Abstract
α-Synuclein is a chaperone-like protein implicated in Parkinson’s disease (PD). Among α-synuclein’s normal functions is an ability to bind to and stimulate the activity of the protein phosphatase 2A (PP2A) catalytic subunit in vitro and in vivo. PP2A activity is impaired in PD and in dementia with Lewy Bodies in brain regions harboring α-synuclein aggregates. Using PP2A as the readout, we measured PP2A activity in response to α-synuclein, ceramides, and FTY720, and then on the basis of those results, we created new FTY720 compounds. We then measured the effects of those compounds in dopaminergic cells. In addition to stimulating PP2A, all three compounds stimulated the expression of brain derived neurotrophic factor and protected MN9D cells against tumor-necrosis-factor-α-associated cell death. FTY720-C2 appears to be more potent while FTY720-Mitoxy targets mitochondria. Importantly, FTY720 is already FDA approved for treating multiple sclerosis and is used clinically worldwide. Our findings suggest that FTY720 and our new FTY720-based compounds have considerable potential for treating synucleinopathies such as PD.
Keywords: α-Synuclein, BDNF, ceramides, Parkinson’s, PP2A, synucleinopathy, triphenylphosphonium
Protein phosphatase 2A (PP2A) is a ubiquitous heterotrimeric dephosphorylating enzyme consisting of a structural A subunit, a catalytic C subunit, and regulatory B subunits.1 The A and C subunits form a core PP2A enzyme dimer that binds to different regulatory B subunits to affect PP2A localization.2 PP2A is an important modulator of normal neuronal function,3 and the enzyme is found localized to mitochondria.4 We discovered that the catalytic subunit of PP2A interacts with the small chaperone-like protein α-synuclein (α-Syn), in dopaminergic neuronal cells and brain, in a manner to stimulate PP2A activity.5,6 We were also the first to show that α-Syn localizes to mitochondria.7 α-Syn has a tendency to aggregate in neurons to form the pathognomonic lesions named Lewy bodies and Lewy neurites in diseases collectively referred to as synucleinopathies. These include Parkinson’s disease (PD), dementia with Lewy Bodies (DLB), multiple system atrophy (MSA), and Alzheimer’s disease.8 PP2A activity is impaired in post-mortem brains from PD, DLB,9 and Alzheimer’s disease.10 In PD, aggregation of α-Syn contributes not only to dysregulation of PP2A9 but also to impaired mitochondrial function and oxidative stress, particularly in dopaminergic neurons of the midbrain substantia nigra pars compacta.11 This raises the possibility that therapies aimed at enhancing PP2A activity and mitochondrial function may be advantageous for treating PD.
We12 and others13 have thus been exploring PP2A as a novel therapeutic target for PD. To this end, we began searching the literature for PP2A-stimulatory compounds and identified FTY720,14 also called fingolimod, a synthetic sphingosine analogue that has been FDA approved as Gilenya, for treating relapsing-remitting multiple sclerosis (MS). FTY720 is given orally and readily crosses the blood–brain barrier15 and data suggest that the drug significantly improves the health of MS patients.16 FTY720 mode of action includes an ability to block the exit of lymphocytes from the lymph nodes, thereby preventing their entry into blood and/or the central nervous system. FTY720 can also be phosphorylated by sphingosine kinase 2 (SPHK2), after which FTY720-P can bind to sphingosine 1 phosphate receptors (S1PRs), which are widely expressed on brain neurons, oligodendrocytes, astrocytes, and microglia. Important new findings demonstrate that phosphorylated and unphosphorylated forms of FTY720 both confer significant neuroprotection in vitro and in vivo.17
Sphingosine-1-phosphate (S1P) and ceramides are biologically active signaling molecules that contribute to cell fate decisions. In contrast to ceramides, which tend to promote cell death, S1P typically enhances cell survival.18 Both sphingosine and FTY720 can be efficiently phosphorylated by SPHK2, allowing them to bind to and activate S1P receptors at the cell surface, although receptor-independent functions have also been reported.19 Importantly, FTY720 is neuroprotective in numerous animal models17,20,21 and has been shown to stimulate the expression of brain derived neurotrophic factor (BDNF),22 which has significant neuroprotective effects on dopaminergic neurons. Together these findings raise the possibility that FTY720 could be a novel therapy for treating synucleinopathies such as PD, perhaps by both neuroprotective and PP2A modulatory effects.
We previously demonstrated that α-Syn stimulates PP2A activity in dopaminergic MN9D cells and PC12 cells as well as in the brains of α-Syn transgenic mice.23 Using recombinant proteins and cell-free assays we herein measured the dose-dependent stimulation of PP2A catalytic subunit by recombinant wild type α-Syn (detailed methods in the Supporting Information). PP2A activity increased in response to α-Syn, with the most potent effect noted for 5.0 μM (Figure 1).
Figure 1.
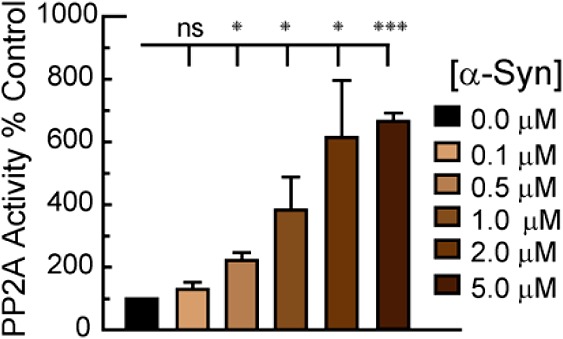
α-Syn stimulates PP2A activity in cell-free assays. Data represent the mean ± SEM of 4–7 experiments performed in duplicate. ns, not significant; * p < 0.05; *** p < 0.001 (ANOVA).
Ceramide is the basic structural unit of all sphingolipids, and both ceramides and sphingolipids play key roles in brain development as well as in neurodegeneration.24 Ceramides are known to stimulate PP2A activity,1,25 so we next assessed PP2A activation in response to different length ceramides to compare their effects to α-Syn. Using 5.0 μM concentrations of ceramide C2, C6, and C10, we saw similar PP2A stimulation in response to C2 and C6, while C10 had little if any effect (Figure 2a). We then assessed a dose response of C2-ceramide on PP2A activation and found similar stimulation of PP2A catalytic subunit in response to 2.5–10 μM concentrations (Figure 2b).
Figure 2.
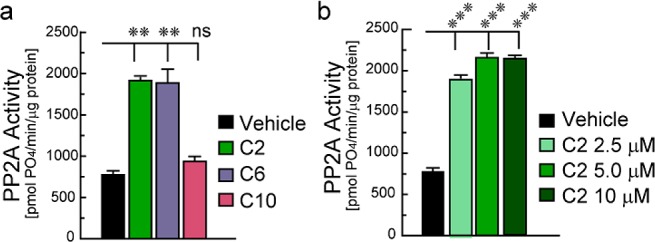
(a) PP2A activity in cell-free assays in response to 5.0 μM C2-, C6-, and C10-ceramides. (b) Dose effect of C2-ceramide on PP2A. Data represent mean ± SEM of 4 independent experiments. ns, not significant; ** p < 0.01; *** p < 0.001 (ANOVA).
As mentioned above, though stimulatory of PP2A activity, ceramides tend to induce neuronal cell death.18,26 In contrast, both S1P and FTY720 tend to be neuroprotective, especially at low doses.27 Others have demonstrated that cellular PP2A activation occurs in response to the parent compound FTY720.28 To assess this in our model systems, we first measured FTY720 effects on PP2A in cell-free assays and then in cultured cells. We found that 5.0 μM FTY720 significantly stimulated the activity of recombinant PP2A catalytic subunit (Figure 3a). Using PC12 cells, we noted that cellular PP2A was significantly more active 30–120 min after 5.0 μM FTY720 treatment as compared to baseline PP2A activity in vehicle treated cells (Figure 3b), verifying PP2A activation by FTY720 in dopaminergic cells.28
Figure 3.
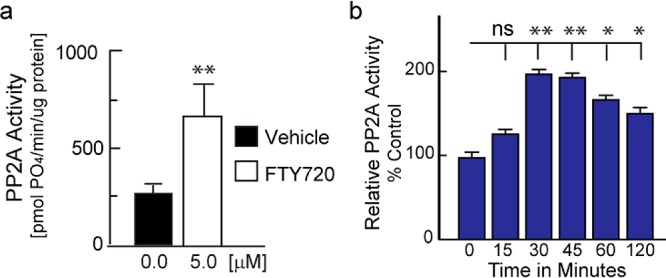
Evaluation of PP2A activity in response to 5.0 μM FTY720 in (a) cell-free assays and (b) PC12 cells. Data represent the mean ± SEM of 3–6 independent experiments performed in duplicate. * p < 0.05; ** p < 0.01 (t test, a; ANOVA, b).
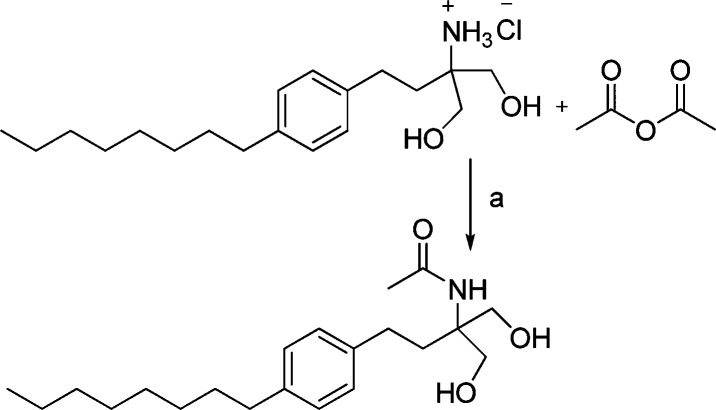
Having determined that PP2A activity was stimulated by FTY720 and, further, that C2 ceramide significantly stimulated PP2A within the range observed for α-Syn (Figure 1), we then elected to modify FTY720 by adding a C2 moiety, not a C2-ceramide, to create the novel compound FTY720-C2, as a potentially more efficacious compound to counteract the abnormalities induced by synucleinopathy in neurodegenerative diseases9 (Scheme 1).
Scheme 1. Generation of FTY720-C2.
Reagents and conditions: (a) CHCl3, NaHCO3 (aq.), 0 °C–rt, 14 h, 88%.
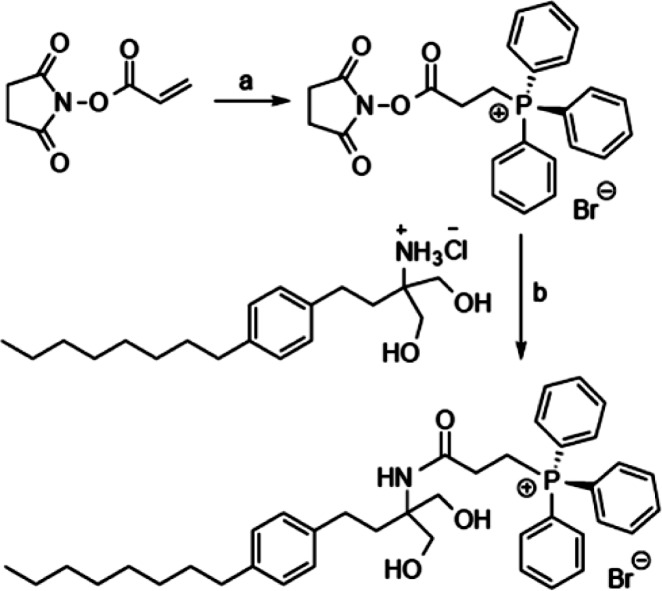
As previously noted, mitochondria are organelles that become severely compromised in PD, and furthermore, PP2A is often found localized to the outer membrane of these organelles.4,29 With a goal of targeting FTY720 to mitochondria we generated beta-triphenylphosphoniumpropanamide-FTY720, which we call FTY720-Mitoxy (Scheme 2). Triphenylphosphonium salts are lipophilic cations that are capable of conveying molecular cargo to mitochondria.30 This localization is thought to occur by the positive charge of a tetravalent phosphonium distributed over the large surface area, provided by the three phenyl groups, which results in a lipophilic species that is able to pass through the cell membrane lipid bilayer followed by an accumulation at the mitochondrion due to the high inner membrane potential of that organelle.
Scheme 2. Generation of FTY720-Mitoxy.
Reagents and conditions: (a) triphenylphosphonium hydrobromide, CH2Cl2, 0 °C–rt, 3 h, 100%; (b) Et3N, CH2Cl2, 0 °C–rt, 23 h, 82%.
Using the new compounds, we next compared the effects of FTY720, C2-ceramide, FTY720-C2, and FTY720-Mitoxy on recombinant PP2A catalytic subunit in cell-free assays. All compounds significantly stimulated recombinant PP2A activity, with ∼8-fold stimulation by C2-ceramide and by the C2 modified FTY720-C2, and ∼4-fold stimulation of PP2A by FTY720 parent compound and mitochondrial localizing FTY720-Mitoxy (Figure 4a). This range of PP2A activation was similar to α-Syn (Figure 1), an endogenous stimulator of PP2A activity in neuronal cells.5,6,9,11 We next confirmed PP2A activation by FTY720, FTY720-C2, and FTY720-Mitoxy in the dopaminergic neuronal cell line, MN9D (Figure 4b).
Figure 4.
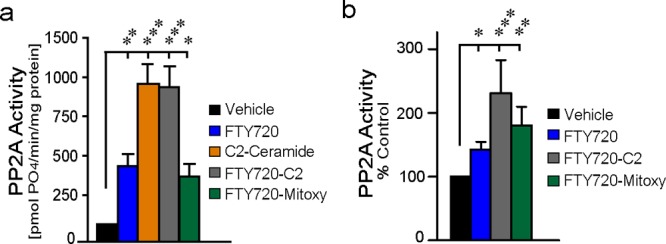
Activation of PP2A in (a) cell free assays using 5.0 μM FTY720, C2-ceramide, FTY720-C2, or FTY720-Mitoxy; and (b) MN9D cells by 5.0 μM FTY720, FTY720-C2, or FTY720-Mitoxy. Data represent the mean ± SEM of 3–4 independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001 (ANOVA).
To assess potential neuroprotective effects in the MN9D cells, we measured the metabolic activity of cells treated 72 h with 0.01–5.0 μM of all FTY720-based compounds. In these longer term cultures by 3 days, cell death was noted in response to >1.5 μM concentrations of all three compounds (not shown), while doses in the range of 0.04–0.16 μM enhanced metabolism (Figure 5a), but cell division was not affected (cell number % control; vehicle 100 ± 4.15; FTY720 98 ± 3.69; FTY720-C2 91.44 ± 2.05; FTY720-Mitoxy 101.37 ± 3.28; p = 0.25, ANOVA).
Figure 5.
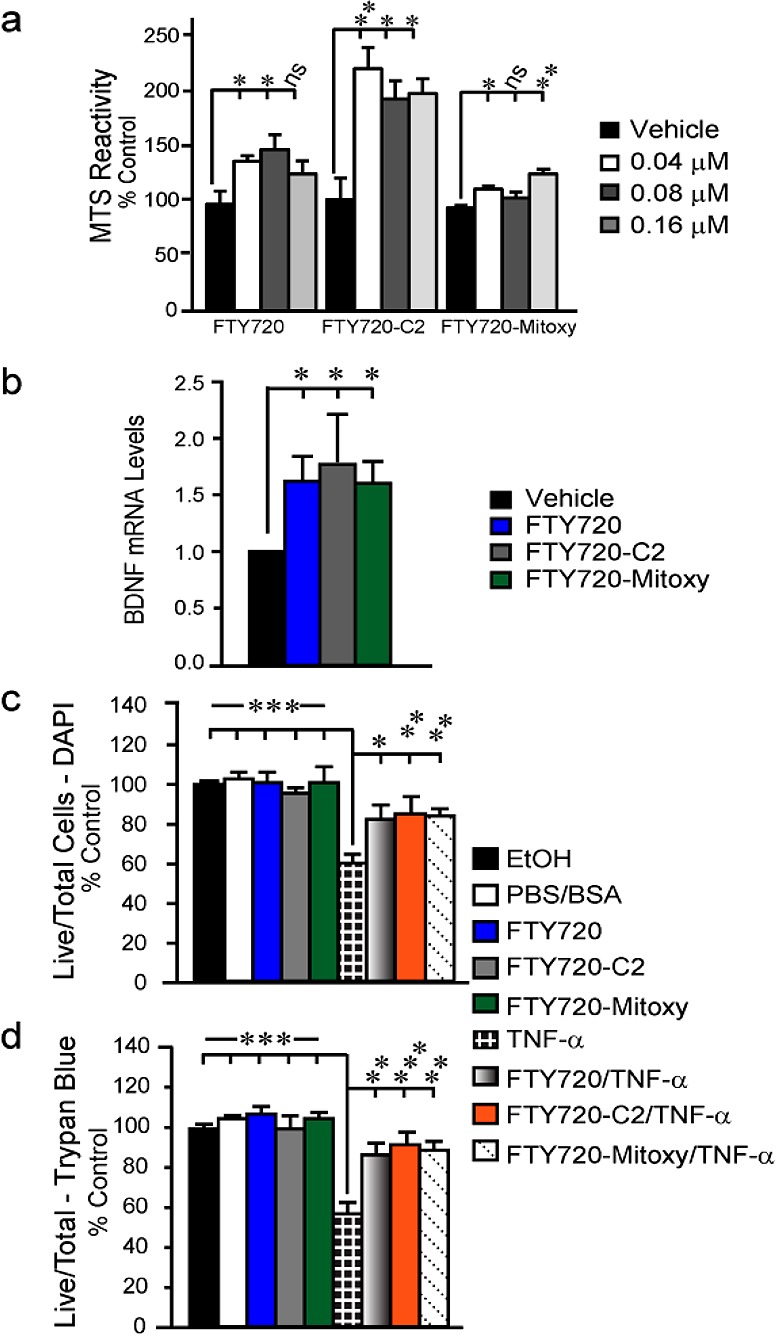
Differentiated MN9D cell response to FTY720, FTY720-C2, and FTY720-Mitoxy as measured by (a) MTS reactivity, (b) quantitative RT-PCR for BDNF mRNA, 2–(ΔΔCt), and (c,d) counts of viable cell after treatment with FTY720-based compounds ± TNF-α and assessed by (c) DAPI and (d) Trypan blue staining. Data are from 3–4 independent experiments; mean ± SEM; ns, not significant; * p < 0.05; ** p < 0.01; *** p < 0.001 (ANOVA).
Because FTY720 can stimulate BDNF expression in neuronal cells by 24 h,22 we measured BDNF mRNA levels at baseline (vehicle) and in response to 24 h treatment with 0.16 μM FTY720, FTY720-C2, or FTY720-Mitoxy (Figure 5b). BDNF mRNA levels were significantly increased by all three FTY720 compounds in MN9D cells. Nevertheless, the data suggested that the FTY720-based compounds have neuroprotective properties. The substantia nigra of PD patients has high levels of tumor necrosis factor alpha (TNF-α), a pro-inflammatory cytokine that induces apoptosis in differentiated MN9D dopaminergic cells.31 We differentiated MN9D cells for 72 h during which we treated with 0.16 μM FTY720, FTY720-C2, or FTY720-Mitoxy ± 1 ng of TNF-α. Cell counts, using established methods,32 verified that all FTY720 compounds significantly blocked toxicity associated with 8 h TNF-α treatment of dopaminergic neuronal cells (Figure 5c,d).
Cumulatively, these findings support further preclinical assessment of all three FTY720 compounds for their potential utility as therapeutics for PD and related neurodegenerative disorders with synucleinopathy.
Acknowledgments
We thank Drs. J. Rosenberg, D. Mitchell, D. Moss, and O. Sundin, Ms. G. Herrera, Mr. I. Segura and Mr. B. Marcus for valuable scientific contributions.
Glossary
Abbreviations Used
- α-Syn
alpha-synuclein
- BDNF
brain derived neurotrophic factor
- DLB
dementia with Lewy bodies
- MS
multiple sclerosis
- MSA
multiple system atrophy
- MTS
methylthiazol-tetrazolium-salt metabolic activity assay
- PP2A
protein phosphatase 2A
- S1P
sphingosine 1 phosphate
- SPHK2
sphingosine kinase 2
- TNF-α
tumor necrosis factor-alpha
Supporting Information Available
Methods for all assays, cell cultures, treatment conditions, and compound synthesis. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
R.G.P. designed and directed experiments. E.V.J. and W.H.G. did PP2A assays. J.V.-M. and H.L. did cell culture and related analyses. S.K. did quantitative RT-PCR and graphical artwork. J.B.A. and R.C. synthesized and confirmed the purity of FTY720-based compounds. The manuscript was written with contributions from all authors who approved the final version.
Author Contributions
∥ (J.V.-M., S.K., and E.V.) These authors contributed equally to this work.
Support was provided by the National Institutes of Health, NINDS [R01-NS42094], TTUHSC Startup, and the Coldwell Foundation (to R.G.P.) and the Ministry of Education of China [BS2010YY036] (to H.L.).
The authors declare no competing financial interest.
Dedication
This work is dedicated to M. J. Fox, R. Byer, and J. Cordy and in memory of L. “Rusty” Lanelli.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Dobrowsky R. T.; Kamibayashi C.; Mumby M. C.; Hannun Y. A. Ceramide activates heterotrimeric protein phosphatase 2A. J. Biol. Chem. 1993, 2682115523–30. [PubMed] [Google Scholar]
- Mumby M. C.; Walter G. Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Physiol. Rev. 1993, 734673–99. [DOI] [PubMed] [Google Scholar]
- Gotz J.; Schild A. Transgenic and knockout models of PP2A. Methods Enzymol. 2003, 366, 390–403. [DOI] [PubMed] [Google Scholar]
- Dagda R. K.; Zaucha J. A.; Wadzinski B. E.; Strack S. A developmentally regulated, neuron-specific splice variant of the variable subunit Bbeta targets protein phosphatase 2A to mitochondria and modulates apoptosis. J. Biol. Chem. 2003, 2782724976–85. [DOI] [PubMed] [Google Scholar]
- Peng X.; Tehranian R.; Dietrich P.; Stefanis L.; Perez R. G. Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J. Cell Sci. 2005, 118Pt 153523–30. [DOI] [PubMed] [Google Scholar]
- Lou H.; Montoya S. E.; Alerte T. N.; Wang J.; Wu J.; Peng X.; Hong C. S.; Friedrich E. E.; Mader S. A.; Pedersen C. J.; Marcus B. S.; McCormack A. L.; Di Monte D. A.; Daubner S. C.; Perez R. G. Serine 129 phosphorylation reduces the ability of alpha-synuclein to regulate tyrosine hydroxylase and protein phosphatase 2A in vitro and in vivo. J. Biol. Chem. 2010, 2852317648–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez R. G.; Waymire J. C.; Lin E.; Liu J. J.; Guo F.; Zigmond M. J. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J. Neurosci. 2002, 2283090–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M.; Spillantini M. G.; Del Tredici K.; Braak H. 100 years of Lewy pathology. Nat. Rev. Neurol. 2013, 9113–24. [DOI] [PubMed] [Google Scholar]
- Wu J.; Lou H.; Alerte T. N.; Stachowski E. K.; Chen J.; Singleton A. B.; Hamilton R. L.; Perez R. G. Lewy-like aggregation of alpha-synuclein reduces protein phosphatase 2A activity in vitro and in vivo. Neuroscience 2012, 207, 288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong C. X.; Singh T. J.; Grundke-Iqbal I.; Iqbal K. Phosphoprotein phosphatase activities in Alzheimer disease brain. J. Neurochem. 1993, 613921–7. [DOI] [PubMed] [Google Scholar]
- Parihar M. S.; Parihar A.; Fujita M.; Hashimoto M.; Ghafourifar P. Alpha-synuclein overexpression and aggregation exacerbates impairment of mitochondrial functions by augmenting oxidative stress in human neuroblastoma cells. Int. J. Biochem. Cell Biol. 2009, 41102015–24. [DOI] [PubMed] [Google Scholar]
- Farrell K. F.; Krishnamachari S.; Villanueva E.; Lou H.; Alerte T. N. M.; Peet E.; Drolet R. E.; Perez R. G. Non-motor parkinsonian pathology in aging A53T α-Synuclein mice is associated with progressive synucleinopathy and altered enzymatic function. J. Neurochem. 2014, 1284536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. W.; Chen W.; Junn E.; Im J. Y.; Grosso H.; Sonsalla P. K.; Feng X.; Ray N.; Fernandez J. R.; Chao Y.; Masliah E.; Voronkov M.; Braithwaite S. P.; Stock J. B.; Mouradian M. M. Enhanced phosphatase activity attenuates alpha-synucleinopathy in a mouse model. J. Neurosci. 2011, 31196963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks J. J.; Santhanam R.; Walker C. J.; Roof S.; Harb J. G.; Ferenchak G.; Eisfeld A. K.; Van Brocklyn J. R.; Briesewitz R.; Saddoughi S. A.; Nagata K.; Bittman R.; Caligiuri M. A.; Abdel-Wahab O.; Levine R.; Arlinghaus R. B.; Quintas-Cardama A.; Goldman J. M.; Apperley J.; Reid A.; Milojkovic D.; Ziolo M. T.; Marcucci G.; Ogretmen B.; Neviani P.; Perrotti D. Antagonistic activities of the immunomodulator and PP2A-activating drug FTY720 (Fingolimod, Gilenya) in Jak2-driven hematologic malignancies. Blood 2013, 122, 1923–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V.; Billich A.; Baumruker T.; Heining P.; Schmouder R.; Francis G.; Aradhye S.; Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discovery 2010, 911883–97. [DOI] [PubMed] [Google Scholar]
- Oderda G.; Archer M.. Fingolimod (Gilenya®) Drug Review. University of Utah College of Pharmacy: Final Report, 2013. [Google Scholar]
- Di Pardo A.; Amico E.; Favellato M.; Castrataro R.; Fucile S.; Squitieri F.; Maglione V. FTY720 (fingolimod) is a neuroprotective and disease-modifying agent in cellular and mouse models of Huntington disease. Hum. Mol. Genet. 2014, 2392251–65. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn J. R.; Williams J. B. The control of the balance between ceramide and sphingosine-1-phosphate by sphingosine kinase: oxidative stress and the seesaw of cell survival and death. Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol. 2012, 163126–36. [DOI] [PubMed] [Google Scholar]
- Sim-Selley L. J.; Goforth P. B.; Mba M. U.; Macdonald T. L.; Lynch K. R.; Milstien S.; Spiegel S.; Satin L. S.; Welch S. P.; Selley D. E. Sphingosine-1-phosphate receptors mediate neuromodulatory functions in the CNS. J. Neurochem. 2009, 11041191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y.; Yemisci M.; Kim H. H.; Yung L. M.; Shin H. K.; Hwang S. K.; Guo S.; Qin T.; Alsharif N.; Brinkmann V.; Liao J. K.; Lo E. H.; Waeber C. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann. Neurol. 2011, 691119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F.; Liu Y.; Li X.; Wang Y.; Wei D.; Jiang W. Fingolimod (FTY720) inhibits neuroinflammation and attenuates spontaneous convulsions in lithium-pilocarpine induced status epilepticus in rat model. Pharmacol., Biochem. Behav. 2012, 1032187–96. [DOI] [PubMed] [Google Scholar]
- Deogracias R.; Yazdani M.; Dekkers M. P.; Guy J.; Ionescu M. C.; Vogt K. E.; Barde Y. A. Fingolimod, a sphingosine-1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. U.S.A. 2012, 1093514230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras J. L.; Perez R. G.. Potential Contribution of Alpha-Synuclein Dysregulation to Parkinson’s Disease Pathology. In Alpha-Synuclein; Kanowitz M. P. a. H. C., Ed.; NOVA Science Publishers: New York, 2014. [Google Scholar]
- Mencarelli C.; Martinez-Martinez P. Ceramide function in the brain: when a slight tilt is enough. Cell. Mol. Life Sci. 2013, 702181–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfant C. E.; Kishikawa K.; Mumby M. C.; Kamibayashi C.; Bielawska A.; Hannun Y. A. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J. Biol. Chem. 1999, 2742920313–7. [DOI] [PubMed] [Google Scholar]
- Toman R. E.; Movsesyan V.; Murthy S. K.; Milstien S.; Spiegel S.; Faden A. I. Ceramide-induced cell death in primary neuronal cultures: upregulation of ceramide levels during neuronal apoptosis. J. Neurosci. Res. 2002, 683323–30. [DOI] [PubMed] [Google Scholar]
- Miron V. E.; Schubart A.; Antel J. P. Central nervous system-directed effects of FTY720 (fingolimod). J. Neurol. Sci. 2008, 2741–213–7. [DOI] [PubMed] [Google Scholar]
- Perrotti D.; Neviani P. Protein phosphatase 2A (PP2A), a drugable tumor suppressor in Ph1(+) leukemias. Cancer Metastasis Rev. 2008, 272159–68. [DOI] [PubMed] [Google Scholar]
- Exner N.; Lutz A. K.; Haass C.; Winklhofer K. F. Mitochondrial dysfunction in Parkinson’s disease: molecular mechanisms and pathophysiological consequences. EMBO J. 2012, 31143038–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reily C.; Mitchell T.; Chacko B. K.; Benavides G.; Murphy M. P.; Darley-Usmar V. Mitochondrially targeted compounds and their impact on cellular bioenergetics. Redox Biol. 2013, 1186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez T. N.; Chen X.; Bandyopadhyay S.; Merrill A. H.; Tansey M. G. Ceramide sphingolipid signaling mediates tumor necrosis factor (TNF)-dependent toxicity via caspase signaling in dopaminergic neurons. Mol. Neurodegener. 2012, 7, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugarte S. D.; Lin E.; Klann E.; Zigmond M. J.; Perez R. G. Effects of GDNF on 6-OHDA-induced death in a dopaminergic cell line: Modulation by inhibitors of PI3 kinase and MEK. J. Neurosci. Res. 2003, 731105–112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.