Abstract
Background: The constellation of metabolic syndrome, although controversial with regard to its clinical usefulness, is epidemiologically related to increased diabetes risk and cardiovascular mortality. Our goal was to investigate the associations among neck circumference (NC), obstructive sleep apnea syndromes (OSAS), and metabolic syndrome in obese men and women sleeping less than 6.5 hr per night.
Methods: This was a cross-sectional study of obese men and premenopausal obese women sleeping less than 6.5 hr per night. We enrolled 120 individuals (92 women), age 40.5±6.9 years and body mass index (BMI) 38.6±6.5 kg/m2. Metabolic syndrome severity was assessed by a score and OSAS was defined as a respiratory disturbance index (RDI) ≥5. Metabolic end endocrine parameters were measured, and sleep duration was determined by actigraphy and validated questionnaires.
Results: Metabolic syndrome was found in 41% and OSAS in 58% (28% had both). Subjects with metabolic syndrome were 3 years older and more often Caucasian; they had higher RDI scores, larger NC, more visceral fat, lower serum adiponectin, higher 24-hr urinary norepinephrine (NE) excretion, and lower growth hormone concentrations. A NC of ≥38 cm had a sensitivity of 54% and 58% and a specificity of 70% and 79% in predicting the presence of metabolic syndrome and OSAS, respectively. RDI, adiponectin, and NC accounted for approximately 30% of the variability in the metabolic syndrome score, as estimated by an age-, gender-, and race-corrected multivariate model (R2=0.376, P<0.001).
Conclusion: Greater NC is associated with OSAS and metabolic syndrome in short-sleeping obese men and premenopausal obese women. Addition of NC to the definition of metabolic syndrome should be considered and needs to be validated in future studies.
Introduction
Metabolic syndrome increases diabetes risk and cardiovascular mortality.1,2 Despite improvements in the awareness of metabolic syndrome, its prevalence has increased over the past decade.3 Sleep has decreased from 9 hr per day in 1910 to 7 hr in 2010,4 possibly because of shift-based and night work, longer work days and commuting time, psychosocial stress, and the advents of artificial light, television, radio, computers, and the internet.5 Chronic sleep deprivation impacts endocrine and metabolic outcomes.6 Acute sleep deprivation activates the hypothalamic–pituitary–adrenal axis, inhibits the growth hormone (GH)/insulin-like growth factor-1 (IGF-1) axis, increases proinflammatory cytokines, and induces insulin resistance.7 Sleep deprivation as a stressor8 may threaten homeostasis, causing metabolic syndrome and other consequences.9 The activation of the sympatho-adrenal system causes abdominal fat accumulation, a source of leptin, adiponectin, and proinflammatory cytokines.5 Decreased plasma leptin and increased ghrelin stimulate appetite and may result in a positive energy balance.10
Sleep alterations and metabolic syndrome often coexist.11 In approximately 1200 subjects, both short and long sleep increased metabolic syndrome odds by 45%.12 Similarly, both short and long sleep increased metabolic syndrome risk in a Korean study.13 Poor sleep quality was associated with increased metabolic syndrome odds in middle-aged men and women.14
The reported prevalence of obstructive sleep apnea syndrome (OSAS) was 24% in men and 9% in women in 1993.15 OSAS and metabolic syndrome have been associated in adolescents,16 middle-aged men,17 men in their sixth decade,18 and in different ethnic groups, including men from Australia19 and men and women from Japan20,21 and China.22 In a case–control study of nonobese Japanese men matched for visceral fat,20 OSAS coexisted with hypertension, dyslipidemia, and hyperglycemia, suggesting that OSAS can be associated with metabolic syndrome in lean males. In a trial of obese patients with severe OSAS, treatment with continuous positive airway pressure (CPAP) reduced blood pressure.23 In contrast, McArdle et al. reported that metabolic syndrome was equally prevalent in obese subjects without OSAS versus obese subjects with OSAS, although these subjects had worse metabolic profiles.19 Metabolic syndrome frequently co-exists with OSAS; however, it does not increase cardiovascular risk when patients received treatment for OSAS.18 In a sample of approximately 1000 Brazilian adults, neck circumference (NC) was related to triglycerides (TGs) and insulin resistance.24 In addition, NC was associated with higher levels of the prothrombotic factor plasminogen activator inhibitor-1 (PAI-1).25 Previously, we reported in this patient cohort that short sleep duration by actigraphy is associated with increased odds of OSAS.26 C-reactive protein (CRP), an accepted marker of inflammation and an independent predictor of cardiovascular risk, is elevated both in metabolic syndrome and in OSAS.27 Finally, several endocrine systems, including the glucorticoid axis and the sympatho-adrenal system, are disrupted in obesity and metabolic syndrome.28
In the present study, we establish the prevalence, describe the features, and identify the mediators of metabolic syndrome in obese adults reporting sleeping less than 6.5 hr per night.
Methods
Study design and participants
The NIDDK Sleep Extension Study is a randomized, controlled study of sleep extension.29 Participants were recruited by advertising for men and premenopausal women aged 18–50 years with a body mass index (BMI) between 30 and 55 kg/m2 who reported sleeping on average less than 6.5 hr per night. The NIDDK Institutional Review Board approved the protocol and each subject signed the informed consent. The study was conducted at the National Institutes of Health (NIH) Clinical Center (CC) (NIDDK protocol 06-DK-0036, ClinicalTrials.gov identifier NCT00261898).
Assessments
Anthropometrics
Height was observed to the nearest centimeter using a wall-mounted stadiometer (SECA 242, SECA North America East, Hanover, MD) and weight was observed using a stand-on scale with the subject in a hospital gown to the nearest 1/10th of a kilogram (SR555 SR Scales, SR Instruments, INC, Tonawanda, NY). Waist circumference (WC) was determined by placing the measuring tape in a horizontal plane around the abdomen above the uppermost lateral border of the right iliac crest at the end of a normal expiration. If this site could not be determined, the maximum circumference was observed at or near the level of the umbilicus. NC was measured at the minimal circumference with the subjects' head in the Frankfurt Horizontal Plane.
Body composition measurements
Dual-energy X-ray absorptiometry (DXA) was performed with a Hologic DXA QDR 4500 (Hologic Inc., Bedford, MA). Abdominal fat content and distribution were measured at L2–L3 and L4–L5, using a HiSpeed Advantage CT/I scanner (GE Medical Systems, Milwaukee, WI) and analyzed on a SUN workstation using the MEDx image analysis software package (Sensor System, Sterling, VA). Conventional (nonhelical) 10-mm-thick X-ray abdominal computed tomography (CT) images limited to the L2–L3 and L4–L5 levels were obtained at 120 kVp, with mAs adjusted according to patient size. Fully automatic processing of these images30 resulted in measurements of visceral and subcutaneous adipose tissue areas, which were summed and normalized by the imaged volume to estimate subcutaneous and visceral abdominal fat.
Sleep measures
Sleep was estimated by wrist actigraphy (Actiwatch-64)31 worn continuously for 2 weeks (average 13±2 days, median 13, range 5–15). Two measures were included: Sleep duration, the amount of nighttime sleep obtained during the 24-hr period; and sleep efficiency, the percentage of time in bed spent sleeping.
Sleep disordered breathing was assessed using a device (Apnea Risk Evaluation System, Advanced Brain Monitoring Inc., Carlsbad, CA)31 estimating the respiratory disturbance index (RDI), the number of apneas and hypopneas per hour of sleep. Apnea was defined as cessation of airflow for 10 sec, and hypopnea as airflow <50% with a 3.5 or greater percentage desaturation and 1% resaturation.31 Hypopneas were also determined as a minimum 1% desaturation and resaturation plus at least one surrogate arousal indicator (head movement, changes in snoring, or changes in pulse rate). The device provided mean blood oxygen saturation (SpO2), and percentage of time that SpO2 was <90%. OSAS was defined as having an RDI ≥5.
Participants completed the Pittsburgh Sleep Quality Index (PSQI), a validated 21-item questionnaire with inquiries about sleep, including quality over the past month. Scores were dichotomized at ≤5 or >5, the conventional threshold for poor sleep quality.32 Daytime sleepiness was assessed by the Epworth Sleepiness Scale (ESS), a validated 8-item questionnaire.33 Higher scores represent increased daytime sleepiness; values >10 indicate abnormal sleepiness. Participants received instructions for maintaining sleep diaries for the following 2 weeks and for wearing an actigraphy monitor (Actiwatch-64, Mini Mitter/Respironics/Philips, Bend, OR) over that same period of time.
Definition of metabolic syndrome
Metabolic syndrome was defined by at least three of the following criteria34: (1) Fasting plasma TGs ≥150 mg/dL; (2) fasting plasma glucose (FPG) ≥100 mg/dL; (3) fasting high-density lipoprotein cholesterol (HDL-C), <40 mg/dL in men and <50 mg/dL in women; (4) systolic (SBP) and/or diastolic blood pressure (DBP) ≥130/85 mmHg; (5) central obesity (WC ≥102 cm in men and ≥88 cm in women). If individuals were taking medication for any of these conditions, they were considered qualifying for that component, irrespective of the obtained measurement values.
To assess metabolic syndrome severity, a score (range, 0–57), taking into account age, gender, fasting glucose, HDL-C, TGs, hypertension, and BMI was computed by giving a specific weight to each individual component of metabolic syndrome.35
Clinical laboratory analyses
Morning fasting venous blood was obtained. Plasma glucose and serum TGs, total cholesterol, low-density lipoprotein cholesterol (LDL-C), and HDL-C were measured on a Dimension Vista 1500 analyzer (Siemens Health Diagnostics, Deerfield, IL). Plasma adrenocorticotropic hormone (ACTH) and serum cortisol, insulin, IGF-1, and GH were measured with chemiluminescence immunoassays (Immulite 2000 and 2500; Siemens). The 24-hr urinary free cortisol was measured by liquid chromatography-tandem mass spectrometry, and 24-hr urinary catecholamines [epinephrine (EPI), norepinephrine (NE), and dopamine] were measured by high-performance liquid chromatography. Serum leptin was measured with an enzyme-linked immunosorbent assay (ELISA) kit from Millipore (Billerica MA). The minimum detectable concentration was 0.5 ng/mL (intra-CV, 3.7%; inter-coefficient of variation, 4.0%). Serum adiponectin was measured by ELISA (Human Total Adiponectin Quantikine Kit, R&D Systems, Minneapolis, MN) (intra-CV, 3.5%; and inter-CV, 6.5%). The minimum detectable concentration was 0.25 ng/mL. CRP concentrations were measured with a high-sensitivity chemiluminescence immunometric assay with a detection limit of 0.1 mg/L (Immulite 2000, Siemens).
Statistical analysis
Values are mean±standard deviation (SD) or median with interquartile if distribution was skewed. Normally distributed continuous variables were assessed by t-test; the Wilcoxon rank test was used for skewed variables. Associations were examined using Pearson or Spearman rank correlation coefficients, depending on data distribution. Post hoc tests were conducted using the Bonferroni correction of significance.
We compared WC, visceral fat by CT, and NC in detecting the presence of metabolic syndrome and OSAS. We performed sensitivity and specificity analyses using logistic regression and constructed receiver operating characteristic (ROC) curves. The optimal cutoff value was defined by the Youden index (sensitivity+specificity−1). We fitted a multiple regression model using those variables of physiological value that were related to each other in univariate linear regressions at a P value of≤0.10.
Results
Demographic, anthropometric, and cardiovascular characteristics according to metabolic syndrome diagnosis
Subjects with metabolic syndrome (n=49) were 3 years older (Table 1). Metabolic syndrome was more common in whites (53%) than blacks (31%). Individuals with metabolic syndrome had a larger NC and 30% more visceral fat, but body weight, BMI, WC, lean body mass, and subcutaneous fat were similar to individuals without metabolic syndrome. SBP and DBP, fasting glucose and insulin, homeostasis model assessment (HOMA) index, TGs were higher, and HDL-C was lower in the metabolic syndrome group. For the whole sample, the metabolic syndrome score ranged from 6 to 44, with an average of 19.2±8.1. Smoking and medication use was similar.
Table 1.
Anthropometric and Metabolic Syndrome Characteristics of the Study Population
| With metabolic syndrome (n=49) | Without metabolic syndrome (n=71) | P value | |
|---|---|---|---|
| Age (years) | 42.7±6.5 | 39.2±6.7 | 0.005 |
| Sex (M/F) (n) | 14/35 | 14/57 | 0.261 |
| Race (white/black/other) (n) | 22/22/5 | 19/49/3 | 0.027 |
| Body weight (kg) | 108±20.7 | 107±20.2 | 0.830 |
| BMI (kg/m2) | 38.5±6.2 | 38.5±6.4 | 0.953 |
| Waist circumference (all) (cm) | 114.4±11.7 | 113.3±14.1 | 0.634 |
| Neck circumference (cm) | 40.1±3.8 | 38.5±3.8 | 0.026 |
| Lean body mass (kg) | 63.1±12.9 | 59.6±11.1 | 0.134 |
| Body fat (%) | 39.8±6.9 | 42.2±6.9 | 0.078 |
| Visceral fat (cm3) | 395.5±151.5 | 298.6±160.3 | 0.002 |
| Subcutaneous fat (cm3) | 872.0±315.2 | 952.3±302.3 | 0.160 |
| Systolic blood pressure (mmHg) | 128.7±12.4 | 120.8±11.3 | <0.001 |
| Diastolic blood pressure (mmHg) | 76.3±10.1 | 72.5±9.4 | 0.034 |
| Fasting glucose (mg/dL) | 94.7±16.2 | 86.6±8.1 | 0.002 |
| Fasting insulin (mU/L) | 12.8±7.2 | 9.8±7.2 | 0.027 |
| HOMA index | 3.0±1.9 | 2.1±1.7 | 0.002 |
| Triglycerides (mg/dL) | 121.9±65.0 | 81.7±36.7 | <0.001 |
| Total cholesterol (mg/dL) | 181.9±43.9 | 178.5±39.2 | 0.618 |
| HDL-C (all) (mg/dL) | 43.2±6.8 | 54.4±14.0 | <0.001 |
| HDL-C (men) (mg/dL) | 42.4±7.6 (n=14) | 48.8±9.4 (n=14) | 0.031 |
| HDL-C (women) (mg/dL) | 43.5±6.5 (n=35) | 55.8±14.6 (n=57) | <0.001 |
| LDL-C (mg/dL) | 118.1±37.1 | 110.0±26.5 | 0.162 |
| Metabolic syndrome score | 24.0±8.7 | 15.9±5.7 | <0.001 |
| Current smoker (Y/N/Unknown) (n) | 3/44/2 | 7/56/8 | 0.260 |
| Medications (n) | |||
| Psychiatric | 1 | 5 | 0.416a |
| Antidiabetic | 3 | 0 | 0.131a |
| Anti-inflammatory | 4 | 4 | 0.850a |
| Statins | 4 | 3 | 0.601a |
| Antihypertensive | 12 | 15 | 0.829a |
| Intranasal steroids | 4 | 4 | 0.850a |
| Oral contraceptives (women only) | 1 | 7 | 0.183a |
Chi-squared test P value.
Significant P values are in bold.
BMI, body mass index; HOMA, homeostasis model assessment; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Blacks versus whites had lower TGs (80.8±40.5 vs. 126.1±64.9; P<0.001, after Bonferroni correction), total cholesterol (172.4±31.2 vs. 191.6±42.1; P=0.009, adjusted by Bonferroni), and LDL-C (107.5±29.0 vs. 121.4±35.2; adj. P=0.026, after Bonferroni correction). The metabolic syndrome score tended to be lower in blacks versus whites (18.0±8.3 vs. 20.3±7.0; P=0.09, after adjustment for BMI). Metabolic syndrome score was higher in men than in women and, as expected, men had larger NC and WCs than women.
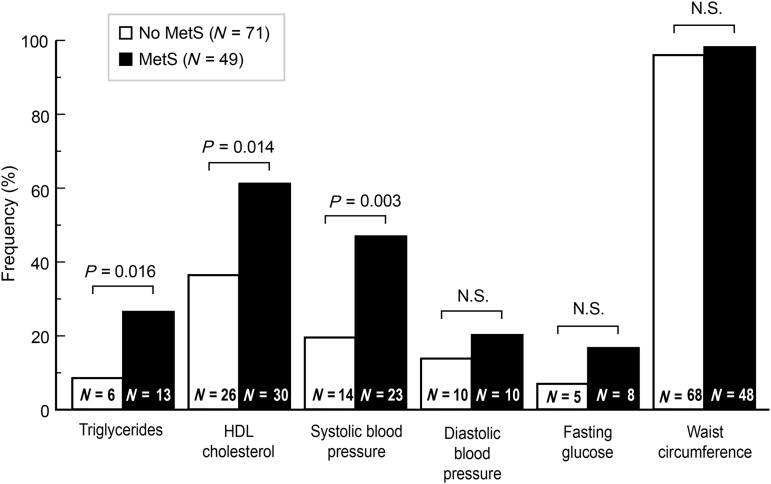
Abnormal values for TGs, HDL-C, and SBP were more common in the metabolic syndrome group, whereas DBP, fasting glucose, and WC were similar (Fig. 1). Only one participant met all five criteria of the metabolic syndrome; 13 participants met four criteria, and 35 participants met three criteria.
FIG. 1.
Percentage and number of participants presenting with individual metabolic syndrome criteria. MetS, metabolic syndrome; HDL, high-density lipoprotein; N.S., not significant.
Sleep characteristics according to metabolic syndrome diagnosis
Subjects with metabolic syndrome had higher RDI and a greater (80% vs. 43%) prevalence of OSAS (Table 2). Other sleep features were similar. On average, participants had a sleep efficiency of about 80% and slept approximately 6.0 hr per night by actigraphy and 6.5 hr by self-report. Sleep quality, as assessed by PSQI, was abnormal in 83% of the participants, whereas 30% reported excessive sleepiness.
Table 2.
Sleep Characteristics of the Study Population
| With metabolic syndrome (n=49) | Without metabolic syndrome (n=71) | P value | |||
|---|---|---|---|---|---|
| RDI score (events/hr) (n=100) | 13.9±14.2 | 10.1±16.3 | 0.015 | ||
| SpO2 (%) (n=100) | 96.2±1.0 | 96.0±2.3 | 0.525 | ||
| OSAS (Y/N) | RDI <5 | RDI >5 | RDI <5 | RDI >5 | |
| 8 | 32 | 34 | 26 | 0.001a | |
| Actigraphy sleep efficiency (%) | 80.9±7.0 | 79.5±5.9 | 0.257 | ||
| Actigraphy sleep duration (min/night) | 363±48 | 352±51 | 0.229 | ||
| Subjective sleep duration (min/night) | 388±50 | 383±49 | 0.638 | ||
| PSQI global score (optimal, 0–5) | 8.1±3.1 | 8.2±2.5 | 0.770 | ||
| PSQI <5 | 10 | 9 | 0.247a | ||
| PSQI >5 | 38 | 61 | |||
| PSQI self-reported (hr) | 5.6±0.9 | 5.7±0.9 | 0.295 | ||
| Epworth sleepiness score (optimal, 0–10) | 7.7±4.3 | 8.7±4.7 | 0.243 | ||
| Epworth score >10 | 35 | 45 | 0.342a | ||
| Epworth score >10 | 12 | 13 | |||
Chi-squared test P value.
Significant P values are in bold.
OSAS, obstructive sleep apnea syndromes; RDI, respiratory disturbance index; PSQI, Pittsburgh Sleep Quality Index.
Hormone levels and CRP according to metabolic syndrome diagnosis
Leptin levels tended to be lower and adiponectin levels were lower in subjects with metabolic syndrome (Table 3). Of note, blacks had lower adiponectin than whites (5115.9±3765.3 vs. 7014.8±5033.6 units; P=0.026, after Bonferroni correction), and tended to have lower leptin levels than whites (57.6±28.6 vs. 47.8±33.6 units; P=0.103, after Bonferroni correction). Subjects with metabolic syndrome had 25% higher 24-hr urinary NE excretion and lower morning serum GH levels. There were no differences in ACTH, cortisol, urinary free cortisol (UFC), urinary EPI and dopamine, and IGF-1. CRP levels were similarly elevated in both groups.
Table 3.
Endocrine Features of the Study Population
| With metabolic syndrome (n=49) | Without metabolic syndrome (n=71) | P value | ||||
|---|---|---|---|---|---|---|
| Leptin (ng/mL) | ||||||
| Mean±SD | 46.7±28.9 | 56.9±31.2 | 0.073 | |||
| Median (IQT)a | 40.8 (24.1–63.4) | 49.6 (33.2–81.1) | 0.058 | |||
| Leptin (ng/mL) | Men | Women | Men | Women | Men | Women |
| 21.4±11.9 | 56.9±27.5 | 27.0±18.7 | 64.3±29.3 | 0.571 | 0.191 | |
| (n=14) | (n=35) | (n=14) | (n=57) | |||
| Adiponectin (ng/mL) | ||||||
| Mean±SD | 5101±3868 | 6210±4460 | 0.161 | |||
| Median (IQT)a | 3682 (2746–5905) | 5093 (3458–7249) | 0.045 | |||
| Adiponectin (ng/mL) | Men | Women | Men | Women | Men | Women |
| 4552±2484 | 5320±4312 | 4398±1983 | 6663±4793 | 0.804 | 0.037a | |
| (n=14) | (n=35) | (n=14) | (n=57) | |||
| ACTH (pg/mL) | 23.3±17.6 | 22.0±17.6 | 0.700 | |||
| Cortisol (μg/dL) | 9.5±3.9 | 9.6±4.6 | 0.920 | |||
| Urinary free cortisol (μg/24 hr) | 19.8±12.6 | 22.1±14.8 | 0.362 | |||
| Urinary NE (μg/24 hr) | 51.8±16.7 | 39.3±18.4 | <0.001 | |||
| Urinary EPI (μg/24 hr) | 4.5±2.9 | 3.9±3.0 | 0.313 | |||
| Urinary dopamine (μg/24 hr) | 266.7±92.9 | 265±90.4 | 0.962 | |||
| Growth hormone (ng/mL) | 0.5±0.9 | 1.0±1.6 | 0.026 | |||
| Log growth hormone | –0.64±0.44 | –0.39±0.59 | 0.013 | |||
| IGF-1 (ng/mL) | 134.6±45.8 | 144.2±51.7 | 0.299 | |||
| C-reactive proteina (mg/L) | 4.23 (1.78–8.54) | 4.15 (1.41–8.45) | 0.924 | |||
| (n=47) | (n=64) | |||||
| Log CRP | 0.56±0.42 | 0.54±0.51 | 0.809 | |||
Values are reported as median with interquartile range due to skewed distribution.
To convert gravimetric units for hormones to SI units, use the following conversion factors:
ACTH (corticotropin), pg/mL * 0.22=pmol/L; adiponectin, ng/mL * 33.33=pM; cortisol, μg/dL * 27.59=nmol/L;
CRP mg/L * 9.52=nmol/L; dopamine, μg/24 hr * 6.58=nmol/24 hr; epinephrine, μg/24 h * 5.46=nmol/24 hr; growth hormone, ng/mL * 1.00=mg/L; IGF-1, ng/mL * 1.00=mg/L; leptin ng/mL * 1.00=μg/L; norepinephrine, μg/24 hr * 5.91=nmol/24 hr.
Significant P values are in bold.
SD, standard deviation; IQT, interquartiles; ACTH, adrenocorticotropin; NE, norepinephrine; EPI, epinephrine; IGF-1, insulin-like growth factor-1; CRP, C-reactive protein.
Relationship between sleep characteristics, metabolic syndrome criteria, hormones, and WC and NC
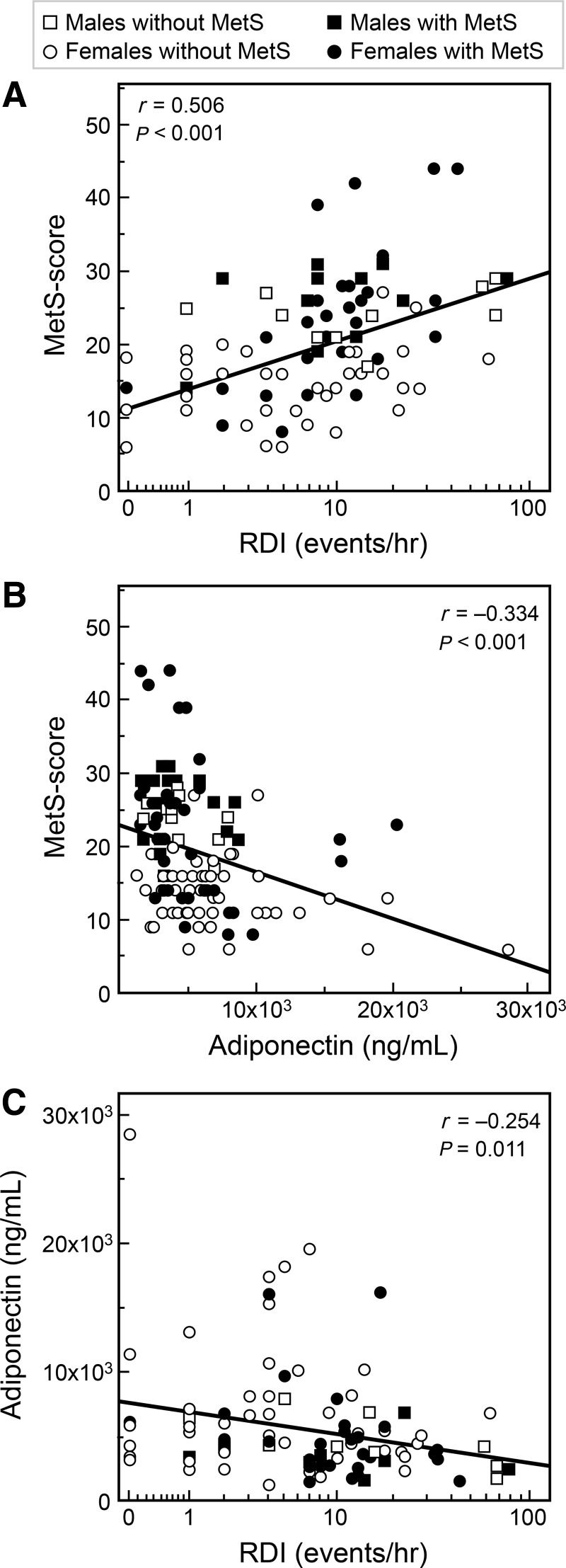
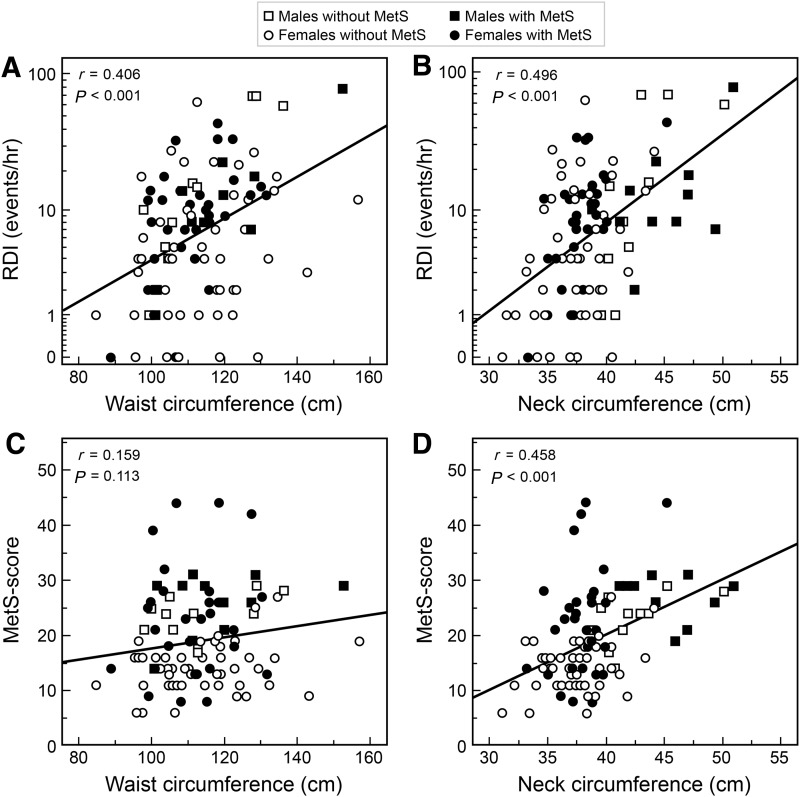
The higher the RDI score and the lower the adiponectin concentrations, the higher the metabolic syndrome score (Fig. 2A, B). RDI and adiponectin were inversely related (Fig. 2C). Figure 3 depicts the relationship between WC and NC versus RDI (upper panels) and versus metabolic syndrome score (lower panels). WC and NC were directly related to RDI. NC was also directly related to the metabolic syndrome score, in contrast to WC (Fig. 3). In addition, NC was directly related to fasting insulin (r=0.476; P<0.001), and HOMA index (r=0.461; P<0.001), and was inversely related to SpO2 (r=−0.354; P<0.001). These correlations remained significant after adjustment for BMI. NC was inversely related to sleep duration by actigraphy (r=−0.211; P=0.029), but this correlation lost significance after adjustment for BMI (partial r=−0.183; P=0.061). Finally, SpO2 and metabolic syndrome score were inversely related (r=−0.207, P=0.039). NC was more strongly related to visceral fat (r=0.674, P<0.001) as compared to WC (r=0.382, P<0.001), the latter being highly associated with subcutaneous fat (r=0.712, P<0.001) as compared to NC that did not show any significant association (r=0.125, P=0.20). When expressed as percent of total abdominal fat, visceral and subcutaneous fat were only related to NC (|r|=0.482, P<0.001) but not WC (|r|=0.062, P=0.52).
FIG. 2.
Relationships between respiratory disturbance index (RDI), metabolic syndrome score, and adiponectin. Note the use of safe logarithmic scale, namely, LOG10(1+RDI) on the x axis. MetS, metabolic syndrome; RDI, respiratory disturbance index.
FIG. 3.
Relationship between waist circumference, neck circumference, and respiratory disturbance index (RDI), as well as waist circumference, neck circumference (NC), and metabolic syndrome score (n=100). Note the use of safe logarithmic scale, namely, LOG10(1+RDI) on the y axis of A and B. MetS, metabolic syndrome; RDI, respiratory disturbance index.
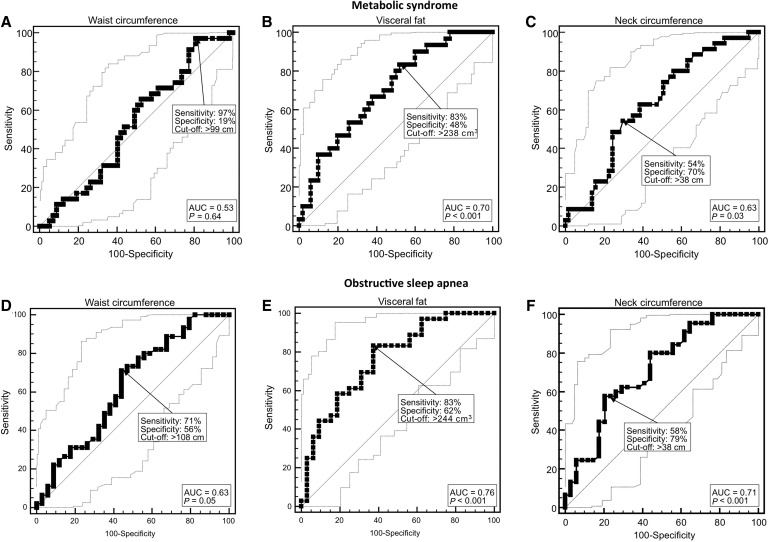
Due to the small number of men, sensitivity and specificity analyses for metabolic syndrome and OSAS by using WC, visceral fat, and NC were performed only in women (Fig. 4). WC was unrelated to metabolic syndrome, whereas both visceral fat and NC were predictive of metabolic syndrome. Visceral fat at the optimal cutoff (238 cm3) resulted in a sensitivity of 83% and a specificity of 48%, and NC at the optimal cutoff (38 cm) resulted in a sensitivity of 54% and a specificity of 70%. WC, visceral fat, and NC were all predictive of OSAS. WC at the optimal cutoff (108 cm) had a diagnostic sensitivity of 71% and a specificity of 56%; visceral fat at the optimal cutoff (244 cm3) resulted in a sensitivity of 83% and a specificity of 62%, whereas NC at the optimal cutoff (38 cm) produced a sensitivity of 58% and a specificity of 79%.
FIG. 4.
Received operating characteristic (ROC) curves for the diagnostic performance of waist circumference, visceral fat, and neck circumference (NC) measurements regarding metabolic syndrome and obstructive sleep apnea syndromes (OSAS) in 92 short-sleeping obese women. Thin lines show 95% confidence intervals, arrows point at the optimal cutoff as defined by the Youden index for diagnostic sensitivity and specificity, and area under the curve (AUC).
We fitted an age/gender/race-corrected, multivariate, forward stepwise predictive model for the metabolic syndrome score (Table 4). The variables selected at P<0.10 level included RDI, SpO2, NC, 24-hr urinary NE, 24-hr urinary dopamine, serum cortisol, GH, leptin, and adiponectin, of which the model retained RDI, adiponectin, and NC. RDI, adiponectin, and NC together accounted for approximately 38% of the variability in metabolic syndrome.
Table 4.
Multivariate, Forward, Stepwise, Age/Gender/Race-Corrected Models of Metabolic Syndrome Score
| Step 1: RDI | Step 2: RDI+adiponectin | Step 3: RDI+adiponectin+neck circumference | |
|---|---|---|---|
| Predictive model of metabolic syndrome score | R2=0.269, | R2=0.349 | R2=0.376 |
| P<0.001 | P<0.001 | P<0.001 | |
| RDIs | β=0.165 | β=0.135 | β=0.096 |
| P=0.001 | P=0.005 | P=0.059 | |
| Partial R=0.343 | Partial R=0.298 | Partial R=0.203 | |
| Adiponectin (ng/mL) | N/A | β=−0.00056 | β=−0.00051 |
| P=0.002 | P=0.004 | ||
| Partial R=−0.332 | Partial R=−0.306 | ||
| Neck circumference (cm) | N/A | N/A | β=0.588 |
| P=0.060 | |||
| Partial R=0.203 |
Each model provides total R2, P value, and unstandardized β coefficient for predictors of metabolic syndrome score.
At each step, unstandardized β coefficients are adjusted for age, gender, and race.
RDI, respiratory disturbance index; N/A, not applicable.
Discussion
Metabolic syndrome was associated with OSAS in obese individuals sleeping less than 6.5 hr. NC was larger in subjects with metabolic syndrome and was directly related to RDI, the metabolic syndrome score, and insulin resistance, and inversely related to sleep duration. Of note, NC was related to the metabolic syndrome score, whereas WC was not. RDI, together with adiponectin and NC, accounted for one-third of the variability of metabolic syndrome, a heterogeneous syndrome. In addition, NC had comparable sensitivity and specificity to WC in predicting OSAS. On the basis of our model, a 10-unit increase in RDI, or a 2000 ng/mL decrease in adiponectin, would worsen the score by 1 point, whereas a 5-cm increase in NC would worsen the metabolic syndrome score by almost 3.5 points.
The importance of metabolic syndrome resides in its ability to predict diabetes and cardiovascular mortality better than the sum of its components.35 A global metabolic syndrome score was validated in the GISSI Study, in which approximately 11,000 patients with recent myocardial infarction were followed for up to 3.5 years.35 In the GISSI Study, a score of 28 elicited the best sensitivity and specificity. Our average metabolic syndrome score was lower, 24, but our subjects were on average 10 years younger.
Our OSAS prevalence was higher than the 22% prevalence reported in a study of short, normal, and long sleepers with an average BMI of 27.12 Many studies have investigated the relationship between OSAS and cardiometabolic parameters.16–18,21,22 In the NHANES, subjects with three or more sleep disorders had a 3.92 odds ratio (OR) of having metabolic syndrome.36 OSAS was independently associated with metabolic parameters in metabolic syndrome, as well as with an increased prevalence of metabolic syndrome.23 Furthermore, OSAS was independently associated with metabolic syndrome, but not with insulin resistance.17 A study from Hong Kong22 reported a five-fold increased risk for metabolic syndrome in individuals with OSAS.
Among the sleep parameters measured in our study, only OSAS differed among subjects with metabolic syndrome and those without it. It is possible that the average sleep duration of our sample, 6 hr, represented a degree of sleep deprivation inadequate to have an impact on metabolic syndrome. In addition, compensatory mechanisms may develop with chronic sleep deprivation. Of note, most of the studies on the effect of sleep deprivation on metabolic syndrome applied acute sleep deprivation.6,10
There were no WC differences between subjects with and subjects without metabolic syndrome, although subjects with metabolic syndrome had 35% more abdominal fat. The imprecision of WC measurement in obese subjects may limit its value in this population. Although CT scan accurately measures visceral fat, its large-scale use would be impractical because of radiation exposure and cost. Subjects with OSAS had a larger NC even if there were no BMI differences between groups. NC was positively related, even after adjustment for BMI, to HOMA index and fasting insulin, as well as RDI and blood oxygen saturation.
NC had higher specificity than WC for OSAS. Of note, NC was negatively related to sleep duration by actigraphy. The fact that NC, independent of BMI, was related to glucose metabolism suggests a relationship with insulin secretion and action. We confirmed the association between NC and HOMA index that had been reported in a smaller sample.37 NC may also be a useful screening tool for cardiovascular risk assessment in pediatric populations.38 These findings are in accord with several previous reports. An independent contributory role for NC to the likelihood of metabolic syndrome has been reported in large samples from Turkey,39 China,40,41 and South Africa.42
Subjects with metabolic syndrome tended to have lower leptin levels and had lower adiponectin levels. Hypoadiponectemia is a risk factor for diabetes and cardiovascular disease.43 Similarly, the leptin/adiponectin ratio is an independent predictor of carotid intima media thickness.44 Urinary NE concentration was higher in subjects with metabolic syndrome, indicating an activation of the sympathetic system. Morning GH levels were also higher in subjects with metabolic syndrome. Night workers secrete more GH during daytime, suggesting compensatory mechanisms.45
Metabolic syndrome was more common in whites than in blacks, and blacks had lower TGs, total cholesterol, and LDL-C. The threshold values currently assigned to TGs and HDL-C may underestimate the metabolic risk in blacks carried by “normal” values for these analytes.46
Because of the cross-sectional design of this analysis, we could not assess causality. We did not use polysomnography that could have documented the presence of OSAS. Of note, actigraphy monitors are less reliable when there are excessive limb movements during sleep, such in subjects with OSAS.47 Single hormonal determinations may have missed changes in circadian hormonal secretion. On the other hand, this cohort of obese sleep-deprived subjects was homogeneous and prospectively assembled, and sleep features were characterized in depth by actigraphy monitors and several validated questionnaires.
We found that even mild OSAS was associated with metabolic syndrome. We have confirmed the association of NC versus OSAS and metabolic syndrome and suggest that NC be routinely measured in obese subjects. Given the relationship between NC and OSAS and the fact that NC measurements can be assessed more readily than OSAS, we concur with the recommendation of including NC in the definition of metabolic syndrome.
Acknowledgments
This work was supported by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases and Clinical Center, National Institutes of Health (NIH). We would like to thank in alphabetical order the following colleagues for their scientific advice and critical suggestions in the development and conduct of the study protocol: Karim Calis, Janet Gershengorn, Gregor Hasler, Emmanuel Mignot, Susan Redline, Terry Phillips, Nancy Sebring, Duncan Wallace, and Elizabeth Wright. We would also like to thank present and past members of the study team: Peter Bailey, Laide Bello, Meredith Coyle, Paula Marincola, Patrick Michaels, Svetlana Primma, Angela Ramer, Rebecca Romero, Megan Sabo, Tanner Slayden, Sara Torvik, Elizabeth Widen, Lydia Williams, and Sam Zuber. We would like to thank Dr. Alex Ling (NIH CC) for analysis of the computer tomography measurements. The bioinformatics support of Frank Pierce (Esprit Health) is gratefully acknowledged. Finally, we are grateful to all of our enthusiastic study participants.
Author Disclosure Statement
The authors have nothing to disclose. None of the authors had a conflict of interest.
References
- 1.Wu SH, Liu Z, Ho SC. Metabolic syndrome and all-cause mortality: A meta-analysis of prospective cohort studies. Eur J Epidemiol 2010;25:375–384 [DOI] [PubMed] [Google Scholar]
- 2.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total cardiovascular disease mortality in middle-aged men. JAMA 2002;288:2709–2716 [DOI] [PubMed] [Google Scholar]
- 3.Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999–2006. Diabetes Care 2011;34:216–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cizza G, Requena M, Galli G, et al. Chronic sleep deprivation and seasonality: Implications for the obesity epidemic. J Endocrinol Invest 2011;34:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siervo M, Wells JCK, Cizza G. The contribution of psychosocial stress to the obesity epidemic: An evolutionary approach. Horm Metab Res 2009;41:261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiegel K, Knutson K, Leproult R, et al. Sleep loss: A novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol 2005;99:2008–2019 [DOI] [PubMed] [Google Scholar]
- 7.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999;354:1435–1439 [DOI] [PubMed] [Google Scholar]
- 8.McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism 2006;55(10 Suppl 2):S20–S23 [DOI] [PubMed] [Google Scholar]
- 9.Kyrou I, Chrousos GP, Tsigos C. Stress, visceral obesity, and metabolic complications. Ann NY Acad Sci 2006;1083:77–110 [DOI] [PubMed] [Google Scholar]
- 10.Spiegel K, Tasali E, Penev P, et al. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004;141:846–850 [DOI] [PubMed] [Google Scholar]
- 11.Cizza G, Skarulis M, Mignot E. A link between short sleep and obesity: Building the evidence for causation. Sleep 2005;10:1217–1220 [DOI] [PubMed] [Google Scholar]
- 12.Hall MH, Muldoon MF, Jennings JR, et al. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep 2008;31:635–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi KM, Lee JS, Park HS, et al. Relationship between sleep duration and the metabolic syndrome: Korean National Health and Nutrition Survey 2001. Int J Obes (Lond) 2008;32:1091–1097 [DOI] [PubMed] [Google Scholar]
- 14.Jennings JR, Muldoon MF, Hall M, et al. Self reported sleep duration is associated with metabolic syndrome. Sleep 2007;30:219–223 [DOI] [PubMed] [Google Scholar]
- 15.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–1235 [DOI] [PubMed] [Google Scholar]
- 16.Redline S, Storfer-Isser A, Rosen CL, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med 2007;176:401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruber A, Horwood F, Sithole J, et al. Obstructive sleep apnoea is independently associated with metabolic syndrome but not the insulin resistance state. Cardiovasc Diabetol 2006;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambrosetti M, Lucioni AM, Conti S, et al. Metabolic syndrome in obstructive sleep apnea and related cardiovascular risk. J Cardiovasc Med 2006;7:826–829 [DOI] [PubMed] [Google Scholar]
- 19.McArdle N, Hillman D, Beilin L, et al. Metabolic risk factors for vascular disease in obstructive sleep apnea. Am J Respir Crit Care Med 2007;175:190–195 [DOI] [PubMed] [Google Scholar]
- 20.Kono M, Tatsumi K, Saibara T, et al. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome. Chest 2007;131:1387–1392 [DOI] [PubMed] [Google Scholar]
- 21.Sasanabe R, Banno K, Otake K, et al. Metabolic syndrome in Japanese patients with obstructive sleep apnea syndrome. Hypertens Res 2006;29:315–322 [DOI] [PubMed] [Google Scholar]
- 22.Lam JC, Lam B, Lam CL, et al. Obstructive sleep apnea and the metabolic syndrome in community-based Chinese adults in Hong Kong. Respir Med 2006;100:980–987 [DOI] [PubMed] [Google Scholar]
- 23.Coughlin SR, Mawdsley L, Mugarza JA, et al. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J 2007;29:720–727 [DOI] [PubMed] [Google Scholar]
- 24.Stabe C, Vasques AC, Lima MM, et al. Neck circumference as a simple tool for identifying the metabolic syndrome and insulin resistance: Results from the Brazilian Metabolic Syndrome Study. Clin Endocrinol (Oxf ) 2013;78:874–881 [DOI] [PubMed] [Google Scholar]
- 25.Jamar G, Pisani LP, Oyama LM, et al. Is the neck circumference an emergent predictor for inflammatory status in obese adults? Int J Clin Pract 2013;67:217–224 [DOI] [PubMed] [Google Scholar]
- 26.Knutson KL, Zhao X, Mattingly M, et al. Predictors of sleep-disordered breathing in obese adults who are chronic short sleepers. Sleep Med 2012;13:484–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, et al. C-reactive protein and obstructive sleep apnea syndrome in children. Front Biosci (Elite Ed) 2012;4:2410–2422 [DOI] [PubMed] [Google Scholar]
- 28.Pasquali R, Vicennati V, Gambineri A, et al. Sex-dependent role of glucocorticoids and androgens in the pathophysiology of human obesity. Int J Obes (Lond) 2008;32:1764–1779 [DOI] [PubMed] [Google Scholar]
- 29.Cizza G, Marincola P, Mattingly M, et al. Treatment of obesity with extension of sleep duration: a randomized, prospective, controlled trial. Clin Trials 2010;7:274–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao J, Sussman DL, Summers RM. Fully automated adipose tissue measurement on abdominal CT. Orlando, FL: SPIE Medical Imaging, 2011 [Google Scholar]
- 31.de Jonge L, Zhao X, Mattingly MS, et al. NIDDK Sleep Extension Study Group Poor sleep quality and sleep apnea are associated with higher resting energy expenditure in obese individuals sleeping less than 6 ½ hours. J Clin Endocrinol Metab 2012;97:2881–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213 [DOI] [PubMed] [Google Scholar]
- 33.Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep 1991;14:540–545 [DOI] [PubMed] [Google Scholar]
- 34.Alberti KG, Eckel RH, Grundy SM, et al.; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 35.Macchia A, Levantesi G, Borrelli G, et al.; Associazione Nazionale Medici Cardiologi Ospedalieri; Istituto di Ricerche Farmacologiche Mario Negri-Consorzio Mario Negri Sud, Santa Maria Imbaro; Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico (GISSI)-Prevenzione Investigators A clinically practicable diagnostic score for metabolic syndrome improves its predictivity of diabetes mellitus: The Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico (GISSI)-Prevenzione scoring. Am Heart J. 2006;151:754..e7–754.e17. [DOI] [PubMed] [Google Scholar]
- 36.Sabanayagam C, Zhang R, Shankar A. Markers of sleep-disordered breathing and metabolic syndrome in a multiethnic sample of US Adults: Results from the National Health and Nutrition Examination Survey 2005–2008. Cardiol Res Pract 2012;2012:630802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang L, Samarasinghe YP, Kane P, et al. Visceral adiposity is closely correlated with neck circumference and represents a significant indicator of insulin resistance in WHO grade III obesity. Clin Endocrinol (Oxf ) 2010;73:197–200 [DOI] [PubMed] [Google Scholar]
- 38.Androutsos O, Grammatikaki E, Moschonis G, et al. Neck circumference: A useful screening tool of cardiovascular risk in children. Pediatr Obes 2012;3:187–195 [DOI] [PubMed] [Google Scholar]
- 39.Onat A, Hergenç G, Yüksel H, et al. Neck circumference as a measure of central obesity: Associations with metabolic syndrome and obstructive sleep apnea syndrome beyond waist circumference. Clin Nutr 2009;1:46–51 [DOI] [PubMed] [Google Scholar]
- 40.Zhou JY, Ge H, Zhu MF, et al. Neck circumference as an independent predictive contributor to cardio-metabolic syndrome. Cardiovasc Diabetol 2013;12:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang GR, Yuan SY, Fu HJ, et al. Neck circumference positively related with central obesity, overweight, and metabolic syndrome in Chinese subjects with type 2 diabetes: Beijing Community Diabetes Study 4. Diabetes Care 2010;33:2465–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoebel S, Malan L, de Ridder JH. Determining cut-off values for neck circumference as a measure of the metabolic syndrome amongst a South African cohort: The SABPA study. Endocrine 2012;42:335–342 [DOI] [PubMed] [Google Scholar]
- 43.Lindsay RS, Funahashi T, Hanson RL, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 2002;360:57–58 [DOI] [PubMed] [Google Scholar]
- 44.Norata GD, Raselli S, Grigore L, et al. Leptin:adiponectin ratio is an independent predictor of intima media thickness of the common carotid artery. Stroke 2007;38:2844–2846 [DOI] [PubMed] [Google Scholar]
- 45.Brandenberger G, Weibel L.The 24-h growth hormone rhythm in men: Sleep and circadian influences questioned. J Sleep Res 2004;13:251–255 [DOI] [PubMed] [Google Scholar]
- 46.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis 2008;196:696–703 [DOI] [PubMed] [Google Scholar]
- 47.Johnson NL, Kirchner HL, Rosen CL, et al. Sleep estimation using wrist actigraphy in adolescents with and without sleep disordered breathing: A comparison of three data modes. Sleep 2007;7:899–905 [DOI] [PMC free article] [PubMed] [Google Scholar]