Summary
Sickle cell disease (SCD) in Saudi patients from the Eastern Province is associated with the Arab-Indian (AI) HBB (β-globin gene) haplotype. The phenotype of AI SCD in children was described as benign and was attributed to their high fetal haemoglobin (HbF). We conducted a hospital-based study to assess the pattern of SCD complications in adults. A total of 104 patients with average age of 27 years were enrolled. Ninety-six percent of these patients reported history of painful crisis; 47% had at least one episode of acute chest syndrome, however, only 15% had two or more episodes; symptomatic osteonecrosis was reported in 18%; priapism in 17%; overt stroke in 6%; none had leg ulcers. The majority of patients had persistent splenomegaly and 66% had gallstones. Half of the patients co-inherited α-thalassaemia and about one third had glucose-6-phosphate dehydrogenase deficiency. Higher HbF correlated with higher rate of splenic sequestration but not with other phenotypes. The phenotype of adult patients with AI SCD is not benign despite their relatively high HbF level. This is probably due to the continued decline in HbF level in adults and the heterocellular and variable distribution of HbF amongst F-cells.
Keywords: Sickle cell disease, Saudi Arabia, Phenotype, Arab-Indian Haplotype, Fetal Haemoglobin
Introduction
Fifty years have passed since the first description of sickle cell disease (SCD) in Saudi Arabia (Lehmann, et al 1963). When the HbS mutation was associated with the Arab-Indian (AI) HBB (β-globin gene ) haplotype, HbS homozygotes or compound heterozygotes for HbS and β0 thalassaemia were reported to have a milder phenotype than patients with a HbS mutation of African origin (Padmos, et al 1991, Pembrey, et al 1980, Perrine, et al 1972, Perrine, et al 1978). This was attributed to their high fetal haemoglobin (HbF) level. In some reports, SCD in the Eastern Province of Saudi Arabia, where the AI haplotype might have originated, was labelled as “benign” (Perrine, et al 1972, Perrine, et al 1978). Most patients in these reports were children, raising the question of whether the “benign” course of AI haplotype SCD extended to adults. Some studies suggested worsening of the disease as patients passed the first decade of life (Adekile, et al 2007, Adekile 2011, Marouf, et al 2003a, Marouf, et al 2003b). We studied the prevalence of SCD complications in Saudi patients with the AI haplotype whose average age was about 27 years, and examined the association between common complications and selected clinical and laboratory variables. We compared our results to African American patients and Saudi SCD patients from the Southwestern Province where the HbS mutation was associated with African HBB haplotypes. SCD in Saudi adult patients is not “benign”, and id probably due to the heterocellular and variable distribution of HbF amongst F-cells.
Methods
Inclusion criteria
Patients (HbSS and HbS-β0 thalassaemia) with the AI haplotype, older than 12 years of age who presented to participating institutions in Al-Hofuf, Eastern Province, Saudi Arabia (Figure 1) in either the clinic or inpatient setting from July 2010 were enrolled after informed consent was obtained. The study was approved by the institutional review boards of all participating institutions.
Figure 1.
Map of Saudi Arabia showing the city of Al Hofuf (Al-Ahsa) in the Eastern Province where sickle cell disease (SCD) patients of the Arab-Indian haplotype were recruited for the current study. Saudi SCD patients from Southwestern Province (SWP) have the African origin HBB haplotypes, most commonly Benin.
Laboratory evaluation
Steady state blood counts and erythrocyte glucose-6-phosphate dehydrogenase (G6PD) level were obtained as part of the clinical evaluation. HbF was measured by high performance liquid chromatography (HPLC) at the time of study enrollment. The Gentra Puregene blood kit was used for DNA extraction. AI haplotype was determined as previously described (Ngo, et al 2013). We screened for common α-thalassaemia deletions (α3.7 and α4.2) and non-deletional poly A signal mutation (αT Saudi) as described (Tan, et al 2001, Viprakasit, et al 2002).
Data collection
A comprehensive medical history and physical examination was undertaken and medical records reviewed. Age, gender, medications (including hydroxycarbamide), history of asthma and blood pressure measurement at time of enrollment were recorded. Duration and dose of hydroxycarbamide were not recorded. SCD complications that were recorded and analysed included acute vaso-occlusive painful events that occurred in the previous year and required hospital admission, visit to emergency department or administration of pain medication at home (including crises that were self-reported or documented in the patient's chart); osteonecrosis proven by x-ray or magnetic resonance imaging (MRI) in symptomatic patients; acute chest syndrome, defined as an acute respiratory illness with a new pulmonary infiltrate on chest x-ray; overt stroke confirmed by MRI/magnetic resonance angiogram (MRA) in symptomatic patients; gallstones confirmed by ultrasound during routine screening or in symptomatic patients; splenic sequestration, defined as acute enlargement of spleen that was associated with a fall in haemoglobin level; persistent splenomegaly, defined as presence of palpable spleen at the time of assessment ; systemic infections, such as meningitis, bacteraemia, osteomyelitis, or septic arthritis, confirmed by positive culture or by MRI in osteomyelitis; priapism, defined as a sustained unwanted painful erection; leg ulcers.
Statistical analysis
Clinical and laboratory characteristics were evaluated for association with SCD-related complications. As these analyses are descriptive and exploratory in nature, p=0.05 was used as a threshold for nominal significance in all cases. Crude associations between outcomes and continuous variables were assessed using t-tests. For categorical variables, chi-square tests were used. SCD complications considered include pain, osteonecrosis, gallstones, acute chest syndrome, splenic sequestration, priapism, thrombosis, stroke, sepsis, and persistent splenomegaly. Each of these outcomes was assessed for association with age, gender, hydroxycarbamide use, history of asthma or hypertension, G6PD deficiency, white blood count (WBC), haemoglobin (Hb), mean corpuscular volume (MCV), HbF, total bilirubin, and co-inheritance of α thalassaemia. Logistic regression was also performed in order to assess the relationship between each of the outcomes and HbF, adjusting for age and gender. All statistical analyses were conducted using R statistical software (R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org).
Results
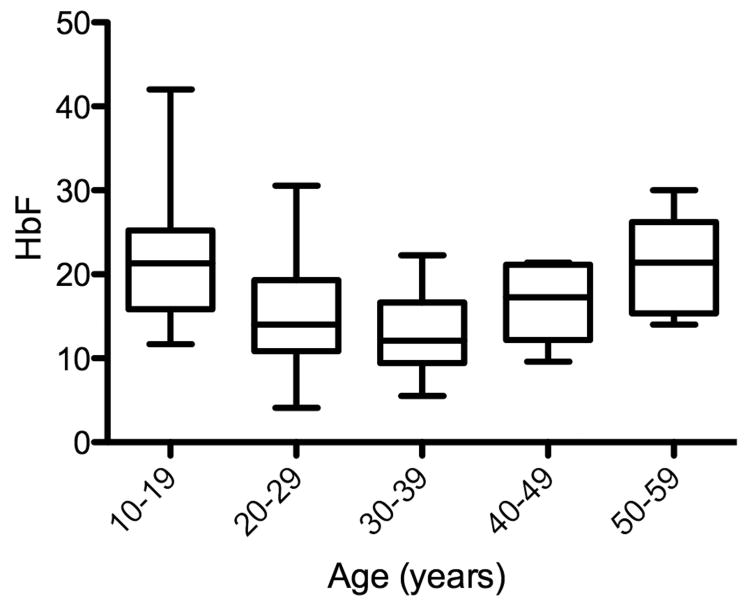
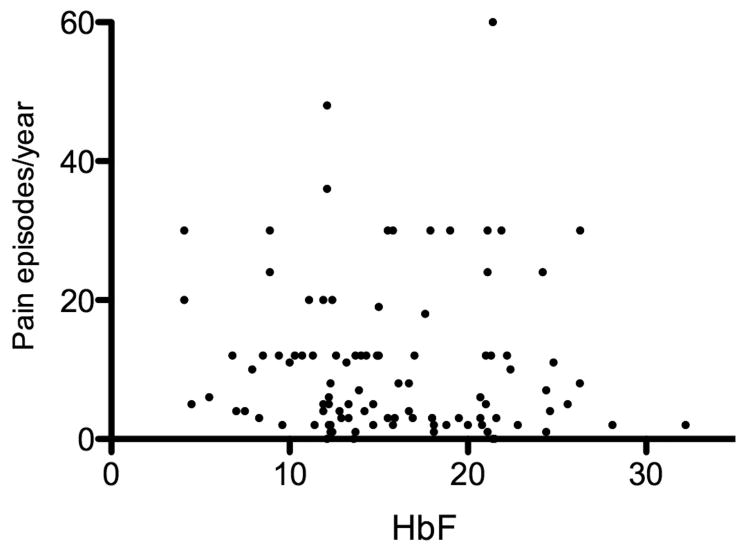
Clinical characteristics are shown in Table I and the laboratory findings are reported in Table II. Nearly 27% of patients were prescribed hydroxycarbamide. HbF level stratified by age of participants, in those who were not receiving hydroxycarbamide, is shown in Figure 2. The mean total Hb and HbF were 92 g/l and 15.6%, respectively. Acute painful episodes occurring within one year prior to joining the study were reported in 96% of patients. HbF level measured at enrollment was not associated with number of pain crisis (Figure 3). Forty-seven percent of cases had at least one episode of acute chest syndrome but only 15% had two or more episodes. The majority of patients had a palpable spleen and 66% had gallstones. All cases of osteonecrosis were in the hip, except two cases in the shoulder. Osteomyelitis or septic arthritis was observed in 8 patients and 13 patients had bacteraemia caused by organisms such as Klebsiella pneumonia, Salmonella, Staphylococcus aureus or epidermidis. About one-third of patients tested had G6PD deficiency and 51 % had α-thalassaemia.
Table I.
Clinical characteristics of Saudi patients with sickle cell disease of Arab-Indian Haplotype (n=104).
| Characteristics | Number of patients (%) |
|---|---|
|
| |
| Gender ratio: Male/Female | 47/57 (45/55) |
|
| |
| Mean age, years ± SD (range) | 26.9±10.6 (12-54) |
|
| |
| Folic acid supplement | 100 (96) |
|
| |
| Penicillin prophylaxis | 3 (3) |
|
| |
| Hydroxycarbamide use | 28 (27) |
|
| |
| History of painful crisis | 100 (96) |
|
| |
| Median pain episodes/year (range) | 6 (0-60) |
|
| |
| Osteonecrosis | 19 (18) |
|
| |
| Acute chest syndrome | |
| At least one episode | 49 (47) |
| Recurrent (≥2) | 16 (15) |
|
| |
| Gall stones/Cholecystectomy | 69 (66)/39 (38) |
|
| |
| Splenic sequestration | 16 (15) |
|
| |
| Splenectomy | 20 (19) |
|
| |
| Priapism | 8 (17) |
|
| |
| Leg ulcers | none |
|
| |
| Serious infections | 18 (17) |
|
| |
| Deep venous thrombosis | 4 (4) |
|
| |
| Overt stroke | 6 (6) |
|
| |
| Persistent splenomegaly n=59 | 42 (71) |
Table II.
Laboratory characteristics of Saudi patients with sickle cell disease of Arab-Indian Haplotype (n=104).
| Characteristics | n (%) or Mean ± SD (range) |
|---|---|
|
| |
| HbSS/HbS-β0 | 74/30 (71/29) |
|
| |
| WBC (× 109/l) | 12.7±7.6 (2.6-52.5) |
|
| |
| Hb (g/l) | 92±17 (37-147) |
|
| |
| MCV (fl) | 76±11 (49-97) |
|
| |
| Reticulocytes % | 9.1±5.1 (2.6-17.0) |
|
| |
| HbF | 15.6±5.7 (4.1-32.2) |
|
| |
| Alpha thalassaemia n=80 | 41 (51) |
| αα/−α3.7 | 7 |
| −α3.7/−α3.7 | 16 |
| αα/−α4.2 | 1 |
| −α3.7/αTSaudiα | 1 |
| αα/αTSaudiα | 15 |
| αTSaudiα/αTSaudiα | 1 |
|
| |
| G6PD deficiency n=46 | 13 (28) |
|
| |
| Total bilirubin μmol/l | 47±72 (5.4-534) |
|
| |
| Serum creatinine μmol/l | 41±17 (4-102) |
|
| |
| LDH (iu/l) n=86 | 432±235 (146-1762) |
Abbreviations: SD=standard deviation; WBC=white blood cells count; Hb=haemoglobin concentration; MCV=mean corpuscular volume; HbF=fetal haemoglobin; G6PD= glucose-6-phosphate dehydrogenase; LDH=lactate dehydrogenase.
Figure 2.
HbF trend in relation to age in sickle cell disease patients with Arab-Indian haplotype who are not receiving hydroxycarbamide. Median HbF levels vary significantly between different age groups (P=0.0005).
Figure 3.
Number of pain episodes per year relative to HbF level. The number of painful episode was not associated with HbF (R2=0.003).
Variables that were associated with different SCD subphenotypes are shown in Table III. HbF level was higher among patients with a history of splenic sequestration, which was probably related to the preservation of splenic function, though this association did not persist after adjustment for age and gender (p=0.051) (Supplemental Table I). As expected, older patients had experienced more osteonecrosis and stroke events. Patients with co-existing systemic hypertension, defined as blood pressure >140/90, had higher rate of osteonecrosis and overt stroke. All 6 patients who had stroke were female. G6PD deficiency was observed more among cases with history of osteonecrosis, and this remained significant after adjusting for other variables.
Table III.
Variables associated significantly (P<0.05) with sickle cell disease phenotype in Saudi patients with Arab-Indian haplotype.
| Clinical History | Variable | Phenotype + n (%) or mean±SD | Phenotype − n (%) or mean±SD | P value |
|---|---|---|---|---|
|
| ||||
| Osteonecrosis | Age | 32.9±13.4 years | 25.1±9.6 years | 0.026 |
| G6PD deficiency | 7 (64) | 6 (18) | 0.008 | |
| Hypertension | 6 (33) | 4 (5) | 0.003 | |
|
| ||||
| Splenic sequestration | HbF | 18.2±5.3 | 15.0±5.7 | 0.041 |
|
| ||||
| Priapism | Hydroxycarbamide | 5 (63) | 6 (18) | 0.011 |
| use | 85±27 g/l | 101±17 g/l | 0.045 | |
| Hb | ||||
|
| ||||
| Gallstones | Total bilirubin | 55±86 μmol/l | 30±15 μmol/l | 0.023 |
|
| ||||
| Thrombosis | Hydroxycarbamide use | 4 (100) | 24 (26) | 0.006 |
|
| ||||
| Overt Stroke | Age | 38.0±13.3 years | 26.3±10.5 | 0.012 |
| Female gender | 6 (100) | 41 (49) | 0.029 | |
| Hypertension | 3 (50) | 7 (8) | 0.017 | |
Abbreviations: SD=standard deviation; G6PD= glucose-6-phosphate dehydrogenase; Hb=haemoglobin concentration;
Discussion
Adults with the AI haplotype of SCD have stable HbF levels that are 3-times higher than those in African American patients and 1.5-times higher than Saudi Arabs with the Benin HBB haplotype. Nevertheless, the phenotype of their disease is not “benign” (Alsultan, et al 2012a, Alsultan, et al 2011). Table IV provides a summary of previous reports of clinical events in AI haplotype patients and a comparison with other patient groups. Except for the absence of leg ulcers, reduced prevalence of overt stroke and higher rate of persistent splenomegaly, the differences between adult AI haplotype and African haplotype patients were minor. Absence of leg ulcers was also observed in Saudi patients with African origin haplotypes, raising the possibility of the lack of an environmental trigger in Saudi Arabia compared with tropical Jamaica where patients have a higher rate of leg ulcers (Alsultan, et al 2012b, Nolan, et al 2006, Powars, et al 2005, Serjeant 1974). A formal study of mortality in the AI haplotype has not been done so it is not possible to compare survival in AI haplotype patients with other patient groups. In one analysis, the leading cause of death in Saudi patients was acute chest syndrome, followed by sepsis (Al-Suleiman, et al 2005, Al-Suliman, et al 2006). Adult patients with AI haplotype SCD bear some resemblance to patients with HbSC, given their higher hemoglobin levels, less haemolysis and a lower incidence of stroke and leg ulcers. Most children with AI haplotype SCD maintain near normal splenic function compared with African haplotype patients who commonly have early auto-splenectomy; this is probably related to the sustained high HbF in this population (Adekile 2011, Al-Awamy, et al 1984, Al-Jam'a, et al 2000, Alsultan, et al 2012b, Babiker, et al 1985, Mallouh, et al 1984). Persistent splenomegaly and hypersplenism sometimes require splenectomy (Al-Salem 2006, Al-Salem, et al 1996, Mallouh and Salamah 1985). In the first four years of life, the risk of infection was similar in AI SCD patients and normal Saudi controls (el Mouzan, et al 1989). Acute chest syndrome was observed in nearly half of our patients and was recurrent in 15%, slightly higher than previous reports (Padmos, et al 1991a, Pembrey, et al 1980, Perrine, et al 1978). Overt stroke was rare in our patients; however, silent brain infarct has been reported to be higher in adults with AI haplotype (Adekile, et al 2002, Marouf, et al 2003a).
Table IV.
Comparison of sickle cell disease patients with the Arab-Indian haplotype and those with African origin haplotype.
| Variable | SCD with AI haplotype | Saudi SCD (African origin haplotype) (Alsultan, et al 2012b) | African American SCD studies | ||||
|---|---|---|---|---|---|---|---|
| Current study | Perrine, et al (1978) | Pembrey, et al (1980) | Padmos, et al (1991) | Kuwaiti studies | |||
| Number | 104 | 270 | 15 | 33 | 159 | ||
| Average age | 26.9 years | 80% <15 years | 12 years | 22.4 years | 17.8 years | ||
| Male/Female (%) | 45/55 | 48/52 | 44/56 | 51/49 | 48/52 | ||
| Pain | 96% | Rare < 3 years | 13% hospitalized for pain | 36% > three admissions for pain | Rare < 4 years Commonest cause of admission (Akar and Adekile 2008) | 98% | 61% (Platt, et al 1991) |
| Osteonecrosis | 18% | 4.4% between 10-19 years | n/a | 31% (only hip AVN was evaluated) | 25%(children) 50% (adults) (Adekile, et al 2001, Marouf, et al 2003b) | 14% | 10-21% (Milner, et al 1991, Powars, et al 2005) |
| Acute chest syndrome | 47% but only 15% ≥2 episodes | 30% | 26% | 15% | 22% | 38-50% (Castro, et al 1994, Gill, et al 1995, Powars, et al 2005) | |
| Gallstones | 66% | 8% using oral cholecystography | n/a | n/a | 34% | 28% (Powars, et al 2005, Rennels, et al 1984) | |
| Splenic sequestration | 15% | n/a | n/a | 3% | 23% | 10-20% (Gill, et al 1995, Powars, et al 2005) | |
| Priapism | 17% | 0.8% | n/a | 3% | 6% | 13-35% (Fowler, et al 1991, Powars, et al 2005) | |
| Leg ulcers | none | none | n/a | none | none | 10% (Nolan, et al 2006, Powars, et al 2005) | |
| Serious infections | 17% | 4.1% | n/a | 9% | 11.5% | 12% (Powars, et al 2005) | |
| Stroke | 6% overt stroke | 0.9% overt stroke | n/a | n/a | Silent infarct 3.3% (children) Silent infarct 20% (adults) (Adekile, et al 2002, Marouf, et al 2003a) | 7.5% overt stroke | 11% overt stroke (Powars, et al 2005) About 30% silent infarct (DeBaun, et al 2012) |
| Persistent splenomegaly | 71% | n/a | n/a | 53% | 11% | Autosplenectomy in majority (Frempong & Steinberg2001) | |
| HbSS/HbS-β0 | both | HbSS only | HbS-β0 only | both | both | both | both |
| Hb (g/d) | 92 | 95 | 93 | 102 | 105 (Adekile, et al 2007) | 86 | 86 (Lettre, et al 2008) |
| MCV (fl) | 76 | n/a | n/a | 82.4 | 78.9 (Adekile, et al 2007) | 81 | 89.4 (Lettre, et al 2008) |
| Reticulocytes% | 9.1 | 6.2 | 5.8 | 5.9 | 7.2 | ||
| HbF % | 15.6 | 22 | 17.3 | 13.3 | 28.9 <5 years 21.5 >15 years (Adekile, et al 2007) | 11.4 | 6.4 (Lettre, et al 2008) |
| α-thalassaemia | 51% | n/a | n/a | 62% | 42% (Adekile, et al 2007) | 38% | 30% (Steinberg and Embury 1986) |
| G6PD deficiency | 28% | n/a | n/a | 21% | 2.5% | 10% (Steinberg, et al 1988) | |
SCD=sickle cell disease; G6PD= glucose-6-phosphate dehydrogenase; MCV.=mean corpuscular volume; n/a= not reported or not applicable.
Painful episodes during the year prior to commencement on study were not associated with HbF (Figure 3). Previous studies of pain in SCD with African origins of the HbS mutation reported that HbF was associated with a reduction of painful episodes (Steinberg and Sebastiani 2012), and HbF levels were not associated with painful events, acute chest syndrome, or survival in an earlier study of young African Americans with SCD (Powars, et al 1980). Our failure to find the expected relationship between HbF and pain might be due to the short period (1 year) chosen in which to enumerate painful episodes, a relative small patient sample compared with previous studies in African Americans, ascertainment bias and the narrow and Gaussian distribution of HbF in our patients. It might also be that the definitions of painful episodes have changed over time, as has accessibility to medical care. HbF in patients with the AI haplotype is near 30% throughout the first 5-10 years of life and afterwards stabilizes at a mean level of 15-20% (Adekile, et al 2007, Pembrey, et al 1978) (Figure 2). This probably explains the benign course of the disease during early childhood and the change in phenotype in adults (Adekile 2011). With a mean haemolysate HbF of 15-20%, why is the protective effect of HbF not maintained in all adults? DeoxyHbS polymerization is prevented at a concentration of about 9-12 pg HbF/F-cell (Poillon, et al 1993). The number of F-cells containing this polymer-sparing concentration of HbF is the major determinant of the protective potential of HbF (Maier-Redelsperger, et al 1994, Poillon, et al 1993). Individuals with HbS-hereditary persistence of fetal Hb (HPFH) are nearly normal haematologically and clinically. Their HbF is uniformly distributed amongst all their erythrocytes and each of their cells should contain about 10 pg of HbF. This amount of HbF has been calculated to inhibit HbF polymerization at physiological venous and capillary O2 saturations of 40-70% (Maier-Redelsperger, et al 1994). SCD with the AI haplotype, in contrast to HbS-HPFH, has a heterocellular distribution of HbF with a widely variable F-cell proportion, which ranges between 30 and 90% (Ngo, et al 2012, Pembrey, et al 1978). Depending on the distribution of HbF/F-cell, some cells will be protected from HbS polymerization and its consequences, while others will not.
This was not a prospective study and patients were restricted to those who attended clinic or were hospitalized. This might overestimate the prevalence of SCD complications. The patients enrolled represented about 9% of the clinic population in Alhassa where the number of SCD patients is estimated to be about 6000 (Al-Qurashi, et al 2008). Testing for certain complications, such as osteonecrosis, was restricted to symptomatic patients and so the very early stages of this complication might be missed. We also analysed the influence of HbF, measured at time of study admission, on different SCD complications; this analysis might be biased because HbF level could have varied during the period of the complication of interest. Adult patients with SCD patients in the Eastern Province of Saudi Arabia who have high HbF levels appear to have a frequency of complications comparable to other SCD populations, albeit with a higher rate of splenomegaly, rare overt strokes and absence of leg ulcers. In these particular patients, HbF appeared to have limited influence on their phenotype. This is probably a result of inconsistent distribution of protective levels of HbF amongst F-cells. Future studies are needed to study the influence of HbF on various organ functions in this population and on the overall risk of mortality.
Supplementary Material
Acknowledgments
This work was supported by King Abdulaziz City for Science and Technology Grant (KACST ARP-30-367) and National Institutes of Health Grants R01 HL87681 (MHS), RC2 L101212 (MHS), 5T32 HL007501 (PG).
Footnotes
Author contributions: A Alsultan: designed the research study; A Alsultan, Mohamed Alabdulaali, Ahmed Alsuliman, Hazem Ghabbour: performed the research; Paula Griffin and Paola Sebastiani: analysed the data; A Alsultan,Paola Sebastiani, Walid Albuali, Amein Alali, David Chui, Martin Steinberg: wrote and edited the manuscript
References
- Adekile A, Al-Kandari M, Haider M, Rajaa M, D'Souza M, Sukumaran J. Hemoglobin F concentration as a function of age in Kuwaiti sickle cell disease patients. Medical principles and practice : international journal of the Kuwait University, Health Science Centre. 2007;16:286–290. doi: 10.1159/000102151. [DOI] [PubMed] [Google Scholar]
- Adekile AD. Limitations of Hb F as a phenotypic modifier in sickle cell disease: study of Kuwaiti Arab patients. Hemoglobin. 2011;35:607–617. doi: 10.3109/03630269.2011.617230. [DOI] [PubMed] [Google Scholar]
- Adekile AD, Gupta R, Yacoub F, Sinan T, Al-Bloushi M, Haider MZ. Avascular necrosis of the hip in children with sickle cell disease and high Hb F: magnetic resonance imaging findings and influence of alpha-thalassemia trait. Acta haematologica. 2001;105:27–31. doi: 10.1159/000046529. [DOI] [PubMed] [Google Scholar]
- Adekile AD, Yacoub F, Gupta R, Sinan T, Haider MZ, Habeeb Y, Al-Bloushi M, Moosa A. Silent brain infarcts are rare in Kuwaiti children with sickle cell disease and high Hb F. American journal of hematology. 2002;70:228–231. doi: 10.1002/ajh.10143. [DOI] [PubMed] [Google Scholar]
- Akar NA, Adekile A. Ten-year review of hospital admissions among children with sickle cell disease in Kuwait. Medical principles and practice : international journal of the Kuwait University, Health Science Centre. 2008;17:404–408. doi: 10.1159/000141506. [DOI] [PubMed] [Google Scholar]
- Al-Awamy B, Wilson WA, Pearson HA. Splenic function in sickle cell disease in the Eastern Province of Saudi Arabia. The Journal of pediatrics. 1984;104:714–717. doi: 10.1016/s0022-3476(84)80950-6. [DOI] [PubMed] [Google Scholar]
- Al-Jam'a AH, Al-Dabbous IA, Chirala SK, Al-Majid H, Al-Ali J. Splenic function in sickle cell anemia patients in Qatif, Saudi Arabia. American journal of hematology. 2000;63:68–73. doi: 10.1002/(sici)1096-8652(200002)63:2<68::aid-ajh2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Al-Qurashi MM, El-Mouzan MI, Al-Herbish AS, Al-Salloum AA, Al-Omar AA. The prevalence of sickle cell disease in Saudi children and adolescents. A community-based survey. Saudi Med J. 2008;29:1480–1483. [PubMed] [Google Scholar]
- Al-Salem AH. Indications and complications of splenectomy for children with sickle cell disease. J Pediatr Surg. 2006;41:1909–1915. doi: 10.1016/j.jpedsurg.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Al-Salem AH, Qaisaruddin S, Nasserallah Z, al Dabbous I, al Jam'a A. Splenectomy in patients with sickle-cell disease. Am J Surg. 1996;172:254–258. doi: 10.1016/S0002-9610(96)00158-4. [DOI] [PubMed] [Google Scholar]
- Al-Suleiman A, Aziz G, Bagshia M, El Liathi S, Homrany H. Acute chest syndrome in adult sickle cell disease in eastern Saudi Arabia. Annals of Saudi medicine. 2005;25:53–55. doi: 10.5144/0256-4947.2005.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Suliman A, Elsarraf NA, Baqishi M, Homrany H, Bousbiah J, Farouk E. Patterns of mortality in adult sickle cell disease in the Al-Hasa region of Saudi Arabia. Annals of Saudi medicine. 2006;26:487–488. doi: 10.5144/0256-4947.2006.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsultan A, Solovieff N, Aleem A, AlGahtani FH, Al-Shehri A, Osman ME, Kurban K, Bahakim H, Al-Momen AK, Baldwin CT, Chui DH, Steinberg MH. Fetal hemoglobin in sickle cell anemia: Saudi patients from the Southwestern province have similar HBB haplotypes but higher HbF levels than African Americans. American Journal of Hematology. 2011;86:612–614. doi: 10.1002/ajh.22032. [DOI] [PubMed] [Google Scholar]
- Alsultan A, Ngo DA, Farrell JJ, Akinsheye I, Solovieff N, Ghabbour HA, Al-Ali A, Alsuliman A, Al-Baghshi M, Albu-Ali W, Alabdulaali M, Baldwin CT, Farrer LA, Luo H, Melista E, Safaya S, Nwaru M, Jr, Chui DH, Steinberg MH. A functional promoter polymorphism of the delta-globin gene is a specific marker of the Arab-Indian haplotype. American journal of hematology. 2012a;87:824–826. doi: 10.1002/ajh.23239. [DOI] [PubMed] [Google Scholar]
- Alsultan A, Aleem A, Ghabbour H, AlGahtani FH, Al-Shehri A, Osman ME, Kurban K, Alsultan MS, Bahakim H, Al-Momen AM. Sickle cell disease subphenotypes in patients from Southwestern Province of Saudi Arabia. Journal of pediatric hematology/oncology : official journal of the American Society of Pediatric Hematology/Oncology. 2012b;34:79–84. doi: 10.1097/MPH.0b013e3182422844. [DOI] [PubMed] [Google Scholar]
- Babiker MA, el-Hazmi MA, Al-Jobori AM, Obeid H, Bahakim HM. Splenic function in children with sickle cell disease: two different patterns in Saudi Arabia. Scand J Haematol. 1985;35:191–193. doi: 10.1111/j.1600-0609.1985.tb01570.x. [DOI] [PubMed] [Google Scholar]
- Castro O, Brambilla DJ, Thorington B, Reindorf CA, Scott RB, Gillette P, Vera JC, Levy PS. The acute chest syndrome in sickle cell disease: incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood. 1994;84:643–649. [PubMed] [Google Scholar]
- DeBaun MR, Armstrong FD, McKinstry RC, Ware RE, Vichinsky E, Kirkham FJ. Silent cerebral infarcts: a review on a prevalent and progressive cause of neurologic injury in sickle cell anemia. Blood. 2012;119:4587–4596. doi: 10.1182/blood-2011-02-272682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Mouzan MI, al Awamy BH, Absood G. Infections and sickle cell disease in Eastern Saudi Arabian children. Am J Dis Child. 1989;143:205–207. doi: 10.1001/archpedi.1989.02150140099028. [DOI] [PubMed] [Google Scholar]
- Fowler JE, Jr, Koshy M, Strub M, Chinn SK. Priapism associated with the sickle cell hemoglobinopathies: prevalence, natural history and sequelae. The Journal of urology. 1991;145:65–68. doi: 10.1016/s0022-5347(17)38248-4. [DOI] [PubMed] [Google Scholar]
- Frempong KO, Steinberg MH. Clinical Aspects of Sickle Cell Anemia in Adults and Children. In: Steinberg MH, F B, Higgs DR, Nagel RL, editors. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management. Cambridge University Press; Cambridge: 2001. pp. 611–710. [Google Scholar]
- Gill FM, Sleeper LA, Weiner SJ, Brown AK, Bellevue R, Grover R, Pegelow CH, Vichinsky E. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood. 1995;86:776–783. [PubMed] [Google Scholar]
- Lehmann H, Maranjian G, Mourant AE. Distribution of sickle-cell hemoglobin in Saudi Arabia. Nature. 1963;198:492–493. doi: 10.1038/198492b0. [DOI] [PubMed] [Google Scholar]
- Lettre G, Sankaran VG, Bezerra MA, Araujo AS, Uda M, Sanna S, Cao A, Schlessinger D, Costa FF, Hirschhorn JN, Orkin SH. DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11869–11874. doi: 10.1073/pnas.0804799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier-Redelsperger M, Noguchi CT, de Montalembert M, Rodgers GP, Schechter AN, Gourbil A, Blanchard D, Jais JP, Ducrocq R, Peltier JY, et al. Variation in fetal hemoglobin parameters and predicted hemoglobin S polymerization in sickle cell children in the first two years of life: Parisian Prospective Study on Sickle Cell Disease. Blood. 1994;84:3182–3188. [PubMed] [Google Scholar]
- Mallouh A, Burke GM, Salamah M, Ahmad MS. Splenic function in Saudi children with sickle cell disease. Annals of tropical paediatrics. 1984;4:87–91. doi: 10.1080/02724936.1984.11748315. [DOI] [PubMed] [Google Scholar]
- Mallouh AA, Salamah MM. Hypersplenism in homozygous sickle-cell disease in Saudi Arabia. Annals of tropical paediatrics. 1985;5:143–146. doi: 10.1080/02724936.1985.11748380. [DOI] [PubMed] [Google Scholar]
- Marouf R, Gupta R, Haider MZ, Adekile AD. Silent brain infarcts in adult Kuwaiti sickle cell disease patients. American Journal of Hematology. 2003a;73:240–243. doi: 10.1002/ajh.10376. [DOI] [PubMed] [Google Scholar]
- Marouf R, Gupta R, Haider MZ, Al-Wazzan H, Adekile AD. Avascular necrosis of the femoral head in adult Kuwaiti sickle cell disease patients. Acta Haematologica. 2003b;110:11–15. doi: 10.1159/000072406. [DOI] [PubMed] [Google Scholar]
- Milner PF, Kraus AP, Sebes JI, Sleeper LA, Dukes KA, Embury SH, Bellevue R, Koshy M, Moohr JW, Smith J. Sickle cell disease as a cause of osteonecrosis of the femoral head. The New England journal of medicine. 1991;325:1476–1481. doi: 10.1056/NEJM199111213252104. [DOI] [PubMed] [Google Scholar]
- Ngo D, Bae H, Steinberg MH, Sebastiani P, Solovieff N, Baldwin CT, Melista E, Safaya S, Farrer LA, Al-Suliman AM, Albuali WH, Al Bagshi MH, Naserullah Z, Akinsheye I, Gallagher P, Luo HY, Chui DH, Farrell JJ, Al-Ali AK, Alsultan A. Fetal hemoglobin in sickle cell anemia: genetic studies of the Arab-Indian haplotype. Blood cells, molecules & diseases. 2013;51:22–26. doi: 10.1016/j.bcmd.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo DA, Aygun B, Akinsheye I, Hankins JS, Bhan I, Luo HY, Steinberg MH, Chui DH. Fetal haemoglobin levels and haematological characteristics of compound heterozygotes for haemoglobin S and deletional hereditary persistence of fetal haemoglobin. British journal of haematology. 2012;156:259–264. doi: 10.1111/j.1365-2141.2011.08916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan VG, Adewoye A, Baldwin C, Wang L, Ma Q, Wyszynski DF, Farrell JJ, Sebastiani P, Farrer LA, Steinberg MH. Sickle cell leg ulcers: associations with haemolysis and SNPs in Klotho, TEK and genes of the TGF-beta/BMP pathway. British journal of haematology. 2006;133:570–578. doi: 10.1111/j.1365-2141.2006.06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmos MA, Roberts GT, Sackey K, Kulozik A, Bail S, Morris JS, Serjeant BE, Serjeant GR. Two different forms of homozygous sickle cell disease occur in Saudi Arabia. British journal of haematology. 1991;79:93–98. doi: 10.1111/j.1365-2141.1991.tb08013.x. [DOI] [PubMed] [Google Scholar]
- Pembrey ME, Wood WG, Weatherall DJ, Perrine RP. Fetal haemoglobin production and the sickle gene in the oases of Eastern Saudi Arabia. British journal of haematology. 1978;40:415–429. doi: 10.1111/j.1365-2141.1978.tb05813.x. [DOI] [PubMed] [Google Scholar]
- Pembrey ME, Perrine RP, Wood WG, Weatherall DJ. Sickle beta 0 thalassemia in Eastern Saudi Arabia. Am J Hum Genet. 1980;32:26–41. [PMC free article] [PubMed] [Google Scholar]
- Perrine RP, Brown MJ, Clegg JB, Weatherall DJ, May A. Benign sickle-cell anaemia. Lancet. 1972;2:1163–1167. doi: 10.1016/s0140-6736(72)92592-5. [DOI] [PubMed] [Google Scholar]
- Perrine RP, Pembrey ME, John P, Perrine S, Shoup F. Natural history of sickle cell anemia in Saudi Arabs. A study of 270 subjects. Ann Intern Med. 1978;88:1–6. doi: 10.7326/0003-4819-88-1-1. [DOI] [PubMed] [Google Scholar]
- Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, Kinney TR. Pain in sickle cell disease. Rates and risk factors. The New England journal of medicine. 1991;325:11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- Poillon WN, Kim BC, Rodgers GP, Noguchi CT, Schechter AN. Sparing effect of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S at physiologic ligand saturations. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5039–5043. doi: 10.1073/pnas.90.11.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powars DR, Schroeder WA, Weiss JN, Chan LS, Azen SP. Lack of influence of fetal hemoglobin levels or erythrocyte indices on the severity of sickle cell anemia. J Clin Invest. 1980;65:732–740. doi: 10.1172/JCI109720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine. 2005;84:363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- Rennels MB, Dunne MG, Grossman NJ, Schwartz AD. Cholelithiasis in patients with major sickle hemoglobinopathies. Am J Dis Child. 1984;138:66–67. doi: 10.1001/archpedi.1984.02140390054016. [DOI] [PubMed] [Google Scholar]
- Serjeant GR. Leg ulceration in sickle cell anemia. Archives of internal medicine. 1974;133:690–694. [PubMed] [Google Scholar]
- Steinberg MH, Embury SH. Alpha-thalassemia in blacks: genetic and clinical aspects and interactions with the sickle hemoglobin gene. Blood. 1986;68:985–990. [PubMed] [Google Scholar]
- Steinberg MH, Sebastiani P. Genetic modifiers of sickle cell disease. American journal of hematology. 2012 doi: 10.1002/ajh.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MH, West MS, Gallagher D, Mentzer W. Effects of glucose-6-phosphate dehydrogenase deficiency upon sickle cell anemia. Blood. 1988;71:748–752. [PubMed] [Google Scholar]
- Tan AS, Quah TC, Low PS, Chong SS. A rapid and reliable 7-deletion multiplex polymerase chain reaction assay for alpha-thalassemia. Blood. 2001;98:250–251. doi: 10.1182/blood.v98.1.250. [DOI] [PubMed] [Google Scholar]
- Viprakasit V, Green S, Height S, Ayyub H, Higgs DR. Hb H hydrops fetalis syndrome associated with the interaction of two common determinants of alpha thalassaemia (--MED/(alpha)TSaudi(alpha)) British journal of haematology. 2002;117:759–762. doi: 10.1046/j.1365-2141.2002.03427.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.