Abstract
Soy isoflavones, genistein and daidzein, are widely consumed in soy-based foods and dietary supplements for their putative health benefits; however, evidence for potential adverse effects has been obtained from experimental animal studies. An important prerequisite for understanding the pharmacodynamics of isoflavones is better information about pharmacokinetics and bioavailability. This study determined the bioavailability of genistein and daidzein in a mouse model by comparing plasma pharmacokinetics of their aglycone and conjugated forms following administration of identical doses (1.2 mg/kg genistein and 0.55 mg/kg daidzein) by either an intravenous injection (IV) or gavage of the aglycones in 90% aqueous solution vs. a bolus administration of equimolar doses delivered in a food pellet prepared using commercial soy protein isolate (SPI) as the isoflavone source. The bioavailability of genistein and daidzein were equivalent for the gavage and dietary routes of administration despite the use of isoflavone aglycones in the former and SPI-derived glucosides in the latter. While absorption of total isoflavones was nearly quantitative from both oral routes (>84% of AUCs for IV), presystemic and systemic Phase II conjugation greatly attenuated internal exposures to the receptor-active aglycone isoflavones (9–14% for genistein and 29–34% for daidzein based on AUCs for IV). These results show that SPI is an efficient isoflavone delivery vehicle capable of providing significant proportions of the total dose into the circulation in the active aglycone form for distribution to receptor-bearing tissues and subsequent pharmacological effects that determine possible health benefits and/or risks.
Keywords: Isoflavone, mice, bioavailability, soy protein isolate, pharmacokinetics, genistein
INTRODUCTION
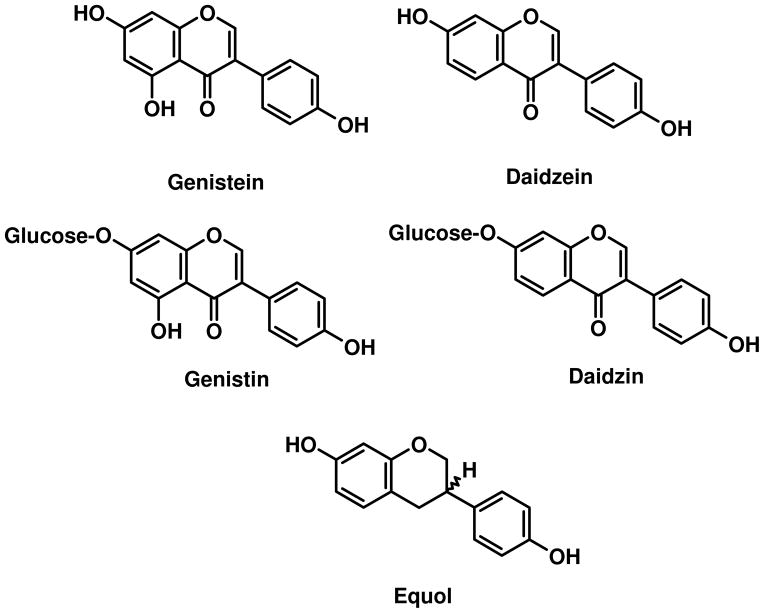
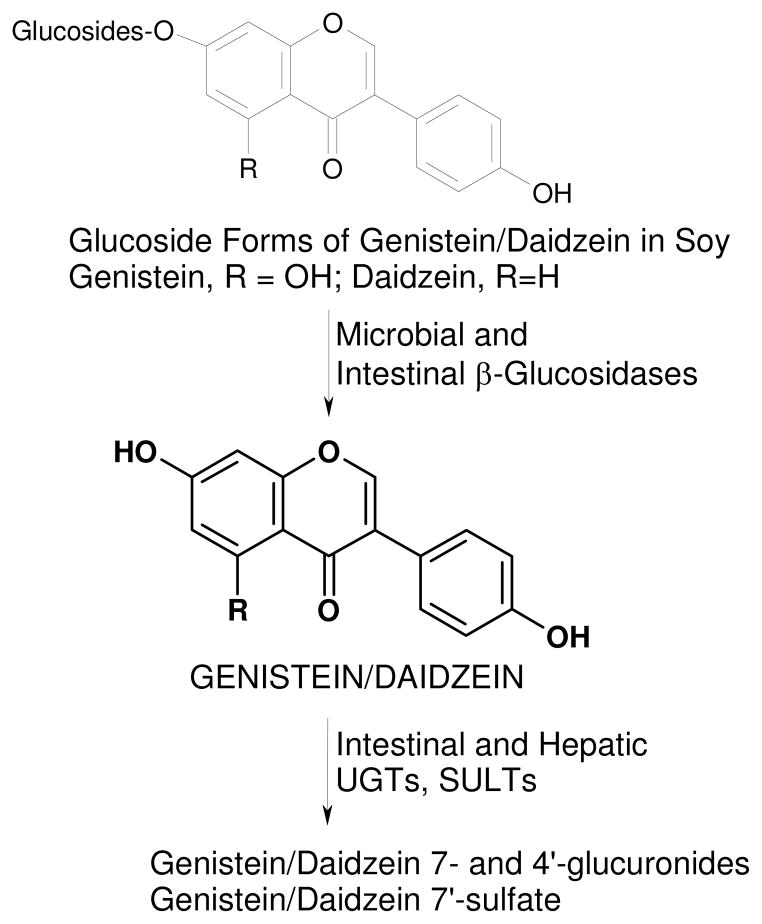
Bioavailability of isoflavones (Scheme 1) from soy-based foods and dietary supplements is a critical component in understanding possible beneficial and adverse health effects in animal models and humans. There is general agreement that absorption in the rodent and human gut proceeds by rapid release of aglycones through the action of bacterial and intestinal β-glucosidases following ingestion of isoflavone glucosides present in whole soy foods (1–5) (Scheme 2). While there is evidence for delivery of small amounts of intact isoflavone glucosides into the circulation in rodents (6, 7) and humans (2), efficient Phase II conjugation within the enterocyte and secretion of glucuronides into the blood greatly limit the amount of biologically active aglycone available for distribution to the tissues (i.e., bioavailable). There is strong evidence in rodents for secretion of isoflavone glucuronides into the bile and enterohepatic recirculation prior to elimination into both feces and urine (7–10).
Scheme 1.
Structures of Isoflavones, their β-D-Glucosides, and Equol
Scheme 2.
Metabolic Transformations of Dietary Isoflavones that Affect Bioavailability
These observations made it imperative to understand better how the food matrix can affect important isoflavone pharmacokinetic factors in an animal model. In the current study, we chose to evaluate the effects of soy protein isolate (SPI) because it is a major commercial ingredient used in the manufacture of many modern soy-based foods and dietary supplements, including infant formula. For these reasons, the current study examined the absolute bioavailability of genistein and daidzein, both as aglycones and total conjugated forms, in plasma of female Balb/c mice. This was achieved by comparing the plasma pharmacokinetics from IV and gavage administration of genistein and daidzein aglycones in solution with bolus feeding of equimolar doses of isoflavone glucosides in a SPI-containing food matrix that is comparable to many popular soy foods, such as high-protein “power bars”.
The experimental design chosen in this study also specifically addresses common methodological deficiencies found in many studies of the pharmacokinetics of soy isoflavones. First, low doses of isoflavone glucosides relevant to food intake were chosen to minimize complications due to non-linear pharmacokinetics often encountered as dose increases and second, both aglycone and total isoflavone concentrations were measured in plasma. To accomplish these goals, analytical methodology based on LC/MS/MS and isotope dilution was employed to assure sensitivity adequate to quantify circulating aglycones, which are the receptor-binding forms, and their inactive conjugated forms.
MATERIALS AND METHODS
Chemicals and reagents
HPLC-grade methanol and acetonitrile, and reagent grade hydrochloric acid, glacial acetic acid and ammonium acetate were from Fisher Scientific (Fair Lawn, NJ). Isoflavone standards, daidzin, glycitin, genistein, daidzein, glycitein, genistein and the internal standard, formononetin, were purchased from different commercial vendors and their purity was determined and confirmed using LC-MS, NMR and LC-UV. Briefly, highly purified chemicals (CAS) were purchased: genistin (529-59-9), glycitein (40957-83-3) and formononetin (485-72-3) from Indofine Chemical Co., Hillsborough, NJ; daidzin (552-66-9) and glycitin (40246-10-4) from Sigma-Aldrich Chem., St Louis, MO. Both, genistein (446-72-0) and daidzein (486-66-8) were synthesized in the Helferich laboratory to >98% purity as previously described (11). Standard solutions at 1–2 mg/ml were prepared in dimethyl sulfoxide (DMSO, Sigma-Aldrich Chemical; St Louis, MO) and stored in darkness at −20 °C. Working standard solutions were prepared on the day of the analysis by dilution of the stock solutions with aqueous methanol and mobile phase A. All solutions were filtered through a 0.45 μm Acrodisc syringe filter (PALL Co.; East Hills, NY) prior to reverse phase HPLC separation. The purity of the isoflavone aglycones and glucosides stock solutions was checked with maximum absorption and known molar extinction coefficients as described in Nurmi et al. (12). Eighteen separate samples of commercial SPIs were purchased from different manufacturers and distributors (Table 1) within a 2-year period (2005–2007). A sample of SPI was prepared using mild alkali conditions as described by Wu et al. (13) from soy flour (Bakers soy flour, ADM, Decatur, IL).
Table 1.
Isoflavone profile in different SPIs.
| SPI | Daidzein | Glycitein | Genistein | Total | Daidzein | Glycitein | Genistein | Gluc.a | Aglyc. |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Product Name
|
mg/g
|
%
|
|||||||
| ADM 161 b | 0.399 | 0.092 | 0.672 | 1.162 | 34.3 | 7.9 | 57.8 | 96.8 | 3.2 |
| ADM 674 b | 0.524 | 0.161 | 0.677 | 1.362 | 38.5 | 11.8 | 49.7 | 96.4 | 3.6 |
| Ardex-F b | 0.093 | 0.039 | 0.288 | 0.420 | 22.2 | 9.2 | 68.5 | 79.2 | 20.8 |
| Ardex b | 0.100 | 0.048 | 0.437 | 0.585 | 17.1 | 8.2 | 74.7 | 83.2 | 16.8 |
| Profam 648 b | 0.101 | 0.035 | 0.251 | 0.387 | 26.1 | 9.1 | 64.7 | 65.2 | 34.8 |
| Profam 781 b | 0.100 | 0.051 | 0.309 | 0.459 | 21.7 | 11.0 | 67.3 | 78.4 | 21.6 |
| Profam 873 b | 0.325 | 0.083 | 0.820 | 1.229 | 26.5 | 6.8 | 66.8 | 86.9 | 13.1 |
| Profam 892 b | 0.290 | 0.075 | 0.607 | 0.972 | 29.8 | 7.7 | 62.5 | 90.3 | 9.7 |
| Profam 982 b | 0.106 | 0.037 | 0.312 | 0.456 | 23.3 | 8.2 | 68.5 | 80.1 | 19.9 |
| Soy flour (SF) b | 0.491 | 0.150 | 0.734 | 1.375 | 35.7 | 10.9 | 53.4 | 98.2 | 1.8 |
| SPI from SF c | 0.522 | 0.118 | 1.171 | 1.812 | 28.8 | 6.5 | 64.6 | 97.8 | 2.2 |
| Solpro 910 d | 0.288 | 0.091 | 0.902 | 1.281 | 22.5 | 7.1 | 70.4 | 89.1 | 10.9 |
| Solpro 950 d | 0.311 | 0.090 | 0.972 | 1.373 | 22.6 | 6.6 | 70.8 | 89.7 | 10.3 |
| SNE-L e | 0.106 | 0.026 | 0.195 | 0.328 | 32.4 | 7.9 | 59.6 | 23.0 | 77.0 |
| SNE-NO e | 0.067 | 0.030 | 0.162 | 0.259 | 26.0 | 11.4 | 62.6 | 92.6 | 7.4 |
| SNE-O e | 0.355 | 0.061 | 1.243 | 1.658 | 21.4 | 3.7 | 75.0 | 81.8 | 18.2 |
| Prolisse f | 0.203 | 0.045 | 0.399 | 0.647 | 31.3 | 7.0 | 61.7 | 18.9 | 81.1 |
| H0206 PTI g | 0.849 | 0.174 | 1.260 | 2.282 | 37.2 | 7.6 | 55.2 | 80.6 | 19.4 |
| Protient 6300 h | 0.086 | 0.020 | 0.236 | 0.341 | 25.1 | 5.7 | 69.2 | 90.4 | 9.6 |
| Protein Drink i | 0.619 | 0.084 | 1.159 | 1.861 | 33.3 | 4.5 | 62.3 | 92.8 | 7.2 |
|
| |||||||||
| Minimum | 0.067 | 0.020 | 0.162 | 0.259 | 17.1 | 3.7 | 49.7 | 18.9 | 1.8 |
| Maximum | 0.849 | 0.174 | 1.260 | 2.282 | 38.5 | 11.8 | 75.0 | 98.2 | 81.1 |
| Mean | 0.297 | 0.075 | 0.640 | 1.013 | 27.8 | 7.9 | 64.3 | 80.6 | 19.4 |
| SD | 0.218 | 0.046 | 0.377 | 0.610 | 6.0 | 2.2 | 6.8 | 22.0 | 22.0 |
| CV% | 73.3 | 60.7 | 58.9 | 60.3 | 21.7 | 27.5 | 10.5 | 27.3 | 113.2 |
Glucoside (Gluc.) or Aglycone (Aglyc.).
From Archer Daniels Midland (ADM; Decatur, IL).
From Helferich Laboratory.
From Solbar, Ltd (Ashdod, Israel).
SNE, Soy-N-Ergy, plus Lecithin, Non-Organic, Organic from American Health & Nutrition (Ann Arbor, MI).).
From Cargill, Inc. (Minneapolis, MN).
From Protein Technologies International, Inc. (St. Louis, MO).
From Protient, Inc. (St. Paul, MN).
Iso-Rich Soy™ high protein drink where soy protein isolate is the main ingredient (>90%) from Jarrow Formulas (Los Angeles, CA).
Analysis of isoflavones in foods
Soy products were analyzed at least in duplicate for isoflavone composition using mild acid hydrolysis conditions as previously described (14). Acid hydrolysis solution was prepared from absolute ethyl alcohol (200 proof, AAPER; Shelbyville, KY), double deionized water and concentrated HCl (12.1 M) in a 64:16:20 (v/v/v), respectively, for a final HCl concentration of 2.4 M. Isoflavones were extracted in aqueous methanol, separated by reverse phase HPLC with gradient elution and quantified using coulometric detection against pure standards.
Non-hydrolytic analysis. Briefly, 100 mg of each sample (in duplicates) were placed in 15 ml centrifuge tubes. An aliquot (10 μl) of formononetin internal standard solution (2.5 mg/ml in DMSO) was spiked on top of the sample, before adding 10 ml of aqueous (80%) methanol. Samples were mixed until materials were suspended in solution and placed in ultrasound water bath (Fisher Scientific; Fair Lawn, NJ) for 30 minutes. Upon sonication, samples were diluted (1:2; v/v) in separate borosilicate glass tubes with mobile phase A, filtered with 0.22 μm Acrodisc syringe filter (PALL Co.; East Hills, NY) and injected (10 μl) into HPLC 100 μl sample loop.
Acid hydrolysis analysis. Mild acid hydrolysis conditions were used to convert isoflavones from their malonyl and acetyl esters to their β-glucoside forms as the basis to calculate total glucoside and aglycone content for each sample. Each sample (100 mg in duplicate) was accurately weighed into ~70 ml tall PTFE screw-capped tubes. An aliquot (10 μl) of formononetin internal standard solution (2.5 mg/ml in DMSO) was spiked on top of the sample before addition of 10 ml acid hydrolysis solution. A small stirring bar was added and tubes were tightly capped. Samples were mixed until materials were suspended in solution. Samples were heated in a stirring-heating block unit controlled with a thermostat set 80 °C for 1 (partial hydrolysis) or 5 hours (complete hydrolysis). Upon termination, samples were diluted (1:3; v/v) in separate borosilicate glass tubes with mobile phase A, filtered through 0.22 μm Acrodisc syringe filters directly into 4 ml amber vials and injected (10 μl) into HPLC 100 μl sample loop.
Chromatographic conditions were adapted from those described by Peñalvo et al. (14). Isoflavone determination was carried out using HPLC with the multi-channel coulometric array detection system (ESA Inc.; Chelmsford, MA) consisting of two solvent pumps (model 582), autosampler model 717-Plus with cooling unit (Waters; Milford, MA) and a CoulArray detector (model 5600) with 2 analytical cells in series, each with four porous graphite electrodes set at 450, 490, 545, 580, 620, 690, 760, 800 mV, in the same order. Analytes were separated using a Zorbax RX C18 column (150 mm × 4.6 mm, 5 μm, 80 Å) from MAC-MAD Analytical (Chadds Ford, PA); protected with a guard column packed with C18 material (Upchurch Sci. Inc., Oak Harbor, WA). Samples were kept, injected, and separated at room temperature (~23 °C). Gradient elution was used for complete separation of the analytes and consisted of two eluents: mobile phase A (MPA), 50 mM ammonium acetate buffer pH 4.5 and MeOH (90:10 v/v), respectively; and MPB, 50 mM ammonium acetate buffer pH 4.5, MeOH and ACN (20:30:50 v/v/v), respectively. Gradient elution of isoflavones was carried out at 1 ml/min in the following pattern: 0–6 min at 15% MPB, increase to 18% MPB in 10 min, keep at 18% MPB for 4 min, increase to 25% MPB in 7 min, keep at 25% MPB for 4 min, increase to 38% MPB in 4 min, keep at 38% MPB for 5 min, increase to 42% MPB in 7 min, increase to 90% MPB in 7 min, keep at 90% MPB for 1 min, reduce to initial conditions in 4 min and equilibrate for 8 min; for a total run time of 67 min including 8 min of equilibration. Controlled data acquisition and processing was performed using CoulArray software (v. 2.0). Quantification from the sum of peak heights was performed using calibration curves generated from 4-point serial dilutions of single standards run in triplicates. Unknown peaks were matched to standards on the basis of retention time (± 3%) as well as response ratio between adjacent channels (± 35%), and manually inspected to ensure correct assignment.
Diet Formulation
American Institute of Nutrition 93 growth (AIN 93G) semi-purified diet, with corn oil substituting soybean oil, was selected as a basal diet for control animals, which meets all the nutritional requirements of mice (15, 16). SPI with its endogenous isoflavone content was selected to evaluate the pharmacokinetics of genistein and daidzein in mice after consumption of a suitable high-protein food. Chocolate-based pellets, with or without SPI, were prepared by grinding commercial chocolate cookies and adding 20 mg SPI (20% by weight). Pellets weighed ~100 mg (± 2 mg) each and were compounded to a pie shape (diameter ~6.8 mm and height ~2.64 mm) using a specially designed stainless steel shaping device and a hammer. Pellets without SPI were used to train the mice to consume the entire meal as a bolus. The same SPI without isoflavones was used to prepare pellets for the control group. Isoflavones were removed from SPI after ethanol washing and was confirmed to have negligible amounts of isoflavones (data not shown). This high-protein food was formulated similar to commercially available high-protein bar products. According to our calculations and using the USDA Food Composition database (17) and available composition tables from vendors, each 100 mg pellet contained protein (17%), carbohydrates (59%) and fats (17%); which delivered ~1.8 kJ/pellet representing ~5.7% of the daily energy requirements for mice used in this study (15, 16).
Animal Handling Procedures
Female Balb/c mice (16.7 ± 1.2 g) were purchased from the National Cancer Institute (Bethesda, MD) and delivered at 30 days of age. Animals were housed individually and kept on a 12 h dark/12 h light cycle. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Illinois. Animals were changed to AIN 93G basal diets on the day after arrival. Mice were trained over a 2-week period to consume the chocolate-based pellets (without SPI) in a bolus manner (i.e., within a few minutes). At the end of the training period, animals were randomized by weight and assigned to either the control or treatment group. Animals in the control group (n = 6) received pellets prepared with ethanol-washed SPI. The treatment group consisted of 42 mice (i.e., 6 mice in each of 7 time points) and received cookie snacks prepared with intact SPI each containing 0.009 ± 0.001 mg and 0.019 ± 0.003 mg of daidzein and genistein, respectively; delivering respective doses of 0.55 and 1.16 mg/kg bw. On the day of sample collection, body weights were recorded, and mice were appropriately dosed, and blood samples were collected by cardiac puncture at 30, 60, 120, 240, 480, 720, and 1440 min after the feeding period. Control animals were euthanized at 30 min after consumption of control pellets. Plasma was prepared by collection of blood into EDTA containing micro-centrifuge tubes and further centrifugation at 2000 rpm (30 min, 4 °C). Samples were kept at −80 °C until further analyses.
Pharmacokinetic evaluations of intravenous and gavage administrations were also conducted using female Balb/c mice similarly obtained from the National Cancer Institute. Procedures involving care and handling of these mice were reviewed and approved by the NCTR Laboratory Animal Care and Use Committee. Body weights were 18.7 ± 0.87 g (n = 107) and did not significantly differ between control, gavage, and IV groups. Dosing solutions were prepared less than 24 hr before administration by dissolving genistein (4.6 mg) and daidzein (2.1 mg) in 4 ml DMSO and then diluted to 40 ml with water to make a clear solution. A dose of 1.2 mg/kg genistein and 0.55 mg/kg daidzein was administered by either IV injection into the tail vein or by gavage using a dosing solution containing 115 μg/ml genistein and 55 μg/ml daidzein with 10 μl administered for every g body weight. The same dosing solution was used for IV and gavage administrations. Following dosing, 6 mice were sacrificed at each of the time points (5, 15, 30, 60, 120, 240, 360, 480 and 1080 min for IV and gavage) by CO2 asphyxiation and blood was collected immediately by cardiac puncture. Blood samples were placed in purple top tubes, plasma was produced by centrifugation, and samples were stored at −80 °C until analyzed in a single batch.
Quantification of Plasma Isoflavones
Plasma concentrations of total isoflavones were determined after complete enzymatic hydrolysis using a H. pomatia preparation containing glucuronidase, sulfatase, and glucosidase activities using a previously validated LC-ES/MS/MS method based on isotope dilution quantification of genistein, daidzein, and equol (18). Isoflavone aglycones were determined identically except without enzymatic deconjugation. Method detection limits (s/n ratio 3) for genistein, daidzein, and equol were approximately 0.005 μM for analysis of 10 μl plasma (total isoflavones) and 0.001 μM for analysis of 50 μl plasma (aglycone isoflavones). Intra- and inter-day precision was in the range of 3–13% relative standard deviation and accuracy was in the range of 88–99% (18). Quality control analyses were also performed during every sample set and included the analysis of blank and spiked serum samples (including glucuronidase/sulfatase), blank injections, and injections of authentic standards.
Pharmacokinetic analysis
Model-independent pharmacokinetic analysis was performed using PK Solutions 2.0 software (Summit Research Services, Montrose, CO). Parameters reported include: t1/2 elim = elimination half-time; AUC0-∞ = area under the time-concentration curve from zero to infinity; Vd = volume of distribution; Cl, total plasma clearance; bioavailability = AUC oral/AUC IV.
Statistical Analysis
Data are shown as the means ± SD. All statistical analyses were conducted using SAS software (SAS Institute Inc., Cary, NC). Group comparisons were made using the two-sided t-test. All reported P values are two-tailed and differences at P < 0.05 were considered statistically significant.
RESULTS
In this study we first determined the total quantity of isoflavones and their profile in multiple commercial SPIs and one SPI made in our laboratory from defatted soy flour. We found that both the content and profile of isoflavones in SPIs, even in those from the same supplier, are variable with average aglycone equivalents in mg/g of SPI of 0.297 ± 0.218 mg/g, 0.075 ± 0.046, and 0.640 ± 0.377 (mean ± SD) for daidzein, glycitein and genistein, respectively (Table 1). Similarly, the variation in the profile of isoflavones, total glycosides vs. total aglycones (80.6 ± 22.0 % vs. 19.4 ± 22.0%), was quite large with most of the SPIs, with the exception of two, containing isoflavones in their original glycoside form as is common with most unfermented soy foods (19). Nonetheless, the relative relationship among aglycone equivalents in all SPI’s was more constant with averages of 27.8 ± 6.0 %, 7.9 ± 2.2 % and 64.3 ± 6.8 % (mean ± SD) for daidzein, glycitein and genistein, respectively. Thus, although the quantity and profile of isoflavones was quite variable, the relative proportion among aglycones was more constant. Next, we selected a SPI product (Solpro 950) based on the amounts and profile of its constituent isoflavones to determine the dose to use for evaluating the pharmacokinetics of genistein and daidzein (1.2 mg/kg bw genistein and 0.55 mg/kg bw daidzein).
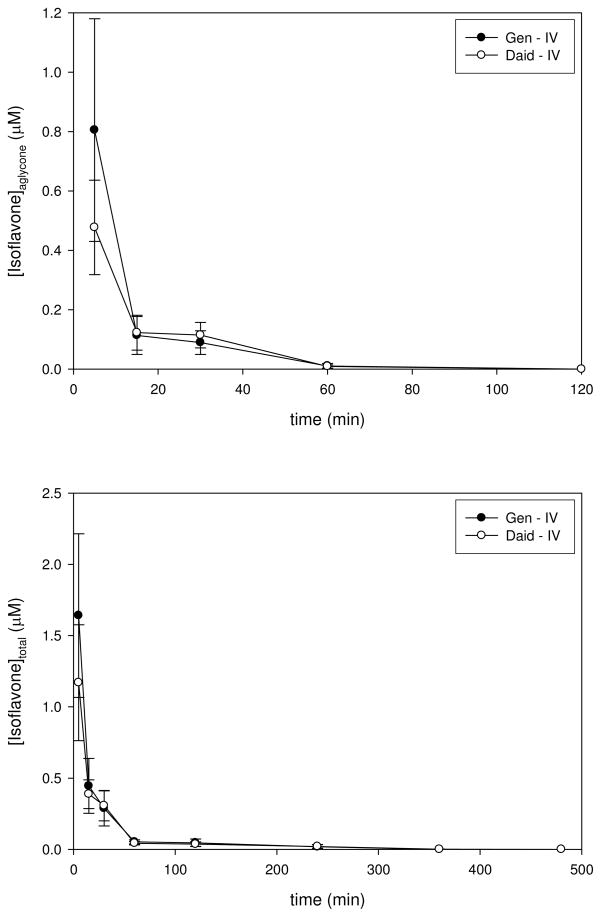
Intravenous (IV) administration of genistein and daidzein produced circulating levels of aglycone and total isoflavones (i.e., conjugates + aglycones) that decreased rapidly (Figure 1, Tables 2 and 3). Half-times for elimination of aglycones were quite fast (10 min) with slower elimination of the total isoflavones (85 and 160 min for genistein and daidzein, respectively). The greater Vd for aglycone genistein relative to daidzein is consistent with its greater lipophilicity and more extensive distribution into tissues. Significant systemic Phase II metabolism was evident because even at the initial time point following IV administration (5 min), only 36–70% of genistein and 36–81% of daidzein were present as aglycones in individual mice. Over the whole exposure period following IV administration, the ratio of AUCs for aglycone/total was 28% for genistein and 27% for daidzein. The similar systemic Phase II metabolism for these compounds as reflected by these AUC percentages is consistent with the comparable catalytic activity shown by human liver microsomes for genistein (kcat/Km=0.093 pmol/min/mg protein) vs. daidzein (kcat/Km=0.11 pmol/min/mg protein) (20). No clear evidence for enterohepatic recirculation (i.e., an apparent “bump” in plasma concentrations late in the profile) was provided by these data; however, the statistical limitations imposed by inter-animal variability from the chosen experimental design of using multiple mice at each time point could mitigate this interpretation.
Figure 1.
Plasma Concentration-Time Profiles for Aglycone and Total Genistein and Daidzein Following Intravenous Administration. The top panel shows a plot of plasma concentrations for aglycone genistein (closed circles) and daidzein (open circles) vs. time following an intravenous injection of 1.2 and 0.55 mg/kg bw, respectively. The bottom panel shows a plot of plasma concentrations for total (i.e., determined following complete enzymatic hydrolysis of conjugated forms) genistein (closed circles) and daidzein (open circles)
Table 2.
Pharmacokinetic parameters for total and aglycone genistein in plasma following administration to mice by different routes. Absolute bioavailability (fraction absorbed) was computed as the ratio of AUC for oral administration to that for intravenous (IV) administration.
| Parameters | IV
|
Gavage
|
SPI
|
|||
|---|---|---|---|---|---|---|
| Total | Aglycone | Total | Aglycone | Total | Aglycone | |
| Dose (μmol/kg bw) | 4.3 | 4.3 | 4.3 | |||
| t1/2 elim (min)1 | 85 | 9.8 | 82 | 14 | 74 | 57 |
| AUC0-∞ (μmol × min/L) | 39 | 11 | 34 | 1.1 | 33 | 1.6 |
| Vd (L/kg bw) | 14 | 5.2 | -- | -- | -- | -- |
| Cl (L/min/kg bw) | 0.11 | 0.37 | -- | -- | -- | -- |
| Bioavailability | -- | -- | 0.89 | 0.094 | 0.86 | 0.14 |
| % Aglycone | 28 | 3.2 | 4.9 | |||
t1/2 elim = elimination half-time; AUC0-∞ = area under the time-concentration curve from zero to infinity; Vd = volume of distribution; Cl, total plasma clearance; bioavailability = AUC oral/AUC IV.
Table 3.
Pharmacokinetic parameters for total and aglycone daidzein in plasma following administration to mice by different routes. Absolute bioavailability (fraction absorbed) was computed as the ratio of AUC for oral administration to that for IV administration.
| Parameters | IV
|
Gavage
|
SPI
|
|||
|---|---|---|---|---|---|---|
| Total | Aglycone | Total | Aglycone | Total | Aglycone | |
| Dose (μmol/g bw) | 2.1 | 2.1 | 2.6 | |||
| t1/2 elim (min) 1 | 160 | 10 | 200 | 60 | 190 | 42 |
| AUC0-∞ (μmol × min/L) | 34 | 9.3 | 42 | 3.1 | 412 | 2.82 |
| Vd (L/kg bw) | 14 | 3.3 | -- | -- | -- | -- |
| Cl (L/min/kg bw) | 0.061 | 0.22 | -- | -- | -- | -- |
| Bioavailability | -- | -- | 1.2 | 0.34 | 1.2 | 0.29 |
| %Aglycone | 27 | 7.4 | 6.7 | |||
t1/2 elim = elimination half-time; AUC0-∞ = area under the time-concentration curve from zero to infinity; Vd = volume of distribution; Cl, total plasma clearance; bioavailability = AUC oral/AUC IV.
Daidzein AUCs were adjusted for the small difference in administered dose for the SPI treatment group.
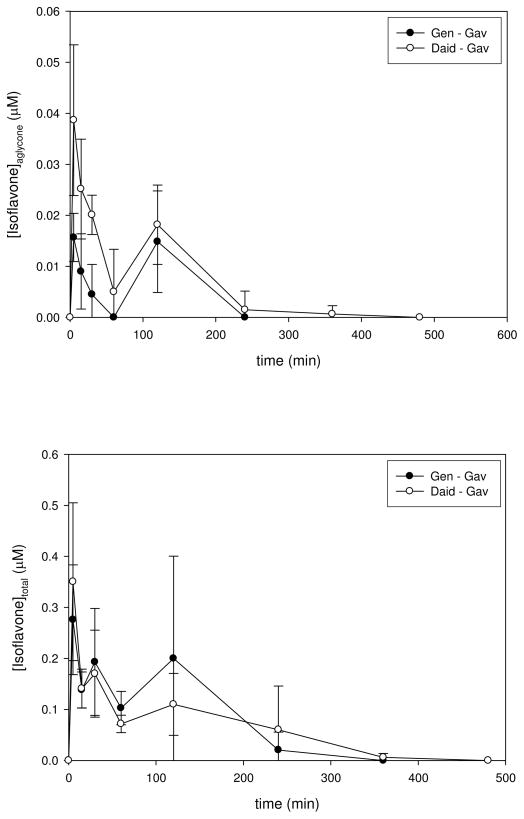
Administration of identical doses of genistein and daidzein by gavage produced more complex plasma concentration-time profiles (Figure 2). The impact of pre-systemic Phase II metabolism in the gut was evident because in individual mice only 3–13% of genistein and 10–15% of daidzein were present as aglycones at the initial sampling point. Over the whole exposure period, the ratio of AUCs for aglycone/total was 3.2% for genistein and 7.4% for daidzein. The changes in relative AUCs between aglycone and total isoflavones from either IV or oral administration suggest that pre-systemic (i.e., gut) metabolism of genistein (28 to 3.2%, respectively) is more extensive than for daidzein (27 to 7.4%, respectively). This conclusion is consistent with the preferential glucuronidation of genistein (kcat/Km = 56 pmol/min/mg protein) over daidzein (not detected, <0.08 pmol/min/mg protein) observed in human colon microsomes (20). The elimination kinetics for aglycone and total genistein and total daidzein following gavage were similar to observed for the IV administration but elimination of aglycone daidzein was 6-fold slower (Tables 2 and 3). In conjunction with the absorption phase following oral administration, the net result was markedly lower peak concentrations relative to IV. The absolute bioavailability (i.e., fraction absorbed = AUC-oral/AUC-IV) was 0.094 for aglycone genistein and 0.89 for total genistein; the absolute bioavailability for aglycone daidzein was 0.34 and essentially quantitative for total daidzein (1.2). Although “bumps” were apparent in the latter part of the composite plasma concentration-time profiles for mean levels of aglycone and total isoflavones, these features could be consistent with the effects of either inter-animal variability, variable absorption from the gut, or enterohepatic recirculation.
Figure 2.
Plasma Concentration-Time Profiles for Aglycone and Total Genistein and Daidzein Following Gavage Administration. The top panel shows a plot of plasma concentrations for aglycone genistein (closed circles) and daidzein (open circles) vs. time following an gavage administration of 1.2 and 0.55 mg/kg bw, respectively. The bottom panel shows a plot of plasma concentrations for total (i.e., determined following complete enzymatic hydrolysis of conjugated forms) genistein (closed circles) and daidzein (open circles)
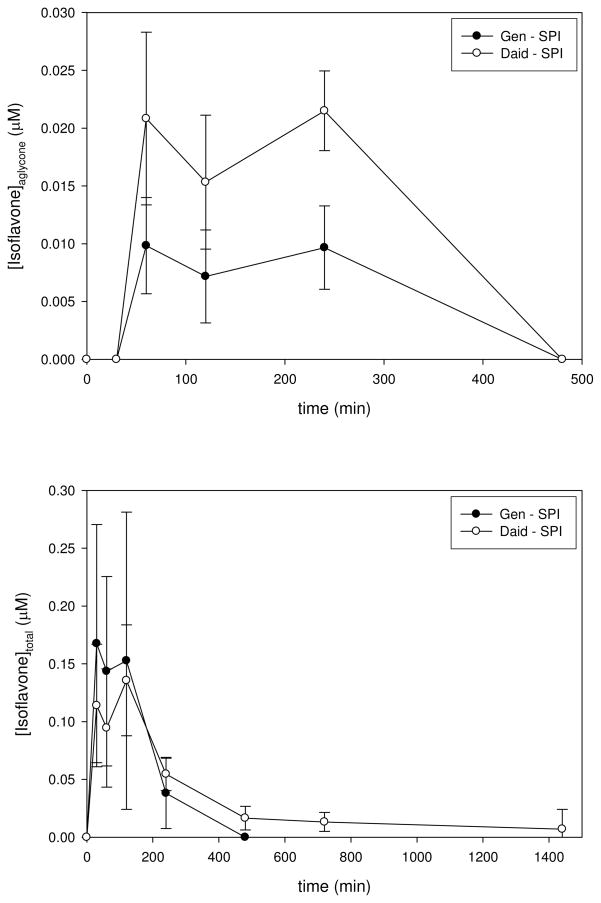
Bolus administration of nearly identical isoflavone doses in the SPI-containing food also produced complex time-concentrations of aglycone and total isoflavones (Figure 3). The pharmacokinetics following administration of the glucosides in the SPI food were similar to those produced by gavage administration of aglycones, although absorption and elimination phases appeared to be somewhat slower resulting in lower peak plasma concentrations. The systemic bioavailability values were quite similar to those obtained from gavage (Tables 2 and 3).
Figure 3.
Plasma Concentration-Time Profiles for Aglycone and Total Genistein and Daidzein Following Bolus Administration of a Soy Protein Isolate-Containing Food Pellet. The top panel shows a plot of plasma concentrations for aglycone genistein (closed circles) and daidzein (open circles) vs. time following consumption of 1.2 and 0.55 mg/kg bw, respectively, from a soy protein isolate-containing food pellet. The bottom panel shows a plot of plasma concentrations for total (i.e., determined following complete enzymatic hydrolysis of conjugated forms) genistein (closed circles) and daidzein (open circles)
Equol, as either total or the aglycone, was not observed in any plasma sample despite a detection limit of approximately 5 nM. These results are at odds with all previous studies, including our own (6), conducted in mice or rats using much higher doses of daidzein, in which equol was consistently observed in plasma at high levels, even from IV administration (10, 21). Given the requirement for gut microflora metabolism to equol and a prolonging effect from enterohepatic recirculation of daidzein (10, 21), it is conceivable that this negative finding might have resulted from monitoring for an insufficient length of time; however, it was deemed unlikely that extending the monitoring interval beyond the time for required for complete systemic elimination of the parent daidzein (18–24 h) could have an impact in a single dose experiment given the rapid clearance of daidzein, its relatively polar nature and the absence of any evidence for sequestration in tissues. This finding suggests that the magnitude of daidzein dose could be an additional factor in determining equol production in vivo.
DISCUSSION
SPI is a highly purified form of soy protein, standardized to contain at least 90% protein on a moisture-free basis. The purified isolate of soy protein has been used for its unique functional properties since the mid 1930’s mostly as an industrial ingredient in the process of making paper coatings and the useful “bean soup” during World War II. Since the early 1960’s, mainly due to its exceptional emulsification, binding, and rheological properties in foods, SPI has steadily entered our food supply becoming an ubiquitous ingredient, from infant formulas (22) to high protein supplements for the elderly (23).
Changes in the content and profile of isoflavones within soybeans and soy products is known to vary mostly due to soy cultivar (24, 25), environmental conditions such as temperature, irrigation and atmospheric CO2 (24, 26, 27), processing (28–31) and storage (32, 33). Among others, processing is a critical factor that determines the final quantity and profile of isoflavones in SPI (30, 34). SPIs produced from ethanol wash have low amounts of total isoflavones and saponins (34). SPIs produced from alkali solubilization and isoelectric precipitation, such as the one prepared in this study, can retain as much as 30% of initial isoflavones present in defatted soy flour. As shown in this study, many SPI manufacturers have different proprietary processing methods to obtain 90% protein (±5%) that will dictate the final functionality of the food ingredient (e.g. use in solid foods or beverages). These methods, along with the initial isoflavone content in the raw material, are main determinants of the large variability in the content and profile of isoflavones in the final product. Although isoflavones are concomitantly isolated during the process of obtaining soy protein, there is no association between the amount of protein and the total amounts and profile of isoflavones. Setchell et al. (35) reported similar large variances in the content and profile of isoflavones for two commercial SPIs collected over a three-year period. Indeed, the differences found in the amount of isoflavones in our study are far larger than this earlier evidence, with ranges as high as 12-fold for specific isoflavones, even in SPIs from the same manufacturer. In contrast, we did not find any SPIs containing glycitein equivalents higher than 11% of total isoflavone content, even in those SPIs with higher aglycone content. Similar variability was also observed in the USDA-Iowa State isoflavone database (36). Indeed, the different amounts for isoflavones described in this study fall within the ranges averaged for SPI in this database. Interestingly, the relative proportions among isoflavones extracted from the USDA database are very similar to the ones presented here.
Given the importance of SPI in many processed food products common in the modern Western diet, the content and bioavailability of isoflavones from SPI is an understudied aspect. The goal of the current study was to use detailed information about the content and form of isoflavones in a food produced from SPI to design and conduct a pharmacokinetic evaluation in a mouse model that would fill data gaps in the literature.
Absolute bioavailability (i.e., the ratio of AUCs for oral and intravenous administrations) of aglycone genistein has been determined only in rats [7–15%, (8); 4.9%, (9); 19–34% (37)]; and aglycone daidzein in rats 13%, (38). The corresponding circulating AUC percentages for total plasma isoflavones following oral vs. IV administration of aglycones have also been determined in rats: a) for genistein, 24–39% (39), 35% (10), and 29–39% (21); b) for genistein glucuronide, 54–73% (37); and c) for daidzein 23–25%, (21); 34%, (10); and for daidzein glucuronide, 47% (38). The percentage of aglycone relative to total isoflavones in plasma (i.e., aglycone + conjugated forms) is typically low (≤5%) in rodent (6, 8, 40–42) and human (43–48) plasma following oral administration. This attenuation of internal exposure to the aglycones results from efficient first-pass metabolism in the gut and liver (20).
The relative bioavailability of purified isoflavone glucosides vs. aglycones has been studied in rodents and humans with conflicting results. Evidence from one rat study showed significantly higher plasma AUCs for total genistein following oral administration of genistin vs. genistein (39). The studies of Sepehr et al. (10, 21) measured total plasma isoflavones in rats following either oral or IV administration of pure aglycones, pure glucosides, or as a glucoside mixture in Novasoy. In young F344 rats, AUC percentages for total plasma genistein, daidzein, and glycitein increased in the order of dosing with pure aglycone < pure glucoside < Novasoy, which approached 100% in some cases (21). For aged F344 rats, AUC percentages for total plasma genistein, daidzein, and glycitein were more comparable between dosing with either aglycone, pure glucoside, or Novasoy (10). Another study showed higher total AUC for isoflavones in portal vein plasma (i.e., no involvement of liver metabolism) following oral aglycone administration (7), and a final one showed no significant differences (49). Oral administration of genistein in its glucoside form (genistin) to neonatal CD-1 mice produced plasma AUCs of total and aglycone genistein that were approximately 3-fold greater than those produced by an equimolar dose of genistein, although oral administration produced internal exposures one half to one sixth lower than that produced by a subcutaneous injection (40). Similarly, in humans dosed orally with either isoflavone glucosides or aglycones, some studies reported higher plasma AUCs for total isoflavones from glucoside administration (44, 46), one showed increased AUC from aglycone administration (43), and others showed no differences (45, 50). It is unclear what study parameters account for this diversity of results, although methodology, animal models, or human population variability bear consideration. A related human study reported relative isoflavone bioavailability following ingestion of soy foods containing primarily glucosides (textured soy protein) vs. one enriched in aglycones (tempeh) (51). In that study, higher plasma total isoflavone AUCs were observed for the aglycone-rich tempeh meal but the impact from differences in food matrices has not been explicitly examined as a potential confounding factor.
The current study sought to build upon our previous work in assessing the pharmacokinetic differences from different soy products using the Balb/c mouse (6). We previously reported the prominent influence of food matrix, and specifically the degree of processing that removed non-isoflavone non-nutritive components, on metabolism, pharmacokinetics, and toxicodynamics (estrogen-dependent tumor growth) of isoflavones in ovariectomized mice (6). In that study, higher plasma AUCs for aglycone genistein were observed following dietary administration of purified genistin as opposed to whole-soy flour. This pharmacokinetic evidence supported a critical role for other non-nutritive components in soy foods and supplements in gut Phase II metabolism and bioavailability of genistein (6), the estrogen receptor-binding form (52).
The current study used a classical pharmacokinetic approach to measure bioavailability of isoflavone glucosides in a food matrix made from a typical SPI product. The selection of a dose level for genistein and daidzein defined by the amounts actually present in many commercial foods makes unnecessary any of the assumptions inherent in extrapolation from higher doses. These studies in a mouse model widely used in pharmacology and toxicology show that bioavailabilities of isoflavone glucosides from a SPI-based food are essentially equivalent to those from gavage of an equimolar dose of the aglycones in aqueous solution. Furthermore, determinations of absolute bioavailability for total isoflavones showed that although absorption from the gut was nearly quantitative following oral administration (>86%), active Phase II metabolism in the gut and liver significantly attenuate internal exposure to the biologically active aglycones (9–14% for genistein and 29–34% for daidzein). Nonetheless, these results show that SPI can efficiently deliver significant proportions of the total administered oral dose into the circulation in the active aglycone form for distribution to receptor-bearing tissues and subsequent pharmacological effects. This clearer definition of internal exposures to the active forms of isoflavones should be considered in discussions of putative beneficial and potentially adverse health effects following consumption of SPI-based foods from the constellation of such products currently available in the marketplace.
Acknowledgments
This research was funded by National Institute on Aging with additional support from National Institute for Complementary and Alternative Medicine, Office of Dietary Supplements, and Women’s Health Initiative (P01 AG024387 to W.G.H., and D.R.D.). The views presented in this article do not necessarily reflect those of the U.S. Food and Drug Administration.
References
- 1.Day AJ, Canada FJ, Diaz JC, Kroon PA, McLauchlan R, Faulds CB, Plumb GW, Morgan MR, Williamson G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000;468(2–3):166–70. doi: 10.1016/s0014-5793(00)01211-4. [DOI] [PubMed] [Google Scholar]
- 2.Hosoda K, Furuta T, Yokokawa A, Ogura K, Hiratsuka A, Ishii K. Plasma profiling of intact isoflavone metabolites by high-performance liquid chromatography and mass spectrometric identification of flavone glycosides daidzin and genistin in human plasma after administration of kinako. Drug Metab Dispos. 2008;36(8):1485–95. doi: 10.1124/dmd.108.021006. [DOI] [PubMed] [Google Scholar]
- 3.Hawksworth G, Drasar BS, Hill MJ. Intestinal bacteria and the hydrolysis of glycosidic bonds. J Med Microbiol. 1971;4(4):451–9. doi: 10.1099/00222615-4-4-451. [DOI] [PubMed] [Google Scholar]
- 4.Chang YC, Nair MG. Metabolism of daidzein and genistein by intestinal bacteria. J Nat Prod. 1995;58(12):1892–6. doi: 10.1021/np50126a014. [DOI] [PubMed] [Google Scholar]
- 5.Sfakianos J, Coward L, Kirk M, Barnes S. Intestinal uptake and biliary excretion of the isoflavone genistein in rats. J Nutr. 1997;127(7):1260–8. doi: 10.1093/jn/127.7.1260. [DOI] [PubMed] [Google Scholar]
- 6.Allred CD, Twaddle NC, Allred KF, Goeppinger TS, Churchwell MI, Ju YH, Helferich WG, Doerge DR. Soy processing affects metabolism and disposition of dietary isoflavones in ovariectomized BALB/c mice. J Agric Food Chem. 2005;53(22):8542–50. doi: 10.1021/jf051246w. [DOI] [PubMed] [Google Scholar]
- 7.Steensma A, Faassen-Peters MA, Noteborn HP, Rietjens IM. Bioavailability of genistein and its glycoside genistin as measured in the portal vein of freely moving unanesthetized rats. J Agric Food Chem. 2006;54(21):8006–12. doi: 10.1021/jf060783t. [DOI] [PubMed] [Google Scholar]
- 8.Coldham NG, Zhang AQ, Key P, Sauer MJ. Absolute bioavailability of [14C] genistein in the rat; plasma pharmacokinetics of parent compound, genistein glucuronide and total radioactivity. Eur J Drug Metab Pharmacokinet. 2002;27(4):249–58. doi: 10.1007/BF03192335. [DOI] [PubMed] [Google Scholar]
- 9.Moon YJ, Sagawa K, Frederick K, Zhang S, Morris ME. Pharmacokinetics and bioavailability of the isoflavone biochanin A in rats. AAPS J. 2006;8(3):E433–42. doi: 10.1208/aapsj080351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sepehr E, Cooke GM, Robertson P, Gilani GS. Effect of glycosidation of isoflavones on their bioavailability and pharmacokinetics in aged male rats. Mol Nutr Food Res. 2009;53 (Suppl 1):S16–26. doi: 10.1002/mnfr.200800170. [DOI] [PubMed] [Google Scholar]
- 11.Chang YC, Nair MG, Santell RC, Helferich WG. Microwave-mediated synthesis of anticarcinogenic isoflavones from soybeans. J Agric Food Chem. 1994;42(9):1869–1871. [Google Scholar]
- 12.Nurmi T, Mazur W, Heinonen S, Kokkonen J, Adlercreutz H. Isoflavone content of the soy based supplements. J Pharm Biomed Anal. 2002;28(1):1–11. doi: 10.1016/s0731-7085(01)00612-4. [DOI] [PubMed] [Google Scholar]
- 13.Wu S, Murphy PA, Johnson LA, Reuber MA, Fratzke AR. Simplified process for soybean glycinin and beta-conglycinin fractionation. J Agric Food Chem. 2000;48(7):2702–8. doi: 10.1021/jf990785w. [DOI] [PubMed] [Google Scholar]
- 14.Penalvo JL, Nurmi T, Adlercreutz H. A simplified HPLC method for total isoflavones in soy products. Food Chem. 2004;87(2):297–305. [Google Scholar]
- 15.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123(11):1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 16.Nutrient Requirements of Laboratory Animals. 4. National Academy Press; Washington, D.C: 1995. p. 192. rev ed. [Google Scholar]
- 17.U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference. Release 22. Nutrient Data Laboratory Home Page. 2009 http://www.ars.usda.gov/nutrientdata.
- 18.Twaddle NC, Churchwell MI, Doerge DR. High-throughput quantification of soy isoflavones in human and rodent blood using liquid chromatography with electrospray mass spectrometry and tandem mass spectrometry detection. J Chromat B Analyt Technol Biomed Life Sci. 2002;777(1–2):139–45. doi: 10.1016/s1570-0232(02)00275-1. [DOI] [PubMed] [Google Scholar]
- 19.Fukutake M, Takahashi M, Ishida K, Kawamura H, Sugimura T, Wakabayashi K. Quantification of genistein and genistin in soybeans and soybean products. Food Chem Toxicol. 1996;34(5):457–461. doi: 10.1016/0278-6915(96)87355-8. [DOI] [PubMed] [Google Scholar]
- 20.Doerge DR, Chang HC, Churchwell MI, Holder CL. Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry. Drug Metab Dispos. 2000;28(3):298–307. [PubMed] [Google Scholar]
- 21.Sepehr E, Cooke G, Robertson P, Gilani GS. Bioavailability of soy isoflavones in rats Part I: application of accurate methodology for studying the effects of gender and source of isoflavones. Mol Nutr Food Res. 2007;51(7):799–812. doi: 10.1002/mnfr.200700083. [DOI] [PubMed] [Google Scholar]
- 22.Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350(9070):23–7. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 23.Castellanos VH, Litchford MD, Campbell WW. Modular protein supplements and their application to long-term care. Nutr Clin Pract. 2006;21(5):485–504. doi: 10.1177/0115426506021005485. [DOI] [PubMed] [Google Scholar]
- 24.Eldridge AC, Kwolek WF. Soybean isoflavones: effect of environment and variety on composition. J Agric Food Chem. 1983;31(2):394–6. doi: 10.1021/jf00116a052. [DOI] [PubMed] [Google Scholar]
- 25.Seguin P, Zheng W, Smith DL, Deng W. Isoflavone content of soybean cultivars grown in eastern Canada. J Sci Food Agric. 2004;84(11):1327–1332. [Google Scholar]
- 26.Kim SH, Jung WS, Ahn JK, Kim JA, Chung IM. Quantitative analysis of the isoflavone content and biological growth of soybean (Glycine max L.) at elevated temperature, CO2 level and N application. J Sci Food Agric. 2005;85(15):2557–2566. [Google Scholar]
- 27.Rasolohery CA, Berger M, Lygin A, Lozovaya V, Nelson RL, Dayde J. Effect of temperature and water availability during late maturation of the soybean seed on germ and cotyledon isoflavone content and composition. J Sci Food Agric. 2008;88(2):218–228. [Google Scholar]
- 28.Aguiar CL, Baptista AS, Walder JM, Tsai SM, Carrao-Panizzi MC, Kitajima EW. Changes in isoflavone profiles of soybean treated with gamma irradiation. Int J Food Sci Nutr. 2009:1–8. doi: 10.1080/09637480701754968. [DOI] [PubMed] [Google Scholar]
- 29.Genovese MI, Barbosa AC, da Pinto MS, Lajolo FM. Commercial soy protein ingredients as isoflavone sources for functional foods. Plant Foods Hum Nutr. 2007;62(2):53–8. doi: 10.1007/s11130-007-0041-0. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Ma Q, Pagadala S, Sherrard MS, Krishman PG. Changes of isoflavones during processing of soy protein isolates. J Am Oil Chem Soc. 1998;75(3):337–341. [Google Scholar]
- 31.Yin LJ, Li LT, Liu H, Saito M, Tatsumi E. Effects of fermentation temperature on the content and composition of isoflavones and beta-glucosidase activity in sufu. Biosci Biotechnol Biochem. 2005;69(2):267–72. doi: 10.1271/bbb.69.267. [DOI] [PubMed] [Google Scholar]
- 32.Huang RY, Chou CC. Stability of Isoflavone Isomers in Steamed Black Soybeans and Black Soybean Koji Stored under Different Conditions. J Agric Food Chem. 2009;57(5):1927–32. doi: 10.1021/jf803702x. [DOI] [PubMed] [Google Scholar]
- 33.Lee SJ, Ahn JK, Kim SH, Kim JT, Han SJ, Jung MY, Chung IM. Variation in isoflavone of soybean cultivars with location and storage duration. J Agric Food Chem. 2003;51(11):3382–9. doi: 10.1021/jf0261405. [DOI] [PubMed] [Google Scholar]
- 34.Lin J, Krishnan PG, Wang C. Retention of Isoflavones and Saponins During the Processing of Soy Protein Isolates. J Am Oil Chem Soc. 2006;83(1):59–63. [Google Scholar]
- 35.Setchell KD, Cole SJ. Variations in isoflavone levels in soy foods and soy protein isolates and issues related to isoflavone databases and food labeling. J Agric Food Chem. 2003;51(14):4146–55. doi: 10.1021/jf026199b. [DOI] [PubMed] [Google Scholar]
- 36.USDA-Iowa State University. Agricultural Research Service. Database on the Isoflavone Content of Foods. Nutrient Data Laboratory; 2002. Web site: http://www.nal.usda.gov/fnic/foodcomp/Data/isoflav/isoflav.html. [Google Scholar]
- 37.Zhou S, Hu Y, Zhang B, Teng Z, Gan H, Yang Z, Wang Q, Huan M, Mei Q. Dose-dependent absorption, metabolism, and excretion of genistein in rats. J Agric Food Chem. 2008;56(18):8354–9. doi: 10.1021/jf801051d. [DOI] [PubMed] [Google Scholar]
- 38.Qiu F, Chen XY, Song B, Zhong DF, Liu CX. Influence of dosage forms on pharmacokinetics of daidzein and its main metabolite daidzein-7-O-glucuronide in rats. Acta Pharmacol Sin. 2005;26(9):1145–52. doi: 10.1111/j.1745-7254.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- 39.Kwon SH, Kang MJ, Huh JS, Ha KW, Lee JR, Lee SK, Lee BS, Han IH, Lee MS, Lee MW, Lee J, Choi YW. Comparison of oral bioavailability of genistein and genistin in rats. Int J Pharm. 2007;337(1–2):148–54. doi: 10.1016/j.ijpharm.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 40.Jefferson WN, Doerge DR, Padilla-Banks E, Woodling KA, Kissling GE, Newbold RR. Oral exposure to genistin, the glycosylated form of genistein, during neonatal life adversely affects the female reproductive system. Environ Health Persp. doi: 10.1289/ehp.0900923. (in press) available via http://dx.doi.org/ [Online 27 Jul 2009] [DOI] [PMC free article] [PubMed]
- 41.Holder CL, Churchwell MI, Doerge DR. Quantification of soy isoflavones, genistein and daidzein, and conjugates in rat blood using LC/ES-MS. J Agric Food Chem. 1999;47(9):3764–70. doi: 10.1021/jf9902651. [DOI] [PubMed] [Google Scholar]
- 42.McClain RM, Wolz E, Davidovich A, Pfannkuch F, Edwards JA, Bausch J. Acute, subchronic and chronic safety studies with genistein in rats. Food Chem Toxicol. 2006;44(1):56–80. doi: 10.1016/j.fct.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 43.Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;130(7):1695–9. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- 44.Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131(4 Suppl):1362S–75S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 45.Richelle M, Pridmore-Merten S, Bodenstab S, Enslen M, Offord EA. Hydrolysis of isoflavone glycosides to aglycones by beta-glycosidase does not alter plasma and urine isoflavone pharmacokinetics in postmenopausal women. J Nutr. 2002;132(9):2587–92. doi: 10.1093/jn/132.9.2587. [DOI] [PubMed] [Google Scholar]
- 46.Rufer CE, Bub A, Moseneder J, Winterhalter P, Sturtz M, Kulling SE. Pharmacokinetics of the soybean isoflavone daidzein in its aglycone and glucoside form: a randomized, double-blind, crossover study. Am J Clin Nutr. 2008;87(5):1314–23. doi: 10.1093/ajcn/87.5.1314. [DOI] [PubMed] [Google Scholar]
- 47.Nielsen IL, Williamson G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr Cancer. 2007;57(1):1–10. doi: 10.1080/01635580701267677. [DOI] [PubMed] [Google Scholar]
- 48.Busby MG, Jeffcoat AR, Bloedon LT, Koch MA, Black T, Dix KJ, Heizer WD, Thomas BF, Hill JM, Crowell JA, Zeisel SH. Clinical characteristics and pharmacokinetics of purified soy isoflavones: single-dose administration to healthy men. Am J Clin Nutr. 2002;75(1):126–36. doi: 10.1093/ajcn/75.1.126. [DOI] [PubMed] [Google Scholar]
- 49.King RA, Broadbent JL, Head RJ. Absorption and excretion of the soy isoflavone genistein in rats. J Nutr. 1996;126(1):176–82. doi: 10.1093/jn/126.1.176. [DOI] [PubMed] [Google Scholar]
- 50.Zubik L, Meydani M. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am J Clin Nutr. 2003;77(6):1459–65. doi: 10.1093/ajcn/77.6.1459. [DOI] [PubMed] [Google Scholar]
- 51.Cassidy A, Brown JE, Hawdon A, Faughnan MS, King LJ, Millward J, Zimmer-Nechemias L, Wolfe B, Setchell KD. Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J Nutr. 2006;136(1):45–51. doi: 10.1093/jn/136.1.45. [DOI] [PubMed] [Google Scholar]
- 52.Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, Helferich WG, Katzenellenbogen JA. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. 2004;12(6):1559–67. doi: 10.1016/j.bmc.2003.11.035. [DOI] [PubMed] [Google Scholar]