Background: Role of AEG-1, an HIV-1 neuropathology-associated gene, in astrocytes is unclear.
Results: AEG-1 regulates astrocyte NF-κB activation and nuclear translocation, increases YY1 expression, and decreases EAAT2 expression and glutamate clearance.
Conclusion: Elevated levels of astrocyte AEG-1 promote neuroinflammation by impairing glutamate clearance and up-regulating NF-κB signal transduction.
Significance: Targeting AEG-1 may be an effective therapy for HIV-1-associated neuroinflammation.
Keywords: Astrocyte, Cancer, Human Immunodeficiency Virus (HIV), Neurodegeneration, Neuroinflammation, AEG-1, HIV-1-associated Neurocognitive Disorders
Abstract
Astrocyte elevated gene-1 (AEG-1), a novel human immunodeficiency virus (HIV)-1 and tumor necrosis factor (TNF)-α-inducible oncogene, has generated significant interest in the field of cancer research as a therapeutic target for many metastatic aggressive tumors. However, little is known about its role in astrocyte responses during HIV-1 central nervous system (CNS) infection and whether it contributes toward the development of HIV-associated neurocognitive disorders (HAND). Therefore, in this study, we investigated changes in AEG-1 CNS expression in HIV-1-infected brain tissues and elucidated a potential mechanism of AEG-1-mediated regulation of HAND. Immunoblotting and immunohistochemical analyses of HIV-1 seropositive and HIV-1 encephalitic human brain tissues revealed significantly elevated levels of AEG-1 protein. Immunohistochemical analyses of HIV-1 Tat transgenic mouse brain tissues also showed a marked increase in AEG-1 staining. Similar to in vivo observations, cultured astrocytes expressing HIV-1 Tat also revealed AEG-1 and cytokine up-regulation. Astrocytes treated with HAND-relevant stimuli, TNF-α, interleukin (IL)-1β, and HIV-1, also significantly induced AEG-1 expression and nuclear translocation via activation of the nuclear factor (NF)-κB pathway. Co-immunoprecipitation studies demonstrated IL-1β- or TNF-α-induced AEG-1 interaction with NF-κB p65 subunit. AEG-1 knockdown decreased NF-κB activation, nuclear translocation, and transcriptional output in TNF-α-treated astrocytes. Moreover, IL-1β treatment of AEG-1-overexpressing astrocytes significantly lowered expression of excitatory amino acid transporter 2, increased expression of excitatory amino acid transporter 2 repressor ying yang 1, and reduced glutamate clearance, a major transducer of excitotoxic neuronal damage. Findings from this study identify a novel transcriptional co-factor function of AEG-1 and further implicate AEG-1 in HAND-associated neuroinflammation.
Introduction
HIV-1 infection is a pandemic affecting 25–40 million people worldwide (1–3). Despite the advent of combined antiretroviral therapy, the neurological complications of HIV-1 infection of the CNS remain a common cause of morbidity. Ranging from mild to severe forms, collectively, these neurological deficits are termed as HIV-associated neurocognitive disorders (HAND)2 (4, 5). In many cases, HAND is associated with the accumulation of infected and/or activated macrophages/microglia in the CNS that secrete pro-inflammatory cytokines, such as IL-1β and TNF-α, chemokines, infectious virions, and potentially neurotoxic viral proteins. These HAND-relevant stimuli can affect neural function and survival by producing excitotoxic damage (6, 7). The molecular mechanisms driving HAND have, however, not been completely elucidated particularly with regard to the role of non-neural cells and the influence of antiretroviral therapy on the process.
In an attempt to identify novel proteins involved in mediating HAND, astrocyte elevated gene-1 (AEG-1), a multifunctional oncogene, was identified as an HIV-1- and TNF-α-inducible transcript in astrocytes (8). Following this discovery, AEG-1 has been extensively studied in the field of cancer research and is recognized as an oncogene implicated in tumor initiation, proliferation, metastasis, angiogenesis, and chemotherapeutic resistance of many metastatic malignancies (9–14). Thus far, the role of AEG-1 in HIV-1-stimulated astrocytes has not been investigated.
Currently, neuroinflammation associated with low level HIV-1 CNS infection is considered as an important histopathological correlate of HAND (15–17). Although HIV-1-associated neuroinflammation is triggered by brain resident microglia and infiltrating macrophages, it is tightly regulated by astrocytes, the most abundant cell type in the brain (15, 18, 19). Interestingly, HIV-1-infected astrocytes themselves do not strongly support virus replication but are important responders and mediators of HAND pathogenesis (19). Upon exposure to HAND-relevant factors, such as IL-1β, TNF-α, and/or HIV-1, astrocytes initiate multiple autocrine and paracrine inflammatory signaling nodes, leading to elevated production of cytokines and chemokines. In turn, these factors can enhance immune cell ingress across the blood-brain barrier, thereby contributing toward immune activation. However, the most important functional perturbation in astrocytes, responsible for HIV-1-associated neurotoxicity, is the deregulation of extracellular glutamate clearance (20, 21). Astrocytes in HAND-positive brain tissues show reduced levels of glutamate transporter and excitatory amino acid transporter 2 (EAAT2) and high levels of extracellular glutamate (22–24). Despite the ubiquitous presence of such dysfunctional astrocytes in the regions of most neuroinflammatory CNS disorders, the intracellular modulatory mechanisms responsible for EAAT2 loss and for driving the interplay between the astrocytes and the neurons during HAND remain largely enigmatic.
Recent studies in malignant gliomas that express very high levels of AEG-1 have implicated AEG-1 in glioma-associated necrosis and neurodegeneration by down-regulation of EAAT2 promoter activity (25, 26). However, AEG-1 induction and regulation of glutamate clearance in noncancerous astrocytes has not been investigated. In this report, we assessed the role of AEG-1 in astrocyte-mediated neurotoxicity and neuroinflammation in response to HIV-1 neuroinvasion and discuss the implications for HIV-1 CNS infection.
EXPERIMENTAL PROCEDURES
Human Postmortem Tissues
Brain tissue lysates from frontal cortex of 12 HIV-1-seropositive (HIV-1+), 11 HIV-1 encephalitic (HIVE) and 12 age-, race-, and gender-matched control subjects were provided by the National NeuroAIDS Tissue Consortium. The selected autopsy cohort characteristics, brain dissections, and protein extraction have been described previously (27). Of the total 12 HIVE postmortem tissues in the cohort, 11 were analyzed because of exclusion of one because of low protein quantities. The controls and HIV-1 tissues were selected based on matching age, race, and gender and excluding the subjects with confounding pathological findings such as bacterial and/or viral infection, cancer, or CNS disease.
HIV-1 Tat Transgenic Mice
Paraffin-embedded brain tissue specimens of wild type and HIV-1 Tat transgenic (GT-tg) mice with or without doxycycline (Dox) treatment, HIV-1 Tat positive (GT-tg + Dox) or HIV-1 Tat negative (GT-tg − Dox), described previously, were provided by Dr. Johnny J. He (28, 29). Briefly, HIV-1 Tat is expressed under the regulation of the astrocyte-specific glial fibrillary acidic protein (GFAP) promoter and is inducible with Dox, permitting astrocyte-specific expression.
Immunohistochemistry
Human postmortem brain tissue sections (formalin-fixed, paraffin-embedded (5 μm)), from frontal cortex, precentral gyrus, were provided by the National NeuroAIDS Tissue Consortium (27). The sections were deparaffinized in xylene and rehydrated through decreasing grades of alcohol up to water. Immunohistochemical analyses were performed by immunofluorescent staining. Briefly, deparaffinized sections, maintained in a humidifier chamber, were blocked at room temperature in 10% normal horse serum containing 0.1% Triton X-100 for 30 min and incubated overnight at 4 °C in blocking buffer with primary antibodies specific to AEG-1 (1:100, rabbit monoclonal; Invitrogen), microglial marker CD68 (1:100, mouse monoclonal, clone KP-1; DAKO, Carpinteria, CA), and GFAP (1:200, chicken polyclonal; Covance Inc., Emeryville, CA). Alexa Fluor secondary antibodies, anti-rabbit (488 nm, green) and anti-chicken (594 nm, red) (1:100; Invitrogen) were utilized to develop the sections. DAPI (1:800, Invitrogen) was used to visualize the nuclei. Fluorescent images were visualized with an ECLIPSE Ti-U microscope using the NLS-Elements BR. 3.0 software (Nikon, Melville, NY).
Isolation, Cultivation, and Treatment of Primary Human Astrocytes
Human astrocytes were isolated from first and early second trimester aborted specimens, obtained from the Birth Defects Laboratory of the University of Washington (Seattle, WA) in full compliance with the ethical guidelines of the National Institutes of Health. The institutional review boards of both the Universities of Washington and North Texas Health Science Center approved the collection of human tissues for research. The Birth Defects Laboratory obtained written consent from all tissue donors. Human astrocytes were isolated as previously described (30). Briefly, brain tissues were dissected and mechanically dissociated. Cell suspensions were centrifuged, suspended in media, and plated at a density of 20 × 106 cells/150 cm2. The adherent astrocytes were treated with trypsin and cultured under similar conditions to enhance the purity of replicating astroglial cells. The astrocyte preparations were routinely >99% pure as measured by immunocytochemical staining for GFAP and CD68 to determine possible microglial contamination and contribution of microglia in inflammatory responses. Astrocytes were treated with IL-1β (20 ng/ml), TNF-α (50 ng/ml), or HIV-1DJV (p24 20 ng/ml) alone or in combination for 24 h in astrocyte medium (DMEM: nutrient mixture F-12 supplemented with 10% FBS, penicillin/streptomycin/neomycin, and fungizone (Invitrogen)) (31).
RNA Extraction and Gene Expression Analyses
RNA was isolated (as described in Ref. 32) from astrocytes after 8 h of treatment or after 48 h of recovery for transfected cells. RNA was reverse-transcribed into cDNA as per the manufacturer's instructions (Invitrogen), and gene expression was quantified by TaqMan 5′ nuclease real time RT-PCR in 30-μl reactions, using a StepOnePlus sequence detection system according to the manufacturer's protocol (Invitrogen). The 30-μl reactions were carried out at 48 °C for 30 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min in 96-well optical, real time RT-PCR plates (Invitrogen). The following TaqMan gene expression assays were used: AEG-1 (catalog no. Hs00757841_m1), IκB kinase (IKK) β (catalog no. Hs00233287_m1), TNF-α (catalog no. Hs00174128 _m1), IL-1β (catalog no. Hs00174097_m1), EAAT2 (catalog no. Hs00188189_m1), and yin-yang (YY1) (catalog no. Hs009987_m1). HIV-1 Tat expression was confirmed by real time RT-PCR using the following HIV-1 Tat specific primers: forward, 5′-GGA AGC ATC CAG GAA GTC AG-3′, and reverse, 5′-GGA GGT GGG TTG CTT TGA TA-3′, with 5′-CCT CCT CAA GGC AGT CAG AC-3′ used as internal probe. GAPDH (catalog no. 4310884E), a ubiquitously expressed housekeeping gene, was used as internal normalizing control. Transcripts were quantified by the comparative CT method and are represented as fold change of control.
Immunofluorescent Cytochemical Analyses
Cultured human astrocytes were fixed after 24 h of post-HAND-relevant stimuli treatment and 72 h for pTat (a plasmid encoding full-length HIV-1 Tat protein)-transfected cells, with 1:1 acetone:methanol (v/v) solution for 20 min at −20 °C and blocked with blocking buffer (2% BSA in 1× PBS containing 0.1% Triton X-100) for 1 h. Cells were then incubated with primary antibodies specific to AEG-1 (1:200; Invitrogen) and GFAP (1:400, Covance Inc.) in blocking buffer overnight at 4 °C, washed, and incubated with Alexa Fluor secondary antibodies, anti-rabbit (488 nm, green) and anti-chicken (594 nm, red) (1:100; Invitrogen). The nuclei were visualized with DAPI (1:800; Invitrogen). Micrographs were obtained on an ECLIPSE Ti-4 using NLS-Elements BR. 3.0 software.
Transfection of Astrocytes
Human astrocytes were transfected with ON-TARGETplus siRNA specific to AEG-1 (siAEG-1), scrambled nontargeting siRNA (siCon; Thermo Scientific, Waltham, MA), empty vector (Con-GFP), AEG-1-GFP (TrueORF cDNA Clones with C-term GFP tag; PrecisionShuttle Vector System, Origene Technologies Inc., Rockville, MD), or pTat available through the National Institutes of Health AIDS Reagents Program, originally contributed by Dr. Eric Verdin (33) (catalog no. 10453) using the AmaxaTM P3 primary cell 96-well Nucleofector kit (Lonza, Walkersville, MD). Briefly, 1.6 million astrocytes were suspended in 20 μl of Nucleofector solution containing siCon or siAEG-1 (100 nm), Con-GFP or AEG-1-GFP (0.125 μg/106 cells), or pTat (0.15 μg/106 cells) and nucleofected using a Nucleofector/Shuttle (Lonza) device. A transfection efficiency of 80% was observed as measured by GFP-positive cells in Con-GFP transfections. Transfected cells were supplemented with astrocyte medium and incubated for 30 min at 37 °C prior to plating. Cell were then cultured in 25-cm2 flasks and allowed to recover for 48 h prior to experimental use.
Western Blot Analyses
Nontransfected or transfected astrocytes were cultured as adherent monolayers in 25-cm2 flasks at a density of 4 × 106 cells/flask and allowed to recover for 24 or 48 h, respectively. Following recovery, cells were treated for 24 h with varying stimuli and whole cell or cytoplasmic, and nuclear protein extracts were isolated using mammalian extraction buffer (Invitrogen) or nuclear and cytoplasmic extraction kit (NE-PER; Thermo Fisher Scientific, Pittsburgh, PA). Cells were collected by scraping in sterile ice-cold PBS to avoid alteration of protein expression on surface of cell membranes. Cytoplasmic and nuclear protein extracts (15 μg) were boiled with 4× NuPAGE lithium dodecyl sulfate loading sample buffer at 100 °C for 5–10 min, resolved by NuPAGE 4–12% bis-tris gel, and subsequently transferred to nitrocellulose membranes using i-Blot (Invitrogen). The membranes were incubated with antibodies against AEG-1 (1:500; Invitrogen) or NF-κB or EAAT2 or YY1 (1:1,000; Cell Signaling Technology, Danvers, MA) or IKKβ (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4 °C, washed, and then incubated with anti-rabbit goat antibody IgG-conjugated to horseradish peroxidase (1:10,000; Bio-Rad) for 2 h at room temperature. The membranes were then developed with SuperSignal west femto substrate (Thermo Fisher Scientific) and imaged in a Fluorochem HD2 Imager (Proteinsimple, Santa Clara, CA). β-Actin (1:4,000; Sigma-Aldrich), GAPDH (1:1,000; Santa Cruz Biotechnology), and lamin A/C (1:3,000; Cell Signaling Technology) were used as loading controls for whole cell, cytoplasmic, and nuclear extracts, respectively.
Co-immunoprecipitation
Twenty-four hours after treatment (as described above), whole cell and cytoplasmic-nuclear extracts were obtained and quantified by Precision Red protein assay (cytoskeleton). Cell lysates (50 μg) were then processed for immunoprecipitation using manufacturer's protocol (protein G magnetic beads; Cell Signaling). Mouse IgG1 magnetic bead conjugates (Cell Signaling) were used as experimental control.
Glutamate Clearance Assay
Transfected astrocytes were plated in 48-well tissue culture plates at a density of 0.15 × 106 cells/well and allowed to recover for 48 h prior to treatment with IL-1β (20 ng/ml) for 24 h. Following treatment, glutamate (400 μm) dissolved in phenol-free astrocyte medium was added into each well, and clearance was assayed at 24 h. The assay was performed and analyzed according to manufacturer's guidelines (Amplex Red glutamic acid/glutamate oxidase assay kit; Invitrogen). Each treatment condition was run in triplicate in multiple astrocyte donors.
CCL2 ELISA
Transfected astrocytes were plated in 48-well tissue culture plates at a density of 0.15 × 106 cells/well and allowed to recover for 48 h prior to treatment with TNF-α (50 ng/ml) for 24 h. Following treatment, CCL2 production was quantified in the cell culture supernatants according to manufacturer's instructions (R&D Systems). A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was performed (as previously described in Ref. 34) to measure cell viability, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide values were used for normalization.
Statistical Analyses
Statistical analyses were performed using Prism 5.0 (GraphPad Software, La Jolla, CA), with one-way analysis of variance and Newman-Keuls post-test for multiple comparisons. Statistical analyses with a paired t test were used for comparisons between siCon and siAEG-1 or mock and pTat. Significance was set at p < 0.05, and the data represent means ± S.E. of the mean.
RESULTS
HIV-1 CNS Infection Induces AEG-1 Expression in the Brain
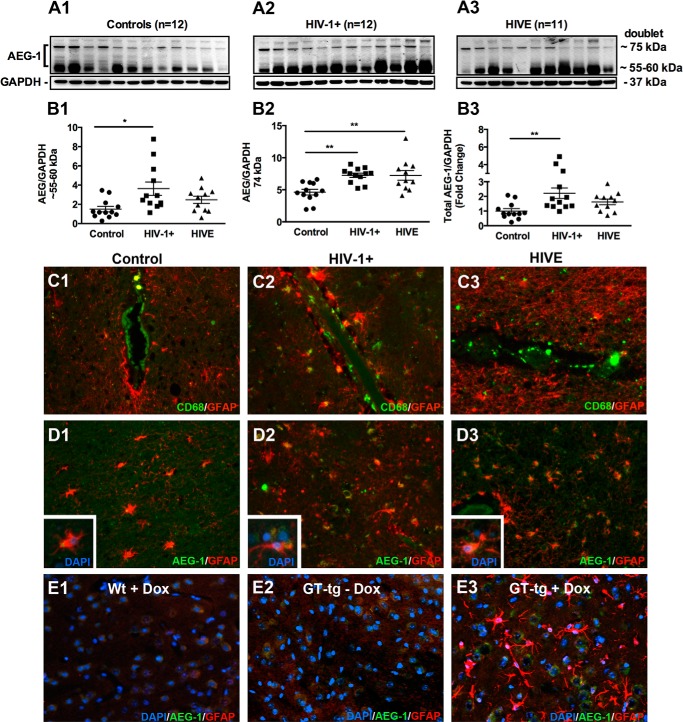
Although AEG-1 was originally identified as an HIV-1- and TNF-α-inducible transcript in human astrocytes (8), its expression in the brain of HIV-1+ and HIVE individuals has not been examined. Therefore, we first analyzed AEG-1 expression in the brain frontal cortex of 23 HIV-1 seropositive patients with or without encephalitis; 11 HIVE and 12 nonencephalitic HIV-1+. Additionally 12 age-, race-, and gender-matched uninfected controls were analyzed in parallel. Immunoblotting for AEG-1 detected the reported AEG-1 doublet with a molecular mass of 74 kDa and an additional noncanonical lower molecular mass band at ∼55–60 kDa (Fig. 1, A1–A3). Densitometry analyses showed a significant induction of the noncanonical AEG-1 protein in HIV-1+ brains (*, p < 0.05; Fig. 1B1) and of canonical AEG-1 74-kDa protein in both HIV-1+ and HIVE brain tissues lysates (**, p < 0.01; Fig. 1B2). Total AEG-1 protein levels were significantly elevated in HIV-1+ brain tissues (**, p < 0.01; Fig. 1B3). Immunohistochemical staining of tissues for CD68, a mononuclear phagocyte marker, demonstrated macrophage/microglial infiltration across the blood-brain barrier into the brain parenchyma in HIV-1+ and HIVE brain tissues but not in control brain tissues (Fig. 1, C1–C3). In parallel, AEG-1 immunostaining demonstrated AEG-1 induction and co-localization with GFAP positive astrocytes in HIV-1+ and HIVE brain tissues compared with control (Fig. 1, D1–D3). Basal and elevated levels of AEG-1 staining intensities were detected in the astrocyte cytoplasmic regions in tissue sections. To further confirm the HIV-1-induced up-regulation of AEG-1, we utilized a previously well characterized GT-tg mouse model, where HIV-1 Tat is expressed under the control of the astrocyte specific GFAP promoter and Dox (28, 29). HIV-1 Tat is a nonstructural viral protein that is secreted from the HIV-1-infected cells and known to cause HIV-1-associated neurotoxicity (35). To determine HIV-1 Tat-induced AEG-1 expression, immunohistochemical analyses of the brain tissue sections of wild type and GT-tg mice with or without Dox treatment were performed. The GT-tg model recapitulates significant neurotoxicity including brain structure alterations, loss of neurons and neuronal processes, and an increase in reactive astrocytes (28, 29). Consistent with this observation, increased astrogliosis was seen upon Dox treatment compared with controls (Fig. 1, E1–E3). A marked increase in AEG-1 staining intensity was detected in GT-tg + Dox mouse brain tissues expressing HIV-1 Tat compared with the wild type + Dox and GT-tg − Dox (Fig. 1E3). Thus, these data demonstrate that AEG-1 expression is elevated in the CNS of HIV-1-infected individuals and correlates with the expression of HIV-1 Tat protein in the brain.
FIGURE 1.
AEG-1 expression is elevated in HIV-1+ brain tissues with or without HIVE. A, brain frontal cortex tissues from age-, race-, and gender-matched noninfected controls (A1), HIV-1+ individuals (A2), and HIVE individuals (A3) were analyzed for AEG-1 expression by immunoblotting. B1–B3, AEG-1 protein expression was elevated in HIV-1+ and HIVE brain tissues (*, p < 0.05; **, p < 0.01). GAPDH was used as loading control. C and D, immunohistochemical analyses of control, HIV-1+ and HIVE human brain tissue sections from precentral gyrus for CD68 (microglial marker, green, C1–C3) and AEG-1 (green; D1–D3) revealed HIV-1-induced neuroinflammation and elevated AEG-1 expression. AEG-1 co-localized with GFAP-positive (red) reactive astrocytes (inset). GFAP (red) was used as an astrocyte marker, and DAPI (blue) was used as a nuclear marker. All micrographs are representative of two individuals from each group (200× original magnification). E, AEG-1 and GFAP immunofluorescence staining of wild type and Dox-inducible HIV-1 Tat transgenic mouse brain tissues; wild type with Dox (Wt + Dox; E1), HIV-1 Tat transgenic without Dox (GT-tg − Dox; E2), and HIV-1 Tat transgenic with Dox (GT-tg + Dox; E3) 4 days post-HIV-1 Tat induction are shown. AEG-1 increased expression and reactive astrogliosis was observed with HIV-1 Tat induction. All micrographs are representative of three individual mice from each group (400× original magnification).
HIV-1 Tat Induces Expression of AEG-1 and Neuroinflammatory Mediators
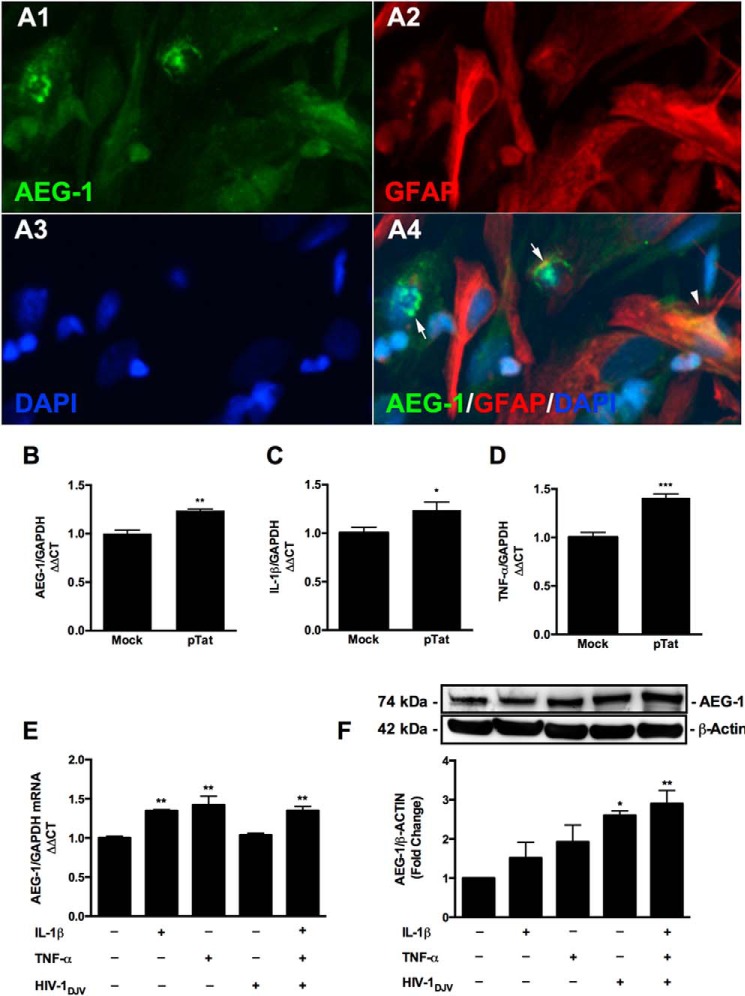
To mimic the GT-tg HIV-1 Tat model in vitro, astrocytes were transfected with a vector expressing the full-length HIV-1 Tat protein (pTat) (33). HIV-1 Tat expression was confirmed by real time RT-PCR 48 h post-transfection. HIV-1 Tat-expressing cells demonstrated AEG-1 localization to the astrocyte cytoplasmic and nuclear compartments (Fig. 2, A1–A4) and significantly induced AEG-1 expression at the mRNA level (**, p < 0.01; Fig. 2B). Furthermore, HIV-1 Tat expression significantly increased mRNA expression of the HAND-relevant inflammatory mediators, IL-1β and TNF-α, as compared with mock transfected controls (*, p < 0.05; Fig. 2C; and ***, p < 0.001; Fig. 2D, respectively). Next, to mimic disease milieu, astrocytes were treated with HIV-1 virions and HAND-relevant inflammatory stimuli. Because HIV-1 neurovirulence is dependent upon the differential ability of the virus to replicate in tissue macrophages (31), we utilized the human brain derived isolate, HIV-1DJV (31). Human astrocytes were treated with HIV-1DJV, IL-1β, and TNF-α, alone and in a triple co-treatment. IL-1β and TNF-α in combination with HIV-1DJV treatment induced a significant increase in AEG-1 mRNA and protein levels (**, p < 0.01; Fig. 2, E and F). Thus, these data show that HAND-relevant stimuli induce AEG-1 expression in human astrocytes.
FIGURE 2.
HIV-1 Tat mediated expression of AEG-1 and neuroinflammatory mediators, as did exogenous HAND-relevant stimuli. Astrocytes were transfected with pTat (0.15 μg/106 cells) and recovered for 48 h. A1–A4, astrocytes were fixed and immunostained for AEG-1 (A1, green), GFAP (A2, red), and DAPI (A3, blue). AEG-1 localized to both the nucleus (A4, arrows) and cytoplasm (arrowhead) in pTat-transfected astrocytes. B–D, AEG-1, IL-1β and TNF-α mRNA levels were determined by real time RT-PCR. pTat-transfected cells significantly increased AEG-1 (B; **, p < 0.01), IL-1β (C; *, p < 0.05), and TNF-α (D; ***, p < 0.001) mRNA levels, as compared with mock. GAPDH was used as a normalizing control. Representative data from three individual donors are shown. E and F, astrocytes were treated with HAND-relevant stimuli, HIV-1DJV, IL-1β (20 ng/ml), TNF-α (50 ng/ml), alone or in combination, and mRNA and proteins were isolated at 8 and 24 h post-treatment. E, IL-1β and TNF-α alone and in combination with HIV-1 DJV significantly induced AEG-1 mRNA levels (**, p < 0.01). F, HIV-1DJV alone and in combination with IL-1β and TNF-α significantly induced AEG-1 expression at the protein level (*, p < 0.05; **, p < 0.01). GAPDH and β-actin levels served as normalizing controls for real time RT-PCR and immunoblotting experiments, respectively. Cumulative (E) and representative (F) data from three individual donors are shown.
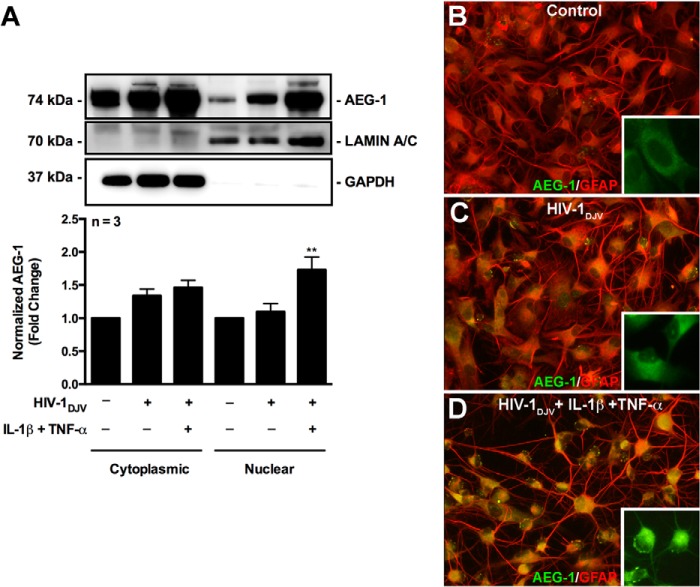
AEG-1 intracellular localization is a key determinant of its cellular function (36–38). Thus, we next investigated whether HAND-relevant stimuli induce AEG-1 differential localization in astrocytes. AEG-1 remained largely cytoplasmic following HIV-1DJV treatment and translocated to the nucleus upon co-treatment with IL-1β and TNF-α (**, p < 0.01; Fig. 3A). Further confirmation by immunocytochemistry showed largely cytoplasmic/perinuclear localization of AEG-1 (green) in untreated control astrocytes (GFAP, red), which showed an increase in staining intensity following HIV-1DJV treatment (Fig. 3, B and C, yellow) with localization of AEG-1 outside the nucleus. However, HIV-1DJV, IL-1β, and TNF-α co-treatment induced AEG-1 translocation to the nucleus (Fig. 3D).
FIGURE 3.
HAND-relevant inflammatory stimuli mediated AEG-1 nuclear localization in human astrocytes. Astrocytes were treated with HIV-1DJV alone or in combination with IL-1β (20 ng/ml) and TNF-α (50 ng/ml) for 24 h, and AEG-1 intracellular localization was analyzed by immunoblotting. A, AEG-1 protein levels were quantified in the cytoplasmic and nuclear compartments of astrocytes, and fold changes to untreated controls were calculated. HAND-relevant stimuli significantly induced AEG-1 nuclear localization (**, p < 0.01). Cytoplasmic GAPDH and nuclear lamin A/C were used as subcellular fraction normalizing controls. Following treatment, astrocytes were fixed and immunostained for GFAP (red) and AEG-1 (green), and AEG-1 intracellular localization was analyzed by immunofluorescence microscopy. B–D, AEG-1 localized to the cytoplasm in untreated control astrocytes (B) and in HIV-1DJV-treated astrocytes (C) but localized to the nucleus in HAND-relevant stimuli-treated astrocytes (D). Representative data from three or more individual donors assayed in triplicate are shown. Immunoblotting data are cumulative of three individual donors.
HIV-1DJV and TNF-α Induces AEG-1 Expression in Astrocytes via NF-κB Pathway
Because our data showed that HAND-relevant stimuli (i.e. HIV-1DJV, IL-1β, and TNF-α) up-regulate AEG-1 in astrocytes, we next focused on investigating specific intracellular signaling pathways that regulate astrocyte AEG-1. IL-1β and TNF-α are canonical activators of NF-κB pathway that phosphorylate NF-κB p65 subunit at Ser536, leading to nuclear translocation (39). Therefore, we investigated whether HAND-relevant stimuli induce AEG-1 expression through the NF-κB pathway. For this, we treated astrocytes with a pharmacological inhibitor of NF-κB pathway, SN50, prior to activation and determined AEG-1 induction. Treatment with HAND-relevant stimuli significantly induced AEG-1 mRNA and protein expression, but NF-κB inhibition with SN50 blocked the HAND-relevant stimuli-induced AEG-1 expression at both mRNA and protein levels (***, p < 0.001; Fig. 4, A and B). Thus, these data show that HAND-relevant stimuli induce AEG-1 expression in human astrocytes via the NF-κB pathway.
FIGURE 4.
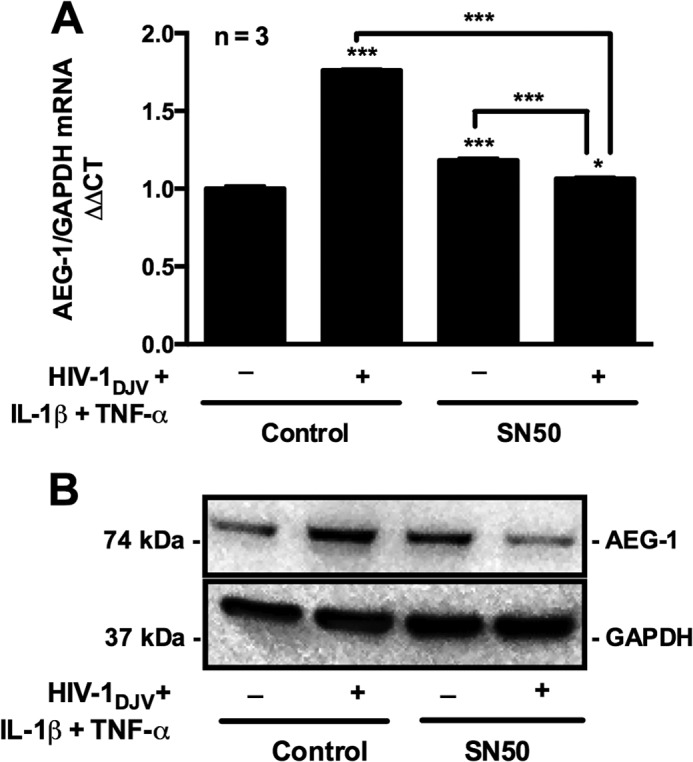
HAND-relevant stimuli signal via the NF-κB pathway to induce AEG-1 expression. Astrocytes were pretreated with NF-κB inhibitor (SN50) or vehicle control for 4 h, and AEG-1 expression was analyzed following 24 h of treatment with HAND-relevant stimuli. A, real time RT-PCR analysis showed significant induction in AEG-1 mRNA levels upon HAND-relevant stimuli treatment, and SN50 pretreatment significantly reduced the response in astrocytes (***, p < 0.001). B, similarly, immunoblotting analysis for AEG-1 protein showed significant induction in AEG-1 protein levels upon treatment with HAND-relevant stimuli and blocked AEG-1 induction in SN50-treated conditions. GAPDH mRNA and protein levels were used as normalizing controls.
Endogenous AEG-1 Regulates NF-κB Signaling in Reactive Astrocytes
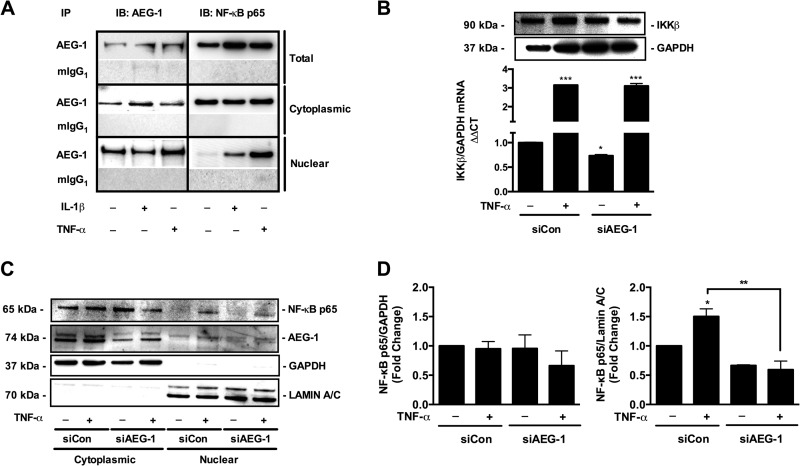
AEG-1 has been previously reported to physically interact with the p65 subunit of NF-κB in gliomas (40). However, whether HAND-relevant stimuli induce AEG-1 interaction with NF-κB and whether AEG-1 can then regulate NF-κB signaling have not been investigated. Because astrocytes mainly respond to pro-inflammatory cytokines IL-1β and TNF-α via activation of the NF-κB pathway, ultimately leading to a reactive phenotype that is characteristic of HAND pathology, we analyzed AEG-1 interaction with NF-κB in response to IL-1β and TNF-α. Although AEG-1 is both cytoplasmic and nuclear in untreated astrocytes, TNF-α treatment enhanced AEG-1 nuclear localization. Under unstimulated conditions, cytoplasmic NF-κB was bound to AEG-1, and upon activation with IL-1β and TNF-α, AEG-1-NF-κB complex translocated to the nucleus (Fig. 5A). Next, to determine whether AEG-1 regulates NF-κB signaling, we analyzed TNF-α-mediated NF-κB pathway signaling in siAEG-1-transfected astrocytes. NF-κB upstream activator kinase IKKβ expression and nuclear NF-κB protein levels were evaluated following TNF-α treatment in siAEG-1-transfected astrocytes. Although, AEG-1 silencing alone significantly reduced IKKβ mRNA levels (*, p < 0.05), it did not affect the IL-1β induction of IKKβ mRNA expression (Fig. 5B). However, AEG-1 silencing reduced the TNT-α-induced IKKβ protein levels (Fig. 5B) and significantly reduced nuclear NF-κB levels in response to TNF-α treatment (*, p < 0.05; Fig. 5, C and D). These data show that AEG-1 regulates NF-κB activation as well as nuclear translocation.
FIGURE 5.
AEG-1 regulated NF-κB signaling in astrocytes. Astrocytes were treated with IL-1β (20 ng/ml) or TNF-α (50 ng/ml) for 24 h, and whole cell, cytoplasmic, and nuclear extracts were obtained. Co-immunoprecipitation analyses were employed to study AEG-1 interaction with NF-κB p65 subunit in the subcellular astrocyte compartments. A, lysates were immunoprecipitated with anti-AEG-1 antibody and immunoblotted for NF-κB p65 subunit. Under untreated conditions, cytoplasmic AEG-1 interacted with the cytoplasmic NF-κB p65 subunit. IL-1β and TNF-α stimulation induced nuclear translocation of NF-κB p65 subunit and AEG-1 and an increase in NF-κB p65 subunit and AEG-1 interaction within the astrocyte nucleus. Mouse IgG1 (mIgG1) was used as a control for the experiment. B, AEG-1-mediated regulation of the NF-κB pathway activation was analyzed by measuring IKKβ expression post-TNF-α treatment and AEG-1 knockdown. Astrocytes were transfected with siAEG-1 or siCon and recovered for 48 h. Post-recovery, cells were treated with TNF-α (50 ng/ml), and IKKβ mRNA and protein levels were analyzed by real time RT-PCR and immunoblotting. TNF-α-induced IKKβ protein up-regulation was decreased in AEG-1 knockdown cells. C, AEG-1 regulation of the TNF-α-stimulated NF-κB pathway was analyzed by immunoblotting for NF-κB p65 subunit in the cytoplasmic and nuclear astrocyte compartments. TNF-α-induced nuclear NF-κB levels were significantly reduced in AEG-1 knockdown cells (*, p < 0.05). D, NF-κB levels were normalized to subcellular loading controls (GAPDH for cytoplasmic and lamin A/C for nuclear extracts), and fold changes to untreated control were calculated. Representative data from three individual donors are shown. IB, immunoblotting; IP, immunoprecipitation.
AEG-1 Overexpression Alters Astrocyte Responses to Inflammation
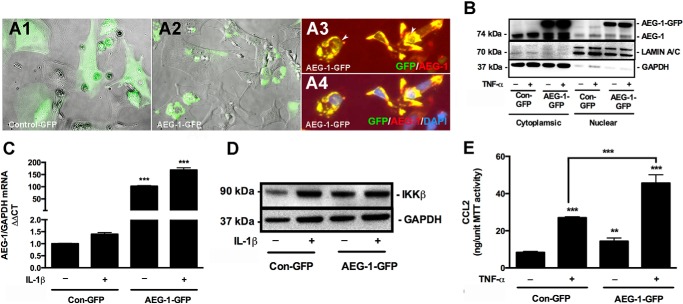
Because AEG-1 is up-regulated in astrocytes following HAND-relevant stimuli treatment, we next investigated downstream effects of AEG-1 up-regulation on astrocyte function. For this purpose, exogenous expression of AEG-1-GFP was utilized to model AEG-1 overexpression conditions in human astrocytes. Unlike Con-GFP that was generally distributed in the cytoplasmic regions of the cell body, exogenous AEG-1-GFP localized to the cytoplasmic perinuclear regions in astrocytes, consistent with endogenous AEG-1 (Fig. 6, A1 and A2). AEG-1-GFP also localized to the astrocyte nucleus when treated with IL-1β (Fig. 6, A3 and A4). An increase in AEG-1 nuclear translocation upon TNF-α treatment was also demonstrated by immunoblotting (Fig. 6B). Next, to evaluate the downstream functional ability of the overexpressed AEG-1, changes in IL-1β/TNF-α-induced NF-κB signaling were analyzed. AEG-1-GFP transfection significantly induced AEG-1 expression, which was exacerbated upon IL-1β treatment (***, p < 0.001; Fig. 6C). AEG-1-GFP transfection was sufficient to induce IKKβ protein expression (Fig. 6D). AEG-1-GFP transfection alone significantly induced production of CCL2, an NF-κB-responsive chemokine, which was exacerbated by TNF-α treatment (**, p < 0.01; ***, p < 0.001; Fig. 6E), thereby demonstrating the functional efficacy of the model. Therefore, this model was used for further characterization of the effect of AEG-1 overexpression on astrocyte responses to HIV-1-induced neuroinflammation.
FIGURE 6.
Development of AEG-1 overexpression model. Astrocytes were transfected with AEG-1-overexpression construct (AEG-1-GFP) or vehicle control (Con-GFP) and recovered for 48 h. AEG-1-GFP localization in transfected astrocytes was assessed by phase contrast and fluorescent microscopy. A1 and A2, unlike Con-GFP (A1), which localized throughout the cell body in the astrocyte cytoplasm, AEG-1-GFP (A2) localized to the perinuclear cytoplasmic regions. A3 and A4, nuclear localization of AEG-1-GFP was detected by immunostaining for AEG-1 (red), GFP (green), and DAPI (blue). Representative phase contrast and fluorescent microscopy overlay photomicrographs are 200× original magnification. B, astrocytes transfected with AEG-1-GFP or Con-GFP were treated with TNF-α (50 ng/ml) for 24 h, and AEG-1-GFP intracellular localization was analyzed by immunoblotting. An increase in nuclear AEG-1-GFP was detected following TNF-α treatment. GAPDH and lamin A/C were used as normalizing controls for cytoplasmic and nuclear extracts, respectively. To determine the functionality of the model, AEG-1-GFP or Con-GFP-transfected astrocytes were treated with IL-1β (20 ng/ml) for 24 h, and changes in AEG-1 and IKKβ expression were quantified by real time RT-PCR and immunoblotting, respectively. C and D, AEG-1 overexpression significantly increased AEG-1 mRNA levels (C; ***, p < 0.001) and IKKβ protein expression with or without IL-1β exposure (D). E, AEG-1 overexpression significantly up-regulated the TNF-α (50 ng/ml)-induced CCL2 production (**, p < 0.01; ***, p < 0.001). GAPDH mRNA and protein levels were used as normalizing controls for real time RT-PCR and immunoblotting analyses, respectively. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was used to normalize CCL2 protein levels. Representative data from three or more individual donors assayed in triplicate are shown.
AEG-1 Reduces Astrocyte Glutamate Clearance
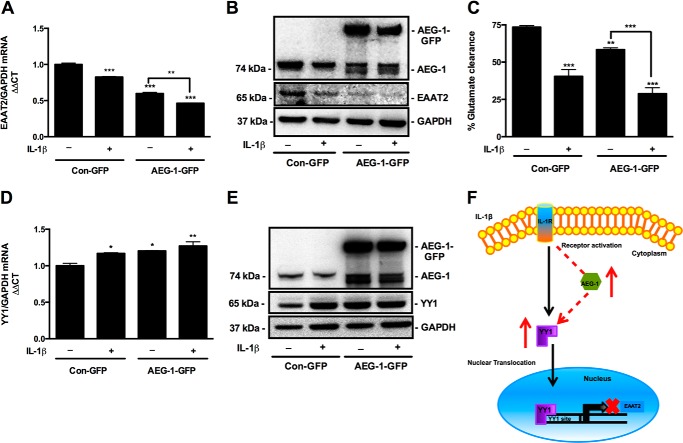
Because astrocyte function of extracellular glutamate clearance is a key regulator of HAND pathogenesis, we investigated the effect of AEG-1 overexpression on astrocyte glutamate clearance. IL-1β significantly reduced EAAT2 mRNA and protein levels. However, AEG-1 overexpression combined with IL-1β treatment demonstrated the highest reduction in EAAT2 mRNA and protein compared with untreated controls (***, p < 0.001; Fig. 7, A and B). AEG-1 overexpression-mediated decrease in EAAT2 mRNA and protein levels translated into reduced glutamate clearance by astrocytes, wherein the AEG-1-overexpressing cells upon IL-1β treatment demonstrated the highest reduction in glutamate clearance, ∼45% lower than that of untreated controls (***, p < 0.001; Fig. 7C). To further investigate the AEG-1-mediated EAAT2 regulation, we determined the effect of AEG-1 overexpression on YY1, an upstream transcriptional repressor of EAAT2 (41). AEG-1 overexpression alone and in combination with IL-1β significantly increased YY1 mRNA and protein levels (**, p < 0.01; Fig. 7, D and E), thereby suggesting a plausible mechanism of AEG-1-mediated EAAT2 regulation. Thus, here we show that HAND-relevant stimulus, IL-1β, induces AEG-1 expression that can lead to YY1 up-regulation and loss of EAAT2 transcription, which in turn reduces the extracellular glutamate clearance (Fig. 7F).
FIGURE 7.
AEG-1 overexpression in astrocytes reduced EAAT2 levels and glutamate clearance by regulating YY1 expression. Astrocytes transfected with AEG-1-GFP or Con-GFP constructs were treated with IL-1β (20 ng/ml) for 24 h, and changes in EAAT2 mRNA and protein levels were analyzed by real time RT-PCR and immunoblotting, respectively. A, AEG-1 overexpression alone significantly reduced EAAT2 mRNA levels and further exacerbated the IL-1β-mediated loss in EAAT2 expression (**, p < 0.01; ***, p < 0.001). B, AEG-1 overexpression alone significantly reduced EAAT2 protein levels and further augmented the IL-1β-mediated decrease in EAAT2 protein levels. C, effect of AEG-1 overexpression on glutamate clearance by astrocytes was studied by fluorometric assay. AEG-1 overexpression alone significantly reduced glutamate clearance and further augmented the IL-1β-mediated impairment in glutamate clearance (***, p < 0.001; **, p < 0.01; *, p < 0.05). D and E, AEG-1 regulation of EAAT2 was analyzed by real time RT-PCR and immunoblotting for YY1. AEG-1 overexpression alone and in combination with IL-1β significantly elevated YY1 mRNA and protein levels (**, p < 0.01). GAPDH was used as a normalizing control for mRNA and protein quantification. Representative data from two individual donors are shown. F, proposed mechanism of AEG-1 regulation of EAAT2. IL-1β activates the NF-κB pathway to induce AEG-1 expression, which further up-regulates YY1 expression leading to EAAT2 down-regulation.
DISCUSSION
The present work identifies AEG-1 as a novel modulator of HAND pathogenesis by regulating astrocyte responses to HIV-1-associated neuroinflammation and further implicates AEG-1 in HIV-1-associated glutamate excitoxicity. Analyses of AEG-1 expression in HIV-1+ and HIVE human brain tissues and GT-tg mouse brain tissues provide evidence for AEG-1 induction upon HIV-1 neuroinvasion. Here, we report AEG-1-mediated regulation of NF-κB signaling in astrocytes and demonstrate the downstream consequences of AEG-1 overexpression on astrocyte inflammatory and neuroprotective functions relevant to HAND. HIV-1 neuroinvasion, an early event in HIV-1 disease pathogenesis, triggers a low level of chronic neuroinflammation because of release of pro-inflammatory cytokines and chemokines by infected and activated cells within the CNS (42, 43). Here, we report AEG-1 induction in the frontal cortex of HIV-1+ and HIVE human brains. These brain regions are known to drive many of the HIV-1-associated neurocognitive deficits that occur in patients with HAND (44). CD68 staining for macrophage infiltration indicated increasing levels of neuroinflammation in response to HIV-1 neuroinvasion. Corresponding AEG-1 expression analyses revealed a similar increase with the disease pathology. AEG-1 co-localization with GFAP-positive reactive astrocytes in the HIVE brain tissues suggests a link between the level of HIV-1-associated neuroinflammation and astrocyte AEG-1 expression. Immunoblotting of frontal cortex brain tissue lysates from HIV-1+ and HIVE patients demonstrated significantly higher levels of the canonical AEG-1 (74 kDa) and also the noncanonical AEG-1 protein, which has been previously reported in certain cell types (45–47). Although the noncanonical AEG-1 protein could be a splice variant or a truncated version of the canonical AEG-1 molecule, the specific origin of this form of AEG-1 remains unclear.
HIV-1 Tat, a neurotoxic nonstructural HIV-1 viral protein, is one of the most commonly detected HIV-1 proteins in the CSF and serum of HIV-1-infected individuals and is considered an important mediator of immune activation and inflammatory processes in the brain of HIV-1-infected individuals (48–51). Therefore, the GT-tg mouse model of HIV-1 CNS infection was utilized for further analyses. The purpose for using the GT-tg mouse model was multifold: first, to confirm the HIV-1-induced AEG-1 CNS expression; second, to identify the specific source of AEG-1 in the brain; and third, to extrapolate the AEG-1 changes related to GFAP-driven HIV-1 Tat protein to in vitro studies for further molecular dissection. Immunocytochemical analyses of brain tissue sections from GT-tg mice demonstrated significant AEG-1 induction with co-localization to GFAP positive reactive astrocytes, similar to human HIV+ and HIVE tissues. It is noteworthy that we also detected AEG-1 staining in cells other than astrocytes in the human and GT-tg mouse brain tissues. However, astrocyte AEG-1 was our primary focus for this study, because AEG-1 was first described as an astrocyte elevated protein of unknown function, demonstrating a significant gap in literature. Furthermore, astrocytes being the most abundant cell type in the brain, AEG-1 regulation in astrocytes may have important disease implications. Further studies to investigate AEG-1 expression and/or function in other neural or non-neural cell types are warranted.
Because the in vivo data demonstrated AEG-1 co-localization to astrocytes, next we performed in vitro studies using cultured human astrocytes. Primary human astrocytes transfected with pTat showed increased mRNA levels of IL-1β and TNF-α, which are consistent with previous reports associated with HIV-1 Tat-induced cytokine elevation in astrocytes (52, 53). Further, the pTat-transfected astrocytes showed elevated AEG-1 mRNA levels and nuclear-cytoplasmic localization of AEG-1 with immunostaining. However, HIV-1 Tat protein in isolation does not mimic the overall scenario in the diseased brain tissues. In the current antiretroviral therapy era, low level neuroinflammation is a better histopathological correlate of HIV-1 neuropathogenesis than HIV-1 virions alone (54). Therefore, to mimic the disease pathology in vitro and to further analyze the specific molecular mechanisms, we treated astrocytes with common HAND-relevant stimuli including the HIV-1 virions and the well established pro-inflammatory cytokines, IL-1β and TNF-α, detected in the brains of HIV-1+ individuals (55). The singular or co-treatment of cultured astrocytes with common HIV-1-induced pro-inflammatory cytokines, IL-1β and TNF-α (56, 57), and exogenous HIV-1 virions, HIV-1DJV, mimicked the astrocyte microenvironment within the CNS of HIV-1+ individuals and provided an appropriate model for AEG-1 analyses. In comparison with HIV-1DJV treatment alone, co-treatment with IL-1β and TNF-α showed a greater increase in AEG-1 mRNA levels. Although HIV-1DJV treatment alone did not significantly change AEG-1 mRNA levels, it significantly increased AEG-1 protein levels. This may be explained by a possible stabilization effect of HIV-1 on AEG-1 protein at the post-translational level as against induction at the transcriptional level. Further, significant induction of AEG-1 in astrocytes by both IL-1β and TNF-α indicates that AEG-1 is an inflammation-inducible gene in astrocytes and that HAND-relevant stimuli alone and in combination are capable of inducing AEG-1.
In cancer cells, intracellular AEG-1 localization is an important determinant of its subsequent functional perturbation and is utilized as a biomarker for disease progression (36–38). Here, we report astrocyte nuclear AEG-1 translocation in response to HAND-relevant stimuli, which further documents a potential role in transcriptional regulation of astrocyte function. However, retention of AEG-1 in the cytoplasmic compartment of the astrocytes in response to HIV-1DJV singular treatment indicates a differential mechanism of AEG-1 regulation by HIV-1 and HIV-1-induced inflammation. However, under physiological disease conditions, the two phenomena are indistinguishable because HIV-1 infection and neuroinflammation go hand-in-hand. Therefore, AEG-1 nuclear translocation in astrocytes will likely depend on the level of inflammation in the astrocyte microenvironment.
Astrocytes respond to inflammation via activation of many inflammatory response pathways, with NF-κB being the most frequently activated signaling cascade (43, 58). Thus, pharmacological inhibition of the NF-κB pathway blocked AEG-1 induction in response to HAND-relevant stimuli, thereby suggesting that the HAND-relevant stimuli signal via the NF-κB pathway to induce AEG-1 expression in astrocytes. Recent oncogenic studies on AEG-1 have proposed a transcriptional co-factor function for AEG-1 and have identified numerous AEG-1 binding partners, such as the p65 subunit of NF-κB (37, 38, 40). However, whether AEG-1 is capable of regulating the NF-κB pathway and whether AEG-1 interacts with NF-κB in other cell types and disease settings was not elucidated. In the present study, we demonstrated the interaction and binding of AEG-1 with the regulatory subunit of NF-κB, p65, in both naïve and activated astrocytes. Further, we illustrated the nuclear translocation of the AEG-1-NF-κB p65 complex in response to TNF-α. Studies in cancer cells have reported a similar type of interaction between AEG-1 and NF-κB p65 subunit after TNF-α treatment (40). In unstimulated astrocytes, we observed that naïve NF-κB p65 remains bound to AEG-1 in the cytoplasm, which then translocates to the nucleus upon inflammatory stimulation. In addition, upon activation of the NF-κB signaling cascade by TNF-α, we detected an increase in AEG-1 bound NF-κB p65 inside the astrocyte nucleus, which further suggests a novel role for AEG-1 in regulating NF-κB signaling. An important upstream canonical regulator of NF-κB signaling is the activator kinase IKKβ (59). The cellular expression levels of IKKβ are important determinants of NF-κB activation; higher levels of IKKβ are indicative of NF-κB activation status and vice versa. Therefore, analyses of IKKβ expression in TNF-α treated AEG-1 knockdown cells was performed, which revealed a significant reduction in TNF-α-induced IKKβ protein levels, as well as a significant decrease in TNF-α-induced nuclear NF-κB p65 levels, thereby demonstrating AEG-1-mediated regulation of astrocyte NF-κB signaling. Although changes in IKKβ mRNA were not statistically significant, AEG-1 knockdown significantly reduced IKKβ protein levels. This may be explained by a plausible post-transcriptional role of AEG-1 in addition to transcriptional regulation at the mRNA level. These results suggest an autocrine model of AEG-1 induction in which HAND-relevant stimuli increase AEG-1 expression via NF-κB pathway activation, and in turn, AEG-1 regulates NF-κB pathway signal transduction by regulating activation and nuclear translocation of NF-κB p65.
Because our studies revealed AEG-1 induction in astrocytes following HAND-relevant stimuli treatment, it is essential to study the downstream effects of AEG-1 up-regulation, which can be best deciphered with AEG-1 ectopic overexpression. Hence, we established AEG-1 overexpression model in cultured human astrocytes using AEG-1-GFP overexpression construct and analyzed astrocyte responses to inflammation. The exogenously expressed AEG-1-GFP localized to the perinuclear regions in addition to the nucleus, similar to endogenous AEG-1 expression in astrocytes. Treatment with TNF-α elevated the AEG-1-GFP levels in the nucleus, thereby indicating that the cellular distribution of ectopically expressed AEG-1 was similar to that of the endogenous protein. Here, we also report that AEG-1 overexpression alone increased the basal levels of IKKβ, the important upstream activator of the NF-κB pathway. Thus, AEG-1 overexpression may prime the astrocytes toward HAND-relevant stimuli-mediated NF-κB activation. Further downstream, AEG-1 overexpression alone significantly increased the production of NF-κB-responsive chemokine CCL2 (60), which plays an important role in the recruitment of immune cells across the blood-brain barrier and also provides a proliferative stimulus for the immune cells and astrocytes (61–64). Although TNF-α treatment induced CCL2 in Con-GFP-transfected cells, the induction was robust in AEG-1-overexpressing cells, ∼5-fold increase compared with control. Because the AEG-1-overexpressing astrocytes treated with TNF-α represent a scenario that is most relevant in the neuroinflammatory disease setting, AEG-1-mediated activation of NF-κB pathway is an important finding.
In addition to chemokine and cytokine production, glutamate clearance is a critical function of astrocytes that is frequently dysregulated during HIV-1 CNS infection and is thus implicated in HAND. Earlier studies have reported the toxicity associated with extracellular glutamate (65). Excitatory amino acid transporter-2 is an important transporter on astrocytes and a major regulator of extracellular glutamate levels (66–68). AEG-1 overexpression alone significantly impaired glutamate clearance by astrocytes via down-regulation of EAAT2 expression. The AEG-1-mediated EAAT2 down-regulation is further exacerbated under conditions of IL-1β exposure, a condition physiologically relevant to disease. Further, AEG-1 overexpression alone increased the expression of YY1, a transcriptional repressor of EAAT2, thereby indicating an indirect upstream modulator of EAAT2 transcription in astrocytes. AEG-1 overexpression alone up-regulated YY1 mRNA and protein levels similar to IL-1β singular treatment. Although a combination of both AEG-1 overexpression and IL-1β treatment failed to demonstrate an additive effect, it showed the most significant YY1 induction. Thus, our studies identify AEG-1 as an important regulator of EAAT2 expression and demonstrate the downstream consequence of AEG-1 overexpression on glutamate clearance by astrocytes. Although the YY1 binding site has been recognized in the AEG-1 protein (25), whether AEG-1 interacts with YY1 under conditions of HIV-1-induced inflammation and whether the YY1-AEG-1 complex down-regulates the EAAT2 promoter activity in reactive astrocytes remain to be investigated. We also note that, similar to the lower molecular mass noncanonical AEG-1 band detected in human brain tissue lysates, an increase in the lower molecular mass AEG-1 band of the canonical AEG-1 doublet was detected in AEG-1-GFP-overexpressing astrocytes (Fig. 7, B and E). Given that the increase in this lower molecular mass band of AEG-1 doublet was detected only in the AEG-1-GFP-expressing cells and not Con-GFP-transfected cells, it suggests that AEG-1 overexpression mimics the conditions present in the HIV-1+ human brain tissues. The origin of the truncated version of AEG-1 needs to be further investigated.
Similar to our results showing AEG-1 regulation of astrocyte glutamate clearance, the AEG-1-mediated loss of EAAT2 has been earlier reported in glioma cell lines and is recognized as an important phenomenon implicated in glioma-associated necrosis (25). The AEG-1-induced reduced glutamate clearance by glioma cells in the tumor microenvironment has been shown to contribute to the necrotic and neurodegenerative effects associated with glial neoplasms. Our studies provide evidence for inflammation-induced AEG-1 expression in astrocytes with subsequent loss of EAAT2, which could contribute to neurodegeneration in a variety of clinical settings including neoplasms and other diseases of the nervous system that have a neuroinflammatory manifestation, such as HAND, traumatic brain injury, toxic metabolites, or autoimmunity.
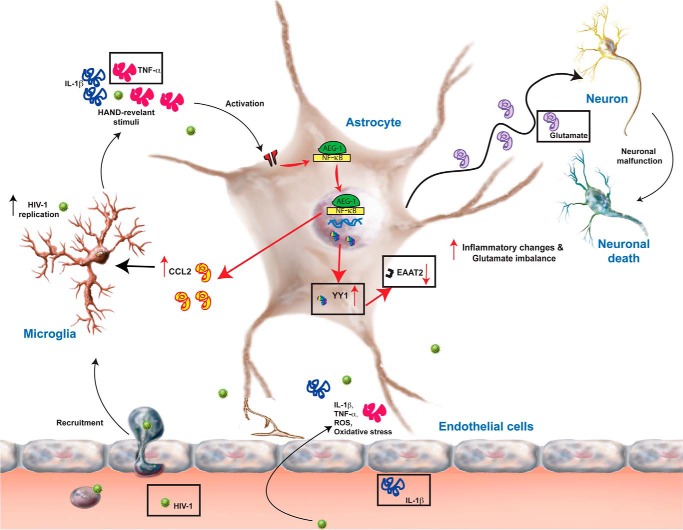
In summary, HIV-1 neuropathogenesis is characterized by presence of inflammatory cytokines and chemokines such as IL-1β and TNF-α along with HIV-1 virions and proteins in the astrocyte microenvironment. These HAND-relevant stimuli activate intracellular signaling cascades leading to impaired glutamate clearance caused by EAAT2 loss and increased CCL2 production. Intracellularly, HAND-relevant stimuli activate the NF-κB pathway and induce increased interaction of NF-κB p65 subunit with cytoplasmic AEG-1, which then translocates to the nucleus to induce the expression of NF-κB-responsive chemokines such as CCL2. HAND-relevant stimuli-induced AEG-1 down-regulates EAAT2 plausibly by up-regulating YY1 expression, thereby leading to reduced glutamate clearance and hence exacerbation of neurotoxicity and neuroinflammation (Fig. 8). Thus, here we identify AEG-1 as a novel regulator of HIV-1-associated neuroinflammation and neurodegeneration via activation of the NF-κB pathway and repression of EAAT2 expression in astrocytes.
FIGURE 8.
AEG-1 regulation of HIV-1 neuropathogenesis. HIV-1 neuropathogenesis is characterized by the presence of inflammatory cytokines and chemokines along with HIV-1 virions and proteins in the microenvironment of astrocytes. These HAND-relevant stimuli activate several intracellular signaling cascades in astrocytes, which modulate the astrocyte microenvironment by impairing their glutamate clearance and/or chemokine and cytokine production. Intracellularly, TNF-α/IL-1β activates the NF-κB pathway and induces increased interaction of NF-κB p65 subunit with cytoplasmic AEG-1, which then translocates to the nucleus to induce the expression of NF-κB-responsive genes such as CCL2 and down-regulates the expression of EAAT2 by up-regulating the expression of YY1, an EAAT2 transcriptional repressor, thereby impairing the glutamate clearance. Thus, here we show that AEG-1 is a novel regulator of HAND pathogenesis by modulating NF-κB-driven cellular processes such as glutamate clearance and chemokine and cytokine production by astrocytes.
Results from our previous study identified a novel role of AEG-1 in regulation of astrocyte responses to neural injury, by regulating astrocyte migration and proliferation during wound healing (69). This report identifies yet another novel facet of AEG-1-mediated regulation of astrocyte function, astrocyte glutamate clearance, and control of neuroinflammation. The results from this study implicate AEG-1 as a potential regulator of astrocyte-driven neuroinflammatory processes in patients with HAND.
Acknowledgments
We appreciate the assistance of the Laboratory of Developmental Biology for providing us with human brain tissues. Jyotsana Singhal assisted with immunoprecipitation and immunoblotting. Lin Tang graciously provided consistent primary human astrocyte cultures.
This work was supported, in whole or in part, by National Institutes of Health Grants R01MH087345 (to A. G. and the JES Edwards Foundation); U01 MH083507, R24NS045091, U01MH083506, R24 MH059745, U01MH083501, R24MH059724, U01MH083500, R24NS038841, and U01MH083545 (to the National NeuroAIDS Tissue Consortium); R01NS072005; and R01MH79886. This work was also supported by National Institutes of Health Award Number 5R24HD0008836 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
- HAND
- HIV-associated neurocognitive disorder
- AEG-1
- astrocyte elevated gene-1
- Dox
- doxycycline
- EAAT2
- excitatory amino acid transporter 2
- GFAP
- glial fibrillary acidic protein
- GT-tg
- Dox-inducible, HIV-1 Tat transgenic mouse
- HIVE
- HIV-1 encephalitis
- HIV-1 Tat
- HIV-1 transactivator of transcription
- IKK
- IκB kinase
- pTat
- plasmid encoding full-length HIV-1 Tat protein
- YY1
- yin yang 1
- siAEG-1
- siRNA specific to AEG-1
- siCon
- scrambled nontargeting siRNA
- HIV-1+
- HIV-1-seropositive.
REFERENCES
- 1. UNAIDS (2008) Report on the Global AIDS epidemic. In XVII International AIDS Conference, Joint United Nations Program on HIV/AIDS, Mexico City, Mexico [Google Scholar]
- 2. Hall H. I., Song R., Rhodes P., Prejean J., An Q., Lee L. M., Karon J., Brookmeyer R., Kaplan E. H., McKenna M. T., Janssen R. S. (2008) Estimation of HIV incidence in the United States. JAMA 300, 520–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ellis R., Langford D., Masliah E. (2007) HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat. Rev. Neurosci. 8, 33–44 [DOI] [PubMed] [Google Scholar]
- 4. Ghafouri M., Amini S., Khalili K., Sawaya B. E. (2006) HIV-1 associated dementia: symptoms and causes. Retrovirology 3, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trujillo J. R., Jaramillo-Rangel G., Ortega-Martinez M., Penalva de Oliveira A. C., Vidal J. E., Bryant J., Gallo R. C. (2005) International NeuroAIDS: prospects of HIV-1 associated neurological complications. Cell Res. 15, 962–969 [DOI] [PubMed] [Google Scholar]
- 6. Navia B. A., Price R. W. (2005) An overview of the clinical and biological features of the AIDS dementia complex. In The Neurology of AIDS (Gendelman H. E., Grant I., Lipton S. A., Swindells S., eds) 2nd Ed., pp. 339–356, Oxford University Press, New York [Google Scholar]
- 7. Kaul M., Garden G. A., Lipton S. A. (2001) Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410, 988–994 [DOI] [PubMed] [Google Scholar]
- 8. Kang D. C., Su Z. Z., Sarkar D., Emdad L., Volsky D. J., Fisher P. B. (2005) Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene 353, 8–15 [DOI] [PubMed] [Google Scholar]
- 9. Sun W., Fan Y. Z., Xi H., Lu X. S., Ye C., Zhang J. T. (2011) Astrocyte elevated gene-1 overexpression in human primary gallbladder carcinomas: an unfavorable and independent prognostic factor. Oncol. Rep. 26, 1133–1142 [DOI] [PubMed] [Google Scholar]
- 10. Liao W. T., Guo L., Zhong Y., Wu Y. H., Li J., Song L. B. (2011) Astrocyte elevated gene-1 (AEG-1) is a marker for aggressive salivary gland carcinoma. J. Transl. Med. 9, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen W., Ke Z., Shi H., Yang S., Wang L. (2010) Overexpression of AEG-1 in renal cell carcinoma and its correlation with tumor nuclear grade and progression. Neoplasma 57, 522–529 [DOI] [PubMed] [Google Scholar]
- 12. Liu L., Wu J., Ying Z., Chen B., Han A., Liang Y., Song L., Yuan J., Li J., Li M. (2010) Astrocyte elevated gene-1 upregulates matrix metalloproteinase-9 and induces human glioma invasion. Cancer Res. 70, 3750–3759 [DOI] [PubMed] [Google Scholar]
- 13. Lee S. G., Jeon H. Y., Su Z. Z., Richards J. E., Vozhilla N., Sarkar D., Van Maerken T., Fisher P. B. (2009) Astrocyte elevated gene-1 contributes to the pathogenesis of neuroblastoma. Oncogene 28, 2476–2484 [DOI] [PubMed] [Google Scholar]
- 14. Li J., Yang L., Song L., Xiong H., Wang L., Yan X., Yuan J., Wu J., Li M. (2009) Astrocyte elevated gene-1 is a proliferation promoter in breast cancer via suppressing transcriptional factor FOXO1. Oncogene 28, 3188–3196 [DOI] [PubMed] [Google Scholar]
- 15. Farina C., Aloisi F., Meinl E. (2007) Astrocytes are active players in cerebral innate immunity. Trends Immunol. 28, 138–145 [DOI] [PubMed] [Google Scholar]
- 16. Aschner M. (1998) Astrocytes as mediators of immune and inflammatory responses in the CNS. Neurotoxicology 19, 269–281 [PubMed] [Google Scholar]
- 17. Dong Y., Benveniste E. N. (2001) Immune function of astrocytes. Glia 36, 180–190 [DOI] [PubMed] [Google Scholar]
- 18. Seifert G., Schilling K., Steinhäuser C. (2006) Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat. Rev. Neurosci. 7, 194–206 [DOI] [PubMed] [Google Scholar]
- 19. Sabri F., Titanji K., De Milito A., Chiodi F. (2003) Astrocyte activation and apoptosis: their roles in the neuropathology of HIV infection. Brain Pathol. 13, 84–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Volterra A., Meldolesi J. (2005) Astrocytes, from brain glue to communication elements: the revolution continues. Nat. Rev. Neurosci. 6, 626–640 [DOI] [PubMed] [Google Scholar]
- 21. López-Bayghen E., Ortega A. (2011) Glial glutamate transporters: new actors in brain signaling. IUBMB Life 63, 816–823 [DOI] [PubMed] [Google Scholar]
- 22. Xing H. Q., Hayakawa H., Gelpi E., Kubota R., Budka H., Izumo S. (2009) Reduced expression of excitatory amino acid transporter 2 and diffuse microglial activation in the cerebral cortex in AIDS cases with or without HIV encephalitis. J. Neuropathol. Exp. Neurol. 68, 199–209 [DOI] [PubMed] [Google Scholar]
- 23. Maragakis N. J., Dykes-Hoberg M., Rothstein J. D. (2004) Altered expression of the glutamate transporter EAAT2b in neurological disease. Ann. Neurol. 55, 469–477 [DOI] [PubMed] [Google Scholar]
- 24. Borjabad A., Brooks A. I., Volsky D. J. (2010) Gene expression profiles of HIV-1-infected glia and brain: toward better understanding of the role of astrocytes in HIV-1-associated neurocognitive disorders. J. Neuroimmune Pharmacol. 5, 44–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee S. G., Kim K., Kegelman T. P., Dash R., Das S. K., Choi J. K., Emdad L., Howlett E. L., Jeon H. Y., Su Z. Z., Yoo B. K., Sarkar D., Kim S. H., Kang D. C., Fisher P. B. (2011) Oncogene AEG-1 promotes glioma-induced neurodegeneration by increasing glutamate excitotoxicity. Cancer Res. 71, 6514–6523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoo B. K., Emdad L., Lee S. G., Su Z. Z., Santhekadur P., Chen D., Gredler R., Fisher P. B., Sarkar D. (2011) Astrocyte elevated gene-1 (AEG-1): a multifunctional regulator of normal and abnormal physiology. Pharmacol. Ther. 130, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nguyen T. P., Soukup V. M., Gelman B. B. (2010) Persistent hijacking of brain proteasomes in HIV-associated dementia. Am. J. Pathol. 176, 893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim B. O., Liu Y., Ruan Y., Xu Z. C., Schantz L., He J. J. (2003) Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am. J. Pathol. 162, 1693–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chang J. R., Mukerjee R., Bagashev A., Del Valle L., Chabrashvili T., Hawkins B. J., He J. J., Sawaya B. E. (2011) HIV-1 Tat protein promotes neuronal dysfunction through disruption of microRNAs. J. Biol. Chem. 286, 41125–41134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gardner J., Borgmann K., Deshpande M. S., Dhar A., Wu L., Persidsky R., Ghorpade A. (2006) Potential mechanisms for astrocyte-TIMP-1 downregulation in chronic inflammatory diseases. J. Neurosci. Res. 83, 1281–1292 [DOI] [PubMed] [Google Scholar]
- 31. Ghorpade A., Nukuna A., Che M., Haggerty S., Persidsky Y., Carter E., Carhart L., Shafer L., Gendelman H. E. (1998) Human immunodeficiency virus neurotropism: an analysis of viral replication and cytopathicity for divergent strains in monocytes and microglia. J. Virol. 72, 3340–3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chadderton T., Wilson C., Bewick M., Gluck S. (1997) Evaluation of three rapid RNA extraction reagents: relevance for use in RT-PCR's and measurement of low level gene expression in clinical samples. Cell Mol Biol. (Noisy-le-grand) 43, 1227–1234 [PubMed] [Google Scholar]
- 33. Ott M., Emiliani S., Van Lint C., Herbein G., Lovett J., Chirmule N., McCloskey T., Pahwa S., Verdin E. (1997) Immune hyperactivation of HIV-1-infected T cells mediated by Tat and the CD28 pathway. Science 275, 1481–1485 [DOI] [PubMed] [Google Scholar]
- 34. Manthrope M., Fagnani R., Skaper S. D., Varon S. (1986) An automated colorimetric microassay for neurotrophic factors. Brain Res. 390, 191–198 [DOI] [PubMed] [Google Scholar]
- 35. Nath A. (2002) Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J. Infect. Dis. 186, (Suppl. 2) S193–S198 [DOI] [PubMed] [Google Scholar]
- 36. Ash S. C., Yang D. Q., Britt D. E. (2008) LYRIC/AEG-1 overexpression modulates BCCIPα protein levels in prostate tumor cells. Biochem. Biophys. Res. Commun. 371, 333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thirkettle H. J., Mills I. G., Whitaker H. C., Neal D. E. (2009) Nuclear LYRIC/AEG-1 interacts with PLZF and relieves PLZF-mediated repression. Oncogene 28, 3663–3670 [DOI] [PubMed] [Google Scholar]
- 38. Thirkettle H. J., Girling J., Warren A. Y., Mills I. G., Sahadevan K., Leung H., Hamdy F., Whitaker H. C., Neal D. E. (2009) LYRIC/AEG-1 is targeted to different subcellular compartments by ubiquitinylation and intrinsic nuclear localization signals. Clin Cancer Res. 15, 3003–3013 [DOI] [PubMed] [Google Scholar]
- 39. Sakurai H., Chiba H., Miyoshi H., Sugita T., Toriumi W. (1999) IκB kinases phosphorylate NF-κB p65 subunit on serine 536 in the transactivation domain. J. Biol. Chem. 274, 30353–30356 [DOI] [PubMed] [Google Scholar]
- 40. Sarkar D., Park E. S., Emdad L., Lee S. G., Su Z. Z., Fisher P. B. (2008) Molecular basis of nuclear factor-κB activation by astrocyte elevated gene-1. Cancer Res. 68, 1478–1484 [DOI] [PubMed] [Google Scholar]
- 41. Karki P., Webb A., Smith K., Johnson J., Jr., Lee K., Son D. S., Aschner M., Lee E. (2014) Yin yang 1 is a repressor of glutamate transporter EAAT2, and it mediates manganese-induced decrease of EAAT2 expression in astrocytes. Mol. Cell. Biol. 34, 1280–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carson M. J., Thrash J. C., Walter B. (2006) The cellular response in neuroinflammation: the role of leukocytes, microglia and astrocytes in neuronal death and survival. Clin. Neurosci. Res. 6, 237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Streit W. J., Mrak R. E., Griffin W. S. (2004) Microglia and neuroinflammation: a pathological perspective. J. Neuroinflammation 1, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. López-Villegas D., Lenkinski R. E., Frank I. (1997) Biochemical changes in the frontal lobe of HIV-infected individuals detected by magnetic resonance spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 94, 9854–9859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Britt D. E., Yang D. F., Yang D. Q., Flanagan D., Callanan H., Lim Y. P., Lin S. H., Hixson D. C. (2004) Identification of a novel protein, LYRIC, localized to tight junctions of polarized epithelial cells. Exp. Cell Res. 300, 134–148 [DOI] [PubMed] [Google Scholar]
- 46. Brown D. M., Ruoslahti E. (2004) Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell 5, 365–374 [DOI] [PubMed] [Google Scholar]
- 47. Sutherland H. G., Lam Y. W., Briers S., Lamond A. I., Bickmore W. A. (2004) 3D3/lyric: a novel transmembrane protein of the endoplasmic reticulum and nuclear envelope, which is also present in the nucleolus. Exp. Cell Res. 294, 94–105 [DOI] [PubMed] [Google Scholar]
- 48. McManus C. M., Weidenheim K., Woodman S. E., Nunez J., Hesselgesser J., Nath A., Berman J. W. (2000) Chemokine and chemokine-receptor expression in human glial elements: induction by the HIV protein, Tat, and chemokine autoregulation. Am. J. Pathol. 156, 1441–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weiss J. M., Nath A., Major E. O., Berman J. W. (1999) HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes. J. Immunol. 163, 2953–2959 [PubMed] [Google Scholar]
- 50. Toborek M., Lee Y. W., Flora G., Pu H., András I. E., Wylegala E., Hennig B., Nath A. (2005) Mechanisms of the blood-brain barrier disruption in HIV-1 infection. Cell Mol Neurobiol 25, 181–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Johnson T. P., Patel K., Johnson K. R., Maric D., Calabresi P. A., Hasbun R., Nath A. (2013) Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc. Natl. Acad. Sci. U.S.A. 110, 13588–13593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kutsch O., Oh J., Nath A., Benveniste E. N. (2000) Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 tat in astrocytes. J. Virol. 74, 9214–9221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nath A., Conant K., Chen P., Scott C., Major E. O. (1999) Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J. Biol. Chem. 274, 17098–17102 [DOI] [PubMed] [Google Scholar]
- 54. Heaton R. K., Franklin D. R., Ellis R. J., McCutchan J. A., Letendre S. L., Leblanc S., Corkran S. H., Duarte N. A., Clifford D. B., Woods S. P., Collier A. C., Marra C. M., Morgello S., Mindt M. R., Taylor M. J., Marcotte T. D., Atkinson J. H., Wolfson T., Gelman B. B., McArthur J. C., Simpson D. M., Abramson I., Gamst A., Fennema-Notestine C., Jernigan T. L., Wong J., Grant I. (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 17, 3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Suryadevara R., Holter S., Borgmann K., Persidsky R., Labenz-Zink C., Persidsky Y., Gendelman H. E., Wu L., Ghorpade A. (2003) Regulation of tissue inhibitor of metalloproteinase-1 by astrocytes: links to HIV-1 dementia. Glia 44, 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wesselingh S. L., Takahashi K., Glass J. D., McArthur J. C., Griffin J. W., Griffin D. E. (1997) Cellular localization of tumor necrosis factor mRNA in neurological tissue from HIV-infected patients by combined reverse transcriptase/polymerase chain reaction in situ hybridization and immunohistochemistry. J. Neuroimmunol. 74, 1–8 [DOI] [PubMed] [Google Scholar]
- 57. Persidsky Y., Buttini M., Limoges J., Bock P., Gendelman H. E. (1997) An analysis of HIV-1-associated inflammatory products in brain tissue of humans and SCID mice with HIV-1 encephalitis. J. Neurovirol. 3, 401–416 [DOI] [PubMed] [Google Scholar]
- 58. Moynagh P. N. (2005) The interleukin-1 signalling pathway in astrocytes: a key contributor to inflammation in the brain. J. Anat. 207, 265–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hayden M. S., Ghosh S. (2004) Signaling to NF-κB. Genes Dev. 18, 2195–2224 [DOI] [PubMed] [Google Scholar]
- 60. Thompson W. L., Van Eldik L. J. (2009) Inflammatory cytokines stimulate the chemokines CCL2/MCP-1 and CCL7/MCP-3 through NFkB and MAPK dependent pathways in rat astrocytes. Brain Res. 1287, 47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Uguccioni M., D'Apuzzo M., Loetscher M., Dewald B., Baggiolini M. (1995) Actions of chemotactic cytokines MCP-1, MCP-1, MCP-3, RANTES, MIP-1a and MIP-1b on human monocytes. Eur. J. Immunol. 25, 64–68 [DOI] [PubMed] [Google Scholar]
- 62. Kasahara T., Yagisawa H., Yamashita K., Yamaguchi Y., Akiyama Y. (1990) IL1 induces proliferation and IL6 mRNA expression in a human astrocytoma cell line: positive and negative modulation by chorela toxin and cAMP. Biochem. Biophys. Res. Commun. 167, 1242–1248. [DOI] [PubMed] [Google Scholar]
- 63. Zheng J. C., Huang Y., Tang K., Cui M., Niemann D., Lopez A., Morgello S., Chen S. (2008) HIV-1-infected and/or immune-activated macrophages regulate astrocyte CXCL8 production through IL-1β and TNF-α: involvement of mitogen-activated protein kinases and protein kinase R. J. Neuroimmunol. 200, 100–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Baggiolini M., Dewald B., Moser B. (1994) Interleukin-8 and related chemotactic cytokine-CXC and CC chemokine. Adv. Immunol. 55, 97–179 [PubMed] [Google Scholar]
- 65. Atlante A., Calissano P., Bobba A., Giannattasio S., Marra E., Passarella S. (2001) Glutamate neurotoxicity, oxidative stress and mitochondria. FEBS Lett. 497, 1–5 [DOI] [PubMed] [Google Scholar]
- 66. Backus K. H., Kettenmann H., Schachner M. (1989) Pharmacological characterization of the glutamate receptor in cultured astrocytes. J. Neurosci. Res. 22, 274–282 [DOI] [PubMed] [Google Scholar]
- 67. Benediktsson A. M., Marrs G. S., Tu J. C., Worley P. F., Rothstein J. D., Bergles D. E., Dailey M. E. (2012) Neuronal activity regulates glutamate transporter dynamics in developing astrocytes. Glia 60, 175–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Biber K., Laurie D. J., Berthele A., Sommer B., Tölle T. R., Gebicke-Härter P. J., van Calker D., Boddeke H. W. (1999) Expression and signaling of group I metabotropic glutamate receptors in astrocytes and microglia. J. Neurochem. 72, 1671–1680 [DOI] [PubMed] [Google Scholar]
- 69. Vartak-Sharma N., Ghorpade A. (2012) Astrocyte elevated gene-1 regulates astrocyte responses to neural injury: implications for reactive astrogliosis and neurodegeneration. J. Neuroinflammation 9, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]