Abstract
Management of acute and chronic vascular disorders of the hand in patients with vasospastic and vaso-occlusive disorders is a complex problem and requires a multidisciplinary approach. The ischemia-related pain, skin ulcerations, and ultimately the threat of digital gangrene require a concerted effort to improve perfusion using a combination of medications and surgery. The purpose of this work is to review our experience over the past 2 decades with this cohort of patients including the variability of the clinical presentation, a method of classification, and a practical treatment philosophy.
Keywords: Ischemia, Hands, Sympathectomy, Digits, Raynaud's, Vasospasm vessel graft, Scleroderma
Introduction
Terminology
A.G. Maurice Raynaud in 1862, as part of his doctoral dissertation, De l'asphyxie locale et de la gangrène symétrique des extrémités, was the first known to describe the phenomenon of vascular spasm with eventual gangrene in the extremities related to the sympathetic nervous system. Over the next 150 years, the clinical community began to distinguish between the patients when associated with an underlying cause—Raynaud’s Syndrome in distinction to those without a known or associated disease—Raynaud’s Disease. This distinction is somewhat contrived and not germane for the purposes of this report. Most, if not all patients with severe symptoms requiring surgical care have an underlying condition, the most common being limited scleroderma (CREST syndrome). However, there is a far more diverse group of associated inflammatory conditions, including rheumatoid arthritis and dermatomyositis. Other causes of severe vascular ischemia includes Buerger’s disease, arteriosclerosis obliterans, and trauma to the digits such as electric shock and frostbite.
Anatomy
Blood flow to the hand is provided by the superficial palmar arch as a branch of the ulnar artery, and the deep palmar arch as a branch of the radial artery. Distinction between the deep and superficial arch is, however, a construct of historical nomenclature, as there are innumerable connections between the 2 branches and wide anatomic variation in the human species. A simpler way to consider the 2 arches is that both are sources of arterial supply, but only 1, the superficial, is available in its entirety for surgical manipulation.
There have been studies that attempt to allocate a percentage of flow, based upon caliber, or using a brief interruption (Allen’s test) and/or angiography images. In 1 study looking at 120 normal subjects, 57 % of the deep arch provided the majority of blood flow to at least 3 digits, the superficial arch provided the majority of flow to at least 3 fingers in 21.5 %, and in the remaining 21.5 %, flow was equal between the arches [1]. However, these studies ignore the near universal fact that ligating the ulnar or radial artery at the wrist, seldom, if ever, leads to the loss of digits in cases of trauma.
The patient who presents with an ischemic finger, which we refer to as a “digit-at-risk”, has started to lose adequate perfusion for simple nutritional flow and we concentrate on the medium size vessels of the palmar arch and common digital arteries out of a practical consideration—this size of vessel is amenable to microsurgical manipulation. The vessels of the forearm are certainly diseased, but pulsatile flow is usually present to the level of the wrist and we have not found grafting or stripping (sympathectomy) of these vessels to be useful.
The pathophysiology leading to this state begins with reversible vasospastic activity, mediated through the sympathetic nervous system. In an unpredictable manner, fixed perivascular fibrosis and advential constriction gradually reduces perfusion and is not reversible. The median sized vessels, specifically the superficial palmar arch and the common digital arteries with a diameter ranging from 1.5–2 mm, are diseased and amenable to surgical manipulation. The smaller vessels, at the level of the digital artery and beyond are also diseased, but are so small that we have not found these useful to alter. Therefore, the most common vessels that require dissection or grafting are the superficial arch, common digital arteries, radial artery into the “snuffbox,” and the princeps pollicis artery [2]. The radial digital artery of the index and the ulnar digital artery of the small finger are usually quite small and, in our experience, of doubtful clinical importance for advential stripping, sympathectomy, or grafting.
Pathophysiology
Ischemia is caused by 1 of 3 factors: vasospasm, occlusion, or a combination of the 2 [3]. Patients with limited scleroderma (LS) typically have vaso-occlusive disease. Vasospasm results from increased sympathetic tone. The occlusion is the result of excessive adventitia around the artery, thereby narrowing the lumen and slowing blood flow, which can eventually lead to thrombosis. Blood flow to the hand serves as both a thermoregulatory control and to provide nutrition to the digits [4]. Ischemia is the result of a loss of both nutritional flow and thermoregulatory control. In response to stress, vasospasm occurs, which leads to a low flow state, which can further lead to thrombosis, further complicating the treatment of these patients [3].
Clinical presentation
Patients with ischemic vascular disorders of the hand present with pain at the finger tip, intolerance to touch at the finger tip, visible discoloration, and paresthesia. There is often a prodromal period of acute sensitivity to changes in temperature. Many patients now despise the overly air-conditioned food markets in the summer, often having to resort to using gloves or mittens, even heavy coats, when shopping during the warmest months. We have also found an uptick in visits to the office when cold weather is sudden and severe, suggesting that the “delta” or vast change in temperature is a contributing factor. Many patients will report episodes of digital ulceration at the finger tip that later resolved. Anxiety also contributes to vasospasm. As a patient begins to worry over losing a finger, the anxiety can exacerbate an already compromised situation. In everyday life, patients with vasospasm will avoid holding cold beverages, retrieving ice from the freezer, or any other activity that creates a cold stress.
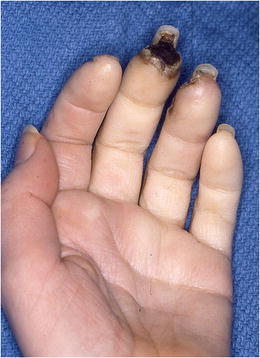
Some patients may present with a history of mild vasospasm that has suddenly and swiftly become acutely painful with very poor or absent perfusion (Fig. 7). The gradual progression of vascular fibrosis can lead to acute thrombosis. This patient requires urgent attention. In this instance, we will perform a regional block, consider IV heparin, and immediate MRA. The urgent surgical options are seldom sympathectomy alone, and usually require a combination of thrombectomy and vein grafting to the level of the common digital artery.
Figure 7.
ᅟ
For the more common presentation, consisting of gradual deterioration, the physical examination of the ischemic hand begins with inspection, looking for the stigmata of calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia (CREST), particularly the cutaneous telangiectasia, small calcifications and mild skin tightness. The very thick, dense sclerodactyly of diffuse scleroderma is uncommon; however, the patient can evolve to this form in especially aggressive cases. Tip ulcerations are common as is atrophy of the pulp.
Clinically, they will present with abnormal Allen test results, pallor, abnormal sweating, anhydrosis, digital ulceration, and gangrene. Occlusive disease is suggested by the presence of unilateral Raynaud’s phenomenon as well as the presence of nonhealing ulcers and gangrene [4]. Patients will present with one of the following clinical scenarios:
Mild episodic cold induced vasospasm, often triphasic—controllable with attention to core temperature and environmental modifications.
Sustained-reversible vasospasm.
Ischemic ulceration without segmental temperature change.
Ischemic ulceration with painful ischemia.
Acute sustained loss of perfusion with or without acral ulceration.
Imminent loss of digit.
Diagnosis
Patients with vasospastic disease of the hand require a complete examination consisting of detailed history, physical exam, laboratory tests, and vascular imaging of the hand [5]. Physical examination should include evaluation of the entire upper extremity from the neck down to the hand. It should also evaluate capillary refill, quality of the skin, peripheral pulse evaluation, Allen’s test, and inspection for ulcers and gangrene. Imaging modalities to evaluate vascular disorders of the hand are divided into those that assess the vascular anatomy and those that determine the adequacy of blood flow [3]. They include color waveform ultrasound, contrast angiography, and magnetic resonance angiography (MRA). Color Doppler sonography is a reliable way to transcutaneously assess blood flow in small vessels [6]. MRA has several advantages over traditional contrast angiography. It limits the patient’s exposure to harmful radiation and reduces the risk of nephrotoxicity and allergic reaction to contrast agent. Conventional contrast angiography requires vasodilators to minimize catheter induced vasospasm in vasospastic disorders of the hand [7]. MRA does not require the use of vasodilators, which may allow for a more accurate evaluation of vasospasm when compared with conventional angiography [8]. MRA findings include hyperemia, severe arterial stenosis, and complete vessel occlusion [9].
Our preferred method of diagnostic imaging is MRA; however, careful attention must be given to proper technique to obtain accurate images. The protocol used at our institution is detailed below. Images are obtained using a 1.5-Tesla clinical magnet (General Electric Health Care, Waukesha, WI) with an 8 channel phased array send and receive surface coil (MEDRAD, Warrendale, PA) centered at the hand. After an intravenous bolus of 0.2 mmol/kg gadodiamide (General Electric Health Care, Waukesha, WI), rapid sequential T1-weighted 3-dimensional gradient-echo images are obtained in multiple phases of contrast enhancement with a total scan time of approximately 5 minutes. Time intensity sensitive reconstruction is performed utilizing time resolved imaging of contrast kinetics (TRICKS, General Electric Health Care, Waukesha, WI), allowing for oversampling of the of center k-space at the deep palmar arch. Images are obtained with a minimum echo time, receiver bandwidth of 62.5 kHz, slice interpolation between 1.4 and 2 mm, and matrix interpolation to 512 × 512 with a field of view of 20 cm2, flip angle of 20°, at 1 excitation. After acquisition of “mask” images without contrast, subsequent subtraction postcontrast images are used to create a 3-dimensional model of the regional vessels.
Treatment
Patients with Raynaud’s are initially managed by their medical doctor. The surgeon isn’t involved until later in the disease. Initial management consists of lifestyle modifications such as smoking cessation, environmental modifications including avoidance of cold temperatures, and biofeedback techniques. An example of a biofeedback technique is a patient consciously increasing their hand temperature and blood flow. Medical management includes calcium channel blockers (nifedipine), TCAs, SSRIs, and prostacyclins. Calcium channel blockers are the first line of medical treatment in vasospastic disorders of the hand [10]. They work by preventing calcium influx into smooth muscle and tempers sympathetically driven vasoconstriction [11]. Thompson et al performed a meta-analysis study that showed Ca channel blockers reduce the number of ischemic attacks. Patients had 8 fewer ischemic events over 2 weeks than patients on placebo [12]. TCAs are postulated to provide both an analgesic effect and a sedative effect. SSRI’s are thought to help re-establish autonomic balance by interfering with the re-uptake of serotonin. Perivascular injection of Botox has been shown to aid in pain relief and healing of ulcers. In 9 of 11 patients, the ulcers spontaneously healed, and the other 2 required small skin graft. All 11 patients had significant pain relief [13]. Referral to a surgeon occurs if patients are refractory to the above conditions.
Surgery is typically reserved for the “digit at risk.” We consider a digit at risk to be one in which, if surgical intervention is not undertaken, the finger will progress to a “point of no return” leaving amputation as the best treatment option. Several surgical options have been described in the treatment of vasospastic disorders of the hand.
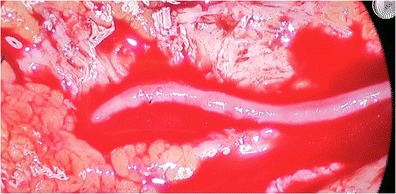
Techniques include proximal cervicothoracic sympathectomy, resection, and ligation of the diseased segment (Leriche sympathectomy), peripheral peri-arterial sympathectomy, peripheral sympathectomy with adventitial stripping, and microvascular construction with or without vein grafts [4]. Proximal cervicothoracic sympathectomy has had variable results, and recurrence is frequently seen [14]. Given the recurrence rate, this procedure is not used at our institution for treatment of vasospastic disorders of the hand. In the 1930s Leriche described removing the diseased portion of the vessel and ligation if there was appropriate collateral flow [15]. This procedure can be used in ulnar artery thrombosis with good collateral flow and retrograde flow from the palmar arterial arch. If there isn’t good collateral flow or there are multiple diseased segments then bypass graft is the optimal treatment choice. Distal sympathectomies were first developed due to the inconsistent results of proximal sympathectomies. Peripheral sympathectomy is performed by removing the sympathetic nerve fibers to the vessel as well as a portion of the adventitia. Flatt in 1980 is first credited with describing this procedure. He advocated removing 3–4 mm of sympathetic fibers and adventitia at the level of the proper digital artery. He advocated that sympathectomy was more effective at more distal levels such as the proper digital artery than proximal levels like the common digital artery [16]. Wilgis then modified this to extend up to 2 cm of adventitial removal and to include the common digital artery. Koman modified this technique by performing adventitial stripping at the level of the radial and ulnar arteries at the wrist over 2.5 cm, as well as the superficial palmar arch [17]. As more patients were treated surgically, it was thought that the patients would benefit not only from a distal sympathectomy, but decompression of the vessel by removal of as much adventitia as possible from the palmar arch out to the proper digital artery just prior to it entering the proximal phalanx [18]. There are times when peripheral sympathectomy is not adequate to improve blood flow distally, requiring microvascular reconstruction with reversed interposition vein graft [19]. In our experience, the decision to proceed with interposition graft is made intraoperatively after sympathectomy is performed. In healthier vessels, after adventitial stripping is completed, the vessel will be noticeably serpentine, and blood can be seen flowing through the vessel. In severely diseased vessels, even after perivascular stripping, the vessel is very rigid with extremely poor flow or no flow at all. Figure 1 demonstrates vessel with good flow after sympathectomy. Figure 2 shows very rigid vessel after sympathectomy. It should be noted that the decision was made not to proceed with reconstruction immediately, but to wait and observe the patient clinically for a few days. She continued to improve and has not required a vein graft up to this point.
Figure 1.
ᅟ
Figure 2.
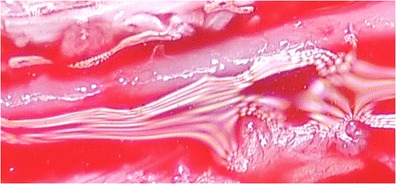
ᅟ
Clinical experience
From 2000 to 2013, a total of 33 patients and 78 digits were treated surgically at our institution with either distal sympathectomy, interposition vein graft, or a combination of the 2 procedures. Patients were referred by their primary care physician or rheumatologist for ischemic pain and a nonhealing ulcer at their fingertips. We prefer an MRA when evaluating these patients. We do not routinely perform sympathetic blocks prior to surgery, as this condition is due to a combination of increased sympathetic tone and mechanical obstruction. Sympathetic block will only relieve the sympathetic tone, and thus, it is not predictive of surgical success. When MR angiography was initially used, a sympathetic block was helpful to provide a more detailed view of the palmar and digital circulation. However, as we have gained experience and modified the MRA techniques, simply warming the patient and their limb has been sufficient.
The decision to proceed to surgery was based upon:
- Unrelieved pain due to ischemia
- slowly developing
- acute onset with threat of imminent loss
Nonhealing ulcers at the tip
Patients were taken to the operating room, using a tourniquet, and under the microscope a bruner type incision was made in the palm extending from the web space of the affected digit, down to the junction of the common digital vessel, and the superficial arch. Using jeweler’s forceps and scissors, the adventitia was carefully stripped from the vessel extending along the entire length of the incision. After an adequate amount of adventitia had been removed, prior to deflation of the tourniquet, patients were given 5000 units of Heparin intravenously. Two minutes postadministration, the tourniquet was deflated and vessels were inspected. Any defects in the vessels from stripping were repaired. Blood flow through the vessels was then examined. If the blood flow was felt to have been improved to allow for adequate flow distally then the wound was irrigated and closed. However, if the blood flow was determined to still be inadequate, a bypass of the diseased vessel using a reverse vein interposition graft was performed at the time of the initial operation. Patients were placed in a postoperative dressing and given 1 more dose of Heparin 5000 units IV. They were admitted to the hospital overnight, placed in a warm room, and started on coumadin with a therapeutic goal of 2.0. They remained on coumadin 6 months postop, or permanently if their clinical situation seemed worrisome.
Postop pain and anxiety should be optimally controlled and we often use our pain management service at the outset. Anxiety can be reduced with chlorpromazine hydrochloride 10 mg every 8 hours for the first 3 days after surgery. This medication is well tolerated and acts as both an anxiolytic and vasodilator. The primary care physician is also encouraged to use more standard anxiolytic medications for these patients in the subsequent weeks. There can often be a period of reperfusion that is painful and also provoke a stress reaction.
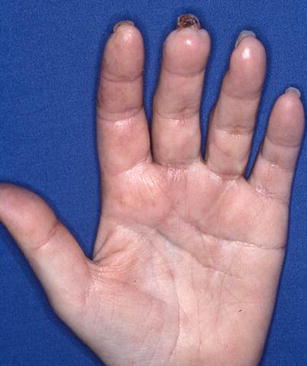
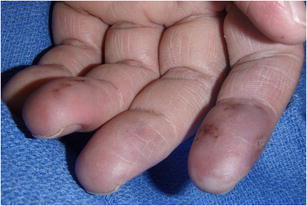
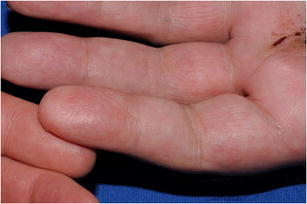
Using the algorithm of lifestyle modification, medical management, sympathectomy, followed by interposition vein graft, all 33 patients had eventual healing of ulcers without amputation (Figs. 3 and 4). Eight patients needed a bypass to obtain adequate blood flow distally. Seven patients required additional irrigation and debridement of the fingertip to remove the necrotic tissue and facilitate healing. Figures 5 and 6 show the before and after of a 55-year-old attorney who presented with an ischemic index finger and required interposition vein grafting. He went on to achieve complete recovery with resolution of his pain and restoration of blood flow to the index finger.
Figure 3.
ᅟ
Figure 4.
ᅟ
Figure 4.
ᅟ
Figure 4.
ᅟ
Discussion
Management of the upper extremity affected with Raynaud’s has traditionally been very difficult and frustrating to manage for physicians. Our understanding of the pathophysiology of this disease process in the hand has been limited, but has improved over the last 2 decades. Initially, Flatt surmised that a sympathectomy distal to the origin of the proper digital artery of about 4 mm was sufficient to treat vasospastic disorders of the hand. The goal of this operation was to decrease sympathetic tone to the vessels supplying blood to the fingers [16]. However, it is now know that in patients with Raynaud’s presenting with ischemic ulcers, that there is a mechanical obstruction as well as increased vasospastic tone. In order to address this, adventitial stripping as well as sympathectomy must be performed from the superficial arch out to the proper digital artery just proximal to the proximal phalanx [18] (Fig. 6). Often times the poor blood flow is found on MRA distal to the level of adventitial stripping. Poiseuille’s Law states that flow is proportional to the radius to the 4th power. By increasing the radius of the artery proximally, the pressure gradient across the artery is increased, thereby increasing the flow distally.
In a study by Ruch et al looking at intermediate follow-up after peri-arterial sympathectomy in scleroderma patients, only 6 of 22 remained ulcer free and the remaining 16 continued to have ischemia with ulcer formation. Mccall et al examined the use of digital artery sympathectomy as a salvage procedure for Severe Ischemia in Raynaud’s in 7 patients. Six of the 7 ulcers healed, and one patient went on to amputation of the digit [20]. In 7 patients with 39 digits with digital ischemia but no gangrene or severe ulceration, peri-arterial sympathectomy was performed and patients were followed for over 10 years. All patients maintained improvement over long-term follow-up [21]. These results question whether surgical intervention should be performed when the digit is ischemic rather than waiting until it is necrotic and gangrenous (Fig. 7).
In looking at long-term results of peri-arterial sympathectomy in patients with digital vasospasm secondary to either autoimmune disease or atherosclerosis, Hartzell et al al showed that the amputation rate for autoimmune disease was 26 % compared with 59 % in those that had atherosclerosis [22]. Although the amputation rate for those with autoimmune disease is much higher than other studies reporting results of sympathectomy, the higher amputation rate in atherosclerotic disease indicates that sympathectomy is ineffective in obstructed vessels or those with poor flow.
In a systematic review of digital sympathectomy for chronic digital ischemia from 1996-2002, patients had an amputation rate of 14 %, ulcer recurrence rate of 18 %, and patients with scleroderma had a postop complication rate of 37 % [23]. The goal in managing these patients is to offer them pain relief as well as improve the blood flow to the digit distally so that amputation may be avoided.
Treatment of the ischemic hand in Raynaud’s phenomenon and disease has traditionally been very difficult to treat. However, as more knowledge has been gained over the years, the approach to treatment has become more straightforward. In treating our patients using the treatment protocol as outlined above with lifestyle modification, medication, sympathectomy, then bypass, we have not had to amputate a single digit. In vessels with disease at more than 1 segment or in those that do not respond to sympathectomy, the physician should have a low threshold to perform interposition vein graft. Using this additional step in treating patients with ischemic disease of the digit has given them good pain relief and, most importantly, allowed us to avoid amputation.
Compliance with Ethics Guidelines
Conflict of Interest
Robert Hotchkiss declares that he has no conflict of interest. Tyler Marks declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
References
- 1.Kleinert JM, Fleming SG, Abel CS, et al. Radial and ulnar artery dominance in normal digits. J Hand Surg [Am] 1989;14:504–508. doi: 10.1016/S0363-5023(89)80012-7. [DOI] [PubMed] [Google Scholar]
- 2.Coleman SS, Anson BJ. Arterial patterns in the hand based upon a study of 750 specimens. Surg Gynecol Obstet. 1961;4:409–424. [PubMed] [Google Scholar]
- 3.Ruch D, Koman A, Smith T. Chronic vascular disorders of the hand. J Hand Surg [Am] 2001;1:73–80. doi: 10.1053/jssh.2001.21780. [DOI] [Google Scholar]
- 4.Troum SJ, Smith TL, Koman LA, Ruch DS. Management of vasospastic disorders of the hand. Clin Past Surg. 1997;24:121–132. [PubMed] [Google Scholar]
- 5.Koman LA, Smith BP, Smith TL. Stress testing in the evaluation of upper extremity perfusion. Hand Clin. 1993;9:59. [PubMed] [Google Scholar]
- 6.Koudsi B, Petti C, Halpern D, Backus A, Nichter L. Assessment of acute microcirculatory changes by color Doppler sonography. J Hand Surg [Am] 1994;19:488–494. doi: 10.1016/0363-5023(94)90068-X. [DOI] [PubMed] [Google Scholar]
- 7.Vogelzang RL. Arteriography of the hand and wrist. Hand Clin. 1991;7:63–86. [PubMed] [Google Scholar]
- 8.Lee VS, Lee HM, Rofsky NM. Magnetic resonance angiography of the hand: a review. Invest Radiol. 1998;33:687–698. doi: 10.1097/00004424-199809000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Stepanksy F, Hecht E, Rivera R, Hirsh L, Taouli B, Kaur M, et al. Angiography of upper extremity vascular disease: pictorial review. Radiographics. 2008;28:e28. doi: 10.1148/radiol.e28. [DOI] [PubMed] [Google Scholar]
- 10.Ruch DS, Holden M, Smith BP, Smith TL, Koman LA. Peri-arterial sympathectomy in scleroderma patients: intermediate-term follow-up. J Hand Surg [Am] 2002;27:258–264. doi: 10.1053/jhsu.2002.29483. [DOI] [PubMed] [Google Scholar]
- 11.Coffman JD. Calcium slows channel antagonists and Raynaud’s phenomenon. Intern Med. 1984;5:107. [Google Scholar]
- 12.Thompson AE, Shea B, Welch V, Fenlon D, Pope JE. Calcium-channel blockers for Raynaud’s phenomenon in systemic sclerosis. Arthritis Rheum. 2001;44:1841–7. [DOI] [PubMed]
- 13.Van Beek AL, Lim PK, Gear AJL, Pritzker MR. Management of Vasospastic Disorders with Botulinum Toxin A. Plast Reconstr Surg. 2007;119:217. [DOI] [PubMed]
- 14.Lowell RC, Gloviczki P, Cherry KJ, Jr, Bower TC, Hallett JW, Jr, Schirger A, et al. Int Angiol. 1993;12:168–172. [PubMed] [Google Scholar]
- 15.Leriche R, Fontaine R, Dupertuis SM. Arterectomy with follow-up on 78 operations. Surg Gynecol Obstet. 1937;64:149. [Google Scholar]
- 16.Flatt AE. Digital artery sympathectomy. J Hand Surg [Am] 1980;5:550–556. doi: 10.1016/S0363-5023(80)80104-3. [DOI] [PubMed] [Google Scholar]
- 17.Koman LA, Smith BP, Pollock FE, Jr, Smith TL, Pollock D, Russell GB. The microcirculatory effects of peripheral sympathectomy. J Hand Surg [Am] 1995;20:709–717. doi: 10.1016/S0363-5023(05)80419-8. [DOI] [PubMed] [Google Scholar]
- 18.Yee AM, Hotchkiss RN, Paget SA. Adventitial stripping: a digit saving procedure in refractory Raynaud’s phenomenon. J Rheumatol. 1998;25:269–276. [PubMed] [Google Scholar]
- 19.Jones NF. Acute and chronic ischemia of the hand: pathophysiology, treatment, and prognosis. J Hand Surg [Am] 1991;16:1074–1083. doi: 10.1016/S0363-5023(10)80072-3. [DOI] [PubMed] [Google Scholar]
- 20.McCall TE, Petersen DP, Wong LB. The use of digital artery sympathectomy as a salvage procedure for severe ischemia of Raynaud’s disease and phenomenon. J Hand Surg [Am] 1999;24:173–177. doi: 10.1053/jhsu.1999.jhsu24a0173. [DOI] [PubMed] [Google Scholar]
- 21.Murata K, Omokawa S, Kobata Y, Tanaka Y, Yajima H, Tamai S. Long-term follow-up peri-arterial sympathectomy for chronic digital ischemia. J Hand Surg (Br) 2012;37:788–793. doi: 10.1177/1753193412441757. [DOI] [PubMed] [Google Scholar]
- 22.Hartzell TL, Makhni EC, Sampson C. Long-term results of peri-arterial sympathectomy. J Hand Surg [Am] 2009;34:1454–1460. doi: 10.1016/j.jhsa.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Kotsis SV, Chung KC. A systematic review of the outcomes of digital sympathectomy for treatment of chronic digital ischemia. J Rheumatol. 2003;30:1788–1792. [PubMed] [Google Scholar]


