Abstract
Although congenital hand anomalies are rare, musculoskeletal clinicians should have a basic understanding of their clinical manifestations and the possibility of concurrent anomalies and syndromes. In this review, we provide a brief overview of the embryology of limb development and the molecular pathways involved. We also summarize the clinical manifestations, diagnostic evaluation, and principles of surgical treatment for radial longitudinal deficiency, thumb hypoplasia, ulnar longitudinal deficiency, central deficiency, syndactyly, polydactyly, and amniotic constriction band. Although one of the main goals of treatment is to provide a functional upper extremity, musculoskeletal clinicians should be aware of the clinical findings that should trigger referral to evaluate for life-threatening syndromes.
Keywords: Congenital hand, Congenital limb, Anomaly, Anomalies, Deformity, Embryology, Radial dysplasia, Radial longitudinal deficiency, Thumb hypoplasia, Ulnar longitudinal deficiency, Ulnar deficiency, Central deficiency, Cleft hand, Syndactyly, Polydactyly, Amniotic constriction band, Constriction band syndrome, Constriction ring syndrome
Introduction
Although congenital hand anomalies are rare, the practicing orthopedic surgeon should be able to recognize the more commonly encountered deformities, initiate evaluation for systemic conditions if necessary, and refer the family to an appropriate clinician for long-term management. Although there are no published population-based studies from the United States, recent epidemiologic studies from Finland, Canada, and Australia have estimated the overall incidence of upper limb anomalies as between 3.4 and 5.3 per 10,000 live births [1••, 2, 3]. The 1-year mortality of live-born infants with congenital upper limb anomalies is 14 %–16 % [1••, 4], emphasizing the importance of prompt recognition of the deformities that are associated with medical syndromes. In these situations, the orthopedic surgeon must facilitate referral to a clinician who can proceed with the appropriate diagnostic evaluations.
Although incremental progress has been made in the clinical treatment of congenital hand anomalies, the most exciting advances in treatment are likely in the future [5, 6••]. Continued advancements in our understanding of the molecular basis of limb development may result in earlier diagnosis and a wider array of treatment options for congenital anomalies [7, 8]. In this paper, we will review the embryology of upper limb development as well as the diagnosis and evaluation of the more commonly encountered congenital hand anomalies.
Embryogenesis of the upper extremity
Embryogenesis of the upper extremity occurs between 4 and 8 weeks after fertilization, and the majority of congenital anomalies occur during this period of time [9]. Limb bud development begins with the lateral migration of 2 layers of mesoderm (somitic and lateral plate) and outgrowth into the overlying ectoderm [7]. Cells from the somitic mesoderm ultimately form the muscle tissue of the limb, while cells from the lateral plate mesoderm form cartilage and bones [7]. The limb bud appears at 26 days and development continues through 47 days after fertilization [10]. Development of the limb is classically considered with respect to its 3 axes of growth (proximal-distal; anterior-posterior/radio-ulnar, and dorsal-ventral), with each axis primarily controlled by distinct, but coordinated molecular pathways.
Proximal-distal axis—development of the proximal-distal axis is controlled by fibroblast growth factors (FGF) secreted by the apical ectodermal ridge (AER) [7]. The AER is a thickened layer of the ectoderm that forms over the distal edge of the limb bud. FGF secreted by the AER stimulates proximal-distal growth of the limb via differentiation of the underlying mesoderm. As part of a complex feedback loop, the signal to produce FGF is supplied by the underlying mesoderm. The AER signaling center is responsible for the differentiation of the underlying mesoderm and the development of the limb in the proximal-to-distal direction. Removal of the AER results in arrest of limb outgrowth. Furthermore, ectopic implantation of the AER results in formation of an extra limb. The AER also contributes to interdigital necrosis, allowing separation of the initially webbed hand [11]. Defects in the AER lead to anomalies such as limb truncation, transverse deficiencies, and syndactyly [9].
Anterior-posterior (radio-ulnar) axis—development in the radio-ulnar axis is patterned by secretion of sonic hedgehog from the zone of polarizing activity (ZPA), a collection of cells along the posterior (ulnar) aspect of the limb bud. Transplantation of the ZPA from the posterior aspect to the anterior aspect of the limb bud causes creation of a mirrored duplication of the ulnar aspect of the hand. In addition to its primary role in development of the radio-ulnar axis, the ZPA also contributes to maintenance of proximal-to-distal limb development and participates in the feedback loop of the AER [7].
Dorsal-ventral axis—development in the dorsal-ventral axis is regulated by the Wingless-type (Wnt) signaling pathway [7, 11] within the dorsal ectoderm. The Wnt pathway induces the underlying mesoderm to develop dorsal characteristics and is blocked in the ventral ectoderm, allowing the development of ventral characteristics. In mice, inactivation of the Wnt signaling pathway results in the development of biventral limbs [7, 11]. The Wnt pathway also contributes to regulation of sonic hedgehog, reflecting the complex interaction and coordination among the pathways responsible for the axes of limb development.
The molecular pathways primarily responsible for each axis work in concert to ensure the proper development of the upper extremity. Errors in any of these signaling pathways can indirectly affect the functioning of the other signaling centers, and may reflect the presence of a multiorgan systemic disorder [11]. Greater understanding of the complex interactions of molecular pathways in limb formation will provide opportunities for the development of innovative biologic treatments to complement existing surgical techniques.
Radial longitudinal deficiency
The incidence of radial longitudinal deficiency (RLD; also referred to as radial club hand) ranges from 0.38 to 1.64 per 10,000 live births [1••, 2, 4, 12]. Although RLD can be passed genetically in autosomal recessive or autosomal dominant forms, most cases of RLD are the result of spontaneous mutations [13••]. Nearly all patients have some form of concurrent congenital musculoskeletal anomaly [1••, 2], most commonly congenital scoliosis [14]. More importantly, one-third of RLD patients have a medical syndrome [14], usually affecting the cardiac, hematologic, and renal systems. The most frequently reported syndromes associated with RLD are thrombocytopenia absent radius (TAR) syndrome; the VACTERL (vertebral defects, anal atresia, cardiac malformation, tracheoesophageal fistula, esophageal atresia, renal anomalies, and limb anomalies) association; Fanconi anemia (pancytopenia associated with bone marrow failure); and Holt-Oram syndrome (atrial or ventricular septal defects) [14]. In Goldfarb’s report of 55 syndromes diagnosed in 164 patients with RLD, there were 25 cases of TAR syndrome, 22 cases of VACTERL, 7 cases of Holt-Oram syndrome, and 1 case of Fanconi anemia. A substantial number of these syndromes were not diagnosed prior to evaluation by the hand surgeon [14].
The manifestations of RLD range from thumb hypoplasia with an intact radius to the complete absence of the radius (Table 1) [15]. RLD is bilateral in 59 % of cases [1••], but it may not be symmetric. Although associated syndromes are more common in advanced stages of RLD, they may occur in the less severe stages. Regardless of the severity of RLD, evaluation in coordination with a pediatrician is required. Diagnostic workup usually consists of thorough physical examination, echocardiography, renal ultrasound, spine radiographs, blood cell count, and a peripheral blood smear. A chromosomal challenge test is used to evaluate for Fanconi anemia, as early diagnosis of this potentially fatal disease can be helpful in providing adequate time for donor matching if bone marrow transport is necessary [11].
Table 1.
Classification of radial longitudinal deficiency (adapted from [15], with permission)
| Type | Thumb anomaly | Carpal anomaly | Distal part of radius | Proximal part of radius |
|---|---|---|---|---|
| N | Absence or hypoplasia | Normal | Normal | Normal |
| 0 | Absence or hypoplasia | Absence, hypoplasia, or coalition | Normal | Normal, radioulnar synostosis, or radial head dislocation |
| 1 | Absence or hypoplasia | Absence, hypoplasia, or coalition | >2 mm shorter than ulna | Normal, radioulnar synostosis, or radial head dislocation |
| 2 | Absence or hypoplasia | Absence, hypoplasia, or coalition | Hypoplasia | Hypoplasia |
| 3 | Absence or hypoplasia | Absence, hypoplasia, or coalition | Absence of physis | Variable hypoplasia |
| 4 | Absence or hypoplasia | Absence, hypoplasia, or coalition | Absence | Absence |
The goal of treatment for RLD is to provide a functional upper extremity with a stable, mobile wrist and adequate forearm length and functional thumb. The absence of bony support on the radial aspect of the forearm leads to radial deviation of the wrist, exacerbating the dysfunction of the already shortened forearm and placing the finger flexors and extensors at a mechanical disadvantage. In cases of advanced RLD with hypoplasia or absence of the radius (Types 3 and 4), surgical “centralization” is often indicated to improve appearance and function of the upper extremity [16•]. The best technique to centralize the wrist relative to the forearm is debatable. Historically, bony procedures were used to secure the repositioned carpus over the ulna. However, concerns regarding recurrent deformities, wrist stiffness, and compromise of ulnar growth after bony centralization [13••, 17••] have prompted the use of alternative techniques that emphasize maintenance of wrist and digit motion [18••]. Following a period of splinting and stretching, Wall and colleagues release the constricted soft tissues along the radial side of the wrist and transpose a flap of ulnar tissue to the deficient radial side [13••]. Continued splinting is used to maintain correction of the deformity. Additional alternative treatments include free microvascular transfer of the proximal fibula [19], or first metatarsophalangeal joint to the radial side of the wrist [20], ulnar lengthening [21•], and use of a ringed or unilateral external fixator for soft tissue correction prior to bony centralization [22, 23]. Shortening and recurrent angular deformity can be addressed with external fixators [24, 25], whereas ulnocarpal arthrodesis is a viable salvage procedure after failed centralization [26]. As with most congenital hand anomalies, there is no uniformly accepted timeframe for surgery, particularly if other aspects of a syndrome must be addressed first. However, it is ideal if the course of surgical treatment can be completed before the child begins school.
Thumb hypoplasia
Thumb hypoplasia is congenital underdevelopment of the thumb (Fig. 1), with manifestations ranging from a slightly smaller thumb to complete absence of a thumb. Thumb hypoplasia may exist independently or in the context of radial longitudinal deficiency. The Manske modification of the Blauth classification is used to describe thumb morphology and to guide treatment (Table 2) [27, 28••]. The Type I thumb is slightly smaller than a typical thumb but is stable and provides good function; no treatment is needed. The Type II thumb has hypoplasia of the intrinsic thenar muscles, narrowing of the first webspace, and deficiency of the ulnar collateral ligament (UCL) at the thumb metacarpal-phalangeal joint. These findings are treated with opponensplasty to regain thumb opposition, soft tissue release of the first webspace, and UCL reconstruction. Although the ring flexor digitorum superficialis (FDS), abductor digiti minimi, or extensor indicis proprius can all be used for opponensplasty, using the ring FDS provides the advantage of using the terminal aspect of the tendon to reconstruct the UCL [28••]. The Type III thumb includes the same findings as the Type II thumb, but also involves loss of the extrinsic muscles and bony hypoplasia. Type III thumbs are further divided based on stability of the thumb carpal-metacarpal (CMC) joint: IIIA thumbs have a stable CMC joint and can be treated similarly to type II thumbs, whereas IIIB thumbs do not have a stable CMC joint and are best treated with pollicization. Type IV thumbs (the “pouce flottant” thumb) have rudimentary elements and are attached to the hand by a small skin bridge, whereas Type V is thumb aplasia with complete loss of all thumb structures and radial carpal bones. Types IV and V are also treated with pollicization.
Fig. 1.

Clinical photograph of bilateral thumb hypoplasia
Table 2.
Blauth classification of thumb hypoplasia (as modified by Manske) with associated treatments (adapted from [28••], with permission)
| Type | Findings | Treatment |
|---|---|---|
| I | Minor generalized hypoplasia | No treatment |
| II | Intrinsic thenar muscle hypoplasia First webspace narrowing UCL insufficiency |
Opponensplasty First web release UCL reconstruction |
| III | Similar findings to Type II, plus: Extrinsic muscle and tendon abnormalities Bone deficiency A: Stable thumb carpal-metacarpal joint B: Unstable thumb carpal-metacarpal joint |
A: Reconstruction B: Pollicization |
| IV | Floating thumb (“pouce flottant”) | Pollicization |
| V | Absent thumb | Pollicization |
UCL ulnar collateral ligament
Pollicization with the index finger is a technically challenging procedure requiring meticulous attention to detail and the ability to manage perioperative complications [29]. The ability of the pollicized thumb to participate in pinch and grasp depends on the preoperative status of the transposed digit, as a stiff index finger is less able to participate in pinch once transposed. Although the pollicized thumb commonly has a more slender appearance than a normal thumb [30], its function into adulthood can be satisfactory [31], albeit with somewhat impaired function, strength, and motion [32, 33•]. In situations in which it is culturally preferred to maintain a 5 finger hand, procedures such as metacarpal lengthening and free microvascular transfer of the second metatarsal-phalangeal joint can be used. However, the results are less reliable than index pollicization [34].
Ulnar longitudinal deficiency
Ulnar longitudinal deficiency (ULD) is approximately 4 times less common than RLD, with an incidence of 0.44 per 10,000 live births [1••]. ULD is not classically associated with life-threatening systemic conditions and often occurs in isolation and unilaterally [1••, 11]. Additional diagnostic evaluation is not necessary in most cases. The entire limb is hypoplastic and is marked by abnormalities of the elbow and forearm. The radius can adopt an ulna-like appearance and may fuse to the distal humerus [11]. The hand and carpus are almost always affected in ULD, with up to 90 % of cases having missing digits and 70 % having thumb abnormalities (specifically, webspace deficiencies, malrotation of the thumb, and thumb syndactyly) [35]. Treatment strategies are centered on assuring appropriate function of the thumb and fingers. Web space deficiencies are treated with soft tissue deepening, thumb malrotation is treated with rotational metacarpal osteotomies, and thumb syndactyly is treated with release [36]. Occasionally, rotational humeral osteotomies are used to better position the distal extremity and the residual cartilaginous ulnar anlage is excised to treat persistent ulnar deviation deformities, though great care must be taken in selecting proper patients [17••, 35].
Central deficiency
Central deficiency (also referred to as typical cleft hand) is also relatively uncommon, with an incidence of 0.52 per 10,000 live births [1••]. Historically, cleft hand was divided into typical and atypical types; however, the latter is now recognized as symbrachydactyly due to differences in embryologic malformation. Central deficiency is commonly inherited in an autosomal dominant pattern, but also may occur spontaneously [37]. Phenotypic expression of central deficiency may vary among generations and may occur with cleft feet and facial clefts. Although no additional diagnostic workup is generally indicated, referral to a geneticist may be helpful for parents inquiring about the heritability of the condition [17••]. In the hand, there is variable absence of the index, middle, and ring rays and central carpus, with a typical appearance of a V-shaped cleft without finger nubbins [11]. There is often syndactyly of the thumb-index and ring-small webspaces. In the most severe cases, there is complete absence of the thumb. Decisions about management of the cleft hand must be handled individually, as families with multiple generations of cleft hands will have a different viewpoint than families affected for the first time [37]. Given the potential stigmatization and social impact of a cleft hand, surgical treatment to improve cosmesis may be considered, taking tremendous care to avoid compromising function. Surgical treatment is generally indicated for progressive deformity of the cleft, deficient first webspace, a malpositioned index ray that interferes with function, and progressive flexion contractures of 1 or more digits [37, 38]. Reconstruction of the first webspace is one of the most challenging aspects of the procedure [17••], as the index finger may require relocation to the ulnar side of the cleft in a manner that avoids malrotation and scissoring in flexion [37, 38]. In addition to first webspace reconstruction and closure of the cleft, removal of transverse bones from within the cleft and release of a deforming syndactyly on the ulnar side of the cleft may be necessary. Given the individuality of each deformity and the surgical treatments, outcomes after surgery for central deficiency are difficult to collectively predict. Patients who have a stable thumb and an appropriate first webspace are most likely to have successful functional outcomes [37].
Syndactyly
Syndactyly is a common congenital anomaly that is characterized by an abnormal connection between adjacent digits [11, 39]. The incidence of syndactyly is approximately 2–3 per 10,000 live births [11]. It occurs more commonly in males than females, and it occurs with equal frequency unilaterally and bilaterally [39]. Syndactyly can be inherited or occur sporadically, but when inherited, it is transmitted in an autosomal dominant pattern with variable expressivity and incomplete penetrance [11].
Specific terminology is used to describe the features of each syndactyly. The extent of involvement of the digit determines whether the syndactyly is complete (complete soft tissue connection between the digits) or incomplete. The type of tissue involved determines whether the syndactyly is simple (soft tissue only) (Fig. 2) or complex (soft tissue and bone). Last, if syndactyly occurs in the setting of a syndrome with extensive bony or soft tissue abnormalities, it is referred to as a complicated syndactyly [11].
Fig. 2.
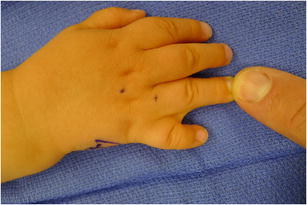
Clinical photograph of a simple syndactyly between the ring and small fingers
Although syndactyly is typically an isolated finding, there are a variety of conditions and syndromes that may be associated with syndactyly, such as Poland’s syndrome and Apert’s syndrome. Clinicians evaluating patients with syndactyly should be aware of these potential associations and should refer patients for further evaluation when appropriate [11, 39]. Poland’s syndrome is commonly associated with syndactyly, and it is characterized by unilateral hypoplasia of the sternal head of the pectoralis major and the entire upper extremity. Similarly, Apert’s syndrome is associated with complex syndactyly as well as craniosynostosis, hypertelorism, exophthalmos, and mild mental retardation [11, 39].
The goal of surgical treatment is to improve hand function by releasing the interconnection between the 2 digits [39]. Over 40 methods have been reported in the literature, and the premise of each method is to develop a deep and wide webspace, as well as cover skin deficits with local soft tissue or skin graft [39, 40]. Surgery is contraindicated in patients with super digits (2 metacarpals supporting 1 digit or 1 metacarpal supporting 2 or more digits); complex syndactyly, in which conjoined fingers move in unison; hands that lack active muscle control; and adults with functional syndactylized digits. Generally, surgery should be performed before the child reaches school age [39].
Polydactyly
Polydactyly, the presence of an extra digit, is the most common congenital hand anomaly [41]. Manifestations of polydactyly are described by the location of the extra digit: pre-axial (radial), central, or post-axial (ulnar).
Pre-axial polydactyly (Fig. 3) is the most common type of polydactyly and occurs most commonly in Caucasian, American Indian, and Asian populations [42•]. Most cases are unilateral, sporadic, and are not associated with systemic syndromes [11]. However, a triphalangeal thumb is linked to an autosomal dominant inheritance pattern, and it may be associated with systemic syndromes such as Holt-Oram syndrome and Fanconi anemia [42•]. Approximately 50 % of cases involve duplicated proximal and distal phalanges that articulate with a bifid metacarpal head, and 90 % of all pre-axial polydactyly cases involve the thumb [11, 42•]. Wassel proposed the most widely used classification system for thumb duplication, dividing this entity into 7 groups based on the level of bifurcation [43]. Treatment of pre-axial polydactyly usually requires removal of the smaller part and reconstruction of the retained component [9].
Fig. 3.
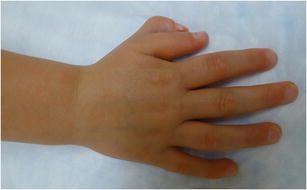
Clinical photograph of a pre-axial polydactyly (thumb duplication)
Post-axial polydactyly (Fig. 4) occurs most commonly in patients of African descent [42•], with cases in Caucasian individuals requiring further evaluation due to concern for an underlying syndrome such as chondroectodermal dysplasia or Ellis-van Creveld syndrome [11]. Post-axial polydactyly is frequently inherited in an autosomal dominant manner with variable penetrance. It is classified as either well-developed (type A) or rudimentary and pedunculated (type B). Type B post-axial polydactyly can be treated with suture ligation of the pedicle in the nursery, whereas Type A requires formal reconstruction with transfer of important anatomical structures, such as the collateral ligaments or muscles to the adjacent finger [9, 11].
Fig. 4.
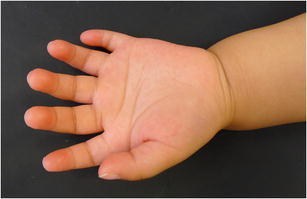
Clinical photograph of a postaxial polydactyly
Central polydactyly is a duplication of a nonborder digit, and it is less prevalent than pre-axial and post-axial polydactyly [11, 42•]. It most commonly occurs in the fourth digit, followed by the third digit [42•]. It may be hidden in a concomitant syndactyly, referred to as a synpolydactyly. Diagnosis requires careful examination and radiographic evidence [11]. The treatment of central polydactyly is individualized to each deformity and should be completed prior to the development of angular deformities and contractures [42•].
Amniotic constriction band
Amniotic constriction band (ACB) is an overarching term used to describe a range of congenital anomalies, including annular constrictions of extremities, oligodactyly, acrosyndactyly, talipes equinovarus, cleft lip and palate, and hemangiomas [44]. There have been over 30 terms (including constriction band syndrome, constriction ring syndrome, and amnion rupture sequence) used to describe this collection of physical findings. This is likely attributable to the lack of consensus on etiology or defining features of ACB [44, 45].
The overall incidence of ACB is estimated at 0.98–1.16 per 10,000 live births [46, 47], whereas the incidence of ACB with amputation of a part of the upper extremity is 0.62 per 10,000 live births [1••]. Risk factors for ACB have not rigorously defined, but associations with maternal illness, young maternal age, maternal drug exposure, prematurity, and low birth weight have been reported [44, 46, 48, 49]. There is limited evidence for familial or hereditary predisposition and the majority of cases are sporadic. However, temporal and geographic clustering has been noted [50]. Although several theories have been proposed, such as an intrinsic germ line abnormality and vascular disruption, there is no universally accepted etiology for the constellation of findings in ACB [44, 49].
The clinical manifestations of ACB can be categorized as 1 of 3 types: disruption, deformation, or malformation. Example of disruptions, defined as the breakdown of normal tissue, in ACB are constriction bands, amputations, and acrosyndactyly. Deformations, which result from abnormal forces on the fetus, may present in ACB as talipes equinovarus, scoliosis, and joint contractures. Malformations are caused by an insult early in gestation and are associated in ACB with body wall defects, internal organ abnormalities, and craniofacial abnormalities [44]. Patterson proposed a classification scheme for the extremity findings of ACB: Type 1 describes simple constriction rings; Type 2 describes constriction ring with distal deformity, including atrophy and lymphedema; Type 3 describes acrosyndactyly or fenestrated syndactyly; and Type 4 describes any type of amputation [51].
ACB classically involves multiple extremities, with the most common deformities being limb or digit amputations, constriction rings, and acrosyndactyly [44]. Usually 3 extremities are involved, with greater involvement of the upper extremity, especially the distal aspects and central digits [47, 52]. Additional findings include talipes equinovarus, which occurs in approximately a third of patients, and less commonly, scoliosis, craniofacial abnormalities, body wall defects, and internal organ abnormalities. Children with amniotic constriction band need to be referred to a clinical geneticist or appropriate subspecialist in order to be evaluated for these associated findings [44].
As with most congenital upper extremity deformities, the management of ACB is individualized and focused on improving function and cosmesis. Circumferential Z-plasty or W-plasty can be used to release deep bands, whereas amputation may be considered in cases of severe ischemia and high risk of osteomyelitis. On-top plasty (partial digital transfer) and bone-lengthening procedures can be performed to restore function in the setting of digital hypoplasia and amputation. Last, digital separation and webspace reconstruction can be performed for acrosyndactyly. One or 2-stage procedures may be required for optimal results, and the timing of surgery is dependent on the disease severity and predicted skeletal growth [49, 53, 54]. In the future, advances in prenatal diagnosis and fetoscopic surgical techniques may allow for in-utero treatment of amniotic constriction band [49].
Conclusions
Although congenital hand anomalies are rare, they may be associated with medical syndromes and may cause significant functional impairment. Detailed history-taking, thorough physical examination and appropriate referral for further diagnostic workup are necessary in evaluating the patient with a congenital hand anomaly. Although classification schemes can provide the basis for treatment for many congenital hand anomalies, treatment should be individualized to the characteristics of each patient and each family. Continued advances in our understanding of the molecular basis of limb development will provide additional opportunities for early diagnosis and innovative treatments.
Acknowledgments
This paper is supported by NIH NIAMS grant T32-AR07281.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Christopher J. Dy declares that he has no conflict of interest. Ishaan Swarup declares that he has no conflict of interest. Aaron Daluiski declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.••.Koskimies E, Lindfors N, Gissler M, Peltonen J, Nietosvaara Y. Congenital upper limb deficiencies and associated malformations in Finland: a population-based study. J Hand Surg [Am] 2011;36:1058–1065. doi: 10.1016/j.jhsa.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Froster UG, Baird PA. Upper limb deficiencies and associated malformations: a population-based study. Am J Med Genet. 1992;44:767–781. doi: 10.1002/ajmg.1320440611. [DOI] [PubMed] [Google Scholar]
- 3.Giele H, Giele C, Bower C, Allison M. The incidence and epidemiology of congenital upper limb anomalies: a total population study. J Hand Surg [Am] 2001;26:628–634. doi: 10.1053/jhsu.2001.26121. [DOI] [PubMed] [Google Scholar]
- 4.Kallen B, Rahmani TM, Winberg J. Infants with congenital limb reduction registered in the Swedish Register of Congenital Malformations. Teratology. 1984;29:73–85. doi: 10.1002/tera.1420290109. [DOI] [PubMed] [Google Scholar]
- 5.Goldfarb CA. Congenital hand surgery: what's new and what's coming. Hand Clin. 2009;25:293–299. doi: 10.1016/j.hcl.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 6.••.Goldfarb CA. Congenital hand anomalies: a review of the literature, 2009‒2012. J Hand Surg [Am] 2013;38:1854–1859. doi: 10.1016/j.jhsa.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Daluiski A, Yi SE, Lyons KM. The molecular control of upper extremity development: implications for congenital hand anomalies. J Hand Surg [Am] 2001;26:8–22. doi: 10.1053/jhsu.2001.9419. [DOI] [PubMed] [Google Scholar]
- 8.Daluiski A. The future impact of molecular control of upper extremity development in hand and upper extremity surgery. Tech Hand Up Extrem Surg. 2001;5:71. doi: 10.1097/00130911-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Seiler JG., III . Essentials of hand surgery. Philadelphia: Lippincott Williams and Wilkins; 2002. [Google Scholar]
- 10.Zaleske DJ. Development of the upper limb. Hand Clin. 1985;1:383–390. [PubMed] [Google Scholar]
- 11.Kozin SH. Upper-extremity congenital anomalies. J Bone Joint Surg Am. 2003;85-A:1564–1576. doi: 10.2106/00004623-200308000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Aro T, Heinonen OP, Saxen L. Incidence and secular trends of congenital limb defects in Finland. Int J Epidemiol. 1982;11:239–244. doi: 10.1093/ije/11.3.239. [DOI] [PubMed] [Google Scholar]
- 13.••.Wall LB, Ezaki M, Oishi SN. Management of congenital radial longitudinal deficiency: controversies and current concepts. Plast Reconstr Surg. 2013;132:122–128. doi: 10.1097/PRS.0b013e318290fca5. [DOI] [PubMed] [Google Scholar]
- 14.Goldfarb CA, Wall L, Manske PR. Radial longitudinal deficiency: the incidence of associated medical and musculoskeletal conditions. J Hand Surg [Am] 2006;31:1176–1182. doi: 10.1016/j.jhsa.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 15.James MA, McCarroll HR, Jr, Manske PR. The spectrum of radial longitudinal deficiency: a modified classification. J Hand Surg [Am] 1999;24:1145–1155. doi: 10.1053/jhsu.1999.1145. [DOI] [PubMed] [Google Scholar]
- 16.•.Kotwal PP, Varshney MK, Soral A. Comparison of surgical treatment and nonoperative management for radial longitudinal deficiency. J Hand Surg Eur. 2012;37:161–169. doi: 10.1177/1753193411413070. [DOI] [PubMed] [Google Scholar]
- 17.••.Bauer AS, Bednar MS, James MA. Disruption of the radial/ulnar axis: congenital longitudinal deficiencies. J Hand Surg [Am] 2013;38:2293–2302. doi: 10.1016/j.jhsa.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 18.••.Ekblom AG, Dahlin LB, Rosberg HE, Wiig M, Werner M, Arner M. Hand function in children with radial longitudinal deficiency. BMC Musculoskelet Disord. 2013;14:116. doi: 10.1186/1471-2474-14-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Innocenti M, Delcroix L, Manfrini M, Ceruso M, Capanna R. Vascularized proximal fibular epiphyseal transfer for distal radial reconstruction. J Bone Joint Surg Am. 2004;86-A:1504–1511. doi: 10.2106/00004623-200407000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Vilkki SK. Vascularized metatarsophalangeal joint transfer for radial hypoplasia. Semin Plast Surg. 2008;22:195–212. doi: 10.1055/s-2008-1081403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.•.Farr S, Petje G, Sadoghi P, Ganger R, Grill F, Girsch W. Radiographic early to midterm results of distraction osteogenesis in radial longitudinal deficiency. J Hand Surg [Am] 2012;37:2313–2319. doi: 10.1016/j.jhsa.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Goldfarb CA, Murtha YM, Gordon JE, Manske PR. Soft-tissue distraction with a ring external fixator before centralization for radial longitudinal deficiency. J Hand Surg [Am] 2006;31:952–959. doi: 10.1016/j.jhsa.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Sabharwal S, Finuoli AL, Ghobadi F. Pre-centralization soft tissue distraction for Bayne type IV congenital radial deficiency in children. J Pediatr Orthop. 2005;25:377–381. doi: 10.1097/01.bpo.0000152907.31293.00. [DOI] [PubMed] [Google Scholar]
- 24.Kawabata H, Shibata T, Masatomi T, Yasui N. Residual deformity in congenital radial club hands after previous centralization of the wrist. Ulnar lengthening and correction by the Ilizarov method. J Bone Joint Surg Br. 1998;80:762–765. doi: 10.1302/0301-620X.80B5.8839. [DOI] [PubMed] [Google Scholar]
- 25.Bhat SB, Kamath AF, Sehgal K, Horn BD, Hosalkar HS. Multi-axial correction system in the treatment of radial club hand. J Child Orthop. 2009;3:493–498. doi: 10.1007/s11832-009-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pike JM, Manske PR, Steffen JA, Goldfarb CA. Ulnocarpal epiphyseal arthrodesis for recurrent deformity after centralization for radial longitudinal deficiency. J Hand Surg [Am] 2010;35:1755–1761. doi: 10.1016/j.jhsa.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Manske PR, McCarroll HR, Jr, James M. Type III-A hypoplastic thumb. J Hand Surg [Am] 1995;20:246–253. doi: 10.1016/S0363-5023(05)80018-8. [DOI] [PubMed] [Google Scholar]
- 28.••.Soldado F, Zlotolow DA, Kozin SH. Thumb hypoplasia. J Hand Surg [Am] 2013;38:1435–1444. doi: 10.1016/j.jhsa.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Goldfarb CA, Monroe E, Steffen J, Manske PR. Incidence and treatment of complications, suboptimal outcomes, and functional deficiencies after pollicization. J Hand Surg [Am] 2009;34:1291–1297. doi: 10.1016/j.jhsa.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Goldfarb CA, Deardorff V, Chia B, Meander A, Manske PR. Objective features and aesthetic outcome of pollicized digits compared with normal thumbs. J Hand Surg [Am] 2007;32:1031–1036. doi: 10.1016/j.jhsa.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 31.Clark DI, Chell J, Davis TR. Pollicisation of the index finger. A 27-year follow-up study. J Bone Joint Surg Br. 1998;80:631–635. doi: 10.1302/0301-620X.80B4.8613. [DOI] [PubMed] [Google Scholar]
- 32.Manske PR, Rotman MB, Dailey LA. Long-term functional results after pollicization for the congenitally deficient thumb. J Hand Surg [Am] 1992;17:1064–1072. doi: 10.1016/S0363-5023(09)91063-2. [DOI] [PubMed] [Google Scholar]
- 33.•.Netscher DT, Aliu O, Sandvall BK, Staines KG, Hamilton KL, Salazar-Reyes H, et al. Functional outcomes of children with index pollicizations for thumb deficiency. J Hand Surg [Am] 2013;38:250–257. doi: 10.1016/j.jhsa.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 34.Riley SA, Burgess RC. Thumb hypoplasia. J Hand Surg [Am] 2009;34:1564–1573. doi: 10.1016/j.jhsa.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 35.Bednar MS, James MA, Light TR. Congenital longitudinal deficiency. J Hand Surg [Am] 2009;34:1739–1747. doi: 10.1016/j.jhsa.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Cole RJ, Manske PR. Classification of ulnar deficiency according to the thumb and first web. J Hand Surg [Am] 1997;22:479–488. doi: 10.1016/S0363-5023(97)80016-0. [DOI] [PubMed] [Google Scholar]
- 37.Kay S, McCombe D, Kozin SH. Deformities of the hand and fingers. In: Wolfe SW, Hotchkiss RN, Pederson WC, Kozin SH, editors. Green's operative hand surgery. 6. Philadelphia: Elsevier; 2010. p. 1303. [Google Scholar]
- 38.Upton J, Taghinia AH. Correction of the typical cleft hand. J Hand Surg [Am] 2010;35:480–485. doi: 10.1016/j.jhsa.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 39.Dao KD, Shin AY, Billings A, Oberg KC, Wood VE. Surgical treatment of congenital syndactyly of the hand. J Am Acad Orthop Surg. 2004;12:39–48. doi: 10.5435/00124635-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Upton J. Congenital anomalies of the hand and forearm. In: McCarthy J, May JJ, Littler J, editors. Plastic surgery: the hand part 2. Philadelphia: WB Saunders; 1990.
- 41.Bunnell S. Surgery of the hand. Philadelphia: Lippincott; 1964. [Google Scholar]
- 42.•.Guo B, Lee SK, Paksima N. Polydactyly: a review. Bull Hosp Jt Dis. 2013;71:17–23. [PubMed] [Google Scholar]
- 43.Wassel HD. The results of surgery for polydactyly of the thumb. A review. Clin Orthop Relat Res. 1969;64:175–193. [PubMed] [Google Scholar]
- 44.Goldfarb CA, Sathienkijkanchai A, Robin NH. Amniotic constriction band: a multidisciplinary assessment of etiology and clinical presentation. J Bone Joint Surg Am. 2009;91(Suppl 4):68–75. doi: 10.2106/JBJS.I.00339. [DOI] [PubMed] [Google Scholar]
- 45.Rayan GM. Amniotic constriction band. J Hand Surg [Am] 2002;27:1110–1111. doi: 10.1053/jhsu.2002.36517. [DOI] [PubMed] [Google Scholar]
- 46.Garza A, Cordero JF, Mulinare J. Epidemiology of the early amnion rupture spectrum of defects. Am J Dis Child. 1988;142:541–544. doi: 10.1001/archpedi.1988.02150050079037. [DOI] [PubMed] [Google Scholar]
- 47.Foulkes GD, Reinker K. Congenital constriction band syndrome: a 70-year experience. J Pediatr Orthop. 1994;14:242–248. doi: 10.1097/01241398-199403000-00021. [DOI] [PubMed] [Google Scholar]
- 48.Miura T. Congenital constriction band syndrome. J Hand Surg [Am] 1984;9A:82–88. doi: 10.1016/S0363-5023(84)80191-4. [DOI] [PubMed] [Google Scholar]
- 49.Kawamura K, Chung KC. Constriction band syndrome. Hand Clin. 2009;25:257–264. doi: 10.1016/j.hcl.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Lockwood C, Ghidini A, Romero R, Hobbins JC. Amniotic band syndrome: reevaluation of its pathogenesis. Am J Obstet Gynecol. 1989;160:1030–1033. doi: 10.1016/0002-9378(89)90153-1. [DOI] [PubMed] [Google Scholar]
- 51.Patterson TJ. Congenital ring-constrictions. Br J Plast Surg. 1961;14:1–31. doi: 10.1016/S0007-1226(61)80002-7. [DOI] [PubMed] [Google Scholar]
- 52.Askins G, Ger E. Congenital constriction band syndrome. J Pediatr Orthop. 1988;8:461–466. doi: 10.1097/01241398-198807000-00016. [DOI] [PubMed] [Google Scholar]
- 53.Gabos PG. Modified technique for the surgical treatment of congenital constriction bands of the arms and legs of infants and children. Orthopedics. 2006;29:401–404. doi: 10.3928/01477447-20060501-10. [DOI] [PubMed] [Google Scholar]
- 54.Upton J, Tan C. Correction of constriction rings. J Hand Surg [Am] 1991;16:947–953. doi: 10.1016/S0363-5023(10)80166-2. [DOI] [PubMed] [Google Scholar]


