Abstract
Glutaminase catalyzes the hydrolysis of glutamine to glutamate and plays a central role in the proliferation of neoplastic cells via glutaminolysis, as well as in the generation of excitotoxic glutamate in central nervous system disorders such as HIV-associated dementia (HAD) and multiple sclerosis. Both glutaminase siRNA and glutaminase inhibition have been shown to be effective in in vitro models of cancer and HAD, suggesting a potential role for small molecule glutaminase inhibitors. However, there are no potent, selective inhibitors of glutaminase currently available. The two prototypical glutaminase inhibitors, BPTES and DON, are either insoluble or non-specific. In a search for more drug-like glutaminase inhibitors, we conducted a screen of 1280 in vivo active drugs (Library of Pharmacologically Active Compounds (LOPAC1280)) and identified ebselen, chelerythrine and (R)-apomorphine. The newly identified inhibitors exhibited 10 to 1500-fold greater affinities than DON and BPTES and over 100-fold increased efficiency of inhibition. Although non-selective, it is noteworthy that the affinity of ebselen for glutaminase is more potent than any other activity yet described. It is possible that the previously reported biological activity seen with these compounds is due, in part, to glutaminase inhibition. Ebselen, chelerythrine and apomorphine complement the armamentarium of compounds to explore the role of glutaminase in disease.
Keywords: Cancer, HIV-associated dementia (HAD), Glutamate, Glutamine, Glutaminase, Kinetics
1. Introduction
Glutamine is a major source of energy for highly proliferative and neoplastic cells via glutaminolysis [1,2]. Glutamine is transported into cells where glutaminases (EC 3.5.1.2) hydrolyze glutamine to glutamate [3]. Glutamate is further catabolized through the tricarboxylic acid cycle to ATP. Glutaminase has been shown to play a critical role in glutaminolysis via a c-Myc regulated process [4]. Glutaminase siRNA, a prototype glutaminase inhibitor and glutamine deprivation have all been shown to cause significant decreases in cell proliferation in various cancer lines [4–9] suggesting a role for small molecule glutaminase inhibitors for the treatment of cancer.
Glutaminase is also thought to play a critical role in the generation of glutamate, a key excitatory neurotransmitter in the CNS [10,11]. HIV-infected macrophages were shown to express increased glutaminase levels and to produce significantly more glutamate that was glutamine dependent [12]. Prototype glutaminase small molecule inhibitors and glutaminase specific siRNA were able to abrogate the increases in glutamate caused by HIV-infected macrophages [13]. These results suggest a fundamental role of glutaminase in HIV-induced neurotoxicity. Glutaminase-mediated glutamate release from microglia was also shown to occur in models of multiple sclerosis [14] and ischemia [15], suggesting glutaminase inhibition could be of broad therapeutic interest for neuroinflammatory and neurodegenerative disorders.
Even though glutaminase inhibition could have therapeutic utility, to date, there are no known potent and selective glutaminase inhibitors available. DON, the earliest known inhibitor of glutaminase, is an active site directed inhibitor [16,17] that has been used as a tool compound to help elucidate potential involvement of glutaminase in HIV-associated dementia (HAD) pathogenesis and multiple sclerosis [12,14]. However, DON is a non-selective toxic reagent that inhibits several glutamine utilizing enzymes [18]. DON also has weak millimolar inhibitory potency in in vitro models of disease [12,14] and is not well tolerated in vivo [19–21]. Elan Pharmaceuticals described a glutaminase inhibitor (Newcomb, US Patent, 2002) termed BPTES with low micromolar potency (Ki = 0.2 μM) and an uncompetitive mode of inhibition [22]. Although more potent than DON, BPTES is not a drug-like compound as it has high molecular weight (534), poor solubility and low bioavailability [23]. Structure Activity Relationship (SAR) studies around BPTES has not yielded significantly better analogs [8,23]. Consequently, in order to identify more drug-like inhibitors, we conducted a screen of a Library of Pharmacologically Active Compounds (LOPAC1280, Sigma) comprising small molecule modulators and approved drugs from all major drug classes. Here, we report on the kinetic characterization of three new inhibitors identified from our screening endeavor and on their direct comparison to DON and BPTES.
2. Materials and methods
2.1. Materials
DON, ebselen, LOPAC1280, sanguinarine chloride, R and S-apomorphine and R(−)-apocodeine hydrochloride were purchased from Sigma (St. Louis, MO, USA). The chloride salts of chelerythrine, nitidine, berberine and norsanguinarine were obtained from LC Laboratories (Woburn, MA, USA), Ontario Chemicals Inc (Guelph, Ontario, Canada), MP Biomedicals (Solon, OH, USA) and Quality Phytochemicals LLC (Brunswick, NJ, USA), respectively and R(−)-propylnorapomorphine hydrochloride was bought from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA). BPTES was synthesized in-house [23]. The plasmid encoding the cDNA for hKGA was graciously provided by Dr. Norman P. Curthoys (Department of Biochemistry and Molecular Biology, Colorado State University, Fort Collins, CO, USA). Finally, mouse liver glutaminase (mLGA) and mouse c-type glutaminase (mGAC) were generous gifts from Dr. Andre Ambrosio (Laboratório Nacional de Biociências, Centro Nacional de Pesquisa em Energia e Materiais, Campinas-SP 13083–970, Campinas, Brazil).
2.2. Methods
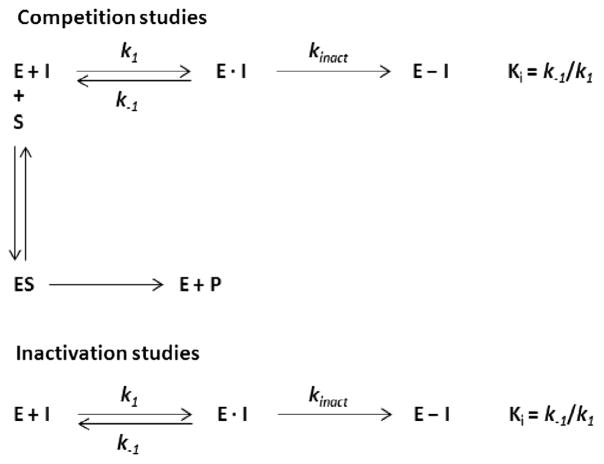
Unless otherwise noted, glutaminase activity in the presence and absence of compounds was determined using the Amplex UltraRed fluorescent assay [24]. A counter-screen was carried to weed out autofluorescent compounds and quenchers and a second counter-screen was performed to eliminate inhibitors of glutamate oxidase and horse radish peroxidase and/or compounds that reacted with Amplex UltraRed. Competition studies (Ki determinations) were carried out with enzyme (E) exposed to substrate (S) and inhibitor (I) at the same time. Km and Vmax for all compounds were determined from glutamine saturation profiles at the various inhibitor concentrations using GraphPad Prism, employing a least-squares fit of the Michaelis–Menten equation: . During inactivation studies (kinact determinations) enzyme was incubated with inhibitor for varying times and the substrate used as a tool to determine the percent of remaining enzyme activity [25]. Due to BPTES’ limited solubility [23], a factor that interferes with fluorescence measurement, the radiolabel assay [26] was used to determine the kinetic parameters of BPTES. [3H]-Glutamine was used as the substrate in this assay. The radiolabel assay was also employed for DON in the inactivation experiment as DON hydrolyzes to glutamate [16], a product of the glutaminase reaction, which results in spurious fluorescence output. Data were analyzed using GraphPad Prism’s non-linear regression analysis, with variable slope, of log [inhibitor] vs. normalized values.
3. Results
3.1. Primary glutaminase screen
The LOPAC1280 were screened for glutaminase activity. Of the 1280 compounds screened, 123 were active (percent inhibition ≥50 and IC50 ≤ 10 μM). After counter screening, 23 were confirmed as bona fide glutaminase inhibitors. From these, ebselen, chelerythrine chloride and R-apomorphine hydrochloride were identified as most potent. Ebselen and chelerythrine chloride inhibited both isoforms of GLS1 (hKGAΔ1 and its splice variant, mGAC) with similar potency and exhibited 5 to 10-fold less activity against GLS2 (mLGA). In contrast, R-apomorphine hydrochloride had similar activity against both GLS1 isoforms and GLS2 (Table 1).
Table 1.
Specificity of ebselen, chelerythrine chloride and apomorphine hydrochloride for GLS1 (hKGAΔ1, mGAC) and GLS2 (mLGA).
| Compound ID | Average IC50 (μM) Against
|
||
|---|---|---|---|
| hKGA | mGAC | mLGA | |
| Ebselen | 0.008 | 0.02 | 0.1 |
| Chelerythrine chloride | 0.03 | 0.07 | 0.3 |
| Apomorphine hydrochloride | 0.4 | 1.0 | 0.3 |
3.2. Kinetic characterization of ebselen, chelerythrine, apomorphine, DON and BPTES
To determine the kinetics of inhibition of ebselen, glutamine saturation experiments were performed in the presence of different concentrations of ebselen (Supplementary: Fig. 1a). As the concentration of inhibitor was increased, the apparent Michaelis constant (Kmapp) increased with a concomitant 6-fold decrease in Vmax. A double reciprocal plot of the data yielded lines with varying slopes that intersected in the second quadrant (Supplementary: Fig. 1b), indicative of mixed non-competitive inhibition. A secondary plot of the slopes for each line of the double reciprocal plot (Kmapp/Vmax) versus inhibitor concentration gave a Ki of approximately 15 nM (Table 2; Supplementary: Fig. 1c).
Table 2.
Competition studies - substrate saturation analysis in the presence of different inhibitor concentrations. (Data are an average of 2–4 determinations.)
| Compound | Structure | Mode of inhibition | Ki, M |
|---|---|---|---|
| Ebselen |
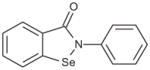
|
Mixed non-competitive | 1.49E–08 |
| Chelerythrine chloride |
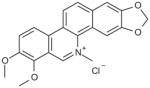
|
Competitive | 2.70E–07 |
| Apomorphine hydrochloride |
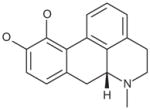
|
Competitive | 2.32E–06 |
| BPTES |
|
Uncompetitive | 1.25E–07 |
| DON |
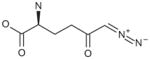
|
Time-dependent | NA |
The mode of inhibition of glutaminase by ebselen was further investigated by performing a time-dependent inhibition experiment [25]. The semilogarithmic plot of the remaining activity of glutaminase versus time showed pseudo-first order kinetics. Additionally, inactivation of glutaminase by ebselen was dependent on both concentration and time of incubation (Supplementary: Fig. 1d). The calculated Ki of ebselen was approximately 14 nM and the kinact of glutaminase by ebselen was 1.32/h (Table 3; Supplementary: Fig. 1e).
Table 3.
Inactivation studies - relative affinities (Ki), rate constant of inactivation (kinact) and second order rate constants (kinact/Ki) of ebselen, chelerythrine chloride, apomorphine hydrochloride, BPTES and DON against glutaminase. (Data are an average of two determinations, at eight concentrations and at six different time points.)
| Compound | Ki, M (calc.) | kinact, h−1 | kinact/Ki, M−1s−1 |
|---|---|---|---|
| Ebselen | 1.44E–08 | 1.31 | 2.52E + 04 |
| Chelerythrine chloride | 3.29E–08 | 3.45 | 2.92E + 04 |
| Apomorphine hydrochloride | 4.17E–09 | 0.20 | 1.32E + 04 |
| BPTES | 2.58E–07 | 0.25 | 2.68E + 02 |
| DON | 5.57E–06 | 0.05 | 2.53E + 00 |
Similar experiments were carried out with chelerythrine, apomorphine and prototype inhibitors, BPTES and DON. Results are shown in tables 2 and 3 (and in Supplementary: Figs. 2, 3, 4 & 5). Competition studies with BPTES showed that Kmapp decreased with a concomitant decrease in Vmax (Supplementary: Fig. 4a). A double reciprocal plot of the data yielded parallel lines (Supplementary: Fig. 4b), indicative of uncompetitive inhibition. A secondary plot of the reciprocal of Kmapp versus inhibitor concentration gave a Ki of 125 nM (Table 2; Supplementary: Fig. 4c). Competition studies could not be carried out with DON since it acts as both a substrate and covalent modifier of glutaminase [16]. Concordantly, inactivation of glutaminase by DON was both concentration and time of incubation dependent with a kinact of 0.05/h and a Ki of 6 μM (Table 3; Supplementary: Fig. 5).
3.3. Analogs of ebselen, chelerythrine and apomorphine
Since ebselen contains a selenium atom, it was of interest to evaluate analogs where the selenium was substituted with sulfur and oxygen, elements within the same group (6A) of the periodic table. The results were in line with the ability of these atoms to oxidize as well as in their leaving group ability; selenium analog being the most potent, followed by the thiol and oxo analogs (Table 4). Substitution of the selenium with a carbon (Group 4A) resulted in loss of activity.
Table 4.
Analogs of ebselen.
| Compound | Molecular Structure | Avg. IC50 (μM) |
|---|---|---|
| Ebselen |
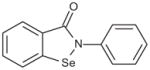
|
0.009 |
| 2-Phenylbenzo[d]isothiazol-3(2H)-one |
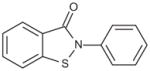
|
0.02 |
| 2-Phenylbenzo[d] isoxazol-3(2H)-one |
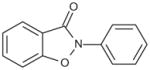
|
20 |
| 2-Phenyl isoindolin-1-one |
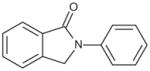
|
>100 |
Four analogs of chelerythrine were tested for glutaminase activity. While both nitidine chloride and sanguinarine chloride retained biological activity, norsanguinarine, a structurally similar analog of sanguinarine lacking the iminium moiety, did not (Table 5). Additionally, berberine chloride, a structurally related analog of chelerythrine, with a positive charge on the nitrogen but containing an additional fused ring instead of the pendant methyl group did not maintain activity. The results confirm the importance of the iminium bond in the biological activity of chelerythrine [27].
Table 5.
Analogs of chelerythrine.
| Compound | Molecular Structure | Avg. IC50 (μM) |
|---|---|---|
| Chelerythrine chloride |
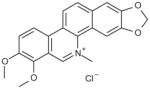
|
0.03 |
| Nitidine chloride |
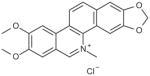
|
9 |
| Berberine chloride |
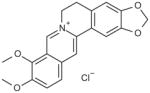
|
>100 |
| Sanguinarine chloride |
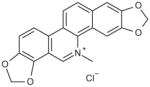
|
0.1 |
| Norsanguinarine chloride |
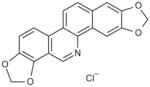
|
>100 |
Glutaminase did not demonstrate enantiomeric selectivity for apomorphine (Table 6). While the propyl analog of apomorphine was slightly weaker, replacing one of the hydroxyl groups with a methoxy group (R(−)-apocodeine hydrochloride) resulted in a 10-fold loss of activity.
Table 6.
Analogs of apomorphine.
| Compound | Molecular Structure | Avg. IC50 (μM) |
|---|---|---|
| R–Apomorphine hydrochloride |
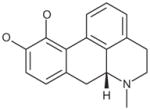
|
0.6 |
| S–Apomorphine hydrochloride |
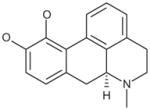
|
0.4 |
| R(−)-Propylnorapomorphine hydrochloride |
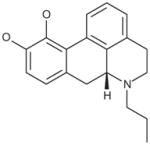
|
0.9 |
| R(−)-Apocodeine hydrochloride |
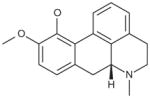
|
4 |
4. Discussion
We conducted a screening of the library of pharmacologically active compounds in order to identify potential, drug-like, glutaminase inhibitors. Here, we report on the kinetic characterization and preliminary analog evaluation of three newly identified glutaminase inhibitors: ebselen, chelerythrine and apomorphine.
To delineate the mechanisms of interaction of ebselen, chelerythrine and apomorphine with glutaminase (hKGAΔ1), we carried out competition and inactivation studies (Scheme 1). During competition studies, the enzyme (E) was exposed to both substrate (S) and inhibitor (I) at the same time. These studies allow the measurement of the equilibrium constant (Ki) between enzyme and inhibitor in the presence of glutamine. During inactivation studies, the enzyme was incubated with the inhibitor for varying times and the substrate subsequently used as a tool to determine the percent of remaining enzyme activity. Inactivation studies allow the measurement of the equilibrium constant (Ki) of a time-dependent inhibitor in addition to the rate of inactivation (kinact) [25].
Scheme 1.
Competition studies indicated mixed non-competitive inhibition for ebselen, competitive inhibition for chelerythrine and apomorphine and uncompetitive inhibition for BPTES (Table 2). Results with BPTES confirmed earlier reports of uncompetitive inhibition [22] as well as an allosteric interaction between BPTES and glutaminase [8,28]. The Ki of DON could not be accurately determined via competition studies. This was most likely due to DON partially acting as a substrate of glutaminase [16].
Remarkably, inactivation studies resulted in a time- and concentration- dependent loss of glutaminase activity for all five inhibitors (Supplementary: Figs. 1d, 2d, 3d, 4d, 5a), suggestive of either irreversible or reversible, slow-binding inhibition. The time-dependent inhibition of BPTES and apomorphine may be attributable to a slow-binding, reversible inhibition as neither BPTES nor apomorphine exhibit reactive moieties. In fact, 3D structure studies confirm a non-reactive, allosteric interaction between BPTES and the enzyme [8,28]. On the other hand, in accordance with earlier reports, we found DON to be time-dependent and irreversible [17]. Conceivably, ebselen and chelerythrine are also irreversible due to the reactive moieties in their structures.
The Ki’s obtained from both competition and inactivation studies were similar for ebselen (15 and 14 nM, respectively), suggesting that the presence or absence of glutamine does not alter the affinity of ebselen for the enzyme (Tables 2 and 3). The same was also true for BPTES (125 and 260 nM, respectively). These data support the idea of covalent adduct formation (ebselen) or slow-binding interaction (BPTES) with glutaminase via an allosteric site. In contrast, the Ki’s obtained from both competition and inactivation studies were dissimilar for both chelerythrine (270 and 30 nM, respectively) and apomorphine (2 μM and 4 nM, respectively). The presence of glutamine makes the apparent affinities (Ki) of chelerythrine and apomorphine weaker, suggesting possible competition for the active site. Alternatively, chelerythrine and apomorphine may bind at an allosteric site, which in turn precludes the binding of glutamine at the active site. An allosteric interaction between chelerythrine and glutaminase is not unlikely considering that the biological activity of chelerythrine is thought to be mediated via adduct formation between the iminium bond of chelerythrine and the thiol groups of the enzyme [27].
The degree of inhibition efficiency, as measured by the second order rate constants (kinact/Ki), suggests chelerythrine to be the most efficient inhibitor (Table 3). The second order rate constant obtained for ebselen against glutaminase was similar to that published previously for its radical scavenging activity [29]. While apomorphine’s kinact was the lowest amongst the three new inhibitors, its efficiency of inhibition was comparable to that of ebselen and chelerythrine due to its highest affinity. In contrast, BPTES’ efficiency of inhibition was two orders of magnitude lower than ebselen, chelerythrine and apomorphine but still two orders of magnitude better than that of DON.
Ebselen has been shown to form a selenenylsulfide (–Se–S–) linkage with the cysteine residues of proteins from several different functional classes [30]. While there are no known cysteine residues in the active site of glutaminase, there are many cysteine residues in all isoforms of the enzyme [5,28]. When the selenium moiety in ebselen was replaced by sulfur, oxygen and carbon that have decreasing oxidation potential and leaving group ability, the inhibitory potency decreased accordingly (Table 4). Chelerythrine is also thought to interact covalently via an adduct formation between its iminium moiety and the thiol groups on proteins [27]. When the iminium functionality was retained as with nitidine and sanguinarine, biological activity was also retained. However, when the methyl-iminium bond in sanguinarine was replaced with an imine, as in norsanguinarine, biological activity was lost, implicating the involvement of this functionality in its biological activity (Table 5). A recent publication [31] suggests the involvement of the methylenedioxy groups in the activity of these benzophenanthridine alkaloids. However, both chelerythrine with two dimethoxy molecules and sanguinarine with a methylenedioxy group were similarly active against glutaminase. The activities of both ebselen and chelerythrine against glutaminase maybe brought about by their ability to either confer an inactive tetrameric configuration to the enzyme [22] or to interfere with the tetramerization process [5].
In contrast to the thiol interactions of ebselen and chelerythrine, apomorphine’s activity appears to be dependent on its catechol moiety. While the apomorphine enantiomers were equipotent and the propyl analog slightly weaker, replacing one of the hydroxyl groups of the catechol with a methoxy group resulted in a 10-fold loss of activity (Table 6). Furthermore, the planarity of apomorphine might help it to have the same allosteric effects on glutaminase as BPTES, which is to ‘freeze’ the interface loops so as to prevent transmittance of the conformational change throughout the tetramer [28].
Even so, due to the multiplicity of biological effects and lack of selectivity, ebselen, chelerythrine and apomorphine may not be good prototype inhibitors for glutaminase inhibition in vivo. In studies conducted at the National Center for Biotechnology Information (NCBI), ebselen and chelerythrine were found to be active in 99 out of 597 and 29 out of 207 different assays, respectively [32,33]. Ebselen is a seleno-organic electrophile that has been pharmacologically profiled as an antioxidant [30]. Chelerythrine has a highly polar iminium moiety that is very reactive and subject to nucleophilic attack [34]. Further, ebselen, chelerythrine and apomorphine are known to inhibit various enzymes and/or receptors [34–39].
However, it is noteworthy that ebselen’s activity against glutaminase is 2-fold greater than the most potent activity reported for ebselen (against UDP-glucose 4′-epimerase, a validated drug target for african sleeping sickness) [33]. Ebselen has been extensively used in a variety of experimental animal models of injury/disease [37] and as a neuroprotectant in the treatment of patients following acute ischemic stroke [40–42] and aneurysmal subarachnoid hemorrhage [43]. A clinical trial is currently underway to determine the utility of ebselen in a hearing loss paradigm [44]. Chelerythrine is a quaternary benzophenanthridine alkaloid derived from Chelidonium majus L, a member of the poppy family [45]. Alkaloids derived from the plant have been shown to possess an array of activities including anti-inflammatory, anti-tumor, anti-microbial and anti-viral activity. Recently, several dibenzophenanthridines were shown to have anti-proliferative effects [6]. Apomorphine is being marketed under various trade names for the treatment of Parkinson’s disease [46]. Another study suggests the potential use of apomorphine as a biological marker for heroin dependence disorder [47].
In summary, ebselen, chelerythrine and apomorphine appear to be significantly more efficient (>kinact/Ki) than either DON or BPTES. They are also more soluble than BPTES. To our knowledge, this is the first report that ebselen, chelerythrine and apomorphine are potent and efficient inhibitors of glutaminase and raises the question whether some of the biological activity previously reported with these compounds could be due to ‘off-target’ inhibition of glutaminase.
Acknowledgments
This work was supported in part by the Johns Hopkins Brain Science Institute, and NIH Grants 1-R03-MH093170-01A1 (to BSS) and 1R21NS074151-01 (to TT).
Abbreviations
- Apomorphine
5,6,6a,7-tetrahydro-6-methyl-4H-dibenzo[de,g] quinoline-10,11-diol
- Berberine
5,6-dihydro-9,10-dimethoxy-benzo[g]-[1,3] benzodioxolo[5,6-a]quinolizinium
- BPTES
bis-2-(5-phenylacetimido-1,2,4-thiadiazol- 2-yl)ethyl sulfide
- CNS
central nervous system
- Chelerythrine
1,2-dimethoxy- N-methyl[1,3]benzodioxolo[5,6-c]phenanthridinium
- DON
6-diazo-5-oxo-L-norleucine
- Ebselen
2-phenyl-1,2-benzisoselenazol-3[2H]-one
- GAC
c-type glutaminase
- GLS
glutaminase
- HIV
human immunodeficiency virus
- HRP
horse radish peroxidase
- KGA
kidney-type glutaminase
- LGA
liver-type glutaminase
- Nitidine
2,3-dimethoxy-N-methyl[1,3]benzodioxolo[5,6-c]phenanthridinium
- Norsanguinarine
[1,3]-benzodioxolo[5,6-c]-1,3-dioxolo[4,5-i]phenanthridine
- Sanguinarine
13-methyl-[1,3]-benzodioxolo[5,6-c]-1,3-dioxolo[4,5-i]phenanthridinium
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bbrc.2013.06.110.
References
- 1.DeBerardinis RJ, Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szeliga M, Obara-Michlewska M. Glutamine in neoplastic cells: focus on the expression and roles of glutaminases. Neurochem Int. 2009;55:71–75. doi: 10.1016/j.neuint.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher FA, Kettunen MI, Day SE, Lerche M, Brindle KM. 13C MR spectroscopy measurements of glutaminase activity in human hepatocellular carcinoma cells using hyperpolarized 13C-labeled glutamine. Magn Reson Med. 2008;60:253–257. doi: 10.1002/mrm.21650. [DOI] [PubMed] [Google Scholar]
- 4.Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV. C-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassago A, Ferreira AP, Ferreira IM, Fornezari C, Gomes ER, Greene KS, Pereira HM, Garratt RC, Dias SM, Ambrosio AL. Mitochondrial localization and structure-based phosphate activation mechanism of glutaminase C with implications for cancer metabolism. Proc Natl Acad Sci USA. 2012;109:1092–1097. doi: 10.1073/pnas.1112495109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katt WP, Ramachandran S, Erickson JW, Cerione RA. Dibenzophenanthridines as inhibitors of glutaminase C and cancer cell proliferation. Mol Cancer Ther. 2012;11:1269–1278. doi: 10.1158/1535-7163.MCT-11-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seltzer MJ, Bennett BD, Joshi AD, Gao P, Thomas AG, Ferraris DV, Tsukamoto T, Rojas CJ, Slusher BS, Rabinowitz JD, Dang CV, Riggins GJ. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010;70:8981–8987. doi: 10.1158/0008-5472.CAN-10-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thangavelu K, Pan CQ, Karlberg T, Balaji G, Uttamchandani M, Suresh V, Schuler H, Low BC, Sivaraman J. Structural basis for the allosteric inhibitory mechanism of human kidney-type glutaminase (KGA) and its regulation by Raf-Mek-Erk signaling in cancer cell metabolism. Proc Natl Acad Sci USA. 2012;109:7705–7710. doi: 10.1073/pnas.1116573109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R, Wilson KF, Ambrosio AL, Dias SM, Dang CV, Cerione RA. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18:207–219. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhry FA, Reimer RJ, Edwards RH. The glutamine commute: take the N line and transfer to the A. J Cell Biol. 2002;157:349–355. doi: 10.1083/jcb.200201070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thanki CM, Sugden D, Thomas AJ, Bradford HF. In vivo release from cerebral cortex of [14C]glutamate synthesized from [U-14C]glutamine. J Neurochem. 1983;41:611–617. doi: 10.1111/j.1471-4159.1983.tb04785.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, Lopez AL, Erichsen D, Herek S, Cotter RL, Curthoys NP, Zheng J. Mitochondrial glutaminase enhances extracellular glutamate production in HIV-1-infected macrophages: linkage to HIV-1 associated dementia. J Neurochem. 2004;88:169–180. doi: 10.1046/j.1471-4159.2003.02146.x. [DOI] [PubMed] [Google Scholar]
- 13.Erdmann N, Zhao J, Lopez AL, Herek S, Curthoys N, Hexum TD, Tsukamoto T, Ferraris D, Zheng J. Glutamate production by HIV-1 infected human macrophage is blocked by the inhibition of glutaminase. J Neurochem. 2007;102:539–549. doi: 10.1111/j.1471-4159.2007.04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shijie J, Takeuchi H, Yawata I, Harada Y, Sonobe Y, Doi Y, Liang J, Hua L, Yasuoka S, Zhou Y, Noda M, Kawanokuchi J, Mizuno T, Suzumura A. Blockade of glutamate release from microglia attenuates experimental autoimmune encephalomyelitis in mice. Tohoku J Exp Med. 2009;217:87–92. doi: 10.1620/tjem.217.87. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi H, Jin S, Suzuki H, Doi Y, Liang J, Kawanokuchi J, Mizuno T, Sawada M, Suzumura A. Blockade of microglial glutamate release protects against ischemic brain injury. Exp Neurol. 2008;214:144–146. doi: 10.1016/j.expneurol.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Hartman SC, McGrath TF. Glutaminase A of Escherichia coli. Reactions with the substrate analogue, 6-diazo-5-oxonorleucine. J Biol Chem. 1973;248:8506–8510. [PubMed] [Google Scholar]
- 17.Shapiro RA, Clark VM, Curthoys NP. Inactivation of rat renal phosphate-dependent glutaminase with 6-diazo-5-oxo-L-norleucine. Evidence for interaction at the glutamine binding site. J Biol Chem. 1979;254:2835–2838. [PubMed] [Google Scholar]
- 18.Pinkus LM. Glutamine binding sites. Methods Enzymol. 1977;46:414–427. doi: 10.1016/s0076-6879(77)46049-x. [DOI] [PubMed] [Google Scholar]
- 19.Earhart RH, Koeller JM, Davis HL. Phase I trial of 6-diazo-5-oxo-L-norleucine (DON) administered by 5-day courses. Cancer Treat Rep. 1982;66:1215–1217. [PubMed] [Google Scholar]
- 20.Kovach JS, Eagan RT, Powis G, Rubin J, Creagan ET, Moertel CG. Phase I and pharmacokinetic studies of DON. Cancer Treat Rep. 1981;65:1031–1036. [PubMed] [Google Scholar]
- 21.Sklaroff RB, Casper ES, Magill GB, Young CW. Phase I study of 6-diazo-5-oxo-L-norleucine (DON) Cancer Treat Rep. 1980;64:1247–1251. [PubMed] [Google Scholar]
- 22.Hartwick EW, Curthoys NP. BPTES inhibition of hGA(124–551), a truncated form of human kidney-type glutaminase. J Enzyme Inhib Med Chem. 2012;27:861–867. doi: 10.3109/14756366.2011.622272. [DOI] [PubMed] [Google Scholar]
- 23.Shukla K, Ferraris DV, Thomas AG, Stathis M, Duvall B, Delahanty G, Alt J, Rais R, Rojas C, Gao P, Xiang Y, Dang CV, Slusher BS, Tsukamoto T. Design, synthesis, and pharmacological evaluation of bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide 3 (BPTES) analogs as glutaminase inhibitors. J Med Chem. 2012;55:10551–10563. doi: 10.1021/jm301191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McElroy KE, Bouchard PJ, Harpel MR, Horiuchi KY, Rogers KC, Murphy DJ, Chung TD, Copeland RA. Implementation of a continuous, enzyme-coupled fluorescence assay for high-throughput analysis of glutamate-producing enzymes. Anal Biochem. 2000;284:382–387. doi: 10.1006/abio.2000.4740. [DOI] [PubMed] [Google Scholar]
- 25.Walsh C. Enzymatic Reaction Mechanisms. W.H. Freeman and Company; San Francisco: 1979. [Google Scholar]
- 26.Engler JA, Gottesman JM, Harkins JC, Urazaev AK, Lieberman EM, Grossfeld RM. Properties of glutaminase of crayfish CNS: implications for axon-glia signaling. Neuroscience. 2002;114:699–705. doi: 10.1016/s0306-4522(02)00357-3. [DOI] [PubMed] [Google Scholar]
- 27.Walterova D, Ulrichova J, Preininger V, Simanek V, Lenfeld J, Lasovsky J. Inhibition of liver alanine aminotransferase activity by some benzophenanthridine alkaloids. J Med Chem. 1981;24:1100–1103. doi: 10.1021/jm00141a019. [DOI] [PubMed] [Google Scholar]
- 28.DeLaBarre B, Gross S, Fang C, Gao Y, Jha A, Jiang F, Song JJ, Wei W, Hurov JB. Full-length human glutaminase in complex with an allosteric inhibitor. Biochemistry. 2011;50:10764–10770. doi: 10.1021/bi201613d. [DOI] [PubMed] [Google Scholar]
- 29.Fujisawa S, Kadoma Y. Kinetic studies of the radical-scavenging activity of ebselen, a seleno-organic compound. Anticancer Res. 2005;25:3989–3994. [PubMed] [Google Scholar]
- 30.Sakurai T, Kanayama M, Shibata T, Itoh K, Kobayashi A, Yamamoto M, Uchida K. Ebselen, a seleno-organic antioxidant, as an electrophile. Chem Res Toxicol. 2006;19:1196–1204. doi: 10.1021/tx0601105. [DOI] [PubMed] [Google Scholar]
- 31.Aburai N, Yoshida M, Ohnishi M, Kimura K. Sanguinarine as a potent and specific inhibitor of protein phosphatase 2C in vitro and induces apoptosis via phosphorylation of p38 in HL60 cells. Biosci Biotechnol Biochem. 2010;74:548–552. doi: 10.1271/bbb.90735. [DOI] [PubMed] [Google Scholar]
- 32.Chelerythrine. http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=72311.
- 33.Ebselen. http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?sid=856002.
- 34.Parenty AD, Smith LV, Guthrie KM, Long DL, Plumb J, Brown R, Cronin L. Highly stable phenanthridinium frameworks as a new class of tunable DNA binding agents with cytotoxic properties. J Med Chem. 2005;48:4504–4506. doi: 10.1021/jm050320z. [DOI] [PubMed] [Google Scholar]
- 35.Kaminskyy V, Lin KW, Filyak Y, Stoika R. Differential effect of sanguinarine, chelerythrine and chelidonine on DNA damage and cell viability in primary mouse spleen cells and mouse leukemic cells. Cell Biol Int. 2008;32:271–277. doi: 10.1016/j.cellbi.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I a multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J Pharmacol Exp Ther. 2002;303:791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- 37.Schewe T. Molecular actions of ebselen–an antiinflammatory antioxidant. Gen Pharmacol. 1995;26:1153–1169. doi: 10.1016/0306-3623(95)00003-j. [DOI] [PubMed] [Google Scholar]
- 38.Sweeney JF, Nguyen PK, Atkins KB, Hinshaw DB. Chelerythrine chloride induces rapid polymorphonuclear leukocyte apoptosis through activation of caspase-3. Shock. 2000;13:464–471. doi: 10.1097/00024382-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Walterova D, Ulrichova J, Valka I, Vicar J, Vavreckova C, Taborska E, Harjrader RJ, Meyer DL, Cerna H, Simanek V. Benzo[c]phenanthridine alkaloids sanguinarine and chelerythrine: biological activities and dental care applications. Acta Univ Palacki Olomuc Fac Med. 1995;139:7–16. [PubMed] [Google Scholar]
- 40.Koizumi H, Fujisawa H, Suehiro E, Shirao S, Suzuki M. Neuroprotective effects of ebselen following forebrain ischemia: involvement of glutamate and nitric oxide. Neurol Med Chir (Tokyo) 2011;51:337–343. doi: 10.2176/nmc.51.337. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa A, Yoshimoto T, Kikuchi H, Sano K, Saito I, Yamaguchi T, Yasuhara H. Ebselen in acute middle cerebral artery occlusion: a placebo-controlled, double-blind clinical trial. Cerebrovasc Dis. 1999;9:112–118. doi: 10.1159/000015908. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, Yasuhara H. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen study group. Stroke. 1998;29:12–17. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- 43.Saito I, Asano T, Sano K, Takakura K, Abe H, Yoshimoto T, Kikuchi H, Ohta T, Ishibashi S. Neuroprotective effect of an antioxidant, ebselen, in patients with delayed neurological deficits after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1998;42:269–277. doi: 10.1097/00006123-199802000-00038. discussion 277–8. [DOI] [PubMed] [Google Scholar]
- 44.Ebselen/Hearing-Loss. http://www.prnewswire.com/news-releases/sound-pharmaceuticals-announces-a-new-spi-1005-clinical-trial-involvingpersonal-music-players-123234673.html.
- 45.Colombo ML, Bosisio E. Pharmacological activities of Chelidonium majus L. (Papaveraceae) Pharmacol Res. 1996;33:127–134. doi: 10.1006/phrs.1996.0019. [DOI] [PubMed] [Google Scholar]
- 46.Chaudhuri KR, Clough C. Subcutaneous apomorphine in Parkinson’s disease. BMJ. 1998;316:641. doi: 10.1136/bmj.316.7132.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guardia J, Casas M, Prat G, Trujols J, Segura L, Sanchez-Turet M. The apomorphine test: a biological marker for heroin dependence disorder? Addict Biol. 2002;7:421–426. doi: 10.1080/1355621021000006206. [DOI] [PubMed] [Google Scholar]