Abstract
Background
Although the American Heart Association/American College of Sports Medicine’s Preparticipation Questionnaire (AAPQ) is a recommended pre-exercise cardiovascular screening tool, it has never been systematically evaluated. The purpose of this research is to provide preliminary evidence of its effectiveness among adults aged 40 years or older.
Methods and Results
Under the assumption that respondents would respond to AAPQ items as they responded to NHANES questionnaire responses, we calculated the gender- and age-specific proportions of adult participants in NHANES, 2001–2004 who would be receive a recommendation for physician consultation based on AAPQ referral criteria. Additionally, we compared recommended AAPQ referrals to a similar assessment using the Physical Activity Readiness Questionnaire (PAR-Q) in the study sample. AAPQ referral proportions were higher with older age. Across all age groups 40 years and older, 95.5% (94.3–96.8%) of women and 93.5% (92.2–94.7%) of men in the US would be advised to consult a physician before exercise. Prescription medication use and age were the most commonly selected items. When referral based on AAPQ was compared to that of the PAR-Q, the two screening tools produced similar results for 72.4% of respondents.
Conclusions
These results suggest that more than 90% of US adults aged 40 years or older would receive a recommendation for physician consultation by the AAPQ. Excessive referral may present an unnecessary barrier to exercise adoption and stress the healthcare infrastructure. (228/250)
Keywords: Exercise, Screening, Population-based research
Introduction
It is well-established that regular participation in vigorous and moderate-intensity physical activity lowers the risk of cardiovascular disease (CVD) and CVD mortality1. Paradoxically, the risk of an acute cardiac event, including myocardial infarction or sudden cardiac death (SCD), is increased during a bout of physical activity, although less so in those who are habitually physically active2,3. Occlusive coronary artery disease is the most prevalent underlying pathology precipitating physical activity-associated myocardial infarction and SCD in adults aged 35 years or older2. Accordingly, efforts to identify adults at elevated risk for physical activity-associated cardiovascular complications usually center on assessment of CVD risk factors and/or diagnosis of occult CVD.
Preventing physical activity-associated cardiac events is one argument for preparticipation screening, which is championed by medical and professional organizations4,5. For adults, such screening can take many forms and varies in formality and thoroughness, from physician-led examination and testing, to cardiovascular health assessments by allied-health professionals, to self-administered health questionnaires (henceforth: self-screening). Self-screening is the least formal but arguably the most common preparticipation screening method, the purpose of which is to identify those with cardiovascular disease symptoms that may benefit from a physician consultation before initiating or increasing participation in physical activity of moderate or vigorous intensity. Further, such tools can foster conversations on exercise safety among the respondent, his or her physician, and the exercise professional5. Self-screening questionnaires can be used in a broad range of settings, including health and fitness facilities, sports clubs, personal training studios, and private use among members of the general public who have concerns about exercise safety.
The American College of Sports Medicine (ACSM) Guidelines for Testing and Prescription4 and the American Heart Association (AHA)/ACSM Joint Position Statement: Recommendations for Cardiovascular Screening, Staffing, and Emergency Policies at Health/Fitness Facilities5 are two professional standards that provide guidance on preparticipation exercise self-screening. Both sources endorse self-screening as a standard practice and recommend two suitable self-screening instruments: the Physical Activity Readiness Questionnaire (PAR-Q) and the AHA/ACSM Preparticipation Questionnaire (AAPQ). The PAR-Q (publically available at www.csep.ca/publications) was developed in Canada in the 1970’s and has been systematically evaluated and revised on two occasions6–9. The AAPQ was developed by the Wisconsin branch of the American Heart Association in the late 1980’s5 (Supplemental Table 1) and has not been evaluated in peer-reviewed research.
The unknown validity of the AAPQ presents some particular challenges. The broad categorization of cardiovascular risk and lack of symptom specificity may result in unnecessary recommendations to consult medical providers, despite lack of evidence that physician consultation improves exercise safety1. In addition to unneeded economic and healthcare burden, unwarranted referral to a physician may unnecessarily arouse fears regarding physical activity participation and present a barrier to physical activity adoption. The purpose of this paper is to apply the AAPQ to a representative sample of US adults aged 40 years or older from the National Health and Nutrition Examination Survey (NHANES) and to quantify the gender-and age-specific proportions that would receive recommendations for preparticipation physician consultation. Additionally, we will examine the agreement between the AAPQ and the PAR-Q in the study sample.
Methods
Data Source
Data from NHANES 2001–2002 and 2003–2004 were used for these analyses10. These cycles were chosen because of homogeneity and breadth of health interview items. Complete information regarding NHANES sampling and data collection is publicly available from the Centers for Disease Control and Prevention10. Briefly, NHANES is an ongoing cross-sectional survey of the United States. Although the geographical regions sampled from year to year are not publically available, NHANES uses multistaged, stratified, probability sampling methods to achieve a representative sample of the US, non-institutionalized population. Each year, participants are selected from approximately 15 primary sampling units corresponding to roughly county-level divisions. The final sampling occurs at the household and individual level. Household residents of all ages and abilities are eligible to participate. Among those screened for participation, response rates for the NHANES interviews were high at 84% in 2001–2002 and 79% in 2003–200410. Data collection is continuous and releases occur on two-year intervals. All NHANES procedures are approved by the research ethics review board of the National Center for Health Statistics, and all participants agree to an informed consent. The present analyses were reviewed by the Committee for the Protection of Human Subjects at the University of Texas Health Science Center, Houston, TX, USA, and exempted from oversight.
Variables
Physician referral based on AAPQ
The AAPQ (Supplemental Table 1) consists of 32 items divided into three sections. In the first section, users indicate any prior or current medical conditions, symptoms, or other health issues that may complicate exercise. If any item in this section is selected, the respondent is instructed to consult a physician or healthcare provider and advised to engage in physical activity at a medically-staffed facility. The second section lists common cardiovascular risk factors. If two or more items in this section are selected, the respondent is advised to consult a physician or healthcare provider and to engage in physical activity at a professionally staffed facility for exercise guidance. The final section is for “none of the above,” and results in a recommendation of unrestricted physical activity.
The AAPQ is not specifically included in NHANES assessments but sufficient information can be obtained from NHANES questionnaire modules to complete all but five AAPQ items. An implicit assumption in this approach is that respondents would answer AAPQ items as they answered NHANES questionnaire items, despite being administered under different contexts. NHANES is an interviewer-administered health questionnaire that precedes a physical exam, while the AAPQ is a self-administered screening questionnaire that precedes volitional exercise participation. A matrix of AAPQ items and their NHANES equivalents is shown in Supplemental Table 2. For each AAPQ item, a binary yes/no variable was created, the value of which was determined by the answer(s) to the corresponding NHANES item(s). For example, the variable corresponding to the AAPQ item, “You take prescription medications” was coded “yes” if the respondent answered “yes” to the NHANES item asking “In the past month, have you used or taken medication for which a prescription is needed?” A binary variable was created indicating recommended referral versus not (henceforth: referred versus not referred), based on the AAPQ referral guidelines.
Five items from the AAPQ do not have matching items in NHANES 2001–2004. These items are denoted in Supplemental Table 2 with “n/a” for NHANES section/variable and include 1) history of pacemaker, implantable cardiac defibrillator, or rhythm disturbance; 2) history of heart valvular disease; 3) history of heart transplantation; 4) history of congenital heart disease; and 5) general concerns about the safety of exercise. Notably, four of these five questions deal with conditions commonly treated with prescription medications. Under AAPQ referral criteria, any reported prescribed medication use is sufficient to flag a respondent for referral. Hence, reported prescription medication use was considered a partial proxy for these conditions. No such proxy was available for “concerns about the safety of exercise.”
Physician referral based on PAR-Q
As with the AAPQ, the PAR-Q was not included in NHANES 2001–2004, but completion thereof can be approximated using NHANES responses. A matrix of PAR-Q items and their NHANES equivalents is presented in Supplemental Table 3. Three notable deviations were present. First, PAR-Q item #1 assesses a history of physician-restricted exercise due to heart conditions, but this was not assessed in NHANES 2001–2004. Second, PAR-Q item #3 assesses past-month history of chest pain at rest, but the closest NHANES proxy is ever having chest pain, regardless of activity level. Finally, PAR-Q item #7 asks for any other reason that physical activity may be contraindicated, but no such item is present in NHANES 2001–2004. Scoring of the PAR-Q was conducted in the same manner described above for the AAPQ, with a binary outcome variable for referred versus not referred based on PAR-Q criteria.
Other Variables
Reported age, gender (self-identified in NHANES), race/ethnicity, and education were used as demographic descriptors. Respondents were asked to report the intensity, frequency, and duration of leisure time physical activity over the past 30 days. Respondents could choose all applicable activities from a list of 48 common choices (including “other” as a catch-all). NHANES provided an activity-specific MET value for participation at both vigorous and moderate intensities. Respondents also reported the frequency and duration of walking and/or bicycling for transportation and household activity of at least moderate intensity. Because NHANES did not recommend a MET value for these domains, one was assigned following the Compendium of Physical Activities11. Activity volume with domains (leisure, transport, and household) was calculated in MET-hours per week (MET·H·Wk−1) by multiplying the weekly frequency, usual duration, and MET value. These domain-specific volume estimates were summed to create an estimate of total activity volume. Respondents reporting a minimum of 7.5 MET·H·Wk−1 were classified as meeting physical activity guidelines1 (2.5 hours per week of physical activity at a minimum of 3 METs).
Statistical Methods
Descriptive statistics included mean with standard deviation for age and percentages for categorical variables. To answer the primary research question, the proportion of respondents that would be referred to a physician was calculated across 5-year age groups for both men and women. Gender stratification was performed due to well-established differences in the risk of CVD by age in men versus women12. The association between proportion referred and age group was assessed separately for men and women using Pearson χ2 tests corrected for survey data after Rao and Scott13. The proportion of affirmative answers for each AAPQ item was also calculated across age groups and type of referral (section 1 alone, section 2 alone, or both sections resulting in referral). Associations between item response and age category were assessed with Pearson χ2tests as above.
For comparison purposes, age- and gender-stratified referral proportions were calculated for the PAR-Q, using the same statistical methods noted above for the AAPQ. Cross-classification tables were constructed to analyze the agreement between AAPQ and PAR-Q referral proportions.
All analyses were conducted using STATA v11SE (STATA Corporation, College Station, TX, USA) and utilized survey-specific procedures to account for the complex survey design and produced unbiased variance estimates and 95% confidence intervals. Four-year sample weights were calculated and applied following NHANES analytic guidelines14 (2-year weight ÷ 2).
Results
The combined 2001–2004 NHANES cycles comprised 6,785 adults (3,326 men and 3,459 women) aged 40 years or older. Pregnancy status could not be ascertained for 13% of female respondents. Among 3,003 women aged 40 years or older with known pregnancy status, only 3 reported current pregnancy. Given the low prevalence, the pregnancy item in the AAPQ was ignored to preserve sample size. Table 1 presents descriptive characteristics of respondents with complete questionnaire data (99% of men and 98% of women). When only reported leisure-time physical activity was considered, 36.0% (95% confidence interval: 33.2–38.7) of women and 45.7% (42.5–48.9) of men met physical activity guidelines. When reported transport and household activity were also considered, these values increased to 57.4% (54.7–60.0) and 68.6% (66.2–77.1), respectively.
Table 1.
Descriptive Characteristics of Adult Respondents age 40 years or older in NHANES, 2001–2004
| Women | Men | Total | |
|---|---|---|---|
| N (Full sample, raw) | 3,459 | 3,326 | 6,785 |
| N (Complete data, raw) | 3,385 | 3,276 | 6,661 |
| N (Complete, weighted)* | 57,158,940 | 62,677,130 | 119,836,070 |
| Age (y) | 57.5 (56.8–58.3) | 55.6 (55.2–56.1) | 56.6 (56.1–57.2) |
| Race/Ethnicity (%) | |||
| Non-Hispanic White | 76.7 (72.1–81.2) | 79.0 (74.9–83.1) | 77.8 (73.5–82.0) |
| Non-Hispanic Black | 10.9 (7.9–13.9) | 9.2 (7.1–11.2) | 10.1 (7.6–12.6) |
| All Hispanic | 8.5 (5.1–11.8) | 8.7 (5.4–12.1) | 8.6 (5.3–11.9) |
| Other/Multiracial | 3.9 (2.8–5.0) | 3.1 (1.9–4.2) | 3.5 (2.6–4.4) |
| Education (%) | |||
| Less than high school | 20.3 (17.8–22.8) | 18.1 (16.3–19.9) | 19.2 (17.2–21.2) |
| High school diploma | 27.0 (25.2–28.8) | 25.2 (23.2–27.1) | 26.1 (24.8–27.5) |
| Some college/assoc | 30.8 (28.2–33.4) | 27.9 (26.0–29.7) | 29.4 (27.6–31.2) |
| College degree+ | 21.6 (19.5–23.8) | 28.6 (25.7–31.5) | 25.0 (22.7–27.3) |
| Refused, unk, missing | 0.2 (0.0–0.4) | 0.2 (0.0–0.4) | 0.2 (0.0–0.4) |
| Leisure-time activity | |||
| No LTPA (%) | 43.3 (40.9–45.7) | 37.4 (34.7–40.1) | 40.5 (38.4–42.5) |
| Insufficient (%) | 20.7 (18.6–22.8) | 16.9 (15.5–18.3) | 18.9 (17.6–20.1) |
| Meeting GL (%) | 36.0 (33.2–38.7) | 45.7 (42.5–48.9) | 40.6 (38.0–43.2) |
| Physical activity (all) | |||
| No PA (%) | 23.7 (21.7–25.8) | 15.4 (13.3–17.4) | 19.8 (18.1–21.5) |
| Insufficient (%) | 18.9 (16.9–20.8) | 16.0 (14.3–17.6) | 17.5 (16.1–18.9) |
| Meeting GL (%) | 57.4 (54.7–60.0) | 68.6 (66.2–71.1) | 62.8 (60.5–65.0) |
LTPA = Leisure-time physical activity, GL = guidelines.
All values take into account sampling weights, except raw N. Values are % (95% confidence interval) except age, which is mean (95% confidence interval)
NHANES results are weighted to produce estimates applicable to the general, non-institutionalized US population
Table 2 presents the proportion of US adults that would be referred to a physician based on the AAPQ, stratified by gender and 5-year age groups. Across all ages, 95.5% (94.3–96.8) of women and 93.5% (92.2–94.7) of men in the US would be referred for preparticipation consultation given their responses to NHANES questions. For both genders, the proportion referred would be generally higher with age, peaking at 99.7% (99.4–99.9) among women 70 years or older and 99.5% (98.6–100.0) among men 60–64 years. For both genders, the largest difference between adjacent age groups was between 40–44 years and 45–49 years. For brevity, subsequent analyses utilized 10-year age groups for those aged 50–59 and 60–69 years, while 5-year age groups were retained for those aged 40–44 and 45–49 years.
Table 2.
Percentage of respondents who would be referred to physician using AAPQ, by age and gender, NHANES 1999–2004
| Age Categories (Years) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 40–44 | 45–49 | 50–54 | 55–59 | 60–64 | 65–69 | 70+ | Overall | |
| Women | (n=447) | (n=393) | (n=370) | (n=268) | (n=420) | (n=348) | (n=1138) | (n=3385) |
| 87.0 (82.8–91.1) | 94.4 (91.5–97.3) | 96.0 (94.1–98.0) | 98.2 (96.6–99.7) | 98.1 (96.3–99.9) | 98.4 (96.7–100.2) | 99.7 (99.4–99.9) | 95.5 (94.3–96.8) | |
|
| ||||||||
| Men | (n=447) | (n=406) | (n=386) | (n=261) | (n=384) | (n=341) | (n=1051) | (n=3276) |
| 83.0 (79.0–87.0) | 93.4 (90.1–96.6) | 95.9 (93.5–98.3) | 94.7 (91.2–98.2) | 99.5 (98.6–100.0) | 96.2 (93.4–99.0) | 98.4 (97.5–99.3) | 93.5 (92.2–94.7) | |
|
| ||||||||
| Both Genders | (n=894) | (n=800) | (n=756) | (n=529) | (n=804) | (n=689) | (n=2189) | (n=6661) |
| 85.0 (81.7–88.2) | 93.9 (91.8–96.0) | 96.0 (94.2–97.7) | 96.5 (94.7–98.4) | 98.7 (97.7–99.7) | 97.4 (95.8–99.0) | 99.1 (98.8–99.5) | 94.5 (93.6–95.5) | |
Values are % (95% confidence interval)
The association between referral and age is statistically significant at p<0.0001 for men, women, and combined by Pearson χ2 test.
The proportion of US women (A) and men (B) who would be referred based on AAPQ criteria, stratified by age, and physical activity level is shown in Figure 1. Referral proportions were generally lower for those meeting versus not meeting physical activity guidelines, but no age- or activity-group exhibited a referral proportion below 75%. Notably, even among those aged 40–44 years reporting sufficient activity to meet guidelines, 82.5% (76.8–88.2) of women and 79.8% (74.9–84.7) of men would be referred for physician consultation based on AAPQ items.
Figure 1.
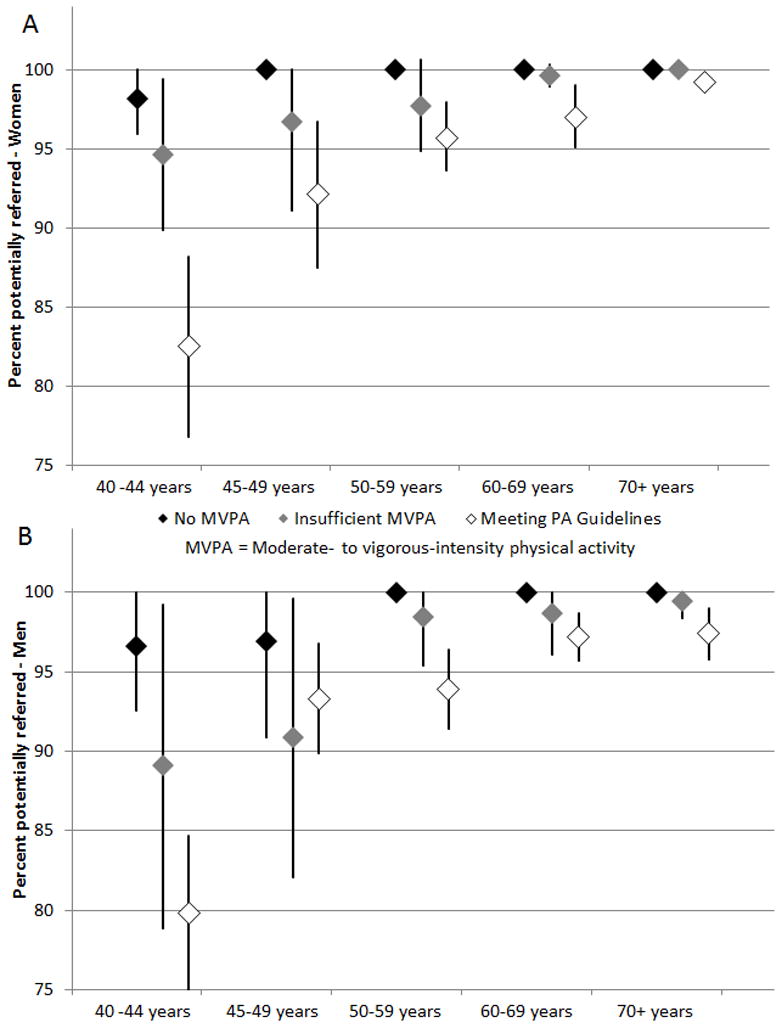
Percentage (with 95% confidence interval) of US women (A) and men (B) ≥ 40 years that would be referred by AAPQ, stratified by age and physical activity, NHANES 2001–2004.
For section 1 of the AAPQ (history, symptoms, and health issues), the most commonly selected item was prescription medication use, with 75.6% (73.7–77.4) of women and 61.5% (58.6–64.4) of men indicating recent use. Within section 2 (cardiovascular risk factors), the most commonly selected items were age in men (76.5% [74.0–78.9]) and age + menopausal status in women (69.5% [67.1–71.9]). Responses to all questions except presence of lung disease, unknown blood pressure, and family history of heart attack were significantly associated with age (not shown). Table 3 presents the proportion of presumed affirmative responses stratified by referral type. For men and women combined, 12.7% of participants would be referred based on section 1 items alone, 11.1% based on section 2 items alone, and 70.7% based on both sections 1 and 2. Among section 1 referrals, the most commonly selected item was prescription medication use (76.6%), followed by musculoskeletal issues (31.0%), and unreasonable breathlessness (23.8%). Among section 2 referrals, age (67.0%), being 20 pounds overweight (47.1%), and reporting unknown cholesterol (45.7%) were the most commonly selected items. Results were similar among referrals based on both sections, where prescription medication use from section 1 (83.7%) and age from section 2 (82.5%) were the most commonly selected items, and nine of 22 items under study would be selected by 40% or more of respondents.
Table 3.
Percentage of respondents with presumed affirmative answers by AAPQ item and referral type, NHANES 2001–2004
| Referral Type
|
|||
|---|---|---|---|
| Section 1 alone | Section 2 Alone | Both Sections | |
| N | 619 | 723 | 5054 |
| % of total | 12.7 (11.4–14.1) | 11.2 (9.9–12.4) | 70.7 (68.4–72.9) |
| % of referrals | 13.5 (12.0–15.0) | 11.8 (10.4–13.2) | 74.7 (72.6–76.9) |
|
| |||
| Section 1 | |||
| History items | |||
| Heart attack | 1.4 (0.8–1.9) | - | 7.9 (6.8–9.0) |
| Coronary heart disease | 2.0 (1.0–2.9) | - | 7.9 (6.7–9.1) |
| Congestive heart failure | 0.4 (0.1–0.7) | - | 5.3 (4.5–6.1) |
| Symptom items | |||
| Chest pain (exertion) | 5.3 (3.0–7.6) | - | 14.3 (12.3–16.3) |
| Breathlessness | 23.8 (19.9–27.8) | - | 47.9 (45.0–50.9) |
| Dizziness/fainting | 22.0 (18.0–25.9) | - | 30.3 (27.8–32.8) |
| Heart medication | 10.2 (7.6–12.8) | - | 50.3 (47.7–52.8) |
| Health issue items | |||
| Diabetes | 5.2 (3.7–6.6) | - | 16.5 (15.0–18.1) |
| Asthma/lung disease | 17.5 (13.7–21.3) | - | 20.3 (18.5–22.1) |
| Lower leg pain | 9.2 (6.2–12.3) | - | 17.4 (15.9–18.9) |
| Musculoskeletal problems | 31.0 (26.5–35.6) | - | 44.5 (42.0–46.9) |
| Prescription medications | 76.6 (72.4–80.7) | - | 83.7 (82.2–85.2) |
| Section 2 | |||
| Age (45 male/55 female) | - | 67.0 (62.9–71.1) | 82.5 (80.8–84.2) |
| Smoking | - | 33.5 (29.4–37.6) | 27.5 (25.6–29.4) |
| High blood pressure | - | 10.5 (7.1–13.9) | 52.2 (49.9–54.5) |
| Unknown blood pressure | - | 1.7 (0.4–2.9) | 0.1 (0.0–0.2) |
| Blood pressure meds | - | 0.7 (0.0–1.4) | 41.0 (38.6–43.4) |
| High cholesterol | - | 21.8 (16.9–26.7) | 47.3 (44.4–50.1) |
| Unknown cholesterol | - | 45.7 (41.1–50.2) | 15.5 (13.8–17.1) |
| Family history | - | 11.1 (8.4–13.8) | 13.9 (12.5–15.4) |
| Inactivity | - | 34.5 (29.1–39.9) | 37.2 (34.8–39.6) |
| Overweight | - | 47.1 (39.8–54.3) | 50.5 (48.9–52.0) |
Values are weighted % (95% confidence interval) except for “N” which is un-weighted count
Table 4 presents the proportion of US adults that would be referred to a physician based on the PAR-Q, stratified by gender and 5-year age groups. Versus comparable results from the AAPQ, the PAR-Q resulted in lower referral proportions in all strata. Notably, for the sample as a whole, 68.4% (66.4–70.4) of respondents would be referred based on the PAR-Q versus 94.5% (93.6–95.5) for the AAPQ. The results of cross-classification are presented in Table 5. For the sample as a whole, 72.4% of participants had matching referral status based on AAPQ and PAR-Q (67.7% [65.5–69.7] referred by both, 4.7% [3.9–5.6] referred by neither).
Table 4.
Percentage of respondents who would be referred to physician using PAR-Q, by age and gender, NHANES 1999–2004
| Age Categories (Years) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 40–44 | 45–49 | 50–54 | 55–59 | 60–64 | 65–69 | 70+ | Overall | |
| Women | (n=447) | (n=393) | (n=370) | (n=268) | (n=420) | (n=348) | (n=1138) | (n=3385) |
| 52.9 (47.0–58.6) | 59.4 (51.5–67.2) | 67.4 (60.4–70.3) | 77.9 (72.3–83.5) | 82.0 (78.1–85.8) | 89.3 (84.9–93.6) | 95.1 (93.6–96.6) | 73.6 (71.5–75.6) | |
|
| ||||||||
| Men | (n=447) | (n=406) | (n=386) | (n=261) | (n=384) | (n=341) | (n=1051) | (n=3276) |
| 44.9 (38.9–50.9) | 46.3 (39.4–53.3) | 62.4 (56.6–68.2) | 59.5 (50.9–68.1) | 74.8 (69.9–79.7) | 82.8 (77.7–87.8) | 90.5 (87.8–93.2) | 62.8 (59.9–65.6) | |
|
| ||||||||
| Both Genders | (n=894) | (n=800) | (n=756) | (n=529) | (n=804) | (n=689) | (n=2189) | (n=6661) |
| 48.8 (44.7–52.9) | 52.9 (47.7–58.1) | 64.8 (59.8–69.8) | 69.3 (63.1–75.4) | 78.6 (75.3–81.9) | 86.2 (83.4–89.1) | 93.2 (91.8–94.6) | 68.4 (66.4–70.4) | |
Values are % (95% confidence interval)
The association between referral and age is statistically significant at p<0.0001 for men, women, and combined by Pearson χ2 test.
Table 5.
Cross classification of AAPQ- and PAR-Q- based referrals by gender: adults 40+ years, NHANES 2001–2004.
| Women | PARQ Referred | PARQ Not Referred |
|---|---|---|
| AAPQ Referred | 73.0 (71.0–75.0) | 22.5 (20.9–24.2) |
| AAPQ Not referred | 0.6 (0.3–1.1) | 3.9 (3.0–5.2) |
| Men | PARQ Referred | PARQ Not Referred |
|---|---|---|
| AAPQ Referred | 61.8 (58.7–64.8) | 31.7 (28.9–34.6) |
| AAPQ Not referred | 1.0 (0.6–1.8) | 5.5 (4.5–6.7) |
| Both Genders | PARQ Referred | PARQ Not Referred |
|---|---|---|
| AAPQ Referred | 67.7 (65.5–69.7) | 26.9 (25.1–28.8) |
| AAPQ Not referred | 0.8 (0.5–1.2) | 4.7 (3.9–5.6) |
Values are weighted % (95% Confidence Interval) of the total sample
Discussion
Self-screening prior to exercise participation is frequently recommended on the assumption that it can identify people at risk of a cardiac-related event that may be precipitated by physical activity. The AAPQ is a self-screening tool that is endorsed by two prominent professional organizations; the American Heart Association and the American College of Sports Medicine. The primary purpose of this research was to estimate the proportion of US adults aged 40 years or older that would receive a recommendation to consult a physician based on the AAPQ before starting an exercise program. These results suggest that if this age group answered the AAPQ items as suggested by their NHANES responses, 95.5% of women and 93.5% of men would be referred.
A preparticipation screening tool that refers over 90% of respondents to a physician is likely ineffective. Given that adult self-screening focuses on risk of CVD, national estimates of CVD prevalence provide perspective for this referral proportion. The National Institutes of Health has estimated that, in 2009, there were approximately 1,537,000 hospital discharges attributed to coronary heart disease in the US15. Under the assumptions that the discharges 1) represented individuals, without readmissions, and 2) were attributed only to adults over 40 (n=139,942,000 adults 40 or older in July 200916), the prevalence in this age range was 1.1%. An effective preparticipation screening tool must be sensitive, to correctly identify those at high risk, and specific, to effectively eliminate those at low risk. By the estimates presented here, the AAPQ fails in the latter task and instead issues near-uniform referrals for adults over 40 years of age. Excessive, non-specific referral for preparticipation consultation is concerning for several reasons. First, there is no evidence that a medical consultation or examination is effective at reducing adverse outcomes associated with physical activity1. In fact, the 2008 Physical Activity Guidelines for Americans state “People without diagnosed chronic conditions (such as diabetes, heart disease, or osteoarthritis) and who do not have symptoms (such as chest pain or pressure, dizziness, or joint pain) do not need to consult a health-care provider about physical activity”(page 39)17. In settings other than pre-activity clearance, large-scale screening of asymptomatic individuals for cardiovascular disease remains controversial. A 2003 systematic review commissioned by the US Preventive Services Task Force found no experimental studies that evaluated the effect of screening asymptomatic adults on subsequent incidence of coronary heart disease18. Further, they concluded that the observational evidence for three common physician-initiated screening tests (resting ECG, exercise treadmill test, and electron beam computed tomography) suggested each can improve risk stratification over traditional evaluation, but each is also accompanied by a large false positive burden, especially in populations with low underlying heart disease prevalence18. Considering that the risk of adverse cardiovascular outcomes with exercise is lessened when activity duration and frequency are progressed gradually, widespread referral is likely unneeded if such a progression is followed.
Second, unnecessary referral to a physician may actually present a barrier to exercise adoption due to the financial and time requirements involved. The prevalence of meeting physical activity guidelines has changed little in the past decade19, suggesting a need to remove versus erect barriers to exercise adoption. The financial barrier may be particularly relevant for those without adequate health insurance. In 2011, the Census Bureau estimated that 15.7% percent of US adults lacked health insurance20. This proportion was higher in Blacks (19.5%) and Hispanics (30.1%)20, two groups disproportionately burdened with chronic disease and arguably with the most to gain from the health-enhancing effects of physical activity21. Placing a potentially unnecessary barrier to exercise adoption could perpetuate noted health disparities among these groups. It should be noted, however, that respondents referred for known, pre-existing conditions are likely to have an established primary care provider; the power of this barrier may be attenuated for them as a simple verbal or electronic consultation may suffice.
Finally, excessive referrals may place an unnecessary burden on health care infrastructure. Evidence suggests that physician workload may already compromise patient care: a 2008 survey of primary care physicians in the US reported that 35.9% consider inadequate time with patients to be a major barrier to providing high-quality medical care22. If public health initiatives are successful in promoting active lifestyles, an influx of patients seeking potentially unnecessary preparticipation consultation could further divert healthcare resources away from more critical areas. This problem may be compounded by false positives resulting from office-based screening techniques such as resting ECG18, which could lead to more invasive and costly diagnostic procedures. As noted above, not everyone referred for a preparticipation consultation would require a full evaluation or examination, so the burden imposed by excessive referral should be interpreted with caution.
The high referral proportion of the AAPQ prompted post-hoc analyses investigating possible revisions based on the present results. First, we attempted to determine if the poor performance was attributable to a small number of influential items by excluding the two most commonly selected items from the AAPQ (prescription medication use and age [+ menopausal status among women]). Once done, referral proportions remained relatively high at 86.7% (85.0–88.3) among women and 84.7% (82.5–86.9) among men. As noted previously, 70.7% (78.4-72.9) of all participants would be referred based on answers to both sections 1 and 2 of the AAPQ, and among these respondents, nine of 22 AAPQ items under study would have a selection prevalence of 40% or greater. The results presented here suggest that particular items or even small sets of items are not to blame for the high referral proportions, rather the overall breadth of the AAPQ may be responsible. Based on these findings, a final analysis was run utilizing only items that assess history or symptoms of CVD (history and symptom items from section 1) and known but untreated hypertension (high blood pressure from section 2 with no reported hypertension medications in NHANES). This tactic resulted in lower referral proportions (70.7% and 60.4% among women and men, respectively) that more closely matched those of the PAR-Q in this population (73.6% of women and 62.8% of men), and may represent a data-driven starting point for future AAPQ revisions.
The above revision strategy resulted in a smaller set of items that more closely assess conditions and symptoms noted in the Physical Activity Guidelines to warrant physician consultation (above). Testing and refinement of these or similar items would yield a tool that is in narrower in scope and in closer agreement to the state-of-the-science for physical activity risk. Results from other areas of medicine suggest that self-reported history items can perform similarly to physician history-taking. In 2003, Reeves, et al. reported that among pre-operative cataract patients, coronary artery disease was reported with 78.3% sensitivity and 91.3% specificity versus physician-led history taking, while diabetes exhibited 92.9% sensitivity and 98.5% specificity23. It is possible that revised phrasing soliciting pertinent history and symptom data could be effective, but proper evaluation studies versus a true gold standard referent would be needed.
This research has several strengths. First, NHANES allows better generalization to the US population than would a locally-sourced convenience sample. A novel application of publicly-available data was used. NHANES is a sizeable investment of public funds, and attempts should be made to maximize its utility. Further, because of the wealth of data available in NHANES, a preliminary evaluation of the AAPQ was possible with no direct research costs. To our knowledge, this is the first attempt to evaluate a screening tool in such a manner.
Several important limitations should be considered when interpreting these data. First, there was no way to quantify how commonly the AAPQ is used, but its continuing presence in the Guidelines for Exercise Testing and Prescription has the potential to reach over 25,000 ACSM-certified exercise professionals across the US and abroad24. Second, five items on the AAPQ did not have direct answers in NHANES and three items regarding coronary artery disease were combined into a single item. While notable, omission of AAPQ items likely underestimates versus overestimates referral proportions (bias towards the null), and medication use served as a proxy indicator for four of the five missing items. Third, there was no way to estimate the reliability of the AAPQ using NHANES data. Internal-consistency measures, such as Cronbach’s α, were applicable due to the multidimensionality of the AAPQ. Other types of reliability, such as test-retest, will be needed in future AAPQ revisions as reliability is a prerequisite to validity. Finally, the assumption that respondents would answer AAPQ items in a manner similar to heir NHANES responses is untestable. It is plausible that knowledge of a possible examination after the NHANES health interview would yield different results than a self-administered screening questionnaire used before volitional participation in exercise.
In conclusion, application of the AAPQ to NHANES data suggests that over 90% of US adults aged ≥40 years would be referred for physician consultation before initiating an exercise program. The high referral proportions are likely due to the breadth of topics covered by the AAPQ, and future revisions should focus on narrower ascertainment of history and symptoms directly relevant to exercise safety. Unless significant revisions are made to the AAPQ, it is of dubious utility among adults aged 40 years or older.
Supplementary Material
Acknowledgments
GPW would like to thank his colleagues in this program for their feedback. The authors would like to thank Dr. Wendy Kohrt for her help on this project.
Funding Sources: GPW was partially supported by the Cancer Education and Career Development Program at the University of Texas School of Public Health, National Cancer Institute/NIH Grant #2 R25 CA57712.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Physical Activity Guidelines Advisory Committee; Department of Health and Human Services, editor. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, D.C: 2008. [DOI] [PubMed] [Google Scholar]
- 2.Kohl HW, 3rd, Powell KE, Gordon NF, Blair SN, Paffenbarger RS., Jr Physical activity, physical fitness, and sudden cardiac death. Epidemiol Rev. 1992;14:37–58. doi: 10.1093/oxfordjournals.epirev.a036091. [DOI] [PubMed] [Google Scholar]
- 3.Dahabreh IJ, Paulus JK. Association of episodic physical and sexual activity with triggering of acute cardiac events: systematic review and meta-analysis. JAMA. 2011;305:1225–1233. doi: 10.1001/jama.2011.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson WR, Gordon NF, Pescatello LS. American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 8. Philadelphia: Lippincott Williams & Wilkins; 2010. [DOI] [PubMed] [Google Scholar]
- 5.Balady GJ, Chaitman B, Driscoll D, Foster C, Froelicher E, Gordon N, Pate R, Rippe J, Bazzarre T. Recommendations for cardiovascular screening, staffing, and emergency policies at health/fitness facilities. Circulation. 1998;97:2283–2293. doi: 10.1161/01.cir.97.22.2283. [DOI] [PubMed] [Google Scholar]
- 6.Jamnik VK, Warburton DE, Makarski J, McKenzie DC, Shephard RJ, Stone JA, Charlesworth S, Gledhill N. Enhancing the effectiveness of clearance for physical activity participation: background and overall process. App Physiol Nutr Me. 2011;36(Suppl 1):S3–S13. doi: 10.1139/h11-044. [DOI] [PubMed] [Google Scholar]
- 7.Shephard RJ, Bailey DA, Mirwald RL. Development of the Canadian Home Fitness Test. Can Med Assoc J. 1976;114:675–679. [PMC free article] [PubMed] [Google Scholar]
- 8.Shephard RJ, Cox MH, Simper K. An analysis of “Par-Q” responses in an office population. Can J Pub Health. 1981;72:37–40. [PubMed] [Google Scholar]
- 9.Cardinal BJ, Esters J, Cardinal MK. Evaluation of the revised physical activity readiness questionnaire in older adults. Med Sci Sports Exerc. 1996;28:468–472. doi: 10.1097/00005768-199604000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. [Accessed March 11, 2013];National Health and Nutrition Examination Survey; Questionnaires, Datasets, and Related Documentation. 2012 http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- 11.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 12.National Cholesterol Education Program Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 13.Rao JNK, Scott AJ. On Chi-Squared Tests for Multiway Contingency Tables with Cell Proportions Estimated from Survey Data. Ann Stat. 1984;12:46–60. [Google Scholar]
- 14.Centers for Disease Control and Prevention. [Accessed July 17, 2012];National Health and Nutrition Examination Survey - Analytic Guidelines. 2011 http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/analytical_guidelines.htm.
- 15.National Institutes of Health, National Heart Lung and Blood Institute. Morbidity & mortality: 2012 chart book on cardiovascular, lung, and blood diseases. Bethesda, MD: 2012. [Google Scholar]
- 16.United States Department of Commerce. [Accessed Jan 28, 2013];Population estimates: Vintage 2009: National tables. 2012 http://www.census.gov/popest/data/historical/2000s/vintage_2009/index.html.
- 17.United States Department of Health and Human Services. [Accessed July 5, 2012];2008 Physical Activity Guidelines for Americans. 2008 www.health.gov/paguidelines/pdf/paguide.pdf.
- 18.Pignone M, Fowler-Brown A, Pletcher M, Tice JA. Screening for Asymptomatic Coronary Artery Disease: A Systematic Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2003. [PubMed] [Google Scholar]
- 19.Carlson SA, Fulton JE, Schoenborn CA, Loustalot F. Trend and prevalence estimates based on the 2008 Physical Activity Guidelines for Americans. Am J Prev Med. 2010;39:305–313. doi: 10.1016/j.amepre.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 20.United States Department of Commerce. [Accessed March 12, 2013];Income, Poverty and Health Insurance Coverage in the United States: 2011. 2012 http://www.census.gov/newsroom/releases/archives/income_wealth/cb12-172.html#tablec.
- 21.Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Pub Health. 2010;100:S186–196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deshpande SP, DeMello J. A comparative analysis of factors that hinder primary care physicians’ and specialist physicians’ ability to provide high-quality care. Health Care Manag (Frederick) 2011;30:172–178. doi: 10.1097/HCM.0b013e318216fa81. [DOI] [PubMed] [Google Scholar]
- 23.Reeves SW, Tielsch JM, Katz J, Bass EB, Schein OD. A self-administered health questionnaire for the preoperative risk stratification of patients undergoing cataract surgery. Am J Opthalmol. 2003;135:599–606. doi: 10.1016/s0002-9394(02)02236-5. [DOI] [PubMed] [Google Scholar]
- 24.American College of Sports Medicine. [Accessed March 12, 2013];ACSM ProFinder. 2011 http://certification.acsm.org/pro-finder.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


