Abstract
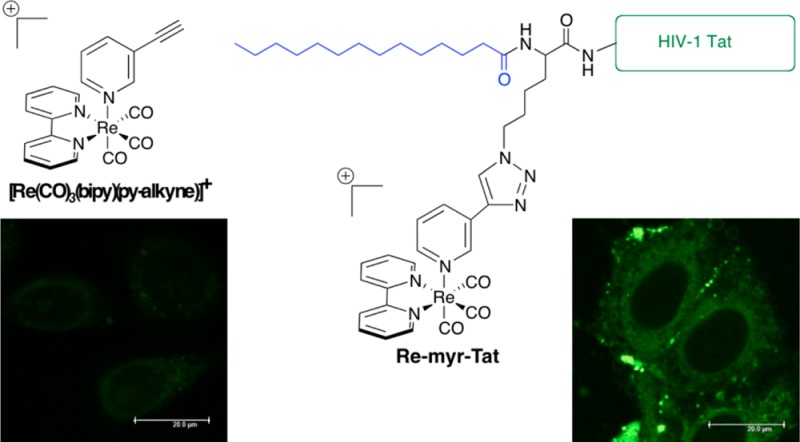
Re(I) tricarbonyl polypyridine-based complexes are particularly attractive metal complexes in the field of inorganic chemical biology due to their luminescent properties, ease of conjugation to targeting biomolecules, and the possibility to prepare their “hot” 99mTc analogues for radioimaging. In this study, we prepared and characterized a novel, “clickable” complex, [Re(2,2′-bipyridine)(3-ethynylpyridine)(CO)3](BF4) ([Re(CO)3(bipy)(py-alkyne)](BF4)), exhibiting the characteristic luminescent properties and moderate cytotoxicity of this general class of compound. Using Cu(I)-catalyzed “click” chemistry, the complex was efficiently attached to a lipidated peptide known to increase cell permeability, namely, the myristoylated HIV-1 Tat peptide (myr-Tat), to give Re-myr-Tat. Fluorescence microscopy localization in human cervical cancer cells (HeLa) confirmed enhanced cellular uptake of Re-myr-Tat compared with [Re(CO)3(bipy)(py-alkyne)](BF4), and cytotoxicity studies showed that this resulted in an increase in potency to a level comparable with cisplatin (13.0 ± 2.0 μM).
Keywords: Anticancer, fluorescence microscopy, medicinal organometallic chemistry, peptide, rhenium complexes
Re(I) tricarbonyl [2 + 1] complexes based on polypyridine-derived ligands, such as (Re(bipy)(L)(CO)3) (L = monodentate ligand), have attracted considerable attention in the past decade as catalysts,1 photosensitizers in photocatalytic water reduction,2,3 and CO-releasing molecules.4 They remain, however, best known for their outstanding photochemical properties (large Stokes shifts, long emission lifetimes, and resistance to photobleaching),5 which make them excellent candidates for cellular imaging and ion sensing applications.6−14 Their use in these areas has been spurred on by their good biocompatibility15 and the possibility to incorporate targeting vectors via the pyridine or bipyridine-based ligands.9,16,17 Significantly, the “hot” 99mTc analogues of these Re compounds could also be prepared, making them promising multimodal agents.16,18−20
While Re(I) tricarbonyl polypyridine-based complexes are normally only moderately toxic, or essentially nontoxic, several other Re(I) compounds have been reported to be as active or even more potent than cisplatin.8,21−28 Given how active many organometallic compounds are known to be against cancer cells lines,29−34 it is surprising that the cytotoxic potential of these Re(I) complexes has not yet been fully explored, especially in light of their aforementioned advantages.
In this work, we aimed to improve the cytotoxicity of a Re(I) compound, namely, [Re(CO)3(bipy)(py-alkyne)]+, whose moderate cytotoxicity was discovered by serendipity in the course of a separate study. To do this, we envisaged enhancing cellular uptake of the complex via conjugation to a myristoylated HIV-1 Tat peptide (Scheme 1). HIV-1 Tat is a membrane translocation sequence from HIV, while myristic acid is a saturated linear fatty acid that naturally occurs as a post-translational protein modification. Both myristic acid and myristoylated Tat peptide were shown to increase the cellular uptake of compounds when conjugated to them.35,36 In this study, the Re complex was appended to an azide-modified myristoylated Tat peptide via “click” chemistry. Over the past few years, click chemistry has been successfully employed to couple other organometallic compounds to peptides, either by solid-phase or solution-phase methods.37−40
Scheme 1. Bioconjugation of [Re(CO)3(bipy)(py-alkyne)]+ to a Myristoylated Tat Peptide.
[Re(CO)3(bipy)(py-alkyne)](BF4) was synthesized following an established literature procedure employed for Re(I) fac-tricarbonyl bipyridyl complexes with a substituted pyridine ligand.41 In brief, the initially formed Re tricarbonyl bipyridyl chloride complex was activated via halide abstraction as the corresponding acetonitrile complex. The acetonitrile ligand was then displaced by 3-ethynylpyridine. The formation and purity of the desired product was confirmed by 1H and 13C NMR, HR-MS (Figures S1–S3, Supporting Information) and elemental analysis. Of note, loss of the monodentate pyridine ligand (m/z 427.0 [M-py]+) during ionization was observed in the MS spectrum (Figure S3, Supporting Information). [Re(CO)3(bipy)(py-alkyne)](BF4) exhibits the typical photophysical properties for this type of Re(I) complex.10,17,25,42−50 The UV–vis absorption spectra showed intense bands at 250–330 nm (absorption coefficients around 15 000 M–1 cm–1) and a weaker shoulder at 350–360 nm (about 4500 M–1cm–1) (Figure S5, Supporting Information). The former are normally attributed to spin-allowed intraligand transitions (1IL = (π → π*) (bipyridine and pyridine), while the latter is assigned to spin-allowed metal-to-ligand charge transfer (1MLCT) [dπ(Re) → π*(bipy)]. Upon irradiation in the MLCT band (355 nm), [Re(CO)3(bipy)(py-alkyne)](BF4) displayed a strong emission centered at 550 nm (Figure S6, Supporting Information). Long, submicrosecond luminescence lifetimes (Table 1) and large Stokes shift indicate the phosphorescent nature of the emission,51 which can be ascribed to a triplet 3MLCT [dπ(Re) → π*(bipy)] transition, as observed for similar complexes.4,12 Typical emission lifetimes and quantum yields of Re(CO)3(bipy)(L) complexes encompass a relatively broad range (0.05–9.6 μs and 0.002–0.59, respectively).52 The values measured for [Re(CO)3(bipy)(py-alkyne)](BF4) are, in fact, very close to those of structurally similar Re(CO)3(bipy)(L) complexes, such as isothiocyanate-substituted pyridine complexes.17 Of note, shorter lifetimes in water compared to acetonitrile can be explained by the greater polarity of water, which stabilizes the 3MLCT excited state, thus lowering its energy and facilitating nonradiative processes.53,54 The complex itself can be solubilized in pure water up to 0.79 ± 0.13 mM (25 °C).
Table 1. Emission Lifetimes and Quantum Yields of [Re(CO)3(bipy)(py-alkyne)](BF4).
| solvent | aerated | degassed | quantum yields (aerated) |
|---|---|---|---|
| H2O | 117 ± 2 ns | 128 ± 3 ns | 0.0048 ± 0.0005 |
| acetonitrile | 168 ± 6 ns | 329 ± 1 ns | 0.011 ± 0.001 |
Since some studies have previously noted the loss of the monodentate pyridine ligand in solution for this type of complex,16,18−20 the stability of [Re(CO)3(bipy)(py-alkyne)](BF4) in water and human blood plasma was assessed. Following an experimental procedure similar to that previously reported by our group,28,55−57 the complex and diazepam (used as an internal standard due to its known stability in blood plasma and water) were incubated in human plasma or double distilled water for up to 72 h. The aqueous phase was then extracted with dichloromethane and the organic phase analyzed by UPLC-MS. Two peaks corresponding to [Re(CO)3(bipy)(py-alkyne)]+ (1.7 min, m/z 530.1 [M]+ and 427.1 [M – ligand]+) and diazepam (2.1 min, m/z 285.2 [M + H]+) could be clearly identified (Figures S9–S12, Supporting Information). The percentage of decomposed [Re(CO)3(bipy)(py-alkyne)]+ was then calculated using diazepam as the internal standard. As shown in Figure 1, decomposition proceeded significantly faster in human blood plasma (half-life of approximately 22 h) than pure water (half-life of approximately 5 days), probably due to the substitution of the pyridine ligand by stonger donor groups present in blood plasma proteins (e.g., histidine or cysteine). These results are consistent with the recent study by the Valliant group, which showed a marked dependence of the pyridine ligand lability on its basicity/leaving group ability.16 The plasma stability of [Re(CO)3(bipy)(py-alkyne)](BF4) is on par with the most stable compounds reported by Valliant and co-workers.16
Figure 1.
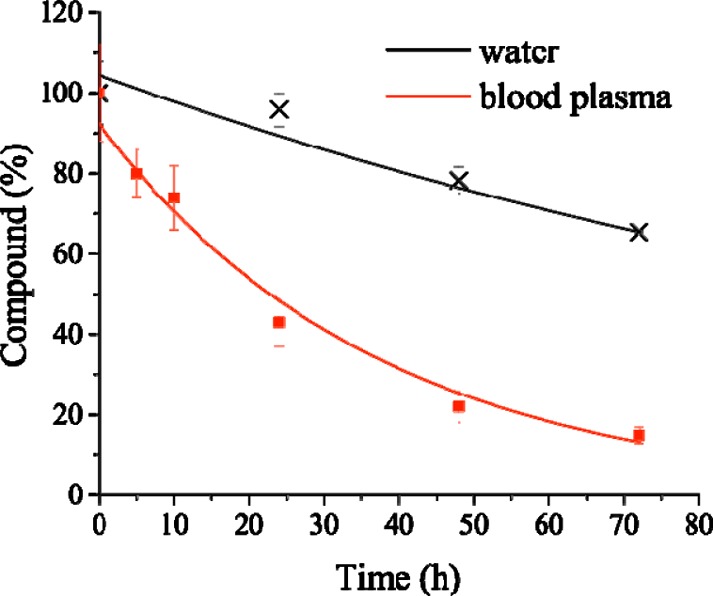
[Re(CO)3(bipy)(py-alkyne)]+ stability in human blood plasma and water (double distilled) at 37 °C.
The azide-modified myristoylated Tat peptide (myr-Tat) was prepared via standard solid-phase peptide synthesis techniques and then purified by preparative HPLC to give a sticky yellow solid, which was unambiguously characterized by HR-MS and HPLC (Figures S13 and S14, Supporting Information). It was then successfully conjugated to [Re(CO)3(bipy)(py-alkyne)](BF4) via Cu(I)-catalyzed “click” chemistry using similar experimental conditions to those reported by Fokin and co-workers.58 The crude compound was purified by preparative HPLC to yield Re-myr-Tat as a pale yellow solid, which was characterized by UPLC-MS and MALDI-TOF (Figures S15 and S16, Supporting Information). A single peak was observed in the UPLC-MS chromatogram, and consistent with the MS spectrum of the complex, both the desired product and fragments resulting from pyridine ligand dissociation appeared in the MS spectrum (m/z 338.9 [M – Re(CO)3(bipy) + 6H]6+, 406.4 [M – Re(CO)3(bipy) + 5H]5+, 491.6 [M + 4H]5+, 507.8 [M – Re(CO)3(bipy) + 4H]4+, 614.2 [M + 3H]4+, and 818.7 [M + 2H]3+). In the MALDI-TOF spectrum, only the fragmented product (m/z 2026.4 [M – Re(CO)3(bipy)+H]+) was detected due to the harsher ionization conditions compared to ESI-MS. The presence of the fac-Re(CO)3 core in the Re-myr-Tat bioconjugate was unambiguously confirmed by the presence of the CO stretching bands in the IR spectrum, which appeared in the same range as that of [Re(CO)3(bipy)(py-alkyne)](BF4) (Figure S17, Supporting Information). The influence of the myr-Tat moiety on the lipophilicity of the resulting Re-myr-Tat bioconjugate was evaluated by measuring the distribution coefficient between octanol and phosphate buffer, pH 7.01 (logD7.01), using a similar procedure to one used by our group for Ru complexes.59 Interestingly, although myr-Tat contains both a long lipophilic fatty acid chain and a highly positively charged peptide sequence, the net effect is an increase in lipophilicity, namely, from a logD7.01 of −0.36 ± 0.05 for [Re(CO)3(bipy)(py-alkyne)]+ to 0.86 ± 0.15 for Re-myr-Tat. This change could potentially improve the cellular uptake of Re-myr-Tat, as higher lipophilicity can favor the entry of compounds into cells and hence enhance the cytotoxicity.59−63
With the desired organometallic complex and bioconjugate in hand, we investigated the intracellular fate of both compounds in human cervical cancer cells (HeLa) by fluorescence microscopy. The cells were first incubated for 2 h with an appropriate concentration of each compound, then fixed with formaldehyde and finally imaged. Figure 2 shows a pronounced difference in emission intensity between the cells treated with [Re(CO)3(bipy)(py-alkyne)](BF4) (B) or Re-myr-Tat (C). Indeed, while the concentration of Re-myr-Tat in the cell medium was five times lower than that of the complex, the cells incubated with Re-myr-Tat appeared much brighter, indicating significantly higher uptake (it is important to keep in mind that the intensity of the emission signal of a compound in the cells sometimes fails to correlate with uptake, as luminescence can be quenched in the cellular environment).28,64 In terms of subcellular localization, the complex and the bioconjugate were visualized in the cytoplasm (although weak signals were sometimes detected in the nucleoli of cells incubated with the complex), a localization typically observed for this type of Re complex.10,12,15,16 Of note, while the HIV-1 Tat peptide sequence has been reported to promote nuclear localization,65 with a myristoylated Tat derivative dual-labeled with Gd-DOTA and fluorescein previously shown to stain both nucleoli and nuclear membranes,36 this was not the case for Re-myr-Tat. Our microscopy images were recordered at a relatively low Re-myr-Tat concentration that left most of the cells alive after 2 h. The Gd-DOTA myr-Tat derivative, however, was imaged at a much higher concentration (260 μM), at which about 60% of the cells were already dying.36 When HeLa cells were incubated with a higher concentration (2.5-fold increase) of Re-myr-Tat, localization in nuclear membrane and nucleoli was also observed (Figure S18, Supporting Information). However, the cells appeared to be already slightly affected by the conjugate at this concentration, so this change in localization could be due to toxin stress.
Figure 2.
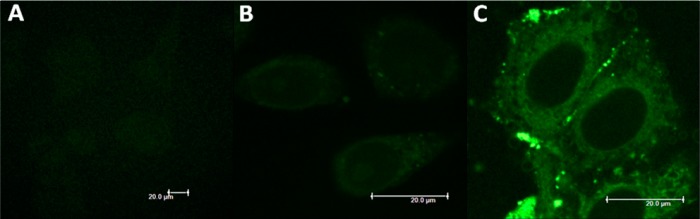
Fluorescence microscopy of HeLa cells fixed after 2 h: (A) untreated cells; (B) treated with [Re(CO)3(bipy)(py-alkyne)](BF4) at 100 μM; and (C) treated with Re-myr-Tat at 20 μM.
Next, the cytotoxicity of [Re(CO)3(bipy)(py-alkyne)](BF4) and Re-myr-Tat toward HeLa cells was evaluated by incubating the cells with increasing concentrations of the compounds for 48 h and quantifying cell viability using the resazurin assay.66 The toxicity of our compounds was compared with that of an established metal-containing anticancer drug, namely, cisplatin. Although several studies report on the cytotoxicity of Re(I) tricarbonyl bipyridine–pyridine complexes, their antiproliferative effect has generally been found to range from nonexistent to moderate; [Re(CO)3(bipy)(py-alkyne)](BF4) does not deviate from this trend (Table 2). A series of similar complexes, namely, Re(phen)(diaminopy)(CO)3, display IC50 values 2–3-fold higher than cisplatin.25 Significantly, however, the coupling of [Re(CO)3(bipy)(py-alkyne)]+ to the myristoylated Tat peptide was found to increase the cytotoxicity of the resulting bioconjugate, bringing it on par with cisplatin.
Table 2. Cytotoxicity Data (IC50 Values) for [Re(CO)3(bipy)(py-alkyne)](BF4), Re-myr-Tat and Cisplatin Towards HeLa Cellsa.
| compd | HeLa IC50 (μM) |
|---|---|
| [Re(CO)3(bipy)(py-alkyne)]+ | 29.9 ± 6.1 |
| Re-myr-Tat | 13.0 ± 2.0 |
| cisplatin | 9.1 ± 2.8 |
Experiments were performed in triplicate.
In conclusion, in this work, we prepared and characterized a new clickable Re(I) complex, which could be successfully conjugated to an azide-containing myristoylated Tat peptide to give Re-myr-Tat. Cytotoxicity and biological studies with HeLa cells revealed that the antiproliferative effect of the complex could be enhanced considerably by the addition of a cell uptake-enhancing biomolecule.
Acknowledgments
The authors thank the Center for Microscopy and Image Analysis (University of Zurich) and PD Dr. Laurent Bigler and Urs Stalder for their help to record MALDI-TOF and HR-ESI spectra.
Glossary
Abbreviations Used
- Bipy
bipyridine
- DOTA
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
- ESI
electrospray ionization
- HeLa
cervical cancer cell line
- HIV
human immunodeficiency virus
- HPLC
high-performance liquid chromatography
- L
ligand
- NMR
nuclear magnetic resonance
- HR-MS
high-resolution mass spectroscopy
- MALDI-TOF
matrix-assisted laser desorption ionization time-of-flight
- MLCT
metal-to-ligand charge transfer
- Py
pyridine
- Tat
trans-activator of transcription (here, a cell-permeating peptide sequence derived from HIV-1 Tat protein)
- UPLC
ultraperformance liquid chromatography
- UV–vis
ultraviolet–visible light
Supporting Information Available
Characterization of the compounds (NMR, MS, UPLC-MS, UV, emission, and IR spectra), emission quantum yields measurements, additional information on stability measurements (UPLC-MS) and fluorescence microscopy images. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by the Swiss National Science Foundation (Professorship No. PP00P2_133568 and Research Grants No. 200021_129910 and No. 200020_146776 to G.G.), the University of Zurich (G.G and S.F.), the Stiftung für wissenschaftliche Forschung of the University of Zurich (G.G and S.F.), the Stiftung zur Krebsbekämpfung (S.F.), the Huggenberger-Bischoff Stiftung (S.F.), the University of Zurich Priority Program (S.F.), and the Australian Research Council through the award of a Future Fellowship to B.G.
The authors declare no competing financial interest.
Supplementary Material
References
- Kutal C.; Weber M. A.; Ferraudi G.; Geiger D. A mechanistic investigation of the photoinduced reduction of carbon dioxide mediated by tricarbonylbromo(2,2′-bipyridine)rhenium(I). Organometallics 1985, 4, 2161–2166. [Google Scholar]
- Probst B.; Rodenberg A.; Guttentag M.; Hamm P.; Alberto R. A highly stable rhenium-cobalt system for photocatalytic H2 production: unraveling the performance-limiting steps. Inorg. Chem. 2010, 49, 6453–6460. [DOI] [PubMed] [Google Scholar]
- Probst B.; Guttentag M.; Rodenberg A.; Hamm P.; Alberto R. Photocatalytic H2 production from water with rhenium and cobalt complexes. Inorg. Chem. 2011, 50, 3404–3412. [DOI] [PubMed] [Google Scholar]
- Pierri A. E.; Pallaoro A.; Wu G.; Ford P. C. A luminescent and biocompatible photocorm. J. Am. Chem. Soc. 2012, 134, 18197–18200. [DOI] [PubMed] [Google Scholar]
- Worl L. A.; Duesing R.; Chen P.; Ciana L. D.; Meyer T. J. Photophysical properties of polypyridyl carbonyl complexes of rhenium(I). J. Chem. Soc., Dalton Trans. 1991, 849–858. [Google Scholar]
- Lo K. K.-W.; Choi A. W.-T.; Law W. H.-T. Applications of luminescent inorganic and organometallic transition metal complexes as biomolecular and cellular probes. Dalton Trans. 2012, 41, 6021–6047. [DOI] [PubMed] [Google Scholar]
- Lo K. K.-W.; Zhang K. Y.; Li S. P.-Y. Recent exploitation of luminescent rhenium(I) tricarbonyl polypyridine complexes as biomolecular and cellular probes. Eur. J. Inorg. Chem. 2011, 3551–3568. [Google Scholar]
- Louie M.-W.; Choi A. W.-T.; Liu H.-W.; Chan B. T.-N.; Lo K. K.-W. Synthesis, emission characteristics, cellular studies, and bioconjugation properties of luminescent rhenium(I) polypyridine complexes with a fluorous pendant. Organometallics 2012, 31, 5844–5855. [Google Scholar]
- Louie M.-W.; Liu H.-W.; Lam M. H.-C.; Lam Y.-W.; Lo K. K.-W. Luminescent rhenium(I) polypyridine complexes appended with an α-d-glucose moiety as novel biomolecular and cellular probes. Chem.—Eur. J. 2011, 17, 8304–8308. [DOI] [PubMed] [Google Scholar]
- Fernàndez-Moreira V.; Ortego M. L.; Williams C. F.; Coogan M. P.; Villacampa M. D.; Gimeno M. C. Bioconjugated rhenium(I) complexes with amino acid derivatives: synthesis, photophysical properties, and cell imaging studies. Organometallics 2012, 31, 5950–5957. [Google Scholar]
- Thorp-Greenwood F. L.; Balasingham R. G.; Coogan M. P. Organometallic complexes of transition metals in luminescent cell imaging applications. J. Organomet. Chem. 2012, 714, 12–21. [Google Scholar]
- Choi A. W.-T.; Poon C.-S.; Liu H.-W.; Cheng H.-K.; Lo K. K.-W. Rhenium(I) polypyridine complexes functionalized with a diaminoaromatic moiety as phosphorescent sensors for nitric oxide. New J. Chem. 2013, 37, 1711–1719. [Google Scholar]
- Clede S.; Lambert F.; Sandt C.; Kascakova S.; Unger M.; Harte E.; Plamont M.-A.; Saint-Fort R.; Deniset-Besseau A.; Gueroui Z.; Hirschmugl C.; Lecomte S.; Dazzi A.; Vessieres A.; Policar C. Detection of an estrogen derivative in two breast cancer cell lines using a single core multimodal probe for imaging (SCoMPI) imaged by a panel of luminescent and vibrational techniques. Analyst 2013, 138, 5627–5638. [DOI] [PubMed] [Google Scholar]
- Clede S.; Lambert F.; Sandt C.; Gueroui Z.; Refregiers M.; Plamont M.-A.; Dumas P.; Vessieres A.; Policar C. A rhenium tris-carbonyl derivative as a single core multimodal probe for imaging (SCoMPI) combining infrared and luminescent properties. Chem. Commun. 2012, 48, 7729–7731. [DOI] [PubMed] [Google Scholar]
- Fernandez-Moreira V.; Thorp-Greenwood F. L.; Amoroso A. J.; Cable J.; Court J. B.; Gray V.; Hayes A. J.; Jenkins R. L.; Kariuki B. M.; Lloyd D.; Millet C. O.; Williams C. F.; Coogan M. P. Uptake and localisation of rhenium fac-tricarbonyl polypyridyls in fluorescent cell imaging experiments. Org. Biomol. Chem. 2010, 8, 3888–3901. [DOI] [PubMed] [Google Scholar]
- Pitchumony T. S.; Banevicius L.; Janzen N.; Zubieta J.; Valliant J. F. Isostructural nuclear and luminescent probes derived from stabilized [2 + 1] rhenium(I)/technetium(I) organometallic complexes. Inorg. Chem. 2013, 52, 13521–13528. [DOI] [PubMed] [Google Scholar]
- Lo K. K.-W.; Ng D. C.-M.; Hui W.-K.; Cheung K.-K. Luminescent rhenium(I) polypyridine complexes with an isothiocyanate moiety-versatile labelling reagents for biomolecules. Dalton Trans. 2001, 2634–2640. [Google Scholar]
- Gottschaldt M.; Koth D.; Müller D.; Klette I.; Rau S.; Görls H.; Schäfer B.; Baum R. P.; Yano S. Synthesis and structure of novel sugar-substituted bipyridine complexes of rhenium and 99m-technetium. Chem.—Eur. J. 2007, 13, 10273–10280. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Chen X. Preparation and characterization of 99mTc(CO)3-BPy-RGD complex as alphav beta-3 integrin receptor-targeted imaging agent. Appl. Radiat. Isot. 2007, 65, 70–78. [DOI] [PubMed] [Google Scholar]
- Alberto R. The chemistry of technetium–water complexes within the manganese triad: challenges and perspectives. Eur. J. Inorg. Chem. 2009, 21–31. [Google Scholar]
- Zhang J.; Vittal J. J.; Henderson W.; Wheaton J. R.; Hall I. H.; Hor T. S. A.; Yan Y. K. Tricarbonylrhenium(I) complexes of phosphine-derivatized amines, amino acids and a model peptide: structures, solution behavior and cytotoxicity. J. Organomet. Chem. 2002, 650, 123–132. [Google Scholar]
- Wang W.; Yan Y. K.; Hor T. S. A.; Vittal J. J.; Wheaton J. R.; Hall I. H. Synthesis, X-ray structures, and cytotoxicity of rhenium(I) carbonyl 2-(dimethylamino)ethoxide complexes. Polyhedron 2002, 21, 1991–1999. [Google Scholar]
- Bartholoma M. D.; Vortherms A. R.; Hillier S.; Ploier B.; Joyal J.; Babich J.; Doyle R. P.; Zubieta J. Synthesis, cytotoxicity, and insight into the mode of action of Re(CO)3 thymidine complexes. ChemMedChem 2010, 5, 1513–1529. [DOI] [PubMed] [Google Scholar]
- Can D.; Peindy N’Dongo H. W.; Spingler B.; Schmutz P.; Raposinho P.; Santos I.; Alberto R. The [(Cp)M(CO)3] (M = Re, 99mTc) building block for imaging agents and bioinorganic probes: perspectives and limitations. Chem. Biodiversity 2012, 9, 1849–1866. [DOI] [PubMed] [Google Scholar]
- Choi A. W.-T.; Louie M.-W.; Li S. P.-Y.; Liu H.-W.; Chan B. T.-N.; Lam T. C.-Y.; Lin A. C.-C.; Cheng S.-H.; Lo K. K.-W. Emissive behavior, cytotoxic activity, cellular uptake, and pegylation properties of new luminescent rhenium(I) polypyridine poly(ethylene glycol) complexes. Inorg. Chem. 2012, 51, 13289–13302. [DOI] [PubMed] [Google Scholar]
- Ho J.; Lee W. Y.; Koh K. J. T.; Lee P. P. F.; Yan Y.-K. Rhenium(I) tricarbonyl complexes of salicylaldehyde semicarbazones: synthesis, crystal structures and cytotoxicity. J. Inorg. Biochem. 2013, 119, 10–20. [DOI] [PubMed] [Google Scholar]
- Viola-Villegas N.; Rabideau A. E.; Cesnavicious J.; Zubieta J.; Doyle R. P. Targeting the folate receptor (FR): imaging and cytotoxicity of Re(I) conjugates in FR-overexpressing cancer cells. ChemMedChem 2008, 3, 1387–1394. [DOI] [PubMed] [Google Scholar]
- Kitanovic I.; Can S.; Alborzinia H.; Kitanovic A.; Pierroz V.; Leonidova A.; Pinto A.; Spingler B.; Ferrari S.; Molteni R.; Steffen A.; Metzler-Nolte N.; Wolfl S.; Gasser G. Photophysical, theoretical and biological evaluation and cell death mechanisms of a Re(I) organometallic complex. Chem.—Eur. J. 2013, 20, 2496–2507. [DOI] [PubMed] [Google Scholar]
- Gasser G.; Ott I.; Metzler-Nolte N. Organometallic anticancer compounds. J. Med. Chem. 2010, 54, 3–25and references therein.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser G.; Metzler-Nolte N. The potential of organometallic complexes in medicinal chemistry. Curr. Opin. Chem. Biol. 2012, 16, 84–91and references therein.. [DOI] [PubMed] [Google Scholar]
- Hartinger C. G.; Metzler-Nolte N.; Dyson P. J. Challenges and opportunities in the development of organometallic anticancer drugs. Organometallics 2012, 31, 5677–5685and references therein.. [Google Scholar]
- Bruijincx P. C. A.; Sadler P. J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206and references therein.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartinger C. G.; Dyson P. J. Bioorganometallic chemistry: from teaching paradigms to medicinal applications. Chem. Soc. Rev. 2009, 38, 391–401and references therein.. [DOI] [PubMed] [Google Scholar]
- Jaouen G.; Metzler-Nolte N.. Medicinal Organometallic Chemistry; Springer-Verlag: Berlin, Germany, 2010. [Google Scholar]
- Nelson A. R.; Borland L.; Allbritton N. L.; Sims C. E. Myristoyl-based transport of peptides into living cells. Biochemistry 2007, 46, 14771–14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturzu A.; Klose U.; Echner H.; Beck A.; Gharabaghi A.; Kalbacher H.; Heckl S. Cellular uptake of cationic gadolinium-DOTA peptide conjugates with and without N-terminal myristoylation. Amino Acids 2009, 37, 249–255. [DOI] [PubMed] [Google Scholar]
- Gasser G.; Hüsken N.; Köster S. D.; Metzler-Nolte N. Synthesis of organometallic PNA oligomers by click chemistry. Chem. Commun. 2008, 3675–4677. [DOI] [PubMed] [Google Scholar]
- Gasser G.; Jäger K. Z.; Bergmann M.; Steinbach R.; Stephan J.; Metzler-Nolte H. N. Preparation, 99mTc-labeling and biodistribution studies of a PNA oligomer containing a new ligand derivative of 2,2′-dipicolylamine. J. Inorg. Biochem. 2010, 104, 1133–1140. [DOI] [PubMed] [Google Scholar]
- Patra M.; Gasser G.; Bobukhov D.; Merz K.; Shtemenko A. V.; Metzler-Nolte N. Sequencial insertion of three different organometallics into a versatile building block containing a PNA backbone. Dalton Trans. 2010, 39, 5617–5619. [DOI] [PubMed] [Google Scholar]
- Pfeiffer H.; Rojas A.; Niesel J.; Schatzschneider U. Sonogashira and ″Click″ reactions in the N-terminal and side chain functionalization of peptides with [Mn(CO)3(tpm)]+-based CO releasing molecules (tpm = tris(pyrazolyl)methane). Dalton Trans. 2009, 4292–4298. [DOI] [PubMed] [Google Scholar]
- Amoroso A. J.; Coogan M. P.; Dunne J. E.; Fernandez-Moreira V.; Hess J. B.; Hayes A. J.; Lloyd D.; Millet C.; Pope S. J. A.; Williams C. Rhenium fac tricarbonyl bisimine complexes: biologically useful fluorochromes for cell imaging applications. Chem. Commun. 2007, 3066–3068. [DOI] [PubMed] [Google Scholar]
- Wrighton M.; Morse D. L. Nature of the lowest excited state in tricarbonylchloro-1,10-phenanthrolinerhenium(I) and related complexes. J. Am. Chem. Soc. 1974, 96, 998–1003. [Google Scholar]
- Caspar J. V.; Meyer T. J. Application of the energy gap law to nonradiative, excited-state decay. J. Phys. Chem. A 1983, 87, 952–957. [Google Scholar]
- Sullivan B. P. Large solvatochromism of metal-to-ligand charge-transfer transitions in organometallic complexes of rhenium(I). J. Phys. Chem. A 1989, 93, 24–26. [Google Scholar]
- Kalyanasundaram K. Luminescence and redox reactions of the metal-to-ligand charge-transfer excited state of tricarbonylchloro-(polypyridyl)rhenium(I) complexes. J. Chem. Soc., Faraday Trans. 1986, 82, 2401–2415. [Google Scholar]
- Tapolsky G.; Duesing R.; Meyer T. J. Synthetic control of excited-state properties in ligand-bridged complexes of rhenium(I). Intramolecular energy transfer by an electron-transfer/energy-transfer cascade. Inorg. Chem. 1990, 29, 2285–2297. [Google Scholar]
- Zipp A. P.; Sacksteder L.; Streich J.; Cook A.; Demas J. N.; DeGraff B. A. Luminescence of rhenium(I) complexes with highly sterically hindered alpha-diimine ligands. Inorg. Chem. 1993, 32, 5629–5632. [Google Scholar]
- Stufkens D. J.; Vlçek A. Jr. Ligand-dependent excited state behaviour of Re(I) and Ru(II) carbonyl-diimine complexes. Coord. Chem. Rev. 1998, 177, 127–179. [Google Scholar]
- Thornton N. B.; Schanze K. S. A chromophore-quencher-based luminescence probe for DNA. Inorg. Chem. 1993, 32, 4994–4995. [Google Scholar]
- Lo K. K.-W.; Hui W.-K.; Ng D. C.-M. Novel rhenium(I) polypyridine biotin complexes that show luminescence enhancement and lifetime elongation upon binding to avidin. J. Am. Chem. Soc. 2002, 124, 9344–9345. [DOI] [PubMed] [Google Scholar]
- Uppal B. S.; Booth R. K.; Ali N.; Lockwood C.; Rice C. R.; Elliott P. I. P. Synthesis and characterisation of luminescent rhenium tricarbonyl complexes with axially coordinated 1,2,3-triazole ligands. Dalton Trans. 2011, 40, 7610–7616. [DOI] [PubMed] [Google Scholar]
- Lo K. K.-W.; Choi A. W.-T.; Law W. H.-T. Applications of luminescent inorganic and organometallic transition metal complexes as biomolecular and cellular probes. Dalton Trans. 2012, 41, 6021–6047. [DOI] [PubMed] [Google Scholar]
- Kuimova M. K.; Alsindi W. Z.; Blake A. J.; Davies E. S.; Lampus D. J.; Matousek P.; McMaster J.; Parker A. W.; Towrie M.; Sun X.-Z.; Wilson C.; George M. W. Probing the solvent dependent photophysics of fac-[Re(CO)3(dppz-X2)Cl] (dppz-X2 = 11,12-X2-dipyrido[3,2-a:2′,3′-c]phenazine); X = CH3, H, F, Cl, CF3). Inorg. Chem. 2008, 47, 9857–9869. [DOI] [PubMed] [Google Scholar]
- Sacksteder L.; Lee M.; Demas J. N.; DeGraff B. A. Long-lived, highly luminescent rhenium(I) complexes as molecular probes: intra- and intermolecular excited-state interactions. J. Am. Chem. Soc. 1993, 115, 8230–8238. [Google Scholar]
- Patra M.; Ingram K.; Pierroz V.; Ferrari S.; Spingler B.; Keiser J.; Gasser G. Ferrocenyl derivatives of the anthelminc praziquantel: design, synthesis and biological evaluation. J. Med. Chem. 2012, 55, 8790–8798. [DOI] [PubMed] [Google Scholar]
- Patra M.; Ingram K.; Pierroz V.; Ferrari S.; Spingler B.; Gasser R. B.; Keiser J.; Gasser G. (η-Praziquantel)Cr(CO)3 derivatives with remarkable in vitro anti-schistosomal activity. Chem.—Eur. J. 2013, 19, 2232–2235. [DOI] [PubMed] [Google Scholar]
- Patra M.; Ingram K.; Leonidova A.; Pierroz V.; Ferrari S.; Robertson M. N.; Todd M. H.; Keiser J.; Gasser G. In vitro metabolic profile and in vivo antischistosomal activity studies of (η-Praziquantel)Cr(CO)3 derivatives. J. Med. Chem. 2013, 56, 9192–9198. [DOI] [PubMed] [Google Scholar]
- Chan T. R.; Hilgraf R.; Sharpless K. B.; Fokin V. V. Polytriazoles as copper(I)-stabilizing ligands in catalysis. Org. Lett. 2004, 6, 2853–2855. [DOI] [PubMed] [Google Scholar]
- Pierroz V.; Joshi T.; Leonidova A.; Mari C.; Schur J.; Ott I.; Spiccia L.; Ferrari S.; Gasser G. Molecular and cellular characterization of the biological effects of ruthenium(II) complexes incorporating 2-pyridyl-2-pyrimidine-4-carboxylic acid. J. Am. Chem. Soc. 2012, 134, 20376–20387. [DOI] [PubMed] [Google Scholar]
- Ghezzi A.; Aceto M.; Cassino C.; Gabano E.; Osella D. Uptake of antitumor platinum(II)-complexes by cancer cells, assayed by inductively coupled plasma mass spectrometry (ICP-MS). J. Inorg. Biochem. 2004, 98, 73–78. [DOI] [PubMed] [Google Scholar]
- Puckett C. A.; Ernst R. J.; Barton J. K. Exploring the cellular accumulation of metal complexes. Dalton Trans. 2010, 39, 1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckett C. A.; Barton J. K. Methods to explore cellular uptake of ruthenium complexes. J. Am. Chem. Soc. 2006, 129, 46–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari C.; Pierroz V.; Rubbiani R.; Patra M.; Hess J.; Spingler B.; Oehninger L.; Schur J.; Ott I.; Salassa L.; Ferrari S.; Gasser G.. DNA intercalating RuII polypyridyl complexes as effective photosensitizers in photodynamic therapy, 2014. Submitted for publication. [DOI] [PubMed]
- Gasser G.; Pinto A.; Neumann S.; Sosniak A. M.; Seitz M.; Merz K.; Heumann R.; Metzler-Nolte N. Synthesis, characterisation and bioimaging of a fluorescent rhenium-containing PNA bioconjugate. Dalton Trans. 2012, 41, 2304–2313. [DOI] [PubMed] [Google Scholar]
- Vivès E.; Brodin P.; Lebleu B. A truncated HIV-1Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 1997, 272, 16010–16017. [DOI] [PubMed] [Google Scholar]
- Ahmed S. A.; Gogal R. M. J.; Walsh J. E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



