Background: Asymmetrical distribution of specific phospholipids between the two leaflets of biological membranes is generated and maintained by transporters.
Results: A mutation in murine Atp11c results in altered morphology and shortened life span of erythrocytes.
Conclusion: Phospholipid transport by ATP11C maintains phospholipid asymmetry in erythrocytes.
Significance: Defects in phospholipid transport across the cell membrane can lead to anemia.
Keywords: Erythrocyte, Lipid Transport, Phosphatidylserine, Phospholipid, Plasma Membrane, ATP11C, Anemia, Flippase, P4 ATPases, Stomatocytosis
Abstract
Transmembrane lipid transporters are believed to establish and maintain phospholipid asymmetry in biological membranes; however, little is known about the in vivo function of the specific transporters involved. Here, we report that developing erythrocytes from mice lacking the putative phosphatidylserine flippase ATP11C showed a lower rate of PS translocation in vitro compared with erythrocytes from wild-type littermates. Furthermore, the mutant mice had an elevated percentage of phosphatidylserine-exposing mature erythrocytes in the periphery. Although erythrocyte development in ATP11C-deficient mice was normal, the mature erythrocytes had an abnormal shape (stomatocytosis), and the life span of mature erythrocytes was shortened relative to that in control littermates, resulting in anemia in the mutant mice. Thus, our findings uncover an essential role for ATP11C in erythrocyte morphology and survival and provide a new candidate for the rare inherited blood disorder stomatocytosis with uncompensated anemia.
Introduction
Mammalian erythrocytes are unique among eukaryotic cells in that they lack nuclei, cytoplasmic structures, and organelles. Because of this, the structural and functional properties of erythrocytes are closely associated with their plasma membranes (1). The erythrocyte plasma membrane consists of a lipid bilayer with an asymmetrical distribution of specific phospholipids between the two leaflets of the bilayer (2). Although phosphatidylcholine and sphingomyelin are concentrated predominantly in the outer monolayer of the erythrocyte membrane, phosphatidylserine (PS)5 and phosphatidylethanolamine are confined mainly to the cytoplasmic leaflet (2). The generation and maintenance of this asymmetric structure are essential for the function and survival of all cells, including erythrocytes (1, 2). The concentration of PS in the cytofacial leaflet of the plasma membrane provides important docking sites for a variety of signaling molecules (3) and, in particular in erythrocytes, plays an essential role in the modulation of the mechanical stability of the membrane through an interaction with the skeletal protein spectrin (4, 5). PS exposure on the surface of erythrocytes acts as an “eat-me” signal to phagocytes and also can lead to adherence of erythrocytes to the vascular endothelium and activation of surface-dependent plasma blood-clotting factors (2), as well as contribute to the anemia observed in a wide variety of blood disorders, including sickle cell anemia and thalassemia (6–10). Three different groups of enzymes are involved in establishing and maintaining phospholipid asymmetry, namely flippases, floppases, and scramblases (11, 12).
Erythrocytes have served as a model system to characterize phospholipid movement across bilayers. Considerable attempts have been made to isolate and identify the major aminophospholipid flippases from red blood cells and other membranes (13–17). Biochemical characterization suggests that the erythrocyte flippase is a Mg2+-dependent, vanadate-sensitive ATPase (13). Studies in yeast suggest that members of the P4-type ATPase family serve as flippases, selectively translocating PS and, to a lesser extent, phosphatidylethanolamine into the cytoplasmic leaflet of cell membranes (11, 12, 18). Although there are 15 members of the P4-type ATPase family in mice (14 members in humans) (12), their biological functions are largely unknown (19), and so far, only a few flippases have been linked to biological functions in mammalians. Among these, mutations in the gene encoding ATP8A2 in humans and mice have been shown to cause neurological disorders (20–22). In addition, ATP8B1 and ATP11C have been implicated in bile secretion in mice, which involves translocation of phospholipids (23, 24). Furthermore, we and others recently discovered that ATP11C, which is encoded on the X chromosome in humans and mice, is required for the development of adult B-cells (25, 26). With regard to erythrocytes, ATP8A1 has been proposed to be the molecular correlate of the erythrocyte flippase activity. The protein was detected in the plasma membrane of mature erythrocytes, and flippase activity was demonstrated upon expression in yeast (27). However, the biochemical characteristics of ATP8A1 are consistent with the flippase from chromaffin granules, which is different from the major flippase activity observed in erythrocytes (28). Consistently, erythrocytes from Atp8a1−/− animals exhibit no increased PS exposure on their surface, most likely due to expression of other aminophospholipid flippases (29). In our initial study on the role of ATP11C in B-cells (25), we also noted a significant reduction in the number of total erythrocytes in the blood of ATP11C mutant mice. Here, we investigated this anemia and demonstrate that the mutant erythrocytes have a shortened life span and an abnormal shape manifesting itself as a pronounced stomatocytosis. Impaired flippase activity during erythropoiesis was confirmed in the mutant mice, and circulating mutant erythrocytes were shown to have elevated surface exposure of PS that increased with the age of the cells.
EXPERIMENTAL PROCEDURES
Mice
The ambrosius mouse strain with an X-linked N-ethyl-N-nitrosourea-induced point mutation in Atp11c has been described previously (25). All animal procedures were approved by the Australian National University Animal Ethics and Experimentation Committee.
Analysis of Hematologic Parameters
An ADVIA 2120 hematology system (Siemens Healthcare Diagnostics) was used for the analysis of blood parameters.
Clinical Chemistry
Serum concentrations of iron parameters were assayed by automated procedures in the Department of Pathology at the Canberra Hospital.
Flow Cytometry
Procedures for surface and annexin V staining have been described previously (25). For flow cytometry analysis, the following antibodies were used: CD71-biotin or CD71-phycoerythrin (clone C2), Ter119-FITC or Ter119-phycoerythrin-Cy7, CD44-allophycocyanin (clone IM7), streptavidin-allophycocyanin (all from BD Biosciences), and annexin V-Pacific Blue (BioLegend).
In Vivo Erythrocyte Life Span Analysis
In vivo life span measurements using carboxyfluorescein diacetate succinimidyl ester (CFSE) in conjunction with mathematical modeling were performed as described previously (30).
Flippase Assay
Flippase activity was assessed as described previously (25) with the modification that 1-palmitoyl-2-(6-((7-nitro-2-1,3-benzoxadiazol-4-yl)amino)hexanoyl)-sn-glycero-3-phosphoserine (NBD-PS; Avanti Polar Lipids) was used in place of 1-palmitoyl-2-(12-((7-nitro-2-1,3-benzoxadiazol-4-yl)amino)dodecanoyl)-sn-glycero-3-phosphoserine, and all incubations were performed at 15 °C instead of 37 °C to avoid nonspecific uptake of the PS analog.
Cation Measurement
Intra- and extracellular Na+ and K+ levels were determined by ultra HPLC (Dionex) linked to a charged aerosol detector (Dionex) as described previously (31). In brief, 10 μl of heparinized blood was separated by centrifugation into erythrocyte and plasma fractions. The erythrocytes were washed once with 250 mm MgSO4 solution to replace extracellular Na+ and K+. The resulting cell pellet and 1 μl of the plasma were added to 200 and 100 μl, respectively, of 20 mm ammonium sulfate (pH 5) and acetonitrile (40:60, v/v) to precipitate proteins. The samples were centrifuged, and 10-μl aliquots of the resulting supernatant solutions were subjected to HPLC analysis.
Scanning Electron Microscopy Analysis
Cells attached to coverslips were fixed with 2% (v/v) glutaraldehyde in 0.1 m sodium cacodylate overnight at 4 °C and post-fixed with 1% (w/v) osmium tetroxide for 1.5 h at room temperature. The samples were dehydrated using a graded ethanol series, critical point-dried, mounted on aluminum stubs, and sputter-coated with gold prior to imaging. Images were taken using a Zeiss Ultra Plus field emission scanning electron microscope.
Osmotic Fragility Test
Peripheral blood from wild-type and ATP11C-deficient mice was diluted to an approximate hematocrit of 10% with PBS, followed by a further 1:10 dilution in NaCl solutions of varying osmolarity (0.1–0.8% (w/v) NaCl in H2O). After incubation for 30 min at room temperature, samples were centrifuged, and the absorbance of the supernatant solutions was measured at 540 nm using a microplate reader (ThermoMax, Molecular Devices). Percentage hemolysis of the cells in each solution was calculated based on the A540 value relative to that obtained for suspended cells (and thereby lysed) in H2O.
Statistical Analysis
For comparison of only two groups, Student's two-tailed t test was used. When multiple experimental groups were compared, one-way analysis of variance followed by pairwise comparison with a Bonferroni post-test were used. All statistical analyses were performed using GraphPad Prism software.
RESULTS AND DISCUSSION
ATP11C Deficiency in Mice Results in Anemia
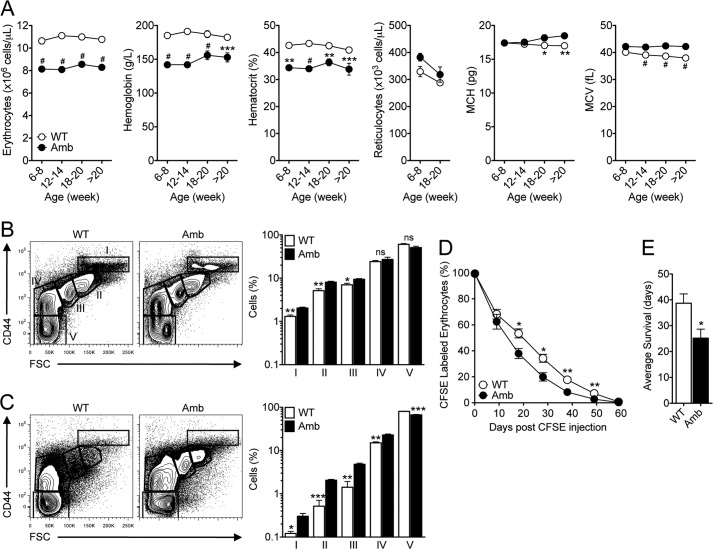
To extend our previous finding of anemia in ATP11C-deficient mice (25), we analyzed different hematologic parameters in the blood of mutant male mice and their control littermates. Mutant mice showed an ∼25% reduction in the number of erythrocytes compared with wild-type littermates in all age groups (Fig. 1A). Although the relative proportion of immature erythrocytes (reticulocytes) was increased from 2.85 ± 0.2% to 4.24 ± 0.2% in the blood of 6–8-week-old ATP11C mutant animals, the absolute number of reticulocytes was not significantly increased (Fig. 1A). Interestingly, erythrocytes from mutant mice were larger, with a higher mean corpuscular volume compared with controls in all age groups (Fig. 1A). Similar results were found in homozygous mutant female mice (data not shown). In contrast, Atp11camb/+ heterozygous female mice had a normal erythrocyte count in the peripheral blood (data not shown). These results reveal that ATP11C deficiency in mice causes anemia and suggest that ATP11C plays a significant role in the development and/or survival of erythrocytes.
FIGURE 1.
Anemia in mice with a point mutation in Atp11c due to a reduced erythrocyte life span despite normal erythropoiesis. A, the graphs show the number of erythrocytes, hemoglobin, hematocrit, the number of reticulocytes, mean corpuscular hemoglobin (MCH), and mean corpuscular volume (MCV) in the blood of Atp11c+/0 (WT, ○) and Atp11camb/0 (Amb, ●) animals of the indicated age groups. The symbols represent means ± S.E., with four to eight mice per genotype in each age group. B and C, representative flow cytometric contour plots of CD44 and forward scatter (FSC) profiles of the bone marrow (B) and spleens (C) of Atp11c+/0 and Atp11camb/0 animals. The plots are pre-gated on CD71+ and Ter119+ erythrocytes. Bar graphs represent means ± S.E. of the percentage of cells in the outlined areas in the flow cytometric plots. Data are representative of at least five independent experiments, with one to four mice per genotype in each. D, the graph shows means ± S.E. of the percentage of CFSE-labeled erythrocytes in the blood of Atp11c+/0 (○) and Atp11camb/0 (●) mice after in vivo labeling. E, average erythrocyte life span calculated by log-normal modeling of the data presented in D. The bar graph represents means ± S.E. of the average survival of erythrocytes. Data are representative of two independent experiments, with four to five mice per genotype in each. Statistical significance was calculated using one-way analysis of variance analysis, followed by the Bonferroni post-test, with the p values comparing mice of each age group shown on the plots (A) or Student's t two-tailed test (B–E). ns, not significant; #, p < 0.0001; ***, p < 0.001; **, p < 0.01; *, p < 0.05.
Normal Erythropoiesis but Reduced Erythrocyte Life Span in ATP11C-deficient Mice
To determine whether ATP11C is required for erythropoiesis, we examined the early stages of erythroid development in bone marrow and spleen. Stepwise analysis of different stages of erythroblast differentiation based on expression of the surface marker CD44 and forward scatter profile (32) showed that ATP11C-deficient mice had essentially normal erythropoiesis in bone marrow and spleen (Fig. 1, B and C). These results indicate that animals deficient in ATP11C have no block in erythrocyte development.
Thus, we next focused on the life span of mature erythrocytes to explain the observed anemia in ATP11C-deficient animals. The life span of erythrocytes was investigated using dilution of a fluorescent label (CFSE) in vivo and mathematical modeling as described previously (30). Erythrocytes from ATP11C-deficient animals exhibited reduced survival compared with cells from their control littermates as determined by the percentage of CFSE-labeled erythrocytes in the blood (Fig. 1D). Erythrocyte survival data showed an average life span of 25.2 ± 3.5 days in mutant animals compared with 38.7 ± 3.6 days in control littermates (presented as means ± S.E., p < 0.05) (Fig. 1E). These data indicate that ATP11C plays an important role in the survival of erythrocytes.
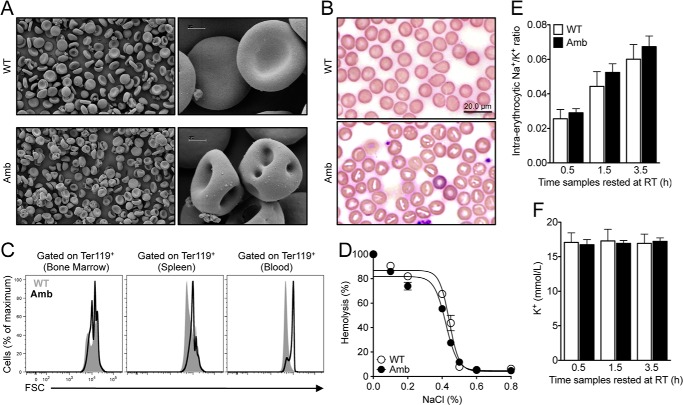
ATP11C-deficient Erythrocytes Form Stomatocytes
The composition and asymmetric distribution of phospholipids in the cell membrane are essential for maintaining the normal biconcave shape of erythrocytes (5). Morphological studies of erythrocytes from the blood of ATP11C-deficient and littermate control mice were performed using scanning electron microcopy. As shown in Fig. 2A, the majority of mutant erythrocytes showed a distinct change in morphology known as stomatocytosis. These results were confirmed with peripheral blood smears (Fig. 2B). Consistent with an increased mean corpuscular volume, flow cytometry analysis revealed that erythrocytes in the blood and spleens from ATP11C-deficient animals were significantly larger than erythrocytes from control animals as determined by an increase in their forward scatter profile (Fig. 2C). This increased size was first apparent in mature erythrocytes in bone marrow, but also in erythrocyte precursors in spleen (Fig. 1, B and C).
FIGURE 2.
Abnormally shaped erythrocytes in the periphery of ATP11C-deficient mice. A, scanning electron microscopy analysis of erythrocytes from the blood of Atp11c+/0 (WT) and Atp11camb/0 (Amb) animals. B, peripheral blood smears from Atp11c+/0 and Atp11camb/0 animals. C, representative overlay histograms of the forward scatter (FSC) profile in Ter119+ erythrocytes from the bone marrow, spleens, and blood of Atp11c+/0 (gray areas) and Atp11camb/0 (black lines) animals. Data are representative of three independent experiments, with four to six mice per genotype in each. D, osmotic fragility of erythrocytes from Atp11c+/0 (○) and Atp11camb/0 (●) mice. The symbols represent means ± S.E. of the percentage of hemolysis. Data are representative of three independent experiments, with four to five mice per genotype in each. E, ratio of intra-erythrocytic Na+ to K+ in Atp11c+/0 and Atp11camb/0 animals analyzed by HPLC within 30 min of blood collection (0.5 h) and after resting at room temperature (RT) for 1.5 and 3.5 h. The bar graph represents means ± S.E. of the ratio of intra-erythrocytic Na+ to K+ content. F, HPLC measurement of K+ in the plasma of Atp11c+/0 and Atp11camb/0 animals within 30 min of blood collection (0.5 h) and after resting at room temperature for 1.5 and 3.5 h. The bar graph represents means ± S.E. of the plasma K+ content. Data are representative of two independent experiments, with four to five mice per genotype in each.
The effect of the mutation on osmotic fragility was assessed by incubating erythrocytes from ATP11C-deficient mice and wild-type littermates in solutions of varying osmolarity and measuring the extent of hemolysis. Erythrocytes from ATP11C-deficient mice displayed a normal hemolysis profile (Fig. 2D), suggesting that their surface-to-volume ratio and cell hydration are comparable with those in erythrocytes from wild-type mice.
No Effect of ATP11C on Na+ and K+ Homeostasis
Stomatocytosis has been associated with altered cation transport across the erythrocyte membrane and, consequently, perturbation of erythrocyte Na+ and K+ homeostasis (33). In some cases, the cation transport abnormality is enhanced upon reduction of the temperature to which the cells are exposed (34). Comparison of the ratio of Na+ to K+ in erythrocytes from ATP11C-deficient mice with that from control mice using ion chromatography revealed no difference between the two groups (Fig. 2E). Measurement of the plasma K+ concentration in blood samples 30 min after blood collection and after incubation for an additional 1 and 3 h at room temperature (to enhance any temperature-dependent K+ leak that might be present) revealed no significant difference in the plasma K+ concentrations in ATP11C-deficient and wild-type mice (Fig. 2F). Therefore, ATP11C deficiency apparently has no significant effect on erythrocyte Na+ and K+ homeostasis.
Hereditary stomatocytosis in humans can cause iron overload (33). We therefore measured serum iron and transferrin in 8–10- and 18–20-week-old ATP11C-deficient mice and their wild-type littermates. Serum iron and transferrin levels were comparable between mutant and wild-type animals and between both age groups (data not shown), thus excluding iron overload as a feature of the stomatocytosis seen in these mice.
Lower PS Flippase Activity in Erythroblasts from ATP11C-deficient Mice
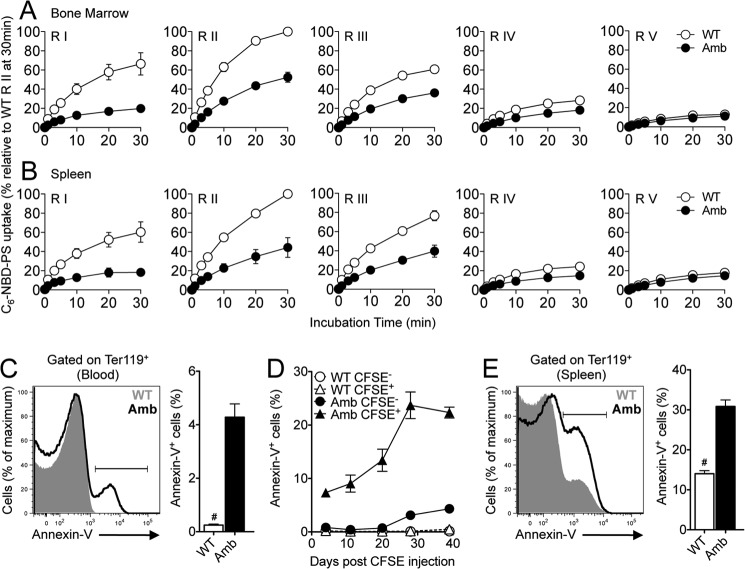
We demonstrated previously that developing B-lymphocytes in ATP11C-deficient mice display impaired PS internalization relative to B-lymphocytes in wild-type mice (25). To investigate whether there is a similar defect in developing or mature erythrocytes, we performed an in vitro flippase activity assay using a fluorescent PS analog (NBD-PS). We first tested flippase activity in the different erythroid stages (proerythroblasts, basophilic erythroblasts, polychromatic erythroblasts, orthochromatic erythroblasts and reticulocytes, and mature red blood cells): gated as regions I–V, respectively, in Fig. 1 (B and C) in bone marrow and spleen, separated on the basis of their CD44 and forward scatter profiles (32). Erythroid precursors (regions I–III) from wild-type mice took up NBD-PS rapidly, whereas orthochromatic erythroblasts and reticulocytes and mature erythrocytes (regions IV and V, respectively) showed a significantly reduced overall NBD-PS uptake (Fig. 3, A and B), indicating that flippase activity decreases with erythrocyte maturity. At all stages, NBD-PS uptake approached equilibrium by 30 min (Fig. 3, A and B). In comparison, the uptake of NBD-PS by ATP11C mutant erythroid precursors (regions I–III) was significantly slower (Fig. 3, A and B). The difference in NBD-PS internalization between wild-type and ATP11C-deficient cells was less marked in the more mature forms (regions IV and V) (Fig. 3, A and B). Similarly, there was a very low uptake of NBD-PS in erythrocytes from peripheral blood of wild-type and ATP11C-deficient mice, with only a minimal reduction in mutant erythrocytes (data not shown).
FIGURE 3.
Reduced flippase activity in mutant erythroblasts and increased PS exposure in mature erythrocytes from ATP11C-deficient animals. A and B, the graphs show C6-NBD-PS uptake after 0-, 1-, 3-, 5-, 10-, 20-, and 30-min incubations in proerythroblasts, basophilic erythroblasts, polychromatic erythroblasts, orthochromatic erythroblasts and reticulocytes, and mature red blood cells (regions (R) I–V, respectively; gated as in Fig. 1, B and C) in the bone marrow (A) and spleens (B) from Atp11c+/0 (WT, ○) and Atp11camb/0 (Amb, ●) mice. The graphs represent means ± S.E. of the percentage of C6-NBD-PS uptake relative to wild-type region II at 30 min, at which the highest rate of PS internalization was observed. Data are pooled from five independent experiments, with one mouse per genotype in each. C, representative overlay histogram of annexin V staining in Ter119+ erythrocytes in the blood of Atp11c+/0 (gray area) and Atp11camb/0 (black line) animals. The bar graph represents means ± S.E. of the percentage of annexin V+ cells in Ter119+ erythrocytes. Data are representative of at least three independent experiments, with four to six mice per genotype in each. D, the graph shows means ± S.E. of the percentage of annexin V+ cells in circulating CFSE− or CFSE+ erythrocytes in the blood of Atp11c+/0 and Atp11camb/0 mice after in vivo labeling. E, representative overlay histogram of annexin V staining in Ter119+ erythrocytes in the spleens of Atp11c+/0 (gray area) and Atp11camb/0 (black line) animals. The bar graph represents means ± S.E. of the percentage of annexin V+ cells in Ter119+ erythrocytes. Data are representative of at least three independent experiments, with four to six mice per genotype in each. Statistical significance was calculated using Student's two-tailed t test. #, p < 0.0001.
Increased PS Exposure on the Surface of ATP11C-deficient Erythrocytes
The appearance of PS in the exoplasmic leaflet of erythrocyte membranes serves as an eat-me signal for the recognition and clearance of erythrocytes by phagocytes (35, 36). To determine whether the defective flippase activity in mutant animals results in increased surface accumulation of PS, erythrocytes from the peripheral blood were stained with annexin V, which binds to PS in the exoplasmic leaflet (37). The percentage of annexin V+ erythrocytes in the blood of ATP11C-deficient mice was 17-fold higher than in control littermates, in which there were virtually no annexin V+ erythrocytes (4.28 ± 0.50% versus 0.25 ± 0.05%, p < 0.0001) (Fig. 3C). We next determined the extent of annexin V binding as a function of erythrocyte age using in vivo CFSE labeling. At all ages tested (newly generated to 50 days of age), there was virtually no annexin V binding by wild-type erythrocytes (Fig. 3D). However, as ATP11C-deficient erythrocytes aged, there was a gradual increase in the number that bound annexin V from 4.3% of newly formed erythrocytes (CFSE−) to >20% of erythrocytes ≥39 days of age (CFSE+) (Fig. 3D). In agreement with the accumulation of PS on aged erythrocytes, we also found a >2-fold increase in the frequency of annexin V+ erythrocytes in the spleens of mutant mice compared with their control littermates (31 ± 2% versus 14 ± 1%, p < 0.0001) (Fig. 3E). In contrast, no difference in annexin V staining was observed on erythroblasts in the bone marrow of wild-type and ATP11C-deficient mice (data not shown). These data demonstrate that ATP11C-deficient erythrocytes accumulate PS on their surface in the peripheral circulation. Significantly reduced in vitro uptake of the fluorescently labeled PS analog NBD-PS by mutant erythroblasts but not by mature erythrocytes is consistent with the fact that the protein is expressed predominantly during erythropoiesis (38). Alternatively, it may also indicate that, in ATP11C-deficient erythrocytes, the reduced rate of PS flipping during the early developmental stages may have a lasting effect on membrane composition, resulting in reduced erythrocyte deformability, increased intravascular damage, and hence PS exposure.
In addition to ATP11C, several other P4-type ATPases are expressed during erythropoiesis, with ATP8A1 and ATP11B being particularly prominent in primitive, fetal, and adult erythroblasts (38). Interestingly, Atp8a1−/− mice exhibited no erythrocyte phenotype, which can be explained by the compensatory expression of ATP8A2 in Atp8a1−/− erythrocytes (29). The observation of reduced but not absent PS translocation in ATP11C-deficient erythroblasts suggests that other translocases may still be present in mutant erythroblasts. In addition to their effect on phospholipid translocation, P4-type ATPases have also been linked to vesicle trafficking (12, 18). This raises the possibility that loss of ATP11C activity may result in defects in membrane or protein trafficking, including trafficking of other translocases like ATP8A1 that are expressed in erythrocytes. Furthermore, lack of ATP11C may lead to a compensatory overexpression of ATP8A1 and other P4-type ATPases that partially compensates for the lack of ATP11C. Differentiation between a direct or more indirect effect of ATP11C defects on PS translocation will require further studies, including the development of better antibodies against all P4-type ATPases, which go beyond the scope of this study.
The abnormal stomatocyte shape of erythrocytes in ATP11C mutant mice has been found to be independent of cation leaks or hydration status and suggests that an alternative mechanism is responsible for the stomatocytosis in these mice. One possibility is a mechanism similar to that responsible for Mediterranean stomatocytosis (39), in which a lack of control over sterol absorption and excretion leads to changes in the plasma lipid composition and presumably the membrane lipid composition of circulating cells. Alterations in the relative lipid composition of the erythrocyte membrane can lead to an expansion or contraction of either the inner or outer leaflet, resulting in altered erythrocyte shape (40–42). In addition, reduced accumulation of PS at the cytofacial leaflet is predicted to interfere with the interaction between skeletal proteins and the membrane (4, 5). It is therefore plausible that a disturbance in these interactions underlies the observed formation of stomatocytes in ATP11C mutant mice.
In conclusion, the results presented in this study reveal an important functional role for ATP11C in erythrocyte biology and raise the question of whether mutations in ATP11C serve as a previously unclassified cause of stomatocytosis in humans. Whether the observed blood phenotypes in ATP11C-deficient mice occur as a result of changes to the erythrocyte membrane composition and/or through changes to the interaction between erythrocytes and other cells, e.g. splenic macrophages, will be an important area for future studies.
Acknowledgments
We thank Jennifer Kofler and Ayla Lorenzo for help in the analysis of hematological parameters, Cathy Gillespie for help in scanning electron microscopy analysis, Anne Prins for staining of blood smears, the staff of the Microscopy and Cytometry Resource Facility at The John Curtin School of Medical Research, and the staff of the Australian Phenomics Facility for animal husbandry.
This work was supported in part by National Health and Medical Research Council Grant GNT1061288.
This article was selected as a Paper of the Week.
- PS
- phosphatidylserine
- CFSE
- carboxyfluorescein diacetate succinimidyl ester
- NBD-PS
- 1-palmitoyl-2-(6-((7-nitro-2-1,3-benzoxadiazol-4-yl)amino)hexanoyl)-sn-glycero-3-phosphoserine.
REFERENCES
- 1. Mohandas N., Gallagher P. G. (2008) Red cell membrane: past, present, and future. Blood 112, 3939–3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daleke D. L. (2008) Regulation of phospholipid asymmetry in the erythrocyte membrane. Curr. Opin. Hematol. 15, 191–195 [DOI] [PubMed] [Google Scholar]
- 3. Leventis P. A., Grinstein S. (2010) The distribution and function of phosphatidylserine in cellular membranes. Annu. Rev. Biophys. 39, 407–427 [DOI] [PubMed] [Google Scholar]
- 4. An X., Guo X., Sum H., Morrow J., Gratzer W., Mohandas N. (2004) Phosphatidylserine binding sites in erythroid spectrin: location and implications for membrane stability. Biochemistry 43, 310–315 [DOI] [PubMed] [Google Scholar]
- 5. Manno S., Takakuwa Y., Mohandas N. (2002) Identification of a functional role for lipid asymmetry in biological membranes: phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc. Natl. Acad. Sci. U.S.A. 99, 1943–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wood B. L., Gibson D. F., Tait J. F. (1996) Increased erythrocyte phosphatidylserine exposure in sickle cell disease: flow-cytometric measurement and clinical associations. Blood 88, 1873–1880 [PubMed] [Google Scholar]
- 7. Kuypers F. A., Yuan J., Lewis R. A., Snyder L. M., Kiefer C. R., Bunyaratvej A., Fucharoen S., Ma L., Styles L., de Jong K., Schrier S. L. (1998) Membrane phospholipid asymmetry in human thalassemia. Blood 91, 3044–3051 [PubMed] [Google Scholar]
- 8. de Jong K., Emerson R. K., Butler J., Bastacky J., Mohandas N., Kuypers F. A. (2001) Short survival of phosphatidylserine-exposing red blood cells in murine sickle cell anemia. Blood 98, 1577–1584 [DOI] [PubMed] [Google Scholar]
- 9. Kean L. S., Brown L. E., Nichols J. W., Mohandas N., Archer D. R., Hsu L. L. (2002) Comparison of mechanisms of anemia in mice with sickle cell disease and beta-thalassemia: peripheral destruction, ineffective erythropoiesis, and phospholipid scramblase-mediated phosphatidylserine exposure. Exp. Hematol. 30, 394–402 [DOI] [PubMed] [Google Scholar]
- 10. Yasin Z., Witting S., Palascak M. B., Joiner C. H., Rucknagel D. L., Franco R. S. (2003) Phosphatidylserine externalization in sickle red blood cells: associations with cell age, density, and hemoglobin F. Blood 102, 365–370 [DOI] [PubMed] [Google Scholar]
- 11. Daleke D. L. (2007) Phospholipid flippases. J. Biol. Chem. 282, 821–825 [DOI] [PubMed] [Google Scholar]
- 12. Sebastian T. T., Baldridge R. D., Xu P., Graham T. R. (2012) Phospholipid flippases: building asymmetric membranes and transport vesicles. Biochim. Biophys. Acta 1821, 1068–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moriyama Y., Nelson N. (1988) Purification and properties of a vanadate- and N-ethylmaleimide-sensitive ATPase from chromaffin granule membranes. J. Biol. Chem. 263, 8521–8527 [PubMed] [Google Scholar]
- 14. Morrot G., Zachowski A., Devaux P. F. (1990) Partial purification and characterization of the human erythrocyte Mg2+-ATPase. A candidate aminophospholipid translocase. FEBS Lett. 266, 29–32 [DOI] [PubMed] [Google Scholar]
- 15. Daleke D. L., Cornely-Moss K., Lyles J., Smith C. M., Zimmerman M. (1992) Identification and characterization of a candidate phosphatidylserine-transporting ATPase. Ann. N.Y. Acad. Sci. 671, 468–470 [DOI] [PubMed] [Google Scholar]
- 16. Auland M. E., Roufogalis B. D., Devaux P. F., Zachowski A. (1994) Reconstitution of ATP-dependent aminophospholipid translocation in proteoliposomes. Proc. Natl. Acad. Sci. U.S.A. 91, 10938–10942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Auland M. E., Morris M. B., Roufogalis B. D. (1994) Separation and characterization of two Mg2+-ATPase activities from the human erythrocyte membrane. Arch. Biochem. Biophys 312, 272–277 [DOI] [PubMed] [Google Scholar]
- 18. Muthusamy B. P., Natarajan P., Zhou X., Graham T. R. (2009) Linking phospholipid flippases to vesicle-mediated protein transport. Biochim. Biophys. Acta 1791, 612–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van der Mark V. A., Elferink R. P., Paulusma C. C. (2013) P4 ATPases: flippases in health and disease. Int. J. Mol. Sci. 14, 7897–7922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cacciagli P., Haddad M. R., Mignon-Ravix C., El-Waly B., Moncla A., Missirian C., Chabrol B., Villard L. (2010) Disruption of the ATP8A2 gene in a patient with a t(10;13) de novo balanced translocation and a severe neurological phenotype. Eur. J. Hum. Genet. 18, 1360–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu X., Libby R. T., de Vries W. N., Smith R. S., Wright D. L., Bronson R. T., Seburn K. L., John S. W. (2012) Mutations in a P-type ATPase gene cause axonal degeneration. PLoS Genet. 8, e1002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Onat O. E., Gulsuner S., Bilguvar K., Nazli Basak A., Topaloglu H., Tan M., Tan U., Gunel M., Ozcelik T. (2013) Missense mutation in the ATPase, aminophospholipid transporter protein ATP8A2 is associated with cerebellar atrophy and quadrupedal locomotion. Eur. J. Hum. Genet. 21, 281–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bull L. N., van Eijk M. J., Pawlikowska L., DeYoung J. A., Juijn J. A., Liao M., Klomp L. W., Lomri N., Berger R., Scharschmidt B. F., Knisely A. S., Houwen R. H., Freimer N. B. (1998) A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat. Genet. 18, 219–224 [DOI] [PubMed] [Google Scholar]
- 24. Siggs O. M., Schnabl B., Webb B., Beutler B. (2011) X-linked cholestasis in mouse due to mutations of the P4-ATPase ATP11C. Proc. Natl. Acad. Sci. U.S.A. 108, 7890–7895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yabas M., Teh C. E., Frankenreiter S., Lal D., Roots C. M., Whittle B., Andrews D. T., Zhang Y., Teoh N. C., Sprent J., Tze L. E., Kucharska E. M., Kofler J., Farell G. C., Bröer S., Goodnow C. C., Enders A. (2011) ATP11C is critical for the internalization of phosphatidylserine and differentiation of B lymphocytes. Nat. Immunol. 12, 441–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Siggs O. M., Arnold C. N., Huber C., Pirie E., Xia Y., Lin P., Nemazee D., Beutler B. (2011) The P4-type ATPase ATP11C is essential for B lymphopoiesis in adult bone marrow. Nat. Immunol. 12, 434–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soupene E., Kuypers F. A. (2006) Identification of an erythroid ATP-dependent aminophospholipid transporter. Br. J. Haematol. 133, 436–438 [DOI] [PubMed] [Google Scholar]
- 28. Daleke D. L., Lyles J. V. (2000) Identification and purification of aminophospholipid flippases. Biochim. Biophys. Acta 1486, 108–127 [DOI] [PubMed] [Google Scholar]
- 29. Levano K., Punia V., Raghunath M., Debata P. R., Curcio G. M., Mogha A., Purkayastha S., McCloskey D., Fata J., Banerjee P. (2012) Atp8a1 deficiency is associated with phosphatidylserine externalization in hippocampus and delayed hippocampus-dependent learning. J. Neurochem. 120, 302–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coupland L. A., Cromer D., Davenport M. P., Parish C. R. (2010) A novel fluorescent-based assay reveals that thrombopoietin signaling and Bcl-XL influence, respectively, platelet and erythrocyte life spans. Exp. Hematol. 38, 453.e1–461.e1 [DOI] [PubMed] [Google Scholar]
- 31. Winterberg M., Rajendran E., Baumeister S., Bietz S., Kirk K., Lingelbach K. (2012) Chemical activation of a high-affinity glutamate transporter in human erythrocytes and its implications for malaria-parasite-induced glutamate uptake. Blood 119, 3604–3612 [DOI] [PubMed] [Google Scholar]
- 32. Chen K., Liu J., Heck S., Chasis J. A., An X., Mohandas N. (2009) Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc. Natl. Acad. Sci. U.S.A. 106, 17413–17418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bruce L. J. (2009) Hereditary stomatocytosis and cation-leaky red cells–recent developments. Blood Cells Mol. Dis. 42, 216–222 [DOI] [PubMed] [Google Scholar]
- 34. Stewart G. W. (2004) Hemolytic disease due to membrane ion channel disorders. Curr. Opin. Hematol. 11, 244–250 [DOI] [PubMed] [Google Scholar]
- 35. McEvoy L., Williamson P., Schlegel R. A. (1986) Membrane phospholipid asymmetry as a determinant of erythrocyte recognition by macrophages. Proc. Natl. Acad. Sci. U.S.A. 83, 3311–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Connor J., Pak C. C., Schroit A. J. (1994) Exposure of phosphatidylserine in the outer leaflet of human red blood cells. Relationship to cell density, cell age, and clearance by mononuclear cells. J. Biol. Chem. 269, 2399–2404 [PubMed] [Google Scholar]
- 37. Koopman G., Reutelingsperger C. P., Kuijten G. A., Keehnen R. M., Pals S. T., van Oers M. H. (1994) Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84, 1415–1420 [PubMed] [Google Scholar]
- 38. Kingsley P. D., Greenfest-Allen E., Frame J. M., Bushnell T. P., Malik J., McGrath K. E., Stoeckert C. J., Palis J. (2013) Ontogeny of erythroid gene expression. Blood 121, e5–e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ducrou W., Kimber R. J. (1969) Stomatocytes, haemolytic anaemia and abdominal pain in Mediterranean migrants. Some examples of a new syndrome? Med. J. Aust. 2, 1087–1091 [PubMed] [Google Scholar]
- 40. Sheetz M. P., Singer S. J. (1974) Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc. Natl. Acad. Sci. U.S.A. 71, 4457–4461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seigneuret M., Devaux P. F. (1984) ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc. Natl. Acad. Sci. U.S.A. 81, 3751–3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Daleke D. L., Huestis W. H. (1985) Incorporation and translocation of aminophospholipids in human erythrocytes. Biochemistry 24, 5406–5416 [DOI] [PubMed] [Google Scholar]