Abstract
By consuming mulberry leaves covered with pollen from nearby genetically engineered, insect-resistant rice lines producing Cry proteins derived from Bacillus thuringiensis (Bt), larvae of the domestic silkworm, Bombyx mori (Linnaeus) (Lepidoptera: Bombyxidae), could be exposed to insecticidal proteins. Laboratory experiments were conducted to assess the potential effects of Cry1C- or Cry2A-producing transgenic rice (T1C-19, T2A-1) pollen on B. mori fitness. In a short-term assay, B. mori larvae were fed mulberry leaves covered with different densities of pollen from Bt rice lines or their corresponding near isoline (control) for the first 3 d and then were fed mulberry leaves without pollen. No effect was detected on any life table parameter, even at 1800 pollen grains/cm2 leaf, which is much higher than the mean natural density of rice pollen on leaves of mulberry trees near paddy fields. In a long-term assay, the larvae were fed Bt and control pollen in the same way but for their entire larval stage (approximately 27 d). Bt pollen densities ≥150 grains/cm2 leaf reduced 14-d larval weight, increased larval development time, and reduced adult eclosion rate. ELISA analyses showed that 72.6% of the Cry protein was still detected in the pollen grains excreted with the feces. The low exposure of silkworm larvae to Cry proteins when feeding Bt rice pollen may be the explanation for the relatively low toxicity detected in the current study. Although the results demonstrate that B. mori larvae are sensitive to Cry1C and Cry2A proteins, the exposure levels that harmed the larvae in the current study are far greater than natural exposure levels. We therefore conclude that consumption of Bt rice pollen will pose a low to negligible risk to B. mori.
Introduction
Rice, Oryza sativa L., is a staple food for more than half of the world's population and for over 65% of the Chinese people [1], [2]. To feed a growing population worldwide, rice production will have to increase by more than 40% by the year 2030 [3]. Similarly, China will need to increase its rice production by at least 20% by 2030 in order to meet its domestic needs [4]. Rice production, however, is constrained by many factors, and insect pests are among the most important [5].
Insect pests that can substantially reduce rice production in China include the following lepidopteran species. Recent research has confirmed that genetic engineering of rice is an efficient strategy for insect pest control. Multiple insect-resistant genetically engineered (IRGE) rice lines have been developed that produce Cry toxins derived from the bacterium Bacillus thuringiensis (Bt), and these IRGE rice lines are very effective against these lepidopteran pests [5]–[7].
Before a novel GE variety is commercialized, its potential risks to the environment and animal and human health must be extensively evaluated, and an important component of the risk assessment concerns the potential effects of IRGE crops on non-target organisms [8], [9]. Many laboratory and field studies have demonstrated that Bt rice represents a negligible threat to non-target arthropods belonging to orders that differ from that of the target pests, i.e., Bt proteins produced in current IRGE rice lines only affect lepidopterans [5], [9]–[14]. On the other hand, non-target lepidopterans could be affected by Bt rice and therefore warrant special attention in the risk assessment of IRGE crops [15]. A non-target lepidopteran of particular concern in China is the silkworm Bombyx mori Linnaeus (Lepidoptera: Bombycidae).
B. mori is an economically and culturally important insect in China, which is a world center of silk production [16]. B. mori larvae feed exclusively on mulberry (Morus atropurpurea Roxb.) leaves. In southeast China, mulberry trees are typically planted near or around rice fields in a planting system that is referred to as mulberry-mixed cropping [17]. Thus, once Bt rice is commercially grown in China, mulberry leaves may be covered with Bt rice pollen. It follows that B. mori larvae could be exposed to Cry proteins if they consume mulberry leaves covered with Bt rice pollen and if Cry proteins are produced in the pollen [18]–[22]. Because B. mori belongs to the same order as the target pests, i.e., the Lepidoptera, it may be sensitive to lepidopteran-active Cry proteins produced by the current Bt rice lines. Thus, before Bt rice lines are approved for commercial use, their potential effects on B. mori should be assessed [5].
In the current study, we developed and used a rice pollen-feeding assay to assess the potential effects of Bt rice pollen containing Cry2A or Cry1C protein on B. mori larvae.
Results
Bt protein contents in rice pollen
No Bt protein was detected in pollen from the control rice (Minghui 63). The mean (±SE) content of Cry2A was 28.15±1.19 µg/g dry weight (DW) in T2A-1 pollen, which was more than 11-fold higher than the content of Cry1C in T1C-19 pollen (2.40±0.08 µg/g DW).
Pollen consumption by B. mori
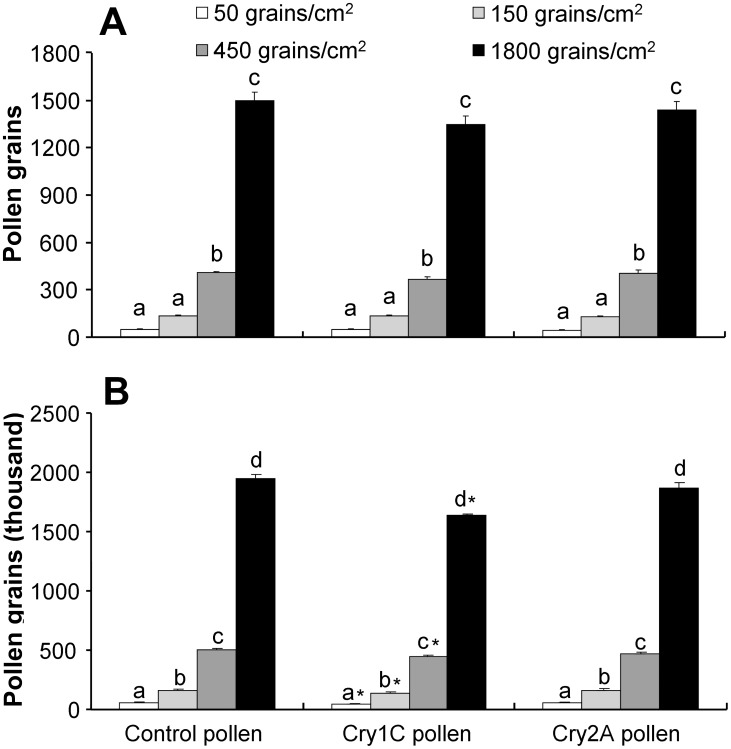
Pollen consumption per larva increased as the density of pollen on the mulberry leaf squares increased (one-way ANOVA; P<0.01 for each type of rice pollen). Pair-wise comparisons by Tukey HSD tests showed significant differences between any two pollen doses in both bioassays except for the two lowest doses in the short-term bioassay (all P<0.001) (Fig. 1A&B).
Figure 1. Rice pollen consumption per Bombyx mori larva.
Lavae were fed mulberry leaves covered with different doses of pollen from Cry1C- or Cry2A-expressing Bt rice or pollen from the corresponding non-transformed varieties for (A) 3 d (short-term assay) and for (B) the entire larval stage (long-term assay). For each pollen type within each assay, bars with different letters are significantly different (one-way ANOVA with Tukey test), while asterisks indicate significant differences between the Bt pollen treatment and the corresponding non-Bt pollen treatment at the same pollen dose (Dunnett test). Values are means + SE, n = 3.
In the short-term bioassay, the number of pollen grains consumed was independent of pollen type (Cry1C, Cry2A, and control rice pollen) (Dunnett test; all P>0.1 at any pollen density) (Fig. 1A). In the long-term assay, however, consumption was lower with Cry1C pollen than with control pollen (P<0.05 for any pollen density) but did not differ (P>0.05 for any pollen density) between Cry2A pollen and control pollen (P>0.05) (Fig. 1B).
Effects of pollen consumption on larval weight
In both bioassays, 14-d larval weight was not affected by increased pollen consumption regardless of the source of the pollen (one-way ANOVA for the short-term assay; control: F = 0.36, df = 17, P = 0.832; Cry1C: F = 0.16, df = 17, P = 0.96; Cry2A: F = 0.33, df = 17, P = 0.86; one-way ANOVA for the long-term assay; control: F = 0.20, df = 17, P = 0.93; Cry1C: F = 2.79, df = 17, P = 0.07; Cry2A: F = 1.96, df = 17, P = 0.16). In the short-term assay at any pollen density, 14-day larval weight did not differ significantly between either Bt treatment and the control (Dunnett's test; P>0.1). In the long-term assay, however, larval weight was significantly lower with Bt rice pollen than with control pollen at the pollen dose of 150 (Cry1C: P = 0.015; Cry2A: P = 0.018), 450 (Cry1C: P = 0.015; Cry2A: P = 0.012), and 1800 grains/cm2 leaf (Cry1C: P = 0.033; Cry2A: P = 0.033) (Table 1).
Table 1. Life table parameters of Bombyx mori larvae when fed pollen from Cry1C- or Cry2A-expressing Bt rice or pollen from the corresponding non-transformed varieties.
| Parameter | No. of pollen grains/cm2 leaf | Short-term assay1 | Long-term assay2 | ||||
| Control pollen | Cry1C pollen | Cry2A pollen | Control pollen | Cry1C pollen | Cry2A pollen | ||
| Larval weight (mg) | 0 | 400.91±14.70 a | 400.91±14.70 a | 400.91±14.70 a | 400.91±14.70 a | 400.91±14.70 a | 400.91±14.70 a |
| 50 | 394.98±13.42 a | 399.64±17.07 a | 389.49±22.92 a | 404.18±12.18 a | 380.03±5.31 a | 384.13±22.84 a | |
| 150 | 410.48±10.80 a | 398.88±4.62 a | 383.40±10.56 a | 415.31±9.41 a | 366.86±8.41 a* | 371.34±6.98 a* | |
| 450 | 396.30±12.12 a | 388.54±23.64 a | 375.96±25.52 a | 410.00 ±11.49 a | 356.02±2.64 a* | 359.17±5.18 a* | |
| 1800 | 385.45±4.09 a | 386.24±18.18 a | 388.89±4.21 a | 412.40±8.82 a | 347.25±17.51 a* | 354.32±4.69 a* | |
| Larval developmental time (d) | 0 | 26.99±0.37 a | 26.99±0.37 a | 26.99±0.37 a | 26.99±0.37 a | 26.99±0.37 a | 26.99±0.37 a |
| 50 | 26.67±0.49 a | 27.00±0.21 a | 27.41±0.16 a | 27.33±0.24 a | 27.70±0.12 a | 27.27±0.12 a | |
| 150 | 26.97±0.28 a | 27.57±0.20 a | 27.67±0.15 a | 27.47±0.19 a | 27.83±0.09 a | 28.07±0.30 b | |
| 450 | 27.60±0.12 a | 27.83±0.13 a | 27.27±0.19 a | 27.57±0.24 a | 28.90±0.15 b* | 28.37±0.15 b | |
| 1800 | 27.30±0.15 a | 27.53±0.07 a | 27.45±0.23 a | 27.90±0.40 a | 29.63±0.15 b* | 29.17±0.35 b | |
| Pupation rate (%) | 0 | 86.67±3.57 a | 86.67±3.57 a | 86.67±3.57 a | 86.67±3.57 a | 86.67±3.57 a | 86.67±3.57 a |
| 50 | 90.00±0.00 a | 85.00±2.89 a | 81.67±1.67 a | 80.00±2.89 a | 80.00±2.89 ab | 85.00±2.89 a | |
| 150 | 83.33±1.67 a | 83.33±4.41 a | 83.33±1.67 a | 81.67±1.67 a | 73.33±1.67 ab | 71.67±3.33 ab | |
| 450 | 78.33±3.33 a | 80.00±5.00 a | 81.67±6.01 a | 76.67±1.67 a | 73.33±1.67 ab | 75.00±2.89 ab | |
| 1800 | 80.00±0.00 a | 81.67±3.33 a | 70.00±2.89 a | 71.67±3.33 a | 66.67±1.67 b | 65.00±2.89 b | |
| Eclosion rate (%) | 0 | 73.33±2.47 a | 73.33±2.47 a | 73.33±2.47 a | 73.33±2.47 a | 73.33±2.47 a | 73.33±2.47 a |
| 50 | 48.33±1.67 b | 53.33±1.67 b | 48.33±4.41 b | 45.00±2.89 b | 38.33±6.01 b | 41.67±4.41 b | |
| 150 | 41.67±4.41 b | 35.00±2.89 c | 28.33±3.33 c | 31.67±4.41 bc | 18.33±6.01 c | 28.33±4.41 bc | |
| 450 | 41.67±6.01 b | 30.00±5.00 c | 31.67±4.41 c | 30.00±2.89 bc | 6.67±1.67 c* | 25.00±2.89 c | |
| 1800 | 33.33±1.67 b | 30.00±2.89 c | - | 23.33±6.01 c | 1.67±1.67 c* | 3.33±1.67 d* | |
Values are means ± SE, n = 3.
Neonates of B. mori were fed mulberry leaves covered with rice pollen for the first 3 d and were then fed mulberry leaves without rice pollen until pupation.
Neonates of B. mori were fed mulberry leaves covered with rice pollen for their entire larval stage.
“-” denotes lost data.
For each parameter, means in a column followed by different letters are significantly different (one-way ANOVAs with Tukey tests for larval weight, pupation and eclosion rate; Kruskal-Wallis Tests followed by Mann-Whitney U-Tests for larval development time).
An asterisk denotes a significant difference between the Bt pollen treatment and the corresponding non-Bt pollen treatment at the same pollen dose (Dunnett test).
Effects of pollen consumption on larval development
Larval developmental time was not significantly affected by the increased pollen consumption of any rice pollen in the short-term assay (Kruskal-Wallis test; control: χ2 = 4.77, P = 0.31; Cry1C: χ2 = 7.88, P = 0.10; Cry2A: χ2 = 2.94, P = 0.57) (Table 1). In the long-term assay, developmental time was not affected by an increase in the density of control pollen (χ2 = 3.63, P = 0.46) but was significantly increased by an increase in the density of both kinds of Bt pollen (χ2 = 14.378, P = 0.006 for Cry1C, and χ2 = 13.769, P = 0.008 for Cry2A) (Table 1). In the short-term assay, pollen type did not significantly affect larval developmental time (Dunnett's test, all P>0.10). In the long-term assay, however, larval developmental time was significantly longer with Cry1C pollen than with control pollen at doses of 450 and 1800 grains/cm2 (both P<0.05). Larval developmental time did not differ between Cry2A and control pollen at any pollen density (all P>0.05) (Table 1).
Effects of pollen consumption on pupation and eclosion rate
In the short-term assay, the pupation rate was not significantly affected by pollen density on the mulberry leaves (one-way ANOVA; control: F = 2.06, df = 17, P = 0.15; Cry1C: F = 0.47, df = 17, P = 0.76; Cry2A: F = 2.70, df = 17, P = 0.08) (Table 1), although the pupation rate tended to drop with the highest density of Cry2A pollen. In the long-term assay, the pupation rate gradually decreased with the increase of pollen density; the decrease was marginally significant with control pollen (F = 3.12, df = 17, P = 0.05), but was significant with both Cry1C pollen (F = 6.58, df = 17, P = 0.004) and Cry2A pollen (F = 6.77, df = 17, P = 0.004).
In both bioassays, eclosion rate of the silkworm significantly decreased as pollen density increased (one-way ANOVA; P<0.001 for any pollen type). In the short-term assay, the eclosion rate was similar with Bt pollen and control pollen (Dunnett's test; P>0.2 for any pollen density). In the long-term assay, however, the eclosion rate was significantly lower with Cry1C pollen than with control pollen at 450 and 1800 grains/cm2 leaf (P = 0.001 and 0.011, respectively) or with Cry2A pollen than with control pollen at 1800 grains/cm2 leaf (P = 0.016) (Table 1).
Fate of Cry1C contained in Bt rice pollen after silkworm gut passage
Pollen grains from Bt rice T1C-19 contained 27.4% less Cry1C protein after passage through the digestive system of B. mori larvae. This difference, however, was not statistically significant (Student's-t test, t = 1.96, df = 4, P = 0.12) (Table 2). Observations under the microscope revealed that the majority of the pollen grains were only partly digested.
Table 2. Cry1C protein content of rice pollen grains before and after passage through the digestive system of Bombyx mori larvae.
| Sample | Cry1C concentration (µg/g dry weight) | No. of pollen grains per mg | Cry1C content per grain (pg) (c x = a x/b x×103) | Lost rate of Cry1C protein (%) (R Cry = (c 1-c 2)/c 1×100) |
| Fresh pollen | 3.62±0.10 (a 1) | 43106.62±1140.13 (b 1) | 0.084±0.002 (c 1) | 27.40 |
| Feces | 0.08±0.03 (a 2) | 1026.01±68.66 (b 2) | 0.061±0.011 (c 2) |
Insects were fed with mulberry leaves covered with T1C-19 rice pollen, and the feces were collected; n = 3.
Values are means ± SE in columns 2 and 4.
Discussion
The risk represented by a Bt crop for a non-target organism depends on the organism's sensitivity to the Bt protein and on the probability that it is exposed to harmful concentrations of that protein in the field [8], [23]. Therefore, when dietary assays are used to assess the effects of Bt pollen on a non-target species, selection of appropriate pollen doses is important and should be based on the pollen densities that the species may encounter in the field [24]. Fan et al. [17] reported that the density of rice pollen deposited on the leaves of mulberry trees near rice fields ranged from 13–199 grains/cm2 with an average density of 93 grains/cm2. Yao et al. [20] reported that the maximum density of rice pollen on mulberry leaves was 1636 grains/cm2, although the probability of that density occurring in the field was only 0.2%. In the current study, the pollen doses tested ranged from 0 to 1800 grains/cm2 of mulberry leaf, which covered the potential pollen densities to which silkworms may be exposed in the field. In addition, both a short-term feeding assay (3 d) and a long-term feeding assay (27 d, covering the entire larval stage) were conducted. In the field, rice anthesis usually lasts 10 to 15 d [20], [21] and pollen is typically shed at a high rate for less than 1 week (Yunhe Li et al., unpublished data). In addition, the silkworm larval period may not totally overlap with rice anthesis, and environmental factors such as rain and wind will also affect rice pollen deposition on mulberry leaves. Therefore the short-term assay may represent an realistic exposure scenario and the long-term assay represents a worst-case scenario. Thus, the assays used in this study are useful for assessing the risk of that Bt rice pollen represents to the silkworm, B. mori.
As expected, the number of pollen grains consumed by B. mori larvae increased as the density of rice pollen on mulberry leaves increased. Interestingly, the larvae in the long-term assay consumed significantly less Cry1C pollen than control pollen even at the low dose of 50 grains/cm2 and even though this density of Cry1C pollen did not affect survival or development. This suggests that the reduced consumption of Cry1C pollen was not caused by harm to the larvae and that Cry1C protein may have antifeedant activity towards B. mori larvae. This was not the case with Cry2A pollen in the long-term assay in that the larvae consumed similar quantities of Cry2A and control pollen.
In both bioassays, the larval weight, development time, and pupation rate were not negatively affected by consumption of control rice pollen even at the highest pollen dose. It seems that consumption of control rice pollen does not affect the normal development of B. mori larvae. Effects seen in the Bt pollen treatments can thus be attributed to the Bt Cry toxins. Surprisingly, however, the eclosion rate of larvae was significantly decreased by consumption of control or Bt pollen even at the lowest dose of 50 grains/cm2 leaf in both bioassays. The biological mechanism underlying this effect is unclear. These results suggest that dietary effects can be specific to certain life table parameters. It follows that as many parameters as possible should be observed in such dietary bioassays.
No adverse effect was detected for Bt pollen in the short-term assay, even at the highest pollen density of 1800 grains/cm2 leaf. In the long-term assay, however, Cry1C or Cry2A pollen negatively affected all of the tested B. mori life table parameters with an exception of pupation rate. Even at a dose of only 150 grains/cm2 leaf, Bt pollen significantly reduced larval weight. This is consistent with Wang et al. [18], who reported that B. mori larval weight but not survival was reduced when the larvae were fed Bt rice pollen containing Cry1Ab toxin. Although we did not statistically compare Cry1C pollen and Cry2A pollen treatments, the data indicate that consumption of Cry1C pollen was more harmful than consumption of Cry2A pollen. For example, B. mori larval developmental time was significantly increased by feeding on Cry1C pollen at 450 and 1800 grains/cm2 leaf but was not increased by feeding on Cry2A pollen even at 1800 grains/cm2 leaf. Our ELISA determination indicated that the Cry2A content in T2A-1 rice pollen was 11-times higher than the Cry1C content in T1C-19 rice pollen. This shows that Cry1C is much more toxic than Cry2A to B. mori larvae, which is also the case for other lepidopterans including the stem borer C. suppressalis, a target pest of the Bt rice lines. In sensitive-insect bioassays, neonate larvae of C. suppressalis were fed for 7 d with artificial diet containing a range of Cry protein concentrations. The EC50 (toxin concentration resulting in 50% weight reduction compared to the control) was 18 ng/mL diet for Cry1C [25] and 1310 ng/mL diet for Cry2A [12]. Previous research has demonstrated that different Cry proteins can have significantly different insecticidal spectra even if they have high homology. For example, Cry1Aa, Cry1Ab, and Cry1Ac have similar structures and belong to the same taxonomic class [26]. In assays with B. mori, however, Cry1Aa was 17-times more toxic than Cry1Ab [27] and 400-times more toxic than Cry1Ac [28]. Based on the EC50 values reported for Cry1C and Cry2A, we would have expected a higher mortality in the silkworm larvae fed with high doses of Bt rice pollen. A likely explanation for the relatively low toxicity is that the rice pollen grains were only partly digested in the larval gut and thus the larvae were only exposed to a fraction of the Cry protein. This was confirmed by the fact that 72.6% of the Cry protein was detected in the pollen grains excreted with the feces.
That cry1C- and cry2A-expressing Bt rice pollen are toxic to the lepidopteran B. mori is not surprising because Cry1 and Cry2 proteins are specifically toxic to lepidopterans. Our results do not indicate, however, that the growing of these Bt rice lines will pose a significant risk to the silk industry for the following reasons. First and as discussed earlier, our long-term pollen exposure assay represents a worst-case scenario, and B. mori larvae are unlikely to be exposed to rice pollen for such a long period [20], [21], [29]. Second, the negative effects were detected only at the relatively high pollen densities of ≥150 grains/cm2 mulberry leaf, which rarely occur in the field. Yao et al. [20] reported a mean of 62.3 grains/cm2 of mulberry leaf at a distance of 0 m from the paddy field edge, and the density steeply declined to 4.0 grains/cm2 of leaf at a distance of 10 m. Third, the mulberry leaves that are fed to B. mori larvae are picked from trees and transferred to a building where the larvae are fed. Thus, the quantity of rice pollen deposited on the leaves would likely be reduced during transport and handling [20]. Fourth, the Bt rice pollen used in our assays was fresh and protected from sunlight and other environmental factors, which would not be the case for Bt rice pollen in the field; in the field, the activity of the Bt proteins in rice pollen may be partially reduced by exposure to rainfall and sunlight before the mulberry leaves are picked and fed to the larvae [20], [30]. Considering all four reasons and the results of our short-term bioassay, we conclude that the impact of T1C-19 and T2A-1 rice pollen on the silkworm, B. mori, is probably minimal.
Although exposure of B. mori larvae to Bt rice pollen is likely to be limited under natural conditions, B. mori has received substantial attention in the risk assessment of Bt rice because of its economic importance [18]–[22], [31]. Wang et al. [18] found that consumption of Bt rice pollen containing Cry1Ab protein at a density of 110 grains/cm2 mulberry leaves during the whole larval development did not affect the survival of the silkworms, but significantly reduced larval weight. Yao et al. [20] reported that Bt rice pollen containing a fusion Cry1Ab/Ac protein had no negative effect on B. mori larvae, when their neonates were exposed to Bt pollen at the density of 3395 grains/cm2 mulberry leaves for 48 h. Likewise, two subsequent studies did not find detrimental effects of Cry1Ab-containing rice pollen from Bt rice lines KMD1 and B1 on B. mori larvae [19], [21]. That different studies have reported differences in the toxicity of Bt pollen containing Lepidoptera-active Cry proteins to B. mori larvae can be explained as follows: i) the studies used rice pollen that contained different types of Bt proteins; ii) the concentrations of Bt proteins in the rice pollen differed; and especially iii) the Bt protein exposure differed among studies [21]. Although Bt rice pollen was found to be toxic to B. mori larvae in some studies, previous researchers have generally concluded that Bt rice pollen poses a negligible risk to this domesticated lepidopteran [5], [21].
Ours is the first study to assess the effects of Cry1C- and Cry2A-containing rice pollen on B. mori larvae. Although the results indicate that the larvae are sensitive to Cry1C and Cry2A proteins contained in T1C-19 and T2A-1 rice pollen, the results also indicate that the Bt rice lines probably represent a low to negligible risk to B. mori larvae because of the limited exposure of the larvae to Bt rice pollen under natural conditions. To guarantee the safety of the silk industry, however, we recommend that mulberry leaves on trees that are near paddy fields planted with Bt rice lines should not be used to feed B. mori larvae or should be washed before they are fed to B. mori larvae.
Materials and Methods
Ethics statement
No specific permits were required for the described field studies. The rice fields from which rice pollen were collected were owned by the author's institute (Institute of Plant Protection, Chinese Academy of Agricultural Sciences, CAAS). These field studies did not involve endangered or protected species.
Insects
A hybrid of B. mori, Liangguang 1, was used in this study. Eggs of B. mori were purchased from the Hainan Silk Development Co., Ltd. (Qiongzhong County, Hainan, China) and were kept in a climatic chamber at 27±0.5°C, 75±5% RH, and 12:12 h L:D photoperiod. Newly hatched larvae (<12 h after hatching) were used for all experiments.
Plant materials
Two transgenic rice varieties, T1C-19 and T2A-1, and their corresponding non-transformed near isoline Minghui 63 were used for the experiments. T2A-1 plants express a synthesized modified cry2A gene and T1C-19 plants express a modified cry1C gene targeting lepidopteran rice pests. Minghui 63 is an elite indica restorer line for cytoplasmic male sterility in China. All rice seeds were kindly provided by Prof. Yongjun Lin (Huazhong Agricultural University, Wuhan).
The rice lines were simultaneously planted in three adjacent plots at the experimental field station of the Institute of Plant Protection, CAAS, near Langfang city, Hebei Province, China (39.5°N, 116.4°E). Each plot was approximately 0.1 hectare, and plots were separated by a 1-m ridge. The rice seeds were sown in a seeding bed on 6 May 2012, and the seedlings were transplanted to the experimental plots on 14 June 2012 when the seedlings were at the four-leaf stage. The plants were cultivated according to the common local agricultural practices but without pesticide sprays.
During rice anthesis from 3 to 13 September 2012, rice pollen was collected daily by shaking the rice tassels in a plastic bag. The collected pollen was air dried at room temperature for 48 h and subsequently passed through a screen with 0.125-mm openings to remove anthers and contaminants. Pollen collected from each rice line was pooled and stored at −80°C until used.
The leaves of mulberry were collected from a mulberry garden at Qiongzhong County, Hainan Province, China. The freshly collected mulberry leaves were washed in water, air dried at room temperature, and stored at 4°C. The leaves were used within 4 days of collection.
Bt protein content in rice pollen
The concentrations of Cry1C and Cry2A proteins in pollen were measured with double-antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) kits from EnviroLogix Inc. (Portland, ME, USA; Catalog No. AP 007and kit lot 140433N for Cry1C, and AP 005 and 202482 for Cry2A). Five samples (5–10 mg) of Bt or control rice pollen were lyophilized and then homogenized in 1 ml of PBST with a micro-mortar and pestle on ice. After centrifugation and appropriate dilution of the supernatants, ELISA was performed according to the manufacturer's instructions. The optical density (OD) values were read with a microplate spectrophotometer (PowerWave XS2, BioTek, USA). The concentrations of Cry1C and Cry2A were calculated by calibrating the OD values to a range of concentrations of standard Cry1C and Cry2Aa samples provided with the kit.
Bioassays
Two bioassays were conducted in a climate chamber at 27±0.5°C, 75±5% RH, and a 12:12 h L:D photoperiod. In a short-term assay, neonates of B. mori were fed cut mulberry leaves (described later in this paragraph) that were covered with rice pollen for the first 3 d and then were fed with cut mulberry leaves without rice pollen for the remainder of the larval period. In a long-term assay, B. mori were fed cut mulberry leaves with rice pollen for their entire larval period. For both assays, a scissors was used to cut mulberry leaves into squares of different sizes. Squares that were 1, 4, 30, and 50 cm2 were used for feeding the first, second, third, and fourth and fifth instar larvae, respectively. Each bioassay included three main treatments: mulberry leaf squares with control rice pollen, Cry1C pollen, or Cry2A pollen. Each main treatment included five doses of rice pollen so that each cm2 of mulberry leaf square contained 0 or about 50, 150, 450, or 1800 pollen grains.
To obtain the different pollen doses on the mulberry leaf squares, the mean weight of a single rice pollen grain was estimated using the method described in Li et al. [32]. Based on the individual weight of a rice pollen grain, the appropriate quantity of rice pollen grains was weighed and placed in a plastic Petri dish. The Petri dish was shaken by hand after a single wet leaf square was placed in the dish. Examination of leaf squares with a stereo-microscope (50×) confirmed that the actual densities of pollen grains that adhered to the leaf surfaces were very similar to the expected doses, and that the pollen grains were relatively evenly distributed.
Plastic boxes (12×7×6 cm for 1st to 3rd instar larvae and 35×25×20 for 4th and 5th instar larvae) with small holes in the lids were used for both feeding assays. A single treated leaf square was placed on a filter paper on the bottom of a box, and 20 randomly selected B. mori neonates were placed on the leaf square. Three replicates and a total of 60 insects were tested for each pollen dose. The number of alive larvae was recorded daily. When a leaf square was almost completely consumed, it was replaced with a new pollen-treated or untreated leaf square. When leaf squares were changed, the uneaten leaf area was calculated using the method of Lang and Vojtech [33]; this information was needed to estimate the mean amount of pollen grains consumed by each larva. When larvae developed into 5th instars and stopped eating, a net was introduced into the plastic dish for larval cocooning. The assays were terminated when all of the insects had developed into adults or died. The following variables were determined: pupation rate, eclosion rate, larval development time, and 14-day larval weight.
Fate of Cry1C contained in Bt rice pollen after silkworm gut passage
To estimate the degree at which silkworm larvae are exposed to Cry protein when Bt rice pollen grains pass through their gut, the mean Cry1C content in pollen grains before ingestion or from pollen grains in the feces of the silkworm larvae was compared. Neonates of B. mori were fed mulberry leaves until the third instar and then starved for 24 h to empty their gut. Subsequently, the larvae were placed in 3 plastic boxes (10 insects per box) and provided with mulberry leaves covered with Bt rice pollen of T1C-19 at a density of >1800 grains/cm2. After 12 h, the silkworm larvae were transferred to new boxes and received the same food. Subsequently, fresh fecal pellets were collected three times at a 2-h interval. All feces collected from each box were pooled as one sample, resulting a total of 3 samples. Meanwhile 3 samples of fresh T1C-19 rice pollen were also obtained. All samples were stored at −80°C. After lyophilization, the concentrations of Cry1C were measured using ELISA as described above.
The total digestion rate of Cry1C in pollen cannot be determined from Bt protein concentrations (Cry protein per dry weight) because the digestion process reduces both the amount of Cry protein and the weight of the pollen grains, and the feces also contained mulberry leaf residues. Therefore, the mean Cry protein content of a single pollen grain before ingestion or present in the feces was assessed. The number of pollen grains in 1.0 mg (dw) rice pollen or feces was estimated. Lyophilized fresh pollen or feces (1.0 mg) were mixed with 300 µl fuchsin acid solution. The pollen grains were counted in each of three 5 µl aliquots of the suspension with a microscope at 50× magnification. The mean number of pollen grains in the aliquots was multiplied by 60 to obtain the number in the whole sample. This procedure was repeated seven to 10 times. Based on the mean number of pollen grains in 1.0 mg per fresh rice pollen and feces and the Cry1C protein concentrations in fresh rice pollen and feces, the Cry1C content in single pollen grains was calculated [13].
Statistical analysis
Student's t-tests were conducted to compare Cry1C and Cry2A contents in rice pollen. For the pollen feeding bioassays, one-way ANOVAs followed by Tukey HSD tests were used to determine how the nature of rice pollen (from non-Bt rice or from Bt rice producing Cry1C and Cry2A) and pollen dose affected pollen consumption, pupation and eclosion rate, and 14-day weight. Because the assumptions for parametric analyses were not met for larval development time (days to pupae), the data were analyzed by Kruskal-Wallis tests, and pair-wise comparisons were further conducted using Mann-Whitney U-tests if significant differences were detected. The Bonferroni correction was applied to correct for 10 pair-wise comparisons leading to an adjusted α = 0.005. At each pollen dose, comparisons were made between each Bt pollen treatment (Cry1C and Cry2A) and the control (non-Bt pollen treatment) using Dunnett tests. The mean Cry1C concentrations in rice pollen grains before and after gut passage were compared using Student's t test.
SPSS 13.0 for Windows was used for all statistical analyses.
Acknowledgments
We thank Yanan Wang for technical assistance and Prof. Yongjun Lin (Huazhong Agricultural University) for kindly providing transgenic rice seeds.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was supported by the National GMO New Variety Breeding Program of PRC (2014ZX08011-02B, 2014ZX08011-001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Food and Agriculture Organization of the United Nations (FAO) (2001) Human energy requirements, Report of a Joint FAO/WHO/UNU Expert Consultation, FAO Food and Nutrition Technical Report Series No 1, Rome. http://www.fao.org/docrep/007/y5686e/y5686e00.htm. Accessed 30 Mar 2014.
- 2. Zhang XF, Wang DY, Fang FP, Zhen YK, Liao XY (2005) Food Safety and Rice Production in China. Reserch of Agricultural Modernization 2: 85–88. [Google Scholar]
- 3. Gurdev SK (2005) What it will take to feed 5.0 bill ion rice consumers in 2030. Plant Molecular Biology 59: 1–6. [DOI] [PubMed] [Google Scholar]
- 4. Peng S, Tang Q, Zou Y (2009) Current status and challenges of rice production in China. Plant Production Science 12: 3–8. [Google Scholar]
- 5. Chen M, Shelton A, Ye GY (2011) Insect-resistant genetically modified rice in China: From research to commercialization. Annual Review of Entomology 56: 81–101. [DOI] [PubMed] [Google Scholar]
- 6. Zhang YJ, Li YH, Zhang Y, Chen Y, Wu KM, et al. (2011) Seasonal expression of Cry1Ab and Cry1Ac proteins in transgenic rice lines and their resistance against striped rice borer Chilo suppressalis (Walker). Environmental Entomology 40: 1323–1330. [DOI] [PubMed] [Google Scholar]
- 7. Wang YN, Zhang L, Li YH, Liu YM, Han LZ, et al. (2014) Expression of Cry1Ab protein in a marker-free transgenic Bt rice line and its efficacy in controlling a target pest, Chilo suppressalis (Lepidoptera: Crambidae). Environmental Entomology 43: 528–536. [DOI] [PubMed] [Google Scholar]
- 8. Romeis J, Bartsch D, Bigler F, Candolfi MP, Gielkens MMC, et al. (2008) Assessment of risk of insect-resistant transgenic crops to nontarget arthropods. Nature Biotechnology 26: 203–208. [DOI] [PubMed] [Google Scholar]
- 9. Li YH, Peng YF, Hallerman EM, Wu KM (2014) Safety management and commercial use of genetically modified crops in China. Plant Cell Reports 33: 565–573. [DOI] [PubMed] [Google Scholar]
- 10. Wang YY, Li YH, Romeis J, Chen XP, Zhang J, et al. (2012) Consumption of Bt rice pollen expressing Cry2Aa does not cause adverse effects on adult Chrysoperla sinica Tjeder (Neuroptera: Chrysopidae). Biological Control 61: 245–251. [Google Scholar]
- 11. Li YH, Wang YY, Romeis J, Liu QS, Lin KJ, et al. (2013) Bt rice expressing Cry2Aa does not cause direct detrimental effects on larvae of Chrysoperla sinica . Ecotoxiology 22: 1413–1421. [DOI] [PubMed] [Google Scholar]
- 12. Li YH, Romeis J, Wu KM, Peng YF (2014) Tier-1 assays for assessing the toxicity of insecticidal proteins produced by genetically engineered plants to non-target arthropods. Insect Science 21: 125–134. [DOI] [PubMed] [Google Scholar]
- 13. Zhang XJ, Li YH, Romeis J, Yin XM, Wu KM, et al. (2014) Use of a pollen-based diet to expose the ladybird beetle Propylea japonica to insecticidal proteins. PLoS ONE 9 1: e85395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li YH, Hu L, Romeis J, Wang YN, Han LZ, et al. (2014) Use of an artificial diet system to study the toxicity of gut-active insecticidal compounds on larvae of the green lacewing Chrysoperla sinica . Biological Control 69: 45–51. [Google Scholar]
- 15. Romeis J, Raybould A, Bigler F, Candolfi MP, Hellmich RL, et al. (2013) Deriving criteria to select arthropod species for laboratory tests to assess the ecological risks from cultivating arthropod-resistant transgenic crops. Chemosphere 90: 901–909. [DOI] [PubMed] [Google Scholar]
- 16. Liu Y, Li Y, Li X, Qin L (2010) The origin and dispersal of the domesticated Chinese oak silkworm, Antheraea pernyi, in China: A reconstruction based on ancient texts. Journal of Insect Science 180: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan L, Wu Y, Pang H, Wu J, Shu Q, et al. (2003) Bt rice pollen distribution on mulberry leaves near rice fields. Acta Ecologica Sinica 23: 826–833. [Google Scholar]
- 18. Wang Z, Ni X, Xu M, Shu Q, Xia Y (2001) The effect on the development of silkworm larvae of transgenic rice pollen with a synthetic cry1Ab gene from Bacillus thuringiensis . Hereditas (Beijing) 23: 463–466. [Google Scholar]
- 19. Wang Z, Shu Q, Cui H, Xu M, Xie X, et al. (2002) The effect of Bt transgenic rice flour on the development of silkworm larvae and the sub-micro-structure of its midgut. Scientia Agricultura Sinica 35: 714–718. [Google Scholar]
- 20. Yao H, Ye G, Jiang C, Fan L, Datta K, et al. (2006) Effect of the pollen of transgenic rice line, TT9-3 with a fused cry1Ab/cry1Ac gene from Bacillus thuringiensis Berliner on non-target domestic silkworm, Bombyx mori Linnaeus (Lepidoptera: Bombyxidae). Applied Entomology and Zoology 41: 339–348. [Google Scholar]
- 21. Yao H, Jiang C, Ye G, Hu C, Peng Y (2008) Toxicological assessment of pollen from different Bt rice lines on Bombyx mori (Lepidoptera: Bombyxidae). Environmental Entomology 37: 825–837. [DOI] [PubMed] [Google Scholar]
- 22. Yuan Z, Yao H, Ye G, Hu C (2006) Survival analysis of the larvae from different hybrids of silkworm, Bombyx mori to Bt rice pollen. Bulletin of Sericulture 3: 23–27. [Google Scholar]
- 23. Raybould A, Caron-Lormier G, Bohan DA (2011) Derivation and interpretation of hazard quotients to assess ecological risks from the cultivation of insect-resistant transgenic crops. Journal of Agricultural and Food Chemistry 59: 5877–5885. [DOI] [PubMed] [Google Scholar]
- 24. Sears MK, Hellmich RL, Stanley-Horn DE, Oberhauser KS, Pleasants JM, et al. (2001) Impact of Bt corn pollen on monarch butterfly populations: A risk assessment. Proceedings of the National Academy of Sciences, USA 98: 11937–11942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li YH, Chen XP, Hu L, Romeis J, Peng YF (2014) Bt rice producing Cry1C protein does not have direct detrimental effects on the green lacewing Chrysoperla sinica (Tjeder). Environmental Toxicology and Chemistry 33: 1391–1397. [DOI] [PubMed] [Google Scholar]
- 26. Crickmore N, Zeigler DR, Feitelson J, Schnepf E, Van Rie J, et al. (1998) Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiology and Molecular Biology Reviews 62: 807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ihara H, Kuroda E, Wadano A, Himeno M (1993) Specific toxicity of δ-endotoxins from Bacillus thuringiensis to Bombyx mori . Bioscience Biotechnology and Biochemistry 57: 200–204. [DOI] [PubMed] [Google Scholar]
- 28. Ge AZ, Shivarova NI, Dean DH (1989) Location of the Bombyx mori specificity domain on a Bacillus thuringiensis delta-endotoxin protein. Proceedings of the National Academy of Sciences, USA 86: 4037–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Julia C, Dingkuhn M (2012) Variation in time of day of anthesis in rice in different climatic environments. European Journal of Agronomy 43: 166–174. [Google Scholar]
- 30. Pusztai M, Fast P, Gringorten L, Kaplan H, Lessard T, et al. (1991) The mechanism of sunlight-mediated inactivation of Bacillus thuringiensis crystals. Journal of Biochemistry 273 Pt 1: 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Niu L, Ma Y, Mannakkara A, Zhao Y, Ma W, et al. (2013) Impact of single and stacked insect-resistant Bt-cotton on the honey bee and silkworm. PLoS ONE 8 9: e72988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li YH, Meissle M, Romeis J (2010) Use of maize pollen by adult Chrysoperla carnea (Neuroptera: Chrysopidae) and fate of Cry proteins in Bt-transgenic varieties. Journal of Insect Physiology 56: 157–164. [DOI] [PubMed] [Google Scholar]
- 33. Lang A, Vojtech E (2006) The effects of pollen consumption of transgenic Bt maize on the common swallowtail, Papilio machaon L. (Lepidoptera, Papilionidae). Basic and Applied Ecology 7: 296–306. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.