ABSTRACT
Postmenopausal women are at a higher risk of ovarian cancer due, in part, to increased levels of gonadotropins such as luteinizing hormone (LH). Gonadotropins and other stimuli are capable of activating two pathways, PKA and PKC, that are altered in ovarian cancer. To determine the role of LH on ovarian cancer, we explored the effects of human chorionic gonadotropin (hCG), an LH mimic, and an activator of the PKC pathway, phorbol-12-myristate 13-acetate (PMA), on ovarian cancer cell-cycle kinetics and apoptosis in Ovcar3 cells. PMA treatment increased cells in the S phase of the cell cycle and initially increased apoptosis after 4 h before diminishing apoptosis after 8 h. Treatment of ovarian cancer cells with hCG had no effect on these parameters. The PKC pathway is known to differentially regulate matrix metalloproteinase (MMP) expression. Results showed that ovarian cancer cells treated with PMA increased MMP7 and MMP10 mRNA levels after 8 h of treatment, and expression remained high after 12 h before decreasing at 24 h. The mRNA expression of extracellular matrix metalloproteinase inducer (BSG), an activator of MMPs, was unaffected by PMA. Due to the role that MMPs play in migration, we investigated the effect of PMA activation of MMPs on ovarian cancer cell migration. The use of the MMP inhibitor GM6001 blocked the increased migratory effects of PMA on ovarian cancer cells. Together, these studies show that activating the PKC pathway causes significant changes in cell cycle kinetics and selective expression of MMPs that are involved in enhancing ovarian cancer cell proliferation and migration.
Keywords: cancer, gene expression, migration, MMPs, ovary
Activation of the PKC pathway increases ovarian cancer migration that is associated with an increase in MMP7 and MMP10.
INTRODUCTION
Ovarian cancer is the leading cause of death in women with gynecological malignancies [1]. The risk of ovarian cancer increases with increased ovulation rate as well as with the use of fertility drugs, while pregnancy and the use of oral contraceptives have been shown to decrease the chances of developing the disease [2]. Infertility drugs, including gonadotropins, have been studied in relation to ovarian cancer risk. The use of infertility drugs and their relationship to ovarian cancer remains an important question since we live in an in vitro fertilization (IVF) era. However, the association between the use of fertility drugs and ovarian cancer remains controversial [3–8].
In addition to exposure to elevated gonadotropins during the reproductive years, postmenopausal women have high levels of gonadotropins such as luteinizing hormone (LH), which has led to the postulate that this elevated gonadotropin may be associated with ovarian cancer [9]. Due to the important implications of elevated gonadotropins in postmenopausal women, the relationship between LH receptors and ovarian cancer has been investigated in ovarian cancer patients, and LH receptors were found to be expressed in five out of seven ovarian borderline tumors, four out of five benign cystadenomas, and about 50% of epithelial ovarian cancers, but none in the germ cell tumors [1]. The impact of LH on the development of ovarian cancer remains an important area of investigation because of the elevated postmenopausal levels of LH and the association of LH receptor expression with ovarian tumors [1].
Cancers typically originate from the misregulation of oncogenes, tumor suppressors, as well as changes in members of the PKC and PKA families and their activation [10, 11]. The PKA pathway is known to be activated by LH and its mimic, human chorionic gonadotropin (hCG), which in turn causes changes in gene expression [12] that ultimately impact the proliferation and invasive capabilities of ovarian cancer cells [9]. Likewise, activation of the PKC pathway by various tumor promoters as well as phorbol-12-myristate 13-acetate (PMA) alters normal physiologic gene expression as well as cell migration, invasion, proliferation, and the cell cycle [13]. The PKC pathway has been implicated in the development and progression of several types of cancer, including breast and ovarian cancer [11]. In fact, previous studies have shown that alterations in the expression of the PKC family members may be used as a biomarker of aggressive forms of ovarian cancer [14]. Activation of the PKC pathway was shown to decrease cisplatin-induced apoptosis and ultimately resistance to cisplatin [15]. Therefore, members of both the PKA and PKC family have been suggested as potential therapeutic ovarian cancer targets [16, 17].
The PKA and PKC pathways are also known to differentially regulate the expression and activity of the MMPs [18]. The matrix metalloproteinases (MMPs) are a family of proteinases that consists of 23 enzymes that are involved in extracellular matrix (ECM) homeostasis. In order for tumor cells to metastasize and invade, it is crucial for these proteinases to disrupt the surrounding matrix [18, 19]. MMPs are known to be important players in matrix degradation necessary for cancer progression [20], and MMP levels may be used to aid in the detection of tumor recurrence [20]. However, little is known about the selective expression of these proteinases in response to PKA and PKC pathway activation in ovarian cancer.
In the present study, we hypothesized that LH would drive ovarian cancer cell progression through the PKA pathway. Alternatively or in combination with LH, activation of the PKC pathway would stimulate ovarian cancer cell growth. To test this hypothesis, we examined the role of the PKA and PKC pathways using hCG or PMA, respectively, on ovarian cancer cell cycle kinetics and apoptosis as well as determined the temporal expression of MMPs in ovarian cancer cells. We also investigated the effects of MMP inhibitors on ovarian cancer cell migration.
MATERIALS AND METHODS
Cells, Media, and Reagents
All cell lines (Ovcar3, CaOv3, and Skov3) and cell culture media were obtained from the American Type Culture Collection. RPMI (Roswell Park Memorial Institute) 1640 was used for Ovcar3 cells, supplemented with 20% fetal bovine serum (FBS), 100 units penicillin, 100 μg streptomycin, and 0.25 μg amphotericin B from Gibco-Invitrogen. CaOv3 and Skov3 cells were grown in Dulbecco modified Eagle medium and McCoy 5A medium, respectively, both supplemented with 10% FBS and antibiotics as above. PMA and hCG were purchased from Sigma-Aldrich. Cells were maintained at 37°C in a 5% CO2 incubator until cells reached the desired confluence described below. The MMP pan inhibitor GM6001 and MMP2/9-specific inhibitor were purchased from Chemicon International and Calbiochem-EMD Biosciences, respectively.
RNA Isolation
In order to examine MMP expression, cells were grown to 60%–90% confluence. Cells were serum starved for 24 h and treated with one of the following: vehicle control (0.1% dimethyl sulfoxide [DMSO]), 1 IU hCG, or 20 nM PMA for 4, 8, 12, or 24 h. Cells were collected and RNA isolated using an RNeasy kit from Qiagen as per the manufacturer's protocol. RNA concentration was determined with a Nanodrop 1000 (Thermo Scientific). One microgram of RNA was reverse transcribed using SuperScript III with Oligo (dT)18 primers for use with real-time PCR according to the manufacturer's protocol (Invitrogen) as previously described [21].
MMP and TIMP mRNA Analysis
Expression of the levels of mRNA for MMPs and TIMPs was analyzed by real-time RT-PCR using SYBR Green (SybrGreen supermix, Invitrogen) and TaqMan (Applied Biosystems Taqman gene expression mastermix) methodologies. For SYBR green, the oligonucleotide primers for MMP2, 7, and 9 and TIMPs are shown in Table 1. GAPDH was used as a control gene. The thermal cycling steps were designed to include 2 min at 50°C, an initial denaturation step for 10 min at 95°C, and then 15 sec at 95°C, 30 sec at 58°C, and 45 sec at 72°C for 45 cycles, followed by 1 min at 95°C, 30 sec at 58°C, and 30 sec at 95°C for ramp dissociation. TaqMan was used to analyze levels of mRNA for MMP8, 10, and 14 and extracellular matrix metalloproteinase inducer (EMMPRIN), which is also known as BSG. TaqMan 20X Gene Expression Assay solutions containing the oligonucleotide primers for human MMP8 (RefSeq: NM_002424.2), human MMP10 (RefSeq: NM_002425.1), human MMP14 (RefSeq: NM_008608.3), human BSG (RefSeq: NM_198589), human MMP11 (RefSeq: NM_005940.3), human LH receptor (RefSeq: NM_000233.3), and human GAPDH (RefSeq: NM_002046.3), an internal endogenous control gene, were purchased from Applied Biosystems. The thermal cycling steps were programmed as follows: 2 min at 50°C to permit AmpErase uracil-N-glycosylase optimal activity, a denaturation step for 10 min at 95°C, and then 15 sec at 95°C and 1 min at 60°C for 45 cycles, followed by 1 min at 95°C, 30 sec at 58°C, and 30 sec at 95°C for ramp dissociation. The relative amount of mRNA in each sample was calculated following the 2−ΔΔCT method and normalized to GAPDH. At least three samples per treatment were analyzed for SYBR green or TaqMan gene expression experiments.
TABLE 1.
Real-time PCR primers.
Apoptosis Assay
Annexin V was used to detect apoptosis. Ovcar3 cells were grown to 60%–90% confluence. Cells were consequently incubated in serum free media for 24 h and treated for an additional 24 h with one of the following: Vehicle control (DMSO), or 20 nM PMA, or 1 IU hCG (160 ng/ml). Cells were then analyzed for apoptosis using the Vybrant Apoptosis Assay kit with Alexa Fluor annexin V/propidium iodide from Invitrogen (Catalog # O11492) as previously described [22]. Briefly, cells were washed, trypsinized, and pelleted by centrifugation (400 × g). The pellet was resuspended in 100 μl annexin V 1× binding buffer, and 5 μl of Alexa Flour 488 annexin V and 1 μl of 100 μg/ml propidium iodide solution was added to the cells and incubated at room temperature for 15 min. Samples were analyzed using flow cytometry readings at 530 nm and >575 nm in the FacsCalibur flow cytometer (Becton Dickson) in the core facility at the University of Kentucky. Each treatment was conducted in triplicate, and the experiment was repeated three times.
Cell Cycle Assay
Changes in cell cycle were measured using propidium iodide staining. Cells were incubated and treated as described above in apoptosis assay. Cell cycle analysis was performed using flow cytometry, as previously described by Vindelov et al. [23] and as routinely performed in our laboratory [22]. Briefly, cells were washed and then incubated at 37°C for 5–10 min with trypsin-ethylenediaminetetraacetic acid and a permeabilizing solution containing trisodium citrate dehydrate, Non-idet P40 (Sigma), spermine HCl (Sigma), and Tris-hydroxymethyl aminoethane. Subsequently, cells were incubated with a solution containing trypsin inhibitor and ribonuclease A for 30 min at 37°C. Propidium iodide in combination with spermine HCl was then added to the cells for 30 min and incubated at 4°C. The suspension was analyzed using a FacsCalibur flow cytometer. Ratios of cells in the G0/G1, S, and G2/M phases of cell cycle were determined on the basis of their DNA content and presented as cell percentage. Each treatment was conducted in triplicate, and the experiment was repeated at least three times.
Cyclic AMP Assay
Changes in cAMP were measured using Promega cAMP-Glo (catalog# V1501). Separate Ovcar3 cultures were plated (2000 cells per well) overnight, and cells were serum starved for 1 h then treated for 20 min with either hCG (n = 5), forskolin (FSK; n = 3), or vehicle control (PBS for hCG, n = 5, or ethanol for FSK, n = 3). FSK treatment was used as a positive control of identical cultures (i.e., for three of the five cultures, cells were split into separate wells, with one set treated with hCG and the corresponding set treated with FSK). Cyclic AMP was measured as described by the manufacturer's protocol.
Cell Migration Assay
Uncoated transwell assays were used to measure cell migration (BD Biocoat control inserts, Franklin Lakes, NJ). Warm, serum-free RPMI 1640 media was added to the bottom well and the interior of the insert (500 μ each). The transwells were incubated in serum-free media to equilibrate for 2 h in a 37°C and 5% CO2 incubator. In the meantime, Ovcar3 cells were seeded at 1.5 × 105 cells suspended in 500 μl serum-free RPMI 1640 media to the top well with vehicle control, 20 nM PMA, 25 or 35 μM GM6001, 50 μM MMP2/9 inhibitor, or a combination of the above. RPMI 1640 media supplemented with 20% FBS was added to the bottom well as an attractant. The plate was incubated for 24 h in a 37°C and 5% CO2 humidified incubator.
After 24 h, cells migrating to the bottom of the membrane were counted. Cells on the top of the membrane were removed by gentle scraping, and the insert was fixed in 70% ethanol and stained with DAPI (4′,6-diamidino-2-phenylindole). Images were taken at 4× and 10× magnification. The number of cells present was determined using Metamorph (Universal Imaging Corp.). The number of migrating cells from each experiment was normalized to the respective control. Three experiments were performed on 3 separate days. At least three assays per treatment were performed.
Statistical Analysis
All data are presented as means ± SEM. One-way or two-way ANOVA was used to test differences among treatments. A Student t-test was performed in two sample comparisons. The number of samples in most cases was at least nine samples. The exact number of samples per experiment is described in the results. If the ANOVA showed significant effects, a Tukey post hoc test was performed in order to identify significant differences among treatments. The means were compared, with P ≤ 0.05 considered significant. Statistical analysis was performed using R software (http://www.r-project.org/) [24].
RESULTS
PMA Stimulated Ovcar3 Cell Cycle Progression
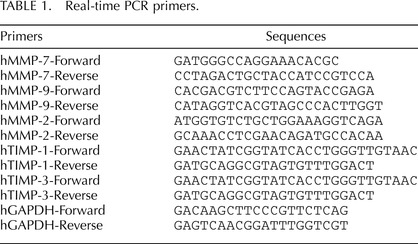
Ovcar3 cells have been shown to express LH receptors [7] and are responsive to PMA stimulation [25, 26]. To determine whether PMA or hCG treatment affects the distribution of ovarian cancer cells in the different phases of the cell cycle, the effects of PMA and hCG on cell cycle progression were assessed in the ovarian cancer cell line Ovcar3. There was a significant increase in cells in the S phase and a decrease in the G0/G1 phase following treatment with 20 nM PMA (Fig. 1, A–C). Cells treated with hCG did not show any change in cell cycle distribution compared to control (Fig. 1, A–C).
FIG. 1.
Ovcar3 cell cycle kinetics after hCG or PMA treatment. Ovcar3 cells were serum starved for 24 h and treated with 1 IU hCG or 20 nM PMA for an additional 24 h. Percentage of the cells in (A) the G0/G1stage of the cell cycle, (B) the G2/M stage of the cell cycle, or (C) the S phase of the cell cycle. Results are the means ± SEM for at least three separate measurements from three individual experiments. Bars that do not share a letter designation are significantly different (P < 0.05).
PMA Causes Differential Changes in Ovcar3 Apoptotic and Viable Cell Ratio
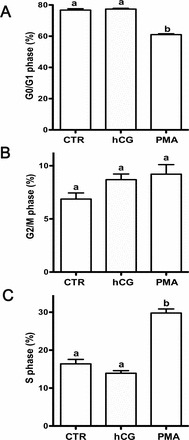
To explore the effects of PMA and hCG on ovarian cancer cell apoptosis, we utilized FACS analysis with an annexin V assay. There was an increase in apoptotic or dead cells after 4 h of treatment with PMA (Fig. 2A). However, after 8 h, PMA led to the presence of fewer apoptotic cells (Fig. 2B), but no changes were observed after 12 h (Fig. 2C). There was an overall increase in nonviable cells over time in culture due to the removal of serum.
FIG. 2.
Apoptosis of Ovcar3 cells after hCG or PMA treatment. Ovcar3 cells were serum starved for 24 h and treated with vehicle control (DMSO), 20 nM PMA, or 1 IU hCG for (A) 4 h, (B) 8 h, or (C) 12 h. Results are the means ± SEM of at least three separate measurements from three individual experiments. White bars represent viable cells; black bars represent cells undergoing early and late apoptosis as well as those that are dead. Bars that do not share a letter or number designation are significantly different (P < 0.05).
Human CG Increased cAMP in Ovcar3 Cells
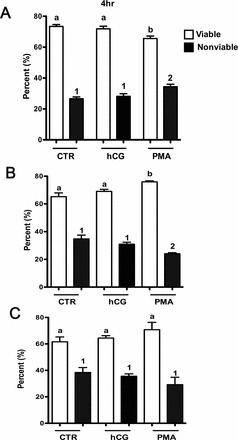
As no changes in cell proliferation were observed in response to hCG, we determined whether LH receptor was expressed in the three cell lines using real-time PCR. LHR mRNA was present in Ovcar3 and CaOv3 cells but was absent in Skov3 (data not shown). The mRNA expression of the LH receptor in Ovcar3 cells remained unchanged after 4, 8, 12, and 24 h of treatment with hCG (data not shown). In order to determine whether the LH receptors in Ovcar3 cells were responsive to hCG, cells were treated with hCG and cAMP levels were measured. After 20 min of hCG treatment, cAMP increased 4-fold (Fig. 3). As a positive control, cells were also treated with FSK, which caused a 9-fold increase compared to control (Fig. 3).
FIG. 3.
Treatment with hCG increases cAMP in Ovcar3 cells. Cells were serum starved for 1 h and treated for 20 min with vehicle control, hCG, or FSK. Human CG increased cAMP activity 4-fold, and FSK increased cAMP by 9-fold. Results are the means ± SEM for three to five measurements from separate Ovcar3 cell cultures. A one-way ANOVA was performed; bars that do not share a letter designation are significantly different within a treatment group (P < 0.05).
PMA Differentially Regulates Members of the Matrix Metalloproteinase Family
MMPs play an essential role in the ability of cancer cells to migrate and invade. As PMA showed more notable effects on ovarian cancer cell cycle and proliferation, we examined the temporal effect of PMA on the mRNA expression of MMP2, 7, 8, 9, 10, 11, and 14. MMP7 is of interest because it influences cancer cell invasiveness and is secreted by ovarian tumor cells [27, 28]. PMA treatment stimulated MMP7 mRNA expression at 8 and 12 h after treatment (Fig. 4A). Similar to MMP7, MMP10 was investigated because it is one of the few MMPs expressed by tumor cells rather than stromal cells and has been shown to be detected in tumors such as lung tumors, but not in their normal counterparts [29]. MMP10 mRNA was elevated at 8, 12, and 24 h after PMA treatment (Fig. 4B). MMP2, 9, and 14 are also correlated with tumor invasiveness [30, 31]. In the present study, MMP9 mRNA expression was elevated at 12 h after PMA treatment (Fig. 4C), whereas MMP2, 8, 11, and 14 did not show any changes in mRNA expression (data not shown). Expression of mRNA for BSG, an MMP activator, did not change; however, its expression was abundant (Fig. 4D). These results indicate that PMA differentially regulates the expression of MMPs in Ovcar3 cells.
FIG. 4.
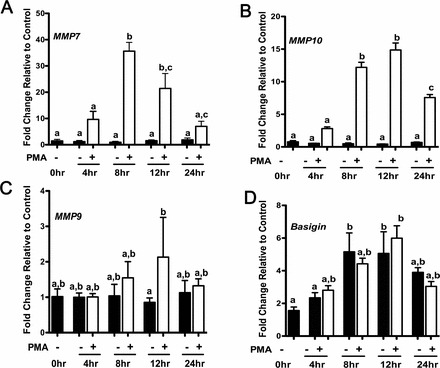
MMP7, MMP10, MMP9, and BSG mRNA expression after temporal PMA treatments. Cells were serum starved for 24 h and treated for an additional 4, 8, 12, or 24 h with vehicle control (DMSO) or 20 nM PMA. Messenger RNA expression was analyzed by real-time PCR at the indicated time points for (A) MMP7, (B) MMP10, (C) MMP9, and (D) BSG (i.e., Basigin) expression. Results are the means ± SEM for three individual measurements. A two-way ANOVA was performed; bars that do not share a letter designation are significantly different within a treatment group (P < 0.05).
The balance between the MMPs and their inhibitors, the TIMPs, determines the fate and composition of the ECM [32]; therefore, we examined the mRNA expression of TIMP1 and TIMP3. Levels of TIMP1 or TIMP3 mRNA did not change across time or treatment (data not shown).
PMA Increased the Migration of Ovcar3 Cells
One of the hallmarks of cancer is the ability of cells to metastasize. Therefore, we examined whether PMA affects Ovcar3 cell migration. Treatment with 20 nM of PMA for 24 h increased Ovcar3 cell migration by about 5-fold compared to that of control (Fig. 5). The MMP pan inhibitor GM6001 compound was capable of inhibiting the PMA induction of ovarian cancer cell migration at 35 uM but had no effect at a lower concentration (Fig. 5). In order to determine whether the decrease in migration observed when cells were treated with GM6001 was due to changes in cell growth, proliferation was measured in the same pool of cells that were plated for the migration assay. The presence of the GM compound in combination with PMA showed no change in cell proliferation after 24 h (data not shown). This indicates that the GM6001 inhibitory effect on migration is not a result of a decrease in Ovcar3 cell proliferation. Since gelatinases have been shown to play a role in cell migration and invasion and since MMP9 was elevated in our study, we explored the role of the gelatinases in Ovcar3 migration using a MMP2/9 inhibitor. The presence of the MMP2/9 inhibitor was not able to reverse the effects of PMA on cell migration. However, when the MMP2/9 inhibitor was used in combination with a lower concentration of the GM6001, cell migration was inhibited (Fig. 5).
FIG. 5.
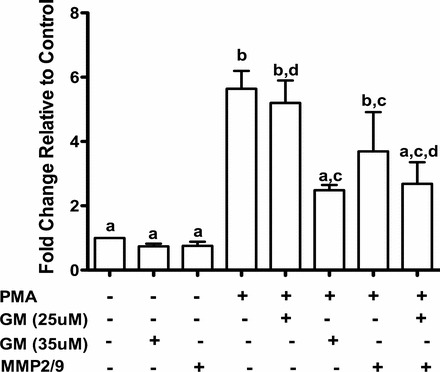
Ovcar3 cell migration with PMA is prevented by MMP inhibitors. Cells were treated with vehicle control (DMSO) or 20 nM PMA in combination with or without 25 μM GM6001, 35 μM GM6001, an MMP2/9 inhibitor, or both MMP inhibitors. Migration was assessed after 24 h of incubation. A two-way ANOVA was performed; bars that do not share a letter designation are significantly different within a treatment group (P < 0.05).
DISCUSSION
Cancer is identified by the presence of six main hallmarks, the most important being “limitless replicating potential” [33]. Proliferation is a balance between cell cycle and apoptosis, which ultimately determines tumor growth. There are several pathways that lead to cell proliferation, most of which are affected in cancer cells. Two of the most commonly altered signaling pathways are the PKA and PKC pathways. We proposed that elevated levels of LH associated with menopause would drive the PKA pathway, resulting in ovarian cancer cell proliferation, as other investigators have reported that hCG causes an increase in ovarian surface epithelium proliferation [34]. The present findings demonstrated that hCG had no effect on ovarian cancer cell cycle progression or apoptosis. These findings, which were somewhat unexpected, led to the investigation of the hCG responsiveness of these cells. We have demonstrated that Ovcar3 and CaOv3 cells contain LH receptors and that hCG administration stimulates up to a 4-fold increase in cAMP, indicating that the lack of an effect is not due to a lack of responsiveness to hCG. However, our findings are in agreement with previous reports showing that treating Ovcar3 cells with hCG had no effect on apoptosis genes such as bcl-2 and bax [1].
We also investigated the effects of PMA, a known activator of the PKC pathway, on cell cycle kinetics and apoptosis. The present findings demonstrated that PMA increased cell proliferation. These changes in proliferation noted in our experiments were due to an increased fraction of cells in the S-phase, and not due to dramatic changes in apoptosis.
Metastasis involves cancer cells migrating from their primary site of origin to distant sites in the body. In order for cancer cells to migrate, they need to escape from the matrix in which they are enclosed. MMPs are proteinases that remodel the ECM and have previously been implicated in cancer metastasis and progression [35]. Furthermore, PMA and hCG are known to regulate the MMPs in the ovary [36]. This led us to explore their expression and regulation in ovarian cancer. We investigated MMP7, also known as matrilysin [37], because it is one of the few MMPs that is secreted by tumor cells rather than stromal cells [37] and has been shown to be expressed in almost all organ tumors in the body. MMP7 overexpression is linked to advanced cancer stages and poor prognosis [30, 38]. In ovarian cancer, MMP7 is elevated in 80% of malignant human ovarian cancer samples, while only 40% of normal or benign samples have elevated MMP7 [39]. MMP7 mRNA levels are also higher in preoperative compared to postoperative patients [27], indicating that it may be used as a biomarker. In in vitro studies using Ovcar3 cells, MMP7 mRNA and protein were highly expressed compared to normal ovarian epithelial cells [30]. In our study, MMP7 mRNA expression was increased almost 40-fold after treatment with the PKC activator PMA. These findings indicate that ovarian tumors with an activated PKC pathway would result in increased MMP7 expression, increasing the tumor's metastatic potential, thereby leading to a more adverse outcome.
Another MMP that was highly upregulated by PMA treatment was a member of the stromelysin family, MMP10. Stromelysins activate other proteinases, such as members of the collagenase family [40]. In our study, MMP10 mRNA was dramatically upregulated 15-fold by PMA, reaching the highest expression after 12 h of treatment. This increased expression may be involved in proteinase activation or may play a role in angiogenesis, since MMP10 has been shown to alter the integrity of the vascular endothelium, thereby regulating angiogenesis and vascularization [41].
MMPs are secreted in an inactive or pro-form and are often activated by other members of the MMP family. MMP7 is sometimes activated by MMP2 and 9 [37], which are known to be involved in cancer progression. MMP2 has been previously shown to control the attachment and adhesion of metastatic ovarian cancer cells to peritoneal surfaces via cleaving ECM proteins and enhancing their binding to integrins [42]. MMP9 was shown to have two potential roles in tumor development, where it acts as a tumor promoter when it is present in the ovarian tumor stroma but prevents tumor advancement when it is expressed in the epithelium [43]. Furthermore, MMP2 expression has been previously correlated to advanced stages of ovarian epithelial tumors [44]. However, controversy exists regarding MMP2 and ovarian cancer, as Gershtein et al. [45] reported a decrease in MMP2 expression, whereas Cheung et al. [46] reported an increase in MMP2 and 9 expression and activity, in CaOv3 cancer cells after treatment with GnRH. These controversial results may be due to differences in the experimental design, the cells being studied, as well as different treatment concentrations. Therefore, we expanded our investigation to explore the temporal regulation of MMP2 and MMP9 expression in Ovcar3 cells. In our study, the expression of MMP2 was not changed; however, in accordance with previous reports, it was expressed in ovarian cancer cells [44]. In our results, MMP9 expression was elevated after 12 h of treatment. This is similar to previous reports showing that MMP9 significantly increased in ovarian tumors [45].
In order to determine potential signals that regulate MMP expression, we investigated the expression of BSG, which has been previously shown to be involved in cancer migration and invasion [47]. BSG is a cell surface glycoprotein that is able to induce MMP expression. In the present study, BSG mRNA levels were very abundant, as indicated by Ct values from the PCR reaction. For example, expression of the control gene Ct values was approximately 18 cycles, whereas BSG expression was seen at 22 cycles. However, the expression of BSG mRNA did not change with PMA treatment across time. It is possible that although BSG mRNA expression remains stable, its activity may be altered [48].
MMPs are known to be involved in cancer migration; therefore, extensive efforts have been directed at synthesizing compounds that inhibit MMPs in order to target this class of proteins in tumor cells. A number of synthetic MMP inhibitors, such as Batimastat, Marimastat and Ilomastat, have been developed and tested in different clinical settings [20, 49]. Interestingly, when we used the pan MMP inhibitor GM6001 (Ilomastat), we were able to inhibit the PMA-stimulated effects on ovarian cancer migration. This is in concordance with the possible role of MMPs in cancer migration, invasion, and angiogenesis [18]. This suggests that the major players in this model of ovarian cancer migration may in fact be MMP10 and MMP7, since the expression of these two MMPs was dramatically increased in response to PMA in our system as compared to the other MMPs. This postulate is supported by the recent report that MMP10 is involved in ovarian cancer invasion [50]. Although GM6001 is widely used to inhibit MMP action, it is important to note that when the GM6001 compound was synthesized and analyzed in 1992, many of the current MMPs had not yet been fully characterized and/or identified, such as MMP10. As such, the specificity of GM6001 to MMP10 has not been reported.
In order to further confirm that the effects we observed with the GM6001 compound are not due to changes in proliferation, cells that were used for the migration assay were also used to measure cell proliferation. Our results demonstrate that there were no changes in proliferation in cells treated with PMA, GM6001, or the combination. However, we did not observe a decrease in PMA-induced migration when a specific MMP2/9 inhibitor was used compared to the pan MMP inhibitor. Our findings are in contrast to previous reports that specific inhibition of MMP2 reversed the stimulatory effect of GnRH on Skov3 ovarian cancer cells [46].
In summary, we demonstrate that PMA increases expression of the MMPs, cell proliferation, and cell migration in ovarian cancer cells. The increase in cell proliferation after PMA treatment is expected since the PKC pathway has been previously shown to regulate cell proliferation in ovarian cancer [51]. However, our findings that MMP7 and MMP10 are highly upregulated following PMA treatment and that an MMP inhibitor blocks PMA-induced cell migration provides evidence that these MMPs may be responsible for the increased migratory phenotype of Ovcar3 cells. Therefore, targeted inhibition of MMP7 and MMP10 may provide possible ovarian cancer therapeutic targets.
Footnotes
Supported by National Institutes of Health Grants P20 RR15592 (T.E.C.) and HD057446 (T.E.C.).
REFERENCES
- Mandai M, Konishi I, Kuroda H, Fujii S. LH/hCG action and development of ovarian cancer—a short review on biological and clinical/epidemiological aspects. Mol Cell Endocrinol 2007; 269: 61 64. [DOI] [PubMed] [Google Scholar]
- Hillard PA. The 5-Minute Obstetrics & Gynecology Consult. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG. Ovarian tumors in a cohort of infertile women. N Engl J Med 1994; 331: 771 776. [DOI] [PubMed] [Google Scholar]
- Venn A, Watson L, Bruinsma F, Giles G, Healy D. Risk of cancer after use of fertility drugs with in-vitro fertilisation. Lancet 1999; 354: 1586 1590. [DOI] [PubMed] [Google Scholar]
- Venn A, Watson L, Lumley J, Giles G, King C, Healy D. Breast and ovarian cancer incidence after infertility and in vitro fertilisation. Lancet 1995; 346: 995 1000. [DOI] [PubMed] [Google Scholar]
- Franceschi S, La Vecchia C, Negri E, Guarneri S, Montella M, Conti E, Parazzini F. Fertility drugs and risk of epithelial ovarian cancer in Italy. Hum Reprod 1994; 9: 1673 1675. [DOI] [PubMed] [Google Scholar]
- Kuroda H, Mandai M, Konishi I, Yura Y, Tsuruta Y, Hamid AA, Nanbu K, Matsushita K, Mori T. Human chorionic gonadotropin (hCG) inhibits cisplatin-induced apoptosis in ovarian cancer cells: possible role of up-regulation of insulin-like growth factor-1 by hCG. Int J Cancer 1998; 76: 571 578. [DOI] [PubMed] [Google Scholar]
- Dor J, Lerner-Geva L, Rabinovici J, Chetrit A, Levran D, Lunenfeld B, Mashiach S, Modan B. Cancer incidence in a cohort of infertile women who underwent in vitro fertilization. Fertil Steril 2002; 77: 324 327. [DOI] [PubMed] [Google Scholar]
- Choi JH, Choi KC, Auersperg N, Leung PC. Gonadotropins activate proteolysis and increase invasion through protein kinase A and phosphatidylinositol 3-kinase pathways in human epithelial ovarian cancer cells. Cancer Res 2006; 66: 3912 3920. [DOI] [PubMed] [Google Scholar]
- Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 2010; 11: 9 22. [DOI] [PubMed] [Google Scholar]
- Lahn M, Kohler G, Sundell K, Su C, Li S, Paterson BM, Bumol TF. Protein kinase C alpha expression in breast and ovarian cancer. Oncology 2004; 67: 1 10. [DOI] [PubMed] [Google Scholar]
- Carletti MZ, Christenson LK. Rapid effects of LH on gene expression in the mural granulosa cells of mouse periovulatory follicles. Reproduction 2009; 137: 843 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Suzuki M, Tanaka Y, Hirashima Y, Terao T. Suppression of urokinase expression and invasiveness by urinary trypsin inhibitor is mediated through inhibition of protein kinase C- and MEK/ERK/c-Jun-dependent signaling pathways. J Biol Chem 2001; 276: 2015 2022. [DOI] [PubMed] [Google Scholar]
- Zhang L, Huang J, Yang N, Liang S, Barchetti A, Giannakakis A, Cadungog MG, O'Brien-Jenkins A, Massobrio M, Roby KF, Katsaros D, Gimotty P, et al. Integrative genomic analysis of protein kinase C (PKC) family identifies PKCiota as a biomarker and potential oncogene in ovarian carcinoma. Cancer Res 2006; 66: 4627 4635. [DOI] [PubMed] [Google Scholar]
- Lee S, Yoon S, Kim DH. A high nuclear basal level of ERK2 phosphorylation contributes to the resistance of cisplatin-resistant human ovarian cancer cells. Gynecol Oncol 2007; 104: 338 344. [DOI] [PubMed] [Google Scholar]
- Naviglio S, Caraglia M, Abbruzzese A, Chiosi E, Di Gesto D, Marra M, Romano M, Sorrentino A, Sorvillo L, Spina A, Illiano G. Protein kinase A as a biological target in cancer therapy. Expert Opin Ther Targets 2009; 13: 83 92. [DOI] [PubMed] [Google Scholar]
- Teicher BA. Protein kinase C as a therapeutic target. Clin Cancer Res 2006; 12: 5336 5345. [DOI] [PubMed] [Google Scholar]
- Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem 2003; 253: 269 285. [DOI] [PubMed] [Google Scholar]
- Schropfer A, Kammerer U, Kapp M, Dietl J, Feix S, Anacker J. Expression pattern of matrix metalloproteinases in human gynecological cancer cell lines. BMC Cancer 2010; 10: 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res 2001; 7: 14 23. [DOI] [PubMed] [Google Scholar]
- Lind AK, Dahm-Kahler P, Weijdegard B, Sundfeldt K, Brannstrom M. Gelatinases and their tissue inhibitors during human ovulation: increased expression of tissue inhibitor of matrix metalloproteinase-1. Mol Hum Reprod 2006; 12: 725 736. [DOI] [PubMed] [Google Scholar]
- Al-Alem L, Southard RC, Kilgore MW, Curry TE. Specific thiazolidinediones inhibit ovarian cancer cell line proliferation and cause cell cycle arrest in a PPARgamma independent manner. PLoS One 2011; 6: e16179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindelov LL, Christensen IJ, Nissen NI. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry 1983; 3: 323 327. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2005. [Google Scholar]
- Debernardis D, Stanzione S, Ottoboni C, Clerico L, Mancuso T, Parodi S, Russo P. Endogenous tumor necrosis factor enhances topoisomerase II inhibitors activity in human ovarian cancer cell lines. J Pharmacol Exp Ther 1996; 279: 84 90. [PubMed] [Google Scholar]
- Gum R, Juarez J, Allgayer H, Mazar A, Wang Y, Boyd D. Stimulation of urokinase-type plasminogen activator receptor expression by PMA requires JNK1-dependent and -independent signaling modules. Oncogene 1998; 17: 213 225. [DOI] [PubMed] [Google Scholar]
- Acar A, Onan A, Coskun U, Uner A, Bagriacik U, Atalay F, Unsal DK, Guner H. Clinical significance of serum MMP-2 and MMP-7 in patients with ovarian cancer. Med Oncol 2008; 25: 279 283. [DOI] [PubMed] [Google Scholar]
- Tanimoto H, Underwood LJ, Shigemasa K, Parmley TH, Wang Y, Yan Y, Clarke J, O'Brien TJ. The matrix metalloprotease pump-1 (MMP-7, Matrilysin): a candidate marker/target for ovarian cancer detection and treatment. Tumor Biol 1999; 20: 88 98. [DOI] [PubMed] [Google Scholar]
- Gill JH, Kirwan IG, Seargent JM, Martin SW, Tijani S, Anikin VA, Mearns AJ, Bibby MC, Anthoney A, Loadman PM. MMP-10 is overexpressed, proteolytically active, and a potential target for therapeutic intervention in human lung carcinomas. Neoplasia 2004; 6: 777 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FQ, So J, Reierstad S, Fishman DA. Matrilysin (MMP-7) promotes invasion of ovarian cancer cells by activation of progelatinase. Int J Cancer 2005; 114: 19 31. [DOI] [PubMed] [Google Scholar]
- Davidson B, Goldberg I, Gotlieb WH, Kopolovic J, Ben-Baruch G, Nesland JM, Berner A, Bryne M, Reich R. High levels of MMP-2, MMP-9, MT1-MMP and TIMP-2 mRNA correlate with poor survival in ovarian carcinoma. Clin Exp Metastasis 1999; 17: 799 808. [DOI] [PubMed] [Google Scholar]
- Chirco R, Liu XW, Jung KK, Kim HR. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev 2006; 25: 99 113. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57 70. [DOI] [PubMed] [Google Scholar]
- Stewart SL, Querec TD, Gruver BN, O'Hare B, Babb JS, Patriotis C. Gonadotropin and steroid hormones stimulate proliferation of the rat ovarian surface epithelium. J Cell Physiol 2004; 198: 119 124. [DOI] [PubMed] [Google Scholar]
- Cai KQ, Yang WL, Capo-Chichi CD, Vanderveer L, Wu H, Godwin AK, Xu XX. Prominent expression of metalloproteinases in early stages of ovarian tumorigenesis. Mol Carcinog 2007; 46: 130 143. [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr,, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 2003; 24: 428 465. [DOI] [PubMed] [Google Scholar]
- Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med (Maywood) 2006; 231: 20 27. [DOI] [PubMed] [Google Scholar]
- Shiomi T, Okada Y. MT1-MMP and MMP-7 in invasion and metastasis of human cancers. Cancer Metastasis Rev 2003; 22: 145 152. [DOI] [PubMed] [Google Scholar]
- Sharp F. Ovarian Cancer 3. New York: Chapman & Hall; 1994. [Google Scholar]
- Barksby HE, Milner JM, Patterson AM, Peake NJ, Hui W, Robson T, Lakey R, Middleton J, Cawston TE, Richards CD, Rowan AD. Matrix metalloproteinase 10 promotion of collagenolysis via procollagenase activation: implications for cartilage degradation in arthritis. Arthritis Rheum 2006; 54: 3244 3253. [DOI] [PubMed] [Google Scholar]
- Chang S, Young BD, Li S, Qi X, Richardson JA, Olson EN. Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell 2006; 126: 321 334. [DOI] [PubMed] [Google Scholar]
- Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest 2008; 118: 1367 1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillanpaa S, Anttila M, Voutilainen K, Ropponen K, Turpeenniemi-Hujanen T, Puistola U, Tammi R, Tammi M, Sironen R, Saarikoski S, Kosma VM. Prognostic significance of matrix metalloproteinase-9 (MMP-9) in epithelial ovarian cancer. Gynecol Oncol 2007; 104: 296 303. [DOI] [PubMed] [Google Scholar]
- Kamel H, Abdelazim I, Habib SM, El Shourbagy MA, Ahmed NS. Immunoexpression of matrix metalloproteinase-2 (MMP-2) in malignant ovarian epithelial tumours. J Obstet Gynaecol Can 2010; 32: 580 586. [DOI] [PubMed] [Google Scholar]
- Gershtein ES, Levkina NV, Digayeva MA, Laktionov KP, Tereshkina IV, Kushlinsky NE. Matrix metalloproteinases 2, 7, and 9 and tissue inhibitor of metalloproteinases-1 in tumors and serum of patients with ovarian neoplasms. Bull Exp Biol Med 2010; 149: 628 631. [DOI] [PubMed] [Google Scholar]
- Cheung LW, Leung PC, Wong AS. Gonadotropin-releasing hormone promotes ovarian cancer cell invasiveness through c-Jun NH2-terminal kinase-mediated activation of matrix metalloproteinase (MMP)-2 and MMP-9. Cancer Res 2006; 66: 10902 10910. [DOI] [PubMed] [Google Scholar]
- Voigt H, Vetter-Kauczok CS, Schrama D, Hofmann UB, Becker JC, Houben R. CD147 impacts angiogenesis and metastasis formation. Cancer Invest 2009; 27: 329 333. [DOI] [PubMed] [Google Scholar]
- Agrawal SM, Yong VW. The many faces of EMMPRIN - roles in neuroinflammation. Biochim Biophys Acta 2011; 1812: 213 219. [DOI] [PubMed] [Google Scholar]
- Brown PD. Ongoing trials with matrix metalloproteinase inhibitors. Expert Opin Investig Drugs 2000; 9: 2167 2177. [DOI] [PubMed] [Google Scholar]
- Solar P, Sytkowski AJ. Differentially expressed genes associated with cisplatin resistance in human ovarian adenocarcinoma cell line A2780. Cancer Lett 2011; 309: 11 18. [DOI] [PubMed] [Google Scholar]
- Mertens-Walker I, Bolitho C, Baxter RC, Marsh DJ. Gonadotropin-induced ovarian cancer cell migration and proliferation require extracellular signal-regulated kinase 1/2 activation regulated by calcium and protein kinase C{delta}. Endocr Relat Cancer 2010; 17: 335 349. [DOI] [PubMed] [Google Scholar]


