Abstract
Exposure to estrogens is suggested to be a risk factor in human breast cancer development. The mechanisms underlying estrogen-induced cancer have not been fully elucidated. Both estrogen receptor (ER)-mediated proliferative processes and ER-independent generation of oxidative stress are suggested to play important roles in estrogen-induced breast carcinogenesis. In the current study, we investigated the role of oxidative stress in breast carcinogenesis using the ACI rat model of mammary tumorigenesis. Female ACI rats were treated with 17β-estradiol (E2), butylated hydroxyanisole (BHA), or a combination of E2 + BHA for up to 240 days. Cotreatment of rats with E2 + BHA reduced estrogen-induced breast tumor development with tumor incidence of 24%, a significant decrease relative to E2 where tumor incidence was 82%. Proliferative changes in the breast tissue of E2 + BHA-treated animals were similar to those observed in E2-treated animals. Tissue levels of 8-isoprostane, a marker of oxidant stress, as well as the activities of antioxidant enzymes including glutathione peroxidase, superoxide dismutase, and catalase were quantified in the breast tissues of rats treated with E2 + BHA and compared to activity levels found in E2-treated animals and respective age-matched controls. Cotreatment with BHA inhibited E2-mediated increases in 8-isoprostane levels as well as activities of antioxidant enzymes. In summary, these data suggest that estrogen-mediated oxidant stress plays a critical role in the development of estrogen-dependent breast cancers and BHA inhibits E2-dependent breast carcinogenesis by decreasing oxidant stress.
Keywords: 17β-estradiol, ACI rat, Animal model of breast cancer, Antioxidant, Breast cancer, Butylated hydroxyanisole, Estrogen, Oxidative stress
INTRODUCTION
Breast cancer represents one of the most common neoplasms among women worldwide, and breast cancer is second only to lung cancer as a leading cause of cancer death in women living in the United States [1–3]. Epidemiological evidence indicates that breast cancer risk is strongly associated with prolonged lifetime exposure to estrogens [4–6]. Indeed, early age at menarche and late age at menopause both increase breast cancer risk, presumably by extending duration of estrogen exposure [7, 8]. Although an extensive body of the literature lends strong support to the involvement of estrogens in breast carcinogenesis, the exact mechanisms by which estrogens exert their carcinogenic effects remain ill defined [9, 10].
Estrogens are suggested to induce mammary carcinogenesis via two distinct mechanisms: the estrogen receptor (ER)-dependent mechanism and the ER-independent mechanism. The ER-dependent pathway of estrogen action encompasses cell growth and proliferation secondary to the binding of estrogen to the ER [10–14]. In addition to stimulation of cell growth in normal cells, estrogens can promote the expansion of estrogen-sensitive neoplastic cells by way of ER-mediated processes. The ER-independent mechanism of estrogen-induced carcinogenesis relies on metabolic activation of endogenous estrogens by cytochrome P450 (Cyp) enzymes to generate highly reactive genotoxic metabolites [10, 12, 15]. These enzymes convert E2 into the catechol estrogens 4-hydroxyestradiol (4-OHE2) and 2-hydroxyestradiol (2-OHE2), respectively [16]. 2-OHE2 is rapidly methylated by catechol-O-methyl transferase and is not genotoxic. In contrast, 4-OHE2 is highly genotoxic. Tumorigenic estrogen metabolites such as 4-OHE2 undergo oxidative metabolism to form catechol estrogen quinones, which readily react with DNA to produce depurinating adducts. If not faithfully repaired, these depurinating adducts have the potential to generate mutations [12, 17, 18]. Furthermore, catechol estrogen quinones undergo oxidative metabolism, and in the process, produce free radicals and reactive oxygen species, that are capable of damaging cellular biomolecules, such as DNA, protein, and lipids [11, 19–21]. Recent data also suggest mitochondrial involvement in the generation of estrogen-associated reactive oxygen species (ROS) [22–24]. The ER-dependent and ER-independent pathways of estrogen action are suspected to act synergistically to exacerbate DNA damage, produce gene mutation, and promote aberrant regulation of gene expression [16, 19, 25, 26].
The present study was intended to examine the impact of oxidative stress, an integral part of the ER-independent pathway of estrogen action, on breast carcinogenesis in vivo using the ACI rat model. The antioxidant BHA was used to assess the impact of oxidative stress on breast tumor development. Female ACI rats were treated with BHA, E2, or E2 + BHA for 7, 15, 120, or 240 days. Treatment of ovary-intact female ACI rats with E2 results in the development of mammary tumors with 80–100% incidence within 6–7 months. Despite the sensitivity of ACI rats to E2-induced tumors, these rats are very resistant to the development of spontaneous breast tumors without E2 treatment [27]. Mammary tumors produced in this model have been shown to be adenocarcinoma in nature with invasive features observed in some. Moreover, mammary tumors observed in this model system share several pertinent characteristics with human mammary tumors [28–34]. Our data demonstrate that BHA inhibits E2-induced breast carcinogenesis and decreases E2-associated increases in oxidative stress. Taken together, our data suggest that oxidant stress plays an important role in the pathogenesis of estrogen-mediated breast cancer.
MATERIALS AND METHODS
Treatment of Animals and Histopathologic Analysis
Female ACI rats (4 weeks of age; Harlan Sprague Dawley, Indianapolis, IN) were housed in our animal facility under controlled temperature, humidity, and lighting conditions. After a one-week acclimatization period, rats were divided in four groups: control, E2, BHA and E2 + BHA. Rats in the E2 and E2 + BHA treatment groups were implanted subcutaneously with E2 pellets (3 mg E2 + 17 mg cholesterol). The control and BHA only groups were implanted with pellets containing 17 mg cholesterol only. E2 and cholesterol pellets were prepared using a pellet press as described previously [4, 35, 36]. E2 and control group of animals were fed with purifiedAIN76A phytoestrogen-free diet (Dyets, Bethlehem, PA), whereas E2 + BHA and the BHA only group of animals were fed with AIN76A diet containing 0.7% (w/w) BHA (Dyets, Bethlehem, PA). Water was given ad libitum to all the animals. Each of the four exposure groups was divided into four subgroups containing at least 10 rats in each subgroup, and these rats underwent treatments for 7, 15, 120, or 240 days, respectively. Animals were palpated twice weekly to monitor mammary tumor development. At the end of each of the above-mentioned time periods, animals were anesthetized using isoflurane and euthanized. Mammary tissues and tumors as well as liver, uterus, kidney, lung, and brain tissues were removed and snap-frozen in liquid nitrogen for further studies. Frozen tissues were stored at −70°C. A portion of the excised mammary and other tissues was stored in 10% buffered formalin for histopathologic analyses. The formalin-fixed tissue was embedded in paraffin, and sections of 4–5 μm thickness were cut. Paraffin-embedded sections of the mammary, liver, brain, uterus, kidney, lung, and ovary were stained with hematoxylin and eosin for histopathologic evaluations. Tumor incidence and the number of tumor nodules per rat were counted at the time of dissection. All the mammary tissue sections from the treatment groups were examined for tumor development and histopathologic changes.
Analysis of 8-isoPGF2α Levels
Total 8-iso-prostane F2α (8-isoPGF2α) levels in the mammary and liver tissue of female ACI rats were quantified using a direct 8-isoPGF2α enzyme immunoassay kit obtained from Assay Designs (Ann Arbor, MI), according to the suppliers instructions, as described previously [4]. Briefly, rat mammary tissue, tumor, and liver tissue (50–100 mg) were homogenized in cold PBS (pH 7.4), containing 0.005% butylated hydroxytolulene. Homogenization was carried out in the TissueLyser (Qiagen, Valencia, CA) at 29 cycles per second for 4 min. Protein concentrations from the homogenates were determined using the Pierce BCA protein assay kit (Pierce, Rockford, IL). For determination of 8-isoPGF2α level, 100 μL of the homogenate from each tissue was hydrolyzed by incubation with 25 μL of 10 N NaOH at 45°C for 2 h. The reaction mixture was cooled on ice for 5 min, neutralized with 25 μL of 12 N HCl, and centrifuged for 5 min at 4°C. Fifty microliter of the hydrolyzed/neutralized supernatant sample was then used in the 96-well format 8-isoPGF2α assay. Samples were incubated with the 8-isoPGF2α antibody for 18 h at 4°C. The contents of the wells were emptied after incubation and washed with wash buffer. After complete removal of wash buffer from the wells, the color was developed by incubation with 200 μL of p-nitrophenyl phosphate for 45 min, at room temperature. The reaction was stopped by the addition of 50 μL of stop solution, and the plate was read at 405 nm. Standards were run on each plate, and standard curve was generated by measuring the optical density of 160–100,000 pg/mL of 8-isoPGF2α standards that were processed simultaneously with the unknown samples. Data are expressed as mean 8-isoPGF2α pg/μg protein ± SEM. Fold changes were calculated by comparing 8-isoPGF2α levels detected in the mammary or liver tissue of treated animals to levels in the respective tissue of age-matched control animals.
Analysis of Superoxide Dismutase, Glutathione Peroxidae, and Catalase Activities
Superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase (CAT) activities were quantified using commercially available kits from Cayman Chemical Company (Ann Arbor, MI) and as reported recently [37]. Briefly, the SOD activity was quantified using a tetrazolium salt for detection of superoxide radicals, generated by xanthine oxidase and hypoxanthine. Approximately, 50 mg of mammary or liver tissue was homogenized in 50 mM phosphate buffer, containing 1 mM EDTA, 210 mM mannitol, and 70 mM sucrose. After homogenization, homogenates were centrifuged at 1500 g for 5 min at 4°C. The supernatant was removed, and the protein concentration was measured using the BCA Protein Assay kit (Pierce, Rockland, IL). Standards were run on each plate along with the unknown samples for generation of standard curve. The absorbance of the sample and standard wells were measured at 450 nm, using a plate reader (Versamax Microplate Reader, Molecular Devices, Sunnyvale, CA). SOD activity is reported as units/μg protein. The GPx assay kit measures GPx activity by way of a coupled reaction with glutathione reductase. This assay is based on the production of oxidized glutathione during reduction of hydroperoxide by GPx and then recycling to its reduced state by glutathione reductase and NADPH. The oxidation of NADPH to NADP+ causes a decrease in the absorbance at 340 nm. The rate of decrease in the absorbance of the samples at 340 nm is directly proportional to the GPx activity in the sample. At the time of analysis, approximately 50 mg of mammary or liver tissue was homogenized in 50 mM phosphate buffer, containing 1 mM EDTA, 210 mM mannitol, and 70 mM sucrose. After homogenization, homogenates were centrifuged at 10,000×g for 15 min at 4°C. Seven time points with a gap of 1 min were obtained to accurately assess the decrease in absorbance at 340 nm. GPx activity was reported as nmol/min/μg protein. The determination of CAT activity is based on the reaction of the catalase enzyme with methanol, in the presence of an optimal concentration of H2O2. The formaldehyde produced is measured spectrophotometrically at 540 nm using 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole (Purpald) as the chromogen. Purpald specifically forms a bicyclic heterocycle with aldehydes, which changes from colorless to purple following oxidation. At the time of analysis, approximately 50 mg of mammary or liver tissue was homogenized in 50 mM phosphate buffer, containing 1 mM EDTA, 210 mM mannitol, and 70 mM sucrose. After homogenization, homogenates were centrifuged at 10,000×g for 15 min at 4°C. Standards were run simultaneously on each plate (Versamax Microplate Reader, Molecular Devices, Sunnyvale, CA). Catalase activity is reported as nmol/min/μg protein. Fold changes in SOD, GPx, and CAT activity were calculated by comparing GPx activity in the mammary, liver tissue of treated groups to the activity measured in the respective tissue of age-matched respective controls.
Statistical Analyses
Statistical analysis was performed by using Sigma Plot 8.0 (Systat Software, San Jose, CA). Fisher’s exact test was used to compare tumor incidence between two treatment groups, or between a treatment group and a specific control group, where tumor incidence was estimated as a proportion of tumor occurrence of the animals in an experimental group. 8-isoPGF2α, SOD, CAT, and GPx assays were all done using at least eight samples from eight different animals in each treatment group. The unpaired t-test analysis was used to calculate p values for comparisons of 8-iso-PGF2α levels, SOD, CAT, and GPx activity between the treated animals and their respective age-matched controls. A p value < 0.05 was considered significant.
RESULTS
BHA Decreases the Incidence and Increases the Latency of E2-Induced Mammary Tumors
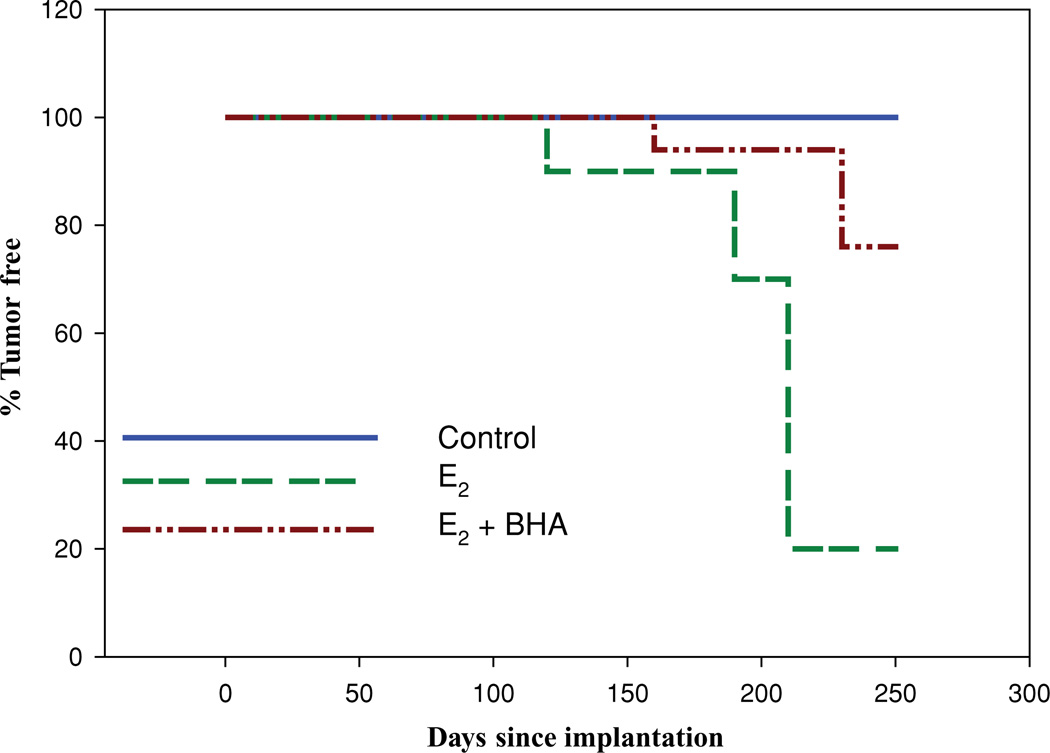
Neither cholesterol control nor BHA-treated control rats developed mammary tumors. Mammary tumor incidence in rats cotreated with E2 and BHA was 24% after 8 months of treatment. The first palpable tumor in this group did not appear until day 169 (Table 1). In contrast, in the E2-treated group, the first palpable breast tumors have been shown to appear after 128 days of treatment and mammary tumor incidence was equal to 82% after 8 months of E2 exposure [37]. BHA treatment significantly increases tumor latency (Figure 1). Treatment with E2 + BHA was associated with a decrease in tumor multiplicity compared to the E2 group (Table 1). In the E2 + BHA group, one tumor nodule per tumor-bearing animal was observed, whereas in the E2 treatment group an average of 3.1 ± 0.7 tumor nodules was present in tumor-bearing rats. Histopathologic examination of mammary tissue from the control and BHA groups revealed normal lobular architecture with branched ducts and normal distribution of fat tissue (Figure 2). Mammary tissues in E2 + BHA-treated rats showed ductal hyperplasia with increased expansion of terminal lobular units accompanied by compression of and expansion into the surrounding fat tissue. The proliferation changes are similar to the hyperplastic changes observed in rats treated with E2 only (Figure 2). Both mammary ductal carcinoma in situ and microinvasive carcinoma were observed in the E2 + BHA-treated group. In most cases, the carcinomas showed mixed morphology, consisting of solid patterns with or without comedo-necrosis. Cribriform and cystic-papillary pattern were also present in some cases (Figure 3). Microscopically, there were no differences in tumor morphology between E2 or E2 + BHA-treated animals except for more invasiveness in E2-treated mammary tumors. Mammary tumors in E2-treated rats showed evidence of invasion of surrounding tissues by breakdown of the basal membrane of the affected tubule-lobular units or ductal units with atypical clusters and poorly formed glands invading the surrounding stroma, whereas animals cotreated with E2 + BHA harbored mammary tumors that were mostly encapsulated and rarely invasive in nature (Figure 3). Histopathological examination of tissue sections from nontarget organ liver of control and E2-treated rats as well as E2 + BHA group and their respective control BHA group did not show any evidence of tumor or dysplasia (data not shown).
TABLE 1.
Effect of E2 and BHA on Mammary Tumor Development
| Treatment Group | n | Tumor Incidence (%) |
Tumor Multiplicity |
Appearance of First Tumor (day) |
|---|---|---|---|---|
| Control | 10 | 0 | NA | NA |
| Estradiol (E2) | 11 | 82 | 3.1± 0.7 | 128 |
| BHA | 17 | 0 | NA | NA |
| BHA + E2 | 17 | 24* | 1.0* | 169 |
Column 1 lists different treatments each group of animals received. The number of animals per group (n) is listed in column 2. Percent tumor incidence after 240 days treatment period is listed in column 3, and the average number of tumors per tumor-bearing animal (tumor multiplicity) is listed in column 4. Column 5 contains the day on which the first tumor appeared in each group (appearance of first tumor).
Indicates significant difference (p < 0.05) between the E2 and E2 + BHA treated group.
FIGURE 1.
BHA exposure increases the latency of E2-induced breast tumors. Female ACI rats were treated with cholesterol, E2, cholesterol + BHA or E2 + BHA as described in the Methods section. Kaplan–Meier survival curves for tumor occurrence were plotted for each treatment group, and the log rank test was used to detect differences in tumor latency curves between groups. Animals in the control groups (cholesterol or cholesterol + BHA) did not develop any tumors and are represented by the same line on the graph (control).
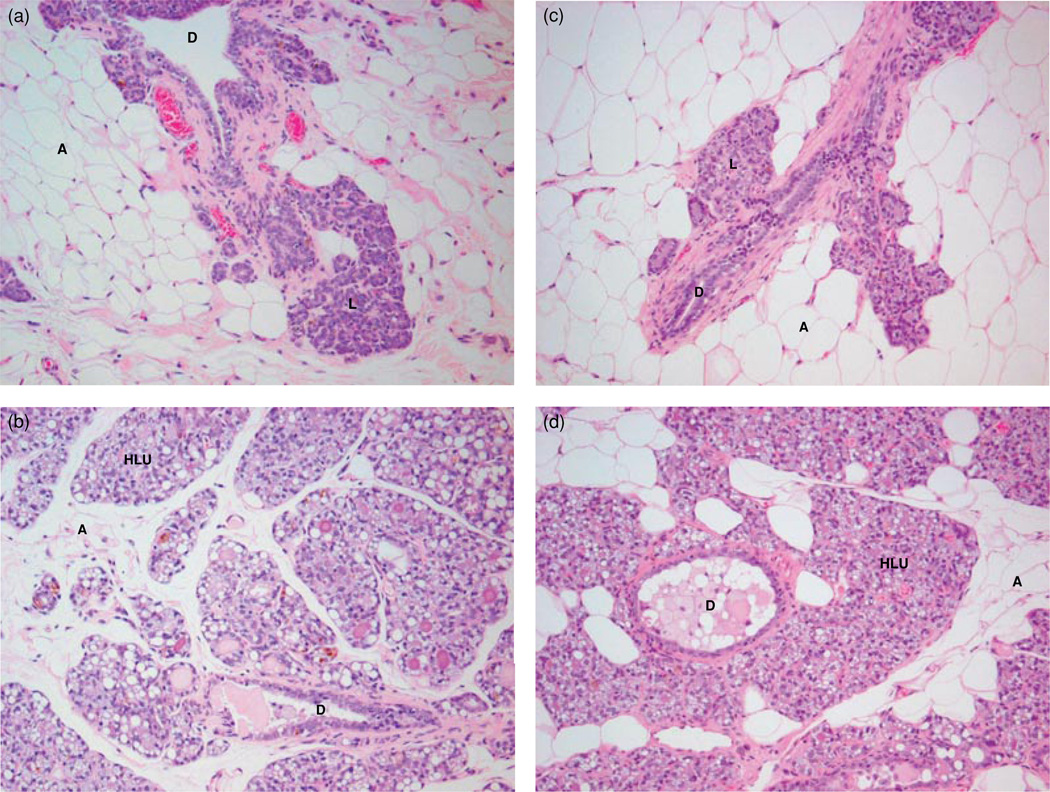
FIGURE 2.
Comparison of histopathology of mammary tissue from control rats to E2 or E2 + BHA-treated rats after 240 days of exposure (100×). Female ACI rats were treated with E2 or E2 + BHA for 240 days. Animals in the E2 or E2 + BHA groups were implanted with E2 pellets (s.c., 3 mg E2 + 17 mg cholesterol) for 240 days. Cholesterol and cholesterol + BHA controls were implanted with pellets containing 17 mg cholesterol only. BHA-treated rats were fed on BHA (0.7% w/w)mixed with diet. Histopathologic analyses were performed on mammary tissue of all rats treated with E2, BHA, and E2 + BHA. (a) The mammary gland of a representative control ACI rat shows normal lobular architecture (L) with branched ducts (D) and normal distribution of fat tissue/adipocytes (A); (b) E2-treated mammary gland shows increased proliferation with dilated ducts containing inspissated secretions (D) and increased proliferation and expansion of terminal lobular units (HLU) accompanied by compression of and expansion into the surrounding fat tissue/adipocytes (A); (c) the mammary gland of BHA only treated rats shows normal lobular architecture (L) with branched ducts (D) and normal distribution of fat tissue (A); (d) mammary from E2 + BHA-exposed rat is not significantly different from E2-exposed mammary tissue and displays increased proliferation with dilated ducts containing inspissated secretions (D) and increased proliferation and expansion of terminal lobular units (HLU) accompanied by compression of and expansion into the surrounding fat tissue (A).
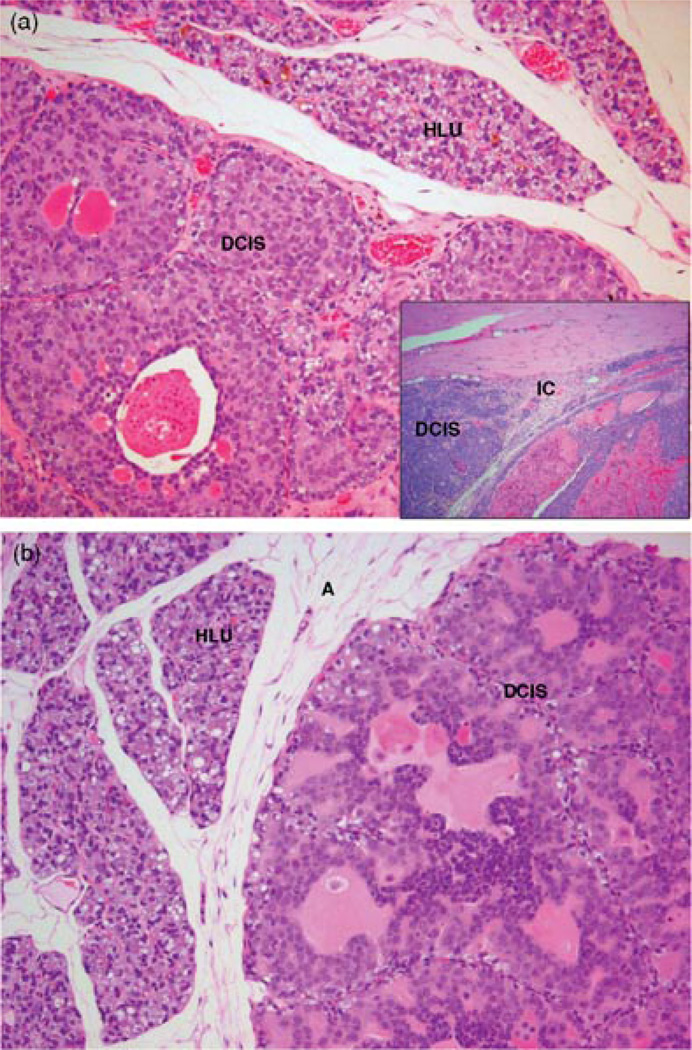
FIGURE 3.
Comparison of mammary tumors observed after 240 days of E2 or E2 + BHA treatment. Female ACI rats were treated with E2 or E2 + BHA for 240 days as described in the Methods section. (a) Mammary tissue from a rat treated with E2 shows ductal carcinoma in situ (DCIS) containing inspissated secretions in ducts (100×); Inset: Invasive adenocarcinoma was observed in mammary tissue of E2-treated rats (100×); (b) mammary tissue from an animal treated with E2 + BHA shows DCIS (100×). Hyperplastic lobules (HLU) and compressed surrounding fat tissue are also present.
BHA Reduces E2-Associated 8-isoPGF2α Formation
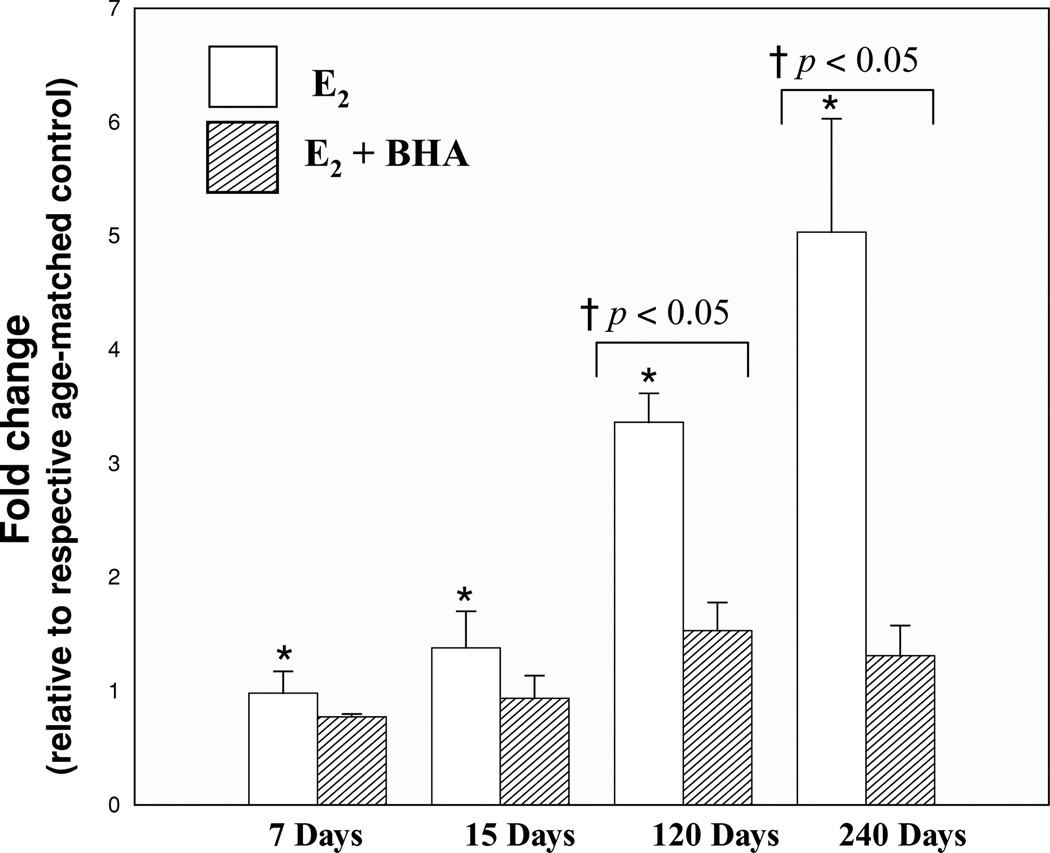
Total 8-isoPGF2α levels were quantified in the mammary and liver tissues of animals from the experimental (E2 and E2 + BHA) and control (cholesterol and BHA) groups. 8-isoPGF2α is an established marker of oxidative stress [38–41]. Fold changes in 8-isoPGF2α levels in mammary and liver tissues were calculated by comparing the levels in experimental groups to the levels in mammary and liver tissue in their respective control groups. No significant differences in liver 8-isoPGF2α levels were detected between control animals and those treated with E2 or E2 + BHA (data not shown). BHA treatment suppressed E2-associated increases in 8-isoPGF2α formation. Rats cotreated with BHA and E2 displayed significantly lower fold change in mammary 8-isoPGF2α levels relative to rats exposed to E2 alone (Figure 4). After 120 and 240 days exposure to E2, 8-isoPGF2α levels in mammary tissue reached approximately three- and five-fold higher, respectively, than controls, indicating a significant increase in 8-isoPGF2α levels. However, in animals cotreated with BHA and E2, mammary 8-isoPGF2α levels were only increased 1.5- and 1.3-fold over control levels after 120 and 240 days of treatment, respectively (Figure 4) indicating about two- and four-fold decreased following treatment with BHA.
FIGURE 4.
BHA inhibits E2-induced 8-isoPGF2α formation. Female ACI rats were treated with cholesterol, E2, cholesterol + BHA, or E2 + BHA as described in Methods section. 8-isoPGF2α levels were measured in mammary tissue from animals in each of these groups. 8-isoPGF2α levels were assessed in mammary tumor as well. Fold changes were calculated for E2-treated animals relative to age-matched cholesterol-treated controls. Fold changes were calculated for E2 + BHA-treated animals relative to age-matched BHA-treated controls. Fold change data for tumor tissue were determined by comparing 8-isoPGF2α levels in tumor tissue to levels detected in nontumor mammary tissue from age-matched control rats. These data are reported as an average of values obtained for at least eight different animals ± SEM. *indicates a p value < 0.05 relative to each group’s respective controls. †indicates a p value < 0.05 when comparing E2 and E2 + BHA groups.
BHA Reduces E2-Induced Alterations in the Activities of Antioxidant Enzymes
The activities of the antioxidant enzymes in a particular tissue depict the status of oxidative stress in that particular tissue or organ. The activities of antioxidant enzymes SOD, CAT, and GPx were quantified in the mammary tissue, mammary tumor, and liver tissue of rats treated with E2 or E2 + BHA, as well as in their respective control groups. SOD and GPx enzyme activity levels in mammary and tumor tissues were significantly different between E2 and cholesterol control group at 240 days treatment time point (Table 2). Cotreatment of rats with BHA and E2 reduced E2-induced changes in enzymatic activity. No significant differences were observed in the activities of antioxidant enzymes, SOD, CAT, and GPx between E2 + BHA and BHA alone when treated for 15, 120, and 240 days that suggests antioxidant role of BHA in E2 + BHA-treated group (Table 2). There were significant decreases in the fold changes of SOD and CAT enzymes activities in the E2 + BHA group compared to the E2-treated group after 240 days of treatment (Table 2).CAT activity in E2 + BHA mammary tissues was also significantly lower after 7 and 120 days of treatment. SOD activity in the E2-treated group was elevated 3.1-fold relative to control mammary tissue after 240 days of treatment. However, in rats exposed to E2 + BHA for 240 days, mammary tissue SOD activity was significantly reduced compared to the E2-treated group (Table 2). SOD and CAT activities were not significantly different in mammary tumors between E2 + BHA and E2-treated animals. No alterations in GPx activity were detected in mammary tissues or mammary tumors from animals in E2 + BHA groups. There was no significant difference in SOD, CAT, and GPx activity in liver tissues between E2 and their control group as well as between the E2 + BHA and the BHA group (data not shown).
TABLE 2.
Fold Changes in Antioxidant Enzyme Activities in Mammary and Tumor Tissue in the E2 and E2 + BHA-Exposed Group
| SOD |
CAT |
GPx |
||||
|---|---|---|---|---|---|---|
| Treatment Group | E2 | E2 + BHA | E2 | E2 + BHA | E2 | E2 + BHA |
| 7 days | 0.9 ± 0.3 | 0.8 ± 0.2 | 1.0 ± 0.1† | 0.6 ± 0.04* | 1.5 ± 0.3 | 1.0 ± 0.2 |
| 15 days | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.2 | 0.7 ± 0.09 | 1.1 ± 0.3 | 1.2 ± 0.6 |
| 120 days | 1.5 ± 0.2 | 1.2 ± 0.2 | 0.9 ± 0.1† | 0.6 ± 0.03 | 1.0 ± 0.2 | 0.8 ± 0.1 |
| 240 days | 3.1 ± 0.8*,† | 0.8 ± 0.2 | 1.2 ± 0.1† | 0.6 ± 0.06 | 1.4 ± 0.2 | 0.4 ± 0.2 |
| Tumor | 1.8 ± 0.4* | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.2 | 2.5 ± 0.4* | 0.9 ± 0.2 |
The activity of the antioxidant enzymes SOD, CAT, and GPx were measured in mammary tissue from animals in the control, BHA, E2 and E2 + BHA groups. Quantification of enzyme activity was performed on mammary tissue from animals in each group after 7, 15, 120, and 240 days of exposure as described in the Methods section. Enzyme activity was measured in mammary tumor tissue as well. Fold changes were calculated for E2 and E2+ BHA group relative to respective age-matched controls. Data are reported as an average of values obtained from at least eight different animals ± SE except in E2 + BHA-treated tumor group in which only four animals had tumor.
Indicates a significant difference (p < 0.05) compared to respective controls, whereas
indicates the significant difference (p < 0.05) between E2 and E2 + BHA-treated groups.
DISCUSSION
A number of epidemiological studies indicate that estrogen use is associated with breast carcinogenesis [11, 42, 43]. Despite extensive investigation, the carcinogenic mechanism(s) underlying estrogen action remain unresolved. Tumor induction is suggested to depend on estrogen metabolism and oxidative stress resulting from the redox cycling of estrogen metabolites as well as on the ER-dependent pathway of estrogen-induced cell growth [4, 11, 14, 44–50]. Metabolic activation of estrogens to catechol estrogens has been shown to be a prerequisite for the generation of oxidative stress [13].
In the present study, we used the ACI rat model of breast cancer to examine the role of oxidative stress in estrogen-mediated breast carcinogenesis. BHA, a known antioxidant, was used to examine whether decreasing oxidative stress could prevent E2-induced breast carcinogenesis. BHA is one of the several widely used antioxidant food additives that have been suggested to provide protection against chemical carcinogens and reduce toxicity. The exact mechanism for the anticarcinogenic activity of BHA administered subsequent to carcinogen administration remains unknown [51]. The anticarcinogenic activity is often ascribed to the ability of BHA to act as a free radical scavenger and/or induce “phase II” metabolic enzymes, those primarily involved with conjugation and detoxification reactions, as opposed to the cytochrome P450, containing-mixed function oxidases that perform the “phase I” oxidation reactions with a resulting decrease in the production of ultimate carcinogenic species [52–55]. BHA also alters the activity of a variety of enzymes involved in xenobiotics metabolism [52, 56, 57].
Histopathologic changes in mammary tissue were examined by a pathologist and oxidative stress markers, such as 8-isoPGF2α and the activities of antioxidant enzymes, were measured in mammary and liver tissues from E2 and E2 + BHA-treated rats, as well as in their respective controls. BHA treatment significantly reduced tumor incidence (Table 1) and increased tumor latency (Figure 1). In a previous study, decrease in the incidence of E2-induced kidney carcinogenesis in Syrian hamster on BHA administration has been demonstrated [58]. One of the explanations of increased tumor latency and decreased tumor incidence in the BHA-treated group may be a result of decreased oxidant stress in this group. Oxidant stress is considered to be essential for the production of DNA damage and mutations [59, 60]. Therefore, there may be a less chance of accumulation of genetic mutation and thus tumor induction in the E2 + BHA group as compared to E2-treated rats.
No apparent histopathologic changes were detected in liver, kidney, uterus, lung, and brain tissue in any of the treatment group (data not shown). BHA administration had no apparent effect on cellular proliferation of the mammary gland as histopathologic analyses showed no difference in cellular morphology between control and the BHA-treated group or between E2 and E2 + BHA-treated groups (Figure 2). These results suggest that BHA did not interfere with ER-dependent pathway in estrogen carcinogenesis. However, our experimental data indicate that BHA reduces E2-associated elevations in oxidative stress in breast tissue of ACI rats. Mammary tissue from rats cotreated with E2 and BHA for 120 and 240 days displayed significantly lower levels of 8-isoPGF2α, a marker of lipid peroxidation, relative to rats treated with E2 only (Figure 4). The E2-treated group showed an increase in antioxidant enzyme activities, which may be as a result of oxidative stress in this group that was decreased in the BHA-treated group. Increased SOD activity and no change in CAT activity in the E2-treated group may lead to more hydrogen peroxide accumulation and thus results in increased oxidant stress but BHA treatment decreases E2-induced increase in SOD and downregulate the CAT activity with no change in the GPx activity (Table 2). Overall, our results demonstrate that animals treated with E2 + BHA have less oxidative stress compared to E2 alone treated group and BHA inhibits the E2-induced breast carcinogenicity. The present studies strongly support the role of oxidative stress in estrogen-mediated breast carcinogenesis.
Acknowledgments
Contract Grant Sponsor: National Institutes of Health.
Contract Grant Numbers: ES009089 and CA 109551.
Footnotes
The authors have no conflict of interest at this time.
REFERENCES
- 1.Galati G, O’Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic Biol Med. 2004;37(3):287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 2.Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21(3):427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 3.Ju YH, Allred KF, Allred CD, Helferich WG. Genistein stimulates growth of human breast cancer cells in a novel, postmenopausal animal model, with low plasma estradiol concentrations. Carcinogenesis. 2006;27(6):1292–1299. doi: 10.1093/carcin/bgi370. [DOI] [PubMed] [Google Scholar]
- 4.Bhat HK, Calaf G, Hei TK, Loya T, Vadgama JV. Critical role of oxidative stress in estrogen-induced carcinogenesis. Proc Natl Acad Sci USA. 2003;100(7):3913–3918. doi: 10.1073/pnas.0437929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R, Muti P, Rogan E, Russo J, Santen R, et al. Catechol estrogen quinones as initiators of breast and other human cancers: Implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta. 2006;1766(1):63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 6.International Agency for Research on Cancer (IARC) Monographs on the evaluation of carcinogenic risks to humans: Overall evaluations of carcinogenicity, an updating of IARC monographs volumes 1 to 42. Supplement 7. Lyon, France: International Agency for Research on Cancer; 1987. [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer (IARC) Monographs on the evaluation of carcinogenic risks to humans: Hormonal contraception and postmenopausal hormone therapy. Vol. 72. Lyon, France: International Agency for Research on Cancer; 1999. pp. 474–530. [Google Scholar]
- 8.NTP. National Toxicology Program. Research Triangle Park, NC: 2002. Report on Carcinogens. [Google Scholar]
- 9.Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 10.McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ. 2000;321(7261):624–628. doi: 10.1136/bmj.321.7261.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344(4):276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 12.Liehr JG, Han X, Bhat HK. 32P-postlabelling in studies of hormonal carcinogenesis. IARC Sci Publ. 1993;124:149–155. [PubMed] [Google Scholar]
- 13.Patel MM, Bhat HK. Differential oxidant potential of carcinogenic and weakly carcinogenic estrogens: Involvement of metabolic activation and cytochrome P450. J Biochem Mol Toxicol. 2004;18(1):37–42. doi: 10.1002/jbt.20005. [DOI] [PubMed] [Google Scholar]
- 14.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354(3):270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 15.Cavalieri EL, Rogan EG. A unifying mechanism in the initiation of cancer and other diseases by catechol quinones. Ann NY Acad Sci. 2004;1028:247–257. doi: 10.1196/annals.1322.029. [DOI] [PubMed] [Google Scholar]
- 16.Cavalieri EL, Stack DE, Devanesan PD, Todorovic R, Dwivedy I, Higginbotham S, Johansson SL, Patil KD, Gross ML, Gooden JK, et al. Molecular origin of cancer: Catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc Natl Acad Sci USA. 1997;94(20):10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao K, Stack DE, Ramanathan R, Gross ML, Rogan EG, Cavalieri EL. Synthesis and structure elucidation of estrogen quinones conjugated with cysteine N-acetylcysteine, and glutathione. Chem Res Toxicol. 1998;11(8):909–916. doi: 10.1021/tx9702291. [DOI] [PubMed] [Google Scholar]
- 18.Singh KP, Roy D. Somatic mutations in stilbene estrogen-induced Syrian hamster kidney tumors identified by DNA fingerprinting. J Carcinog. 2004;3(1):4. doi: 10.1186/1477-3163-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Estrogens as endogenous genotoxic agents—DNA adducts and mutations. J Natl Cancer Inst Monogr. 2000;2000(27):75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- 20.Liehr JG, Fang WF, Sirbasku DA, Ari-Ulubelen A. Carcinogenicity of catechol estrogens in Syrian hamsters. J Steroid Biochem. 1986;24(1):353–356. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- 21.Roy D, Liehr JG. Estrogen, DNA damage and mutations. Mutat Res. 1999;424(1–2):107–115. doi: 10.1016/s0027-5107(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 22.Felty Q, Singh KP, Roy D. Estrogen-induced G1/S transition of G0-arrested estrogen-dependent breast cancer cells is regulated by mitochondrial oxidant signaling. Oncogene. 2005;24(31):4883–4893. doi: 10.1038/sj.onc.1208667. [DOI] [PubMed] [Google Scholar]
- 23.Felty Q, Xiong WC, Sun D, Sarkar S, Singh KP, Parkash J, Roy D. Estrogen-induced mitochondrial reactive oxygen species as signal-transducing messengers. Biochemistry. 2005;44(18):6900–6909. doi: 10.1021/bi047629p. [DOI] [PubMed] [Google Scholar]
- 24.Roy D, Cai Q, Felty Q, Narayan S. Estrogen-induced generation of reactive oxygen and nitrogen species, gene damage, and estrogen-dependent cancers. J Toxicol Environ Health B: Crit Rev. 2007;10(4):235–257. doi: 10.1080/15287390600974924. [DOI] [PubMed] [Google Scholar]
- 25.Liehr JG, Avitts TA, Randerath E, Randerath K. Estrogen-induced endogenous DNA adduction: Possible mechanism of hormonal cancer. Proc Natl Acad Sci USA. 1986;83(14):5301–5305. doi: 10.1073/pnas.83.14.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yager JD. Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr. 2000;2000(27):67–73. doi: 10.1093/oxfordjournals.jncimonographs.a024245. [DOI] [PubMed] [Google Scholar]
- 27.Turan VK, Sanchez RI, Li JJ, Li SA, Reuhl KR, Thomas PE, Conney AH, Gallo MA, Kauffman FC, Mesia-Vela S. The effects of steroidal estrogens in ACI rat mammary carcinogenesis: 17β-estradiol, 2-hydroxyestradiol, 4-hydroxyestradiol, 16α-hydroxyestradiol, and 4-hydroxyestrone. J Endocrinol. 2004;183(1):91–99. doi: 10.1677/joe.1.05802. [DOI] [PubMed] [Google Scholar]
- 28.Arnerlov C, Emdin SO, Cajander S, Bengtsson NO, Tavelin B, Roos G. Intratumoral variations in DNA ploidy and s-phase fraction in human breast cancer. Anal Cell Pathol. 2001;23(1):21–28. doi: 10.1155/2001/430674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li JJ, Papa D, Davis MF, Weroha SJ, Aldaz CM, El-Bayoumy K, Ballenger J, Tawfik O, Li SA. Ploidy differences between hormone- and chemical carcinogen-induced rat mammary neoplasms: Comparison to invasive human ductal breast cancer. Mol Carcinog. 2002;33(1):56–65. doi: 10.1002/mc.10022. [DOI] [PubMed] [Google Scholar]
- 30.Li JJ, Weroha SJ, Lingle WL, Papa D, Salisbury JL, Li SA. Estrogen mediates aurora-A overexpression, centrosome amplification, chromosomal instability, and breast cancer in female ACI rats. Proc Natl Acad Sci USA. 2004;101(52):18123–18128. doi: 10.1073/pnas.0408273101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li SA, Weroha SJ, Tawfik O, Li JJ. Prevention of solely estrogen-induced mammary tumors in female ACI rats by tamoxifen: Evidence for estrogen receptor mediation. J Endocrinol. 2002;175(2):297–305. doi: 10.1677/joe.0.1750297. [DOI] [PubMed] [Google Scholar]
- 32.Makris A, Allred DC, Powles TJ, Dowsett M, Fernando IN, Trott PA, Ashley SE, Ormerod MG, Titley JC, Osborne CK. Cytological evaluation of biological prognostic markers from primary breast carcinomas. Breast Cancer Res Treat. 1997;44(1):65–74. doi: 10.1023/a:1005717924761. [DOI] [PubMed] [Google Scholar]
- 33.Shull JD, Spady TJ, Snyder MC, Johansson SL, Pennington KL. Ovary-intact, but not ovariectomized female ACI rats treated with 17β-estradiol rapidly develop mammary carcinoma. Carcinogenesis. 1997;18(8):1595–1601. doi: 10.1093/carcin/18.8.1595. [DOI] [PubMed] [Google Scholar]
- 34.Weroha SJ, Li SA, Tawfik O, Li JJ. Overexpression of cyclins D1 and D3 during estrogen-induced breast oncogenesis in female ACI rats. Carcinogenesis. 2006;27(3):491–498. doi: 10.1093/carcin/bgi278. [DOI] [PubMed] [Google Scholar]
- 35.Han X, Liehr JG. DNA single-strand breaks in kidneys of Syrian hamsters treated with steroidal estrogens: Hormone-induced free radical damage preceding renal malignancy. Carcinogenesis. 1994;15(5):997–1000. doi: 10.1093/carcin/15.5.997. [DOI] [PubMed] [Google Scholar]
- 36.Wang MY, Liehr JG. Induction by estrogens of lipid peroxidation and lipid peroxide-derived malonaldehyde-DNA adducts in male Syrian hamsters: Role of lipid peroxidation in estrogen-induced kidney carcinogenesis. Carcinogenesis. 1995;16(8):1941–1945. doi: 10.1093/carcin/16.8.1941. [DOI] [PubMed] [Google Scholar]
- 37.Mense SM, Remotti F, Bhan A, Singh B, El-Tamer M, Hei TK, Bhat HK. Estrogen-induced breast cancer: Alterations in breast morphology and oxidative stress as a function of estrogen exposure. Toxicol Appl Pharmacol. 2008;232(1):78–85. doi: 10.1016/j.taap.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milne GL, Yin H, Brooks JD, Sanchez S, Jackson Roberts L, II, Morrow JD. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods Enzymol. 2007;433:113–126. doi: 10.1016/S0076-6879(07)33006-1. [DOI] [PubMed] [Google Scholar]
- 39.Montuschi P, Barnes PJ, Roberts LJ., II Isoprostanes: Markers and mediators of oxidative stress. FASEB J. 2004;18(15):1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 40.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., II A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci USA. 1990;87(23):9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pratico D, Lawson JA, FitzGerald GA. Cyclooxygenase-dependent formation of the isoprostane, 8-epi prostaglandin F2 alpha. J Biol Chem. 1995;270(17):9800–9808. doi: 10.1074/jbc.270.17.9800. [DOI] [PubMed] [Google Scholar]
- 42.Henderson BE, Ross RK, Pike MC, Casagrande JT. Endogenous hormones as a major factor in human cancer. Cancer Res. 1982;42(8):3232–3239. [PubMed] [Google Scholar]
- 43.Lupulescu A. Estrogen use and cancer incidence: A review. Cancer Invest. 1995;13(3):287–295. doi: 10.3109/07357909509094464. [DOI] [PubMed] [Google Scholar]
- 44.Cavalieri E, Rogan E. Catechol quinones of estrogens in the initiation of breast, prostate, and other human cancers: Keynote lecture. Ann NY Acad Sci. 2006;1089:286–301. doi: 10.1196/annals.1386.042. [DOI] [PubMed] [Google Scholar]
- 45.Cavalieri EL, Rogan EG, Chakravarti D. Initiation of cancer and other diseases by catechol ortho-quinones: A unifying mechanism. Cell Mol Life Sci. 2002;59(4):665–681. doi: 10.1007/s00018-002-8456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jefcoate CR, Liehr JG, Santen RJ, Sutter TR, Yager JD, Yue W, Santner SJ, Tekmal R, Demers L, Pauley R, et al. Tissue-specific synthesis and oxidative metabolism of estrogens. J Natl Cancer Inst Monogr. 2000;2000(27):95–112. doi: 10.1093/oxfordjournals.jncimonographs.a024248. [DOI] [PubMed] [Google Scholar]
- 47.Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocr Rev. 2000;21(1):40–54. doi: 10.1210/edrv.21.1.0386. [DOI] [PubMed] [Google Scholar]
- 48.Liehr JG, Roy D. Free radical generation by redox cycling of estrogens. Free Radic Biol Med. 1990;8(4):415–423. doi: 10.1016/0891-5849(90)90108-u. [DOI] [PubMed] [Google Scholar]
- 49.Liehr JGHX, Bhat HK. In: Postlabelling methods for detection of DNA adducts. Phillips DH, Castegnaro M, Bartsch H, editors. Lyon, France: IARC Scientific Publications; 1993. pp. 149–155. [Google Scholar]
- 50.Weisz J, Bui QD, Roy D, Liehr JG. Elevated 4-hydroxylation of estradiol by hamster kidney microsomes: A potential pathway of metabolic activation of estrogens. Endocrinology. 1992;131(2):655–661. doi: 10.1210/endo.131.2.1386303. [DOI] [PubMed] [Google Scholar]
- 51.McCormick DL, Major N, Moon RC. Inhibition of 7,12-dimethylbenz(a)anthracene-induced rat mammary carcinogenesis by concomitant or postcarcinogen antioxidant exposure. Cancer Res. 1984;44(7):2858–2863. [PubMed] [Google Scholar]
- 52.Cha YN, Heine HS. Comparative effects of dietary administration of 2(3)-tert-butyl-4-hydroxyanisole and 3,5-di-tert-butyl-4-hydroxytoluene on several hepatic enzyme activities in mice and rats. Cancer Res. 1982;42(7):2609–2615. [PubMed] [Google Scholar]
- 53.Iverson F. In vivo studies on butylated hydroxyanisole. Food Chem Toxicol. 1999;37(9–10):993–997. doi: 10.1016/s0278-6915(99)00091-5. [DOI] [PubMed] [Google Scholar]
- 54.Lam LK, Wattenberg LW. Effects of butylated hydroxyanisole on the metabolism of benzo[a]pyrene by mouse liver microsomes. J Natl Cancer Inst. 1977;58(2):413–417. doi: 10.1093/jnci/58.2.413. [DOI] [PubMed] [Google Scholar]
- 55.Sydor W, Jr, Chou MW, Yang SK, Yang CS. Regioselective inhibition of benzo[a]pyrene metabolism by butylated hydroxyanisole. Carcinogenesis. 1983;4(2):131–136. doi: 10.1093/carcin/4.2.131. [DOI] [PubMed] [Google Scholar]
- 56.Dock L, Cha YN, Jernstrom B, Moldeus P. Differential effects of dietary BHA on hepatic enzyme activities and benzo[a]pyrene metabolism in male and female NMRI mice. Carcinogenesis. 1982;3(1):15–19. doi: 10.1093/carcin/3.1.15. [DOI] [PubMed] [Google Scholar]
- 57.Marnett LJ, Reed GA, Johnson JT. Prostaglandin synthetase dependent benzo(a)pyrene oxidation: Products of the oxidation and inhibition of their formation by antioxidants. Biochem Biophys Res Commun. 1977;79(2):569–576. doi: 10.1016/0006-291x(77)90195-4. [DOI] [PubMed] [Google Scholar]
- 58.Roy D, Liehr JG. Inhibition of estrogen-induced kidney carcinogenesis in Syrian hamsters by modulators of estrogen metabolism. Carcinogenesis. 1990;11(4):567–570. doi: 10.1093/carcin/11.4.567. [DOI] [PubMed] [Google Scholar]
- 59.Cadet J, Delatour T, Douki T, Gasparutto D, Pouget JP, Ravanat JL, Sauvaigo S. Hydroxyl radicals and DNA base damage. Mutat Res. 1999;424(1–2):9–21. doi: 10.1016/s0027-5107(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y. Bulky DNA lesions induced by reactive oxygen species. Chem Res Toxicol. 2008;21(2):276–281. doi: 10.1021/tx700411g. [DOI] [PubMed] [Google Scholar]