Abstract
Impaired synaptic plasticity is implicated in the functional decline of the nervous system associated with ageing. Understanding the structure of ageing synapses is essential to understanding the functions of these synapses and their role in the ageing nervous system. In this review, we summarize studies on ageing synapses in vertebrates and invertebrates, focusing on changes in morphology and ultrastructure. We cover different parts of the nervous system, including the brain, the retina, the cochlea, and the neuromuscular junction. The morphological characteristics of aged synapses could shed light on the underlying molecular changes and their functional consequences.
Keywords: Synapse, aging, postsynaptic density, neurotransmitter vesicles, presynaptic bouton, dendritic spine
1. Introduction
One inevitable part of ageing is a decline in the functions of our nervous system, a decline that can affect everything from learning and memory to hearing and vision. The proper function of the nervous system depends on the underlying structure of neuronal networks, which includes the morphology of their axons, dendrites and synapses. Over one hundred years ago, Sir Charles Sherrington first coined the word synapse to connote the physical relation – or the connection – between neurons (Foster and Sherrington, 1897). Since then, we have learned a great deal about how the synapse performs its role, connecting neurons via electrochemical neurotransmission and serving as the focal point of cell-cell signaling in the nervous system. A synapse, in the simplest sense, is typically composed of two main parts: a presynaptic compartment or terminal from the signal-sending neuron and a postsynaptic terminal from the signal-receiving neuron (Fig. 1; see Stewart et al., 2014). The presynaptic terminal harbors clusters of neurotransmitter-filled synaptic vesicles that are stunningly uniform in their size and shape. The acceptor postsynaptic terminal contains neurotransmitter-specific receptors, arranged on the membrane surface in a position to respond to neurotransmitter released from the presynaptic terminal.
Figure 1.
Generalized diagram of typical synapses of the vertebrate brain. Presynaptic terminals can be a terminal bouton or en passant along the axon. Common examples include excitatory ones with round vesicles (green) containing the neurotransmitter, glutamate, and inhibitory ones with pleomorphic vesicles (red) containing the neurotransmitter, GABA, and they can form on dendrite spines (sp) or directly on the dendrite shaft (den). The area of vesicle release along the presynaptic membrane is called the active zone. The space between the pre- and postsynaptic processes is the synaptic cleft (cl) and the postsynaptic membrane, which contains the receptors, has an associated region of dense material called the postsynaptic density (psd) that is thick (asymmetric synapse) in excitatory and thin (symmetric synapse) in inhibitory types. A terminal can sometime form synapses on more than one postsynaptic process (multisynaptic bouton; left bouton in figure) and typically contains mitochondria (mit) and various reticular and vesicular structures (not shown). During ageing, changes are seen in the shape, size and number of all of these various components of synapses in animals, as described in the text.
The maintenance of this precise arrangement of the synaptic terminals requires a complex protein network. In the presynaptic terminal, there is an expansive array of proteins. These presynaptic proteins are expressed in precise amounts, situated at specific locations, and designated for specific functions (Takamori et al., 2006; Siksou et al., 2011). Similarly, in the postsynaptic terminal, there is a compendium of proteins (Sheng and Kim, 2011; Chen and Sabatini, 2012). Some of these proteins serve as neurotransmitter receptors and their downstream signaling molecules, whereas others function as the anchor for the receptors or the scaffold for the construction of the postsynaptic terminals. Together, these synaptic protein components constitute a dedicated system to ensure accurate, efficient and reliable synaptic transmission.
Age-related abnormalities of synaptic transmission have been documented in ageing studies of animal models and humans subjects (Peters et al., 2008; Dumitriu et al., 2010; Luebke et al., 2007; 2010). In this review we focus on structural changes that occur in synapses during ageing, i.e., the period that is considered normal old age for a particular animal. We discuss the morphological and structural alterations observed in the synapses of the ageing vertebrate nervous system, taking into account commonalities in the brain and several other parts of the nervous system. We also compare age-related changes in the synapses of several invertebrate model systems. We refer the reader to Morrison and Baxter (2012) and Yeoman et al. (2012) for comprehensive reviews of synaptic functions in ageing neurons, and Sheng et al. (2012) and Picconi et al. (2012) as examples of reviews of abnormal synapses observed in age-related diseases (and see section 9).
2. Vertebrate forebrain
2A. Synapse number and distribution
Two approaches are often used to study synapse number and distribution in the nervous system. One approach is focused on the overall level of a synapse-specific protein, inferring the number or the state of the synapses from a given brain area. For example, when assessed by immunoblot analysis, the levels of synaptic vesicle proteins such as synaptophysin and SNAP-25 are significantly reduced in the hippocampus of 14–24 month-old rats compared to 2 month-old rats (Canas et al., 2009). Because synaptophysin and SNAP-25 are expressed in the presynaptic terminal, this finding implies an age-related decrease in the number of presynaptic terminals and may also point to a decrease in the amount of these proteins in the terminals. Another study using a combination of proteomic and immunoblot analysis, finds that the level of postsynaptic proteins, in addition to several presynaptic proteins, is also decreased in the hippocampus of older (26 months) rats (VanGuilder et. al., 2010). This observation suggests that both presynaptic and postsynaptic terminals are affected in the ageing hippocampus.
The second approach is to study individual synaptic terminals using light or electron microscopy. This approach is useful for revealing spatial information of affected synapses in a specific neuronal circuit within a defined anatomical area. Using immunolabeled synaptophysin as an indicator, for instance, one study finds that the number of synaptic terminals in hippocampal CA3 lacunosum moleculare area is significantly reduced in aged rats with spatial learning deficits and, remarkably, that the extent of the reduction correlates with the severity of the learning deficits (Smith et al., 2000). This finding supports the notion derived from similar studies that the loss of hippocampal synapses directly contributes to age-related cognitive impairment (Bondareff and Geinisman, 1976; McWilliams and Lynch, 1984; Geinisman et al., 1986; 1992). Note that these changes in synapse number probably represent changes in overall complexity of the individual neurons, but maybe not a change in neuron number; most studies indicate that ageing in the prefrontal cortex and hippocampus does not involve a substantial loss in neuron number, although there are exceptions (Rapp and Gallagher, 1996; Hara and Morrison, 2014). Geinisman et al. (1986, 1992) used electron microscopy to analyze the density of different types of synaptic contacts in the hippocampal dentate gyrus of aged rats. For axodendritic synapses, there is no apparent difference among young rats, old rats with normal memory, and aged rats with impaired memory. For axospinous synapses, there is no difference between young and old rats with normal memory. However, aged rats with impaired memory capacity show a significant loss of axospinous synapses (Geinisman et al., 1986,1992). Quantitative electron microscope-based analysis of synapses in the middle frontal cortex (Brodmann area 9) of human subjects ranging in age from 20 to 89 years, reveals no effects of ageing on synapse numbers (Scheff et al., 2001), suggesting that certain neuronal populations maintain normal synaptic connections throughout life. Consistent with variability among neuronal populations in the vulnerability of their synapses to ageing, a study of young and old Fischer 344 rats indicates that ageing results in a reduction of synaptic density in the lateral septal nucleus, but not the medial septal nucleus, of some but not all old animals (Scheff et al., 1991). Similarly, differences in synaptic loss can vary among subregions of the cerebral cortex and hippocampus (reviewed in Hara and Morrison, 2014). From 5 to 30 years old, the prefrontal cortex (area 46) of monkeys shows a 30% decrease in excitatory (asymmetric)+inhibitory (symmetric) synapses in layers II/III, and synapse density is correlated with performance on memory tests and with the cognitive impairment index (Peters et al., 2008). In contrast, there is less loss of synapses in layer V, and this consists only of loss of asymmetric synapses, and does not seem to correlate with performance on cognitive tests (Peters et al., 2008). Rats show significant age-related losses of axospinous synapses in parts of the dentate gyrus and CA3 region, while synapse density does not change with ageing in the CA1 regions (reviewed in Hara and Morrison, 2014). A region specific loss also is seen in the ageing mouse olfactory bulb, comparing mice of various ages from 2 to 24 months (Richard et al, 2010). While the laminar and cellular organization remains stable with ageing, there is a significant decrease in synaptic density in the glomerular layer but not the external plexiform layer; this alteration of the circuitry may lead to age-related changes in odor discrimination. Interestingly, differential decreases with ageing in layer and cell size and functional changes may vary greatly among strains of mice and rats (discussed in Mirich et al., 2002), so a definite correlation of synaptic changes with ageing deficits requires further study.
Recently, a study using in vivo two-photon imaging showed that the axonal terminals in the somatosensory cortex of aged mice (22–24 months old, and showing cognitive impairment) have much higher rates of turnover as well as increased rates of size change compared to those in young mice (4–6 months; Grillo et al., 2013). This decreased “synaptic tenacity” in aged mice seems contradictory to most reports that aged animals have decreased synapse number and plasticity. Thus, cognitive impairment in aged animals may occur because the maintenance of stable synaptic connections in the ageing brain is compromised.
In some parts of the brain, ageing synapses may be derived from neurons that actually were formed in the adult. Adult neurogenesis is known to occur in at least 2 places. Neuroblasts derived from the adult subventricular zone (SVZ) of the lateral ventricle migrate to the olfactory bulb where most of them become granule cells and a few become periglomerular cells (Sawada and Sawamoto, 2013). Also, in the hippocampus dentate gyrus, new adult granule cells are generated from the subgranular zone (Drew et al., 2013). There is evidence for adult neurogenesis in the dentate gyrus in old animals, including >2 year-old rodents, 15 year old dogs, and 72 year old humans (reviewed in Leuner et al., 2007). Adult-born neurons of the mouse olfactory bulb show experience-dependent plasticity when the mice are examined at about 6 months old (Livneh and Mizrahi, 2012). In the hippocampus dentate gyrus in mice about 2 months old, adult-born granule cells form mossy fiber terminal synapses with CA3 pyramidal cells within 2 weeks; these synapses mature in 2 months and remain stable at 4 months (Faulkner et al., 2008). Spinogenesis on adult-born neurons may start with attraction of a dendritic filopodium to a pre-existing synapse between an axon terminal and a spine from another neuron; the filopodium then matures into a spine while the other spine retracts (Toni and Sultan, 2011). Thus, studies of ageing synapses, at least in the olfactory bulb and hippocampus, would need to take into consideration both the synapses from neurons formed in postnatal development, and the ones that are formed continuously in the maturing adult animal. Unfortunately, the morphology of ageing synapses that are derived from adult-born neurons has not been studied. Several studies indicate that adult neurogenesis decreases in the ageing brain; in the SVZ of rodents, the greatest decline (by more than half) occurs between young adult (2–6 months) and middle age (6–14 months); adult neurogenesis then seems to level off in old age (studied up to 2 years; Hamilton et al., 2013). Similarly, monkeys (marmosets) show a significant decline in neurogenesis in both the dentate gyrus and SVZ by middle age (3.5–7 years old) compared to young one (1.5–3 years; Leuner et al, 2007). At this point, studies on changes in synaptic circuitry in old age can only speculate about how a decrease in adult neurogenesis in aged animals can affect this. For example, Richard et al. (2010) looked at changing synaptic densities during aging (as discussed above), and note that the granule cell number in the olfactory bulb remains stable throughout ageing. So, during ageing, there might be a changing balance between addition and depletion of granule cells, to maintain cell number while changing synaptic density.
2B. Presynaptic terminals
Ultrastructural studies using electron microscopy reveal some age-related changes in both the type of terminal and in substructures within the terminal. Terminal types include single-synapse boutons, multiple-synapse boutons (Fig. 1), and boutons lacking apparent synaptic contacts with postsynaptic processes (nonsynaptic boutons; Hara et al., 2011). Aged female monkeys have fewer boutons with multiple synapses and fewer synaptic contacts per multiple synapse in the dentate gyrus. Yet they have twice as many nonsynaptic boutons. The latter correlate with age-associated memory impairment (Hara et al., 2011; 2012). Nonsynaptic boutons are probably terminals that have lost their spine synapses, and this suggests that the boutons persist while the spines tend to be lost in the ageing brain. But this finding in aged monkeys is inconsistent with the study described above from Grillo et al. (2013) who report that it is the bouton that is unstable in aged mice, rather than the spine. Terminals also can be classified (Fig. 1) as 1) asymmetric excitatory, with round vesicles, wide clefts, and thick, asymmetric density structure, and 2) symmetric inhibitory, with pleomorphic vesicles (mixture of round and flattened vesicle profiles in sections) and thin, symmetric density structure. In layer 2/3 of the frontal cortex of ageing monkeys, there is a 30% reduction in the density of asymmetric excitatory and symmetric inhibitory synapses as a function of age (Peters et al., 2008). While both types of synapses are decreased by similar amounts, only the loss of asymmetric excitatory synapses is strongly correlated with cognitive impairment in ageing monkeys (Peters et al., 2008).
Presynaptic terminal ultrastructure is characterized chiefly by the presence of large numbers (typically) of synaptic vesicles, as well as a variety of other organelles including mitochondria, endosomes, autophagosomes, and endoplasmic reticulum. All of these components are involved in the release of neurotransmitter, and these might be expected to change during ageing. However, there is little change in the ultrastructure within synaptic terminals in the primate frontal cortex in animals up to 30 years old (Peters et al., 2008). Similarly, in the parietal cortex of rats from 6 to 17 months of age, there are no significant changes in terminal surface area or in the number of vesicles per terminal (Adams and Jones, 1982). However, some changes have been reported: thus, Applegate and Landfield (1988) find a significant decrease in distant vesicle density (i.e., vesicles more than 150 nm from the active zone) in the hippocampus of 24–27 month old rats compared to 3 to 5 month old ones. And Soghomonian et al. (2010) find an increase in the number of synaptic vesicles in inhibitory axosomatic synaptic terminals in the prefrontal cortex of aged monkeys.
Presynaptic mitochondria presumably provide the energy needed for neurotransmitter release at the terminal and for restoration of Na+ and Ca2+ gradients after the membrane depolarization that triggers neurotransmitter release. Bertoni-Freddari et al. (2007) find no change in synaptic mitochondria in the frontal and temporal cortices of aged monkeys. Similarly, Soghomonian et al. (2010) find no change in mitochondria in inhibitory axodendritic synapses in the prefrontal cortex of aged monkeys. However, they do find larger mitochondria in inhibitory axosomatic terminals in this area. In contrast, no changes in mitochondria are seen in axosomatic terminals on Betz cells of area 4 motor cortex in aged monkeys (Tigges et al., 1992). Overall, this suggests that some basic, minimal mitochondrial participation is necessary for any normal synaptic function, even if that function is reduced in aged animals (Martinez et al., 1996; see also sections 5–7 for other descriptions of changes in mitochondria).
Few other changes in organelles have been described with normal ageing. Adams and Jones (1982) find fewer tubular cisternae in presynaptic terminals of aged rats. This could suggest either less protein trafficking or less ion transport, depending on their nature, and would require further clarification of their identity. Autophagosomes and associated structures in the brain are affected by ageing (Keller et al., 2004) and are typical organelles found in some presynaptic terminals (Petralia et al., 2013); they could clear away cell debris in healthy terminals or aid in degradation of inactive terminals. However, most studies have concentrated on their roles in age-related neurodegenerative diseases such as Parkinson’s, Huntington’s and Alzheimer’s (Yang et al., 2013) and little seems to be known about changes in the normally ageing presynaptic terminal.
2C. Postsynaptic Spine
Spine structure and function is varied and complex in the mammalian forebrain and shows different ageing patterns depending on the region. This is reviewed in general by Morrison and Baxter (2012) and Hara and Morrison (2014) and we will concentrate here on specific changes in morphology. Most spines studied have a distinct head on a thin neck, although short stubby spines also can be common (Fig. 2). Spines can be classified in more detail based on the structure of their postsynaptic density (PSD). Thin spines with small heads typically have small uniform PSDs without distinct breaks and are called non-perforated (Fig. 2; Nicholson et al., 2004). Large spines have large heads and often are described as mushroom-like. These usually have perforated PSDs that are further classified as fenestrated, horseshoe-shaped or segmented, depending on the elaboration and extent of the perforation (Fig. 2). The segmented types are the largest; they have 2 or more separate PSDs and associated active zones; they often extend membranous spinules between the segments and into the presynaptic terminal (Fig. 2), and they usually have a spine apparatus (complex reticular organelle) in the spine (Geinisman et al. 1992; Ganeshina et al., 2004b; Nicholson et al., 2004).
Figure 2.
In mammals (left), synaptic spine shape (beige) varies from thin to stubby to mushroom-shaped (based on Harris et al., 1992, and Geinisman, 2000). The postsynaptic density (black) in mushroom-shaped spines is usually perforated to various degrees: from fenestrated (Fen) to horseshoe-shaped (HS) to completely segmented into two or more pieces. In this diagram, a membrane partition (dark beige) is illustrated only for the completely segmented example, but partitions may be present on any kind of perforated spine depending on the stage in plasticity (e.g., inductive versus maintenance phase of long-term potentiation, as discussed by Geinisman, 2000). Spines in honeybees (right) vary less in shape than those in mammals, and show a gradual enlargement of the head and consequent shortening of the narrow part of the neck (stem) as the honeybee ages and changes its duties in the colony, from newly emerged (New), to nurse to forager. Spine shapes are based on light microscope studies of Coss et al. (1980). Mammalian and honeybee spines are not to scale. Presynaptic terminals are excluded from the drawings for clarity of the postsynaptic structures.
Spine shape is related to plasticity and excitatory activity of the spine synapse (e.g., as during long-term potentiation; see Horak et al., 2014 for general review), e.g., the number of AMPA receptors in the adult hippocampus is higher in the larger, more complex spines (Ganeshina et al., 2004a, b). In addition to changes in receptor numbers, long-term potentiation of synapses may induce changes in spine structure: stubby spines and shaft synapses may change into thin spines and then change further by elongation and development of concave spine heads, while mushroom spines also may enlarge and acquire more concave spine heads (Popov et al., 2004). Popov et al. (2004) did not see a change in the proportion of synapses on mushroom spines, although there were decreases in synapses on stubby spines and dendritic shafts. This might favor a model where there is some type of definitive separation between the development of long thin spines and that of the large, perforated, mushroom spines. But it is not clear at this point if long, thin spines and large, perforated spines represent two different lineages of unrelated spine types, or if there is a complete continuum of changes among all spine types (Geinisman et al, 2000; Popov et al., 2004; Stewart et al., 2014). When synaptogenesis is induced by blocking synaptic transmission in acute hippocampal slices from ~2 month old rats, there is a proliferation of short filopodia (as opposed to the longer filopodia typically seen in early development) and stubby spines along mature dendrites (Petrak et al., 2005). In a related study, there are increased numbers of immature stubby and mature mushroom spines, but not mature thin spines in hippocampal slices from ~2 month old rats compared to the hippocampus from perfused animals (Kirov et al., 1999). Again, this might imply that mature thin spines are a separate class of spines. Of course, newly generated mature thin and mushroom spines both probably go through a stubby spine stage (and maybe a filopodial stage before that; Fiala et al., 1998; Bourne and Harris, 2008). Long-term potentiation may effect the full range of development of spine size and shape, in a continuum, with developmental differences that depend on the phase -inductive versus maintenance – of the potentiation, as discussed in detail by Geinisman (2000)(Fig. 2). It also is not clear if there is substantial turnover of synapses during potentiation or if most change involves changes in spine type and size (Sora and Harris, 1998; Toni et al, 1999; Geinisman et al, 2000; Popov et al., 2004; Stewart et al., 2014).
Changes with ageing alone must be distinguished from changes related to impairment of learning and memory (i.e., some old individuals have better learning and memory than others) and hormonal changes (Morrison and Baxter, 2012). Interestingly, there may be a broad difference in the trend with ageing between the prefrontal cortex and the hippocampus, as noted by Morrison and Baxter (2012) and Hara and Morrison (2014); the ageing prefrontal cortex tends to lose more thin spines that perhaps confer greater potential for plasticity, and are “linked to variability in cognitive function,” while the ageing hippocampus tends to lose the large, complex (perforated mushroom) synapses that help control established memories and learning circuits. Again, this suggests that there could be some class differences in the lines of maturing thin versus mushroom spines, as we discussed above.
For example, in the rat prefrontal cortex, spine loss between middle (12 months) and old (20 months) age is due largely to loss of thin spines; these changes are not affected much by stress, suggesting that ageing spines have less plasticity (Bloss et al., 2011). Two studies in area 46 of the ageing monkey prefrontal cortex show that about 1/3 of spine synapses are lost (Peters et al., 2008; Dumitriu et al., 2010) and most of this loss is in the thin spines (about 46% decrease), and the remaining thin spines are the biggest ones (Dumitriu et al., 2010). Furthermore, there is an almost complete correlation between the increased volume of thin spines and reduced cognitive performance. However, in contrast to these findings, Mostany et al. (2013) used chronic in vivo two-photon imaging of dendritic spines and axonal boutons in the somatosensory cortex, and find an abundance of thin spines only in juvenile mice (<1 month), while ratios of stubby, thin, and mushroom spines do not vary among young (3–5 months), mature (8–15 months) and old (>20 months) mice. They also find that there is no spine loss in old mice, but spines tend to be smaller, with less long-term retention of stable spines. Thus, the general conclusion is similar to that proposed by others, i.e., that old synapses and circuits tend to be less plastic overall.
In the dentate gyrus in the hippocampus of ageing rats (28 months), there is a significant loss of non-perforated synaptic spines, and at least a trend of similar loss in perforated spines, but no loss in synapses on dendritic shafts (Geinisman et al., 1992). Furthermore, in the CA1 stratum radiatum in the hippocampus of ageing rats (27 months), there is a significant reduction in the PSD area (by ~23%) of the large, segmented synapses in learning-unimpaired animals (Nicholson et al., 2004). More changes are seen in ageing animals with learning impairments. Thus, in the dentate gyrus of memory-impaired aged rats, there is a loss of perforated synapses (Geinisman et al., 1986), and this loss is profound (by ~27–44%) for all three kinds of perforated synapses in learning-impaired rats (Nicholson et al., 2004). Similarly, Hongpaisan et al. (2013) find that both the total density and mushroom spine density in the CA1 stratum radiatum are lower in age-impaired old rats compared to age-unimpaired or young rats. Interestingly, activation (using treatment with activators) of protein kinase C, which is believed to affect synaptogenesis, increases mushroom spine formation and improves spatial memory retention in old rats. In contrast to the findings in rats, no effect of ageing alone is seen for synapse density, PSD length, or perforated synapse percentage in the dentate gyrus of ageing monkeys (22–35 years; Hara et al., 2012). However, these authors do find a lower density of perforated synapses in the outer molecular layer of the dentate gyrus in peri/post-menopausal animals versus pre-menopausal ones. Hara et al. (2011) also find that ageing peri/postmenopausal monkeys have fewer multiple synapse boutons (see also section 2B) contacting one or more perforated synapse spines compared to aged premenopausal monkeys. Interestingly, estrogen can induce spine formation in ageing female monkeys but not in ageing female rats (discussed in Morrison and Baxter, 2012). Hara et al. (2012) also find that synapse density and perforated synapse density in the outer molecular layer are correlated with recognition memory.
3. Brainstem, cerebellum and spinal cord
There is relatively little published work on morphological changes in synapses during normal ageing in parts of the brain outside of the forebrain, or in the spinal cord, retina, cochlea, or in the nervous systems of invertebrates (sections 2–6.). Here we present the information that is available, to show how these cases may be similar to or different from that described in the previous sections on the forebrain. Because of its sporadic nature, it would be difficult to make many generalizations or draw broad conclusions; but we have included comparative comments where a pattern is clear (e.g., see Fig. 2).
Ageing rats (24–33 months) show distinct evidence of degeneration of axons, dendrites and nerve terminals in the medial nucleus of the trapezoid body, which is an auditory nucleus that receives unusual giant terminals, the calyces of Held, on principal cell somas; the axons of these terminals originate from the contralateral ventral cochlear nucleus (Casey and Feldman, 1985; 1988). Signs of degeneration in the axons include membranous debris and large filamentous structures. The calyx becomes more electron dense and contains unusual vacuoles and multivesicular bodies, and extracellular spaces between the calyx and the postsynaptic soma become prevalent. The surface area of the postsynaptic soma covered by synapses decreases from ~62% at 3 months to ~44% at 27– 33 months, and this loss is due to a decrease in the number of terminals derived from the calyx; other kinds of terminals are not lost, nor does the synapse length change for any kind of terminal including the calycine ones.
In the cerebellum, Purkinje cells show similar age-related changes in rats (~26 months; Rogers et al., 1984; Chen and Hillman, 1999) and monkeys (20 years; Nandy, 1981); synapses on Purkinje cell dendrites decrease in number and lipofuscin bodies become more prominent. Also, in the rat, small and intermediate-size dendrites show abundant vacuolar profiles and membrane swirls, and overall synaptic density in the molecular layer decreases by ~17%. However, there is a 10% increase in synaptic contact length and a 9% increase in spine head area, as well as a 66% increase in synaptic terminal volume, due largely to an increase in the number of vesicles. The authors, Chen and Hillman (1999) suggest that these increases are compensation for the losses in synapse number with ageing. Alternatively, this represents an increase in large spines and reduction of smaller and more plastic ones, as discussed for the forebrain in the section above.
The impact of ageing on synapses of the spinal cord has been infrequently studied. In both the rat (monoaminergic innervation − 3 versus 24 months; Ranson et al., 2003) and chicken (serotonin-mediated innervation – up to 2 years; Chen et al., 1997), innervation decreases with age in the ventral horn, yet it is retained or even increases during ageing in other areas, such as for 5-HT positive fibers in the dorsal horn of the chicken. Comparison of axosomatic and axodendritic synaptic density from hatching to 2 years in lamina I of the dorsal horn and lamina IX of the ventral horn shows opposite changes in synaptic density, with an increase in I and a decrease in IX (e.g., for the latter, axodendritic density changes from ~24 to ~13 synapses per 200 square micrometers).
4. Retina
Ageing mice (3–5 months versus 24–28 months) show a variety of changes in neuron structure and synaptic connections in the retina, with some types of neurons showing more changes than others (Samuel et al., 2011). There is a 17% decrease in synaptic density in the inner plexiform layer, based on immunolabeling for the presynaptic protein, Bassoon; further examination with other markers indicates that the decrease involves several kinds of synapses (Samuel et al., 2011). Also, there is a decrease in the dendritic field areas of the retinal ganglion cells, although these dendritic arbors maintain their distribution in the inner plexiform layer. These ganglion cells maintain the proper distribution of their axon terminals in the superior colliculus in the brain, but there is a 30% decrease in axon terminal area, as well as a decrease in axon density in this nucleus (Samuel et al., 2011). In ageing humans (up to 90 years), there is a 30% decrease in the number of rods in the central retina, but interestingly, rod inner segments increase in size by 13.5%, compensating for the loss in rod numbers (Curcio et al., 1993). In the ageing mouse retina, rod bipolar cell dendrites and horizontal cell arborizations sprout new neuronal processes that make aberrant, ectopic extensions into the outer nuclear layer; normally cell processes from these neurons would be limited to the outer plexiform layer where they form synapses with terminals from photoreceptor neurons (Liets et al., 2006; Terzibasi et al., 2009; Samuel et al., 2011). Light and electron microscope immunocytochemistry indicate that these processes can form ribbon synapses with rod cells and have postsynaptic mGluR6 (metabotropic glutamate receptor). Similar ectopic extensions are seen in the ageing human retina (68–77 years) and indicate that some retinal neuronal plasticity is retained in old age (Eliasieh et al., 2007).
5. Cochlea
Studies on the ageing cochlea have centered on the C57BL/6 mouse that shows significant hearing deficits within the first year; this mouse has been used as a model of the typical hearing loss seen in most elderly people. Mammals have two kinds of auditory hair cells, inner and outer (IHC and OHC), that synapse on afferent terminals originating from spiral ganglion neurons, which send their axons to the brain. IHCs directly transduce sound into nerve impulses at their afferent synapse, while OHCs have a modulatory effect. In addition, efferent axon terminals from certain brain neurons innervate OHCs directly and IHCs indirectly via the afferents. In less than 12 months, there is a profound loss of OHCs in these mice, while IHC cell density remains stable (Stamataki et al., 2006). Nevertheless, IHCs in older mice have fewer afferent synapses than seen in younger animals (Stamataki et al., 2006; Lauer et al., 2012). The remaining afferent terminals are enlarged, with a larger volume of mitochondria, and enlarged postsynaptic densities as well as enlarged presynaptic bodies (Stamataki et al., 2006). Changes of efferent terminals in older mice occur for both OHCs and IHCs. There is a prominent decrease in efferent synaptic terminals to OHCs and this decrease is not directly correlated with OHC cell loss such that efferent synapses may occur without OHCs and OHCs may be present without the efferents (this study used a mutant mouse that expresses yellow fluorescent protein in these efferents; Fu et al., 2010). There is also increased direct efferent innervation of IHCs in older mice; these efferent terminal contacts with the IHC soma show evidence of being functional efferent synapses, including the presence of presynaptic vesicles and postsynaptic subsurface cisternae (Lauer et al., 2012). A general decrease in efferent function at both OHCs and IHCs is indicated by a progressive decrease in the synaptic vesicle protein, synaptophysin, at efferent terminals on these hair cells (Bartolome et al., 2009). Immunolabeling for synaptophysin is completely absent from OHCs of the basal turn of the cochlea by 21 months of age and from the apical turn by 24 months; a similar progression is seen for IHCs but low levels of labeling remain in the apical turn in 24 month-old mice.
6. Invertebrate Nervous Systems
Little is known about ageing in nervous systems of lower metazoan groups. Hydra (Hydra vulgaris) does not seem to age and it maintains normal reproductive rates throughout its life, suggesting that it is potentially immortal (Martinez, 1998). Terman and Brunk (2005) note that: “Although hydras can live indefinitely, their neurons are short-lived and continuously replaced by new nerve cells originating from differentiating stem cells.” Thus, they conclude that hydras probably cannot store information for extended time periods; this may explain why their behavior is limited primarily to non-associative learning, including habituation and sensitization. The hypothesis then is that ageing in the nervous system is an unavoidable consequence of evolving more long-lived neurons and their connections to facilitate the more complex behavior patterns (i.e., requiring long-term memory) found in “higher” animals (Terman and Brunk, 2005); but this may not be the whole answer since some more complex animals live long with little ageing, as discussed later in this section and sections 7 and 9. Many basal metazoans also form clonal colonies of interconnected organisms such as corals that may show signs of ageing and mortality in portions of the colony, while the colony as a whole survives (Irikawa et al., 2011). Flatworms, such as planarians, possess large numbers of totipotent stem cells and can rejuvenate their bodies in response to injury or following fission for asexual reproduction. If the head is removed, newly generated neurons form a new brain in a few days and grow axons and dendrites that form connections with cells in the rest of the body (Nishimura et al., 2007). At least some flatworms go through alternating phases of de-growth and growth, and actual metabolic ageing may be absent (Mouton et al., 2011). However, other flatworms have predictable signs of ageing; Macrostomum lignano reaches old age in less than a year on average, showing increased deformities and the growth of cysts that cause reduction of mesodermal structures including nervous tissue (Crucke, 2010).
The nematode, Caenorhabditis elegans, lives only about 3 weeks and shows distinct signs of ageing in the nervous system (Toth et al., 2012), although neurons remain alive and nerve cords remain intact (Herndon et al., 2002). Touch receptor neurons show new processes growing from the somas, and new branches off of the main sensory dendrite (Toth et al., 2012). In addition, neuronal processes often have a wavy appearance (as opposed to being straight in the young ones), and may also have a beaded appearance. Mitochondria change their distribution with age and include locations in many of the beadings and at branch sites. Also, the major synaptic neuropils, found in the nerve ring and ventral ganglion, show reductions in synaptic terminal size and depletion of synaptic vesicles, as well as reduced presynaptic density size. Interestingly, these reductions are more prominent in “decrepit,” nearly paralyzed individuals compared to those that maintain locomotory vigor in their “old age.” Similarly, 35-day old animals of a related species, C. briggsae that start to lose their ability to move (i.e., just prior to death) show profound breakdown of nerve tissue, including the accumulation of large, membranous structures (Epstein et al., 1972). In another study of C. elegans, mutations of a regulator of the early endosomal Rab protein, RAB-5 affects neuronal endosomal structures and synaptic vesicle accumulation and causes the animals to age faster (Sann et al., 2012).
In higher invertebrates, old age typically is accompanied by a reduction of neurons and/or neuronal processes. The polychaete worm, Ophryotrocha labronica, shows a reduction in neuron number and partial substitution of the brain region with connective tissue in old age (3 months; Franchini and Ottaviani, 2007). The hermaphroditic pond snail Lymnaea stagnalis can live almost 2 years in captivity but egg-laying declines in less than a year and so these animals apparently live for several months after female reproduction mostly ends (Janse et al., 1996). Egg laying is controlled by peptidergic neuroendocrine caudodorsal cells in the cerebral ganglia that are electrically coupled and also receive chemical synapses on spines on branches of the axon; the neuropeptides are released via nonsynaptic endings in the nervous and neurohemal tissue. In ageing animals, axon branches are reduced and some are lost completely, and this loss is correlated with reduced egg laying (Janse et al., 1996, 1999). In addition, axon terminals appear to be swollen and extend into the perineurium and are strongly immunoreactive for neuropeptides, suggesting that there is impaired neuropeptide release in these old animals; preliminary ultrastructural studies indicate that secretory vesicles in these may fuse into irregular masses (Janse et al., 1996). Similarly, in the ageing (30 days) fruit fly, Drosophila melanogaster, the numbers of side branches and varicosities in the axon of a mechanosensory neuron are reduced compared to young flies (Corfas and Dudai, 1991). Curiously, there are more side branches and varicosities in these axons in mutants with impaired memory (impairment of learning and the cAMP cascade). Ageing female crickets (Teleogryllus oceanicus) lose the ascending axon of the omega auditory interneuron; 75% of crickets less than 2 weeks old have ascending axons while only 25–35% of crickets over 1 month old have them (Atkins and Pollack, 1986). Strangely, this axon is lost while auditory behavior remains active, so the consequence of its loss is not clear.
Age-related changes in spine synapse morphology on the dendrites of spiny Kenyon cells in the brains of worker honeybees (Coss et al., 1980) are somewhat reminiscent of changes seen in spines in the ageing vertebrate forebrain. As noted above, the ageing forebrain, especially the frontal cortex, can show a loss of thin spines in favor of short, thick, mushroom-like ones. Similarly, ageing honeybees have larger spines with shorter necks (although not mushroom-shaped; Fig. 2). This shape change is correlated with the change in worker duties, from nursing when they are young, to foraging when they are old.
7. Neuromuscular Junctions
Neuromuscular junctions (NMJs) are a special class of synapse between a presynaptic neuron terminal and a postsynaptic muscle fiber, and thus they bridge a connection between two different tissues/organs. The best-known examples, found in vertebrates and many invertebrates, involve the projection of a long axon from a motor neuron of the central nervous system (CNS) that extends to the surface of a muscle fiber, where it forms the synaptic junction. However, in other kinds of invertebrates, such as nematodes (e.g., C. elegans), the postsynaptic muscle fiber extends a long process (or arm) to the CNS where the NMJ is formed. Acetylcholine is the neurotransmitter deployed at vertebrate and nematode NMJs, while glutamate is used at NMJs of some invertebrates such as Drosophila.
There is considerable variation in findings in the abundant literature on ageing NMJs in vertebrates (reviewed by Larsson and Ansved, 1995), and this may be due partly to the effects of diet and exercise on the condition of the NMJs and neuromuscular function in old animals (Fahim, 1997; Vandervoort, 2002; Valdez et al., 2010; Nishimune et al., 2012 (Fig. 3)). For example, a typical old laboratory rat will tend to be fat and sedentary and will show more profound negative changes in neuromuscular function with old age, as compared with other rat and mouse strains (Banker et al., 1983). Thus, the CBF-1 mouse remains physically active and healthy in its old age (2–3 years), and shows relatively few changes in neuromuscular function in a variety of muscles examined in the study (Banker et al., 1983). Nevertheless, ultrastructural analyses carried out on one of these muscles (30 and 34 months) show prominent decreases in synaptic vesicle number, nerve terminal area and areas of postsynaptic folds lacking nerve terminals. Thus, the authors concluded that “these profound structural changes are either not functionally significant or are well compensated”. The degree of change of NMJs in ageing may depend in some cases on the kind of muscles and muscle fibers innervated. Thus, Prakash and Sieck (1998) find that, in rat diaphragm muscle, type IIx and IIb fibers show increase in nerve terminal and end-plate areas and numbers, suggesting that individual NMJs may fragment during ageing; in contrast, type I and IIa fibers show little change. Ageing laryngeal muscle NMJs in rats resemble denervated ones, with reduced terminal area, and areas of acetylcholine receptor labeling that lack the corresponding nerve terminals (Connor et al., 2002). Similarly, Valdez et al. (2010) find significant structural alterations in NMJs in C57BL/6 and CD-1 (and a Thy1-YFP transgenic line) mice, evident at 18 months and profound by 24 months. These include axonal swelling and sproutings and synaptic detachments; acetylcholine receptor (AChR) sites are sometimes partially denervated and often fragmented into small islands. In another study, similar changes as these are induced in younger mutant mice (6–8 months) with reduced expression of trkB, a receptor for brain-derived neurotrophic factor (BDNF) that is coexpressed with AChR at the NMJ (Kulakowski et al., 2011), suggesting that reduced BDNF signaling may be important in the ageing process of neuromuscular structures. Changes with ageing also are evident in presynaptic active zone proteins such as Bassoon; the latter shows decreases both in puncta density and signal intensity in NMJs of 27 month-old mice (Chen et al., 2012).
Figure 3.
The NMJ of old rats (2 years) shows profound changes in structure compared to young rats (56 days), and these can be ameliorated by exercise (2 months). Note the increase in NMJ size as indicated by the markers for the nerve terminal (NF+SV2=neurofilament+synaptic vesicle protein SV2) and the acetylcholine receptors (AChR) of the postsynaptic membrane. The severe loss of the presynaptic active zone protein, Bassoon, with ageing is rescued with exercise. Scale bar is 10 micrometers. This is reprinted from figure 3A in Nishimune et al. (2012); see this paper for more details.
Ageing neuromuscular function in invertebrates has been studied in nematodes and Drosophila, which can be ideal model systems for studying ageing since these animals have short life spans and age quickly, thus allowing ageing experiments to be completed in a short time (Augustin and Partridge, 2009). Ageing C. elegans develop sarcopenia, i.e., a progressive loss of muscle mass and function, similar to humans (Herndon et al., 2002). Ageing also affects function at the NMJ. Thus, animals 6–10 days old (mid-life and early late-life) show locomotory deficits that improve significantly after treatment with the muscarinic agonist, arecoline; this agonist affects presynaptic muscarinic acetylcholine receptors to stimulate acetylcholine release, which activates postsynaptic nicotinic acetylcholine receptors (Glenn et al., 2004). However, specific changes with ageing at the NMJ are unknown; structural changes at the NMJ have not been examined, and a GABA receptor found at NMJs of C. elegans shows relatively constant labeling patterns throughout life (Herndon et al., 2002). At least one study looked at NMJs during normal ageing in Drosophila (Beramendi et al., 2007). As for C. elegans and humans, ageing muscles show signs of degeneration, including mitochondrial malformations, prevalent autophagosomes, and sarcomeric structural irregularities (Beramendi et al., 2007; also reviewed in Augustin and Partridge, 2009). NMJs in ageing flies are shorter and thinner overall than those of younger flies, but individual boutons are larger and have some larger vesicles (i.e., more variable vesicle size) and more mitochondria and vacuoles; moreover, parts of the subsynaptic reticulum of the postsynaptic muscle cell may be missing, leaving the presynaptic membrane “naked” in places, and thus directly apposed to the extracellular matrix (Beramendi et al., 2007).
While nematodes and Drosophila can make good invertebrate models for understanding human neuromuscular ageing because of their short lifespan, they may be not so useful in some ways because they avoid some problems that are only a consequence of a long lifespan. For example, they lack cells that could function like mammalian satellite cells, which are important for muscle regeneration and rejuvenation (Augustin and Partridge, 2009); these capacities may be less important to an animal that lives only a couple of weeks (C. elegans) or months (Drosophila). So considering this, it might be useful to study NMJs in an invertebrate animal that lives as long as humans, 50–100 years, such as the lobster, Homarus americanus (Pearce et al., 1985; Govind, 1992). However, unlike humans, lobsters continue to grow throughout life, reaching sexual maturity when they weigh less than 500 g, and obtaining weights of 10 kg or more by their 50th birthday, yet still showing few signs of conventional ageing. Pearce et al. (1985) compared the multi-terminal innervation of a single excitor axon onto a particular muscle in 0.5 and 5 kg animals (~20 years difference). During this time, nerve terminals increase 2x in number and almost 4x in length, while the numbers of synapses and presynaptic dense bars (indicating active zones) increase about 4x. Also, as the number of dense bars increase, there is an increase in the number and size of perforations (spaces separating the pre- and postsynaptic membranes of the terminals) near these dense bars; presumably these perforations represent areas of synaptic membrane recycling. Thus, lobsters appear to grow and maintain functional NMJs concomitant with overall body growth, living many decades with little sign of ageing.
Another invertebrate, the mollusk Aplysia californica, has a more modest lifespan of 12–14 months, but also like the lobster, shows little sign of ageing in the NMJs (~250 days old; Peretz et al., 1984). However, changes in NMJs with age vary, depending on the level of use of the associated muscles. Thus, the NMJ between the L7 motor neuron and medial pinnule longitudinal muscle has an increased contact length and decreased cleft width with age, correlated with reduced facilitation that results in decreased muscle contraction with age. This muscle is involved in the defensive gill withdrawal reflex, which is of less importance to an older, larger individual and so its use may dwindle with age. In contrast, the NMJ between the LDG1 motor neuron and the efferent vessel longitudinal muscle does not show consistent changes in contact length or cleft width with age. Contraction rates of this muscle remain about the same throughout life, probably because it controls the crucial respiratory movements of the gill.
Thus, for both vertebrates and invertebrates, muscle usage may be better correlated with NMJ structural and functional state than ageing alone.
8. Dietary restriction and synapse structure
Dietary energy restriction can extend the lifespan of a wide range of animals including worms, flies and mammals (Mercken et al., 2012). Conversely, excessive energy intake promotes age-related diseases that are the major causes of mortality in mammals including diabetes, cancers, cardiovascular disease and stroke (Pi-Sunyer, 2009). Dietary energy restriction can counteract the adverse effects of ageing on synaptic plasticity and associated functions in multiple regions of the nervous system, and can ameliorate disease-related neurodegenerative processes and symptoms in animal models of Alzheimer’s, Parkinson’s and Huntington’s diseases (Bruce-Keller et al., 1999; Duan et al., 2003; Maswood et al., 2004; Halagappa et al., 2007; and see Mattson, 2012 for review). Although limited, the available data suggest that energy restriction can prevent, while obesity and diabetes exacerbate, age-related alterations in synaptic structure (Box 1).
Box 1. Energy intake, ageing and synapses.
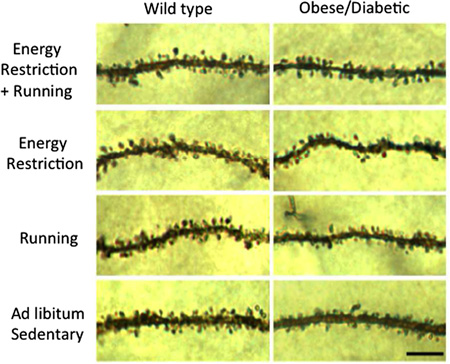
In rats, long-term caloric restriction results in the maintenance of numbers of synapses and size of synapses during ageing compared to control rats fed ad libitum (Bertoni-Freddari et al., 1999). In mice, dietary energy restrictions increase the density of postsynaptic spines on dendrites of hippocampal dentate granule neurons, whereas diabetes reduces dendritic spine density (Stranahan et al., 2009; and see Box 1 Figure). Dendritic spine density and total number of axospinous synapses on dentate gyrus-granule neuron dendrites increase significantly in rats subjected to daily caloric restriction for 36 weeks (Andrade et al., 2002). Rats susceptible to diet-induced obesity exhibit greater numbers of inhibitory synapses on arcuate nucleus proopiomelanocortin (POMC) neurons compared with rats resistant to diet-induced obesity (Horvath et al., 2010). When fed a high-fat diet, obesity prone rats exhibit a loss of synapses on POMC neurons and associated gliosis, whereas resistant rats do not.
Box 1 Figure Caption. Dietary energy restriction and running increase synaptic spine density on hippocampal dentate gyrus-granule neuron dendrites, and attenuate obesity/diabetes-induced synaptic spine loss. Modified from Stranahan et al., 2009.
9. Summary and conclusions
It does appear that some animals have discovered the fountain of youth. As discussed here, some simple animals have large numbers of totipotent stem cells and may renew themselves, including their nervous system, continuously and without experiencing the deterioration of old age. But some more complex animals, including both invertebrate and vertebrate (Finch, 2009) species, can live long with little signs of ageing. Thus, the neuromuscular junctions of lobsters retain their normal structure for many years – equal to human lifetimes but maybe without the concomitant ageing effects. In fact, some marine clams can show negligible ageing for lifetimes of over 500 years, possibly due to their “general resistance to multiplex stressors” (Ungvari et al., 2013). On the other hand, other creatures have neurons and synapses that develop the deterioration of old age in days (C. elegans) or weeks (Drosophila) or months (mice and rats) and make excellent models for studying human ageing within a reasonable experimental study period (Augustin and Partridge, 2009; Wilson et al., 2013). There are general trends for ageing neurons and synapses that are seen in both vertebrate and invertebrate types (Morrison and Baxter, 2012; Peters and Kemper 2012; Yeoman et al., 2012; this review). Typically, there is a reduction in neuronal arborization, spine density and connections. But changes can vary with different areas of the nervous system; examples that we discussed include the loss of more thin spines in the prefrontal cortex (= decrease in plasticity) versus the loss of mushroom spines in the hippocampus (=decrease in established memories and learning circuits); another example is in the mollusk nervous system, where synapses in some circuits show degenerative changes and others do not, probably dependent on functional needs that extend into old age (this review and Yeoman et al., 2012). And in some cases, such as in the vertebrate retina and cochlea, new connections form in old age, at least in some cases due to compensation for losses in neurons or connections.
In this review, we have concentrated on the structure of the normal ageing synapse, mainly to limit the size of the review. We have ignored a large body of literature on structural and functional changes that occur with those diseases that are associated with old age, such as Alzheimer’s, Parkinson’s, ALS, hearing impairment, etc. There are other excellent reviews on these topics (Stranahan and Mattson, 2010; Sheng et al., 2012; Picconi et al., 2012; Adalbert and Coleman, 2013; Moser et al., 2013; Sery et al., 2013) including those that concentrate on invertebrate models (White et al., 2010; Ewald and Li, 2012; Jaiswal et al., 2012). There also are many studies that concentrate on the positive effects on ageing synapses, of environmental factors such as diet, as discussed in section 8 (Mattson et al., 2003; Mattson, 2012; Mercken et al., 2012), and exercise (see our discussion on the neuromuscular junction), and general environmental enrichment (Mora et al., 2007; Mora, 2013).
Studies of structural changes of synapses will continue to be a major part of research on ageing. Since normal synapses form the basis for learning, memory and actions, so too their loss or deterioration are central to understanding the ageing brain.
Highlights.
Ageing brains show reductions in neuronal dendritic arborization, and spine and synapse densities.
In some brain regions new growth and synaptic remodeling may compensate for lost synapses.
Ageing synapses in some organisms or regions of the nervous system exhibit no discernible structural changes during ageing.
The NMJs of old mice exhibit multiple alterations compared to NMJs of young mice including swollen axons, detachment of synapses and fragmentation of postsynaptic specializations; caloric restriction initiated in early adulthood attenuates age-related pre-and post-synaptic alterations (Valdez et al., 2010). Excessive energy intake and diabetes may accelerate age-related alterations of NMJs. Thus, in a mouse model of type 1 diabetes, motor neuron axon terminals exhibit a reduction in synaptic vesicles and have morphologically abnormal mitochondria, and mitochondrial and sarcoplasmic reticulum abnormalities are evident in muscle cells (Fahim et al., 2000). In a mouse model of type 2 diabetes caused by hyperphagia, axons innervating the extraocular muscles exhibit inclusion bodies, and postsynaptic muscle cells exhibit hypertrophied endplates and pseudopod-like extensions (Pachter, 1986).
Studies of the effects of energy restriction and excess on synaptic plasticity in animal models of normal ageing and age-related neurodegenerative disorders have elucidated possible molecular cellular mechanisms by which synapses respond to ageing. During ageing there is an accumulation of oxidatively modified proteins, lipids and nucleic acids in brain cells in general, and synaptic terminals, in particular (Martinez et al., 1996; Mattson and Magnus, 2006). In addition, impaired neuronal bioenergetics and inflammation-like processes involving glial and immune cells occur during normal brain ageing and are exacerbated in neurodegenerative disorders such as Alzheimer’s disease (Kapogiannis and Mattson, 2011; Lynch, 2010; Rao et al., 2012). Oxidative stress, metabolic compromise and neuroinflammation may render synapses vulnerable to degeneration by promoting excessive calcium influx and accumulation in synapses (Foster, 2007; Mattson, 2007). A general mechanism whereby dietary energy restriction can mitigate age-related structural and functional deficits in synapses is by triggering adaptive cellular stress responses including the up-regulation of neurotrophic factor signaling pathways, protein chaperones and antioxidant enzymes (Arumugam et al., 2010; Stranahan and Mattson, 2012). Energy restriction can also suppress local inflammatory processes in multiple brain regions (Arumugam et al., 2010). On the other hand, excessive energy intake, obesity and diabetes may adversely affect synapses by inhibiting the production of neurotrophic factors and disabling cellular defenses against oxidative and metabolic stress (Stranahan and Mattson, 2012).
Acknowledgements
This work was supported by the Intramural Research Programs of the NIA/NIH and NIDCD/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adalbert R, Coleman MP. Axon pathology in age-related neurodegenerative disorders. Neuropathol. Appl. Neurobiol. 2012 Oct 10; doi: 10.1111/j.1365-2990.2012.01308.x. 2012. [DOI] [PubMed] [Google Scholar]
- Adams I, Jones DG. Quantitative ultrastructural changes in rat cortical synapses during early-, mid- and late-adulthood. Brain Res. 1982;239:349–363. doi: 10.1016/0006-8993(82)90514-5. [DOI] [PubMed] [Google Scholar]
- Andrade JP, Lukoyanov NV, Paula-Barbosa MM. Chronic food restriction is associated with subtle dendritic alterations in granule cells of the rat hippocampal formation. Hippocampus. 2002;12:149–164. doi: 10.1002/hipo.1102. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Ageand energy intake interact to modify cell stress pathways and stroke outcome. Ann.Neurol. 2010;67:41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate MD, Landfield PW. Synaptic vesicle redistribution during hippocampal frequency potentiation and depression in young and aged rats. J Neurosci. 1988;8:1096–1111. doi: 10.1523/JNEUROSCI.08-04-01096.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins G, Pollack GS. Age-dependent occurrence of an ascending axon on the omega neuron of the cricket, Teleogryllus oceanicus . J. Comp Neurol. 1986;243:527–534. doi: 10.1002/cne.902430407. [DOI] [PubMed] [Google Scholar]
- Augustin H, Partridge L. Invertebrate models of age-related muscle degeneration. Biochem. Biophys. Acta. 2009;1790:1084–1094. doi: 10.1016/j.bbagen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Banker BQ, Kelly SS, Robbins N. Neuromuscular transmission and correlative morphology in young and old mice. J. Physiol. 1983;339:355–377. doi: 10.1113/jphysiol.1983.sp014721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolome MV, Zuluaga P, Carricondo F, Gil-Loyzaga P. Immunocytochemical detection of synaptophysin in C57BL/6 mice cochlea during aging process. Brain Res Rev. 2009;60:341–348. doi: 10.1016/j.brainresrev.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Beramendi A, Peron S, Casanova G, Reggiani C, Cantera R. Neuromuscular junction in abdominal muscles of Drosophila melanogaster during adulthood and aging. J. Comp. Neurol. 2007;501:498–508. doi: 10.1002/cne.21253. [DOI] [PubMed] [Google Scholar]
- Bertoni-Freddari C, Fattoretti P, Caselli U, Casoli T, Di Stefano G, Algeri S. Dietary restriction modulates synaptic structural dynamics in the ageing hippocampus. Age (Omaha) 1999;22:107–113. doi: 10.1007/s11357-999-0013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoni-Freddari C, Fattoretti P, Giorgetti B, Grossi Y, Balietti M, Casoli T, Di Stefano G, Perretta G. Preservation of mitochondrial volume homeostasis at the early stages of age-related synaptic deterioration. Ann. NY Acad. Sci. 2007;1096:138–146. doi: 10.1196/annals.1397.079. [DOI] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, Ohm DT, Yuk FJ, Wadsworth S, Saardi KM, McEwen BS, Morrison JH. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. J. Neurosci. 2011;31:7831–7839. doi: 10.1523/JNEUROSCI.0839-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondareff W, Geinisman Y. Loss of synapses in the dentate gyrus of the senescent rat. Amer. J. Anat. 1976;145:129–136. doi: 10.1002/aja.1001450110. [DOI] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu. Rev. Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- Canas PM, Duarte JM, Rodrigues RJ, Kofalvi A, Cunha RA. Modification upon aging of the density of presynaptic modulation systems in the hippocampus. Neurobiol. Aging. 2009;30:1877–1884. doi: 10.1016/j.neurobiolaging.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Casey MA, Feldman ML. Aging in the rat medial nucleus of the trapezoid body. II. Electron microscopy. J Comp Neurol. 1985;232:401–413. doi: 10.1002/cne.902320311. [DOI] [PubMed] [Google Scholar]
- Casey MA, Feldman ML. Age-related loss of synaptic terminals in the rat medial nucleus of the trapezoid body. Neuroscience. 1988;24:189–194. doi: 10.1016/0306-4522(88)90322-3. [DOI] [PubMed] [Google Scholar]
- Chen J, Mizushige T, Nishimune H. Active zone density is conserved during synaptic growth but impaired in aged mice. J. Comp. Neurol. 2012;520:434–452. doi: 10.1002/cne.22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Hamaguchi K, Hamada S, Okado N. Regional differences of serotonin-mediated synaptic plasticity in the chicken spinal cord with development and aging. J Neural Transpl. Plast. 1997;6:41–48. doi: 10.1155/NP.1997.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Hillman DE. Dying-back of Purkinje cell dendrites with synapse loss in aging rats. J. Neurocytol. 1999;28:187–196. doi: 10.1023/a:1007015721754. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sabatini BL. Signaling in dendritic spines and spine microdomains. Curr. Opin. Neurobiol. 2012;22:389–396. doi: 10.1016/j.conb.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor NP, Suzuki T, Lee K, Sewall GK, Heisey DM. Neuromuscular junction changes in aged rat thyroarytenoid muscle. Annals Otol. Rhinol. Laryngol. 2002;111:579–586. doi: 10.1177/000348940211100703. [DOI] [PubMed] [Google Scholar]
- Corfas G, Dudai Y. Morphology of a sensory neuron in Drosophila is abnormal in memory mutants and changes during aging. Proc. Natl. Acad. Sci. USA. 1991;88:7252–7256. doi: 10.1073/pnas.88.16.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss RG, Brandon JG, Globus A. Changes in morphology of dendritic spines on honeybee calycal interneurons associated with cumulative nursing and foraging experiences. Brain Res. 1980;192:49–59. doi: 10.1016/0006-8993(80)91007-0. [DOI] [PubMed] [Google Scholar]
- Crucke J. Characterization of the Macrostomum lignano ageing phenotype. Thesis, Universiteit Gent. 2010:1–65. [Google Scholar]
- Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Investig Opthamol Visual Sci. 1993;34:3278–3296. [PubMed] [Google Scholar]
- Drew LJ, Fusi S, Hen R. Adult neurogenesis in the mammalian hippocampus: Why the dentate gyrus? Learn. Mem. 2013;20:710–729. doi: 10.1101/lm.026542.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc. Natl. Acad. Sci. USA. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J. Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasieh K, Liets LC, Chalupa LM. Cellular reorganization in the human retina during normal aging. Investig Opthamol Vis. Sci. 2007;48:2824–2830. doi: 10.1167/iovs.06-1228. [DOI] [PubMed] [Google Scholar]
- Epstein J, Himmelhoch S, Gershon D. Studies of ageing in nematodes III. Electronmicroscopical studies on age-associated cellular damage. Mech. Ag. Dev. 1972;1:245–255. [Google Scholar]
- Ewald CY, Li C. Caenorhabditis elegans as a model organism to study APP function. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2012;217:397–411. doi: 10.1007/s00221-011-2905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim MA. Endurance exercise modulates neuromuscular junction of C57BL/6NNia aging mice. J. Appl. Physiol. 1997;83:59–66. doi: 10.1152/jappl.1997.83.1.59. [DOI] [PubMed] [Google Scholar]
- Fahim MA, Hasan MY, Alshuaib WB. Early morphological remodeling of neuromuscular junction in a murine model of diabetes. J. Appl. Physiol. 2000;89:2235–2240. doi: 10.1152/jappl.2000.89.6.2235. [DOI] [PubMed] [Google Scholar]
- Faulkner RL, Jang M-H, Liu X-B, Duan X, Sailor KA, Kim JY, Ge S, Jones EG, Ming G, Song H, Cheng H-J. Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proc. Nat. Acad. Sci. 2008;105:14157–14162. doi: 10.1073/pnas.0806658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J. Neurosci. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE. Update on slow aging and negligible senescence--a mini-review. Gerontology. 2009;55:307–313. doi: 10.1159/000215589. [DOI] [PubMed] [Google Scholar]
- Foster M, Sherrington CS. A Textbook of Physiology, Part 3. New York: MacMillan; 1897. [Google Scholar]
- Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell. 2007;6:319–325. doi: 10.1111/j.1474-9726.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- Franchini A, Ottaviani E. IL-6 immunoreactivity changes during aging in the polychaete Ophryotrocha labronica (Polychaeta: Dorvilleidae) Tis. Cell. 2007;39:27–34. doi: 10.1016/j.tice.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Fu B, Le Prell C, Simmons D, Lei D, Schrader A, Chen AB, Bao J. Age-related synaptic loss of the medial olivocochlear efferent innervation. Mol. Neurodegen. 2010;5:53. doi: 10.1186/1750-1326-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Differences in the expression of AMPA and NMDA receptors between axospinous perforated and nonperforated synapses are related to the configuration and size of postsynaptic densities. J. Comp. Neurol. 2004a;468:86–95. doi: 10.1002/cne.10950. [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Synapses with a segmented, completely partitioned postsynaptic density express more AMPA receptors than other axospinous synaptic junctions. Neuroscience. 2004b;125:615–623. doi: 10.1016/j.neuroscience.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Geinisman Y. Structural synaptic modifications associated with hippocampal LTP and behavioral learning. Cerebral Cortex. 2000;10:952–962. doi: 10.1093/cercor/10.10.952. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F. Loss of perforated synapses in the dentate gyrus: morphological substrate of memory deficit in aged rats. Proc. Natl. Acad. Sci. USA. 1986;83:3027–3031. doi: 10.1073/pnas.83.9.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F. Loss of perforated synapses in the dentate gyrus: morphological substrate of memory deficit in aged rats. Proc. Natl. Acad. Sci. USA. 1986;83:3027–3031. doi: 10.1073/pnas.83.9.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Glenn CF, Chow DK, David L, Cooke CA, Gami MS, Iser WB, Hanselman KB, Goldberg IG, Wolkow CA. Behavioral deficits during early stages of aging in Caenorhabditis elegans result from locomotory deficits possibly linked to muscle frailty. J. Gerontol. 2004;59:1251–1260. doi: 10.1093/gerona/59.12.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind CK. Age-related remodeling of lobster neuromuscular terminals. Exp. Gerontol. 1992;27:63–74. doi: 10.1016/0531-5565(92)90029-y. [DOI] [PubMed] [Google Scholar]
- Grillo FW, Song S, Teles-Grilo Ruivo LM, Huang L, Gao G, Knott GW, Maco B, Ferretti V, Thompson D, Little GE, De Paola V. Increased axonal bouton dynamics in the aging mouse cortex. Proc. Natl. Acad. Sci. USA. 2013;110:E1514–E1523. doi: 10.1073/pnas.1218731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer's disease. Neurobiol. Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Hamilton LK, Joppé SE, Cochard LM, Fernandes KJL. Aging and neurogenesis in the adult forebrain: what we have learned and where we should go from here. Eur. J. Neurosci. 2013;37:1978–1986. doi: 10.1111/ejn.12207. [DOI] [PubMed] [Google Scholar]
- Hara Y, Morrison JH. Synaptic correlates of aging and cognitive decline. In: Pickel V, Segal M, editors. The Synapse: Structure and function. Elsevier; 2014. pp. 301–342. [Google Scholar]
- Hara Y, Park CS, Janssen WG, Punsoni M, Rapp PR, Morrison JH. Synaptic characteristics of dentate gyrus axonal boutons and their relationships with aging, menopause, and memory in female rhesus monkeys. J. Neurosci. 2011;31:7737–7744. doi: 10.1523/JNEUROSCI.0822-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Park CS, Janssen WG, Roberts MT, Morrison JH, Rapp PR. Synaptic correlates of memory and menopause in the hippocampal dentate gyrus in rhesus monkeys. Neurobiol. Aging. 2012;33(421):e417–e428. doi: 10.1016/j.neurobiolaging.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J. Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing. C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Hongpaisan J, Xu C, Sen A, Nelson TJ, Alkon DL. PKC activation during training restores mushroom spine synapses and memory in the aged rat. Neurobiol. Dis. 2013;55:44–62. doi: 10.1016/j.nbd.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Horak M, Seabold GK, Petralia RS. Trafficking of glutamate receptors and associated proteins in synaptic plasticity. In: Pickel V, Segal M, editors. In The Synapse: Structure and Function. Elsevier: 2014. pp. 221–279. [Google Scholar]
- Horvath TL, Sarman B, García-Cáceres C, Enriori PJ, Sotonyi P, Shanabrough M, Borok E, Argente J, Chowen JA, Perez-Tilve D, Pfluger PT, Brönneke HS, Levin BE, Diano S, Cowley MA, Tschöp MH. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc. Natl. Acad. Sci. U S A. 2010;107:14875–14880. doi: 10.1073/pnas.1004282107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irikawa A, Casareto BE, Suzuki Y, Agostini S, Hidaka M, van Woesik R. Growth anomalies on Acropora cytherea corals. Marine Pollut. Bull. 2011;62:1702–1707. doi: 10.1016/j.marpolbul.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Jaiswal M, Sandoval H, Zhang K, Bayat V, Bellen HJ. Probing mechanisms that underlie human neurodegenerative diseases in Drosophila. Ann Rev Genet. 2012;46:371–396. doi: 10.1146/annurev-genet-110711-155456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse C, Peretz B, van der Roest M, Dubelaar EJ. Excitability and branching of neuroendocrine cells during reproductive senescence. Neurobiol Aging. 1999;20:675–683. doi: 10.1016/s0197-4580(99)00021-4. [DOI] [PubMed] [Google Scholar]
- Janse C, van der Roest M, Jansen RF, Montagne-Wajer C, Boer HH. Atrophy and degeneration of peptidergic neurons and cessation of egg laying in the aging pond snail Lymnaea stagnalis. J. Neurobiol. 1996;29:202–212. doi: 10.1002/(SICI)1097-4695(199602)29:2<202::AID-NEU6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer's disease. Lancet Neurol. 2011;10:187–198. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Dimayuga E, Chen Q, Thorpe J, Gee J, Ding Q. Autophagy, proteasomes, lipofuscin, and oxidative stress in the aging brain. Int. J. Biochem. Cell Biol. 2004;36:2376–2391. doi: 10.1016/j.biocel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Kirov SA, Sorra KE, Harris KM. Slices have more synapses than perfusion-fixed hippocampus from both young and mature rats. J. Neurosci. 1999;19:2876–2886. doi: 10.1523/JNEUROSCI.19-08-02876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulakowski SA, Parker SD, Personius KE. Reduced TrkB expression results in precocious age-like changes in neuromuscular structure, neurotransmission, and muscle function. J. Appl. Physiol. 2011;111:844–852. doi: 10.1152/japplphysiol.00070.2011. [DOI] [PubMed] [Google Scholar]
- Larsson L, Ansved T. Effects of ageing on the motor unit. Prog. Neurobiol. 1995;45:397–458. doi: 10.1016/0301-0082(95)98601-z. [DOI] [PubMed] [Google Scholar]
- Lauer AM, Fuchs PA, Ryugo DK, Francis HW. Efferent synapses return to inner hair cells in the aging cochlea. Neurobiol. Aging. 2012;33:2892–2902. doi: 10.1016/j.neurobiolaging.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Kozorovitskiy Y, Gross CG, Gould E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc. Nat. Acad. Sci. 2007;104:17169–17173. doi: 10.1073/pnas.0708228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liets LC, Eliasieh K, van der List DA, Chalupa LM. Dendrites of rod bipolar cells sprout in normal aging retina. Proc. Natl. Acad. Sci. USA. 2006;103:12156–12160. doi: 10.1073/pnas.0605211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh Y, Mizrahi A. Experience-dependent plasticity of mature adult-born neurons. Nat. Neurosci. 2012;15:26–28. doi: 10.1038/nn.2980. [DOI] [PubMed] [Google Scholar]
- Luebke JI, Amatrudo JM. Age-related increase of sI(AHP) in prefrontal pyramidal cells of monkeys: relationship to cognition. Neurobiol. Aging. 2012;33:1085–1095. doi: 10.1016/j.neurobiolaging.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke JI, Chang YM. Effects of aging on the electrophysiological properties of layer 5 pyramidal cells in the monkey prefrontal cortex. Neuroscience. 2007;150:556–562. doi: 10.1016/j.neuroscience.2007.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. Age-related neuroinflammatory changes negatively impact on neuronal function. Front. Aging Neurosci. 2010 Jan;4:1–6. doi: 10.3389/neuro.24.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez DE. Mortality patterns suggest lack of senescence in hydra. Exp. Gerontol. 1998;33:217–225. doi: 10.1016/s0531-5565(97)00113-7. [DOI] [PubMed] [Google Scholar]
- Martinez M, Hernández AI, Martínez N, Ferrándiz ML. Age-related increase in oxidized proteins in mouse synaptic mitochondria. Brain Res. 1996;731:246–248. doi: 10.1016/0006-8993(96)00708-1. [DOI] [PubMed] [Google Scholar]
- Maswood N, Young J, Tilmont E, Zhang Z, Gash DM, Gerhardt GA, Grondin R, Roth GS, Mattison J, Lane MA, Carson RE, Cohen RM, Mouton PR, Quigley C, Mattson MP, Ingram DK. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson's disease. Proc. Natl. Acad. Sci. U S A. 2004;101:18171–18176. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Guo Z. Meal size and frequency affect neuronal plasticity and vulnerability to disease: cellular and molecular mechanisms. J. Neurochem. 2003;84:417–431. doi: 10.1046/j.1471-4159.2003.01586.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat. Rev. Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams JR, Lynch G. Synaptic density and axonal sprouting in rat hippocampus: stability in adulthood and decline in late adulthood. Brain Res. 1984;294:152–156. doi: 10.1016/0006-8993(84)91321-0. [DOI] [PubMed] [Google Scholar]
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res. Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirich JM, Williams NC, Berlau DJ, Brunjes PC. Comparative study of aging in the mouse olfactory bulb. J. Comp. Neurol. 2002;454:361–372. doi: 10.1002/cne.10426. [DOI] [PubMed] [Google Scholar]
- Mora F. Successful brain aging: plasticity, environmental enrichment, and lifestyle. Dialogues Clin. Neurosci. 2013;15:45–52. doi: 10.31887/DCNS.2013.15.1/fmora. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora F, Segovia G, del Arco A. Aging, plasticity and environmental enrichment: structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res. Rev. 2007;55:78–88. doi: 10.1016/j.brainresrev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat. Rev. Neurosci. 2012;13:240–250. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Predoehl F, Starr A. Review of Hair Cell Synapse Defects in Sensorineural Hearing Impairment. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2013 doi: 10.1097/MAO.0b013e3182814d4a. [DOI] [PubMed] [Google Scholar]
- Mostany R, Anstey JE, Crump KL, Maco B, Knott G, Portera-Cailliau C. Altered synaptic dynamics during normal brain aging. J. Neurosci. 2013;33:4094–4104. doi: 10.1523/JNEUROSCI.4825-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton S, Willems M, Houthoofd W, Bert W, Braeckman BP. Lack of metabolic ageing in the long-lived flatworm Schmidtea polychroa. Exp. Gerontol. 2011;46:755–761. doi: 10.1016/j.exger.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Nandy K. Morphological changes in the cerebellar cortex of aging Macaca nemestrina . Neurobiol. Aging. 1981;2:61–64. doi: 10.1016/0197-4580(81)90061-0. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Yoshida R, Berry RW, Gallagher M, Geinisman Y. Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments. J. Neurosci. 2004;24:7648–7653. doi: 10.1523/JNEUROSCI.1725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Kitamura Y, Inoue T, Umesono Y, Sano S, Yoshimoto K, Inden M, Takata K, Taniguchi T, Shimohama S, Agata K. Reconstruction of dopaminergic neural network and locomotion function in planarian regenerates. Dev. Neurobiol. 2007;67:1059–1078. doi: 10.1002/dneu.20377. [DOI] [PubMed] [Google Scholar]
- Nishimune H, Numata T, Chen J, Aoki Y, Wang Y, Starr MP, Mori Y, Stanford JA. Active zone protein Bassoon co-localizes with presynaptic calcium channel, modifies channel function, and recovers from aging related loss by exercise. PloS One. 2012;7:e38029. doi: 10.1371/journal.pone.0038029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachter BR. Structural alterations of the intramuscular nerves and junctional region in extraocular muscles of C57BL/Ks (db/db) diabetic mice. Acta Neuropathol. 1986;72:164–169. doi: 10.1007/BF00685979. [DOI] [PubMed] [Google Scholar]
- Pearce J, Govind CK, Meiss DE. Growth-related features of lobster neuromuscular terminals. Acta Neuropathol. 1985;353:215–228. doi: 10.1016/0165-3806(85)90210-x. [DOI] [PubMed] [Google Scholar]
- Peretz B, Romanenko A, Markesbery W. Functional history of two motor neurons and the morphometry of their neuromuscular junctions in the gill of Aplysia: evidence for differential aging. Proc. Natl. Acad. Sci. USA. 1984;81:4232–4236. doi: 10.1073/pnas.81.13.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Kemper T. A review of the structural alterations in the cerebral hemispheres of the aging rhesus monkey. Neurobiol. Aging. 2012;33:2357–2372. doi: 10.1016/j.neurobiolaging.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152:970–981. doi: 10.1016/j.neuroscience.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrak LJ, Harris KM, Kirov SA. Synaptogenesis on mature hippocampal dendrites occurs via filopodia and immature spines during blocked synaptic transmission. J. Comp. Neurol. 2005;484:183–190. doi: 10.1002/cne.20468. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Schwartz CM, Wang YX, Kawamoto EM, Mattson MP, Yao PJ. Sonic hedgehog promotes autophagy in hippocampal neurons. Biology open. 2013;2:499–504. doi: 10.1242/bio.20134275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picconi B, Piccoli G, Calabresi P. Synaptic dysfunction in Parkinson's disease. Adv. Exp. Med. Biol. 2012;970:553–572. doi: 10.1007/978-3-7091-0932-8_24. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer X. The medical risks of obesity. Postgrad. Med. 2009;121:21–33. doi: 10.3810/pgm.2009.11.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov VI, Davies HA, Rogachevsky VV, Patrushev IV, Errington ML, Gabbott PLA, Bliss TVP, Stewart MG. Remodelling of synaptic morphology but unchanged synaptic density during late phase long-term potentiation (LTP): A serial section electron micrograph study in the dentate gyrus in the anaesthetized rat. Neuroscience. 2004;128:251–262. doi: 10.1016/j.neuroscience.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Sieck GC. Age-related remodeling of neuromuscular junctions on type-identified diaphragm fibers. Muscle Nerve. 1998;21:887–895. doi: 10.1002/(sici)1097-4598(199807)21:7<887::aid-mus6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Ranson RN, Dodds AL, Smith MJ, Santer RM, Watson AH. Age-associated changes in the monoaminergic innervation of rat lumbosacral spinal cord. Brain Res. 2003;972:149–158. doi: 10.1016/s0006-8993(03)02521-6. [DOI] [PubMed] [Google Scholar]
- Rao JS, Kellom M, Kim HW, Rapoport SI, Reese EA. Neuroinflammation and synaptic loss. Neurochem. Res. 2012;37:903–910. doi: 10.1007/s11064-012-0708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc. Nat. Acad. Sci. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard MB, Taylor SR, Greer CA. Age-induced disruption of selective olfactory bulb synaptic circuits. Proc. Nat. Acad. Sci. 2010;107:15613–15618. doi: 10.1073/pnas.1007931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Zornetzer SF, Bloom FE, Mervis RE. Senescent microstructural changes in rat cerebellum. Brain Res. 1984;292:23–32. doi: 10.1016/0006-8993(84)90886-2. [DOI] [PubMed] [Google Scholar]
- Samuel MA, Zhang Y, Meister M, Sanes JR. Age-related alterations in neurons of the mouse retina. J. Neurosci. 2011;31:16033–16044. doi: 10.1523/JNEUROSCI.3580-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sann SB, Crane MM, Lu H, Jin Y. Rabx-5 regulates RAB-5 early endosomal compartments and synaptic vesicles in C. elegans. PloS One. 2012;7:e37930. doi: 10.1371/journal.pone.0037930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Sawamoto K. Mechanisms of neurogenesis in the normal and injured adult brain. Keio J. Med. 2013;62:13–28. doi: 10.2302/kjm.2012-0005-re. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Scott SA, DeKosky ST. Quantitation of synaptic density in the septal nuclei of young and aged Fischer 344 rats. Neurobiol. Aging. 1991;12:3–12. doi: 10.1016/0197-4580(91)90032-f. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Sparks DL. Quantitative assessment of possible age-related change in synaptic numbers in the human frontal cortex. Neurobiol. Aging. 2001;22:355–365. doi: 10.1016/s0197-4580(01)00222-6. [DOI] [PubMed] [Google Scholar]
- Sery O, Povova J, Misek I, Pesak L, Janout V. Molecular mechanisms of neuropathological changes in Alzheimer's disease: a review. Folia neuropathologica / Association of Polish Neuropathologists and Medical Research Centre, Polish Academy of Sciences. 2013;51:1–9. doi: 10.5114/fn.2013.34190. [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim E. The postsynaptic organization of synapses. Cold Spring Harb. Persp. Biol. 2011 Dec;1(3):12. doi: 10.1101/cshperspect.a005678. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Sabatini BL, Sudhof TC. Synapses and Alzheimer's disease. Cold Spring Harb. Persp. Biol. 2012 May;1(4):5. doi: 10.1101/cshperspect.a005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siksou L, Triller A, Marty S. Ultrastructural organization of presynaptic terminals. Curr. Opin. Neurobiol. 2011;21:261–268. doi: 10.1016/j.conb.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J. Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghomonian JJ, Sethares C, Peters A. Effects of age on axon terminals forming axosomatic and axodendritic inhibitory synapses in prefrontal cortex. Neuroscience. 2010;168:74–81. doi: 10.1016/j.neuroscience.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorra KE, Harris KM. Stability in synapse number and size at 2 hr after long-term potentiation in hippocampal area CA1. J. Neurosci. 1998;18:658–671. doi: 10.1523/JNEUROSCI.18-02-00658.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamataki S, Francis HW, Lehar M, May BJ, Ryugo DK. Synaptic alterations at inner hair cells precede spiral ganglion cell loss in aging C57BL/6J mice. Hearing Res. 2006;221:104–118. doi: 10.1016/j.heares.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Stewart MG, Popov VI, Kraev IV, Medvedev N, Davies HA. Structure and complexity of the synapse and dendritic spine. In: Pickel V, Segal M, editors. The Synapse: Structure and function. Elsevier: 2014. pp. 1–20. [Google Scholar]
- Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Mattson MP. Selective vulnerability of neurons in layer II of the entorhinal cortex during aging and Alzheimer's disease. Neural Plast. 2010:108190. doi: 10.1155/2010/108190. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Mattson MP. Recruiting adaptive cellular stress responses for successful brain ageing. Nat. Rev. Neurosci. 2012;13:209–216. doi: 10.1038/nrn3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, Muller SA, Rammner B, Grater F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmuller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Terman A, Brunk UT. Is aging the price for memory? Biogerontology. 2005;6:205–210. doi: 10.1007/s10522-005-7956-3. [DOI] [PubMed] [Google Scholar]
- Terzibasi E, Calamusa M, Novelli E, Domenici L, Strettoi E, Cellerino A. Age-dependent remodelling of retinal circuitry. Neurobiol. Aging. 2009;30:819–828. doi: 10.1016/j.neurobiolaging.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Tigges J, Herndon JG, Peters A. Axon terminals on Betz cell somata of area 4 in rhesus monkey throughout adulthood. Anat. Rec. 1992;232:305–315. doi: 10.1002/ar.1092320216. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs P-A, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Toni N, Sultan S. Synapse formation on adult-born hippocampal neurons. Eur. J. Neurosci. 2011;33:1062–1068. doi: 10.1111/j.1460-9568.2011.07604.x. [DOI] [PubMed] [Google Scholar]
- Toth ML, Melentijevic I, Shah L, Bhatia A, Lu K, Talwar A, Naji H, Ibanez-Ventoso C, Ghose P, Jevince A, Xue J, Herndon LA, Bhanot G, Rongo C, Hall DH, Driscoll M. Neurite sprouting and synapse deterioration in the aging Caenorhabditis elegans nervous system. J. Neurosci. 2012;32:8778–8790. doi: 10.1523/JNEUROSCI.1494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Sosnowska D, Mason JB, Gruber H, Lee SW, Schwartz TS, Brown MK, Storm NJ, Fortney K, Sowa J, Byrne AB, Kurz T, Levy E, Sonntag WE, Austad SN, Csiszar A, Ridgway I. Resistance to genotoxic stresses in Arctica islandica, the longest-living non-colonial animal: Is extreme longevity associated with a multistress resistance phenotype? J. Gerontol. 2013;68:521–529. doi: 10.1093/gerona/gls193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Tapia JC, Kang H, Clemenson GD, Jr, Gage FH, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc. Natl. Acad. Sci. USA. 2010;107:14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- VanGuilder HD, Yan H, Farley JA, Sonntag WE, Freeman WM. Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome. J. Neurochem. 2010;113:1577–1588. doi: 10.1111/j.1471-4159.2010.06719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KE, Humphrey DM, Hirth F. The dopaminergic system in the aging brain of Drosophila. Front. Neurosci. 2010;4:205. doi: 10.3389/fnins.2010.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Mattson MP, Zou S. Invertebrates as workhorses for aging and intervention studies. Ageing Res. Rev. 2013;12:402–403. doi: 10.1016/j.arr.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Yang Y, Coleman M, Zhang L, Zheng X, Yue Z. Autophagy in axonal and dendritic degeneration. Trends Neurosci. 2013;36:418–428. doi: 10.1016/j.tins.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoman M, Scutt G, Faragher R. Insights into CNS ageing from animal models of senescence. Nat. Rev. Neurosci. 2012;13:435–445. doi: 10.1038/nrn3230. [DOI] [PubMed] [Google Scholar]





