SUMMARY
Proliferation of the self-renewing epithelium of the gastric corpus occurs almost exclusively in the isthmus of the glands, from where cells migrate bi-directionally towards pit and base. The isthmus is therefore generally viewed as the stem cell zone. We find that the stem cell marker Troy is expressed at the gland base by a small subpopulation of fully differentiated chief cells. By lineage tracing using a Troy-eGFP-ires-CreERT2 allele, single marked chief cells are shown to generate entirely labeled gastric units over periods of months. This phenomenon accelerates upon tissue damage. Troy+ chief cells can be cultured to generate long-lived gastric organoids. Troy marks a specific subset of chief cells that display plasticity in that they are capable of replenishing entire gastric units, essentially serving as quiescent ‘reserve’ stem cells. These observations challenge the notion that stem cell hierarchies represent a 'one-way street'.
INTRODUCTION
The gastric epithelium is a physiologically self-renewing tissue (Mills and Shivdasani, 2011). Anatomically, the stomach can be divided into three parts: the forestomach (in mice) or the cardiac region (in humans), the corpus and the pyloric region. Invaginations from the inner surface called gastric units or glands, penetrate deep into the mucosa and contain distinct cell lineages. In the corpus, the main body of the stomach, gastric units are subdivided further into four distinct zones based on the presence of characteristic cell types. Short-lived (2–3 days) surface mucous cells are the main cell type of the uppermost segment, the pit. Directly below the pit, the isthmus contains immature, fast-dividing cells. Below this, the neck region contains mucous neck cells that are thought to trans-differentiate into chief cells in a period of weeks (Goldenring et al., 2011; Mills and Shivdasani, 2011). Chief cells populate the base and produce digestive enzymes. Scattered throughout all regions are acid-producing parietal cells and rare, hormone-secreting enteroendocrine cells. Chief and parietal cells are long-lived, with an estimated turnover rate of months (Karam and Leblond, 1993a).
Lineage-tracing studies using chemical mutagenesis (Bjerknes and Cheng, 2002) or genetic tracing from the Sox2 locus (Arnold et al., 2011) have demonstrated the existence of multipotent stem cells in the epithelium. As Sox2 positive (Sox2+) cells are scattered throughout the isthmus as well as in lower parts of the gastric unit, it is not clear if all or only a subset of the Sox2+ cells can induce stem cell-like lineage tracing events. Additional markers have been proposed (but not proven by definitive experiments such as e.g. lineage tracing) for gastric stem cells (Mills and Shivdasani, 2011; Qiao and Gumucio, 2011).
We have recently shown that Lgr5 marks adult stem cells in the pyloric region of the stomach (Barker et al., 2010). Lgr5+ stem cells express a Wnt target gene program, are located at the bottom of pyloric glands and are capable of long-term renewal of the epithelium. A second pyloric stem cell has been revealed by lineage tracing using a Villin promoter-driven Cre transgene, which identifies a quiescent stem cell of unknown identity that only becomes apparent upon Interferon-γ stimulation (Qiao et al., 2007). Neither of these two studies identified stem cells in the much larger gastric corpus. Based on the predominant location of proliferative cells in the isthmus of the corpus units, it is generally believed that the isthmus represents the stem cell zone of the corpus epithelium (Karam and Leblond, 1993a).
RESULTS
Identification of Troy as a stem cell marker in multiple adult tissues
Following the identification of Lgr5 as a marker of adult stem cell populations in small intestine and colon, we have established transcriptional profiles of Lgr5+ stem cells (Munoz et al., 2012; van der Flier et al., 2009). One of the genes that closely followed the expression pattern of Lgr5 in intestinal crypts was Troy (encoded by Tnfrsf19). Troy potentially functions as a receptor for lymphotoxin A (Hashimoto et al., 2008). It is highly homologous to two other Tnfrsf members, Xedar and Edar. Troy knock-out mice are viable and fertile without an obvious phenotype (Shao et al., 2005). A recent study has confirmed that Troy marks intestinal stem cells (Fafilek et al., 2012). Interestingly, Troy expression does not correlate with Lgr5 expression in non-intestinal Lgr5+ stem cell populations (Barker et al., 2010; Jaks et al., 2008). As Troy may mark novel Lgr5-independent sets of adult stem cells, we generated a Troy-eGFP-ires-CreERT2 knock-in mouse line (Troy-ki), in which eGFP and CreERT2 are under the control of endogenous Troy-regulatory sequences (Fig. S1A).
Expression of eGFP occurred in crypt base columnar cells, the Lgr5-positive stem cells of small intestine (Fig. S1B). In vivo lineage tracing performed in Troy-ki mice crossed with the R26R-LacZ Cre reporter strain resulted in typical 'ribbons', confirming recently published data (Fafilek et al., 2012) (Fig. S1C). As expected, lineage tracing was not observed in Lgr5+ stem cell compartments that were Troy negative, i.e. in the colon or gastric pylorus. However, tracing events occurred in the gastric corpus, kidney, liver, lung and brain. For here, we focused on the gastric corpus.
Troy is expressed specifically at the base of gastric corpus units
Troy-eGFP expression was readily detectable at the base of each gastric corpus unit (Fig. 1A,B), faithfully recapitulating the endogenous expression of Troy. Single molecule in situ hybridization (Itzkovitz et al., 2012) detected Troy mRNA message in chief and parietal cells at glands bases, whereas cells of the same types, yet located higher up towards the neck region, were Troy-negative (Fig. S1D). Of note, the muscle layer of the stomach also expressed Troy (Fig. 1B, white arrow). Double-immunofluorescent stainings confirmed the expression of Troy-eGFP in chief and parietal cells at the gland base. Troy-eGFP+ cells co-labeled either with H+K+-ATPase, a marker for parietal cells, or with gastric intrinsic factor (Gif), a marker for chief cells in mice (Fig. 1C,D), whereas the third cell type present at the bottom of corpus glands, the enteroendocrine cell, was Troy-negative (Fig. 1E).
Fig. 1. Troy is expressed in chief and parietal cells at the base of corpus glands.
(A) Confocal image showing Troy-eGFP expression at the base of corpus glands in a Troy-ki mouse. Projection of six 1µm spaced z-stacks. (B) Troy-eGFP is expressed at gland bottoms throughout the corpus. Black arrows point to the base of the epithelial lining, white arrows to the muscle layer. (C–E) Confocal microscopy reveals that Troy-eGFP+ cells are co-expressing either the parietal cell marker H+K+-ATPase (C) or the chief cell marker gastric intrinsic factor (D). Basal enteroendocrine cells marked by chromogranin A are Troy-eGFP− (E). (F) Electron microscopy of cryo immunogold labeled Troy-eGFP+ cells. 15 nm Gold label corresponding to eGFP, visible as black dots. Both chief and parietal cells at the gland base express eGFP and positive cells possess characteristics of maturation specific to that lineage. See also Fig. S1.
Next, electron microscopy was employed to resolve the ultrastructure of Troy+ cells. Cryo-immunogold labeling detected the eGFP marker in both chief and parietal cells at the gland base (Fig. 1F). Quantification showed an average of 3.9 and 3.5 eGFP-gold particles/1 µm2 in chief cells and parietal cells, respectively. No eGFP-gold label was detected in the same cell types higher up in the gastric unit or in enteroendocrine cells at the gland bottom (Fig. S1E,F). The marked cells showed characteristics of mature chief and parietal cells, i.e. extending basal rER cisternae and light homogeneous secretory granules in chief cells and a central nucleus surrounded by the intracellular canaliculus and mitochondria-filled cytoplasm in parietal cells (Karam, 1993; Karam and Leblond, 1993b).
Lineage tracing reveals that Troy+ cells act as multi-potent stem cells of the gastric corpus
Lineage tracing initiated in 8 week-old mice induced single LacZ+ cells at the bottoms of corpus glands one day post-induction (p.i.) (Fig. 2A). A slow clonal expansion over time was apparent. Indeed, historic labeling experiments with tritiated thymidine (3H-TdR) have indicated that proliferating cells with chief cell characteristics exist at the gland bottom (Chen and Withers, 1975; Willems et al., 1972). We performed triple-immunofluorescent stainings for Ki67 and Pepsinogen C together with a membrane marker, and confirmed the existence of rare, dividing chief cells at the bottom of glands (Fig. S2A). On average, 3.2±0.78% of corpus gland sections contained a proliferative cell at the gland base (Fig. S2B). Thus, despite the fact that the isthmus appears to be the principle zone of proliferation, a second zone with the capability to proliferate exists at the bottom of glands.
Fig. 2. Troy+ cells generate all lineages.
(A) Expression of the Rosa-LacZ reporter gene in Troy+ cells one day p.i. and followed for 1, 3, 6 month and 1.5 years p.i. (B) Whole-mount LacZ staining of Troy-ki stomach 6 month p.i. Lineage tracing is evident in the corpus; pylorus is negative. (C) The position of LacZ+ cells quantified for 100 tracing clones at 1 day, 1 month and 3 month p.i.. (D) Numbers of LacZ+ cells in 100 tracing clones counted at 1 day, 1 month, 3 month and 1.5 years p.i. as percentage of clones with <3, 3–15 and >15 cells. (E–H) Double/Triple immuno-fluorescence stainings for corpus lineage markers on Troy-ki;Rosa-YFP mice: Chief cells (Pepsinogen C, E), mucus neck cells (GSII, F), parietal cells (VEGFB, G) and isthmus cells (Ki67, H). See also Fig. S2 and S3.
Eventually, lineage tracing yielded clones of LacZ+ cells spanning the entire length of a gland. This was rarely observed at 4 weeks of tracing, yet became more frequent from 3 month onward (Fig. 2A,B). Of note, no tracing was detected in the pyloric region, consistent with the absence of Troy-eGFP+ cells (Fig. 2B). Entirely traced gastric units persisted at least up to 1.5 years, the latest time point examined (Fig. 2A). Besides the expanding clones, a fraction of cells remained as single LacZ+ cells at the bottom of glands (Fig. S3A, arrows). Staining for the parietal cell marker H+K+-ATPase on tissue sections from six-month tracing experiments revealed that these non-expanding single LacZ+ cells were parietal cells (Fig. S3B), while all early expanding clones contained chief cells (Fig. S3C). Control non-induced Troy-ki/R26R-LacZ did not show LacZ positive cells at any time point (Fig. S3D). Furthermore, induction of lineage tracing according to our standard protocol did not result in parietal cell loss, unlike what has been reported in other models (Huh et al., 2012) (Fig. S3E).
Quantification of clone size in tracing clones containing only parietal cells and clones containing chief cells (only chief cells or mixed chief/parietal clones) was performed. Pure parietal cell clones did not grow; the average clone size after 1 week and 3 month was 1.1 and 1.3±0.1 cells, respectively. On the other hand, clones containing chief cells increased in size from an average of 1.9±0.1 to 3.6±0.8 cells/clone at 3 month. This suggested that growth of clones originated from the chief cell population. The position of LacZ+ cells was quantified for 100 glands over a 3-month time course (Fig. 2C). At day one p.i., we never detected a LacZ+ cell above position 10 from the bottom (this position is still >10 cell positions below the lower isthmus region). Progressively, the number of LacZ+ cells higher up in the gland increased. The clone size was quantified in 100 glands over a 1.5 year time course p.i. An increase in clone size could be observed (Fig. 2D). The rate of expansion was relatively slow, with an average doubling time of clone size of around 50 days. This fitted well with the observation that chief cells double in two months, as analyzed by grain count analysis after 3H-TdR administration (Willems et al., 1972).
Within LacZ+ ribbons that had grown beyond the isthmus, all differentiated cell types of the gastric corpus were detected, i.e. chief cells, neck cells, parietal cells, proliferating isthmus cells, pit cells and enteroendocrine cells (Fig. 2E–H, S3F). We concluded that a Troy+ cell with the capacity to generate all stomach epithelial cell types exists at the bottom of glands in the adult gastric corpus.
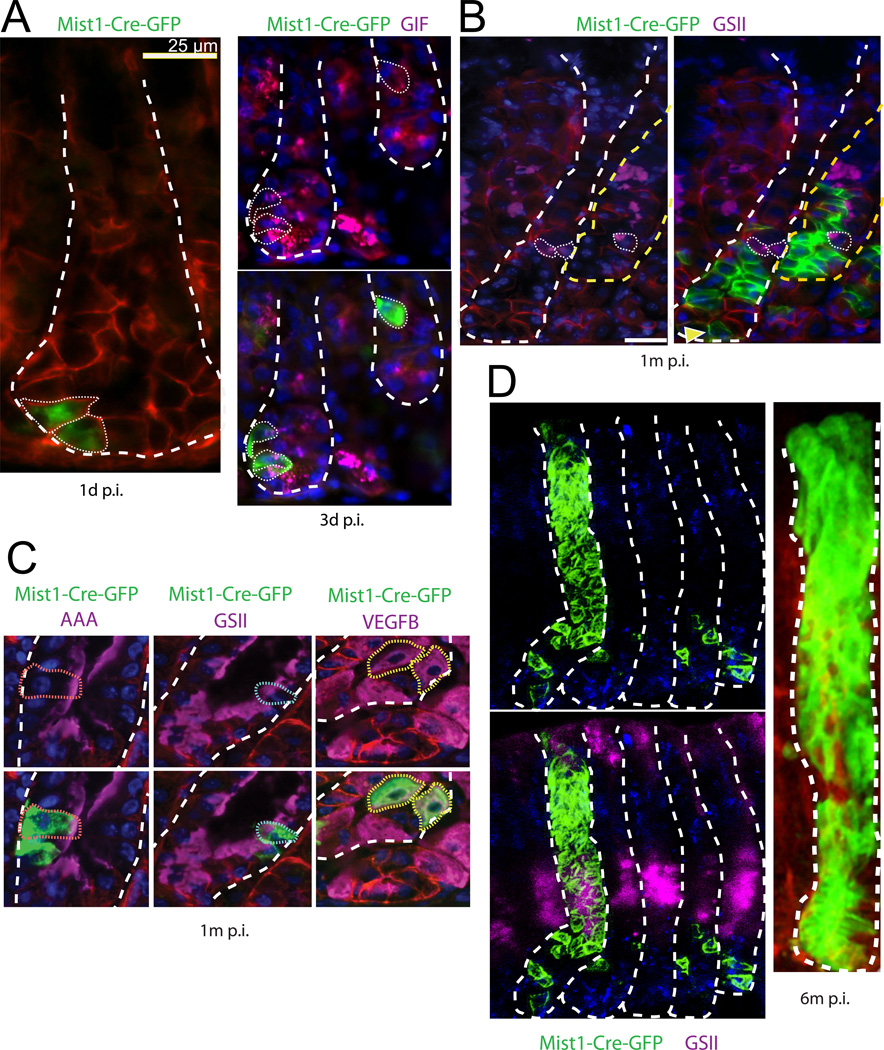
We repeated the tracing experiments using the Cre knock-in line Mist1-CreERT2, which is expressed only in mature chief cells (Nam et al., 2010; Shi et al., 2009). These mice were crossed with the Rosa-mTmG reporter line (Fig. 3). Induction of Cre activity led to the labeling of 1–3 GFP+ chief cells in the gland base (Fig. 3A). One month later, rare tracing events were observed (Fig. 3B). Traced clones consisted mainly of chief cells and were located at gland bottoms, typically containing at least one cell located at the very base, overlapping with the Troy expression domain (Fig. 3B, arrow). Already within these early tracing units, all principal differentiated cell types of the gastric epithelium were detected (Fig. 3C). Lineage tracing proceeded as observed for the Troy locus. At 6 month, tracing units spanning entire corpus glands were readily detectable (Fig. 3D).
Fig. 3. Mist1+ cells generate all lineages.
(A) One and three days following Mist1-CreERT2 induction (p.i.), typical corpus gastric units show 1–3 Rosa-mTmG reporter positive epithelial cells, which label with the chief cell marker gastric instrinsic factor (GIF). (B) One month p.i., the vast majority of Mist1 lineage traced cells (green) are basal, GSII (purple) negative chief cells. (C) Occasionally, at 1 month p.i. Mist1+ cells generate AAA positive pit cells, GSII positive mucus neck cell and VEGFB positive parietal cells. (D) At 6 months p.i., entirely marked corpus units can be detected, encompassing the GSII-marked mucous neck cells (staining of luminal cells is background), the isthmus and the pit region. Units are visualized as Z-stack reconstructions from thick tissue sections using Zeiss ApoTome (left) and multiphoton (right) technology.
Troy+ chief cells express markers of chief cells and Wnt-driven stem cells
We FACS-sorted Troy+ chief cells (described below; see Fig 6A) to analyze their transcriptional program. Troy+ chief cells comprised around 0.9%, while Troy+ parietal cells accounted for 0.5% of all epithelial corpus gland cells. Three independent sorts were performed and mRNA analyzed on microarrays. On average, Troy mRNA was found to be 7.3-fold enriched in sorted GFP+ chief cells compared to whole corpus glands, further validating the recombinant Troy allele.
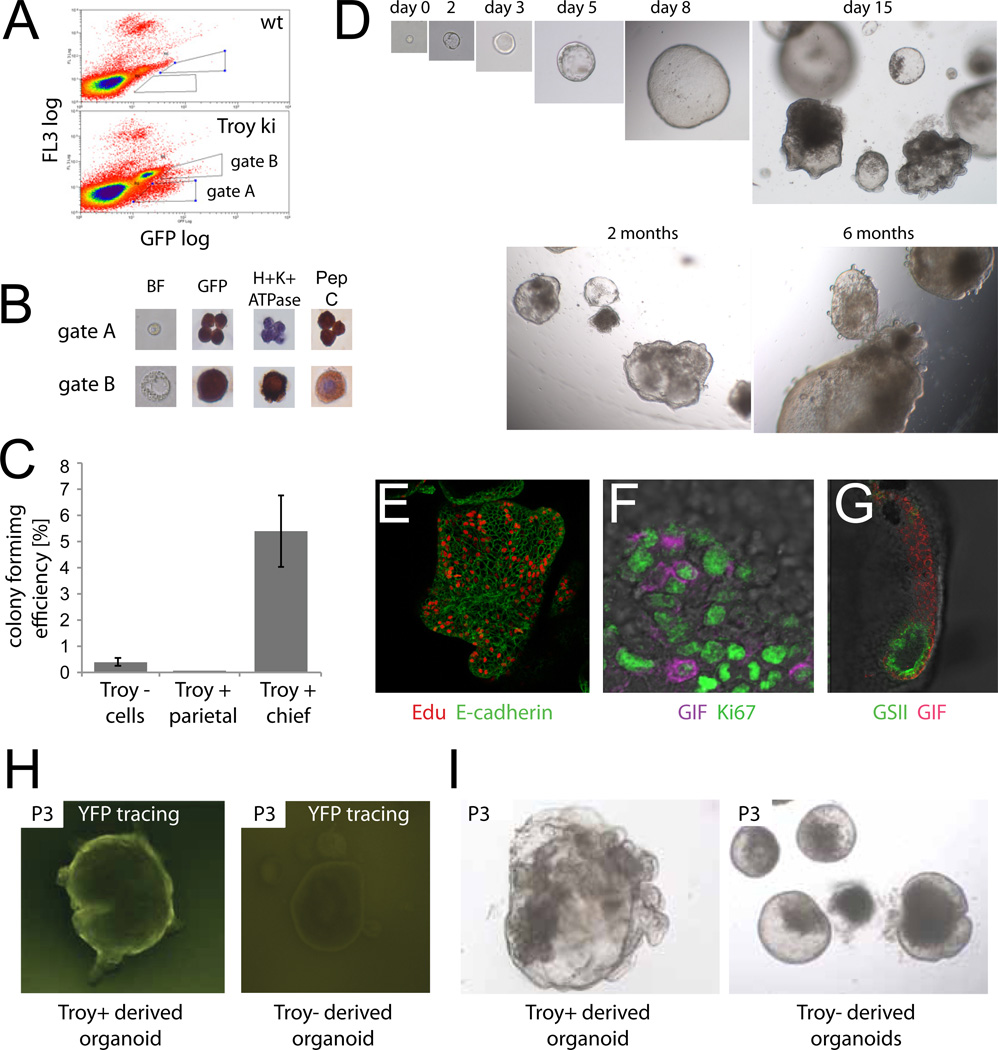
Fig. 6. Single Troy+ cells generate gastric organoids in vitro.
(A) Two different populations of Troy-eGFP+ cells can be distinguished by FACS (gate A and gate B). (B) Sorted 'gate A' Troy-eGFP+ cells stain with chief cell marker pepsinogen C,. Gate B (large) Troy-eGFP+ cells express parietal cell marker H+K+-ATPase. (C) Colony formation efficiency of Troy+ chief and parietal cells as well as Troy negative cells. The number of organoids was counted at day 7 after seeding. Data are represented as mean ± SEM of 180 seeded wells. (D) Representative example of a single sorted and cultured Troy+ chief cell. Organoids where passaged weekly and grown for >6 months. (E) Organoids are highly proliferative. Edu: proliferative cells; cell borders marked by E-cadherin. (F) Dividing (Ki67 positive) chief cells (gastric intrinsic factor (GIF) positive) occur frequently. (G) Mucus neck cells (GSII positive) cells form a coherent distinct domain. (H) Organoids from single sorted Troy-GFP+ and Troy-GFP− cells from Troy-ki/Rosa-YFP mice were induced by Tamoxifen during the first 16h of culture. Troy-GFP+ derived organoids were homogenously YFP positive, while Troy-GFP− derived organoids were YFP negative. (I) Troy-GFP+ consistently showed a more complex growing behavior then Troy-GFP− derived organoids. See also Fig. S5.
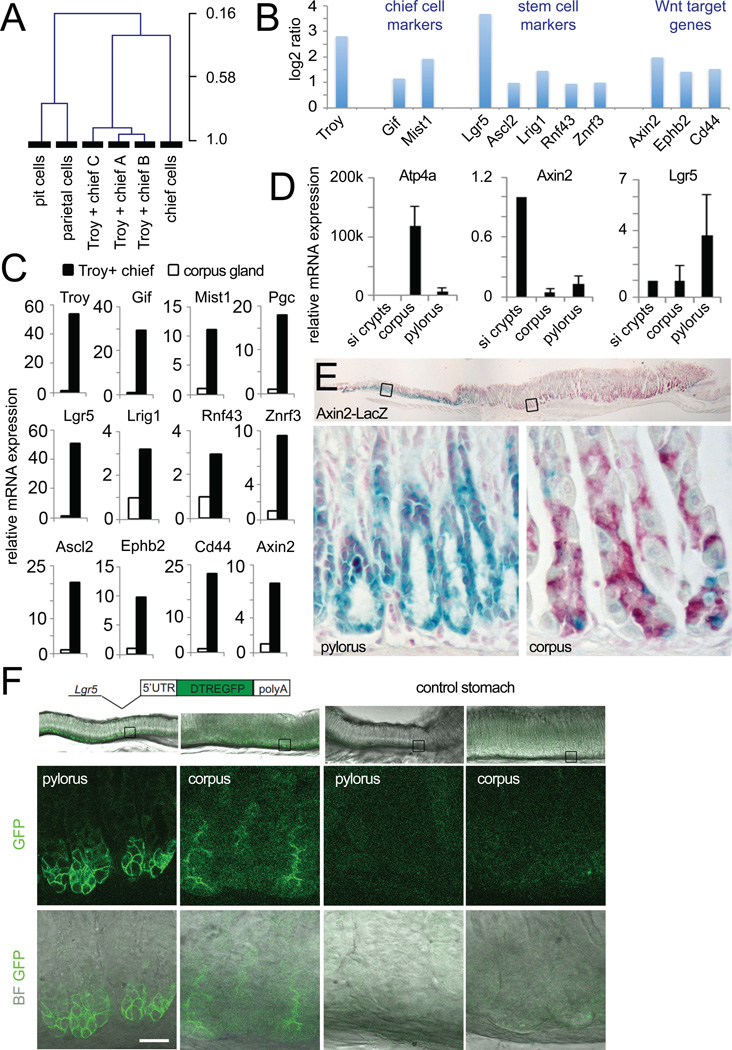
We first compared our arrays by unsupervised hierarchical clustering to arrays representing the three main lineages of the stomach (pit, parietal and chief cells) (Ramsey et al., 2007). The Troy+ arrays clustered together with the chief cell array, while the pit and parietal cell arrays separated in a different tree (Fig. 4A). The overall chief cell signature was therefore maintained in the Troy+ subpopulation. Troy+ chief cells showed enrichment for the chief cell markers Gif and Mist1 (Fig. 4B). We also detected enrichment in genes previously detected in small intestinal or pyloric stem cells, i.e. Lgr5, Ascl2, Lrig1 and Rnf43/Znrf3 (Barker et al., 2007; Koo et al., 2012; van der Flier et al., 2009; Wong et al., 2012). In addition to the Wnt target genes Lgr5, Ascl2 and Rnf43/Znrf3, several other well-characterized Wnt target genes were expressed, i.e. Axin2, EphB2 and Cd44 (Van der Flier et al., 2007) (Fig. 4B). Overall, 113 genes were found to be more than 2-fold enriched in Troy+ chief cells compared to whole corpus glands (Table S1).
Fig. 4. Transcriptional profile of Troy+ chief cells.
(A) Unsupervised hierarchical clustering joins the Troy+ chief cell arrays with a chief cell array prepared from laser-captured chief cells, while pit and parietal cell arrays cluster in a separate tree (data taken from Gene Expression Omnibus dataset GSE5018 (Ramsey et al., 2007). (B) Log2 ratio of selected genes comparing the Troy+ chief cell arrays to arrays from whole corpus glands. Troy+ chief cells show enrichment of marker genes for chief, digestive tract stem cells and Wnt target genes. (C) qPCRs performed on a separately sorted set of Troy+ chief cells and compared to corpus glands (set to 1) confirms enrichment of all marker genes depicted in B in Troy+ chief cells. Data are represented as mean +/− SEM of 3 qPCRs. (D) Comparison of the expression level of the Wnt target gene Axin2 and the stem cell marker Lgr5 between small intestine, gastric corpus and pylorus. Data are represented as mean +/− SEM of 3 qPCRs. (E) LacZ staining of the Axin2-LacZ reporter mouse documents expression of Axin2 in a few gland bottom cells in the gastric corpus (nuclear red counter stain). (F) Endogenous eGFP expression in the Lgr5-DTR:eGFP reporter mouse visualizes Lgr5 expression at the bottom of gastric corpus glands. See also Fig. S4 and Table S1.
The detection of active Wnt signaling in the adult gastric corpus was surprising, as this pathway has until now only been associated with the pyloric region (Mills and Shivdasani, 2011). The enrichment of the Wnt signature and intestinal stem cell marker genes in Troy+ chief cells was first confirmed by qPCR (Fig. 4C). Next, we compared the levels of Wnt activity between corpus glands, pylorus and small intestine by performing qPCR of Axin2 (Fig. 4D). The overall level of Wnt activity was highest in the small intestine, 10-fold lower in the pylorus, and lowest in corpus glands. The intestinal and pyloric stem cell marker Lgr5 was detected at similar levels in small intestine and corpus, while being highest in the pyloric region. Axin2-LacZ mice (Lustig et al., 2002) showed strong LacZ positivity at the base of pyloric glands. In addition, these mice revealed Axin2-positive cells in the corpus (Fig. 4E). As the Lgr5-eGFP-ires-CreERT2 mouse (Barker et al., 2007) did not show Lgr5 expression in the corpus region, contrary to our array and qPCR data, we examined expression in an independent Lgr5 reporter, the Lgr5-DTR:eGFP mouse (Tian et al., 2011). In this line, Lgr5 expression was readily detectable at the base of corpus glands (Fig. 4F).
We used gene set enrichment analysis (GSEA) to statistically test if Troy+ chief cells express only a few marker genes (Fig. 4B,C) or -more broadly- the signature of intestinal/pyloric stem cells, chief cells and of the Wnt pathway. GSEA revealed significant enrichment of all four gene sets in Troy+ chief cells (Fig. S4A–D). Gene sets for parietal and pit cells were negatively correlated with Troy+ chief cells (Fig. S4E,F). In summary, the transcriptional program of Troy+ chief cells combines chief cell-specific genes with genes previously described as Wnt-dependent stem cell genes.
Single Troy+ chief cells can form long-lived organoids that differentiate towards mucus neck and pit cells
We have previously established a long-term culture system that allows unlimited expansion from single Lgr5+ pyloric stem cells (Barker et al., 2010). To test if similar epithelial organoid cultures can be established from the corpus epithelium, we attempted to culture freshly isolated, entire corpus units. Within a few hours after seeding, the units disaggregated. Subsequently, rare cells typically located at the bottom of the gland units started to proliferate to form cystic structures (Fig. 5A). To investigate if the cultures were derived from Troy+ cells, we induced tracing in vivo in Troy-ki crossed with Rosa-YFP Cre reporter mice 3 days before the isolation of glands. We then followed the expression of YFP in the developing organoids in vitro. Initially, YFP and GFP signals overlapped (Fig. 5B). In the course of a week, the YFP-tracing clones continuously expanded, contributing significantly to the cell mass of growing organoids (Fig. 5C).
Fig. 5. Lineage tracing from in vivo to in vitro reveals Troy+ cells as the origin of corpus organoids.
(A) Freshly isolated corpus unit 4h after seeding. The unit already starts to degenerate. Note the small cystic structure at the bottom of the gland (arrow). (B) Tracing was induced in Troy-ki/Rosa-YFP reporter mice three days before isolation of corpus glands. Two days after seeding, GFP and YFP signal still overlap, indicating that the tracing clone was still restricted to the Troy+ population. (C) One week after seeding, the YFP clones had expanded and occupied large parts of the organoid.
We next tested if single Troy+ cells isolated from gastric units of the corpus were also capable of generating organoids. Two populations of Troy+ cells, different in scatter properties, were observed using FACS (Fig. 6A). Stainings for lineage markers on sorted cells showed that the 'small cell'-population (gate A) consisted of chief cells, while the population containing larger cells (gate B) represented parietal cells (Fig. 6B). Troy+ chief cells consistently grew out into organoids (colony forming efficiency 5.4%) (Fig. 6C). We followed a single sorted chief cell over a period of 6 month, by weekly passaging at a 1:6 ratio (Fig. 6D). No change in growth behavior was apparent over the culture period. Similarly, single sorted Mist1+ chief cells could initiate organoid growth (Fig. S5A,B). In contrast, Troy+ parietal cells died within 1–2 days of culture (Fig. 6C). Immunohistochemistry demonstrated a high proliferative activity of Troy+ derived organoids (Fig. 6E). Double stainings revealed the presence of proliferative chief cells (Fig. 6F). Besides chief cells, we noted a distinct population of cells expressing the epitope for GSII, a marker for mucus neck cells (Fig. 6G). A similar expression pattern of mucous neck, chief and proliferation markers has been described in a 2D chief cell-culture (Tashima et al., 2009).
A small proportion of Troy− cells (0.4%) was also able to generate corpus organoids (Fig. 6C). To exclude contamination of Troy− by Troy+ cells we examined Troy− derived organoids from Troy-ki/Rosa-YFP mice (Fig. 6H). FACS isolated single Troy− and Troy+ cells were induced in vitro with tamoxifen right after initiation of culture. No YFP was detected in Troy− derived organoids, while YFP was expressed throughout Troy+ derived organoids. Phenotypically, Troy− derived organoids showed a more cystic growth behavior with fewer buddings during the first four passages (Fig. 6I). Troy mRNA expression was hardly detectable in Troy− derived organoids (Fig. S5C). During subsequent passages, growth characteristics of Troy− and Troy+ derived organoids became indistinguishable. Of note, only 2/3 of initially growing Troy− derived organoids could be propagated for more than 3 passages, while all Troy+ derived organoids grew without constraints.
To further test the differentiation capacity of the Troy+ derived organoids, we removed several mitogenic growth factors (Fgf10, Noggin and Wnt3a) from the culture medium (termed ERG medium). Microarraying of organoids derived from a single Troy+ chief cell grown in normal (termed ENRGFW) medium revealed the expression of markers of intestinal and pyloric stem cells (i.e. Lgr5, Ascl2, Rnf43 and Troy), chief cells (i.e. Mist1, Gif and Pgc) as well as markers for proliferative cells (i.e. Ccnb2 and Ki67) (Fig. S5D,F) (Barker et al., 2007; Koo et al., 2012; van der Flier et al., 2009). Upon withdrawal of the mitogenic factors, a profound upregulation of markers for pit cells (i.e. Muc5ac and Gkn1) was detected (Fig. S5E,G), indicating that Troy+ chief cells have the capability to generate pit cells, the second mucus-secreting cell lineage in the gastric corpus. We did not detect expression of markers for parietal or enteroendocrine lineages.
Troy+ stem cells are activated by depletion of the proliferating isthmus compartment
Compared to the rate of tracing from Lgr5+ cells in the pylorus, tracing from the Troy+ cells followed much slower kinetics. Although occasional tracings spanning entire gastric units could be found within one month p.i., most tracings progressed slowly (Fig. 2A and S3C). Proliferating chief cells were rare in the bottom part of glands (Fig. S2A,B), whereas the isthmus region was constantly cycling (Fig. 7A). Troy+ stem cells therefore appeared largely dispensable for physiological renewal of the corpus epithelium, rather acting like a ‘reserve’ stem cell population.
Fig. 7. Troy+ chief/stem cells activated by depletion of cycling isthmus cells.
(A–F) 5-FU treatment ablates proliferating cells in the isthmus. Control (A, B, C) and 5-FU treated (D, E, F) mice analyzed for Ki-67 (A, D, for proliferating cells), GFP (B, E, for Troy+ cells), and cleaved caspase 3 (C, F, for apoptotic cells). 5-FU induces apoptosis of cycling isthmus cells. (G–H) Accelerated expansion of Troy initiated lineage tracing upon tissue damage. Control (G) and 5-FU treated (H) mice were analyzed 1 month p.i.. Representative examples of Troy tracings (i, ii) and whole mount pictures from the gastric lumen (iii). Circles indicate LacZ+ glands reaching the lumen. (I) The number of tracings that reach the lumen was counted one month p.i. in 5-FU treated vs. untreated mice and shows a 6-fold increase (p<0.0001, t-test). Data are represented as mean +/− SEM of ten sections. (J) The number of LacZ+ cells in 100 tracing clones counted 1 month after 5-FU treatment. Percentage of clones with one, two or more than two cells is represented. (K) Time scheme of 5-FU experiment. Troy+ cells were labeled on day 0 by tamoxifen induction. Proliferative cells were subsequently ablated by 5-FU treatment three days later and tissues analyzed at 1 month p.i.
To test this hypothesis, we selectively killed the proliferative cells in the gastric corpus using 5-Fluoruracil (5-FU). This approach has been successfully employed in the bone marrow (Lerner and Harrison, 1990). We induced tracing by tamoxifen administration followed by injection of a single dose of 5-FU three days later in the treatment (but not the control) group (Fig. 7K). A single dose of 150mg/kg was enough to completely abolish proliferation in the corpus epithelium (Fig. 7D) two days post 5-FU injection, whereas Troy+ cells, consistent with their slow-cycling nature, appeared unaffected (Fig. 7B,E). We did not detect any significant change in the composition of the other lineages (Fig. S3E). Apoptosis was mainly observed in the isthmus region (Fig. 7C,F).
The first sign of a contribution of Troy+ chief cells to regeneration after 5-FU was seen after 7 days. The percentage of glands with proliferating, Ki67 positive cells at the gland bottom increased 3 fold from 3.2±0.8% to 10.5±0.9% (Fig. S2B). Cell cycle dynamics at the gland bottom were further analyzed using a double thymidine-analogue label-retention experiment. Administration of BrdU (2 weeks of labeling followed by a 3-week chase) and EdU (3× within 6h before sacrifice) resulted in BrdU retaining cells at gland bottoms (Fig. S2C). These cells could be induced to proliferate again upon 5-FU damage, resulting in BrdU;Edu double positive cells (Fig. S2C, right panel). Dividing gland bottom cells therefore can re-enter the cell cycle.
Four weeks after 5-FU treatment, accelerated expansion of LacZ+ clones was evident (Fig. 7G–J). The number of tracing events that reached the gastric lumen increased 6-fold after 5-FU treatment, as compared to the untreated controls (Fig. 7Hiii,I). Quantification of the size of tracing units clearly showed a shift towards larger units in 5-FU treated mice (Fig. 7J).
DISCUSSION
In this study, we assess Troy as a marker of cells that contribute to tissue renewal in the gastric corpus. We find that Troy is expressed by a small subset of chief cells and parietal cells located at the gland base. Under steady state conditions, these cells phenotypically fulfill all requirements of differentiated cells. It was recognized 40 years ago that rare cells with chief cell characteristics and located at the bottom of corpus glands can incorporate radiolabeled thymidine (Chen and Withers, 1975; Willems et al., 1972). Indeed, when Troy+ chief cells are cultured in vitro, they vigorously proliferate, while initially maintaining a chief cell-specific gene expression profile. Thus, albeit fully mature, Troy+ chief cells have the capacity to undergo cell division. This phenomenon is not without precedent. Mature hepatocytes, for instance, are also capable of re-entering the cell cycle upon partial hepatectomy and resume cell division (Fausto, 2000; Michalopoulos and DeFrances, 1997). Adult Schwann cells have recently been shown to possess a degree of plasticity. When infected with leprosy bacilli, differentiated Schwann cells are induced to convert towards a mesenchymal progenitor/stem-like phenotype (Masaki et al., 2013).
Troy+ chief cells eventually produce all epithelial lineages present in the corpus in vivo and can generate stomach organoid cultures that can be differentiated towards the mucus-producing cell lineages of the neck and pit in vitro. Their slowly cycling nature as well as their potential to be activated upon selective killing of the highly proliferative isthmus cells is reminiscent of quiescent/‘reserve’ tissue stem cells (Li and Clevers, 2010). Troy+ chief cells are unique amongst chief cells in expressing a large number of Wnt target genes. This implies that a source of Wnt is located near the gland bottom, much like in the pylorus, small intestine and colon. It will be interesting in the future to dissect the factors that build the niche for Troy+ chief cells. Besides the underlying mesenchyme, a potential niche cell candidate are the Troy+ parietal cells intermingled between the Troy+ chief cells at the gland bottom.
How do these Troy+ stem cells compare to previously identified gastric stem or progenitor cells in the corpus? In contrast with Troy+ cells, Sox2+ stem cells are scattered within and below the isthmus and do not express known differentiation markers (Arnold et al., 2011). In our hands, almost all epithelial cells within the normal stomach as well as in the organoids express Sox2 (Fig. S6). We also do see clear expression of Sox2 in Troy+ chief cells in our array data with no difference compared to whole corpus glands (p=0.41). Within the isthmus, Tff2-expressing cells have been demonstrated to represent progenitors of chief and parietal cells, but not of mucous-secreting pit cells and enteroendocrine cells (Quante et al., 2010). Besides the immediate labeling of parietal cells, Tff2-driven lineage tracing results in the labeling of mucous neck cells and -later- in labeling of chief cells. This delay agrees with the view that chief cells can be generated by trans-differentiation of mucous neck cells (Goldenring et al., 2011). Although this has not been unequivocally proven by lineage tracing of mucous neck cells, substantial indirect evidence exists to support this concept (Goldenring et al., 2011). This evidence is based on findings that, firstly, nucleotide analogs after administration are initially seen in mucous neck cells and only afterwards in chief cells (Karam and Leblond, 1993b). Secondly, the deletion of the chief cell transcription factor Mist1 or its upstream transcriptional regulator Xbp1 results in the accumulation of cells with mixed chief-neck cell characteristics, while neck cells are normal, indicating a block in trans-differentiation (Bredemeyer et al., 2009; Huh et al., 2010; Ramsey et al., 2007). The current finding might constitute a reversion of this process, a re-transformation into mucous neck cells and then into isthmus cells.
In parallel to our observation of Troy+ chief cell activation upon 5-FU treatment, chief cells at the bottom of glands reportedly are activated upon the specific loss of parietal cells (Bredemeyer et al., 2009; Nomura et al., 2004). Such loss leads to the generation of a metaplastic cell lineage derived from chief cells, called SPEM (spasmolytic polypeptide expressing metaplasia) (Nam et al., 2010). This activation of chief cells is another example of the capacity of mature chief cells to re-enter the cell cycle. The generation of the SPEM cell lineage can also be seen as a reverse transformation of chief cells towards mucous neck cells, as the SPEM lineage expresses markers of both lineages (Capoccia et al., 2013). Chief cells thus react upon damage with proliferation and with changes in their differentiation state. Our lineage tracing-based observations of stem cell-like behavior of chief cells might thus constitute a physiological equivalent of the SPEM process.
What might explain this unusual phenomenon? The proposed location of the more active, 'workhorse' stem cell population in the isthmus is unique compared to other stem cell niches in the gastrointestinal tract. The stem cells of the pylorus, small intestine and colon all reside at the bottom of epithelial invaginations, furthest away from the potentially harmful contents of the lumen. The isthmus is thus less well protected against damaging agents. A quiescent stem-like cell, such as the Troy+ chief cell at the bottom, might serve as back-up to a vulnerable, active stem cell niche located closer to the lumen.
Taken together, Troy+ chief cells at gland bottoms can serve as quiescent stem-like cells in the epithelium of the gastric corpus. As has been proposed for other self-renewing tissues (Li and Clevers, 2010), the gastric corpus thus appears to contain two stem cell populations: an actively dividing population located in the isthmus (that remains to be specifically identified) and a small population of ‘reserve’ stem-like chief cells, marked by Troy, at the gland base. The unique property of the Troy+ cell as a fully differentiated cell with the capability to act as a multi-potent stem cell represents a surprising example of plasticity in epithelial stem cell biology.
EXPERIMENTAL PROCEDURES
Details on procedures can be found in the Supplementary Data.
Mice and treatments
Troy-ki mice were generated by homologous recombination in embryonic stem cells targeting an eGFP-ires-CreERT2 cassette at the translational start site of Tnfrsf19 (Fig. S1A). Cre-recombinase was activated in Troy-ki+/ki;RosaLacZ reporter+/Rep mice by injecting IP 5mg/20g mouse weight tamoxifen (Sigma, T5648). For 5-FU treatment, mice were injected with 150mg/kg of 5FU (Sigma, F6627) by IP injection. Mist1-CreERT2 mice (Shi et al., 2009) were crossed to the Rosa-mTmG reporter line (Muzumdar et al., 2007). Tamoxifen (1mg/20g; Sigma) was injected intraperitoneally every other day for a week (3 injections total) to induce GFP induction.
Confocal analysis of eGFP expression in near native tissue sections
Vibratome sections were prepared from Troy-ki and Lgr5-DTR:eGFP stomachs and analyzed for eGFP expression by confocal microscopy.
Single molecule mRNA in situ hybridization
Single molecule mRNA in situ hybridization was performed as described before (Raj et al., 2008). Probe libraries consisted of typically 48 probes 20bp of length complementary to the coding sequence.
Detection of β-galactosidase activity and quantification
Detection of β-galactosidase was performed by X-Gal staining. One hundred gastric units were counted and the position of LacZ+ cells from the bottom of glands noted 1 day, 1 and 3 month p.i. Quantification of LacZ+ clone size was performed in 100 gastric units at 1 day, 1 and 3 month and 1.5 year p.i. For the number of tracings that reached the lumen, LacZ+ clones in ten adjacent sections were counted.
Immunohistochemistry (IHC) and confocal imaging
Paraffin and cryo-sections were prepared according to standard protocols. For primary and secondary antibody details see Supplementary Data. Mist1-CreERT2 tracings were imaged using either Apotome 2 optical sectioning on a Zeiss Axiovert or a custom-built two photon microscope (Kao et al., 2010).
Immunoelectron Microscopy
GFP expression was detected using Cryo-immuno gold staining (Peters et al., 2006).
Gastric unit isolation and FACS
Gastric units were isolated by incubation with EDTA and single cells prepared by trypsinization. Troy-eGFP+ large and small cells as well as Mist1-eGFP+ cells (after induction of Mist1-CreERT2;Rosa-mTmG mice) could be readily differentiated by flow cytometry and gating on FL3 and GFP (Fig. 6A) or Tomato-Red and GFP (Fig. S5A), respectively.
Gastric corpus organoid culture
Whole gastric glands, FACS isolated single Troy-eGFP+ chief or parietal cells and Mist1-eGFP+ cells were cultured in Matrigel using EGF, Gastrin, FGF10, Noggin, Wnt3a and R-spondin supplemented culture medium (ENRGFW medium) and passaged weekly. Differentiation towards the pit cell lineage was induced by growing the organoids in Fgf10-, Noggin- and Wnt-free medium (ERG medium). Tamoxifen was added to the culture medium to induce in vitro lineage tracing. YFP and eGFP were subsequently visualized and recorded in live organoids using confocal microscopy (Leica, SP5).
BrdU and EdU labeling
BrdU labeling was administered using osmotic pumps. Edu was injected 6, 4 and 2h before sacrifice. Paraffin sections were stained with an anti-BrdU antibody, EdU detected by a Click-iT reaction, and visualized by confocal microscopy.
Microarray analysis
Expression profiling was performed on Affymetrix chips using sorted Troy+ chief cells (gate A in Fig. 6), whole corpus glands, and organoids grown in either ENRGFW or ERG medium and analyzed using the R2 web application (http://r2.amc.nl). Array data is available at Gene Expression Omnibus (GEO) under the accession number GSE44060.
Supplementary Material
HIGHLIGHTS.
A subset of quiescent, differentiated chief cells expresses Troy
Troy+ chief cells can generate all differentiated lineages of the gastric epithelium.
Troy+ chief cells act as “reserve” stem cells upon challenge of tissue homeostasis.
Troy+ chief cells can initiate long-term in vitro cultures.
ACKNOWLEDGEMENTS
This work was supported by grants from the Centre for Biomedical Genetics (CBG) to D.E.S., the European Research Council (EU/232814-StemCellMark), the National Research Foundation of Korea (NRF2011-357-C00093) and the Wellcome Trust (097922/C/11/Z) to B.-K.K., a EU Marie Curie Fellowship (EU/236954-ICSC-Lgr5) to M.H, by the Cancer Genomics Center (CGCII) to O.B., a EU Marie Curie Fellowshio (EU/300686-InfO) to S.B., a grant from TI Pharma (T3-106) to J.H.v.E, the European Research Council (EU/Health-F4-2007-200720) to M.v.d.W and DK094989, 2P30 DK052574 to JCM. We would like to thank Mark J. Miller and the Washington University School of Medicine In vivo Imaging Core (IVIC) for help with 2-photon imaging, the Washington University Digestive Disease Core Center (DDRCC) Advanced Imaging and Tissue Analysis Core and Shradha Khurana for help in figure preparation, James Goldenring for constructive discussion and Frederic J. de Sauvage for Lgr5-DTR:eGFP mice.
D.E.S, B.-K.K. and H.C. conceived, designed and analyzed the experiments. D.E.S. constructed the Troy-ki mouse. D.E.S. and B.-K.K. performed the lineage tracing experiments and analyzed the data. Organoid experiments were conceived, performed and analyzed by M.H. Mist1 experiments were conceived, performed and analyzed by G.S., J.H.G. and J.C.M. Confocal microscopy was performed by O.B. Single molecule mRNA in situ data was generated by A.L. Electron microscopy was performed by P.K and P.J.P. Analysis of Lgr5-eGFP:DTR, Axin2-LacZ mice and qPCRs were performed by S.B. Bioinformatic support was provided by J.K. Data interpretation was aided by J.H.v.E. and M.v.d.W. The manuscript was written by D.E.S, B.-K.K. and H.C. and commented on by all other authors.
REFERENCES
- Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Sengupta S, Seandel M, Geijsen N, Hochedlinger K. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H. Multipotential stem cells in adult mouse gastric epithelium. Am J Physiol Gastrointest Liver Physiol. 2002;283:G767–G777. doi: 10.1152/ajpgi.00415.2001. [DOI] [PubMed] [Google Scholar]
- Bredemeyer AJ, Geahlen JH, Weis VG, Huh WJ, Zinselmeyer BH, Srivatsan S, Miller MJ, Shaw AS, Mills JC. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev Biol. 2009;325:211–224. doi: 10.1016/j.ydbio.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capoccia BJ, Jin RU, Kong YY, Peek RM, Jr, Fassan M, Rugge M, Mills JC. The ubiquitin ligase Mindbomb 1 coordinates gastrointestinal secretory cell maturation. The Journal of clinical investigation. 2013;123:1475–1491. doi: 10.1172/JCI65703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KY, Withers HR. Proliferative capability of parietal and zymogen cells. J Anat. 1975;120:421–432. [PMC free article] [PubMed] [Google Scholar]
- Fafilek B, Krausova M, Vojtechova M, Pospichalova V, Tumova L, Sloncova E, Huranova M, Stancikova J, Hlavata A, Svec J, et al. Troy, a Tumor Necrosis Factor Receptor Family Member, Interacts with Lgr5 to Inhibit Wnt Signaling in Intestinal Stem Cells. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.10.048. [DOI] [PubMed] [Google Scholar]
- Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Goldenring JR, Nam KT, Mills JC. The origin of pre-neoplastic metaplasia in the stomach: chief cells emerge from the Mist. Exp Cell Res. 2011;317:2759–2764. doi: 10.1016/j.yexcr.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Schlessinger D, Cui CY. Troy binding to lymphotoxin-alpha activates NF kappa B mediated transcription. Cell Cycle. 2008;7:106–111. doi: 10.4161/cc.7.1.5135. [DOI] [PubMed] [Google Scholar]
- Huh WJ, Esen E, Geahlen JH, Bredemeyer AJ, Lee AH, Shi G, Konieczny SF, Glimcher LH, Mills JC. XBP1 controls maturation of gastric zymogenic cells by induction of MIST1 and expansion of the rough endoplasmic reticulum. Gastroenterology. 2010;139:2038–2049. doi: 10.1053/j.gastro.2010.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh WJ, Khurana SS, Geahlen JH, Kohli K, Waller RA, Mills JC. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology. 2012;142:21–24. e27. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzkovitz S, Lyubimova A, Blat IC, Maynard M, van Es J, Lees J, Jacks T, Clevers H, van Oudenaarden A. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nature cell biology. 2012;14:106–114. doi: 10.1038/ncb2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Kao JY, Zhang M, Miller MJ, Mills JC, Wang B, Liu M, Eaton KA, Zou W, Berndt BE, Cole TS, et al. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology. 2010;138:1046–1054. doi: 10.1053/j.gastro.2009.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam SM. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. The Anatomical record. 1993;236:314–332. doi: 10.1002/ar.1092360205. [DOI] [PubMed] [Google Scholar]
- Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993a;236:259–279. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec. 1993b;236:297–313. doi: 10.1002/ar.1092360204. [DOI] [PubMed] [Google Scholar]
- Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, van Es JH, Mohammed S, Heck AJ, Maurice MM, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012 doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- Lerner C, Harrison DE. 5-Fluorouracil spares hemopoietic stem cells responsible for long-term repopulation. Exp Hematol. 1990;18:114–118. [PubMed] [Google Scholar]
- Li J, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Molecular and cellular biology. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki T, Qu J, Cholewa-Waclaw J, Burr K, Raaum R, Rambukkana A. Reprogramming Adult Schwann Cells to Stem Cell-like Cells by Leprosy Bacilli Promotes Dissemination of Infection. Cell. 2013;152:51–67. doi: 10.1016/j.cell.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- Mills JC, Shivdasani RA. Gastric epithelial stem cells. Gastroenterology. 2011;140:412–424. doi: 10.1053/j.gastro.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S, et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent '+4' cell markers. The EMBO journal. 2012 doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Nam KT, Lee HJ, Sousa JF, Weis VG, O'Neal RL, Finke PE, Romero-Gallo J, Shi G, Mills JC, Peek RM, Jr, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037. e2029. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S, Baxter T, Yamaguchi H, Leys C, Vartapetian AB, Fox JG, Lee JR, Wang TC, Goldenring JR. Spasmolytic polypeptide expressing metaplasia to preneoplasia in H. felis-infected mice. Gastroenterology. 2004;127:582–594. doi: 10.1053/j.gastro.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Peters PJ, Bos E, Griekspoor A. Cryo-immunogold electron microscopy. Curr Protoc Cell Biol. 2006;Chapter 4(Unit 4):7. doi: 10.1002/0471143030.cb0407s30. [DOI] [PubMed] [Google Scholar]
- Qiao XT, Gumucio DL. Current molecular markers for gastric progenitor cells and gastric cancer stem cells. J Gastroenterol. 2011;46:855–865. doi: 10.1007/s00535-011-0413-y. [DOI] [PubMed] [Google Scholar]
- Qiao XT, Ziel JW, McKimpson W, Madison BB, Todisco A, Merchant JL, Samuelson LC, Gumucio DL. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology. 2007;133:1989–1998. doi: 10.1053/j.gastro.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quante M, Marrache F, Goldenring JR, Wang TC. TFF2 mRNA transcript expression marks a gland progenitor cell of the gastric oxyntic mucosa. Gastroenterology. 2010;139:2018–2027. e2012. doi: 10.1053/j.gastro.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat Methods. 2008;5:877–879. doi: 10.1038/nmeth.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007;134:211–222. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- Shao Z, Browning JL, Lee X, Scott ML, Shulga-Morskaya S, Allaire N, Thill G, Levesque M, Sah D, McCoy JM, et al. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Shi G, Zhu L, Sun Y, Bettencourt R, Damsz B, Hruban RH, Konieczny SF. Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology. 2009;136:1368–1378. doi: 10.1053/j.gastro.2008.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashima K, Zhang S, Ragasa R, Nakamura E, Seo JH, Muvaffak A, Hagen SJ. Hepatocyte growth factor regulates the development of highly pure cultured chief cells from rat stomach by stimulating chief cell proliferation in vitro. American journal of physiology Gastrointestinal and liver physiology. 2009;296:G319–G329. doi: 10.1152/ajpgi.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, Van Gijn ME, Suijkerbuijk S, Van de Wetering M, Marra G, et al. The Intestinal Wnt/TCF Signature. Gastroenterology. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–912. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Willems G, Galand P, Vansteenkiste Y, Zeitoun P. Cell population kinetics of zymogen and parietal cells in the stomach of mice. Z Zellforsch Mikrosk Anat. 1972;134:505–518. doi: 10.1007/BF00307670. [DOI] [PubMed] [Google Scholar]
- Wong VW, Stange DE, Page ME, Buczacki S, Wabik A, Itami S, van de Wetering M, Poulsom R, Wright NA, Trotter MW, et al. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nature cell biology. 2012;14:401–408. doi: 10.1038/ncb2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.