Ischemic injury to the myocardium is likely the most frequent but certainly the most deadly contemporary wound in humans. The lack of oxygen jeopardizes cardiac resident cells, which include myocytes, fibroblasts, endothelial cells and cardiac macrophages. These cells then die or react by releasing danger signals which systemically alert the immune system1. The most numerous responders are myeloid cells, including neutrophils, monocytes and macrophages. The past decade elucidated the time line of response, in which consistent high-rate inflammatory neutrophil and monocyte recruitment dominate the first hours to days1-3. After day 4, inflammation begins to resolve and recruited monocytes give rise to less inflammatory reparative macrophages4. These two phases, a first inflammatory and a second resolution phase, are essential for infarct healing5, 6. In mice with atherosclerosis7 and likely in patients1, lack or delay of the inflammation resolution phase brings about infarct rupture, infarct expansion, and ultimately heart failure. In patients with acute MI, systemic markers of inflammation correlate with higher mortality1. These insights have triggered an interesting question with potentially high therapeutic value: what brings about the switch from Phase 1 to Phase 2? In other words, what are the mechanisms that initiate resolution of infarct inflammation, shielding the reparative actions of wound healing in the heart from inflammatory immune cells? Are these mechanisms local or systemic, macrophage-intrinsic or not? And how can we harness them therapeutically to support infarct healing and prevent post-MI heart failure? In the current issue of Circulation Research, Weirather et al.8 present data suggesting that T regulatory cells (Tregs) are involved in the transition from infarct inflammation towards resolution.
Tregs constitute a subset of CD4+ T cells whose role, in simple terms, is to suppress (or regulate) immune inflammatory function. Until the discovery of Foxp3 as the transcription factor determining Treg identity9, investigators focused on CD25, the high affinity receptor for IL-2, as a defining feature of these cells. Ontogenic and functional studies have identified thymus-derived naturally occurring Tregs as Foxp3+ cells that circulate in the blood in low numbers in the steady state, as well as induced Tregs that arise from mature T cell subsets during certain situations, such as tolerance induction, producing large amounts of the cytokine IL-10 10. Treg's suppressive function has been known for years and represents a double-edged sword. On the one hand, in conditions such as allergy and autoimmune disease, harnessing the regulatory ability of T cells can be beneficial and therapeutically desirable. On the other, in conditions such as cancer, where tumor cell eradication is the goal, Tregs are an obstacle to effective immunotherapy.
Previous work from the same and other groups had indicated that T11, 12 and B13 lymphocytes participate in the response to myocardial infarction. Although Tregs have received attention in other areas of cardiovascular disease14, their role in the biphasic monocyte/macrophage response that follows myocardial infarction has remained unknown. In the current manuscript, Weirather et al. demonstrate the expansion of Tregs in the mediastinal lymph nodes draining the heart, and in the infarct itself. On day 7 after coronary ligation, ∼150 Tregs accumulate per mg infarct tissue which, compared to the ∼ 50,000 macrophages accumulating per mg infarct tissue5, 15 represents a very small Treg-to-macrophage ratio. This clearly eliminates Tregs as “foot soldiers” of the response. However, even a small number of leukocytes can be important. The question then is, does this accumulation matter?
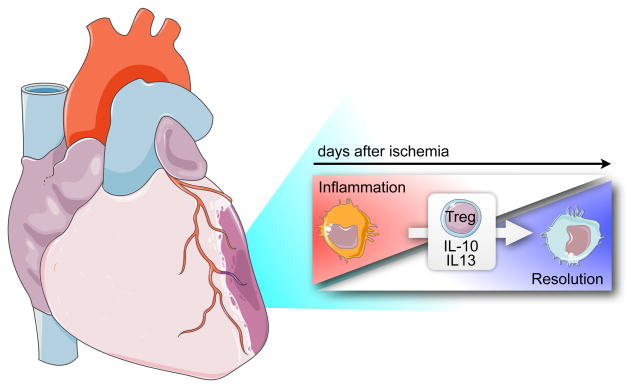
Weirather et al. induced myocardial infarction in Foxp3DTR mice, which allowed the investigators to deplete Tregs. In these mice, infarct size was increased, resolution of infarct inflammation was impaired, and neutrophil and Ly6Chigh monocyte numbers were augmented. Moreover, the gene expression profile from macrophages isolated from 5 day old infarcts was biased towards inflammation, and factors typically associated with healing, such as TGFβ, osteopontin and transglutaminase factor XIII were decreased. The latter was previously identified as a prominent marker of inflammatory classical mouse monocytes by the ImmGen consortium (www.immgen.org), highlighting that the simple M1/M2 categorization of macrophages is far from precise, and suggesting that local factors may change specific genes in a manner that overrides the M1/2 classification. Taken together, absence of Tregs led to impaired transition towards resolution of inflammation, resulting in the persistence of inflammatory macrophages and delayed healing (Figure).
Figure 1.
Tregs promote transition of macrophage phenotype after myocardial infarction and their lack leads to impaired infarct healing.
When Tregs were depleted with a CD25 antibody 8 days prior to MI, post-MI survival was impaired, which did not occur in Foxp3DTR mice. However, antibody depletion led to more numerous neutrophil and Ly6Chigh monocyte in infarcts. Treatment with a superagonistic anti-CD28 antibody expanded Tregs 2-fold in blood and heart-draining lymph nodes, shifted the macrophage gene expression towards inflammation resolution, improved infarct matrix repair, and reduced infarct mortality. Co-culture experiments suggested that Tregs may modulate macrophage phenotype in vitro via secretion of IL-10 and IL-13.
Collectively, the Frantz group describe a very interesting observation in 2 different models of Treg depletion and one model of Treg activation: an altered abundance of these cells correlated with an altered macrophage phenotype and outcome post MI. The study adds to the increasing evidence that heart macrophages are important in the evolution of heart failure, and reports a novel, potentially clinically important mechanism of how cardiac macrophage phenotype is steered. The data raise a number of interesting questions. For example, where and how do Tregs interact with myeloid cells? On the one hand, recent studies indicate that IL-10 is a critical homeostatic factor for macrophages16. On the other hand, the very low absolute numbers of Tregs vis-a-vis myeloid cells in the infarct raises the question of whether additional interactions are important elsewhere. Can Tregs influence the supply of cells in other locations? Previously, Tregs were shown to influence the action of hematopoietic progenitors in the bone marrow17. Can Tregs interact with monocyte progenitors and change their phenotype before they enter inflamed tissues? Recent studies have shown that cell intrinsic factors such as the orphan nuclear hormone receptor, nuclear receptor subfamily 4, group a, member 1 (Nr4a1) expressed in monocytes, regulates the emergence of Ly6Clow monocytes in blood and regulates the infarct macrophage phenotype4, 18. Another regulator of macrophage phenotype after MI is the master transcription factor IRF519. In light of the Treg data discussed above, it would be interesting to explore if these mechanisms are coupled or independent. Overall, it is likely that the infarct macrophage phenotype results from an integration of multiple inputs, including cell-intrinsic, systemic, and local factors. Going forward, we will need to identify viable avenues for a therapeutic intervention that modulates infarct inflammation and prevents post-MI heart failure. Future work will show if Treg activation is such an option for patients with myocardial infarction.
Acknowledgments
Sources of Funding: This work as supported by NHLBI grants R01HL114477, R01HL095629 (to M.N.), and R01HL095612, R56AI104695 (to F.K.S.).
Footnotes
Disclosure Section: The authors declare no conflict of interest.
References
- 1.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339(6116):161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11(5):255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frantz S, Bauersachs J, Ertl G. Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc Res. 2009;81(3):474–481. doi: 10.1093/cvr/cvn292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherer-Crosbie M, Hedrick CC, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Ly-6Chigh Monocytes Depend on Nr4a1 to Balance Both Inflammatory and Reparative Phases in the Infarcted Myocardium. Circ Res. 2014;114(10):1611–1622. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204(12):3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121(22):2437–2445. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55(15):1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weirather J, Hofmann U, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+CD4+ T Cells Improve Healing after Myocardial Infarction by Modulating Monocyte/Macrophage Differentiation. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- 9.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 10.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol. 2010;176(5):2177–2187. doi: 10.2353/ajpath.2010.090759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, Kerkau T, Frantz S. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125(13):1652–1663. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 13.Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guerin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L, Dumeau E, Kotti S, Bruneval P, Charo IF, Binder CJ, Danchin N, Tedgui A, Tedder TF, Silvestre JS, Mallat Z. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013;19(10):1273–1280. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ait-Oufella H, Sage AP, Mallat Z, Tedgui A. Adaptive (T and B cells) immunity and control by dendritic cells in atherosclerosis. Circ Res. 2014;114(10):1640–1660. doi: 10.1161/CIRCRESAHA.114.302761. [DOI] [PubMed] [Google Scholar]
- 15.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209(1):123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zigmond E, Bernshtein B, Friedlander G, Walker CR, Yona S, Kim KW, Brenner O, Krauthgamer R, Varol C, Muller W, Jung S. Macrophage-Restricted Interleukin-10 Receptor Deficiency, but Not IL-10 Deficiency, Causes Severe Spontaneous Colitis. Immunity. 2014 doi: 10.1016/j.immuni.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, Gao W, Saito TI, Lo Celso C, Tsuyuzaki H, Sato T, Cote D, Sykes M, Strom TB, Scadden DT, Lin CP. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474(7350):216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol. 2011;12(8):778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courties G, Heidt T, Sebas M, Iwamoto Y, Jeon D, Truelove J, Tricot B, Wojtkiewicz G, Dutta P, Sager HB, Borodovsky A, Novobrantseva T, Klebanov B, Fitzgerald K, Anderson DG, Libby P, Swirski FK, Weissleder R, Nahrendorf M. In Vivo Silencing of the Transcription Factor IRF5 Reprograms the Macrophage Phenotype and Improves Infarct Healing. J Am Coll Cardiol. 2014;63(15):1556–1566. doi: 10.1016/j.jacc.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]