Abstract
Thrombin receptor activation was explored in human epidermal keratinocytes and human dermal fibroblasts, cells that are actively involved in skin tissue repair. The effects of thrombin, trypsin, and the receptor agonist peptides SFLLRN and TFRIFD were assessed in inositolphospholipid hydrolysis and calcium mobilization studies. Thrombin and SFLLRN stimulated fibroblasts in both assays to a similar extent, whereas TFRIFD was less potent. Trypsin demonstrated weak efficacy in these assays in comparison with thrombin. Results in fibroblasts were consistent with human platelet thrombin receptor activation. Keratinocytes, however, exhibited a distinct profile, with trypsin being a far better activator of inositolphospholipid hydrolysis and calcium mobilization than thrombin. Furthermore, SFLLRN was more efficacious than thrombin, whereas no response was observed with TFRIFD. Since our data indicated that keratinocytes possess a trypsin-sensitive receptor, we addressed the possibility that these cells express the human homologue of the newly described murine protease-activated receptor, PAR-2 [Nystedt, S., Emilsson, K., Wahlestedt, C. & Sundelin, J. (1994) Proc. Natl. Acad. Sci. USA 91, 9208-9212]. PAR-2 is activated by nanomolar concentrations of trypsin and possesses the tethered ligand sequence SLIGRL. SLIGRL was found to be equipotent with SFLLRN in activating keratinocyte inositolphospholipid hydrolysis and calcium mobilization. Desensitization studies indicated that SFLLRN, SLIGRL, and trypsin activate a common receptor, PAR-2. Northern blot analyses detected a transcript of PAR-2 in total RNA from keratinocytes but not fibroblasts. Levels of thrombin receptor message were equivalent in the two cell types. Our results indicate that human keratinocytes possess PAR-2, suggesting a potential role for this receptor in tissue repair and/or skin-related disorders.
Full text
PDF
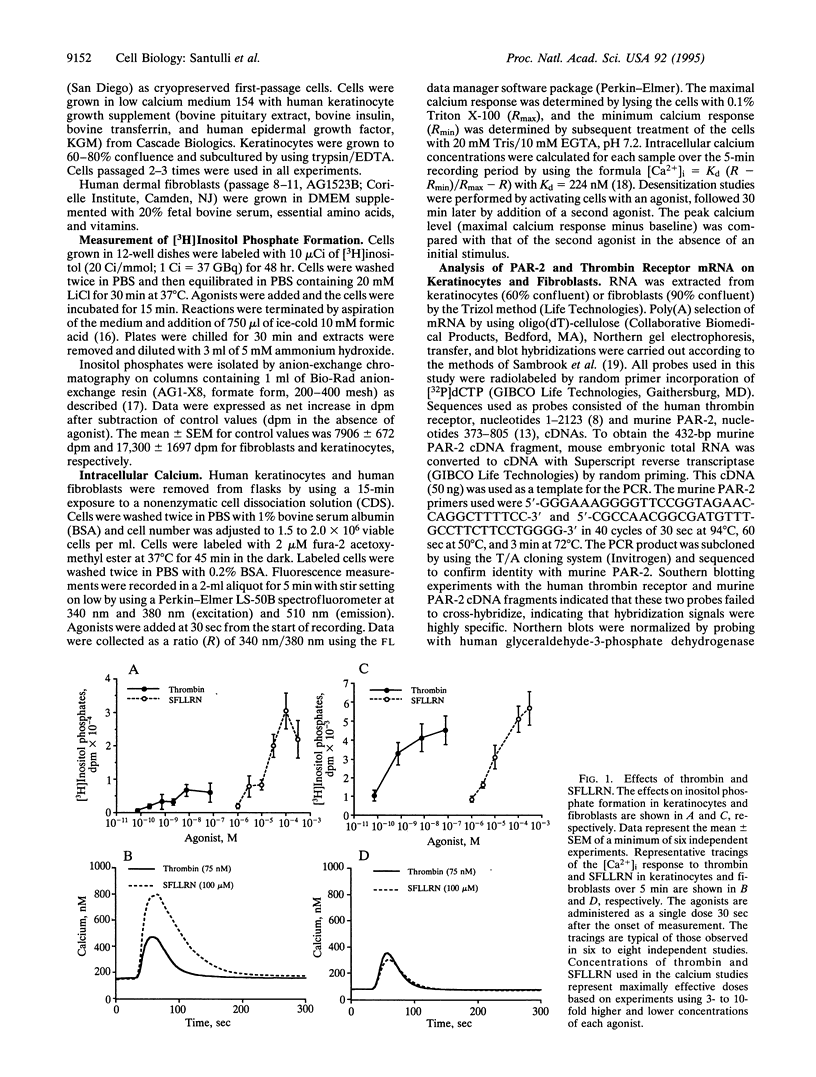
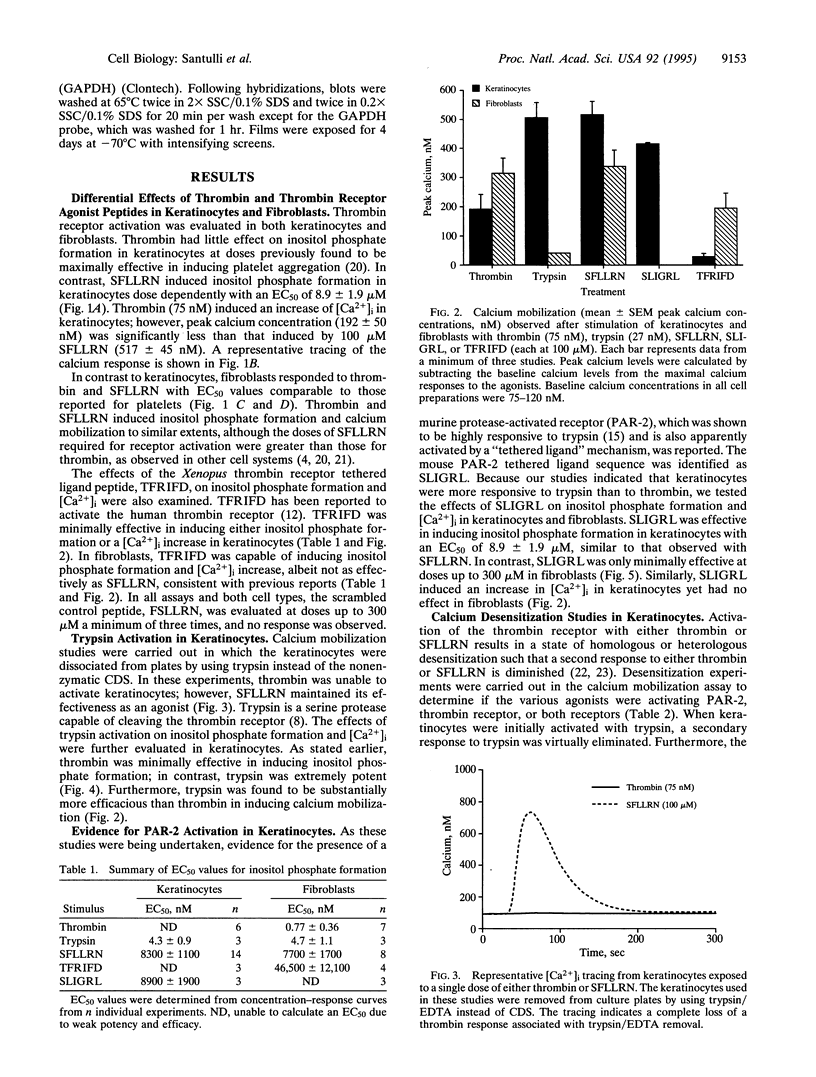
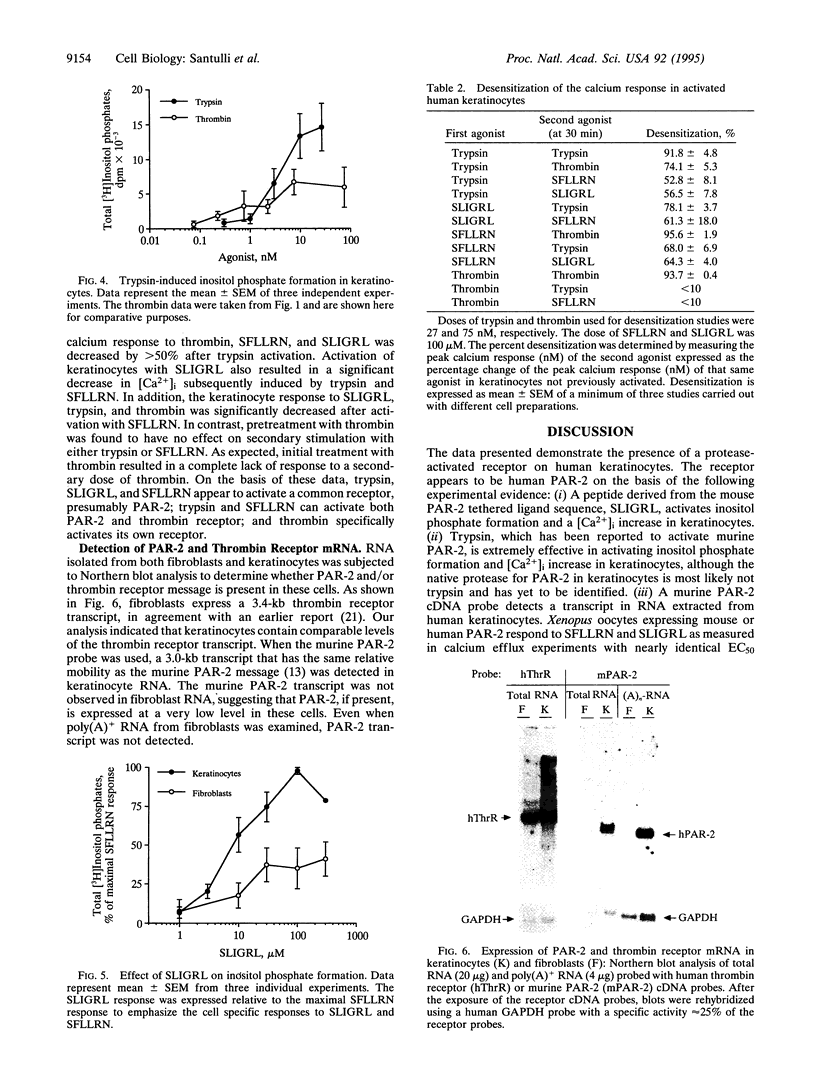

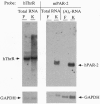
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brass L. F., Pizarro S., Ahuja M., Belmonte E., Blanchard N., Stadel J. M., Hoxie J. A. Changes in the structure and function of the human thrombin receptor during receptor activation, internalization, and recycling. J Biol Chem. 1994 Jan 28;269(4):2943–2952. [PubMed] [Google Scholar]
- Coughlin S. R. Protease-activated receptors start a family. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9200–9202. doi: 10.1073/pnas.91.20.9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Hawkins P. T., Irvine R. F. Inositol 1,3,4,5-tetrakisphosphate and not phosphatidylinositol 3,4-bisphosphate is the probable precursor of inositol 1,3,4-trisphosphate in agonist-stimulated parotid gland. Biochem J. 1986 Sep 1;238(2):501–506. doi: 10.1042/bj2380501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerszten R. E., Chen J., Ishii M., Ishii K., Wang L., Nanevicz T., Turck C. W., Vu T. K., Coughlin S. R. Specificity of the thrombin receptor for agonist peptide is defined by its extracellular surface. Nature. 1994 Apr 14;368(6472):648–651. doi: 10.1038/368648a0. [DOI] [PubMed] [Google Scholar]
- Glenn K. C., Carney D. H., Fenton J. W., 2nd, Cunningham D. D. Thrombin active site regions required for fibroblast receptor binding and initiation of cell division. J Biol Chem. 1980 Jul 25;255(14):6609–6616. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hein L., Ishii K., Coughlin S. R., Kobilka B. K. Intracellular targeting and trafficking of thrombin receptors. A novel mechanism for resensitization of a G protein-coupled receptor. J Biol Chem. 1994 Nov 4;269(44):27719–27726. [PubMed] [Google Scholar]
- Hung D. T., Vu T. H., Nelken N. A., Coughlin S. R. Thrombin-induced events in non-platelet cells are mediated by the unique proteolytic mechanism established for the cloned platelet thrombin receptor. J Cell Biol. 1992 Feb;116(3):827–832. doi: 10.1083/jcb.116.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani H., Hayashi T., Nouchi N., Ohyanagi S., Hashimoto K., Shimizu M., Suzuki K. Functional and immunoreactive thrombomodulin expressed by keratinocytes. J Invest Dermatol. 1994 Dec;103(6):825–828. doi: 10.1111/1523-1747.ep12413560. [DOI] [PubMed] [Google Scholar]
- Nystedt S., Emilsson K., Wahlestedt C., Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjanpelto P. Proteases stimulate proliferation of human fibroblasts. J Cell Physiol. 1977 Jun;91(3):387–392. doi: 10.1002/jcp.1040910308. [DOI] [PubMed] [Google Scholar]
- Raife T. J., Lager D. J., Madison K. C., Piette W. W., Howard E. J., Sturm M. T., Chen Y., Lentz S. R. Thrombomodulin expression by human keratinocytes. Induction of cofactor activity during epidermal differentiation. J Clin Invest. 1994 Apr;93(4):1846–1851. doi: 10.1172/JCI117171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen U. B., Vouret-Craviari V., Jallat S., Schlesinger Y., Pagès G., Pavirani A., Lecocq J. P., Pouysségur J., Van Obberghen-Schilling E. cDNA cloning and expression of a hamster alpha-thrombin receptor coupled to Ca2+ mobilization. FEBS Lett. 1991 Aug 19;288(1-2):123–128. doi: 10.1016/0014-5793(91)81017-3. [DOI] [PubMed] [Google Scholar]
- Scarborough R. M., Naughton M. A., Teng W., Hung D. T., Rose J., Vu T. K., Wheaton V. I., Turck C. W., Coughlin S. R. Tethered ligand agonist peptides. Structural requirements for thrombin receptor activation reveal mechanism of proteolytic unmasking of agonist function. J Biol Chem. 1992 Jul 5;267(19):13146–13149. [PubMed] [Google Scholar]
- Seuwen K., Lagarde A., Pouysségur J. Deregulation of hamster fibroblast proliferation by mutated ras oncogenes is not mediated by constitutive activation of phosphoinositide-specific phospholipase C. EMBO J. 1988 Jan;7(1):161–168. doi: 10.1002/j.1460-2075.1988.tb02796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talwar H. S., Fisher G. J., Harris V. A., Voorhees J. J. Agonist-induced hydrolysis of phosphoinositides and formation of 1,2-diacylglycerol in adult human keratinocytes. J Invest Dermatol. 1989 Aug;93(2):241–245. doi: 10.1111/1523-1747.ep12277581. [DOI] [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Chambard J. C., Paris S., L'Allemain G., Pouysségur J. alpha-Thrombin-induced early mitogenic signalling events and G0 to S-phase transition of fibroblasts require continual external stimulation. EMBO J. 1985 Nov;4(11):2927–2932. doi: 10.1002/j.1460-2075.1985.tb04025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Weaver V. M., Lach B., Walker P. R., Sikorska M. Role of proteolysis in apoptosis: involvement of serine proteases in internucleosomal DNA fragmentation in immature thymocytes. Biochem Cell Biol. 1993 Sep-Oct;71(9-10):488–500. doi: 10.1139/o93-071. [DOI] [PubMed] [Google Scholar]
- Zacharias U., He C. J., Nguyen G., Sraer J. D., Rondeau E. Long-term effects of thrombin require sustained activation of the functional thrombin receptor. FEBS Lett. 1993 Nov 15;334(2):225–228. doi: 10.1016/0014-5793(93)81716-d. [DOI] [PubMed] [Google Scholar]
- Zhong C., Hayzer D. J., Corson M. A., Runge M. S. Molecular cloning of the rat vascular smooth muscle thrombin receptor. Evidence for in vitro regulation by basic fibroblast growth factor. J Biol Chem. 1992 Aug 25;267(24):16975–16979. [PubMed] [Google Scholar]