Summary
Functional testing of dermal papilla (DP) signaling inputs into hair follicle (HF) morphogenesis and regeneration is becoming possible with the advent of new Cre lines. Targeted deletion of the signature genes in early DP precursors has revealed significant signaling redundancy during HF morphogenesis. Furthermore, the DP lineage commitment program can be exploited for generating highly inductive DP cells to be used in HF bioengineering assays.
Novel genetic tools: dermal papilla in the spotlight
The early availability of keratin-specific genetic tools provided epithelial biologists nearly a two-decade head start over those who studied mesenchymal aspects of HF function. Indeed, careful gene ablation and overexpression studies have now brought our understanding of follicular bulge stem cells to a level that rivals that of hematopoetic stem cells. Owing to these studies, the HF now holds the position of a model system for many stem cell investigators.
At the same time, classic tissue recombination and transplantation experiments have revealed critical roles for mesenchymal dermal papilla (DP) in regulating various aspects of HF biology, such as regenerative cycling. Moreover, recent clinical data indicate that human scalp HFs affected by androgenic alopecia contain a largely intact population of bulge stem cells and that the primary defect is in DP signaling, rendering it unable to support long-lasting phases of hair growth. However, it was not until recently that the molecular identity of DP signaling input into hair morphogenesis and growth was revealed. New DP-specific genetic tools now make it possible to study HF signaling networks during development and during adulthood.
The paper by Grisanti et al. (2013b) of this issue is the latest in a series of studies that use specificity of expression of the T-box transcription factor Tbx18 in DP precursor cells in embryonic dermal condensates for genetic labeling, isolation, and genetic targeting of precursor cells during the early stages of HF morphogenesis. Indeed, Tbx18-driven Cre knock-in lines, as well as the Tbx18H2BGFP reporter become active at embryonic day 14.5 coinciding with the early event of dermal condensation during morphogenesis of primary HFs in mice (Clavel et al., 2012; Grisanti et al., 2013a). This specificity of Tbx18Cre for embryonic DP progenitors enables the investigation of their role in signaling networks during primary HF development. Both constitutive and inducible Tbx18Cre can be used for efficient DP-specific gene ablation, such as Sox2, as shown in Clavel et al. (2012), and Enpp2, as shown by Grisanti et al. (2013b).
Because Tbx18 is activated in dermal condensations as they are being specified by placode signals, genetic targeting of dermal progenitors at earlier stages can be achieved with alternative Cre lines, such as Dermo1-Cre (Fu and Hsu, 2013), En1-Cre (Chen et al., 2012), Prx1-Cre (Woo et al., 2012) and HoxB6-CreERT1 (Chen et al., 2012). In contrast, genetic targeting of DPs in fully formed HFs can be achieved with Corin-Cre (Enshell-Seijffers et al., 2010). Taken together, these Cre lines now make it possible to study the signaling regulation of HF development and cycling using a DP centric approach.
DP signature genes: which ones matter the most?
Unique properties of DP cells, most distinctly, their ability to induce formation of new HFs when combined with competent epithelial cells, have long Q2 fascinated hair researchers. Moreover, reconstitution of new HFs from patient-specific inductive DP and competent epithelial cells has long been considered a viable therapeutic strategy for restoring hair growth. Yet, although mouse DP cells often demonstrate impressive inductive abilities in HF reconstitution assays, adult and even fetal human DP cells are less prone to induction. Thus, protocols aimed at increasing the inductive abilities of human adult DP cells have to be developed. An alternative strategy for obtaining highly inductive DP cell populations could potentially be to generate early embryonic-like DP precursors from patient-derived iPS cells or from patient’s adult cells, such as dermal fibroblasts, through their direct reprogramming. For such iPS- and reprogramming-based HF therapeutics to become a useful approach, much work has to be done to understand the basic biology of the highly specialized DP cell lineage commitment program. To this end, newly developed DP-specific cell sorting and Cre-targeting strategies now make it possible to establish functionally relevant DP signature genes and master regulators of the DP lineage commitment program.
DP lineage can best be understood in the context of a stereotypical cell line- age program (Figure 1). Ontogeny of any given cell type starts with the commitment event, when precursor cells choose a new fate following exposure to one or several morphogenetic cues from their microenvironment. This leads to relatively stable gene expression changes mediated by distinct transcription factors, known as lineage master regulators. Commitment to the DP lineage likely occurs following exposure of embryonic skin mesenchyme to Fgf20 produced by the epithelial placode (Huh et al., 2013), whereas cells of early dermal condensations can be viewed as already committed to the DP fate. Following commitment, progenitors undergo lineage maturation, during which they progressively acquire terminally differentiated characteristics. Depending on the cell type, lineage maturation can be accompanied by additional rounds of fate selection between alternative sublineages, such as in case of hematopoietic cells. Lineage maturation also can proceed without further divergent choices, such as in case of skeletal muscle or cartilage. Maturation of committed DP precursors gives rise to DP and dermal sheath cell types of an adult HF (Grisanti et al., 2013a).
Figure 1. Dermal papillae lineage program.
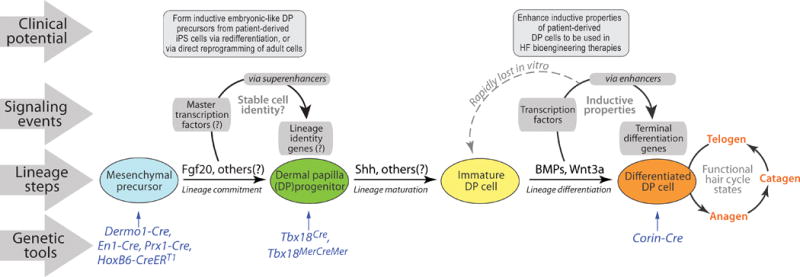
DP cells form during embryonic HF development from initially uncommitted mesenchymal precursors. Formation of the DP lineage likely follows a stereotypical cell lineage program, which starts with the commitment event. While DP lineage commitment is likely associated with stable gene expression changes, signaling characteristics of differentiated DP cells are unstable and can be rapidly lost in culture. Signaling networks of DP lineage can now be studied using several stage-specific Cre lines. Mechanisms of the DP lineage commitment and differentiation can be exploited for developing optimized HF bioengineering therapies for alopecia (listed at the top).
The differentiated state of adult DP cells is dependent on continuous signaling feedback from the surrounding microenvironment of the HF, most distinctly, from epithelial matrix cells during anagen phase and from secondary hair germ and bulge stem cells during telogen phase. As the signaling input differs greatly between anagen and telogen phases of the hair growth cycle, DP transitions between functionally distinct active (anagen) and quiescent (telogen) states (Rendl et al., 2005; Greco et al., 2009). Rendl et al. (2008) and others have shown that DP cells quickly lose their differentiated properties when taken out of the context of their HF microenvironment. Indeed, freshly sorted DP cells lose all but a handful of genes from their original in vivo expression signature after only one passage in vitro. This coincides with the loss of their inductive abilities in HF reconstitution assays (Rendl et al., 2008). Thus, comparative expression profiling of freshly isolated versus cultured DP cells can yield valuable insight into the regulatory aspects of the DP lineage.
The core identity characteristics of any given cell lineage become established during their initial commitment, and they include one or several master transcription factors acting via the so- called superenhancers to activate expression of their downstream target genes. These genes, in turn, endow cells with their baseline lineage identity characteristics stably and highly (Figure 1). Activation of such master transcription factors can be exploited for instructing iPS cells or adult-differentiated cells to undergo specification toward a new desirable cell type via their redifferentiation or direct reprogramming, respectively. Indeed, iPS cell redifferentiation and direct reprogramming protocols have now been established for various cell lineages and, in some cases, such as for insulin-producing pancreatic beta-cells, such protocols have been optimized for high efficiency. Similar approaches for achieving inductive DP precursors are, thus, highly desirable from the clinical point of view.
Transcriptional regulation via superenhancers is inherently stable, and master transcription factors autoregulate themselves via positive feedback loops. Therefore, they should resist downregulation under stress conditions, such as in vitro cell culture. Indeed, although some DP signature transcription factors, such as Alx3, Alx4, and Hey1 are downregulated rapidly in culture, others, such as homeobox protein Sox2 and zinc-finger protein Snai2, remain expressed at high levels (Rendl et al., 2008). On the basis of these and other characteristics, Sox2 was regarded as a putative master regulator of the DP lineage in all but zigzag HFs (in the latter, HF-type Sox2 is distinctly absent from DP). Recently, Clavel et al. (2012) were able to test the functional importance of Sox2 in this context by ablating its expression genetically in early dermal condensates. Surprisingly, despite specific and efficient Sox2 ablation in newly committed DP precursors, HF morphogenesis proceeded largely undisturbed and Sox2-deficient precursors matured into adult, albeit dysfunctional, DP cells. These data reveal either a redundancy in Sox2 function during DP lineage specification or a leading role in this process for an, as of yet unidentified, transcription factor(s).
A recent study by Woo et al. (2012) indicates that other transcription factors, such as Sox18, can potentially compensate for Sox2 function. Sox2, however, has an additional, non- compensated role in the DP cells. Comprehensive analysis of Tbx18Cre; Sox2−/− mutant HFs has revealed its essential role in fine-tuning BMP signa ling activity in the overlying matrix, where it acts to regulate upward migration of hair shaft progenitors (Clavel et al., 2012).
In an effort to evaluate the functional significance of other key DP signature genes, Grisanti et al. (2013b) of this issue report on the Tbx18Cre-mediated ablation of Enpp2 (aka Autotaxin), an enzyme participating in the production of lysophosphatidic acid (LPA) and the highest differentially expressed gene in adult DPs compared with adult dermal fibroblasts. LPA signals via several LPA receptors, which, in HFs, have complex and overlapping distribution patterns spanning both epithelial and mesenchymal compartments. Following careful examination, the authors showed that Enpp2 is dispensable for HF morphogenesis and DP lineage maturation. Although surprising on the surface, this result echoes the effect of Sox2 ablation (Clavel et al., 2012), and it speaks in favor of redundancy at the level of highly enriched DP signaling regulators. Indeed, data by Grisanti et al. (2013b) suggest that Enpp2 deletion can be compensated functionally by LIPH, a phospholipase that can generate LPA via a pathway alternative to Enpp2.
Importantly, studies by Clavel et al. (2012) and Grisanti et al. (2013b) of this issue map out a novel genetic strategy for systematically examining the functional significance of DP signature genes both in its own lineage specification and, more broadly, in HF morphogenesis. Future gene ablation studies using DP-specific Cre lines will undoubtedly reveal the molecular identity of DP lineage master regu- lators. An in-depth understanding of the DP lineage commitment program will help devise protocols for achieving highly inductive patient-specific DP cells and improve prospects for HF bioengineering therapies for alopecia.
Clinical Implications.
Activating DP transcriptional master regulators will enable the production of patient-specific DP cells from induced pluripotent stem (iPS) cells.
Strategies can be devised for using iPS-derived DP cells to bioengineer new human HFs that are resistant to androgenic alopecia.
Candidate regulators of DP inductive properties can now be tested in gene ablation studies using DP-specific Cre lines.
References
- Chen D, Jarrell A, Guo C, Lang R, Atit R. Dermal β-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development. 2012;139:1522–1533. doi: 10.1242/dev.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel C, Grisanti L, Zemla R, Rezza A, Barros R, Sennett R, Mazloom AR, Chung CY, Cai X, Cai CL, Pevny L, Nicolis S, Ma’ayan A, Rendl M. Sox2 in the dermal papilla niche controls hair growth by fine-tuning BMP signaling in differentiating hair shaft progenitors. Dev Cell. 2012;23:981–994. doi: 10.1016/j.devcel.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. Beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Hsu W. Epidermal Wnt Controls Hair Follicle Induction by Orchestrating Dynamic Signaling Crosstalk between the Epidermis and Dermis. J Invest Dermatol. 2013;133:890–898. doi: 10.1038/jid.2012.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanti L, Clavel C, Cai X, Rezza A, Tsai SY, Sennett R, Mumau M, Cai CL, Rendl M. Tbx18 targets dermal condensates for labeling, isolation, and gene ablation during embryonic hair follicle formation. J Invest Dermatol. 2013a;133:344–353. doi: 10.1038/jid.2012.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanti L, Rezza A, Clavel C, Sennett R, Rendl M. Enpp2/Autotaxin in dermal papilla precursors is dispensable for hair follicle morphogenesis. J Invest Dermatol. 2013b doi: 10.1038/jid.2013.140. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh SH, Närhi K, Lindfors PH, Häärä O, Yang L, Ornitz DM, Mikkola ML. Fgf20 governs formation of primary and secondary dermal condensations in developing hair follicles. Genes Dev. 2013;27:450–458. doi: 10.1101/gad.198945.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–557. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo WM, Zhen HH, Oro AE. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes Dev. 2012;26:1235–1246. doi: 10.1101/gad.187401.112. [DOI] [PMC free article] [PubMed] [Google Scholar]


