29 ± 0.04, respectively. BDNF and NT-3 levels in the spinal cord and bladder of the control groups at POD14 were not different from those of the control groups at POD4 (data not shown).
FIG. 5.
BDNF and NT-3 levels by ELISA in the spinal cord and bladder after spinal cord injury. (A) BDNF levels in spinal cord decreased gradually to below the normal range 14 days after spinal cord injury. (B) NT-3 levels in the spinal cord did not change. (C) BDNF levels in the bladder increased at 4 days after spinal cord injury and then decreased to the normal range after 14 days. (D) NT-3 levels in the bladder did not change. Linear regression analysis was performed (A, B, and D) and nonlinear regression analysis with the quadratic model was applied (C). Groups with asterisks were different from the control group (p < 0.05) by one-way ANOVA with the Bonferroni post-hoc test.
Curve estimation analysis was performed before regression analysis to delineate the changes of BDNF and NT-3 levels over time. The optimal model of the BDNF level in the spinal cord and NT-3 levels in the spinal cord and bladder was linear, whereas that of the BDNF level in the bladder was quadratic. However, NT-3 levels in the spinal cord and bladder did not differ at any period, as determined by one-way ANOVA. The BDNF level in the spinal cord decreased gradually to levels far below normal, but the BDNF level in the bladder peaked at POD4 and then returned to normal. Therefore, by POD56, only the BDNF level in the spinal cord was outside the normal range of the control group.
Correlation among bladder function, locomotion, and levels of neurotrophic factors
All numeric parameters of the urodynamic study, bladder volume, locomotor scores, and BDNF, and NT-3 levels in the spinal cord and bladder at POD4, POD14, POD28, and POD56 were used for Pearson's partial correlation analysis. Experimental groups were used as control variables to exclude the period after spinal cord injury when analyzing the partial correlation. Table 2 shows that the BDNF levels in the spinal cord and bladder after spinal cord injury correlated negatively with the BBB score and positively with bladder volume (p < 0.05 and p < 0.01, respectively).
Table 2.
Correlations Among Bladder Function, Locomotor Function, and ELISA
| BBB score | Coupling score | Micturition frequency | Maximal pressure | Bladder volume | BDNF in SC | NT-3 in SC | BDNF in bladder | NT-3 in bladder | |
|---|---|---|---|---|---|---|---|---|---|
| BBB score | – | 0.680** | 0.240 | 0.270 | −0.613** | −0.577* | −0.431 | −0.890** | −0.132 |
| Coupling score | 0.688** | – | −0.183 | 0.086 | −0.398 | −0.106 | −0.306 | −0.426 | −0.396 |
| Micturition frequency | 0.240 | −0.183 | – | 0.412 | 0.123 | −0.031 | 0.232 | −0.340 | 0.078 |
| Maximal pressure | 0.270 | 0.086 | 0.412 | – | −0.159 | −0.105 | 0.175 | −0.269 | −0.142 |
| Bladder volume | −0.613** | −0.398 | 0.123 | −0.159 | – | 0.543* | 0.378 | 0.648** | 0.001 |
| BDNF in SC | −0.577* | −0.106 | −0.031 | −0.105 | 0.543* | – | 0.485* | 0.672** | 0.020 |
| NT-3 in SC | −0.431 | −0.306 | 0.232 | 0.175 | 0.378 | 0.485* | – | 0.243 | −0.106 |
| BDNF in bladder | −0.890** | −0.426 | −0.340 | −0.269 | 0.648** | 0.672** | 0.243 | – | 0.097 |
| NT-3 in bladder | −0.132 | −0.396 | 0.078 | −0.142 | 0.001 | 0.020 | −0.106 | 0.097 | – |
p < 0.05; **p < 0.01 by Pearson's partial correlation analysis with control of experimental groups.
SC, spinal cord.
The bladder volume, locomotor function, and BDNF/NT3 levels were also compared to the voiding patterns revealed by the urodynamic studies (i.e., flaccid, hyperreflexic, normal voiding patterns; Table 3). Whereas bladder volume and coupling score differed among rats with different voiding patterns (p < 0.05), NT3 levels did not. BBB score and BDNF levels in spinal cord of rats with a flaccid voiding pattern were different from those whose voiding pattern was normal (p < 0.05). BDNF levels in the bladder were also different in the animals with flaccid and hyperreflexic voiding patterns (p < 0.05).
Table 3.
Comparison of Bladder Volume, Locomotor Function, and ELISA Results Among the Different Voiding Patterns
| Flaccid (n = 5) | Hyperreflexia (n = 10) | Normal (n = 10) | P value | Post-hoc | |
|---|---|---|---|---|---|
| BBB score | 4.6 ± 6.26 | 12.4 ± 1.71 | 14.0 | 0.000* | Flaccid<>normal, hyperreflexia |
| Coupling score | 17.36 ± 33.15 | 57.31 ± 27.29 | 100 | 0.000* | Flaccid<>hyperreflexia<>normal |
| Bladder volume (mm3) | 3547.75 ± 1632.33 | 2204.04 ± 633.81 | 577.74 ± 224.14 | 0.000* | Flaccid<>hyperreflexia<>normal |
| BDNF in SC | 2.32 ± 0.41 | 1.95 ± 0.26 | 1.60 ± 0.13 | 0.001* | Flaccid<>normal, hyperreflexia |
| NT-3 in SC | 3.19 ± 0.04 | 3.21 ± 0.15 | 3.18 ± 0.04 | 0.482 | ns |
| BDNF in bladder | 1.35 ± 0.60 | 0.79 ± 0.19 | 0.93 ± 0.83 | 0.039* | Flaccid<>hyperreflexia |
| NT-3 in bladder | 1.30 ± 0.21 | 1.42 ± 0.36 | 1.53 ± 0.32 | 0.474 | ns |
p < 0.05 by one-way ANOVA with the Bonferroni post-hoc test.
SC, spinal cord; ns, not significant.
Discussion
To our knowledge the present study is the first attempt to correlate changes in bladder function with locomotor function and levels of neurotrophic factors in contused spinal cords. Our results indicate that the somatic and autonomic nervous systems recovered together, and are consistent with the findings of a previous study that evaluated bladder function with a noninvasive ultrasonographic method instead of UDS (Keirstead et al., 2005). However we found neurotrophic factors did not change in correlation with the recovery of bladder or locomotor function. The spontaneous recovery of locomotor function, as indicated by the BBB score, progressed for 8 weeks after mild contusion injury. The forelimb/hindlimb coupling score also reflected the locomotor recovery well, and correlated well with the recovery of the bladder. Notably, however, the standard deviation of the coupling score was higher than that of the BBB score because scores below 10 or above 14 of the BBB score were recorded as 0 and 100%, respectively, and, unlike the BBB score, the coupling score was not correlated with bladder volume or BDNF levels in the spinal cord and bladder.
We studied a mild contusion injury because contusion is the most common type of spinal cord trauma in humans, and because spontaneous recovery of gait and bladder function is greater with this form of spinal cord trauma than after moderate or severe contusion (Basso et al., 1996; Pikov and Wrathall, 2001). The recovery mechanisms after mild injury are likely to differ from those that act after more severe types of spinal cord injury, but we do not believe that these mechanisms are relevant only to recovery from spinal shock. For example, spinal shock lasts for about 3 weeks even after complete transection spinal cord injury (i.e., the most severe spinal cord injury; Shaker et al., 2003), whereas we found that both urodynamic and locomotor dysfunction lasts for at least 8 weeks after injury and that mild but obvious damage persists in the spinal cords even at POD56. The protracted recovery that we observed after mild contusion injury is therefore likely to be due to mechanisms that contribute to recovery after more injuries than just those that cause spinal shock.
Although we observed that the level of neurotrophic factors did not correlate with changes in the functional recovery of micturition and locomotion, we do not underestimate the potential roles of BDNF and NT-3 in the spontaneous recovery from spinal cord contusion. BDNF and NT-3 have shown to play important roles in the development, function, and regeneration of both peripheral and central nervous systems (Barde, 1989; Carroll et al., 1998; von Meyenburg et al., 1998; Bradbury et al., 1999; Markus et al., 2002; Gomez-Pinilla et al., 2007; Marcol et al., 2007). Administration of BDNF or NT-3 is known to enhance recovery of the injured spinal cord and neurogenic bladder. Continuous intramedullary infusion of BDNF, for example, enhanced functional recovery in rats with compressed spinal cords (Namiki et al., 2000). NT-3 promoted axonal sprouting acutely across the midline in injured spinal cord (Chen et al., 2006) and reduced glial scar formation (Taylor et al., 2006). Genetically modified fibroblasts that release both BDNF and NT-3 promoted recovery of the neurogenic bladder following spinal cord injury in rats (Mitsui et al., 2005). Earlier studies also reported that locomotor and bladder function partially recovered after administration of exogenous BDNF and NT-3 (Shumsky et al., 2003).
Some of the previous studies showed that the level of BDNF increases acutely as well as chronically in completely transected spinal cords of rats (Zvarova et al., 2004; Li et al., 2007). We, however, observed a gradual decline in the level of BDNF in the contused spinal cord, as others have reported previously (Ikeda et al., 2001). An explanation of the discrepancy could be differences in injury models. In support of this notion, severe, but not mild, compression of the spinal cord increased BDNF levels in mouse spinal cord (Uchida et al., 2003). It is also possible that BDNF-binding receptors are altered in different ways by various types of injury and that these changes affect levels of BDNF level in the spinal cord (Widenfalk et al., 2001).
The NT-3 level in the spinal cord did not change over time after contusion injury. This result is in agreement with the reports of Tokumine and colleagues (2003) and Nakamura and Bregman (2001) who measured the NT-3 levels within 2 weeks after injury, and of Hutchinson and colleagues (2004) who measured the NT-3 level 7 weeks after injury. We also found that NT-3 levels in the spinal cord and bladder were not correlated with the spontaneous recovery of somatic and autonomic functions.
We found that the BDNF level in the bladder, unlike that in the spinal cord, increased slightly above normal acutely and then gradually returned to normal in proportion to a decrease in bladder volume. BDNF levels were also distinctly different in flaccid and hyperreflexic bladders. The activity of BDNF-binding tyrosine kinase B receptor in the lumbosacral dorsal root ganglia was found to be increased acutely after spinal cord injury (Qiao and Vizzard, 2005). It is therefore possible that the increase of this receptor may induce an upregulation of BDNF in the bladder and may also cause the initial recovery of the bladder from a flaccid to a hyperreflexic pattern. In support of this notion, Vizzard found that the NGF in the bladder decreased gradually in the completely transected spinal cord, whereas NGF and BDNF increased in the bladder, which they attributed to the retrograde transport of NGF to the dorsal root ganglia (Vizzard, 2000). BDNF was also found to be transported retrogradely to dorsal root ganglia and spinal cord motor neurons after peripheral nerve injury (DiStefano et al., 1992; Curtis et al., 1998) and possibly after spinal cord injury (Qiao and Vizzard, 2002). It is conceivable therefore that retrograde transport of BDNF increased the level of BDNF in the bladder, contributing to the reorganization of micturition reflexes.
Mechanisms other than neurotrophic factors may also be involved in the recovery of bladder function after spinal cord injury. For example, nitric oxide synthase was found to increase in the sacral parasympathetic nucleus after spinal cord injury and formed nitric oxide, which contributed to bladder hyperreflexia and facilitated the micturition reflex elicited by nociceptive bladder afferents at the spinal level (Vizzard, 1997). Spinal cord injury also caused an increase in pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide expression in bladder afferent cells of dorsal root ganglia and in lumbosacral spinal cord; these compounds may have a role in the control of micturition altered by injury (Zvarova et al., 2005; Yoshiyama and de Groat, 2008). Lastly, indirect propriospinal relay connections that partially restore supraspinal control of locomotion after incomplete spinal cord injury may play a similar role in the recovery of micturition (Courtine et al., 2008). These alternative connections can reverse bladder dysfunction without restoration of the interrupted long supraspinal pathways that control micturition when the spinal cord is intact. Further studies are necessary to explore the identity of the factors or mechanisms that play important roles in spontaneous recovery of bladder and locomotor function after mild spinal contusion injury.
Acknowledgments
This study was supported by grants received in 2005 from the Institute of Life Science, Dankook University Medical Center. We thank Dr. Seong Hoon Byun for surgical assistance and animal care, and Dr. Alan Tessler for editing the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- Barde Y.A. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Bradbury E.J. Khemani S. King V.R. Priestley J.V. McMahon S.B. NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur. J. Neurosci. 1999;11:3873–3883. doi: 10.1046/j.1460-9568.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- Bregman B.S. McAtee M. Dai H.N. Kuhn P.L. Neurotrophic factors increase axonal growth after spinal cord injury and transplantation in the adult rat. Exp. Neurol. 1997;148:475–494. doi: 10.1006/exnr.1997.6705. [DOI] [PubMed] [Google Scholar]
- Carroll P. Lewin G.R. Koltzenburg M. Toyka K.V. Thoenen H. A role for BDNF in mechanosensation. Nat. Neurosci. 1998;1:42–46. doi: 10.1038/242. [DOI] [PubMed] [Google Scholar]
- Chen Q. Zhou L. Shine H.D. Expression of neurotrophin-3 promotes axonal plasticity in the acute but not chronic injured spinal cord. J. Neurotrauma. 2006;23:1254–1260. doi: 10.1089/neu.2006.23.1254. [DOI] [PubMed] [Google Scholar]
- Courtine G. Song B. Roy R.R. Zhong H. Herrmann J.E. Ao Y. Qi J. Edgerton V.R. Sofroniew M.V. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis R. Tonra J.R. Stark J.L. Adryan K.M. Park J.S. Cliffer K.D. Lindsay R.M. DiStefano P.S. Neuronal injury increases retrograde axonal transport of the neurotrophins to spinal sensory neurons and motor neurons via multiple receptor mechanisms. Mol. Cell. Neurosci. 1998;12:105–118. doi: 10.1006/mcne.1998.0704. [DOI] [PubMed] [Google Scholar]
- DiStefano P.S. Friedman B. Radziejewski C. Alexander C. Boland P. Schick C.M. Lindsay R.M. Wiegand S.J. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- Fouad K. Schnell L. Bunge M.B. Schwab M.E. Liebscher T. Pearse D.D. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J. Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Leoni M.E. Esclarin De Ruz A. Management of urinary tract infection in patients with spinal cord injuries. Clin. Microbiol. Infect. 2003;9:780–785. doi: 10.1046/j.1469-0691.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F. Huie J.R. Ying Z. Ferguson A.R. Crown E.D. Baumbauer K.M. Edgerton V.R. Grau J.W. BDNF and learning: Evidence that instrumental training promotes learning within the spinal cord by up-regulating BDNF expression. Neuroscience. 2007;148:893–906. doi: 10.1016/j.neuroscience.2007.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson K.J. Gomez-Pinilla F. Crowe M.J. Ying Z. Basso D.M. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- Ikeda O. Murakami M. Ino H. Yamazaki M. Nemoto T. Koda M. Nakayama C. Moriya H. Acute up-regulation of brain-derived neurotrophic factor expression resulting from experimentally induced injury in the rat spinal cord. Acta Neuropathol. 2001;102:239–245. doi: 10.1007/s004010000357. [DOI] [PubMed] [Google Scholar]
- Keirstead H.S. Fedulov V. Cloutier F. Steward O. Duel B.P. A noninvasive ultrasonographic method to evaluate bladder function recovery in spinal cord injured rats. Exp. Neurol. 2005;194:120–127. doi: 10.1016/j.expneurol.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Li X.L. Zhang W. Zhou X. Wang X.Y. Zhang H.T. Qin D.X. Zhang H. Li Q. Li M. Wang T.H. Temporal changes in the expression of some neurotrophins in spinal cord transected adult rats. Neuropeptides. 2007;41:135–143. doi: 10.1016/j.npep.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Marcol W. Kotulska K. Larysz-Brysz M. Kowalik J.L. BDNF contributes to animal model neuropathic pain after peripheral nerve transection. Neurosurg. Rev. 2007;30:235–243. doi: 10.1007/s10143-007-0085-5. [DOI] [PubMed] [Google Scholar]
- Markus A. Patel T.D. Snider W.D. Neurotrophic factors and axonal growth. Curr. Opin. Neurobiol. 2002;12:523–531. doi: 10.1016/s0959-4388(02)00372-0. [DOI] [PubMed] [Google Scholar]
- Mitsui T. Fischer I. Shumsky J.S. Murray M. Transplants of fibroblasts expressing BDNF and NT-3 promote recovery of bladder and hindlimb function following spinal contusion injury in rats. Exp. Neurol. 2005;194:410–431. doi: 10.1016/j.expneurol.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Nakamura M. Bregman B.S. Differences in neurotrophic factor gene expression profiles between neonate and adult rat spinal cord after injury. Exp. Neurol. 2001;169:407–415. doi: 10.1006/exnr.2001.7670. [DOI] [PubMed] [Google Scholar]
- Namiki J. Kojima A. Tator C.H. Effect of brain-derived neurotrophic factor, nerve growth factor, and neurotrophin-3 on functional recovery and regeneration after spinal cord injury in adult rats. J. Neurotrauma. 2000;17:1219–1231. doi: 10.1089/neu.2000.17.1219. [DOI] [PubMed] [Google Scholar]
- Oh S.J. Ku J.H. Jeon H.G. Shin H.I. Paik N.J. Yoo T. Health-related quality of life of patients using clean intermittent catheterization for neurogenic bladder secondary to spinal cord injury. Urology. 2005;65:306–310. doi: 10.1016/j.urology.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Pikov V. Wrathall J.R. Coordination of the bladder detrusor and the external urethral sphincter in a rat model of spinal cord injury: effect of injury severity. J. Neurosci. 2001;21:559–569. doi: 10.1523/JNEUROSCI.21-02-00559.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L. Vizzard M.A. Up-regulation of tyrosine kinase (Trka, Trkb) receptor expression and phosphorylation in lumbosacral dorsal root ganglia after chronic spinal cord (T8-T10) injury. J. Comp. Neurol. 2002;449:217–230. doi: 10.1002/cne.10283. [DOI] [PubMed] [Google Scholar]
- Qiao L. Vizzard M.A. Spinal cord injury-induced expression of TrkA, TrkB, phosphorylated CREB, and c-Jun in rat lumbosacral dorsal root ganglia. J. Comp. Neurol. 2005;482:142–154. doi: 10.1002/cne.20394. [DOI] [PubMed] [Google Scholar]
- Schurch B. Schmid D.M. Kaegi K. Value of sensory examination in predicting bladder function in patients with T12-L1 fractures and spinal cord injury. Arch. Phys. Med. Rehabil. 2003;84:83–89. doi: 10.1053/apmr.2003.50007. [DOI] [PubMed] [Google Scholar]
- Shaker H. Mourad M.S. Elbialy M.H. Elhilali M. Urinary bladder hyperreflexia: a rat animal model. Neurourol. Urodyn. 2003;22:693–698. doi: 10.1002/nau.10147. [DOI] [PubMed] [Google Scholar]
- Shumsky J.S. Tobias C.A. Tumolo M. Long W.D. Giszter S.F. Murray M. Delayed transplantation of fibroblasts genetically modified to secrete BDNF and NT-3 into a spinal cord injury site is associated with limited recovery of function. Exp. Neurol. 2003;184:114–130. doi: 10.1016/s0014-4886(03)00398-4. [DOI] [PubMed] [Google Scholar]
- Smith C.P. Somogyi G.T. Bird E.T. Chancellor M.B. Boone T.B. Neurogenic bladder model for spinal cord injury: spinal cord microdialysis and chronic urodynamics. Brain Res. Brain Res. Protoc. 2002;9:57–64. doi: 10.1016/s1385-299x(01)00137-4. [DOI] [PubMed] [Google Scholar]
- Takahara Y. Maeda M. Nakatani T. Kiyama H. Transient suppression of the vesicular acetylcholine transporter in urinary bladder pathways following spinal cord injury. Brain Res. 2007;1137:20–28. doi: 10.1016/j.brainres.2006.12.042. [DOI] [PubMed] [Google Scholar]
- Taylor S.J. Rosenzweig E.S. McDonald J.W., III Sakiyama-Elbert S.E. Delivery of neurotrophin-3 from fibrin enhances neuronal fiber sprouting after spinal cord injury. J. Contr. Release. 2006;113:226–235. doi: 10.1016/j.jconrel.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumine J. Kakinohana O. Cizkova D. Smith D.W. Marsala M. Changes in spinal GDNF, BDNF, and NT-3 expression after transient spinal cord ischemia in the rat. J. Neurosci. Res. 2003;74:552–561. doi: 10.1002/jnr.10760. [DOI] [PubMed] [Google Scholar]
- Uchida K. Baba H. Maezawa Y. Furukawa S. Omiya M. Kokubo Y. Kubota C. andNakajima H. Increased expression of neurotrophins and their receptors in the mechanically compressed spinal cord of the spinal hyperostotic mouse (twy/twy) Acta Neuropathol. 2003;106:29–36. doi: 10.1007/s00401-003-0691-4. [DOI] [PubMed] [Google Scholar]
- Vizzard M.A. Increased expression of neuronal nitric oxide synthase in bladder afferent and spinal neurons following spinal cord injury. Dev. Neurosci. 1997;19:232–246. doi: 10.1159/000111212. [DOI] [PubMed] [Google Scholar]
- Vizzard M.A. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp. Neurol. 2000;161:273–284. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- von Meyenburg J. Brösamle C. Metz G.A. Schwab M.E. Regeneration and sprouting of chronically injured corticospinal tract fibers in adult rats promoted by NT-3 and the mAb IN-1, which neutralizes myelin-associated neurite growth inhibitors. Exp. Neurol. 1998;154:583–594. doi: 10.1006/exnr.1998.6912. [DOI] [PubMed] [Google Scholar]
- Watanabe T. Vaccaro A.R. Kumon H. Welch W.C. Rivas D.A. Chancellor M.B. High incidence of occult neurogenic bladder dysfunction in neurologically intact patients with thoracolumbar spinal injuries. J. Urol. 1998;159:965–968. [PubMed] [Google Scholar]
- Weiss D.J. Fried G.W. Chancellor M.B. Herbison G.J. Ditunno J.F., Jr. Staas W.E., Jr. Spinal cord injury and bladder recovery. Arch. Phys. Med. Rehabil. 1996;77:1133–1135. doi: 10.1016/s0003-9993(96)90135-5. [DOI] [PubMed] [Google Scholar]
- Widenfalk J. Lundstromer K. Jubran M. Brene S. Olson L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J. Neurosci. 2001;21:3457–3475. doi: 10.1523/JNEUROSCI.21-10-03457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyndaele J.J. Correlation between clinical neurological data and urodynamic function in spinal cord injured patients. Spinal Cord. 1997;35:213–216. doi: 10.1038/sj.sc.3100391. [DOI] [PubMed] [Google Scholar]
- Yoshiyama M. de Groat W.C. The role of vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide in the neural pathways controlling the lower urinary tract. J. Mol. Neurosci. 2008;36:227–240. doi: 10.1007/s12031-008-9090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvarova K. Dunleavy J.D. Vizzard M.A. Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury. Exp. Neurol. 2005;192:46–59. doi: 10.1016/j.expneurol.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Zvarova K. Murray E. Vizzard M.A. Changes in galanin immunoreactivity in rat lumbosacral spinal cord and dorsal root ganglia after spinal cord injury. J. Comp. Neurol. 2004;475:590–603. doi: 10.1002/cne.20195. [DOI] [PubMed] [Google Scholar]