Background: It has been reported that the kinase activity of Cdk5-p35 is activated by Tyr-15 phosphorylation of Cdk5.
Results: Phosphorylation at Tyr-15 occurs on free Cdk5 and is inhibited when Cdk5 is coexpressed with Cdk5 activators.
Conclusion: Phosphorylation at Tyr-15 does not activate Cdk5-p35.
Significance: Phosphorylation of Tyr-15 is not the activation mechanism of Cdk5-p35 in neurons.
Keywords: Cyclin, Cyclin-dependent Kinase (Cdk), neuron, Protein Phosphorylation, Protein-tyrosine Kinase (Tyrosine Kinase), Cdk5, Activation Mechanism, Fyn, p35
Abstract
Cdk5 is a member of the cyclin-dependent kinase (Cdk) family. In contrast to other Cdks that promote cell proliferation, Cdk5 plays a role in regulating various neuronal functions, including neuronal migration, synaptic activity, and neuron death. Cdks responsible for cell proliferation need phosphorylation in the activation loop for activation in addition to binding a regulatory subunit cyclin. Cdk5, however, is activated only by binding to its activator, p35 or p39. Furthermore, in contrast to Cdk1 and Cdk2, which are inhibited by phosphorylation at Tyr-15, the kinase activity of Cdk5 is reported to be stimulated when phosphorylated at Tyr-15 by Src family kinases or receptor-type tyrosine kinases. We investigated the activation mechanism of Cdk5 by phosphorylation at Tyr-15. Unexpectedly, however, it was found that Tyr-15 phosphorylation occurred only on monomeric Cdk5, and the coexpression of activators, p35/p25, p39, or Cyclin I, inhibited the phosphorylation. In neuron cultures, too, the activation of Fyn tyrosine kinase did not increase Tyr-15 phosphorylation of Cdk5. Further, phospho-Cdk5 at Tyr-15 was not detected in the p35-bound Cdk5. In contrast, expression of active Fyn increased p35 in neurons. These results indicate that phosphorylation at Tyr-15 is not an activation mechanism of Cdk5 but, rather, indicate that tyrosine kinases could activate Cdk5 by increasing the protein amount of p35. These results call for reinvestigation of how Cdk5 is regulated downstream of Src family kinases or receptor tyrosine kinases in neurons, which is an important signaling cascade in a variety of neuronal activities.
Introduction
Cyclin-dependent kinases (Cdks)2 are a family of serine/threonine kinases that mainly function in cell cycle progression. Cell cycle-associated Cdks, which are expressed in proliferating cells, require cyclin binding for activation (1). In addition to cyclin binding, Cdk activity is regulated by multiple phosphorylation on the Cdk itself. The activation mechanism of Cdk1-cyclin B (CycB) has been investigated extensively. Cdk1 is phosphorylated at Thr-161 in the activation loop by Cdk activation kinase upon binding to CycB. This phosphorylation is a prerequisite for activation (2). Concomitantly, Cdk1 is phosphorylated at Thr-14 and Tyr-15 in the ATP-binding G-loop by Wee1 or Myt1 kinase (3). The latter phosphorylation keeps the Cdk1-CycB complex in an inactive state until the onset of M phase, when these sites are dephosphorylated by the phosphatase Cdc25 (4). Although many Cdks have the conserved TY sequence in the N-terminal region (5), the role of this residue has not been investigated for other Cdks except Cdk1 and Cdk2 and Cdk4-Cdk6.
Cdk5 is a unique Cdk that plays a role in various neuronal activities unrelated to the cell cycle (6–8) because the Cdk5 activation subunit, p35 or p39, is expressed predominantly in postmitotic neurons (9–11). The activation mechanism also differs from that of Cdks involved in the cell cycle (Cdk1, Cdk2, Cdk4, and Cdk6). Cdk5 can be activated only by binding to p35 or p39 without phosphorylation in the activation T-loop. Furthermore, in contrast to Cdks involved in the cell cycle, Cdk5 activity has been reported to be stimulated by phosphorylation at Tyr-15 by non-receptor-type tyrosine kinases such as c-Abl and Fyn or receptor-type tyrosine kinases such as TrkB and EphA4 (12–16). Activation of Cdk5-p35 by Tyr-15 phosphorylation may play a role in neurite and spine retraction, dendrite outgrowth, and neuron death (12–16). Thus, phosphorylation of Tyr-15 appears to be important for various Cdk5 functions. However, it is not known how Tyr-15 phosphorylation of Cdk5 is regulated. Cdk5 has been shown to be phosphorylated by c-Abl and then activated by binding p35 (12). However, whether Cdk5 is activated similarly by other tyrosine kinases has not been investigated. Cdk5 has a ternary structure similar to Cdk2, with an amino acid sequence identity of about 60% (17–20). It would be interesting, therefore, to understand why Cdks involved in the cell cycle and neuronal Cdk5 are oppositely regulated by phosphorylation at Tyr-15. To address this issue, we investigated the effect of Tyr-15 phosphorylation on Cdk5 activation using the COS-7 cell overexpression system and cultured neurons.
EXPERIMENTAL PROCEDURES
Chemicals and Antibodies
Anti-phospho-Tyr-15 Cdk5, catalog no. ab63550 (referred to as anti-phospho-Tyr-15 Cdk5 (ab) in this work), was purchased from Abcam (Cambridge, MA). Anti-phospho-Tyr-15 Cdk5, catalog no. sc-12918 (referred to as anti-phospho-Tyr-15 Cdk5 (sc)), was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-Tyr-15 Cdk1, catalog no. 9111 (referred to as anti-phospho-Tyr-15 Cdk1 (cs)) was obtained from Cell Signaling Technology (Danvers, MA). Antibodies against Cdk5 DC17, Cdk5 C8, p35 C19, and Fyn FYN3 were from Santa Cruz Biotechnology. Anti-actin A2066, anti-FLAG M2, and anti-Myc 9E10 were obtained from Sigma. Anti-Myc 4A6 was obtained from Millipore (Billerica, MA). Anti-phospho-MAPK, catalog no. 9101, and anti-phospho-Src family kinase (SFK), catalog no. 2101, were from Cell Signaling Technology. Anti-Src clone 327 was purchased from Calbiochem. HRP-conjugated goat anti-mouse IgG, HRP-conjugated swine anti-rabbit IgG, and HRP-conjugated rabbit anti-goat IgG were from Dako (Glostrup, Denmark). Alexa Fluor 488-conjugated goat anti-mouse IgG was from Invitrogen. Semaphorin 3A (Sema3A) and Ephrin-A1 were from R&D Systems (Pune, Maharashtra, India). BDNF was purchased from BioVision (Mountain View, CA). Protein A-Sepharose was obtained from GE Healthcare. [γ-32P]ATP was from PerkinElmer Life Sciences. The Crosslink immunoprecipitation kit was obtained from Pierce. Pervanadate was freshly prepared by mixing Na3VO4 and H2O2 for 10 min at 37 °C.
Plasmid Construction
pCMV5-mouse Cdk5, pCMV2-FLAG-mouse Cdk5, pCMV5-kinase-negative (kn) Cdk5 D144N, pCMV5-mouse p35, pcDNA3-mouse p35-myc, pcDNA3-mouse p25-myc, pCAGGS-GFP, pCAGGS-DsRed, pCMV5-FLAG-p39 activation domain (AD), and pCMV2-FLAG-Cyclin I have been described previously (21–27). pME-Fyn, constitutively active (ca) pME-Fyn Y531F, kinase-negative pME-Fyn K299M, and pcDNA-caSrc Y530F were provided by T. Tezuka and T. Yamamoto at the University of Tokyo (28). pCMV2-FLAG-Cdk5 Y15F (Tyr-15 mutated to Phe) was constructed by PCR with the QuikChange site-directed mutagenesis kit (Stratagene, Santa Clara, CA) using pCMV2-FLAG-mouse Cdk5 as a template with primers 5′-GAAGGCACCTTTGGAACTGTG-3′ (forward) and 5′-CACAGTTCCAAAGGTGCCTTC-3′ (reverse). pCMV2-FLAG-Cdk5 Y15E (Tyr-15 mutated to Glu) was constructed by PCR using primers 5′-ATTGGGGAAGGCACCGAAGGAACTGTG-3′ (forward) and 5′-CACAGTTCCTTCGGTGCCTTCCCCAAT-3′ (reverse). pCMV2-FLAG-Cdk5 Y15A (Tyr-15 mutated to Ala) was constructed by PCR using primers 5′-ATTGGGGAAGGCACCGCTGGAACTGTG-3′ (forward) and 5′-CACAGTTCCAGCGGTGCCTTCCCCAAT-3′ (reverse). pCMV2-FLAG-Cdk5 T14A (Thr-14 mutated to Ala) was constructed by PCR using primers 5′-ATTGGGGAAGGCGCCTATGGAACTGTG-3′ (forward) and 5′-CACAGTTCCATAGGCGCCTTCCCCAATC-3′ (reverse).
Cell Culture and Transfection
COS-7 cells were maintained in Dulbecco's modified Eagle's medium containing 50 units/ml penicillin and 100 μg/ml streptomycin and supplemented with 10% fetal bovine serum. Primary cortical neurons were prepared from brains of ICR mice (Sankyo Lab, Tokyo, Japan) at embryonic day (E) 15 or 16. Mice were handled in accordance with institutional guidelines and housed in a pathogen-free environment on a 12-h light/dark cycle. The experimental protocol was approved by the ethics committee of the Tokyo Metropolitan University. Cortical neurons were maintained in Neurobasal medium supplemented with B-27 and 0.5 mm l-glutamine (29). In some cases, the cells were treated with 1 nm Sema3A, 5 μg/ml Ephrin-A1, or 10 ng/ml BDNF for 30 min. COS-7 cells were transfected with combinations of plasmids encoding Cdk5, p35, or Fyn using PolyFect transfection reagent (Qiagen, Hilden, Germany), HilyMax (Dojindo, Kumamoto, Japan), or Lipofectamine 2000 (Invitrogen) according to the protocol of the manufacturer. In some cases, the cells were treated with 100 μm pervanadate for 10 min or 10 μg/ml cycloheximide for 3 h from 24 h after transfection. Cells were harvested in lysis buffer (20 mm HEPES (pH 7.4), 0.1 mm EDTA, 0.1 mm EGTA, 100 mm NaCl, 2 mm MgCl2, 0.5% (w/v) Nonidet P-40, 1 mm NaF, 1 mm Na3VO4, 0.4 mm 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, 10 μg/ml leupeptin, and 1 mm dithiothreitol).
Immunoprecipitation and Kinase Assay of Cdk5
COS-7 cells transfected with the indicated plasmids were lysed in Laemmli sample buffer or the lysis buffer mentioned above. E18.5 mouse brains (ICR) were homogenized in 20 mm HEPES (pH 7.4), 0.1 mm EDTA, 0.1 mm EGTA, 150 mm NaCl, 2 mm MgCl2, 1 mm Na3VO4, 0.4 mm 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, 10 μg/ml leupeptin, and 1 mm dithiothreitol with a Teflon pestle homogenizer. The lysates or homogenates were centrifuged at 15,000 × g for 20 min, and the supernatants were used for immunoprecipitation of Cdk5 with anti-Cdk5 (C8) or anti-p35 (C19). In some cases, immunoprecipitation was performed with anti-Cdk5 (C8) or anti-p35 (C19) that had been cross-linked to protein A-Sepharose beads using the Pierce Crosslink IP kit according to the protocol of the manufacturer. The cell extracts were incubated with 1.5 μg of antibody and 20 μl of protein A-Sepharose beads and rotated overnight at 4 °C. The beads were washed with washing buffer (25 mm Tris-HCl (pH 7.5), 0.1 mm EDTA, 0.1 mm EGTA, 500 mm NaCl, 0.5% Nonidet P-40, and 1 mm dithiothreitol) five times. The kinase activity of Cdk5 was measured with histone H1 as a substrate in kinase buffer (10 mm MOPS (pH 6.8), 1 mm MgCl2, 0.1 mm EDTA, and 0.1 mm EGTA) at 37 °C for 30 min. After SDS-PAGE, phosphorylation was visualized by autoradiography with an imaging plate (FujiFilm, Tokyo, Japan).
Immunofluorescent Staining and Real-time Observation
Cortical neurons were transfected with DsRed, p35-myc, and caFyn by a calcium phosphate method at 5 days in vitro (DIV), as described previously (30), and, 48 h after transfection, neurons were immunostained with anti-myc 4A6, followed by Alexa Fluor 488-conjugated secondary antibodies. Fluorescent images were captured with an LSM510 or LSM710 confocal microscope (Carl Zeiss, Oberkochen, Germany). For Sema3A-induced growth cone retraction experiments, cortical neurons were transfected with pCAGGS-EGFP at 3 DIV by Lipofectamine 2000. Time-lapse observation was performed at intervals of 5 min after addition of 5 nm Sema3A at 5 DIV using an LSM710 confocal microscope (Carl Zeiss).
SDS-PAGE and Immunoblotting
After SDS-PAGE using a 10% or 12.5% polyacrylamide gel, proteins were analyzed by immunoblotting. In some cases, phosphorylation of Cdk5 was analyzed by Phos-tag SDS-PAGE, which was performed with a 10% polyacrylamide gel containing 10 μm Phos-tag acrylamide (Wako, Osaka, Japan) and 20 μm MnCl2, as described previously (31). Proteins in conventional SDS-PAGE gels or Phos-tag gels were transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA) using a semidry or submarine transfer apparatus. The membranes were blotted with primary antibodies. Following washing, the membranes were incubated with relevant secondary antibodies. The proteins were detected with ECL (GE Healthcare, Bioscience) or Millipore Immobilon Western chemiluminescent horseradish peroxidase substrate.
RESULTS
Coexpression of Cdk5 Activators Suppresses Tyr-15 Phosphorylation of Cdk5 in COS-7 Cells
Anti-phospho-Tyr-15 antibodies were used to detect phosphorylation of Cdk5 at Tyr-15. We confirmed the specificity of three available antibodies: anti-phospho-Tyr-15 of Cdk5 (ab) and (ca) and anti-phospho-Tyr-15 of Cdk1 (cs). Indeed, all of these antibodies recognized Cdk5 (Fig. 1A, lane 3) but not its Phe mutant (Fig. 1A, lane 4) when coexpressed with constitutively active (ca) Fyn, a non-receptor tyrosine kinase, confirming that Cdk5 is phosphorylated at Tyr-15 by caFyn and that these antibodies specifically recognize phosphorylated Tyr-15 of Cdk5. Cdk5 expressed in the absence of caFyn showed no or reduced reaction with these antibodies (Fig. 1A, lane 2), indicating that COS-7 cells do not express Tyr kinases strong enough to phosphorylate Tyr-15 of Cdk5.
FIGURE 1.
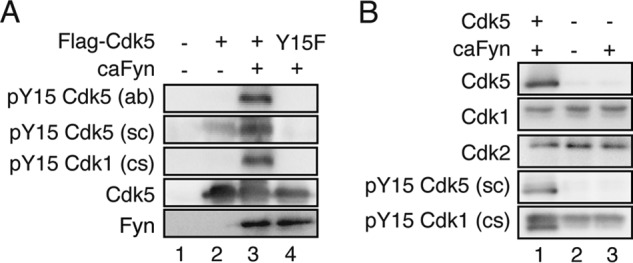
Reactivity and specificity of anti-phospho-Tyr-15 Cdk5 antibodies. A, Cdk5 or Cdk5 Y15F was cotransfected into COS-7 cells with ca Fyn. Cell lysates were immunoblotted with anti-phospho-Tyr-15 Cdk5 (ab) or (sc), anti-phospho-Tyr-15 Cdk1 (cs), anti-Cdk5 C8, and anti-Fyn. B, anti-phospho-Tyr-15 Cdk5 antibody does not react with Cdk1 and Cdk2. caFyn was transfected in COS-7 cells with or without Cdk5. Cell lysates were immunoblotted with anti-Cdk5 C8, anti-Cdk1, anti-Cdk2, anti-phospho-Tyr-15 Cdk5 (sc), and anti-phospho-Tyr-15 Cdk1 (cs).
Here we used COS-7 cells as a cellular expression system to examine Tyr-15 phosphorylation of Cdk5. COS-7 cells express other Cdk family members, such as Cdk1 and Cdk2. To exclude the possibility that anti-phospho-Tyr-15 of Cdk5 reacted with them, we examined the reactivity of anti-phospho-Tyr-15 of Cdk5 with Cdk1 or Cdk2 (Fig. 1B). Cdk1 and Cdk2 moved at a slightly higher molecular mass region. Although they were recognized by anti-phospho-Tyr-15 of Cdk1 (cs), anti-phospho-Tyr-15 of Cdk5 (sc) reacted with only Cdk5 but not Cdk1 and Cdk2.
First, we investigated the effect of Cdk5 activator expression on Tyr-15 phosphorylation of Cdk5. Cdk5 and caFyn or caSrc were cotransfected into COS-7 cells with or without p35. Unexpectedly, the reaction of Cdk5 with any anti-phospho-Tyr-15 antibodies was reduced greatly when p35 was coexpressed (Fig. 2, A and B, lanes 5). The same result was obtained when these proteins were expressed in HEK293 cells (data not shown).
FIGURE 2.
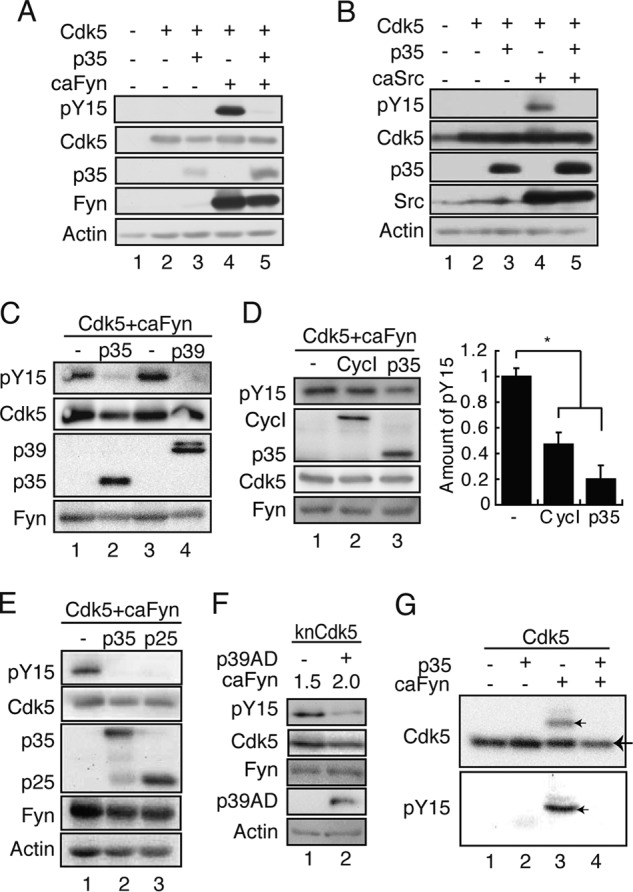
Inhibition of Tyr-15 phosphorylation of Cdk5 by Cdk5 activators. A, p35 inhibits Tyr-15 phosphorylation of Cdk5 by Fyn. Cdk5 and caFyn were cotransfected into COS-7 cells with or without p35. Cell lysates were immunoblotted with antibodies against phospho-Tyr-15 (pY15) of Cdk5 (ab), Cdk5, p35, Fyn, and actin. B, p35 inhibits Tyr-15 phosphorylation of Cdk5 by Src. Cdk5 and caSrc were cotransfected into COS-7 cells with or without p35. Cell lysates were immunoblotted with antibodies against phospho-Tyr-15 of Cdk5 (ab), Cdk5, p35, Src, and actin. C, p39 inhibits Tyr-15 phosphorylation of Cdk5. Cdk5 was cotransfected into COS-7 cells with caFyn in the presence or absence of p35 or p39. Cell lysates were immunoblotted with antibodies against phospho-Tyr-15 Cdk5 (ab), Cdk5, myc for p35 or p39, and Fyn. D, Cyclin I (CycI) inhibits Tyr-15 phosphorylation of Cdk5. Cdk5 was cotransfected into COS-7 cells with caFyn in the presence or absence of p35 or Cyclin I. Cell lysates were immunoblotted with antibodies against phospho-Tyr-15 Cdk5 (ab), Cdk5, FLAG for p35 or Cyclin I, and Fyn. The quantification of Tyr-15 phosphorylation of Cdk5 is shown in right panel (mean ± S.E., n = 3). *, p < 0.05; Student's t test. E, effect of p25 on Tyr-15 phosphorylation of Cdk5. Cdk5 and caFyn were cotransfected into COS-7 cells with p35-myc or p25-myc, a C-terminal fragment of p35. Cell lysates were immunoblotted with antibodies to phospho-Tyr-15 of Cdk5 (ab), Cdk5, myc for p35 and p25, Fyn, and actin. F, effect of p39 AD on Tyr-15 phosphorylation of Cdk5. knCdk5 and different levels of caFyn were cotransfected into COS-7 cells with or without FLAG-p39 AD. Cell lysates were immunoblotted with antibodies to phospho-Tyr-15 of Cdk5 (ab), Cdk5, FLAG for p39 AD, Fyn, and actin. G, detection of phospho-Tyr-15 of Cdk5 with Phos-tag SDS-PAGE. COS-7 cells were transfected with Cdk5 in the presence or absence of p35 and/or caFyn. Cell lysates were subjected to Phos-tag SDS-PAGE, followed by immunoblotting with anti-Cdk5 C8 (top panel) and anti-phospho-Tyr-15 Cdk5 (ab) (bottom panel). A big arrow indicates unphosphorylated Cdk5, and small arrows indicate Tyr-15-phosphorylated Cdk5.
We examined whether inhibition of Cdk5 Tyr-15 phosphorylation was specific to the p35 activator by using another Cdk5 activator, p39. p39 also suppressed Tyr-15 phosphorylation by caFyn (Fig. 2C). Cyclin I has been reported recently to bind (27, 32) and activate Cdk5 (32). We tested the effect of Cyclin I on Tyr-15 phosphorylation of Cdk5. Coexpression of Cyclin I inhibited phosphorylation of Cdk5 at Tyr-15. However, stronger inhibition of Tyr-15 phosphorylation was obtained with p35 (Fig. 2D). Cdk5 is known to be abnormally activated by p25, the N-terminal truncation form of p35, which is produced by calpain cleavage when neurons undergo cell death. We wondered whether Tyr-15 phosphorylation of Cdk5 may have any relationship to the activation of Cdk5 by p25 and examined the effect of p25 expression on Tyr-15 phosphorylation of Cdk5 by caFyn (Fig. 2E). p25, in addition to p35, decreased the phosphorylation of Cdk5 at Tyr-15. This result suggests that the binding of the activators is sufficient to suppress Tyr-15 phosphorylation of Cdk5. To test this possibility, we used the AD, which is the minimum size of the activators being capable of activating Cdk5. Because the expression levels of the p35 AD (amino acids 147–291 of p35) were extremely low, the p39 AD (amino acids 182–327 of p39) was used. The p39 AD still suppressed Tyr-15 phosphorylation of Cdk5 (Fig. 2F).
In the above experiments, we used anti-phospho-Tyr-15 antibodies to detect Cdk5 phosphorylation. To detect phosphorylated Tyr-15 with a phosphorylation-independent Cdk5 antibody, we employed Phos-tag SDS-PAGE, a method developed recently. Because of the phospho-protein-binding ability of Phos-tag in SDS-PAGE, phosphorylated proteins are extraordinarily retarded during electrophoresis (31, 33). Cdk5 cotransfected with caFyn in the presence or absence of p35 was analyzed with Phos-tag SDS-PAGE, followed by immunoblotting with anti-Cdk5 C8 (Fig. 2G). A retarded C8-reactive band was found only when Cdk5 alone was coexpressed with caFyn (Fig. 2G, top panel). Immunoblotting with anti-phospho-Tyr-15 Cdk5 (ab) confirmed that the band was a Cdk5 species phosphorylated at Tyr-15 (Fig. 2G, bottom panel). We can estimate the extent of the phosphorylation because both phosphorylated Cdk5 and unphosphorylated Cdk5 can be detected with the same antibody. Cdk5 phosphorylated at Tyr-15 constituted 19.3 ± 3.5% (n = 6) of total Cdk5 when coexpressed with caFyn. These results indicate that a portion of Cdk5 was phosphorylated at Tyr-15 when Cdk5 alone was coexpressed with caFyn.
The Binding of p35 Inhibits Tyr-15 Phosphorylation of Cdk5
Phosphorylation of Cdk5 at Tyr-15 was suppressed in COS-7 cells when the Cdk5 activators were coexpressed (Fig. 2). To further confirm the activator-dependent inhibition of Cdk5 phosphorylation, phosphorylation at Tyr-15 was examined with increasing expression of p35. Tyr-15 phosphorylation decreased with increasing amounts of p35 (Fig. 3A). The simplest interpretation of these results is that Cdk5 was not phosphorylated by Fyn when complexed with p35. However, there were several other alternatives that could explain the p35-dependent suppression of Tyr-15 phosphorylation. We examined and eliminated those possibilities.
FIGURE 3.
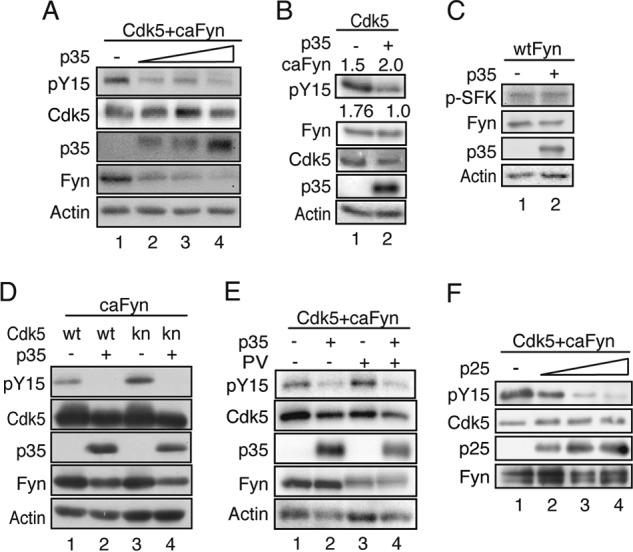
The binding of p35 inhibits Tyr-15 phosphorylation of Cdk5. A, Tyr-15 phosphorylation of Cdk5 with increasing expression of p35. Cdk5 and caFyn were cotransfected into COS-7 cells with increasing amounts of p35 (0–1 μg of plasmid). Cell lysates were immunoblotted with antibodies to phospho-Tyr-15 of Cdk5 (ab) (pY15), Cdk5, p35, Fyn, and actin. B, inhibition of Tyr-15 phosphorylation of Cdk5 by p35 is not due to decreased expression of caFyn. COS-7 cells were transfected with Cdk5 and different amounts of caFyn (1.5 or 2.0 μg of plasmid) in the absence (lane 1) or presence (lane 2) of p35. Cell lysates were immunoblotted with antibodies to phospho-Tyr-15 of Cdk5 (ab), Fyn, Cdk5, p35, and Actin. The ratio of Tyr-15 phosphorylation of Cdk5 is shown under the blot. Data are mean of three independent experiments. C, effect of p35 expression on Fyn activity. Cdk5 and WT Fyn were cotransfected into COS-7 cells without (lane 1) or with p35 (lane 2). Cell lysates were immunoblotted with antibodies against phospho-Tyr416 of Fyn (p-SFK) for Fyn activity, Fyn, p35, and actin. D, kinase-negative Cdk5 was phosphorylated at Tyr-15 by caFyn in the absence of p35. WT Cdk5 (wt) or knCdk5 D144N (kn) was cotransfected with or without p35 into COS-7 cells in the presence of caFyn. Cell lysates were immunoblotted with antibodies to phospho-Tyr-15 of Cdk5 (ab), Cdk5, p35, Fyn, and actin. E, effect of pervanadate (PV) on Tyr-15 phosphorylation of Cdk5. Cdk5 and caFyn were cotransfected into COS-7 cells with or without p35. Cells were treated with 100 μm pervanadate or vehicle for 10 min. Phosphorylation of Tyr-15 in Cdk5 was detected by immunoblotting with antibodies to phospho-Tyr-15 of Cdk5 (ab). Blots of Cdk5, p35, Fyn, and actin are also shown. F, in vitro inhibition of Tyr-15 phosphorylation of Cdk5 by p25. Cdk5-His was incubated in vitro with different amounts of p25-His at 4 °C for 1 h and then incubated with FLAG-caFyn, which was prepared from COS-7 cells by immunoprecipitation at 37 °C for 1.5 h in the presence of ATP. The mixture was immunoblotted with antibodies to phospho-Tyr-15 of Cdk5 (sc), Cdk5, p25, and Fyn.
First, we noticed that overexpression of p35 decreased the amount of Fyn protein (Fig. 3A). A decrease in phospho-Tyr-15 Cdk5 by p35 could, therefore, be due to decreased expression, i.e. the activity, of Fyn. We tested this possibility by controlling the protein amount of expressed caFyn. By changing the plasmid concentrations of caFyn in transfection, we could obtain similar expression levels of caFyn in the absence and presence of p35 (Fig. 3B). Despite similar caFyn expression, higher levels of phospho-Tyr-15 were detected in the absence of p35 (Fig. 3B, lane 1, 1.76 ± 0.08, n = 3), indicating that the decrease in Cdk5 phosphorylation was not simply due to Fyn expression reduced by p35. However, it was still possible that Fyn activity might be inhibited by interaction with p35 even though the expression levels were similar. Fyn activity was measured with autophosphorylation of Fyn at Tyr-416 in the presence or absence of p35. Tyr-416 phosphorylation was not affected by coexpression of p35 (Fig. 3C, p-SFK).
A third possibility is that Fyn activity may be down-regulated by the kinase activity of Cdk5-p35. Although Cdk5 activity reportedly does not affect c-Abl tyrosine kinase activity (12), we tested it here by using the kn mutants of Cdk5, D144N, and K33T. Both kn mutants were phosphorylated by Fyn in the absence of p35, and the phosphorylation was inhibited by coexpression of p35 (Fig. 3D, lanes 3 and 4, and data not shown), as was observed with WT Cdk5. The same result was reported with in vitro phosphorylation of Cdk5 D144N-p25 by c-Abl (20).
The next possibility was that p35 stimulates dephosphorylation of Tyr-15 of Cdk5, resulting in the apparent reduction in Cdk5 phosphorylation. To test this possibility, we used pervanadate, a tyrosine phosphatase inhibitor. Coexpression of p35 inhibited Cdk5 phosphorylation at Tyr-15 even in the presence of pervanadate (Fig. 3E, PV).
The experiments above were performed in cultured cells by overexpression of proteins. Unidentified cellular factors might mediate the inhibition of Tyr-15 phosphorylation of Cdk5 in the presence of the activator proteins. To show the direct inhibition, we conducted in vitro experiments. Cdk5 and p25 were prepared separately from Sf9 cells, and, after formation of the Cdk5-p25 complex in vitro, they were incubated with caFyn, which was prepared from COS-7 cells by immunoprecipitation. Phosphorylation of Cdk5 at Tyr-15 was decreased by increasing the protein amounts of p25 (Fig. 3F). Together, these results suggest that Cdk5, when complexed with its activators, is not phosphorylated by Src family tyrosine kinases.
Fyn Increases Cdk5 Activity by Stabilizing p35 in COS-7 Cells
Cdk5 has been reported to be phosphorylated at Tyr-15 by c-Abl in a ternary complex of Cdk5-Cables-c-Abl, and then Tyr-15-phosphorylated Cdk5 is activated by binding to p35 (12). Therefore, we tested whether phospho-Tyr-15 Cdk5 binds p35 in our experimental paradigm. To observe the binding of phospho-Tyr-15 Cdk5 to p35, we reduced p35 expression so that a portion of Cdk5 was phosphorylated at Tyr-15 even in the presence of p35 (Fig. 3A). Cdk5 should be expressed in excess of p35 in this condition. Then, we isolated p35 by immunoprecipitation with anti-p35 under conditions in which all p35 was precipitated. To directly compare phosphorylated Cdk5 in the p35 immunoprecipitate and its supernatant, we adjusted the protein amount of Cdk5 in the blot (Fig. 4A). Tyr-15-phosphorylated Cdk5 was not found in the p35 immunoprecipitate. These results indicate that phospho-Tyr-15 Cdk5 does not bind to p35 more than unphosphorylated Cdk5 does.
FIGURE 4.
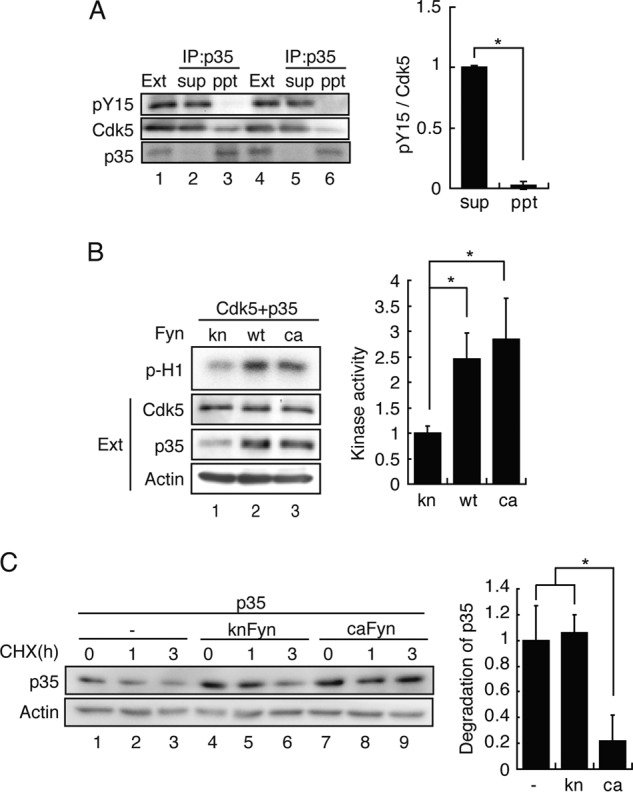
Fyn activates Cdk5 by increasing expression of p35 but not by Tyr-15 phosphorylation in COS-7 cells. A, Tyr-15-phosphorylated Cdk5 did not bind to p35. COS-7 cells were transfected with Cdk5, p35, and caFyn. The Cdk5-p35 complex was immunoprecipitated (IP) with anti-p35. The p35 immunoprecipitate (ppt) and the supernatant (sup) were immunoblotted with antibodies to phospho-Tyr-15 of Cdk5 (ab) (pY15), Cdk5, and p35. Duplicates are shown in lanes 1–3 and lanes 4–6. Tyr-15 phosphorylation of Cdk5 is shown in the top row of the left panel, and the quantification is shown in the right panel. Data are mean ± S.E. (n = 3). *, p < 0.05; Student's t test. Ext, extract. B, Cdk5 and p35 were cotransfected into COS-7 cells with either kn, WT, or ca Fyn. Cdk5 was immunoprecipitated with anti-Cdk5, and then the kinase activity was measured by histone H1 phosphorylation (p-H1). Quantification of the kinase activity is shown in right panel. Data are mean ± S.E. (n = 3). *, p < 0.05; Student's t test. C, effect of Fyn activity on degradation of p35. p35 was cotransfected into COS-7 cells with either empty vector (−), knFyn, or caFyn. Cells were treated with 10 μg/ml cycloheximide (CHX) for 1 and 3 h 24 h after transfection. Cell lysates were immunoblotted with antibodies to p35 and actin. The extent of p35 degradation, which is the difference in p35 between 0 and 3 h, is shown as the ratio to that of the control. Data are mean ± S.E. (n = 3). *, p < 0.05; Student's t test.
The higher Cdk5 activity was obtained previously by coexpression of Fyn in cultured cells. We confirmed the results by coexpression of Cdk5-p35 with kinase inactive or active Fyn. The total kinase activity of Cdk5-p35 was measured with immunoprecipitated Cdk5 by histone H1 phosphorylation. Cdk5 activity was in fact increased 3-fold by coexpression with WT Fyn or caFyn but not with knFyn (Fig. 4B). We confirmed that monomeric Cdk5 with phospho-Tyr-15 did not have kinase activity (data not shown). Cdk5 activity is mainly determined by the amount of p35 (34), but it was not examined previously under experimental conditions where phosphorylation at Tyr-15 was reported. We looked at p35 expressed in COS-7 cells in the presence of ca or kn Fyn. The amount of p35 increased in COS-7 cells expressing WT Fyn or caFyn compared with knFyn (Fig. 4B). These results suggest that the activation of Cdk5 is due to the increased p35 but not Tyr-15 phosphorylation, at least in the cell culture overexpression system.
p35 is a protein with a short half-life (24, 34). To ask how Fyn increases p35 protein, we measured the synthesis and degradation of p35 in the presence of MG132 proteasome inhibitor and cycloheximide protein synthesis inhibitor, respectively. The degradation of p35 was reduced in the presence of caFyn compared with that in its absence or the presence of knFyn (Fig. 4C), whereas synthesis was not changed (data not shown). The results suggest that Fyn activates Cdk5 activity by stabilizing p35 in COS-7 cells.
Tyr-15 Is a Critical Amino Acid for Cdk5 Activity
To further examine the role, if any, of phosphorylation at Tyr-15 in Cdk5 activity, we used another approach: introduction of a negative charge at Tyr-15. We constructed a Glu mutant (Y15E) as well as Phe (Y15F) and Ala (Y15A) unphosphorylatable mutants, at Tyr-15 of Cdk5. These Cdk5 mutants and p35 were expressed in COS-7 cells and immunoprecipitated with anti-Cdk5. We used FLAG-tagged Cdk5 WT (WT Cdk5), Y15F, Y15E, and Y15A, whereas knCdk5 D144N was untagged. Therefore, knCdk5 showed faster migration on SDS-PAGE (Fig. 5A, lane 1) than the other FLAG-tagged Cdk5s. All Cdk5 mutants bound to p35 similarly (Fig. 5A). The band of p35 became diffuse by phosphorylation with Cdk5 when p35 was coexpressed with WT Cdk5 (Fig. 5A, lane 2). In contrast, p35 appeared as a distinct band when coexpressed with Cdk5 mutants (Fig. 5A, lanes 3–5), suggesting lower kinase activity. The histone H1 phosphorylation assay was conducted in vitro with immunoprecipitated Cdk5 using [γ-32P]ATP. As expected, WT Cdk5-p35 showed strong kinase activity but knCdk5-p35 did not. Despite binding to p35, Cdk5 mutants showed only moderate kinase activity (Fig. 5B). We confirmed the results using Phos-tag SDS-PAGE. p35 is phosphorylated at Ser-8 and Thr-138 by Cdk5 when coexpressed in COS-7 cells (22). p35 phosphorylated at Ser-8 shows a markedly delayed migration on Phos-tag SDS-PAGE (31). From the ratio of Ser-8-phosphorylated to Ser-8-unphosphorylated p35, we could estimate the approximate kinase activity of Cdk5 in cells. Although all p35 coexpressed with WT Cdk5 migrated to the position of Ser-8-phosphorylated p35 (Fig. 5C, WT), p35 coexpressed with knCdk5 migrated faster (Fig. 5C, kn). p35 coexpressed with Cdk5-Y15F, Y15E, or Y15A split into two bands, Ser-8-phosphorylated and Ser-8-unphosphorylated, indicating partial activation. The ratio of phospho-Ser-8 p35 to total p35 resembled the Cdk5 activity measured by histone H1 phosphorylation. These results indicate that Tyr-15 is an important amino acid for the kinase activity of Cdk5 and that its modification impairs kinase activity.
FIGURE 5.
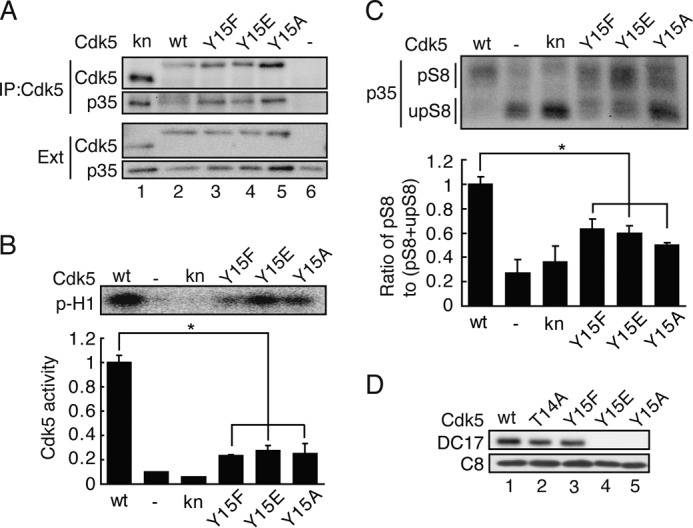
Effect of mutation at Tyr-15 on the kinase activity of Cdk5. A, kn Cdk5 D144N; WT Cdk5; or Phe, Glu, or Ala mutants of Cdk5 at Tyr-15 (Y15F, Y15E, or Y15A) was coexpressed with p35 in COS-7 cells. After immunoprecipitation (IP) with anti-Cdk5 C8, the binding of p35 was detected by immunoblotting with anti-p35. Ext, extract. B, the kinase activity of Cdk5 and its mutants was measured with anti-Cdk5 immunoprecipitates using histone H1 as a substrate. The autoradiogram is shown in the top panel, and quantification is shown in the bottom panel. Data are mean ± S.E. (n = 3). *, p < 0.05; Student's t test. C, the Cdk5 activity estimated by Ser-8 phosphorylation using Phos-tag SDS-PAGE. Phosphorylation of p35 at Ser-8 by Cdk5 was used as a marker of in situ Cdk5 activity. The Cdk5 construct, WT, kn Cdk5 D144N, the Phe mutant at Tyr-15, the Glu mutant at Tyr-15, or the Ala mutant at Tyr-15 was coexpressed with p35 in COS-7 cells. The cell lysates were immunoblotted with anti-p35 C19 after Phos-tag SDS-PAGE. The upper band is Ser-8-phosphorylated p35 (pS8), and the lower band correspond to unphosphorylated p35 (upS8). The kinase activity of Cdk5 was estimated in the bottom panel by the ratio of pS8 to total p35 (pS8 + upS8. Data are mean ± S.E. (n = 3). *, p < 0.05; Student's t test. D, the epitope of monoclonal anti-Cdk5 DC17 on Cdk5. The Cdk5 construct, WT, T14A, Y15F, Y15E, or Y15A was expressed in COS-7 cells with p35. The cell lysates were subjected to immunoblotting with the anti-Cdk5 antibodies DC17 and C8.
While we were doing these experiments, we found that DC17, a monoclonal antibody against Cdk5 that has been used frequently, recognizes the region around Tyr-15 as its epitope. DC17 showed a weaker reaction with the Cdk5-T14A and Cdk5-Y15F mutants and no reaction with the Cdk5-Y15E and Cdk5-Y15A mutants (Fig. 5D). Thus, an aromatic amino acid at position 15 may be required for recognition by DC17 anti-Cdk5.
Tyr-15 Phosphorylation Is Not Involved in the Activation of Cdk5 in Cultured Neurons
Many papers have reported Cdk5 activation by Tyr-15 phosphorylation in neurons and brains. This conclusion has been made mostly on the basis of immunoblotting or immunostaining data with anti-phospho-Tyr-15 Cdk5. Then, we tested whether these antibodies can be used to selectively detect Tyr-15 phosphorylation of Cdk5 in the extracts of neurons or brains. To make the results unambiguous, we used neurons prepared from Cdk5-deficient mouse brains as well as WT mouse brains. The position of Cdk5 is shown in immunoblots of WT (+/+) neuron extracts, and its absence is shown in that of Cdk5−/− neurons (Fig. 6A, Cdk5) (35). No clear reaction was found at the position of Cdk5 in blots of WT and Cdk5−/− neuron extracts with anti-phospho-Tyr-15 (sc) (Fig. 6A, pY15 (sc)). The smeared bands are found to decrease in extracts of Cdk5−/− neurons in blots with anti-phospho-Tyr-15 (ab) (Fig. 6A, vertical bar in pY15 (ab)), but they appeared at a little higher molecular weight region than Cdk5. Thus, in contrast to Cdk5 overexpressed in COS-7 cells, it was difficult to detect Tyr-15 phosphorylation of Cdk5 in whole neuron lysates with these antibodies. Then, we isolated Cdk5 from the brain extracts by immunoprecipitation with anti-Cdk5 C8, followed by immunoblotting with anti-phospho-Tyr-15 (sc). A specific reaction was detected in the Cdk5 immunoprecipitate (Fig. 6B, lane 6) but not in the control IgG immunoprecipitate (Fig. 6B, lane 8). The increase in the reaction by incubation with ATP and vanadate supported that the band was Tyr-15-phosphorylated Cdk5 (Fig. 6B, lane 7). Next, we examined whether Tyr-15-phosphorylated Cdk5 binds p35 in the brain extracts. Although anti-Cdk5 precipitated phospho-Tyr-15 Cdk5, no phospho-Tyr-15 reaction was observed in anti-p35 immunoprecipitates (Fig. 6C).
FIGURE 6.
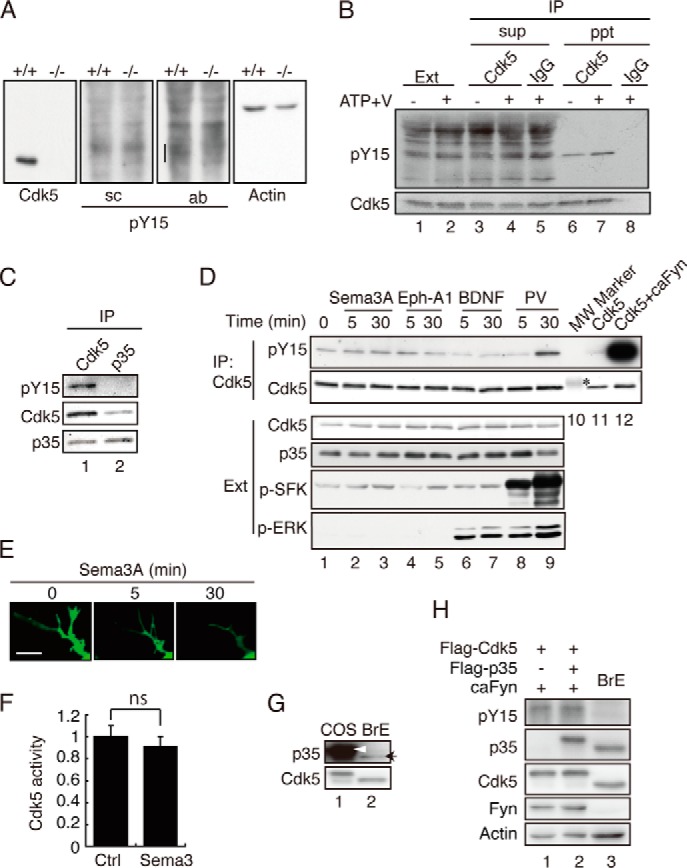
Tyr-15 phosphorylation of Cdk5 in neurons treated with Sema3A, Ephrin-A1, or BDNF. A, immunoblots of neuronal lysates with anti-phospho-Tyr-15 Cdk5 (pY15). Mouse cortical neurons prepared from wild-type (+/+) or Cdk5 knockout (−/−) mouse brains were immunoblotted with antibodies to Cdk5 C8, anti-phospho-Tyr-15 of Cdk5 (sc) and (ab), and actin. B, an immunoblot of anti-Cdk5 C8 immunoprecipitates with anti-phospho-Tyr-15 (sc). Cdk5 was immunoprecipitated (IP) from the extract (Ext) of mouse brains at embryonic day18.5, which was treated with Na3VO4 (V) and ATP. The supernatant (sup) or immunoprecipitate (ppt) was immunoblotted with anti-Cdk5 C8 or control IgG, followed by immunoblotting with anti-phospho-Tyr-15 Cdk5 (sc) (top panel) or anti-Cdk5 C8 (bottom panel). C, phospho-Cdk5 at Tyr-15 did not coimmunoprecipitate with anti-p35. Cultured neuronal lysates were immunoprecipitated with anti-Cdk5 C8 or anti-p35 C19, and Cdk5 phosphorylated at Tyr-15 was detected with anti-phospho-Tyr-15 Cdk5 (sc). D, Tyr-15 phosphorylation in neurons treated with Sema3A, Ephrin-A1, or BDNF. Mouse cortical neurons were treated with Sema3A, Ephrin-A1 (Eph-A1), BDNF, or 100 μm pervanadate (PV) for the indicated times. Cell lysates were immunoblotted with antibodies to Cdk5 (third panel), p35 (fourth panel), anti-phospho-SFK (fifth panel), or phospho-Thr-202/Tyr-204 of ERK (p-MAPK, sixth panel). Phosphorylation of Tyr-15 on Cdk5 was detected with anti-phospho-Tyr-15 Cdk5 (sc) (first panel) after immunoprecipitation with anti-Cdk5 C8 (second panel). Lane 10 is a molecular weight (MW) marker. The asterisk indicates carbonic anhydrase at 32.2 kDa. Lanes 11 and 12 are Cdk5 coexpressed with or without caFyn in HEK293 cells for reference. E, the effect of Sema3A on neurite retraction. Neurites of mouse cortical neurons expressing EGFP were observed by time-lapse imaging at intervals of 5 min after addition of Sema3A. Scale bar = 5 μm. F, kinase activity of Cdk5 after Sema3A treatment. Cdk5 was prepared from cultured neurons treated with Sema3A for 30 min by immunoprecipitation, and its kinase activity was measured by phosphorylation of histone H1. Data are mean ± S.E. (n = 3). ns, not significant; Student's t test. Ctrl, control. G, the protein ratio of p35 and Cdk5 in COS-7 cells and mouse brain extract. FLAG-Cdk5 and FLAG-p35 were transfected into COS-7 cells using 1 μg of plasmid for each of Cdk5 and p35, the same as in Fig. 2. Immunoblotting was performed by adjusting Cdk5 approximately (bottom panel). FLAG-p35 expressed in COS-7 cells is indicated by an arrowhead, and p35 in the brain extract (BrE) is indicated by an arrow (top panel). H, the effect of p35 at the low expression levels (similar to the brain extract) on Tyr-15 phosphorylation of Cdk5. COS-7 cells were transfected with FLAG-Cdk5 and caFyn with or without FLAG-p35 as shown in Fig. 2A, except for the reduced plasmid amount of p35 (1/40). Phosphorylation of Tyr-15 in Cdk5 was detected by immunoblotting with antibodies to phospho-Tyr-15 of Cdk5 (ab). Blotting of Cdk5, p35, Fyn, and actin is also shown.
Various stimuli, such as Sema3A, Ephrin-A1, BDNF, and amyloid β, have been reported to increase phosphorylation of Cdk5 at Tyr-15 in cultured neurons (13–15). We set up the experiments to confirm those results in our neuron cultures. Cortical neurons at 4 DIV were treated with Sema3A, Ephrin-A1, or BDNF, and Tyr-15 phosphorylation was examined with immunoblotting with anti-phospho-Tyr-15 (sc) after immunoprecipitation. Activation of downstream signaling of these ligands was confirmed with phospho-SFK for Sema3A and Ephrin-A1 treatment (Fig. 6D, p-SFK), neurite retraction for Sema3A treatment (Fig. 6E), and phospho-ERK (phospho-MAPK) for BDNF treatment (Fig. 6D, p-ERK). Nevertheless, Tyr-15 phosphorylation in Cdk5 was not increased clearly in neurons treated with any of these ligands (Fig. 6D, lanes 2–7). In contrast, the pervanadate treatment, which nonspecifically enhances Tyr phosphorylation by inhibiting Tyr phosphatases, clearly increased phospho-Tyr-15 reaction (Fig. 6D, lanes 8 and 9). Even in pervanadate-treated neurons, Tyr-15 phosphorylation was extremely low compared with that obtained in cultured cells by coexpression with caFyn (Fig. 6D, lane 12). Additionally, we did not see a clear increase in p35 protein in neurons treated with Sema3A, Eph-A1, or BDNF (Fig. 6D, p35) nor an increase in histone H1 kinase activity of Cdk5 immunoprecipitated with anti-Cdk5 from Sema3A-treated neurons (Fig. 6F).
Several previous results suggest an excess amount of Cdk5 over p35 in neurons or brain (36–38). However, extremely low Tyr-15 phosphorylation in cultured neurons, compared with that in COS-7 cells, may suggest that a pool size of free Cdk5 is not so large. To know the amount of free Cdk5 that can be phosphorylated at Tyr-15 in brain extracts and COS-7 cells transfected with both Cdk5 and p35 (the same plasmid amount as in Fig. 2), we compared the relative ratio of Cdk5 and p35 by immunoblotting. When the protein amount of Cdk5 was adjusted (Fig. 6G, bottom panel), it was shown that COS-7 cells expressed p35 in significantly higher amounts than brain (Fig. 6G, top panel). A 40× reduction of the p35 plasmid used for transfection led to similar expression levels in the brain extract (Fig. 6H). Such a small expression of p35 did not affect the Tyr-15 phosphorylation of Cdk5 by caFyn (Fig. 6H). The result that only the trace amount of Cdk5 was phosphorylated at Tyr-15 in a large pool of free Cdk5 suggests that Tyr-15 phosphorylation is a nonphysiological, minor reaction.
Active Fyn Increases p35 Expression in Cultured Neurons
Extracellular ligands, such as Sema3A, Eph-A1, or BDNF, failed to increase p35 in cultured neurons (Fig. 6C), whereas coexpression of active Fyn increased the protein amount of p35 in COS-7 cells (Fig. 4B). We thought that these ligands might stimulate downstream tyrosine kinases insufficiently to increase p35 to the detectable levels by immunoblotting of the total neuron lysates. Therefore, we assessed whether expression of caFyn increases p35 in neurons. Because there is no proper antibody available for immunostaining of endogenous p35, we expressed p35-myc together with caFyn. It was shown that neurons expressing caFyn displayed stronger anti-myc staining (Fig. 7A). Quantification indicates an 1.8-fold increase in p35 compared with transfection with an empty vector (Fig. 7B). These results suggest that tyrosine kinase signaling can activate Cdk5 by increasing p35 in neurons.
FIGURE 7.
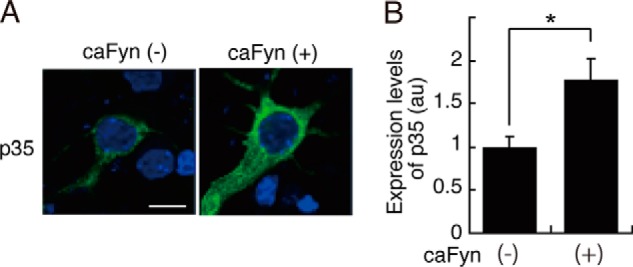
Levels of p35 in neurons coexpressing caFyn. A, mouse brain cortical neurons were transfected with p35-myc in the absence (−) or presence (+) of caFyn at 5 DIV, and expression levels of p35 were estimated at 7 DIV by immunofluorescent staining with anti-myc antibody. B, quantification of p35 staining. Data are mean ± S.E. (n = 27 for caFyn (−, and n = 31 for caFyn (+)). *, p < 0.05; Student's t test. Scale bar = 10 μm.
DISCUSSION
All Cdks in the human and mouse genomes have been recently grouped and numbered on the basis of the amino acid sequence around the N-terminal cyclin-binding region (5). There are 20 Cdks in the mouse genome and 21 Cdks in the human genome. Tyr-15 is ∼30 residues upstream of the cyclin-binding region in the small N-terminal lobe of Cdk1, Cdk2, and Cdk5. Tyr-15 constitutes the roof of the ATP binding pocket, which is in the cleft between the N-terminal and C-terminal lobes (1, 5). The equivalent Tyr residue is found in 17 of 20 mouse Cdks (5), indicating the importance of Tyr at this position for kinase activity. Phosphorylation at Tyr-15 acts oppositely on the kinase activity of Cdk1 and Cdk5: inhibition for Cdk1 and Cdk2 and activation for Cdk5. In this study, contrary to previous studies, we found that Tyr-15 phosphorylation is not involved in Cdk5 activation. It is very important to clarify the role of Tyr-15 phosphorylation for the activation mechanism of Cdk5 as well as other members of Cdks, which will be investigated in the future. Here, we discuss why Tyr-15 phosphorylation was interpreted to activate Cdk5.
p35 binding is necessary and sufficient for Cdk5 activation. If Tyr-15 phosphorylation induces further stimulation of the kinase activity, Cdk5 complexed with p35 should be phosphorylated at Tyr-15. However, we could not detect Tyr-15 phosphorylation of Cdk5 in the Cdk5-p35 complex under our experimental conditions. By contrast, p35 coexpression inhibited Tyr-15 phosphorylation of Cdk5. Phosphorylation at Tyr-15 occurred on inactive Cdk5, in agreement with the first report by Zukerberg et al. (12). They showed that Cdk5, in a trimeric complex with c-Abl and Cables but not p35, is phosphorylated by c-Abl in cells (12). However, subsequent studies demonstrated stimulation of Cdk5 by tyrosine kinases under various cellular circumstances (13–16) but have not shown how Cdk5 is phosphorylated and how Cdk5 activity is stimulated. Moreover, many recent reports demonstrate Tyr-15 phosphorylation-induced Cdk5 activation only by immunoblotting or immunostaining of brain or neurons with anti-phospho-Tyr-15 antibodies, which are not sensitive enough to detect phospho-Tyr-15 Cdk5 without immunoprecipitation with anti-Cdk5 antibody.
We examined the results of early several reports (12–16) that analyzed the role of Tyr-15 phosphorylation on Cdk5 activation and found no substantial discrepancies between their results and our results using cultured cell lines. For example, Cdk5 is phosphorylated at Tyr-15 by c-Abl or Fyn, even in the presence of p35 (12, 13), which was also seen in our case. Cdk5 is usually expressed at higher levels than p35 because of a labile property of p35 (36–38). Therefore, the amounts of Cdk5 protein are more abundant than that of p35 when overexpressed in cultured cells. The ratio of Cdk5 and p35 is much greater in neurons (Fig. 6G). There are two pools of Cdk5 in neurons, Cdk5 complexed with p35 and free monomeric Cdk5 (39). Only a proportion of monomeric Cdk5 was phosphorylated at Tyr-15. Previous data showing Tyr-15 phosphorylation were obtained by immunoblotting of Cdk5 immunoprecipitates with anti-phospho-Tyr-15 (13, 15, 16). Because Cdk5 immunoprecipitates contain both free Cdk5 and Cdk5 bound to p35, it is hard to determine which of them is phosphorylated. Thus, in a strict sense, Tyr-15 phosphorylation of Cdk5 bound to p35 has not been shown previously. In contrast, all of our current results indicate that Tyr-15 phosphorylation occurs only on free Cdk5 and not on Cdk5 complexed with p35.
Another issue is whether Tyr-15-phosphorylated Cdk5 can bind p35. Zukerberg et al. (12) proposed a model in which Cdk5 complexed with c-Abl and Cables is phosphorylated by c-Abl, and then phospho-Cdk5 exchanges its binding partner from c-Abl and Cables to p35 for activation. This was on the basis of several results, such as lower kinase activity of Cdk5 Y15F and higher activity and higher phospho-Tyr-15 phosphorylation of Cdk5 in COS-7 cells when cotransfected with c-Abl and Cables. Although the experiments were performed carefully, those are correlative lines of evidence, and one cannot definitively conclude that Cdk5 phosphorylated at Tyr-15 forms a complex with p35 that has higher kinase activity. Zukerberg et al. (12) also showed the presence of phospho-Tyr-15 Cdk5 in the p35 immunoprecipitate from E15 brain lysates, but its amount was considerably less than that found in Cdk5 immunoprecipitates. The latter results can also be interpreted to mean that most of phospho-Tyr-15 Cdk5 is monomeric. In fact, we found marginal phospho-Tyr-15 Cdk5 in the anti-p35 immunoprecipitates. These results suggest that the binding affinity of phospho-Tyr-15 Cdk5 for p35, if any, is weaker than unphosphorylated Cdk5 in cells.
The important question is the activation of Cdk5. Previously, Cdk5 activation has been demonstrated mainly by coexpression with tyrosine kinases in cultured cell lines (12, 13). Our results are in agreement with this. Cdk5 activity was stimulated more than 2-fold in COS-7 cells by coexpression with active Fyn. The difference is interpretation. The previous reports concluded that activation of Cdk5 was due to Tyr-15 phosphorylation, whereas we demonstrate increased p35 expression. The previous reports assessed Tyr-15 phosphorylation and Cdk5 activation in separate experiments. Tyr-15 phosphorylation of Cdk5 was not compared directly in the presence or absence of p35, and expression levels of p35 were not measured. In contrast, we found that coexpression of active Fyn increased the levels of p35 in COS-7 cells (Fig. 4B). Increased expression of p35 can explain the Fyn or Abl-induced Cdk5 activation reported previously.
We think that the similar mechanism takes place in neurons, although the situation is not clear. We could not detect the activation of Cdk5 in cortical neurons treated with Sema3A. To our knowledge, there is only one report that describes the activation of Cdk5 in cortical neurons treated by Sema3A (40), but it is a statement under discussion without data. However, we could not detect an increased expression of p35 by immunoblotting of total cell lysates after treatment with Sema3A, Ephrin-1A, or BDNF. In contrast, p35 was increased in neurons when caFyn was cotransfected. These results may suggest that Cdk5 is activated by stabilization of p35 downstream of tyrosine kinase signals, but because these signals may function locally at the region where the receptors accumulate, the activation of Cdk5 or increase in p35 expression by physiological ligands has not been reported.
Previous studies indicated that Cdk5 mediates Sema3A signaling in growth cone retraction, Ephrin-A1 signaling in dendritic spine retraction, and BDNF signaling in dendritic growth (13–15). Our results do not necessarily rule out the possibility that Cdk5-p35 is downstream of Src family kinases or receptor-type tyrosine kinases, but only demonstrate that Tyr-15 phosphorylation is not its activation mechanism. There could be cross-talk at several levels of those signaling pathways. One possible mechanism may keep active Cdk5 complexes in the cellular region stimulated, otherwise, Cdk5 activity would be down-regulated by degradation of p35. Alternatively, tyrosine kinase signals may recruit the active Cdk5-p35 complex to the stimulated region. Because of the lack of an appropriate antibody for detailed cellular localization of endogenous p35, however, we cannot currently examine this possibility. In any case, further analysis is required to clarify the activation mechanism of Cdk5 in neurons, an important issue concerning tyrosine kinase-mediated signaling pathways.
Acknowledgments
We thank Drs. T. Tezuka and M. Yamamoto for Fyn and Src expression plasmids and Govinda Sharma for reading the manuscript.
This work was supported by grants-in-aid for scientific research on a priority area from MEXT of Japan (to S. H.).
- Cdk
- cyclin-dependent kinase
- SFK
- Src family kinase
- kn
- kinase-negative
- AD
- activation domain
- ca
- constitutively active
- DIV
- days in vitro.
REFERENCES
- 1. Morgan D. O. (1995) Principles of CDK regulation. Nature 374, 131–134 [DOI] [PubMed] [Google Scholar]
- 2. Ducommun B., Brambilla P., Félix M. A., Franza B. R., Jr., Karsenti E., Draetta G. (1991) Cdc2 phosphorylation is required for its interaction with cyclin. EMBO J. 10, 3311–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atherton-Fessler S., Liu F., Gabrielli B., Lee M. S., Peng C.Y., Piwnica-Worms H. (1994) Cell cycle regulation of the p34cdc2 inhibitory kinases. Mol. Biol. Cell 5, 989–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strausfeld U., Labbé J. C., Fesquet D., Cavadore J. C., Picard A., Sadhu K., Russell P., Dorée M. (1991) Dephosphorylation and activation of a p34cdc2/cyclin B complex in vitro by human CDC25 protein. Nature 351, 242–245 [DOI] [PubMed] [Google Scholar]
- 5. Malumbres M., Harlow E., Hunt T., Hunter T., Lahti J. M., Manning G., Morgan D. O., Tsai L. H., Wolgemuth D. J. (2009) Cyclin-dependent kinases: a family portrait. Nat. Cell Biol. 11, 1275–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barnett D. G., Bibb J. A. (2011) The role of Cdk5 in cognition and neuropsychiatric and neurological pathology. Brain Res. Bull. 85, 9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dhavan R., Tsai L. H. (2001) A decade of CDK5. Nat. Rev. Mol. Cell Biol. 2, 749–759 [DOI] [PubMed] [Google Scholar]
- 8. Cheung Z. H., Ip N. Y. (2007) The roles of cyclin-dependent kinase 5 in dendrite and synapse development. Biotechnol. J. 2, 949–957 [DOI] [PubMed] [Google Scholar]
- 9. Lew J., Huang Q. Q., Qi Z., Winkfein R. J., Aebersold R., Hunt T., Wang J. H. (1994) A brain-specific activator of cyclin-dependent kinase 5. Nature 371, 423–426 [DOI] [PubMed] [Google Scholar]
- 10. Tsai L. H., Delalle I., Caviness V. S., Jr., Chae T., Harlow E. (1994) p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature 371, 419–423 [DOI] [PubMed] [Google Scholar]
- 11. Uchida T., Ishiguro K., Ohnuma J., Takamatsu M., Yonekura S., Imahori K. (1994) Precursor of cdk5 activator, the 23 kDa subunit of Tau protein kinase II: its sequence and developmental change in brain. FEBS Lett. 355, 35–40 [DOI] [PubMed] [Google Scholar]
- 12. Zukerberg L. R., Patrick G. N., Nikolic M., Humbert S., Wu C. L., Lanier L. M., Gertler F. B., Vidal M., Van Etten R. A., Tsai L. H. (2000) Cables links Cdk5 and c-Abl and facilitates Cdk5 tyrosine phosphorylation, kinase upregulation, and neurite outgrowth. Neuron 26, 633–646 [DOI] [PubMed] [Google Scholar]
- 13. Sasaki Y., Cheng C., Uchida Y., Nakajima O., Ohshima T., Yagi T., Taniguchi M., Nakayama T., Kishida R., Kudo Y., Ohno S., Nakamura F., Goshima Y. (2002) Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron 35, 907–920 [DOI] [PubMed] [Google Scholar]
- 14. Fu W. Y., Chen Y., Sahin M., Zhao X. S., Shi L., Bikoff J. B., Lai K. O., Yung W. H., Fu A. K., Greenberg M. E., Ip N.Y. (2007) Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat. Neurosci. 10, 67–76 [DOI] [PubMed] [Google Scholar]
- 15. Cheung Z. H., Chin W. H., Chen Y., Ng Y. P., Ip N. Y. (2007) Cdk5 is involved in BDNF-stimulated dendritic growth in hippocampal neurons. PLoS Biol. 5, e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin H., Lin T. Y., Juang J. L. (2007) Abl deregulates Cdk5 kinase activity and subcellular localization in Drosophila neurodegeneration. Cell Death Differ. 14, 607–615 [DOI] [PubMed] [Google Scholar]
- 17. Meyerson M., Enders G. H., Wu C. L., Su L. K., Gorka C., Nelson C., Harlow E., Tsai L. H. (1992) A family of human Cdc2-related protein kinases. EMBO J. 11, 2909–2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Bondt H. L., Rosenblatt J., Jancarik J., Jones H. D., Morgan D. O., Kim S. H. (1993) Crystal structure of cyclin-dependent kinase 2. Nature 363, 595–602 [DOI] [PubMed] [Google Scholar]
- 19. Tarricone C., Dhavan R., Peng J., Areces L. B., Tsai L. H., Musacchio A. (2001) Structure and regulation of the CDK5-p25(nck5a) complex. Mol. Cell 8, 657–669 [DOI] [PubMed] [Google Scholar]
- 20. Mapelli M., Massimiliano L., Crovace C., Seeliger M. A., Tsai L. H., Meijer L., Musacchio A. (2005) Mechanism of CDK5/p25 binding by CDK inhibitors. J. Med. Chem. 48, 671–679 [DOI] [PubMed] [Google Scholar]
- 21. Yamada M., Saito T., Sato Y., Kawai Y., Sekigawa A., Hamazumi Y., Asada A., Wada M., Doi H., Hisanaga S. (2007) Cdk5-p39 is a labile complex with the similar substrate specificity to Cdk5-p35. J. Neurochem. 102, 1477–1487 [DOI] [PubMed] [Google Scholar]
- 22. Kamei H., Saito T., Ozawa M., Fujita Y., Asada A., Bibb J. A., Saido T. C., Sorimachi H., Hisanaga S. (2007) Suppression of calpain-dependent cleavage of the CDK5 activator p35 to p25 by site-specific phosphorylation. J. Biol. Chem. 282, 1687–1694 [DOI] [PubMed] [Google Scholar]
- 23. Asada A., Yamamoto N., Gohda M., Saito T., Hayashi N., Hisanaga S. (2008) Myristoylation of p39 and p35 is a determinant of cytoplasmic or nuclear localization of active cyclin-dependent kinase 5 complexes. J. Neurochem. 106, 1325–1336 [DOI] [PubMed] [Google Scholar]
- 24. Minegishi S., Asada A., Miyauchi S., Fuchigami T., Saito T., Hisanaga S. (2010) Membrane association facilitates degradation and cleavage of the cyclin-dependent kinase 5 activators p35 and p39. Biochemistry 49, 5482–5493 [DOI] [PubMed] [Google Scholar]
- 25. Saito T., Yano M., Kawai Y., Asada A., Wada M., Doi H., Hisanaga S. (2013) Structural basis for the different stability and activity between the Cdk5 complexes with p35 and p39 activators. J. Biol. Chem. 288, 32433–32439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tanabe K., Yamazaki H., Inaguma Y., Asada A., Kimura T., Takahashi J., Taoka M., Ohshima T., Furuichi T., Isobe T., Nagata K., Shirao T., Hisanaga S., (2014) Phosphorylation of drebrin by cyclin-dependent kinase 5 and its role in neuronal migration. PLoS ONE 9, e92291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagano T., Hashimoto T., Nakashima A., Hisanaga S., Kikkawa U., Kamada S. (2013) Cyclin I is involved in the regulation of cell cycle progression. Cell Cycle 12, 2617–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hisatsune C., Umemori H., Mishina M., Yamamoto T. (1999) Phosphorylation-dependent interaction of the N-methyl-d-aspartate receptor ϵ 2 subunit with phosphatidylinositol 3-kinase. Genes Cells 4, 657–666 [DOI] [PubMed] [Google Scholar]
- 29. Saito T., Onuki R., Fujita Y., Kusakawa G., Ishiguro K., Bibb J. A., Kishimoto T., Hisanaga S. (2003) Developmental regulation of the proteolysis of the p35 cyclin-dependent kinase 5 activator by phosphorylation. J. Neurosci. 23, 1189–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaminosono S., Saito T., Oyama F., Ohshima T., Asada A., Nagai Y., Nukina N., Hisanaga S. (2008) Suppression of mutant Huntingtin aggregate formation by Cdk5/p35 through the effect on microtubule stability. J. Neurosci. 28, 8747–8755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hosokawa T., Saito T., Asada A., Fukunaga K., Hisanaga S. (2010) Quantitative measurement of in vivo phosphorylation states of Cdk5 activator p35 by Phos-tag SDS-PAGE. Mol. Cell Proteomics 9, 1133–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brinkkoetter P. T., Olivier P., Wu J. S., Henderson S., Krofft R. D., Pippin J. W., Hockenbery D., Roberts J. M., Shankland S. J. (2009) Cyclin I activates Cdk5 and regulates expression of Bcl-2 and Bcl-XL in postmitotic mouse cells. J. Clin. Invest. 119, 3089–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kinoshita E., Kinoshita-Kikuta E., Takiyama K., Koike T. (2006) Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol. Cell Proteomics 5, 749–757 [DOI] [PubMed] [Google Scholar]
- 34. Hisanaga S., Endo R. (2010) Regulation and role of cyclin-dependent kinase activity in neuronal survival and death. J. Neurochem. 115, 1309–1321 [DOI] [PubMed] [Google Scholar]
- 35. Ohshima T., Ward J. M., Huh C. G., Longenecker G., Veeranna, Pant H. C., Brady R. O., Martin L. J., Kulkarni A. B. (1996) Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl. Acad. Sci. U.S.A. 93, 11173–11178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee K.Y., Rosales J. L., Tang D., Wang J. H. (1996) Interaction of cyclin-dependent kinase 5 (Cdk5) and neuronal Cdk5 activator in bovine brain. J. Biol. Chem. 271, 1538–1543 [DOI] [PubMed] [Google Scholar]
- 37. Zhu Y. S., Saito T., Asada A., Maekawa S., Hisanaga S. (2005) Activation of latent cyclin-dependent kinase 5 (Cdk5)-p35 complexes by membrane dissociation. J. Neurochem. 94, 1535–1545 [DOI] [PubMed] [Google Scholar]
- 38. Patrick G. N., Zhou P., Kwon Y. T., Howley P. M., Tsai L. H. (1998) p35, the neuronal-specific activator of cyclin-dependent kinase 5 (Cdk5) is degraded by the ubiquitin-proteasome pathway. J. Biol. Chem. 273, 24057–24064 [DOI] [PubMed] [Google Scholar]
- 39. Nikolic M., Chou M. M., Lu W., Mayer B. J., Tsai L. H. (1998) The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature 395, 194–198 [DOI] [PubMed] [Google Scholar]
- 40. Chen G., Sima J., Jin M., Wang K. Y., Xue X. J., Zheng W., Ding Y. Q., Yuan X. B. (2008) Semaphorin-3A guides radial migration of cortical neurons during development. Nat. Neurosci. 11, 36–44 [DOI] [PubMed] [Google Scholar]


