Abstract
Abuse of buprenorphine (BUP) by the intravenous (IV) route has been documented in several studies, and reports of intranasal (IN) abuse are increasing. However, no studies have directly compared the effects of BUP when it is administered intranasally and intravenously. The present secondary analysis used data from two separate studies to compare the reinforcing and subjective effects of IV and IN buprenorphine. One study evaluated IV buprenorphine (N=13) and the other evaluated IN buprenorphine (N=12). Participants were maintained on 2 mg sublingual (SL) BUP and tested with each intranasal or intravenous buprenorphine test dose (0 mg, 2 mg, 4 mg, 8 mg, and 16 mg). During morning laboratory sessions, participants received money (US $20) and sample doses of IN or IV BUP, and then completed subjective effects questionnaires. Later that day, they completed a self-administration task to receive 10% portions of the drug and/or money they previously sampled. In general, positive subjective ratings for both IV and IN BUP were significantly greater than placebo, with IV BUP having a greater effect than IN BUP. All active BUP doses (IV and IN) maintained significantly higher progressive ratio breakpoint values than placebo, but breakpoint values for IV BUP were greater than for IN BUP. Buprenorphine is an effective maintenance treatment for opioid dependence, valued for its ability to reduce the positive subjective effects of other opioids. Nevertheless, the present data demonstrate that in participants maintained on a low dose of SL BUP, the medication itself has abuse liability when used intravenously or intranasally.
Keywords: Intranasal, Intravenous, Self administration, Buprenorphine, Opioid, Abuse Liability
1. Introduction
Opioid abuse is a major public health problem in the United States and around the world (SAMHSA, 2010; UNODC, 2012). Maintenance treatment with the partial mu (μ) opioid agonist buprenorphine has been shown to reduce the morbidity and mortality associated with opioid abuse (Mattick et al., 2008). With a superior safety profile in comparison to methadone, buprenorphine treatment quickly gained popularity and the availability of BUP around the world has steadily increased (Auriacombe et al., 2004; Carrieri et al., 2006; Maxwell et al., 2010; Walsh et al., 1994). In spite of its clinical utility, buprenorphine itself has abuse liability and diversion to illicit use has been observed (Johanson et al., 2012).
Originally it was believed that buprenorphine had relatively low abuse liability because of its partial μ agonist profile (Jasinksi, 1978; Mello and Mendelsen, 1985, Walsh et al., 1994, 1995). Yet the abuse of buprenorphine has been noted in Europe (Alho et al., 2007; Auriacombe et al., 2001; Carrieri et al., 2006; Hakansson et al., 2007; Obadia et al., 2001; Roux et al., 2008a,b; Vidal-Trecan et al., 2003), Australia, and South East Asia (Chua and Lee, 2006; Horyniak et al., 2011; Jenkinson et al., 2005; Lee, 2006; Nielson et al., 2007; Vicknasingam et al., 2010). Consistent with the epidemiological data, laboratory studies have shown that when it is injected (intramuscularly or intravenously) BUP can produce robust opioid-like effects, similar to other potent μ agonists (Bedi et al., 1998; Comer et al., 2002; Duke et al., 2010; Strain et al., 1997; Zacny et al., 1997). For example, intramuscular administration of buprenorphine to opioid-dependent participants (maintained on sublingual BUP) produces significant increases in subjective ratings of: drug “liking”, “good” drug effect, and “high” (Duke et al., 2010). Similar findings have been reported using intravenously administered buprenorphine in recently detoxified heroin-users (Comer et al., 2005) and buprenorphine-maintained heroin users (Comer et al., 2010).
Although several epidemiological and laboratory studies of injected buprenorphine have been conducted, relatively few studies have examined abuse of buprenorphine by the intranasal route, despite the growing number of reports that the medication is being abused in this manner. For example, studies have reported that the incidence of intranasal BUP abuse is notable in Europe (Hakansson et al., 2007; Roux et al., 2008b). In a rural area of the U.S., a recent investigation on prescription opioid abuse found intranasal buprenorphine abuse to be almost nine times more prevalent than intravenous abuse (Young et al., 2010), while another study reported roughly equivalent rates of IV and IN BUP abuse (Nordman et al., 2012). Only one laboratory study has investigated the pharmacodynamic effects of intranasal buprenorphine (Middleton et al., 2011). These investigators found that in non-dependent, intranasal opioid abusers, IN BUP produced dose-related increases in ratings of drug liking and street value (Middleton et al., 2011). Whether the same profile would be observed in opioid-dependent individuals is unclear, however.
The purpose of the present secondary data analysis was to utilize unpublished data from two separate investigations in order to compare the abuse liability of BUP when it is administered via the IV and IN routes to BUP-dependent heroin users. Employing a same-day sample and choice self-administration procedure, the subjective and reinforcing effects of IV and IN BUP were quantified in order to assess their abuse liability (Comer et al., 2008; Jones and Comer, 2013). In addition, physiological and cognitive responses were also observed. This study may allow us to better understand the differential prevalence of intranasal and intravenous buprenorphine abuse.
2. Methods
2.1 Participants
Participants were required to be physically and mentally healthy intravenous or intranasal heroin users between the ages of 21 and 45 (IV study) or 55 (IN study) years. All participants were required to meet DSM-IV criteria for opioid abuse and physical dependence. Potential participants were excluded from the studies if they were seeking treatment for their drug use, physiologically dependent on alcohol or illicit drugs (other than opioids), or had a severe Axis I psychiatric diagnosis (other than opioid, nicotine or caffeine dependence).
As compensation, participants were paid $25/day with a $25/day bonus for completing the study. In addition to the per diem payment, participants had the opportunity to earn money during the experimental sessions ($20 per sample session plus up to $20 per self-administration session, described below). Following completion of subsequent study procedures, participants were discharged from the hospital, and provided with referrals for drug treatment if they were interested. Opioid detoxification on the inpatient unit was also available to all participants at the end of the studies.
2.2 Procedures
Participants were recruited from the New York City metropolitan area through various print media advertisements. In one study, the effects of IV BUP were evaluated and in a separate study, the effects of IN BUP were examined. Those respondents who met study inclusion/exclusion criteria, based upon the initial telephone interview, were scheduled to come to the New York State Psychiatric Institute for additional screening procedures. Screening consisted of both self-report and clinical interviews administered by a team of research assistants, psychologists, nurses, and physicians. Assessments were made of drug use, medical history and general health (hematology, blood chemistry panel, liver and thyroid functioning, urinalysis, syphilis serology). A semi-structured psychiatric interview and physical examination were performed by a physician. An 11-panel rapid urine drug screen assessed recent use of: amphetamine, barbiturates, benzodiazepines, buprenorphine, cocaine, methadone, methamphetamine, opiates, oxycodone, PCP, and THC. Women were tested for pregnancy by measuring serum hCG levels. Naloxone challenge was used to determine current opioid use in participants who met DSM-IV opioid dependence criteria. In this procedure we administer an intramuscular dose of naloxone (between 0.2–0.8 mg) and observe for opioid withdrawal symptoms. Participants also had the option of presenting themselves to clinical staff in a state of opioid withdrawal. Potential participants who were screened for the study were most often excluded for medical or psychiatric concerns.
Once enrolled, participants resided on a locked inpatient unit during the study. During the first week after admission, they were stabilized on 2 mg of sublingual (SL) BUP, which was administered at approximately 8 p.m. The 2 mg dose was chosen in order to prevent withdrawal, but minimize the ability of the sublingual dose to alter the effects of the parenteral dose. During the first week after admission into the hospital, participants were treated for emergent withdrawal symptoms with various supplemental medications until withdrawal symptoms dissipated based on self-report and clinician observations. During the second week after admission into the hospital, while still being maintained on 2 mg SL BUP, each participant was tested with each intranasal or intravenous buprenorphine dose in ascending order (2 mg, 4 mg, 8 mg, and 16 mg). One dose was tested on each day, and a placebo (Pbo, 0 mg) dose was randomly inserted into this order. Doses were administered at approximately 11 a.m. during a morning sample session and again at approximately 3 p.m. during an afternoon choice session (see below).
The previously unpublished data presented currently were part of a buprenorphine self-administration “qualification phase.” Participants who self-administered more active buprenorphine than placebo qualified for a subsequent series of laboratory sessions that were designed to compare the effects of placebo, buprenorphine, buprenorphine/naloxone, heroin, and naloxone alone. The design of the qualification phases for these two studies were identical, except for the route of buprenorphine administration, and are described in further detail below. Data from the parent IV study have been published (Comer et al., 2010), while the parent IN study is under review. In the present paper, data collected from all participants were included in the analysis regardless of whether they qualified for the parent study.
Qualification phase testing consisted of two types of laboratory sessions, the first of which was conducted approximately 15 hr after administration of the SL BUP maintenance dose. During a “sample” session, participants received $20 and one of the challenge doses [Placebo (0 mg), BUP 2 mg, 4 mg, 8 mg, 16 mg] administered either intravenously or intranasally. Subjective, performance, and physiological effects were measured before and repeatedly after drug administration. The sample session was followed a few hours later by a self-administration or “choice” session. During the choice session, participants were given the opportunity to work for either the dose of drug that was given during the sample session or money. An alternative approach to conducting the sample and choice sessions on the same day would be to complete the choice session on the following day. The investigators opted to complete both sessions on the same day because it more closely mimics the pattern of use on the “street,” and our previous experience with the pharmacology of buprenorphine made us confident that carry-over drug effects from the sample to the choice session would be minimal (Comer et al., 2010).
Sample Session
At approximately 10 a.m., participants were brought to the laboratory to complete a sample session. Forty minutes (min) prior to drug administration, physiological monitoring began. A pulse oximeter continuously measured arterial oxygen saturation (%SpO2); heart rate, systolic blood pressure, and diastolic blood pressure were measured every 5 minutes throughout the session and for an hour following dosing. Participants received money (US $20) and the full doses of the IV or IN challenge drug at 0 min, which occurred at approximately 11 a.m. During the IN study, participants were instructed to insufflate the entire dose within a 30-second period in one or both nostrils, and during the IV study, the entire intravenous solution was infused over the course of 30 seconds. At various time points throughout the session, pupil diameter was measured and participants completed subjective effects batteries and performance tasks (Table 1).
Table 1.
Time points at which physiological, performance and subjective measurements were taken throughout the sample session.
| Time (mins) | |
|---|---|
| −40 | Physiological monitoring (oxygen saturation, blood pressure), Pupils, DSST, DAT, VAS, SOWS |
| 0 | Participants received sample drug dose and money |
| 5 | Pupils, VAS, DEQ |
| 10–15a | Pupils, DSST, DAT, VAS, DEQ |
| 40–45a | Pupils, VAS, DEQ |
| 60 | Pupils, DSST, DAT, VAS, DEQ |
Due to differences between the study protocols, these time points varied slightly between the two studies.
Choice Sessions
At approximately 2 p.m., participants were brought to the laboratory to complete a choice session. The baseline assessments during each choice session were identical to those used in the sample session; participants then completed a self-administration task to obtain portions of the dose of drug or money they had received earlier in the day (see details below).
2.3 Assessments
Participant Characteristics
Participant demographics were ascertained from: a telephone interview by a research assistant, clinical interview by research psychologists, and a mental examination by a psychiatrist. Urine toxicology results collected during the various screening visits were also compiled and assessed.
Subjective Effects
Three questionnaires were used to assess subjective responses: one assessed opioid withdrawal symptoms, and two assessed a range of possible drug effects (positive and negative). An additional questionnaire was used in the IN study to assess the effects of nasal insufflation of the powder.
The 16-item Subjective Opioid Withdrawal Scale (SOWS) was used to identify the presence and severity of opioid withdrawal symptoms (Handelsman et al., 1987). Each question was rated on a scale from 0 [‘Not At All’] to 4 [‘Extremely’]. The sum score ranged between 0 and 64.
A 26-item visual analog scale (VAS) was used to assess subjective and physiological drug effects such as: I feel a “Good Drug Effect” and I feel “High”. Participants rated each item on the scale from ‘Not at all’ (0 mm) to ‘Extremely’ (100 mm). In addition, a 6-item drug effects questionnaire (DEQ) was used to measure drug effects (strength of drug effects, good effects, bad effects, willingness to take the drug again, drug liking, and similarity to other drugs). Participants selected among a series of possible answers ranging from 0 [‘No Drug Effects at All’] to 4 [‘Very Strong Effects’]. The drug liking question ranged from −4 [‘Dislike Very Much’] to 4 [‘Like Very Much’].
Participants in the IN study also completed a questionnaire designed to assess specific aspects of how their nose and throat felt after each intranasal drug administration (Middleton et al., 2011). Participants rated the presence of 9 sensations such as “burning,” “stinging,” and “pain” on a scale from 0 [‘Not At All’] to 4 [‘Extremely’]. Because this questionnaire was added later in the intranasal study, only a subset of the participants (N= 6) had completed it at the time this analysis was performed.
Reinforcing Effects
The reinforcing effects of each Bup dose given by each route, was assessed using a Drug vs Money self-administration procedure. For this assessment, the primary dependent variable is the drug’s “breakpoint,” which is the largest ratio value completed for drug. Drugs that maintain larger breakpoint values (i.e., drugs for which participants are willing to perform more work to receive) are considered to have greater abuse liability (Katz, 1990).
Cognitive/Performance Effects
The performance battery consisted of two tasks: a 3-min digit-symbol substitution task (DSST) and a 10-min divided attention task (DAT; Table 1). Custom-made software was used for these performance tasks (see Comer et al., 1999 for details). Briefly, the digit-symbol substitution task consisted of nine 3-row by 3-column squares (with one black square per row) displayed across the top of the computer screen. A randomly generated number indicated which of the nine patterns should be emulated on a keypad by the participant on a particular trial. Participants were required to emulate as many patterns as possible by entering the pattern associated with randomly generated numbers appearing on the bottom of the screen. The divided attention task consisted of concurrent pursuit-tracking and vigilance components. Participants tracked a moving stimulus on the video screen using the mouse and also signaled when a small black square appeared at any of the four corners of the video screen. The distance between the cursor and moving stimulus was measured, as was the speed of the moving stimulus (with greater accuracy, the stimulus moved at a faster rate).
Physiological Effects
Pupil diameter was measured using a NeurOptics™ Pupillometer under ambient lighting conditions. Miosis was used as a physiological indicator of mu agonist effects, and assessed prior to drug administration and at various intervals following.
2.4 Drugs
Buprenorphine tablets (2 mg/day) for sublingual use and buprenorphine HCl powder for IV and IN use were obtained from Murty Pharmaceuticals (Lexington, KY) and Reckitt-Benckiser Pharmaceuticals Inc. (Richmond, VA) through the Research Triangle Institute. The doses (2, 4, 8, and 16 mg) were chosen based on previous studies of IV and IN buprenorphine conducted in our laboratory as well as in other laboratories (Comer and Collins, 2002; Comer et al., 2005; Middleton et al., 2011; Umbricht et al., 2004). A maximum dose of 16 mg buprenorphine was selected because it is considered by many to be the optimal dose for daily clinical maintenance (Carrieri et al., 2006; Johnson et al., 2003). Intranasal placebo consisted of lactose powder and intravenous placebo was saline. A constant weight of 100 mg of powder was used during the IN study and a constant volume of 1 ml solution was administered during the IV study at each dose administration in order to maintain the blind.
Naloxone HCl (Narcan) for injection was obtained from the International Medication System Limited Amphastar (South Elmonte, CA). All drugs were prepared by the New York State Psychiatric Institute Pharmacy and administered by a physician.
2.6 Statistical Analyses
Participant Characteristics
Continuous and categorical variables were initially summarized descriptively. T-tests were employed to examine differences between the intranasal and intravenous samples for continuous variables, whereas the Pearson X2 statistic was calculated to assess categorical differences between the groups.
Drug Effects
Mixed-model analyses of variance (ANOVA) tests were used to compare mean peak drug effects among the 5 buprenorphine doses and between the two routes of administration. In these analyses the buprenorphine dose (Dose) served as the within-subjects factor, while the between-subjects factor was the route of buprenorphine administration (Route). In cases where a significant or trending (p< 0.10) main effect or interaction was found, planned comparisons (T-tests) were used to compare the effects of corresponding buprenorphine doses when administered via the intranasal and intravenous routes. Any comparison where homogeneity was violated was not included in the results (no comparisons violated homogeneity).
In order to further explore where mean peak differences occurred, separate repeated-measures ANOVAs were performed for each route of administration comparing peak drug effects among the various doses. In cases where a significant or trending (p<0.10) effect was found, planned comparisons were employed to determine which doses significantly differed from one another. For the sake of clarity and brevity, the results of the OMNIBUS tests have been omitted and only the final planned comparisons are reported in the results section.
Both the maximal drug effect for each measure (peak= increase, or trough=decrease) and time-course of drug effect were analyzed for all relevant measures. A peak or trough measurement was taken from across all of the time points, post drug administration (sample session). The data presented in section 3.3 explain why we primarily report and discussion peak/trough data. For final analyses, the significance level of α was set at 0.05. All data analyses were performed using SPSS version 18 (SPSS, 2009) and SuperANOVA (Gagnon et al., 1990).
3. Results
3.1 Participant Characteristics
Intravenous Sample
Sixteen participants were enrolled into this study. Of those, 13 were included in this analysis (9M, 4F; 7 White, 3 Black, and 3 Latino). Of the 3 participants who did not complete the study, 1 withdrew due to personal issues, 1 was dropped due to inability to follow unit rules, and another for unreported methadone use. The mean age of those included in the data analysis was 36.4 years. All participants reported daily intravenous heroin use (Table 2). The mean daily amount spent on heroin was $67.50 (range: $30– $145), and mean duration of use was 11.0 years (range: 2–32 years).
Table 2.
Select demographic characteristics for the IV and IN participant samples.
| IV | IN | |
|---|---|---|
| Sex | 9M, 4F | 11M, 1F |
| Ethnicity | 7 Caucasian 3 Latino 3 Afr-American |
4 Caucasian 3 Afr-American 2 Multiracial 2 Latino 1 Asian |
| Age ± SEM (yrs) | 36.4 ± 1.9 | 36.5 ± 1.5 |
| Duration of Heroin Use ± SEM (yrs) | 11.0 ± 1.2 | 13.6 ± 1.7 |
| Amount Spent/Day on Heroin ± SEM ($) | 67.50 ± 9.1 | 70.40 ± 7.8 |
Intranasal Sample
At the time this analysis was completed, 20 participants had been enrolled into the study. Of those, complete data sets were obtained from 12 participants for inclusion in this analysis (11M, 1F; 4 White, 3 Black, 2 Latino, 2 Multiracial, 1 Asian). Of the 8 participants who did not complete the study, 5 withdrew due to personal issues, 2 were dropped due to inappropriate behavior on the inpatient unit, and 1 participant was dropped after AST/ALT levels rose to 3 times the upper limit of normal. The mean age of those included in the data analysis was 36.5 years. The majority were daily intranasal heroin users (83%, 10 of 12), but 2 reported current intravenous use with a history of intranasal use. The mean daily amount spent on heroin was $70.40 (range: $20–$170), and mean duration of use was 13.6 years (range: 6–26 years). No significant group differences were found for any of the demographic variables. Urine toxicology data collected during screening suggested that in addition to opioids, participants from both groups sporadically used non-opioid drugs, most commonly, cocaine (none of the enrolled participants met criteria for dependence on non-opioid drugs).
3.2 Subjective Effects
Subjective Opioid Withdrawal Scales (SOWS)
At the beginning of each laboratory session (sample and choice), subjective ratings of opioid withdrawal were minimal [SOWS scores of less than 10 (range: 0–64)]. Means of all measures are shown in Table 3 as a function of BUP dose (0 mg, 2 mg, 4 mg, 8 mg, and 16 mg) and route of administration (IV, IN).
Table 3.
Mean peak (± SEM) subjective measures as a function of BUP dose and route of administration.
| IV | IN | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 mg | 2 mg | 4 mg | 8 mg | 16 mg | 0 mg | 2 mg | 4 mg | 8 mg | 16 mg | |
| Dependent Measure | ||||||||||
| SOWS Sum (0–64) | ||||||||||
| Sample Session Baseline | 7.4 (1.6) | 8.8 (2.2) | 6.4 (0.8) | 6.4 (1.5) | 5.7 (1.1) | 4.6 (0.6) | 4.5 (0.8) | 4.8 (0.7) | 5.2 (2.0) | 2.7 (0.6) |
| Choice Session Baseline | 3.74 (.89) | 3.60 (.90) | 3.57 (.84) | 3.76 (.67) | 4.74 (2.2) | 3.59 (.88) | 3.92 (1.71) | 3.29 (.91) | 4.15 (1.78) | 2.1 (.74) |
| VAS (0– 100) | ||||||||||
| Anxious | 6.4 (3.3) | 10.5 (4.9) | 12.2 (7.0) | 6.4 (3.4) | 2.3 (1.5) | 22.2# (2.3) | 15.8 (1.5) | 19.4 (1.5) | 23.2# (6.0) | 17.2# (3.4) |
| Bad | .21 (.21) | 2.1 (1.2) | 1.57 (1.4) | .14 (.14) | 3.6 (3.4) | 2.7# (2.7) | .38 (.38) | 1.9 (1.3) | 5.8 (5.7) | 6.0 (3.9) |
| Energetic | 24.1 (7.80 | 41.0 (7.3) | 37.0 (8.7) | 35.1 (8.1) | 31.3 (8.2) | 29.5 (9.1) | 33.8 (8.0) | 40.1 (10.1) | 35.8 (9.7) | 33.9 (9.6) |
| Gooseflesh | .43 (.36) | 2.4 (1.4) | 1.4 (1.2) | .28 (.28) | .14 (.14) | 2.2 (1.5) | 1.6 (1.3) | 4.5 (3.7) | 7.5 (6.1) | 0.0 (0.0) |
| High Quality | 5.6 (3.5) | 27.5 (8.2) | 37.3 (8.8) | 30.4 (9.0) | 35.6 (6.9) | 11.7 (6.1) | 11.2 (5.7) | 15.2 (6.9) | 16.5 (7.1) | 25.2 (9.1) |
| Irritable | 11.0 (6.6) | 5.3 (3.4) | 3.5 (1.7) | 13.8 (7.6) | 16.0 8.2) | 9.9 (3.8) | 8.5 (4.2) | 10.0 (6.5) | 9.1 (5.0) | 7.4 (4.3) |
| Liked the Dose | 1.6 (1.4) | 38.0 (9.5) | 40.8 (9.1) | 34.2 (9.3) | 43.9 (7.6) | 16.3# (6.9) | 11.5# (5.8) | 15.1# (6.3) | 17.5# (6.7) | 28.2# (9.8) |
| Mellow | 25.1 (5.8) | 49.3 (7.0) | 35.5 (8.3) | 31.6 (6.2) | 44.3 (6.2) | 36.5 (8.2) | 32.5 (9.4) | 36.9 (9.4) | 30.1 (8.4) | 40.5 (10.4) |
| Nauseated | 0.6 (0.5) | 5.4 (4.5) | 6.1 (5.8) | 4.1 (3.7) | 1.2 (0.8) | 3.6 (3.5) | 0.5 (0.5) | 0.8 (0.5) | 0.0 (0.0) | 3.9 (3.8) |
| Potent | 1.7 (1.3) | 25.4 (8.3) | 34.3 (8.6) | 29.4 (8.8) | 38.0 (8.6) | 9.7# (5.2) | 10.6# (5.6) | 17.1# (6.6) | 15.4# (7.3) | 25.3 (9.1) |
| Sedated | 1.9 (1.7) | 19.1 (5.8) | 26.9 (8.3) | 16.0 (6.9) | 30.4 (8.9) | 10.2# (5.6) | 7.6 (4.1) | 7.3# (4.0) | 15.6 (6.8) | 22.0 (8.8) |
| Sleepy | 24.1 (8.4) | 21.0 (7.1) | 13.2 (5.8) | 15.8 (5.5) | 16.5 (7.4) | 10.7 (5.8) | 16.4 (6.5) | 21.0 (8.3) | 18.9 (7.5) | 23.9 (8.1) |
| Social | 29.8 (6.3) | 50.5 (9.1) | 41.4 (7.0) | 38.2 (7.3) | 37.9 (7.1) | 32.7 (8.5) | 41.3 (8.6) | 43.8 (9.8) | 30.1 (8.8) | 35.5 (8.3) |
| Stimulated | 9.9 (5.6) | 29.0 (9.6) | 31.8 (9.6) | 23.5 (8.5) | 25.7 (9.1) | 13.8 (7.9) | 19.4 (8.7) | 25.2 (9.9) | 19.3 (6.7) | 24.1 (8.5) |
| Talkative | 29.6 (7.4) | 40.6 (8.7) | 41.6 (6.9) | 35.8 (7.9) | 32.3 (8.3) | 29.0 (8.5) | 32.6 (10.5) | 38.2 (10.4) | 25.0 (8.6) | 33.9 (9.7) |
| Would Pay | 0.0 (0.0) | 5.6 (1.6) | 8.2 (1.5) | 6.1 (1.5) | 8.8 (1.6) | 1.0 (0.5) | 1.4# (0.6) | 1.3# (0.7) | 4.1 (1.5) | 6.5 (2.2) |
| DEQ (0–4) | ||||||||||
| Bad | 0.1 (0.1) | 0.1 (0.2) | 0.2 (0.2) | 0.1 (0.1) | 0.9 (0.7) | 0.2 (0.1) | 0.1 (0.1) | 0.0 (0.0) | 0.1 (0.1) | 0.3 (0.2) |
| Good | 0.2 (0.4) | 1.5 (0.3) | 2.0 (0.3) | 1.6 (0.3) | 2.4 (0.6) | 0.5 (0.2) | 0.6 (0.2) | 1.0 (0.3) | 1.1 (0.3) | 1.5 (0.4) |
| Like | −0.4 (0.4) | 1.4 (0.5) | 2.4 (0.3) | 1.8 (0.3) | 2.4 (0.6) | −0.5 (0.4) | 0.2 (0.5) | 0.5# (0.5) | 0.6# (0.5) | 0.9# (0.6) |
| Strong | 0.4 (0.2) | 1.4 (0.3) | 2.1 (0.3) | 1.7 (0.3) | 2.5 (0.6) | 0.8 (0.2) | 0.9 (0.3) | 1.2 (0.3) | 1.0 (0.2) | 1.5 (0.3) |
| Take Again | 0.1 (0.4) | 1.6 (0.4) | 2.3 (0.3) | 1.8 (0.3) | 2.6 (0.6) | 0.7 (0.2) | 0.5# (0.2) | 0.9# (0.3) | 1.1 (0.4) | 1.2# (0.3) |
Bolded numbers indicates a significant difference from 0 mg, and
indicates a significant difference between IN and IV dosing.
Time Course
The time course of drug effects varied between the routes of administration. Typically, peak effects occurred 5 min after IV BUP administration. In contrast, peak drug effects following IN administration of BUP, were typically found later in the session (45, 60 minutes post-drug administration). As such, for subsequent direct comparisons between IV and IN dosing conditions, only peak or trough drug effects are reported.
Strength of Drug Effect
Peak DEQ ratings of “strong” drug effect significantly increased after IV administration of all of the active doses of BUP (vs. 0 mg; all p’s <0.01). However, no significant differences were found among the active BUP doses. None of the active doses of IN BUP significantly altered ratings of “strong” drug effect in comparison to placebo. Direct IV to IN comparisons between identical doses revealed that the strength of the drug effect was significantly greater when 4 mg and 16 mg of BUP were administered intravenously as opposed to intranasally (p < 0.05 and 0.01: respectively). This pattern of results was repeated when we examined the VAS rating of “Strong” drug effect.
A similar pattern was found for VAS assessments of “potent” drug effect. Planned contrasts revealed that IV administration of all of the active doses of BUP significantly increased ratings when compared to placebo (p’s < 0.01) with no significant differences among the active BUP doses. None of the active doses of IN BUP significantly altered VAS ratings of “potent” in comparison to placebo. Interestingly, when directly compared across IV and IN conditions, only the 4 mg of IV BUP produced a significantly more “potent” drug effect compared to 4 mg of IN BUP (p < 0.05).
Positive Drug Effect
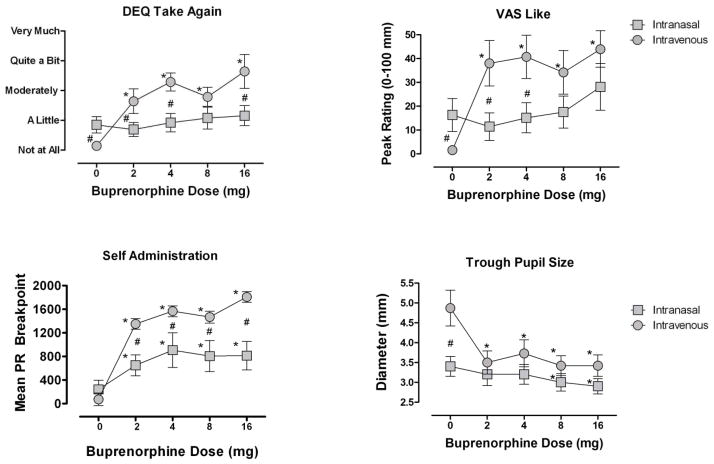
When examining peak DEQ assessments of “Would Take Again” (Figure 1), ANOVA revealed that peak ratings on this measure differed significantly among the 5 doses. All active IV doses increased ratings significantly above placebo (p’s < 0.01) but no difference among the active BUP doses was found. No differences on ratings of “Would Take Again” were found among the IN doses.
Figure 1.
Mean peak (± SEM) DEQ ratings of “Would Take Again,” VAS ratings of “Liked the Dose,” progressive ratio breakpoint, along with mean trough pupil diameter. Data are shown as a function of BUP dose and route of administration.
* Indicates a significant difference from 0 mg, and # indicates a significant difference between IN and IV dosing.
ANOVA performed on the DEQ measure of “Good” drug effect revealed that peak ratings differed significantly among the 5 doses. All active doses increased ratings significantly above placebo (p’s < 0.01). This analysis for the IN group found no overall difference among the doses, however but the highest dose of IN BUP significantly increased DEQ ratings of “Good” drug effect compared to placebo (0 mg vs. 16 mg, p < 0.05).
A similar pattern was found for VAS ratings of I feel “High”: peak VAS ratings on this measure did significantly differ among the 5 doses administered intravenously. Planned comparisons among the doses revealed that ratings significantly increased after IV administration of all of the active doses when compared to placebo (p’s < 0.01), but the active doses failed to differ significantly from one another. Similar analysis for the IN group failed to show a significant main effect of Dose. However, planned contrasts showed that IN BUP 16 mg significantly increased rating of “High” relative to placebo (p < 0.05). Comparing between the IV and IN doses, the 2 mg and 4 mg doses of IV BUP produced greater ratings of “High” (p’s < 0.05).
All active doses of IV BUP significantly increased peak VAS ratings of “Liked the Dose” compared to placebo (p’s < 0.01, Figure 1). For the IN group, the main effect of Dose was not significant, but the highest IN BUP dose (16 mg) significantly increased ratings of drug liking compared to placebo (p < 0.05). Planned contrasts directly comparing the equal doses of IV and IN BUP revealed that the 2, 4 and 8 mg doses of IV BUP produced greater effects (p’s < 0.05).
Aversive Drug Effect
VAS and DEQ subjective ratings of “Bad” drug effect did not significantly differ between placebo and active doses of IV and IN BUP on any of the analyses performed. None of the participants reported that any of the test doses were “difficult to snort,” though several adverse effects of insufflation were reported. The most commonly reported effects of snorting the doses were “burning,” “tingling,” “stinging,” “dry mouth,” and “thirsty.” No significant differences in the magnitude of these effects were observed among the doses, and all of the participants reporting experiencing “a little bit” of each measure for the BUP/lactose combination and lactose powder alone
3.3 Reinforcing Effects: Self-Administration
All active doses of IV BUP were self-administered significantly more than placebo (p’s < 0.01) but did not differ among the active BUP doses themselves. All active doses of IN BUP were also self-administered more than placebo (p’s < 0.05) but again, no differences were observed among the active BUP doses (Figure 1). Planned comparisons revealed that breakpoint values for IV BUP were higher than for IN BUP in all dose comparisons except for the 8 mg dose (2 and 4 mg, p < 0.05; 8 mg, p = 0.07; 16 mg, p < 0.01). Among IV users, only 1 participant failed to self-administer an active dose of BUP more than placebo. Among the IN users, 5 participants did not choose an active BUP dose more than they chose placebo.
3.4 Cognitive/Performance Effects
No significant effects of BUP Dose, Route, or Dose by Route interactions were found on any of the DAT outcome measures. ANOVA also revealed no significant effects of Dose or Dose by Route interactions for the dependent variables of the DSST.
3.5 Physiological Effects: Miosis
Comparing trough pupil diameter, ANOVA revealed that all active doses of IV BUP (2 mg, 4 mg, 8 mg, and 16 mg) significantly decreased pupil size when compared to placebo (p’s < 0.01) but the active doses did not significantly differ from one another (Figure 1). For the IN group, only the two highest doses (BUP 8 mg, 16 mg) significantly decreased pupil diameter relative to placebo (p <0.05 and 0.01: respectively). Planned comparisons revealed that when directly comparing each dose of BUP between the two routes, the IV and IN groups only differed at 0 mg (p< 0.05).
4. Discussion
Intravenously administered buprenorphine significantly increased subjective reports of positive drug-related effects. Larger doses of intranasal buprenorphine also increased positive subjective effects but the magnitude was typically less than IV doses. Active doses of IN buprenorphine were self-administered more than placebo, despite the fact that smaller doses often failed to significantly increase positive subjective effects. Other studies have shown that there can be a dissociation between the subjective and reinforcing effects of opioids. In one such study, heroin-dependent participants did not report subjective effects of low doses of morphine that differed from placebo, yet the dose maintained responding (Lamb et al., 1991, see also Comer et al., 2008). These data demonstrate that the reinforcing effects of opioids are not necessarily causally related only to their euphoric/positive subjective effects, and highlight the importance of employing both techniques in assessments of abuse liability.
All active doses of IV buprenorphine were self-administered, with IV BUP producing greater reinforcing effects than IN BUP. No significant cognitive impairment or self-reported adverse events were found for either route of BUP administration. Interestingly, active doses of IV and IN BUP produced a similar degree of miosis (an indicator of μ receptor activation). As both groups appeared to have experienced equivalent levels of μ activation, this suggests that the more robust positive subjective and reinforcing effects of IV BUP were due to its faster onset of effects. The dose-to-dose comparisons of the positive subjective and reinforcing effects of IV and IN buprenorphine suggest that IV BUP has greater abuse liability compared to IN BUP. However, it is possible that IN BUP is simply less potent than IV BUP. If this is the case, larger doses of IN BUP (i.e., doses greater than 16 mg) may produce effects that are of similar magnitude as the doses of IV BUP shown in the present study (Lindhardt et al., 2001; Lloyd-Jones et al., 1980; Middleton et al., 2001). Future studies will need to examine this possibility.
Two additional caveats must be mentioned with respect to the direct comparison between IV and IN BUP. The first concerns our determination of “peak” effects of the intranasally administered doses. The effect of intranasal BUP typically increased over the course of the sample session until the final point of observation (60 min after drug administration). This allows for the possibility that drug effects could have continued to increase at subsequent time points. Therefore, due to the limited 60-minute window of assessment, “peak” drug effects for the intranasal dose may not have been captured. However, Middleton and colleagues (2011) reported that peak effects for 2 mg and 8 mg of intranasal buprenorphine typically occurred between 40 and 90 minutes after drug administration, so it is likely that peak or near-peak drug effects were captured in the present study with IN BUP.
Second, pupil diameter was significantly greater after administration of IV compared to IN placebo (Figure 1). This finding could be interpreted as either greater withdrawal effects in the IV group or greater agonist effects in the IN group. The latter possibility is unlikely since the last agonist administration occurred when 2 mg SL BUP was administered the previous evening. The former possibility is also unlikely because opioid withdrawal symptoms were low (< 10 out of a possible maximum of 64) for both groups at the beginning of the sample session (Table 3). When we carefully reviewed the individual data for the IV group, we discovered that 4 of the 13 participants consistently had larger pupil diameters at baseline prior to drug administration and during placebo sessions, but their other responses at baseline (including symptoms of withdrawal) and their responses to active drug were not different from the other participants. We have observed in our many years of conducting this type of research that some participants have larger pupil diameters than others. It appears based on our evaluation of the data set as a whole that the larger pupil diameter observed under placebo conditions for the IV group may simply reflect individual variability in this particular endpoint.
Another important consideration when comparing IV and IN BUP is that when administered intranasally, BUP may be aversive. In fact, participants reported that IN BUP produced a “stinging” sensation in the nose and sinuses (Middleton et al., 2011). The current sample of intranasal users also reported slight “stinging” and “burning” in their nose and throat resulting from insufflation of the buprenorphine and lactose power, particularly at the 16 mg dose (although these ratings did not differ significantly from placebo). These aversive effects may have resulted in the lower positive subjective and reinforcing effects observed in the present study. They may also explain the smaller pupil diameter observed after placebo administration in the IN compared to the IV group. That is, the “stinging” and “burning” sensations may have acted as distinctive interoceptive cues that became associated with the effects of the drug. Thus, it is possible that the lactose powder may have acted as a conditioned reinforcer, resulting in a greater miotic effect after IN administration. This possibility is supported by research demonstrating stronger cue reactivity among heroin inhalers compared to injectors (Liu et al., 2011).
Another limitation of the present study is that the intranasal buprenorphine administration procedures that we used reduced the generalizability of our results. This study employed a buprenorphine powder (combined with lactose) in lieu of a crushed buprenorphine tablet. While this procedure allowed our participants to avoid the insufflation of excipient particles found in the SL buprenorphine tablets, crushing of the SL tablet is how the drug is most often prepared for IN use in the natural environment. As a result, our findings concerning the subjective experience of snorting buprenorphine may differ from what users typically experience.
Differences in how buprenorphine was prepared for intranasal delivery also may have contributed to differences between our findings and those from similar studies. The magnitude of the subjective effects found in the current study is smaller in comparison to a previous clinical investigation. Using crushed SL BUP tablets, Middleton and colleagues (2011) reported that 2 and 8 mg of intranasal BUP resulted in peak VAS “High” ratings of 24.7 mm and 39.3 mm, respectively. In the present study the same doses of IN BUP produced peak VAS “High” ratings of 12.3 mm and 22.0 mm, respectively. However, since the investigation by Middleton and colleagues (2011) was performed in non-dependent opioid abusers, our use of sublingual BUP as a maintenance medication is mostly likely the strongest contributor to the attenuated IN BUP effect observed in the present study. Further evidence in support of this idea can be found if the current data are compared to a previous investigation by our group using detoxified heroin users. Comer and colleagues (2005) reported that 2 mg of intravenously administered BUP produced VAS ratings of “High” and “Potent” of approximately 35.0 mm and 22.5 mm (respectively), similar to what was found in the current study (36.0 mm and 25.0 mm). In contrast, the 2005 study found that the 8 mg dose of IV BUP increased ratings on both these measures to between 50.0 – 55.0 mm. This is nearly double of what was observed at present with this dose of IV BUP (high = 29.0 mm, potent = 31.0 mm).
When the current results are examined within the context of the Middleton et al., 2011 and Comer et al., 2005 experiments, it appears as though the SL buprenorphine maintenance may have antagonized the subjective effects of IV and IN BUP. We recognized this possibility and attempted to minimize the effects of SL BUP maintenance by planning the lab sessions at a time when the effects of the maintenance dose would be at their nadir. Despite these efforts, the data suggest that buprenorphine maintenance did attenuate the effects of BUP during the laboratory sessions and therefore may be somewhat protective against the recreational use of IV and IN BUP. Nevertheless, the self-administration data indicate that BUP may be used recreationally via both routes in patients maintained on a low dose of sublingual buprenorphine.
The SL buprenorphine maintenance may have also contributed to another interesting finding in the present study: the flat dose-response relationship among the active buprenorphine doses. Increasing the buprenorphine dose 8-fold only resulted in small and statistically insignificant increases in positive subjective ratings and PR breakpoints. Another study using buprenorphine-maintained volunteers (Strain et al., 1997) similarly found that VAS ratings of “Drug Effect,” “High” and “Good Effect” did not differ significantly among active doses of intramuscular BUP (4, 8, 16 mg).
The fact that participants were BUP-maintained cannot be the only factor responsible for the flat dose-response function observed in the current study, however. Other clinical studies have reported a lack of a BUP dose-response effect in participants who were not BUP-maintained. For example, Umbricht and colleagues (2004) administered equivalent doses of intravenous BUP (0, 2, 4, 8, 12, and 16 mg) to non-dependent participants and found no significant differences among the active doses for VAS measurements of “Drug Effect,” “Liking,” and “Good Effect.” A ceiling effect has been observed repeatedly with buprenorphine, which supports its characterization as a partial μ receptor agonist (for a review see Walsh and Eissenberg, 2003). Future studies are needed to determine which variables and/or parametric conditions moderate the slope of the buprenorphine dose-response curve. These factors have important implications concerning the abuse liability and safe/efficacious medical dosing of this drug.
In sum, this investigation adds credence to the long-standing argument for the utility of properly used buprenorphine as medication-assisted therapy, which may deter recreational opioid use (Comer et al., 2005; Mello et al. 1982, 1983; Mello and Mendelson, 1980). The combined BUP + naloxone formulation is primarily used in the U.S. as a means of reducing abuse and diversion. As generic formulations of buprenorphine alone are introduced in the U.S, they may gain popularity among health care professionals and drug users as they did in France where they saw a 7.5 fold increase in the number of generic users in just two years after their introduction (Nordmann et al., 2012). Accordingly, the present study suggests the need for vigilance, particularly concerning IV abuse of the mono-product. Although the data demonstrate the potential of IN abuse of Bup, the IV route appears to have a greater abuse liability. Given the other adverse effects of IV drug use (i.e., transmission of blood-borne pathogens); the health consequences for these individuals may be greater. In any case, the field should continue to develop and assess the utility of buprenorphine formulations designed to reduce these forms of misuse, such as long-acting injectable depot formulations and subcutaneous implants.
Highlights.
IV BUP increased reports of positive drug effects, and was self-administered.
The magnitude of IN Bup’s positive effects were typically less than IV doses.
All active doses of IN Bup were self-administered.
IN or IV BUP retains its abuse potential in participants on a low dose of SL BUP.
Acknowledgments
Funding for these studies was provided by Reckitt-Benckiser Pharmaceuticals (intranasal study) and the Schering-Plough Corporation (intravenous study). However, the sponsors had no role in study design or data collection, analysis or interpretation. The sponsors also had no involvement in the decision to submit this report for publication or in the writing of it, other than to review the paper for scientific accuracy. Financial support for the preparation of this manuscript was provided by the National Institute on Drug Abuse (R01DA016759 to SDC and K01DA030446 to JDJ).
Over the past three years SDC and JDJ have received compensation (in the form of partial salary support) from investigator-initiated studies supported by Reckitt-Benckiser Pharmaceuticals, Schering-Plough Corporation, Johnson & Johnson Pharmaceutical Research & Development, Endo Pharmaceuticals, and MediciNova. In addition, SDC has served as a consultant to the following companies: AstraZeneca, Grunenthal USA, Guidepoint Global, Mallinckrodt, Neuromed, Orexo, and Pfizer.
The medical assistance of Maria Sullivan, MD, PhD, Jeanne Manubay, MD, Shanthi Mogali, MD, Janet Murray, RN, Claudia Tindall, RN, and Audrey Perez, RN, along with the technical assistance of Ziva Cooper, PhD, Suzanne Vosburg, PhD, Perrine Roux, PhD, Ellen Walker, PhD, Jessica Fogel, BS, Paula Askalasky, BS, Brian Wade, BS, and Andrew Segoshi, BS, are gratefully acknowledged.
Footnotes
Disclosures
Only the authors listed are responsible for the content and preparation of this manuscript. All authors contributed significantly to this work and have read and approved this manuscript for publication.
Gabriela Madera has no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alho H, Sinclair D, Vuori E, Holopainen A. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88:75–8. doi: 10.1016/j.drugalcdep.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Aitken CK, Higgs PG, Hellard ME. Buprenorphine injection in Melbourne, Australia—an update. Drug Alcohol Rev 2008. 2008;27:197–9. doi: 10.1080/09595230701829553. [DOI] [PubMed] [Google Scholar]
- Auriacombe M, Fatsea M, Dubernet J, Daulouede J, Tignol J. French field experience with buprenorphine. Am J Addict. 2004;13(Suppl 1):S17–28. doi: 10.1080/10550490490440780. [DOI] [PubMed] [Google Scholar]
- Bedi NS, Ray R, Jain R, Dhar NK. Abuse liability of buprenorphine-a study among experienced drug users. Indian J Physiol Pharmacol. 1998;42(1):95–100. [PubMed] [Google Scholar]
- Carrieri MP, Amass L, Lucas GM, Vlahov D, Wodak A, Woody GE. Buprenorphine use: the international experience. Clin Infect Dis. 2006;43:S197–215. doi: 10.1086/508184. [DOI] [PubMed] [Google Scholar]
- Chua SM, Lee TS. Abuse and prescription buprenorphine, regulatory controls. Ann Acad Med Singapore. 2006;35:492–95. [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Choice between money and intranasal heroin in morphine-maintained humans. Behav Pharmacol. 1997;8:677–90. doi: 10.1097/00008877-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, MacArthur RB, Fischman MW. Comparison of intravenous and intranasal heroin self-administration by morphine-maintained humans. Psychopharmacol. 1999;143:327–38. doi: 10.1007/s002130050956. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED. Self-administration of intravenous buprenorphine and the buprenorphine/naloxone combination by recently detoxified heroin abusers. J Pharmacol Exp Ther. 2002;303:695–703. doi: 10.1124/jpet.102.038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Vosburg SK, Manubay J, Amass L, Cooper ZD, Saccone P, Kleber HD. Abuse liability of intravenous buprenorphine/naloxone and buprenorphine alone in buprenorphine-maintained intravenous heroin abusers. Addiction. 2010;105:709–18. doi: 10.1111/j.1360-0443.2009.02843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Walker EA. Comparison of intravenous buprenorphine and methadone self-administration by recently detoxified heroin-dependent individuals. J Pharmacol Exp Ther. 2005;315:1320–30. doi: 10.1124/jpet.105.090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ. Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacol. 2008;33:1179–91. doi: 10.1038/sj.npp.1301479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels LB, Nichols DF, Seifert MS, Hock HS. Changes in pupil diameter entrained by cortically initiated changes in attention. Vis Neurosci. 2012;29(2):131–42. doi: 10.1017/S0952523812000077. [DOI] [PubMed] [Google Scholar]
- Duke AN, Correia CJ, Walsh SL, Bigelow GE, Strain EC. Acute effects of intramuscular and sublingual buprenorphine and buprenorphine/naloxone in non-dependent opioid abusers. Psychopharmacol (Berl) 2010;211:303–12. doi: 10.1007/s00213-010-1898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugs-forum.com. Heroin Drip? 2011 Jan 22; Message posted to: http://www.drugs-forum.com/forum/showthread.php?t=173934.
- Epstein DH, Preston KL, Jasinski DR. Abuse liability, behavioral pharmacology, and physical dependence potential of opioids in humans and laboratory animals: lessons from tramadol. Biol Psychol. 2006;73:90–9. doi: 10.1016/j.biopsycho.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank B. An overview of heroin trends in New York City: past, present and future. Mt Sinai J Med. 2000;67(5–6):340–46. [PubMed] [Google Scholar]
- Gagnon J, Roth JM, Carroll M, Haycock KA, Plamondon J, Feldman DS, Simpson J. Superanova accessible general linear modeling. Yale J Biol Med. 1990;63:191–92. [Google Scholar]
- Hakansson A, Medvedeo A, Andersson M, Berglund M. Buprenorphine misuse among heroin and amphetamine users in Malmo, Sweden: purpose of misuse and route of administration. Eur Addicy Res. 2007;13:207–15. doi: 10.1159/000104883. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Cochran KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- Horyniak D, Dietze P, Larance B, Winstock A, Degenhardt L. The prevalence and correlates of buprenorphine inhalation amongst opioid substitution treatment (OST) clients in Australia. Int J Drug Pol. 2011;22:167–71. doi: 10.1016/j.drugpo.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Archives of Gen Psychiatry. 1978;35:501–16. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- Jenkinson RA, Clark NC, Fry CL, Dobbin M. Buprenorphine diversion and injection in Melbourne, Australia: an emerging issue? Addiction. 2005;100:197–205. doi: 10.1111/j.1360-0443.2004.00958.x. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Arfken CL, di Menza S, Schuster CR. Diversion and abuse of buprenorphine: findings from national surveys of treatment patients and physicians. Drug Alcohol Depend. 2012;120:190–95. doi: 10.1016/j.drugalcdep.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70:S59–S77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- Jones JD, Comer SD. A review of human drug self-administration procedures. Behav Pharmacolo. 2013;24(5–6):384–95. doi: 10.1097/FBP.0b013e3283641c3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaa E. Impurities, adulterants and diluents of illicit heroin. Changes during a 12-year period. Forensic Sci Int. 1994;64(2–3):171–79. doi: 10.1016/0379-0738(94)90228-3. [DOI] [PubMed] [Google Scholar]
- Katz JL. Models of relative reinforcing efficacy of drugs and their predictive utility. Behav Pharmacol. 1990;1(4):283–301. doi: 10.1097/00008877-199000140-00003. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Preston KL, Schindler CW, Meisch RA, Davis F, Katz JL, Henningfield JE, Goldberg SR. The reinforcing and subjective effects of morphine in post-addicts: a dose-response study. J Pharmacol Exper Ther. 1991;259(3):1165–73. [PubMed] [Google Scholar]
- Lee CE. Tackling Subutex abuse in Singapore. Singapore Med J. 2006;47:919–21. [PubMed] [Google Scholar]
- Lindhardt K, Bagger M, Andreasen KH, Bechgaard E. Intranasal bioavailability of buprenorphine in rabbit correlated to sheep and man. Int J Pharm. 2001;217(1–2):121–6. doi: 10.1016/s0378-5173(01)00591-9. [DOI] [PubMed] [Google Scholar]
- Lintzeris N, Ritter A, Panjari M, Clark N, Kutin J, Bammer G. Implementing buprenorphine treatment in community settings in Australia: experiences from the Buprenorphine Implementation Trial. Am J Addict. 2004;13:S29–41. doi: 10.1080/10550490490440799. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhou W, Zhang J, Wang Q, Xu J, Gui D. Differences in cigarette smoking behaviors among heroin inhalers versus heroin injectors. Nicotine Tobacco Res. 2011;13(11):1023–8. doi: 10.1093/ntr/ntr115. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones JG, Robinson P, Henson R, Biggs SR, Taylor T. Plasma concentration and disposition of buprenorphine after intravenous and intramuscular doses to baboons. Eur J Drug Metab Pharmacokinet. 1980;5(4):233–9. doi: 10.1007/BF03189469. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008;16:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- Maxwell JC, McCance-Katz EF. Indicators of buprenorphine and methadone use and abuse: what do we know? Am J Addict. 2010;19:73–88. doi: 10.1111/j.1521-0391.2009.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Bree MP, Mendelson JH. Comparison of buprenorphine and methadone effects on opiate self-administration in primates. J Pharmacol Exp Ther. 1983;225:378–86. [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Buprenorphine suppresses heroin use by heroin addicts. Sci. 1980;207:657–9. doi: 10.1126/science.7352279. [DOI] [PubMed] [Google Scholar]
- Mello N, Mendelson J. Behavioral pharmacology of buprenorphine. Drug Alcohol Depend. 1985;14:283–303. doi: 10.1016/0376-8716(85)90062-6. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC. Buprenorphine effects on human heroin self-administration: an operant analysis. J Pharmacol Exp Ther. 1982;223:30–9. [PubMed] [Google Scholar]
- Middleton LS, Nuzzo PA, Lofwall MR, Moody DE, Walsh SL. The pharmacodynamic and pharmacokinetic profile of intranasal crushed buprenorphine and buprenorphine/naloxone tablets in opioid abusers. Addiction. 2011;106(8):1460–73. doi: 10.1111/j.1360-0443.2011.03424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte A, Mandell T, Wilford B, Tennyson J, Boyer E. Diversion of buprenorphine/naloxone coformulated tablets in a region with high prescribing prevalence. J Add Dis. 2009;28:226–31. doi: 10.1080/10550880903014767. [DOI] [PubMed] [Google Scholar]
- Moratti E, Kashanpour H, Lombardelli T, Maisto M. Intravenous misuse of buprenorphine: characteristics and extent among patients undergoing drug maintenance therapy. Clin Drug Investig. 2010;30:3–11. doi: 10.2165/11536020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Nordmann S, Frauger E, Pauly V, Orléans V, Pradel V, Mallaret M, Thirion X, Micallef J. Misuse of buprenorphine maintenance treatment since introduction of its generic forms: OPPIDUM survey. Pharmacoepidemiol Drug Saf. 2012;21:184–90. doi: 10.1002/pds.2263. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Dietze P, Lee N, Dunlop A, Taylor D. Concurrent buprenorphine and benzodiazepine use and self-reported opioid toxicity substitution treatment. Addiction. 2007;102:616–22. doi: 10.1111/j.1360-0443.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- Obadia Y, Perrin V, Feroni I, Vlahov D, Moatti JP. Injecting misuse of buprenorphine among French drug users. Addiction. 2001;96:267–72. doi: 10.1046/j.1360-0443.2001.96226710.x. [DOI] [PubMed] [Google Scholar]
- Roux P, Villes V, Blanche J, Bry D, Spire B, Feroni I, Carrieri MP. Buprenorphine in primary care: risk factors for treatment injection and implications for clinical management. Drug Alcohol Depend. 2008A;97(1–2):105–13. doi: 10.1016/j.drugalcdep.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Roux P, Villes V, Bry D, Spire B, Feroni I, Marcellin F, Carrieri MP. Buprenorphine sniffing as a response to inadequate care in substituted patients: results from the Subazur survey in south-eastern France. Addict Behav. 2008B;33(12):1625–29. doi: 10.1016/j.addbeh.2008.07.018. [DOI] [PubMed] [Google Scholar]
- SPSS I. SPSS 18.0.0 for windows. Chicago, Illinois: 2009. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings (Office of Applied Studies, NSDUH Series H-38A, HHS Publication No. SMA 10-4586 Findings) Rockville, MD: 2010. [Google Scholar]
- Strain EC, Walsh SL, Preston KL, Liebson IA, Bigelow GE. The effects of buprenorphine in buprenorphine-maintained volunteers. Psychopharmacol (Berl) 1997;129:329–38. doi: 10.1007/s002130050199. [DOI] [PubMed] [Google Scholar]
- Umbricht A, Huestis MA, Cone EJ, Preston KL. Effects of high-dose intravenous buprenorphine in experienced opioid abusers. J Clin Psychopharmacol. 2004;24:479–87. doi: 10.1097/01.jcp.0000138766.15858.c6. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. World Drug Report. 2012 Retrieved on June 26th, 2012 from: http://www.unodc.org/documents/data-and-analysis/WDR2012/WDR_2012_web_small.pdf.
- Vicknasingam B, Mazlan M, Schottenfeld RS, Chawarski MC. Injection of buprenorphine and buprenorphine/naloxone tablets in Malaysia. Drug Alcohol Depend. 2010;111:44–9. doi: 10.1016/j.drugalcdep.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Vidal-Trecan G, Varescon I, Nabet N, Boissonnas A. Intravenous use of prescribed sublingual buprenorphine tablets by drug users receiving maintenance therapy in France. Drug Alcohol Depend. 2003;69:175–81. doi: 10.1016/s0376-8716(02)00312-5. [DOI] [PubMed] [Google Scholar]
- Walsh S, Eissenberg T. The clinical pharmacology of buprenorphine: extrapolating from the laboratory to the clinic. Drug Alc Depend. 2003;70:S13–27. doi: 10.1016/s0376-8716(03)00056-5. [DOI] [PubMed] [Google Scholar]
- Walsh S, Preston K, Stitzer M, Cone E, Bigelow G. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–80. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- Walsh S, Preston K, Bigelow G, Stitzer M. Acute administration of buprenorphine in humans: partial agonist and blockage effect. J Pharmacolo Exp Ther. 1995;274:361–72. [PubMed] [Google Scholar]
- Young AM, Havens JR, Leukefeld CG. Route of administration for illicit prescription opioids: a comparison of rural and urban drug users. Harm Red J. 2010;7:24. doi: 10.1186/1477-7517-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Conley K, Galinkin J. Comparing the subjective, psychomotor and physiological effects of intravenous buprenorphine and morphine in healthy volunteers. J Pharmacol Exp Ther. 1997;282:1187–97. [PubMed] [Google Scholar]