Abstract
Background
Sub-Saharan African (SSA) countries are experiencing rapid transitions with increased life expectancy. As a result the burden of age-related conditions such as neurodegenerative diseases might be increasing. We conducted a systematic review of published studies on common neurodegenerative diseases, and HIV-related neurocognitive impairment in SSA, in order to identify research gaps and inform prevention and control solutions.
Methods
We searched MEDLINE via PubMed, ‘Banque de Données de Santé Publique’ and the database of the ‘Institut d’Epidemiologie Neurologique et de Neurologie Tropicale’ from inception to February 2013 for published original studies from SSA on neurodegenerative diseases and HIV-related neurocognitive impairment. Screening and data extraction were conducted by two investigators. Bibliographies and citations of eligible studies were investigated.
Results
In all 144 publications reporting on dementia (n = 49 publications, mainly Alzheimer disease), Parkinsonism (PD, n = 20), HIV-related neurocognitive impairment (n = 47), Huntington disease (HD, n = 19), amyotrophic lateral sclerosis (ALS, n = 15), cerebellar degeneration (n = 4) and Lewy body dementia (n = 1). Of these studies, largely based on prevalent cases from retrospective data on urban populations, half originated from Nigeria and South Africa. The prevalence of dementia (Alzheimer disease) varied between <1% and 10.1% (0.7% and 5.6%) in population-based studies and from <1% to 47.8% in hospital-based studies. Incidence of dementia (Alzheimer disease) ranged from 8.7 to 21.8/1000/year (9.5 to 11.1), and major risk factors were advanced age and female sex. HIV-related neurocognitive impairment’s prevalence (all from hospital-based studies) ranged from <1% to 80%. Population-based prevalence of PD and ALS varied from 10 to 235/100,000, and from 5 to 15/100,000 respectively while that for Huntington disease was 3.5/100,000. Equivalent figures for hospital based studies were the following: PD (0.41 to 7.2%), ALS (0.2 to 8.0/1000), and HD (0.2/100,000 to 46.0/100,000).
Conclusions
The body of literature on neurodegenerative disorders in SSA is large with regard to dementia and HIV-related neurocognitive disorders but limited for other neurodegenerative disorders. Shortcomings include few population-based studies, heterogeneous diagnostic criteria and uneven representation of countries on the continent. There are important knowledge gaps that need urgent action, in order to prepare the sub-continent for the anticipated local surge in neurodegenerative diseases.
Keywords: Neurodegenerative diseases, Parkinsonism, Dementia, HIV-related cognitive impairment, Sub-Saharan Africa
Background
Worldwide, populations are increasingly living longer including in developing countries, where the largest number of elderly people is currently found. In sub-Saharan Africa (SSA) (Figure 1), life expectancy at birth has increased by about 20 years between 1950 and 2010 [1]. During this same period, while the proportion of people aged 60 years and above has remained constant at around 5%, the absolute number in this group has increased by about four folds from 9.4 million in 1950 (total population 179.5 million) to 40.3 million in 2010 (total population 831.5 million). In general, population ageing has been described as a more recent phenomenon in SSA, causing figures for this region to be well below the global average [1]. However, projections suggest that the gap in life expectancy between SSA and the world average, which was around 20 years in 2010, will drop to 10 years by 2050. By this time, about 7.6% of the SSA population (estimated total 2.074 billion) will be aged 60 years and above, which in absolute number will translate into four times the 2010 estimates, and correspond approximately to 156.7 million people [2].
Figure 1.
Sub-Saharan African countries.
Population ageing is considered a global public health success, but also brings about new health challenges in the form of chronic diseases including cardiovascular diseases, cancers, as well as neurodegenerative disorders. A characterization and updated picture of the latter conditions in SSA is particularly important in view of a) the ongoing demographic transition and the resulting surge in the prevalence of neurodegenerative diseases in SSA; b) the successful roll-out of antiretroviral therapies in the region and the potential, yet unknown impact of long-term survival with HIV infection and related treatments on the occurrence of neurodegenerative disorders [3]; and c) lastly, the need for reliable data for health service planning. Recently, there have been efforts to summarize existing data for conditions like Parkinson disease (PD) [4,5] dementia [6,7] or amyotrophic lateral sclerosis [8], but not for other common neurodegenerative disorders, while there are suggestions of possible African distinctiveness in their occurrence and features [9].
We systematically reviewed the published literature on common neurodegenerative disorders and HIV-related neurocognitive impairment among sub-Saharan Africans, with the objective of describing their main features as well as clinical and public health implications.
Methods
Data sources
We searched MEDLINE via PubMed, and the French database ‘Banque des Données en Santé Publique’ (BDSP http://www.bdsp.ehesp.fr) for articles published until February 2013. In addition we searched the database of the ‘Institut d’Epidemiologie Neurologique et de Neurologie Tropicale’ (IENNT). We used a combination of relevant terms to search (in English for PubMed and in French for BDSP and IENNT), which are presented in Additional file 1 (except for IENNT searches for which we used ‘neuroepidemiologie’ and other themes referring to neurodegenerative diseases). Two evaluators (AL and JBE) independently identified articles and sequentially (titles, abstracts, and then full texts) screened them for inclusion (Figure 2). For articles without abstracts or without enough information in the abstract to make a decision, the full text, and where necessary supplemental materials, were reviewed before a decision was made. We supplemented the electronic searches by scanning the references lists of relevant publications, and identifying their citations through the ISI Web of Science, and by hand-searching all issues of the African Journal of Neurological Sciences. Disagreements were solved by consensus or review by a third investigator (APK).
Figure 2.
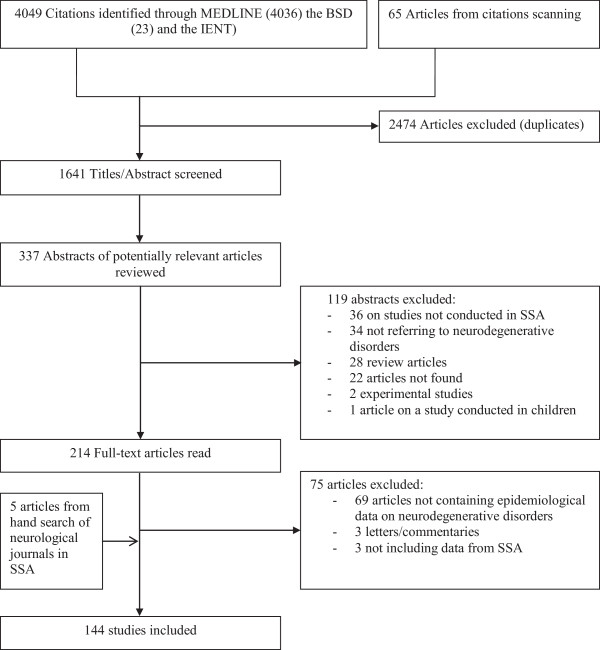
Flow of selection of studies for inclusion.
Study selection
We included studies conducted in a country of the SSA region (Figure 1) that reported on the following neurodegenerative diseases among adults: Alzheimer’s disease, fronto-temporal dementia, Lewy body dementia, vascular dementia, cortico-basal degeneration, multi system atrophy, Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), Huntington disease, cerebellar degeneration, and HIV-related neurocognitive impairment. We made no restriction by study design. We excluded duplicate publications, review articles, studies conducted exclusively in pediatric populations, studies conducted exclusively on migrant populations of African descent living out of the continent. Figure 2 shows the study selection process.
We provide a rigorous appraisal of the overall data and the epidemiological studies in particular, and make recommendations regarding future approaches to measurement, notwithstanding the challenges involved in such undertakings.
Data extraction, assessment, and synthesis
Two reviewers (AL and JBE) independently conducted the data extraction from included studies. We extracted data on study settings, design, population characteristics, measures of disease occurrence (incidence and/or prevalence), and risk factors for the various conditions examined. Given the diversity of neurodegenerative pathologies and the heterogeneity of populations assessed, we did not use a particular framework for the assessment of the quality of studies. However, whenever population-based studies and hospital-based studies had been conducted for a condition, we relied more on the conclusions of population-based studies to address relevant questions, and appropriately reported the results. We conducted a narrative synthesis of the evidence.
Results
The study selection process is shown in Figure 2. A total of 4049 citations were identified through MEDLINE, the IENNT database and BDSP searches; 337 abstracts were evaluated in detail and 214 full-text publications reviewed. The final selection included 144 publications reporting on Parkinsonism (20 studies), dementia (49 publications), HIV-related neurocognitive impairment (47 publications), Huntington disease (19 studies), amyotrophic lateral sclerosis (15 studies), cerebellar degeneration (4 studies) and Lewy body dementia (1 study). These studies were published between 1955 and 2012, with about 50% conducted in only two countries: Nigeria and South Africa.
Parkinson disease, other Lewy body diseases and fronto-temporal dementia
Twenty studies reported on Parkinsonism (Table 1), including five community-based and sixteen hospital-based. Four were case–control in design and all the others were cross-sectional studies, including reviews of medical records. These studies were conducted in seven countries including Nigeria (ten studies), South Africa (four studies), Tanzania (two studies), Ethiopia, Ghana, Cameroon and Zimbabwe (one studies each). The number of participants with PD ranged from two to 32 and the prevalence from ten to 235/100,000 in community-based studies. The number of participants with Parkinsonism ranged from four to 397, and the prevalence of Parkinsonism varied from 0.41 to 7.2% of neurological admissions/consultations in hospital-based studies. The proportion of men among those with PD ranged from 53 to 100%, and age ranged from 30 to >100 years. Age at the clinical onset of the disease ranged from 17 to 90 years. The clinical types of the disease were largely dominated by Parkinson disease (38 to 100%).
Table 1.
Overview of studies on Parkinsonism and risk factors in sub-Saharan African countries
| Author, year of publication | Country | Setting | Design/period of study | Population characteristics | Diagnosis criteria | Prevalence | Profile of parkinsonism patients | Comments |
|---|---|---|---|---|---|---|---|---|
| Bower
[10], 2005 |
Ethiopia |
Hospital |
Cross-sectional 2003-2004 |
720 patients; 109 (15 · 1%) with movement disorders including 71 men; age 52 y. (13–80) |
Not provided |
72/1,000 of all admissions (PD: 64/1,000) |
N:52; PD:88% |
Review of medical files/outpatient neurology clinic. |
| Age (at onset): 57y (30–80) | ||||||||
| Men: 75% | ||||||||
| Akinyemi
[11], 2008 |
Nigeria |
Hospital |
Case–control 2005-2005 |
51 patients (men 37) with PD and 50 controls |
UKPDS Brain Bank criteria |
NA |
N:51; PD: 100% |
22% patients with PD had cognitive dysfunction, with age at PD onset as sole predictor of cognitive dysfunction. |
| Age (at onset): 70y (41–80) | ||||||||
| Men:72% | ||||||||
| Cosnett
[12], 1988 |
South Africa |
Hospital |
Cross-sectional 1979-1985 |
2638 patients |
Clinical (Bradykinesia, rigidity, resting tremor and postural instability) |
5.3/1,000 |
N:14; PD: 100% |
Retrospective review of medical files/outpatient clinic |
| Age: NA |
Blacks: 1.5/1000 |
|||||||
| Men: NA |
Indians: 12.6/1000 |
|||||||
| Whites: 23.1/1000 | ||||||||
| Dotchin
[13], 2008 |
Tanzania |
Community |
Cross-sectional |
161,071 inhabitants |
UKPDS Brain Bank criteria |
Overall: 40/100,000 |
N: 32; PD:100% |
Prevalence is adjusted to UK population. Mean duration 5.1 y |
| Men: 64/100,000 | ||||||||
| women: 20/100,000 |
Age (at onset): 69y (29–90) |
|||||||
| Men: 72% | ||||||||
| Schoenberg
[14], 1988 |
Nigeria |
Community |
Cross-sectional |
Black population aged 40 + 3412 participants |
Clinical |
Age adjusted: 67/100,000 |
N: 2; PD:100% |
|
| Age: NA | ||||||||
| Men: NA | ||||||||
| USA |
Community |
Cross-sectional |
Black population aged 40 + 3521 black participants and 5404 white participants. |
Clinical |
Age adjusted: |
N: 12; PD: 100% |
|
|
| Age: NA | ||||||||
| Blacks: 341/100,000 |
Men: NA |
|||||||
| Whites: 352/100,000 | ||||||||
| Winkler
[15], 2010 |
Tanzania |
Hospital |
Cross-sectional |
n = 8676 patients admitted (740 with neurological diseases) |
UKPDS Brain Bank criteria |
1/1,000 (all patients) |
N: 8; PD:37% |
|
| 2003 |
11/1,000 (Patients with neurological diseases |
|||||||
| Age: ≥32 y | ||||||||
| Men: 100% | ||||||||
| Community |
Cross-sectional |
1569 people, age 50–110 years |
UKPDS Brain Bank criteria |
235/100,000 |
N: 0 |
None of the 18 screened-positive was confirmed as having PD. Poisson distribution used to estimate the prevalence. |
||
| 2003-2005 | ||||||||
| Kengne
[16], 2006 |
Cameroon |
Hospital |
Cross-sectional |
4041 patients in a neurology clinic145 (3.9%) had neurodegenerative diseases |
Not provided |
488/1,000 of all neurodegenerative diseases; 10.1/1,000 of all neurologic consultation |
N: 41; PD 100% |
4 selected neurodegenerative brain disorders: dementia, PD, ALS, chorea |
| 1993-2001 |
Age: 15-84 y |
|||||||
| Men: 73.2% | ||||||||
| Lombard
[17],1978 |
Zimbabwe |
Hospital |
Cross-sectional |
Total patients admitted: 83,453 blacks, 34,952 whites |
Not provided |
Blacks: 0.21/1,000 |
N: 50 (17 blacks) |
Retrospective review of medical files |
| Whites: 2.83/1,000 |
Age/men: NA |
|||||||
| Osuntokun
[18], 1979 |
Nigeria |
Hospital |
Cross-sectional |
217 patients with parkinsonism |
Not provided |
NA |
N: 217; PD 38% |
All patients evaluated by the authors |
| 1966-1976 |
Age: median 51-70 y, |
|||||||
| Men:75% | ||||||||
| Osuntokun
[19], 1987 |
Nigeria |
Community |
Cross-sectional |
Total participants surveyed: 18,954 |
Not provided |
10/100,000 |
N. 2; PD 100% |
Screening Questionnaire developed by author |
| 1985 |
Age/men: NA |
|||||||
| Haylett
[20], 2012 |
South Africa |
Hospital |
Cross-sectional |
229 patients with PD including 163 whites (71%), 45 mixed ancestry (20%), 17 blacks (7%) and 4 Indians (2%) |
UKPDS Brain Bank criteria |
NA |
N: 229; PD 100% |
Mutation in the Parkin gene |
| Age (at onset): 54 y (17–80) |
Homozygous or compound heterozygous mutations: 7 patients |
|||||||
| Heterozygous variant: 7 | ||||||||
| Men: % NA | ||||||||
| Ekenze
[21], 2010 |
Nigeria |
Hospital |
Cross-sectional |
8440 admission in the medical ward; 1249 had neurological diseases (men 640) |
Not specified |
21.9/1000 of al neurological admissions |
N: 14 |
|
| 2003-2007 |
Age ≥ 70 y (71%) |
|||||||
| Men: 28.6% | ||||||||
| Owolabi
[22], 2010 |
Nigeria |
Hospital |
Cross-sectional |
6282 admission in the medical ward; 980 had neurological diseases (men 586) |
Clinical: any 3 out of tremor, rigidity, Akinesia/bradikinesia/postural and instability |
4.1/1,000 of all neurological admissions |
N: 4 |
|
| 2005-2007 |
Age: (50–68) |
|||||||
| Men; 100% | ||||||||
| Okubadejo
[23], 2004 |
Nigeria |
Hospital |
Case–control |
33 participants (men 25, mean age 60 y) with PD and 33 match controls |
Any 3 out of tremor, rigidity, Akinesia/bradikinesia/postural and instability |
NA |
N: 33 |
Case fatality rate was higher in PD (25% vs. 7.1%), Factors associated with increased mortality: advanced age and disease severity |
| Age (at onset): 36-80y | ||||||||
| Men: 75% | ||||||||
| Okubadejo
[24], 2005 |
Nigeria |
Hospital |
Case–control |
28 participants (men 21, mean age 63 y) with PD and 28 match controls |
Any 2 out of tremor, rigidity, Akinesia/bradikinesia/postural and instability, exclusion of other causes of parkinsonism |
NA |
N: 28; PD 100% |
Autonomic dysfunction rate was higher in PD (61% vs. 6%), |
| Age (at onset): 37-76 y | ||||||||
| Men: 76% | ||||||||
| Okubadejo
[25], 2010 |
Nigeria |
Hospital |
Cross-sectional |
124 participants with Parkinsonism in a neurology clinic |
Any 3 of the following: tremors, rigidity, bradykinesia, and postural or gait abnormality |
15/1,000 of all neurological consultations |
N: 98; PD 79% |
Other causes of parkinsonism n(%): Vascular/drug induced/MSA/LBD: 9(35)/5(19)/4(15)/3(11) |
| 1996-2006 | ||||||||
| Age (at onset): 61y Men: 76.5% | ||||||||
| Keyser
[26], 2010 |
South Africa |
Hospital |
Cross-sectional |
154 patients with PD including 51 whites (35%), 45 Afrikaners (31%), 29 mixed ancestry (20%), 17 blacks (12%) and 3 Indians (2%). |
UK Parkinson’s Disease UKPDS Brain Bank criteria |
NA |
N: 154; PD 100% |
16 sequence variants of the PINK1gene identified: 1 homozygous mutation (Y258X), 2 heterozygous missense variants (P305A and E476K), and 13 polymorphisms |
| Age (at onset): 52 y | ||||||||
| Men: 62% | ||||||||
| Van Der Merwe
[27], 2012 |
South Africa |
Hospital |
Cross-sectional |
111 patients with early onset PD (men 71) and 286 with late onset PD (men 62%) from a movement disorder clinic |
UKPDS Brain Bank criteria |
NA |
N: 397; PD 100% |
A positive family history was associated with a younger age at onset. |
| 2007-2011 |
Age (at onset): 57 y Men: 248 |
|||||||
| Femi
[28], 2012 |
Nigeria |
Hospital |
Cross-sectional |
1153 participants in 2 Neurologic clinics; 96 (men: 74) had parkinsonism |
presence of at least three of the four cardinal features of tremors, rigidity, bradykinesia, and postural or gait abnormality |
69.4/1,000 of all neurological consultations |
N: 96; PD (83.3%) |
|
| 2007-2011 | ||||||||
| Age: 58 y | ||||||||
| Men: 63.5% | ||||||||
| Cilia
[29], 2012 |
Ghana |
Hospital |
Case–control |
54 participants with PD and 46 healthy participants |
UKPDS Brain Bank criteria |
NA |
N: 54; PD 100% |
Leucine-rich repeat kinase 2 (LRRK2) gene found in no participants |
| Age (at onset): 59 y (30–83) | ||||||||
| Men: 61% |
NA: Not available; PD: Parkinson’s disease; UK: United Kingdom; USA: United States of America; y: years.
The most commonly used tool to diagnose PD was the UKPDS Brain bank criteria and population-based (hospital-based) prevalence for the studies that applied those criteria ranged from 40 to 235/100,000 (11 to 69.4/1,000 neurological consultations). In general risk factors were not investigated across studies, although one study found that 38% of patients with Parkinsonism had atherosclerosis and 8% had encephalitis [18].
We found three cases of Lewy body dementia in a retrospective study in Nigeria, and one case in a retrospective study in Senegal representing respectively 1.2/100,000 of admission over a period of 10 years [30] and 7.5/1000 of participants in a specialized memory clinic [31].
The prevalence of fronto-temporal dementia has been reported in two hospital-based studies conducted in Neuropsychiatric clinics in Nigeria (prevalence rate: 1.7/100,000 of all admissions) and in Senegal (prevalence rate: 7.5/1000 of all participants evaluated for memory impairment) [30,31].
Dementia
(Table 2) summarizes the 49 publications that reported on dementia. These include 18 hospital-based, 30 community-based publications and one publication from a nursing home. Two were case–control in design, seven were cohort-studies and 40 were cross-sectional, including two autopsy studies. These publications reported on studies conducted in eleven countries: Nigeria (33 publications), Senegal (four publications), Kenya and Tanzania (three publications each), Benin, Central African Republic, Congo republic, (two publications each), South Africa, Cameroon and Zambia (one publication each). In addition, there were seven publications on multicenter studies including African American participants in the USA and participants from African countries [32-37]. The overall study size varied from 56 to 2494 in community-based studies and from 23 to 240,294 in hospital-based investigations. The prevalence of dementia ranged from <1% to 10.1% in population-based studies [32,34-57] and from <1% to 47.8% in hospital-based studies [16,21,30,33,38,58-69].
Table 2.
Overview of studies on dementia and risk factors in sub-Saharan Africa
| Author, year of publication | Country/setting | Design/period of study | Population characteristics | Diagnostic criteria | Incidence | Prevalence (%) | Risk factors |
|---|---|---|---|---|---|---|---|
| Lambo
[58], 1966 |
Nigeria |
Retrospective/Cross-sectional, 1954-1963 |
328 participants (26% ≥60 y.) |
Not provided |
NA |
Senile dementia*: |
NA |
| Hospital | |||||||
| Overall: 26%, Men: 18.9% Women: 30.5% | |||||||
| 75 cases of dementia (21 men) | |||||||
| Ben-Arie
[39], 1983 |
South Africa |
Cross-sectional, 1982 |
139 participants aged ≥65 y. |
MMSE/ICD-8 codes |
NA |
Any (severe) dementia 8.6% (3.6%) |
NA |
| Community | |||||||
| Makanjuola
[59], 1985 |
Nigeria |
Cross-sectional 1979-1982 |
51 (5.2% of new consultations); age ≥60 y. |
ICD-9 codes |
NA |
Dementia 11.2% |
NA |
| Hospital | |||||||
| Gureje
[60], 1989 |
Nigeria |
Cross-sectional, 1984 |
1914 patients; |
ICD- 9 codes |
NA |
No case of dementia |
NA |
| Community | |||||||
| Ogunniyi
[40], 1992 |
Nigeria |
Cross-sectional |
930 participants; age ≥40 y. (293 aged ≥65 y.); No case of dementia |
DSM-III-R criteria |
NA |
No case of dementia |
NA |
| Community | |||||||
| Osuntokun
[61], 1994 |
Nigeria, hospital Autopsy study |
Cross-sectional 1986- 1987 |
111 brains autopsied including 85 patients aged ≤60 y. |
Beta A4 amyloid on brain tissues |
NA |
Heavy/moderate/mild plaque load: 0/6.3/18.9% |
NA |
| Osuntokun
[41], 1995 |
Nigeria, community |
Cross-sectional |
56 subjects (17 with dementia and 12 with AD); age ≥65 y. |
Dementia –CSID |
NA |
APOE ϵ4 allele in dementia/AD/controls 17.6/16.7/20.5%. |
NA |
| AD - NINCDS-ADRDA criteria | |||||||
| Osuntokun
[38], 1995 |
Nigeria, hospital Autopsy study |
Cross-sectional |
198 brains were autopsied |
senile plaque, neurofibrillary tangle, and amyloid vascular degeneration |
NA |
No evidence of NFT or senile plaque |
NA |
| 1986- 1987 |
Including 45 (23%) ≥65 year |
||||||
| Hendrie
[32], 1995 |
Nigeria, community |
Cross-sectional |
2494 participants, age ≥65 y., Dementia -28, AD - 18, VaD - 8. |
Dementia: CSID/DSM-III-R/ICD-10/AD: NINCDS-ADRDA criteria |
NA |
Dementia - Overall/65-74/75-84/≥85 y: |
|
| 1992-1993 |
2.3/0 · 9/2.7/9.6; |
||||||
| AD - 1.4/0.5/1.7/5.9% | |||||||
| Indianapolis-USA, community & nursing home |
Cross-sectional |
2212participants, aged ≥65 y. (community) and 106 (nursing home) |
Dementia: CSID/DSM-III-R/ICD-10/AD: NINCDS-ADRDA criteria |
NA |
Dementia Overall/65-74/75-84/≥85 y: |
NA |
|
| 1992 - 1993 | |||||||
| 8.2/2 · 6/11.4/32.4% | |||||||
| AD -6.2/1.6/8.0/28.8% | |||||||
| Ogeng'o
[33], 1996 |
Tanzania, hospital |
Cross-sectional |
12 Non-demented subjects aged 45–83 y. |
senile plaque, neurofibrillary tangle, and cerebral amyloid angiopathy |
NA |
Amyloid β plaques:17% |
NA |
| 1996 |
Autopsy study |
||||||
| Neurofibrillary Tangles: 17%; Cerebral Amyloid angiopathy: 17% | |||||||
| Kenya, hospital |
Cross-sectional Autopsy study |
20 Non-demented subjects aged 45–70 y. |
Senile plaque, neurofibrillary tangle, and cerebral amyloid angiopathy |
NA |
Amyloid β plaques: 15%; Neurofibrillary Tangles: 15%; Cerebral Amyloid angiopathy: 15% |
NA |
|
| USA-Cleveland, Hospital |
Cross-sectional/Autopsy study |
20 Non-demented subjects aged 48–84 y. |
Senile plaque, neurofibrillary tangle, and cerebral amyloid angiopathy |
NA |
Amyloid β plaques: 20%; Neurofibrillary: 15%; Cerebral Amyloid angiopathy: 20% |
NA |
|
| Ogunniyi
[42], 1997 |
Nigeria, community |
Cross-sectional |
2494 participants aged >65 y screened, 28 with dementia. |
Screening: CSI-D) |
NA |
Any/ AD/ vascular dementia - 1.1/0.7/0.3% |
N A |
| 1992-1994 | |||||||
| Dementia: DSM-III-R and ICD-10 codes | |||||||
| AD: NINCDS-ADRDA | |||||||
| Sayi
[62], 1997 |
Tanzania, hospital |
Cross-sectional |
24 demented and 286 non-demented participants aged 50–89 y. |
Swahili modified MMSE |
NA |
Prevalence of ϵ4 allele of APOE: Demented - 25%; non demented - 21% |
NA |
| Kenya, hospital |
Cross-sectional |
22 demented and 60 non-demented participants aged ≥65 y. |
Swahili modified MMSE |
NA |
Prevalence of ϵ4 allele of APOE: Demented - 42%, non-demented - 27% |
NA |
|
| Baiyewu
[63], 1997 |
Nigeria, Nursing home |
Cross-sectional |
23 participants (in a nursing home) aged 66–102 y.; 11 women |
DSM-III-R/AGECAT |
NA |
Any dementia (AD) - 47 · 8% (26 · 1%) |
NA |
| 1994 | |||||||
| Hall
[34],1998 |
Nigeria, community |
Case–control |
2494 participants; age ≥ 65 y.; |
Screening: CSID |
NA |
18 cases of possible or probable AD1.4% |
age (OR = 1.15; 95% |
| 423 clinically assessed after screening, | |||||||
| CI = 1.12-1.18) and female gender (OR = 13.9; 95% CI = 3.85-50.82) | |||||||
| Dementia: DSM-III-R/ICD-10/AD: NINCDS-ADRDA | |||||||
| USA–Indianapolis, community |
Case–control |
2212 participants; age ≥ 65 y.; |
Screening: CSID |
NA |
Possible/probable AD 6.2% |
age, family history of dementia, education; rural residence |
|
| Dementia: DSM-III-R/ICD-10/AD: NINCDS-ADRDA | |||||||
| 351 clinically assessed after screening,; 49 (men 17) diagnosed with AD | |||||||
| Uwakwe
[70], 2000 |
Nigeria, Hospital |
Cross-sectional |
119 participants; age ≥65 y; 3 had dementia |
Geriatric Mental State and/ICD-10 |
NA |
2.8% |
NA |
| 1995-1996 | |||||||
| Ogunniyi
[43], 2000 |
Nigeria, community |
Cross-sectional 1992-1994 |
2494 participants, age ≥65 y.; 28 with dementia (men: 8) including 18 with AD, 8 with vascular dementia |
Screening: CSID |
NA |
Any dementia 2.3% |
Age (OR: 1.15), female gender (13.9), living with others (OR: 0 · 06) |
| Dementia: DSM-III-R/ICD-10 | |||||||
| AD: NINCDS-ADRDA |
AD: 1.4% |
||||||
| E4 allele in AD (normal subjects) 34.2% (21.8%) | |||||||
| Indianapolis-USA, community |
Cross-sectional |
2212 participants, age ≥65 year; 65 with dementia including 49 with AD, 10 with vascular dementia |
Screening: CSID |
NA |
Dementia (AD) overall/65-74/75-84/≥85 y – 8.2 (6.2)/2.62 (1.58)/ 11.4 (8.0)/32 · 4% (28.8%); |
Age, rural residence, family history of dementia, education |
|
| Dementia: DSM-III-R/ICD-10 | |||||||
| 1992-1994 | |||||||
| AD: NINCDS-ADRDA | |||||||
| Hendrie
[35], 2001 |
Nigeria, community |
Prospective cohort Baseline survey in 1992-1993 |
2459 participants included after the first visit; 1303 (men 461) completed the follow-up; age ≥65 y. |
Screening: CSID |
Dementia: 13.5/1,000 |
NA |
NA |
| Dementia: DSM-III-R/ICD-10 | |||||||
| AD: NINCDS-ADRDA |
AD: 11.5/1000 |
||||||
| USA-Indianapolis, community |
Prospective cohort |
2147 African-Americans included after the first visit; 1321 (men 417) completed the follow-up; age ≥65 y. |
Screening: CSID |
Dementia (AD) |
NA |
NA |
|
| Baseline survey in 1992-1993 | |||||||
| Dementia: DSM-III-R/ICD-10/AD: NINCDS-ADRDA | |||||||
| 32.4/1,000 (25.2/1,000) | |||||||
| Baiyewu
[44], 2002 |
Nigeria, community |
Prospective cohort baseline survey in 1992-1993 |
2487 participants; age ≥65 y.; |
Screening: CSID |
Conversion from CIND to dementia 16 · 1%; From CIND to normal 25 · 3% |
NA |
Sex |
| Dementia: DSM-III-R/ICD-10 | |||||||
| 423 clinically assessed after screening; 152 diagnosed with CIND; 28 (men 7) with dementia, 87 followed up for 2 years. | |||||||
| Perkins
[36], 2002 |
Ibadan-Nigeria community |
Prospective, 1992-1993 |
2487 participants; age ≥65 y; |
Screening: CSID |
NA |
1.8% |
Dementia associated with mortality |
| Dementia: DSM-III-R/ICD-10 | |||||||
| 423clinically assessed after screening | |||||||
| Indianapolis-USA, Community |
Prospective Baseline survey in 1992-1993 |
2212 participants; aged ≥65 y.; |
Screening: CSID |
|
4.9% |
Dementia associated mortality (adjusted RR: 2 · 05) |
|
| 342 clinically assessed after screening | |||||||
| Dementia: DSM-III-R/ICD-10 | |||||||
| Lane
[37], 2003 |
Nigeria Community |
Prospective 8.7 y follow up Baseline 1992-1993 |
968 participants (271 aged ≥75 y.); |
Screening: CSID |
NA |
NA |
ApoEϵ4 alleles not associated with increased mortality |
| 23with dementia at follow-up | |||||||
| Dementia: DSM-III-R/ICD-10 | |||||||
| Indianapolis-USA, Community |
Prospective 9.5 y. Baseline 1992-1993 |
353 participants (17 4 aged ≥75 y.); 17 with dementia at follow-up |
Screening: CSID |
NA |
NA |
ApoEϵ4 associated with increased mortality for patient under 75 year |
|
| Dementia: DSM-III-R/ICD-10 | |||||||
| Ogunniyi
[45], 2005 |
Nigeria, Community |
Cross-sectional/1992- 1998 |
98 demented subjects; age ≥65 y. |
Screening: CSID |
NA |
AD: 82% of all cases |
NA |
| Dementia: DSM-III-R/ICD-10 | |||||||
| VaD: 11.1% of all cases | |||||||
| Kengne
[16], 2006 |
Cameroon, |
Cross sectional, |
4041 neurologic consultations |
Not provided |
NA |
0.4% (all neurologic admission), 19% (neurodegenerative diseases) |
NA |
| Hospital |
1993-2001 |
||||||
| 145 with neurodegenerative diseases | |||||||
| 16 (men 14) with dementia, mean age 67.8 y. | |||||||
| Gureje
[46], 2006 |
Nigeria, Community |
Cross-sectional, 2003-2004 |
2152 participants at baseline with a respondent rate of 74% (1904 participants). Aged 65 year or older. |
adapted 10-Word Delay Recall Test (10-WDRT)10 |
NA |
Overall: 10.1%; |
Female gender, Increasing age, alcohol |
| Female: 14.6% | |||||||
| Men: 7.0% | |||||||
| Gureje
[71], 2006 |
Nigeria Community |
Cross-sectional, |
2245 DNA samples, 830 had a diagnosis |
Screening: CSID |
NA |
Any dementia (16.9% |
E4 allele in AD (normal subjects) 26 · 0% (21 · 7%) |
| Dementia: DSM-III-R/ICD-10 | |||||||
| AD: 14.8% | |||||||
| Ogunniyi
[72], 2006 |
Nigeria, Community |
Case–control |
62 participants with AD (Men 16.1%, mean age 82 y) and 461 non demented (men 33.2%, mean age 77 y) |
Screening: CSID |
NA |
|
Age (OR 1 · 07) |
| Dementia: DSM-III-R/ICD-10/AD: NINCDS-ADRDA | |||||||
| Rural to age (OR 2 · 93) Hypertension (OR 0 · 33) | |||||||
| Indianapolis-USA, Community |
Case–control |
89 participants with AD (men 30.3%, mean age 83 y), mean age 77 y) and 381 non demented (Men 31.2%, mean age 78 y) |
Screening: CSID |
NA |
|
Age (OR 1.09 |
|
| Rural to age (OR 2.08) | |||||||
| Dementia: DSM-III-R/ICD-10/AD: NINCDS-ADRDA |
Alcohol consumption (OR 0.49) |
||||||
| Uwakwe
[64], 2006 |
Nigeria, Community |
Cross-sectional |
30 patients (men 12) with dementia and their caregivers (total 30) |
Not provided |
NA |
N:52; |
|
| 2003-2005 | |||||||
| AD: not provided | |||||||
| Men: 12 | |||||||
| Ochayi
[47], 2006 |
Nigeria, Community |
Cross-sectional 2002 |
280 participants; age ≥65 y.; |
CSID |
NA |
Overall dementia: 6.4% |
Female gender, |
| Lower body mass index, age, NSAIDS | |||||||
| 65-74 year old: 5.2% | |||||||
| 18 (men 2) with dementia |
≥85 year 16%. |
||||||
| Hall
[48], 2006 |
Nigeria, Community |
Cross-sectional |
1075 participants; age ≥ 70 y. 29 (men 5) with AD, |
NINCDS-ADRDA |
NA |
NA |
Total- or LDL- cholesterol in individuals without the APOE-ϵ4 allele |
| Uwakwe
[73], 2009 |
Nigeria, community |
Cross-sectional |
914 (men 432) participants, age ≥65 y; 87 with ≥2 tests memory tests impaired |
Memory impairment assessed by MMS, CISD and 10 word list immediate and delayed recall |
NA |
9.9% |
NA |
| Guerchet
[50], 2009 |
Benin Community |
Cross-sectional |
502 (men 156) participants, aged ≥65 y; 52 with cognitive impairment |
Screening: CSI-D |
NA |
Cognitive impairment |
Age, current depressive disorder, absence of the APOE ϵ 2 |
| Overall: 10.4%; men 7.7 women 11.5% | |||||||
| Dementia: DSM-IV | |||||||
| AD: NINCDS-ADRDA |
|
Dementia Overall: 2.5%, men 0.6% women 3.4% |
|||||
| 13 (men 1) with dementia | |||||||
| Toure
[67], 2009 |
Senegal |
Cross-sectional |
872 participants; age ≥55 y. |
DSM-IV-R |
NA |
Overall 6.6% |
Age, social isolation, history of stroke, epilepsy, family history of dementia, Parkinson’s disease |
| Hospital |
2004-2005 |
58 cases of dementia |
|||||
| Napon
[68], 2009 |
Burkina Fasso |
Cross-sectional |
15815 (2396) out (in) participants; age ≥15 y.; 72 (and 53 inpatients) with dementia; AD: 7; VaD: 19 cases |
DSM-IV |
NA |
outpatients: 0.45% inpatients: 0.22% |
NA |
| Hospital | |||||||
| Guerchet
[49], 2010 |
Central African Republic Community |
Cross-sectional |
509 interviewed; 496 (men 218) included in final sample, age ≥65 y. |
Screening: CSID |
NA |
Overall: 8.1%, men 2.7%, women 12.2% |
NA |
| 2008-2009 | |||||||
| Dementia: DSM-IV | |||||||
| 188 with cognitive impairment and 40 (men 6) with dementia (mean age 76 y.); 33 (men 3) with AD and 7 (men 2) with VaD |
AD: NINCDS-ADRDA Hachinski scale, |
||||||
| Republic of Congo Community |
Cross-sectional |
546 interviewed; 520 (men 198) included in final sample, age ≥65 y. 148 with cognitive impairment and 35 (men 9) with dementia (mean age 79 y.); 24 (men 7) with AD and 11 (men 3) with VaD |
CSID/ DSM-IV and NINCDS-ADRDA Hachinski scale |
|
Overall: 6.7%, men 4.5%, women 8.1% |
NA |
|
| 2008-2009 | |||||||
| Chen
[65], 2010 |
Kenya |
Cross-sectional |
100 participants; age ≥ 65 y. |
CSI-D using a version in Kikuyu. |
NA |
Apo ϵ4 allele frequency: |
NA |
| Hospital | |||||||
| Demented 31.3%, non-demented 32.2% | |||||||
| 84 controls (men 38) and 16 with dementia participants (men 7) | |||||||
| Ekenze
[21], 2010 |
Nigeria |
Cross-sectional |
8440 admissions; 1249 (men 640) with neurological diseases (age range18-83 y.); 38 (men 23) with dementia |
Not specified |
NA |
3% |
NA |
| Hospital |
2003-2007 |
||||||
| Siddiqi
[69], 2009 |
Zambia |
Cross-sectional |
443 inpatients (men 219); median age 39 y., 67 with HIV; 368 outpatients (men 168); median age 39 y., 58 with HIV; 36 with dementia |
Not specified |
NA |
Dementia: |
Dementia in HIV + patients 8 (13.8%) vs. general population 9 (2.9%) (p = 0.002) |
| Overall: 4.4% | |||||||
| Hospital |
2006 |
||||||
| Yusuf
[74], 2011 |
Nigeria Community |
Cross-sectional |
322 participants (men 128); mean age: 75.5 y |
Screening: CSID/CERAD/SDT |
NA |
Dementia: 2.8% |
Age |
| AD: 1.9% | |||||||
| VaD: 0.6% | |||||||
| Dementia: DSM-IV and ICD-10 | |||||||
| 9 cases of dementia (men 3); mean age: 82.4 y |
LBD: McKhan clinical criteria |
||||||
| FTD: McKeith clinical criteria | |||||||
| Gureje
[51], 2011 |
Nigeria, Community |
Prospective Cohort Baseline 2003-2004 |
2,149 participants at baseline |
10-Word Delayed Recall |
21.80/1,000 |
NA |
Poor social engagement, rural residence, low economic status, female gender, age. |
| Test (cut off of 18) | |||||||
| 1,408 at 39 months follow-up; 85 (among ≥65 y.) developed dementia | |||||||
| Ogunniyi
[52], 2011 |
Nigeria Community |
Cohort study |
1559 participants aged > 65 year without dementia a baseline. 136 (men 33) with dementia (mean age 83.1 y.) at follow-up; 255 with MCI |
Dementia: DSM-III-R and ICD-10 |
Dementia: 8.72/1,000/year |
NA |
Low BMI |
| 1992-2007 |
MCI: 16.35/1000/year |
||||||
| Ogunniy
[53], 2011 |
Nigeria Community |
prospective cohort baseline 1992 |
2718 participants interviewed |
Dementia: DSM-III-R and ICD-10 |
Dementia/AD/VaD (per 1,000/year) 11.50/9.50/1.10 |
NA |
Higher SBP, DBP and PP |
| 1753 (age ≥65 y.) in the final sample | |||||||
| 120 (men 30) with dementia (mean age 83.8 y.); 99 with AD; 11 with VaD | |||||||
| Paraïso
[56], |
Benin Community |
Cross-sectional |
1,139 (men 523) participants; age ≥65 y.; 42 (men 13) with dementia (mean age 79 · 1 y) |
Screening: CSI-D |
NA |
Dementia Overall 3.7% men 1.1% women: 2.5% |
NA |
| 2011 | |||||||
| 2008 | |||||||
| Dementia: DSM-IV | |||||||
| 32 with AD, 105 with CIND | |||||||
| AD: NINCDS-ADRDA |
|
AD Overall 2.8% |
|||||
| VD Overall 0.8% | |||||||
| VaD: NINCDS-AIREN | |||||||
| Amoo
[30], |
Nigeria |
Cross-sectional |
240,294 participants |
Dementia: ICD-10 |
NA |
Dementia: 45/100,000 |
NA |
| 2011 | |||||||
| AD; 25 · 8/100,000 | |||||||
| Hospital |
1998-2007 |
VaD: 7 · 4/100,000 |
|||||
| 108 (men 51) with dementia (mean age: 70.1); 62 (men 24) with AD; 18 (men 13) with VaD; 4 (men 2) with mixed forms; |
ADNINCDS – ADRDA |
||||||
| VaD: NINCDS –AIRENS | |||||||
| LBD: McKeith criteria, | |||||||
| FTD: Lund and Manchester Criteria | |||||||
| 4 (men 2) with FTD; 3 (men 0) with DLB; 13 (men 2) with unclassified dementia | |||||||
| Ndiaye
[31], 2011 |
Senegal |
Cross-sectional |
132 patients seen at a memory clinic (men 41, mean age: 67 y |
Screening: “Test du Senegal”/modified HodKinson test |
NA |
MCI: 14.4% |
NA |
| Hospital | |||||||
| 2004-2005 | |||||||
| 57 with dementia; 37 with AD, 10 with VaD, 5 with FTD and 1 with LBD. |
Dementia: 43.2% |
||||||
| AD: 64.7% of all cases of dementia | |||||||
| MCI: Petersen criteria | |||||||
| Dementia: DSM-IV | |||||||
| Coume
[75], 2012 |
Senegal |
Cross-sectional |
872 (men 546) participants aged >55 y; mean age 67 · 2 y |
Test du Senegal |
NA |
Cognitive impairment 10.8% |
NA |
| Hospital |
2004-2005 |
94 (men 65) with cognitive impairment (74 aged > =65 y) |
|||||
| Baiyewu
[54]., 2012 |
Nigeria |
Cross-sectional/2001 and 2004 |
21 (men 4) participants with normal cognition (mean age 82.8 y.) |
Screening: CSID |
NA |
NA |
NA |
| Dementia: DSM-III-R/ICD-10 | |||||||
| Community |
53 (men 4) with cognitive impairment (mean age 80.9); 34 (men 6) with dementia (mean age 83.3 y) |
AD: NINCDS-ADRDA |
|||||
| Toure
[66], 2012 |
Senegal |
Cross-sectional |
507 participants; age ≥65 y. |
Screening: Aging in Senegal Questionnaire |
NA |
8.9% |
advanced age (Age ≥80 y, OR 4.3, 95% CI 1.4-13), illiteracy, epilepsy, family history of dementia |
| 45 with dementia |
DSM-IV-R |
||||||
| Hospital |
2004-2005 |
||||||
| Longdon
[57], 2012 |
Tanzania |
Cross-sectional |
1198 (men 525) participants; age ≥70 y; 78 with dementia |
Screening: CSI-D |
NA |
6.4% |
Advanced age |
| Community |
2010 |
||||||
| DSM-IV-R | |||||||
| Onwuekwe
[76], 2012 |
Nigeria |
Cross-sectional |
135 participants (men: 79), aged between 16–76 y |
MMSE (cut off of 17 for MCI) |
NA |
MCI: 5.9% |
|
| Hospital |
2004 |
||||||
| 8 with MCI | |||||||
| Guerchet
[55], 2012 |
Central African Republic, Congo |
Cross-sectional |
509 interviewed; 496 (men 218) included in final sample; age ≥65 y.; 188 with cognitive impairment |
Dementia: DSM-IV-R/AD: NINCDS-ADRDA |
NA |
Dementia: 7.4% |
Hypertension, low BMI, depressive symptoms, change of residence, age (OR 2.59, 95% CI, early death of one parent, female gender |
| 2008-2009 | |||||||
| Community |
AD: 5.6% |
||||||
| 546 interviewed; 520 (men 198) included in final sample; age ≥65 y.; 148 with cognitive impairment | |||||||
| Overall 75 (men 15) had dementia 18 with vascular dementia |
AD: Alzheimer’s disease; APOE: Apolipoprotein E; ICD: International Classification of Disease; BMI: Body Mass Index; CI: confidence Interval; CIND: Cognitive Impairment and No Dementia; CSID: Community Screening Interview for Dementia; DSM-III-R: Diagnostic and Statistical Manual 3rd edition revised; MMSE: Mini Mental State Examination; NA: Not available; NFT: Neurofibrillary tangle; NINCDS/ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association; OR: Odd ratio; SCEB: short cognitive evaluation battery; USA: United States of America; VaD: Vascular dementia; y: years.
The proportion of men among those with dementia was 7.1 to 69.1%. The mean age of participants ranged from 70.1 to 83.8 years. When provided, age at clinical diagnosis of disease ranged from 80.7 to 83.8 years. Alzheimer disease was the most common form of the disease, representing 57.4 to 89.4 % of all cases [30-32,34,42,45,55,56,63,71,74], followed by vascular dementia 5.7 to 31.0% of cases [30,31,45,56,74]. Four publications in Nigeria provided incidence data for dementia ranging from 8.7 to 21.8 cases per 1000 per year [35,51-53]. Incidence of Alzheimer disease ranged from 9.5 to 11.5 per 1000 per year [35,53].
The most commonly used tool for dementia screening was the Community Screening Interview for Dementia (CSID) questionnaire applied in 20 publications [32,34,36,37,41-43,45-47,49,50,54,56,65,70]. The diagnosis of dementia mainly relied on the DSM-III-R/DSM-IV and ICD-10 classification [30,32,34-37,40,42-46,52-54,63,70]. The diagnosis of Alzheimer’s disease was based on the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA) criteria [30,32,34,35,41,43,48,50,52-56,75]. Population-based studies that used DSM-III/DSM-IV and ICD-10 for dementia reported prevalences ranging from 1.1 to 8.1% [32,35,42,49,55-57,65,67,74] (ref 13, 16, 23, 30, 36–38, 48, 50, 118). Likewise the prevalence of Alzheimer’s disease ranged from 0.7 to 5.6% based on NINCDS/ADRDA criteria [35,42,55].
Risk factors for dementia were reported in 14 publications. The following were associated with an increased risk of dementia: age (twelve publications), female sex (five publications), low body mass index (three publications), anxiety/depression (three publications), hypertension (three publications), social isolation (two publications), lifetime history of alcohol consumption, elevated total- or LDL cholesterol in those without Apo E ϵ4 (one publication), low socio-economic status, history of stroke and family history of dementia (one publication). The following characteristics were inversely associated with dementia: living with others, use of non-steroidal anti-inflammatory drugs and absence of Apo E ϵ2. Some risk factors were more strongly related to the disease. These include age, which increased the risk of dementia by five to 16% across groups [34,43], but this effect was much higher after the age of 60 years, more than 100% increase risk especially after the age of 75 [46,50,51,55,66,67]. Female sex, low level of education (<6 years), rural residence and family history increased the risk of dementia by >100% [34,43,46,55,56,66].
HIV-related neurocognitive impairment
Fifty-one hospital-based studies (47 publications) reported on HIV-related neurocognitive impairment (Table 3), of which ten were case–control, six cohort and 31 cross-sectional. These studies were conducted in 14 countries including South Africa (14 studies), Uganda (eight studies), Nigeria (six studies), Zambia and Kenya (four studies each), Cameroon and Democratic republic of Congo (three studies each) Ethiopia and Malawi (two studies each), Central African Republic, Botswana, Guinea Bissau, Tanzania and Zimbabwe (one study each). A total of 33 out of the 47 selected publications were published during the last 5 years and only 7 before 2000. The absolute number of participants with HIV-related dementia ranged from 0 to 396, with a prevalence ranging from 0% to 80%.
Table 3.
Overview of studies on HIV-related dementia and risk factors in sub-Saharan
| Author, year of publication | Country/setting | Design/study period | Population characteristics | Diagnostic criteria | Prevalence | Risk factors | Comments |
|---|---|---|---|---|---|---|---|
| Belec
[77], 1989 |
Central African republic, Hospital |
Cross-sectional 1987 |
93 HIV + participants; age and sex not specified |
Not reported |
HAND: 3 cases (3.2%) |
NA |
No neuro-imaging or neuropathological studies |
| Howlet
[78], 1989 |
Tanzania, hospital |
Cross-sectional 1985-1988 |
200 (men 129) HIV + participants; mean age: 32 y |
Decline of memory and other functions |
Dementia complex: 54% |
NA |
|
| Turnbull
[79], 1991 |
South Africa |
Cross-sectional 1982-1983 |
27 haemophilic patients with HIV infection |
Battery of neuropsychological tests: Rey complex figure, Babcock story, digit span, WAIS |
HAND: 4 cases (14.8%) |
NA |
|
| Perriëns
[80], 1992 |
Democratic republic of Congo Hospital |
Cross sectional 2008 |
104 (men 48) HIV + participants; mean age: 34.3 y.; 92 (men 53) HIV- participants; mean age 44 y 9 (men 5) HIV + with HAND |
WHO operational criteria/American Academy of neurology criteria |
HIV Associated |Dementia Complex. 8.7% |
NA |
No neuro-imaging study |
| Maj
[81], 1994 |
Kenya Hospital |
Cross sectional 1990-1991 |
65 (men 49) HIV- participants; mean age: 30 y.; 66 (men 42) asymptomatic HIV + participants; mean age 30.7; 72 (men 48) symptomatic HIV + participants; mean age: 33.2 y |
ICD-10/DSM-IV |
Dementia HIV- 0 Asymptomatic HIV + 0 Symptomatic HIV + 6 (%) |
NA |
|
| Democratic republic of Congo Hospital |
85 ( men 48) HIV- participants; mean age: 33.9 y; 52 (men 33) asymptomatic HIV + participants; mean age 32.3 y.; 68 (men 35) symptomatic HIV + participants; mean age: 33.8 y |
ICD-10/DSM-IV |
Dementia HIV- 0 Asymptomatic HIV + 0 Symptomatic HIV + (5.9%) |
NA |
|
||
| Carson
[82], 1998 |
Kenya |
Cross sectional |
78 (men 52) HIV + participants; mean age: 29.9 y.; 138 (men 114) HIV- participants; mean age 29.8 y. |
Revised WAIS, Trails A and Trails B tests, Digit span, Delayed word and d recognition |
NA |
NA |
No difference in neuropsychiatric test performance between HIV + and HIV- |
| Hospital |
1994 |
||||||
| Sebit
[83], 1995 |
Kenya |
Cross sectional |
191 participants, 72 (men 48) symptomatic HIV + (mean age 33.2 y.), 66 (men 42) asymptomatic HIV + (mean age 30.7) and 65 (men 49) HIV- (mean age 30 y.) |
WHO operational criteria/American Academy of neurology criteria |
Mental disorders: |
NA |
No specific data for HIV associated neurocognitive disorders |
| Hospital | |||||||
| 1990-1991 | |||||||
| Symptomatic HIV + 7.1%, Asymptomatic HIV + 4.5%, HIV -0 | |||||||
| Democratic republic of Congo (DRC)/Hospital |
190 participants, 68 (men 35) symptomatic HIV + (mean age 33.8 y.), 52 (men 33) asymptomatic HIV + (mean age 32.3) and 85 (men 48) HIV- (mean age: 33.9 y.) |
WHO operational criteria/American Academy of neurology criteria |
Mental disorders: |
NA |
No specific data for HIV associated neurocognitive disorders |
||
| symptomatic HIV + 5.9%, asymptomatic HIV + 1.9%, HIV– 1.2% | |||||||
| Sacktor
[84], 2006 |
Uganda, Hospital |
Prospective |
23 (men 5) HIV + participants on |
MSK HIV dementia scale IHDS |
Baseline: Subclinical dementia 35% |
NA |
All participants had CD4 count ≤200 cells/mL and an IHDS ≤ 10 (suggestive of HAND) |
| Cohort study | |||||||
| 2004-2005 | |||||||
| HAART (mean age 32.8 y.) | |||||||
| Re-assessment at 3 and 6 months. | |||||||
| Mild dementia 61% | |||||||
| At 3 (6) months: mild dementia 26% (4%) | |||||||
| Sacktor
[85], 2005 |
Uganda, Hospital |
Cross-sectional 2003-2004 |
81 HIV+; mean age: 37 y.; 100 HIV- mean age: 31.4 y; 21 had HIV dementia |
IHDS (cut off ≤10), |
HIV dementia: 31% |
NA |
|
| MSK HIV dementia scale | |||||||
| Modi
[86], 2007 |
South-Africa, Hospital |
Cross-sectional |
506 HIV + (men 203) on HAART; mean age/range: 37 years 193 had HIV associated dementia |
American Academy of Neurology AIDS Task force |
HIV dementia: 38% |
NA |
75% had CD4 below 100 cells/mm3 |
| 2005 | |||||||
| Clifford
[87], 2007 |
Ethiopia, Hospital |
Case–control |
73 (men 67%) HIV + participants (median age 39 y.); |
IHDS |
NA |
NA |
Quantitative neuropsychiatric tests - no difference between groups |
| 2004 | |||||||
| 87 (men 63%) HIV- participants (median age 38 y.) | |||||||
| Odiase
[88], 2007 |
Nigeria, Hospital |
case–control |
96 (men 48) symptomatic HIV + patients (mean age 33.6 y.), |
FePsy computerized neuropsychological test battery |
NA |
NA |
Severity of immune suppression predictive of cognitive decline |
| 2004 | |||||||
| 96 (men 48) asymptomatic HIV + (mean age 31.5 y.); 96 (men 48) HIV- (mean age 32.9 y.) | |||||||
| Wong
[89], 2007 |
Uganda, Hospital |
Cross-sectional |
78 (men 28) HIV + participants (mean age 37 y.); 24 (men 6) with dementia; 100 HIV – participants |
MSK HIV dementia scale |
HIV dementia. 31% |
Age, low CD4 count associated HIV dementia |
|
| 2003-2004 | |||||||
| Robertson
[90], 2007 |
Uganda, Hospital |
Cross-sectional |
110 (men 34) HIV + participants (WHO Stage 2/3/4, n = 21/69/20); mean age 36.7 y.; 49 on HAART |
MSK HIV dementia scale |
NA |
NA |
Pattern of neuropsychological deficits similar to that in western countries. |
| 2003-2004 | |||||||
| 100 (men 60) HIV– controls (mean age 27.5 y.) | |||||||
| Salawu
[91], 2008 |
Nigeria, hospital |
Cross-sectional |
60 HIV + (men 24), asymptomatic, naïve of HAART; mean age 32 y) |
CSID |
56.7% |
No correlation between CD4 count and performance on neuropsychological testing |
|
| 60 HIV- (men 24); mean age: 30.1 y; | |||||||
| 34 had HIV dementia | |||||||
| Singh
[92], 2008 |
South Africa, Hospital |
Cross-sectional |
20 HIV + (men 8) participants; median age 34 y |
IHDS-criteria (cut-off ≤10) |
HAND: 80% |
NA |
CD4 < 200 cells/mm3, older than 18 years and not be delirious. |
| 2007 |
16 had HAND |
||||||
| Säll
[93], 2009 |
South Africa, Hospital |
Retrospective |
38 HIV + admitted to the psychiatric ward with psychiatric symptoms; mmean age 32.4 y |
DSM-IV |
Dementia: 32% |
NA |
|
| 1987-1997 | |||||||
| 12 had dementia | |||||||
| Ganasen
[94], 2008 |
South Africa, Hospital |
Cross-sectional |
474 (men 123) HIV + patients (328 blacks and 135 coloured); mean age 34 y. |
HIV dementia scale |
HAND: 17.1% (IHDS) and 2.3% (MMSE) |
NA |
|
| MMSE | |||||||
| Njamnshi
[95], 2008 |
Cameroon, Hospital |
Case–control study 2006 |
204 (men 64) HIV + participants (mean age 37.2 y.); 204 (men 64) HIV- participants (mean age 37.1 y.) |
IHDS-criteria (cut-off ≤10) |
HAND: |
NA |
|
| HIV+: 21.1% | |||||||
| HIV-: 2.5% | |||||||
| Sacktor
[96], 2009 |
Uganda, Hospital |
Prospective cohort |
102 (men 29) HIV + never treated patients (mean age 34.2 y.) started on Stavudine-based HAART |
IHDS criteria |
Base line: 40% had HIV dementia (33% mild, 7% moderate) |
NA |
|
| MSK HIV dementia scale | |||||||
| 2005-2007 | |||||||
| Follow-up 6 months | |||||||
| 25 (men 15) HIV- (mean age 30.3 y.) | |||||||
| At 3 months: 26%, 23% mild, 3% moderate | |||||||
| At 6 months: 16% (13% mild, 3% moderate | |||||||
| Njamnshi
[97], 2009 |
Cameroon, Hospital |
Cross-sectional |
185 (men 61) HIV + participants (mean age 37 y.); 41 with possible HAND (mean age 37y.) |
IHDS-criteria |
HAND: 22. 2% |
Advanced clinical stage, low CD4 count, and low haemoglobin levels |
|
| 2006 | |||||||
| Sacktor
[98], 2009 |
Uganda, |
Cross-sectional |
60 HIV + never treated participants; 22 with dementia |
IHDS criteria |
Overall: 36.7% |
HIV subtype D associated with increased risk of HIV dementia |
All participants had CD4, count ≤200 cells/mL and an IHDS ≤ 10 (suggestive of HAND) |
| Hospital | |||||||
| 2005-2007 | |||||||
| MSK HIV dementia scale | |||||||
| Nakasujja
[99], 2010 |
Uganda, |
Prospective cohort |
102 HIV + (men 28); mean age: 34.2 y; 70 with cognitive impairment at baseline |
IHDS (cut-off ≤10) |
Base line: 68.6% |
NA |
|
| Hospital | |||||||
| 2005-2007 | |||||||
| neuropsychological tests and MSK HIV dementia scale |
At 3 months: 36% |
||||||
| At 6 months: 30% | |||||||
| Kinyanda
[100], 2011 |
Uganda, |
Cross-sectional |
618 HIV + (men 169), 83% <45 y |
IHDS (cut-off ≤ 10) |
64% |
|
|
| 396 had cognitive disorders | |||||||
| Hospital |
2010 |
||||||
| Choi
[101], 2011 |
Guinea Bissau, |
Case–control |
22 HIV-2 + (men 4)participants mean age for those with CD4 < 350 = 55.1 y, mean age for those with CD4 ≥ 350 = 50.3 y) |
IHDS |
HIV+: 22.7% (CD4 < 350 = 27%, CD4 ≥ 350 = 18%) |
age (β = -0.11) |
|
| Hospital | |||||||
| 45 HIV- controls (men 1); mean age51 · 9 y) |
MSK HIV dementia scale |
||||||
| Control: 11% | |||||||
| Birbeck
[102], 2011 |
Zambia,, |
Cross-sectional |
496 HIV + (men 205) participants screened within 1 week of initiating ART; mean age 38.1 y) |
I\HDS (cutt-off ≤ 10) |
42.1% (IHDS) |
NA |
Low IHDS score was associated with poor adherence to HAART |
| Hospital |
2006-2007 |
||||||
| MMSE (<=22) |
34.4% (zMMSE) |
||||||
| IHDS administered to 440 participants. | |||||||
| 185 had dementia | |||||||
| Joska
[103], 2010 |
South Africa, Hospital |
Cross-sectional |
536 (men 26.7%) HIV + participants (68% blacks, 28% coloured), mean age 34 y. |
HDS (cutt-off ≤ 10) |
HAND: 23.5% |
Age, education, diagnosed duration, post-traumatic stress disorder |
IDHS not yet available by the time of the study |
| Kanmogne
[104], 2010 |
Cameroon |
Case–control |
43 (men 18) HIV- participants (mean age 33.3 y.); 44 (men 17) HIV + participants (mean age 34.9 y.); 22 with AIDs defining conditions, 34% on HAART |
HIV Neurobehavioral Research Center International neuropsychological test battery |
NA |
NA |
|
| Hospital | |||||||
| 2008-2009 | |||||||
| Lawler
[105], 2010 |
Botswana, |
Cross-sectional |
120 (men 60) HIV + patients (mean age 37.5 y.); 97.5% on HAART; |
IHDS-criteria (cut-off ≤9.5) |
HAND: 38% |
NA |
|
| 2008 |
46 with HIV dementia |
||||||
| Hospital | |||||||
| Patel
[106], 2010 |
Malawi, Hospital |
Cross sectional |
179 (men 63) HIV + participants (mean age 36.7 y.); Stage III/IV 90%; 134 on HAART > 6 months; |
IHDS-criteria (cut-off ≤10) |
HAD |
Female gender, low education |
|
| 2007 | |||||||
| 25 (men 14) with HIV dementia | |||||||
| Overall: 14% | |||||||
| Men: 22.2% | |||||||
| Women: 9.5% | |||||||
| Siddiqi
[69], 2009 |
Zambia |
Cross-sectional |
443 (men 219) inpatients (median age 39 y., 67 HIV+); 368 (men 168) outpatients (median age 39 y., 58 HIV+); Overall 36 cases of dementia |
Not specified |
NA |
HIV+: 10.4% |
HIV + patient had a higher frequency of dementia and had dementia at younger age |
| Hospital | |||||||
| HIV-: 3.3% | |||||||
| Ekenze
[21], 2010 |
Nigeria, Hospital |
Cross-sectional |
8440 admissions; 1249 (men 640) with neurological diseases (mean age 45 y.); 44 (men 18) with AIDS dementia complex |
Not specified |
AIDS dementia complex: 3.5% of all neurological admission |
NA |
|
| 2003-2007 | |||||||
| Holguin
[107], 2011 |
Zambia, Hospital |
Case–control |
57 (men 30) HIV- participants (mean age 28 y.); 83 (men 32) HIV + (mean age 34 y.) including 54 naïve of HAART |
IHDS (cut-off ≤ 10) |
HAND = 22% among HIV + naïve of ARV |
NA |
|
| Color Trails Test 1 and | |||||||
| 2008 |
2, Grooved pegboard Test, and Time Gait Test |
||||||
| Joska
[108], 2011 |
South Africa, Hospital |
Case–control |
94 (men 36) HIV- participants (mean age 25.2 y); 96 (men 20) HIV + (mean age 29.8 y) |
IHDS |
NA |
Education associated with IHDS total score |
Validation study of the IHDS |
| 2008 | |||||||
| Obiabo
[109], 2011 |
Nigeria, |
Prospective Cohort study |
69 (men 25) HIV + participants with CD4 < 350 (mean age 36.2 y.); 30 (men 11) HIV- (mean age 36.6 y.) |
CSID and FePsy computerized neuropsychological test battery |
NA |
NA |
HAART improved neuropsychological performances after 12 months of treatment |
| Hospital | |||||||
| Joska
[110], 2011 |
South Africa Hospital |
Cross-sectional |
170 (men 44) HIV + participants (mean age 29.5 y.)never treated; 43 (men 14) with HIV-dementia; 72 (men 19 with MND |
AAN revised criteria |
Mild neurocognitive disorder: 42.4% HIV dementia: 25.4% |
Education, and male gender independent predictors of HIV-dementia |
|
| 2008-2009 | |||||||
| Robertson
[111], 2011 |
Malawi, |
Cross sectional |
133 (men 39) never treated HIV + patients (median age 31 y.) |
Not provided |
MND: 8% |
|
|
| HAD: 0% | |||||||
| Hospital | |||||||
| South Africa, |
167 (men 60) never treated HIV + patients (median age 34 y.) |
Not provided |
MND: 4% |
|
|
||
| HAD: 0% | |||||||
| Hospital | |||||||
| Zimbabwe, Hospital |
80 (men 31) never treated HIV + patients (median age 36 y.) |
Not provided |
MND: 14% |
NA |
860 HIV + HAART naïve patients with CD4 count < 300 cells/mL and KI ≥70% |
||
| HAD: 3% | |||||||
| Robbins
[112], 2011 |
South Africa, |
Cross-sectional |
65 (men 23) HIV + patients on HAART for ≥6 months (mean age 38.5 y) |
IHDS and Xhosa-validated IHDS |
HIV Associated dementia 80% |
Low CD4 counts, alcohol dependency |
|
| Hospital |
2009-2010 |
||||||
| Kwasa
[113], 2012 |
Kenya, |
Cross sectional |
30 (men 17) HIV + patients (mean age 39 y.) |
Neuropsychological test battery MMSE/IHDS (cut-off ≤10) |
HAD 20% |
NA |
|
| Hospital |
6 (men 5)with HAD |
||||||
| Spies
[114], 2012 |
South-Africa, |
Case–control |
35 HIV + without childhood trauma; mean age: 31.5 y |
Neuropsychological test battery |
NA |
NA |
Significant HIV effects for the Hopkins Verbal Learning Test (HVLT) learning and delay trials and the Halstead Category Test (HCT) |
| Hospital |
48 HIV + with childhood trauma; mean age: 31.7 y |
||||||
| 27 HIV- without childhood trauma; mean: 25y | |||||||
| 20 HIV- with childhood trauma; mean age: 27 · 7 y | |||||||
| All participants were women. | |||||||
| Hestad
[115], 2012 |
Zambia, Hospital |
Case–control |
38 HIV + (men 16); mean age: 28.3 y 42 HIV- (men 18); mean age: 28.9 y |
Neuropsychological tests |
NA |
NA |
HIV + individuals performance lower than that of HIV- on verbal fluency, executive function, speed of information processing, verbal episodic memory and motor function |
| Berhe
[116], 2012 |
Ethiopia, |
Cross-sectional |
347 HIV + (men 176) participants; mean age/range: 34.6 y admitted with neurological disorders |
“cognitive and motor abnormalities, CT/MRI showing brain atrophy and other opportunistic infections ruled out” |
HIV encephalopathy: 0.3% |
NA |
|
| Hospital |
Retrospective |
||||||
| 10 had dementia | |||||||
| 2002-2009 | |||||||
| Joska
[117], 2012 |
South Africa, |
Prospective |
166 HIV + participants assessed at baseline, 108 reassessed at one year (82 received HAART) |
Neuropsychological tests |
NA |
Lower level of education |
Improvement on neuropsychological tests for all participants at one year. |
| Average Global deficit score | |||||||
| Hospital | |||||||
| Breuer
[118], 2012 |
South Africa, |
Cross-sectional |
269 HIV + (men 97) participants on HAART for ≥6; months; 34% aged >40 y) |
IHDS (cut-off ≤10.5) |
HAND: 12% |
NA |
|
| Hospital | |||||||
| Hoare
[119], 2012 |
South Africa |
Cross-sectional |
43 stage III HIV + (24 with at least one ϵ4 ApoE allele, men: 8, Age: 29 y and 19 without the ϵ4 ApoE allele, men: 2, Age: 28 y) |
Neuropsychological test battery |
NA |
Performance on Hodgkin Verbal Learning Tool- Revised was poorer in the group with the ϵ4 genotype. |
|
| Participants with the ϵ4 genotype had more white matter injury on MRI. | |||||||
| Hospital | |||||||
| Oshinaike
[120], 2012 |
Nigeria |
Case–control |
208 HIV + (men 71), mean age: 36.8 y |
IHDS (cut off ≤10) |
HAND by MMSE: 2.9% |
Lower CD4 count |
|
| Hospital |
2007-2008 |
||||||
| 121 HIV – (men: 35), mean age:38.0 y |
MMSE (cut off ≤26) |
||||||
| AAN revised criteria (any value below 2SD) | |||||||
| HAND by IHDS: 54.3% | |||||||
| HAND by AAN: 42.3% | |||||||
| Royal [121], 2012 | Nigeria, Hospital | Cross-sectional | 60 (men 23) never treated HIV + participants (mean age 34 y); |
IHDS (cut off ≤10) | 28.8% HIV + individuals scored abnormally |
Low CD4 count, WHO clinical stage of disease | |
| 56 (men 34) HIV- (mean age 29 · 4 y.); 32 had dementia | |||||||
| 16.0% HIV- individuals scored abnormally |
3TC: Lamivudine; AIDS: Acquired Immunodeficiency Syndrome; CD4: cluster of differentiation 4; CSID: Community Screening Interview for Dementia; CT: computerized tomography; DSM-III-R: Diagnostic and Statistical Manual 3rd edition revised; DSM-IV: Diagnostic and Statistical Manual 4th edition; dT4: Didanosine; FePsy: The Ion Psyche Program; HAART: Highly Active Anti-Retroviral Treatment; HAD: HIV Associated Dementia; HAND: HIV Associated Neurocognitive Disorders; HDS: HIV Dementia Scale; HIV: Human Immunodeficiency Virus; ICD-III-R: International Classification of Disease; IHDS: International HIV Dementia Scale; MSK: Memorial Sloan Kettering; MMSE: Mini Mental State Examination; MND: Mild Neurocognitive Disorder; NA: Not available; NVP: Nevirapine; WHO: World Health Organization; y, years; ZDV: Zidovudine.
The diagnostic tools used to identify HIV-related dementia were variable, making comparison between studies less reliable. However, the International HIV Dementia Scale (IHDS) [89,95,97,105,107-110,112,113,120,121] and the Sloan Memorial Kettering scale [86,89,90,98] were frequently used. Studies that used the IHDS reported a prevalence ranging from 21.1 to 80%. The mean/median age of participants ranged from 31 to 40 years for those with HIV-related dementia, and men represented 25% to 56% of this group. In the nine studies that investigated etiological factors, the identified determinants of HIV-related dementia were: low level of CD4 count (four studies), low level of education, and advanced age (three studies), comorbid psychiatric conditions (two studies each), advance clinical stage (two studies), male sex, HIV-subtype and duration of disease (one study each). The most commonly reported risk factors of HIV associated dementia were the level of CD4 count [89,97,112,120,121] and the clinical stage of disease [97,121].
Amyotrophic lateral sclerosis and cerebellar degeneration
Fifteen studies (12 retrospective, 2 cross-sectional and 1 case-series) (Table 4) including 13 hospital and two community-based studies on amyotrophic lateral sclerosis (ALS) have been conducted in 9 SSA countries including Nigeria (four studies), Senegal (three studies), Ethiopia (2 studies), Zimbabwe, Kenya, South Africa, Sudan, Cameroon and Ivory coast (one study each). The number of participants with ALS ranged from two to 73. Two community-based studies provided a prevalence of 15/100,000 and 5/100,000 respectively in Nigeria [19] and in Ethiopia [122]. Five hospital-based studies provided prevalence figures: between 0.2 and 8.0/1000 of all neurologic consultation/admission [16,21,122-126]. The method of ascertainment of ALS was variable across studies, but electromyography was done in four of the fifteen studies included [125-129]. The proportion of men among those with ALS was 57.6 to 100%. The age of those with ALS ranged from 12 to 84 years. When provided, the age at the clinical onset of ALS ranged from 12 to 71 years and the time to diagnosis from 3 months to more than 15 years. In general, risk factors for ALS were not investigated across studies.
Table 4.
Overview of studies on amyotrophic lateral sclerosis risk factors in sub-Sahara Africa
| Author, year of publication | Country/setting | Design/year | Population characteristics | Diagnostic criteria/tools | Prevalence | Risk factors | Comments |
|---|---|---|---|---|---|---|---|
| Wall
[130],1972 |
Zimbabwe |
Retrospective |
13 (men 10) consecutive patients; age 24–55 y. |
Clinical (no ENMG) |
NA |
NA |
6 participants had sensory changes |
| Hospital-based |
1967-1971 |
||||||
| Osuntokun
[126], 1974 |
Nigeria |
Retrospective |
92 patients with MND ALS 73; PMA 10, SMA 9 |
ENMG/Muscle biopsy/ |
21/100,000 |
NA |
Mean age at onset: 39 y |
| Mean duration of disease exceeded 15 y in 8% of participants | |||||||
| Hospital-based |
1958 -1973 |
||||||
| 4 patients with ALS had poliomyelitis in childhood. | |||||||
| Osuntokun
[19], 1987 |
Nigeria |
Cross-sectional |
18954 participants (men 9282); 58% <20 y and 11% > 50 y |
Screening questionnaire developed by the authors |
MND: 15/100,000 |
NA |
|
| Community-based |
1985 |
||||||
| Cosnett
[125], 1989 |
South Africa Hospital-based |
Retrospective Cases collected during 9.5 y. |
59 blacks (mean age 47.4 y.); 16 whites and 2 coloured (mean age 54 y.) 9 Indians (mean age 54 y) |
Clinical and ENMG in 45% |
Blacks/white & coloured/Indians (per 100,000) 0.88/2 · 7/1.4 |
NA |
Mean age of onset: 47 y (blacks) and 54 y (in whites and Indians) |
| 29% of participants not followed up. | |||||||
| Ekenze
[21], 2010 |
Nigeria |
Retrospective |
8440 admissions; 1249 (men 640) with neurological diseases, mean age 45 y.; 10 (men 4) with ALS |
Not specified |
800/100,000 |
NA |
|
| Hospital-based |
2003-2007 |
||||||
| Abdulla
[127], 1997 |
Sudan |
Retrospective: |
28 (men 17) patients with MND; 19 (men 14) with ALS |
Clinical and ENMG |
NA |
Family history of MND in 14% |
Mean age of onset: 40 y |
| Hospital-based |
1993-1995 |
||||||
| Kengne
[16], 2006 |
Cameroon |
Retrospective |
4041 neurologic consultations; 145 with neurodegenerative diseases 10 (men 8) with ALS; mean age 50.9 y. |
Not provided |
12% of all neurodegeneration 250/100,000 of all neurologic consultation |
|
4 selected degenerative brain diseases: Dementia, PD, ALS and chorea |
| Hospital-based |
1993-2001 |
||||||
| Imam
[131], 2004 |
Nigeria |
Retrospective |
16 (men 15) participants; age 16-60 y. |
El Escorial diagnostic criteria for ALS, no ENMG |
NA |
NA |
|
| Hospital-based |
1980-99 |
||||||
| Adam
[129], 1992 |
Kenya |
Retrospective |
47(men 35) participants with MND; |
Clinical (ENMG in 1/3 of participants) |
NA |
NA |
Duration of disease: 5 m to 4 y. |
| Hospital-based |
1978-88 |
Age 13-80 y |
|||||
| 18 had ALS | |||||||
| Tekle-Haimanot
[122], 1990 |
Ethiopia |
Cross-sectional |
60820 participants (men 29412), 59% aged < 20 y |
Screening questionnaire and neurological exam |
5/100,000 |
NA |
A population survey of neurological diseases |
| Community-based |
1986-88 |
3 (2 men) had MND |
|||||
| Harries
[132], 1955 |
Ethiopia |
Case series |
2(all males) participants |
Clinical (no ENMG) |
NA |
NA |
|
| Age 26 and 30 y | |||||||
| Hospital-based |
1954 |
||||||
| Jacquin-cotton
[123], 1970 |
Senegal |
Retrospective |
6100 participants with neurological disorders |
Clinical (No ENMG) |
290/100,000 |
|
A study of patients with paraplegia in a neurological unit |
| Hospital-based |
1960-1969 |
18 (16 men) participants with ALS, age 25-70 y |
|||||
| Piquemal
[124], 1982 |
Ivory coast |
Retrospective |
4000 participants with neurological disorders |
Clinical (no ENMG) |
750/100,000 |
NA |
Duration of disease: 3 m to 5 y. |
| Hospital-based |
1971-80 |
30 (men 22) participants had ALS, 50% aged <40 y |
|||||
| Collomb
[133], 1968 |
Senegal |
Retrospective |
18 (17 men) participants with ALS, age 25-70 y |
Clinical (no ENMG) |
NA |
NA |
Duration of disease: 4 m to 13 y |
| Hospital-based |
1960-68 |
||||||
| Sene
[128], 2004 |
Senegal Hospital- |
Retrospective |
33 (19 men) participants with ALS; |
El Escorial |
|
|
Definite ALS: 57%, |
| Probable: 30%, Possible ALS: 9% | |||||||
| Suspect ALS: 3% age at onset 14–67 y. | |||||||
| (ENMG in half of the patients) |
|||||||
| based | 1999-2000 | Duration of disease: 6 m to 5 y. |
ALS: amyotrophic lateral sclerosis; ENMG: Electroneuromyography; MND: Motor Neuron Disease; NA: Not available; PMA: Progressive muscular atrophy; SMA: Spinal Muscular Atrophy; y: years; m: months.
One retrospective study in Nigeria reported on two cases (a 32 year old male and a 42 year old female) of cerebellar degeneration among 2 · 1 million admissions over a period of 25 year [14]. One study in Rwanda reported on a family of 33 members, with 15 (including eight men, age at onset 12–49 years) having type 2 spino-cerebellar ataxia [134]. A study in Mauritania reported on 12 cases of cerebellar degeneration-based on clinical criteria, including 9 familial cases (including 7 men, aged 3 to 29 years) and 3 apparently sporadic cases (all men, aged 8 to 50 years) [135]. Another clinic-based study of paraplegia in Senegal reported on 7 cases of spino-cerebellar degeneration among 6100 neurological admissions [123].
Huntington disease
Nineteen studies (four community-based studies and 15 hospital-based) investigated Huntington disease; including 8 cross-sectional studies (including reviews of medical records), 10 case series (two to 13 patients), and one case report (Table 5). The studies were conducted in nine countries: South Africa (nine studies), Zimbabwe and Tanzania (two studies each), Nigeria, Mauritius Island, Senegal, Sudan, Togo and Burkina Faso (one study each). The diagnostic of Huntington disease was mostly clinical, based on a constellation of probing clinical elements; however genetic testing was carried out in five studies [136-140]. The absolute number of participants with Huntington disease ranged from one to 481. Only one community-based study provided a prevalence estimate of 3.5/100,000 in South-Africa [141]. The hospital-based prevalence of Huntington disease when reported ranged from 0.2/100,000 to 46.0/100,000 [138,142-146]. No study reported data on the incidence of Huntington disease. Among those with the disease, males represented 42 to 100%, and age varied from <9 years to 80 years. When provided, the age at the clinical onset of the disease ranged from less than one year to 58 years. In general, antecedent risk factors for Huntington disease were not investigated across studies except for a positive family history reported in 58.3 to 100% of cases.
Table 5.
Overview of studies on Huntington disease and risk factors in sub-Sahara African countries
| Author, year of publication | Country | Setting | Design/year of the study | Population characteristics | Diagnostic tool/criteria | Prevalence |
|---|---|---|---|---|---|---|
| Hayden
[141], 1977 |
South Africa |
Community |
Cross-sectional |
26 cases (men 11); age 12–68 y. |
Clinical |
3.5/100,000 |
| Samuels
[147], 1978 |
Zimbabwe |
Community |
Case series |
1 family of HD |
Clinical |
NA |
| 4 cases (men 2) age 14–26 y. | ||||||
| Glass
[148], 1979 |
South Africa |
Community |
Case series |
2 cases of HD (men 1) age 42-52 |
Clinical |
NA |
| Hayden
[142], 1980 |
South Africa |
Community/hospital |
Cross-sectional, |
481 cases (m en 241) of whom 153 (m en 69) alive by the time of the study |
Clinical |
Overall: 0.65/100,000, Whites: 2.22/100,000, Mixed ancestry: 2.17/100,000, Blacks: 0 · 01/100,000 |
| Scrimgeour
[149], 1981 |
Tanzania |
Community |
Case series |
11 cases, aged 25–80 y. |
Clinical |
NA |
| Hayden
[143], 1981 |
Mauritius |
Hospital |
Cross-sectional |
2166 persons, 6 cases of HD (men 3) |
Not provided |
46/100,000 |
| Hayden
[144], 1981 |
South Africa |
Hospital |
Cross-sectional/NR |
17 children (onset before 20 y.) identified during a national survey among of 219 patients |
Not provided |
Overall: 0.6/100,000 |
| Whites: 0.37/100,000 | ||||||
| Mixed ancestry: 0.89/100,000 | ||||||
| Blacks: No case | ||||||
| Hayden
[150], 1982 |
South Africa |
Community/hospital |
Cross-sectional |
157 (men 71) individuals investigated and 328 (women 156, only 3 negro-Africans) deceased individuals with probably HD |
Not specified |
Combined white and black heterozygote frequency = 6 · 7 x 100,000 |
| Scrimgeour
[151], 1982 |
Tanzania |
Hospital |
Case series (National registry) |
7 patients with chorea (1 aged 80 y.) and 50 potential patients with chorea in 23 families |
Not specified |
NA |
| Mean age at onset: 36 y. | ||||||
| Aiyesimoju
[145], 1984 |
Nigeria |
Hospital |
Cross sectional 1957-1982 |
2.1 million patients admitted to the hospital. |
Not specified |
HD: 0.2/100,000 |
| 4 cases (men 3) of HD aged 24–50 y at diagnosis. | ||||||
| Stephany
[146], 1984 |
Senegal |
Hospital |
Cross sectional |
12370 patients seen in a neurologic clinic; 3 (men 2) with HD; age 31–64 y. |
Family history |
24.2/100,000 |
| All patients had movement disorders and neuropsychiatric features | ||||||
| 1960-1980 | ||||||
| Joubert
[136], 1988 |
South Africa |
Community/hospital |
Cross-sectional 1983-1986 |
8 cases in hospital setting (n = 6. all men) and at home (n = 2); |
Clinical/genetic testing/screening for Wilson disease |
NA |
| Age at onset: 8–47 y. | ||||||
| Age at diagnosis: 13–50 y. | ||||||
| Scrimgeour
[152], 1992 |
Zimbabwe |
Hospital |
Case series1991 |
11 cases in a 4 generation of a single family; 2 probable cases |
Clinical |
0.5/100,000 |
| Scrimgeour
[153], 1995 |
Sudan |
Hospital |
Case-report |
1 case of HD: A |
Clinical/MRI |
NA |
| 40 year old black Sudanese man | ||||||
| Grunitzky
[154], 1995 |
Togo |
Hospital |
Case series |
A family including 8 patients with HD and 67 at risk across 6 generations; mean age at onset: 33 y. |
Not specified |
NA |
| Silber
[137], 1998 |
South Africa |
Community |
Case series |
5 families of HD including a total of 7 genetically confirmed cases of HD and 10 clinically suspect cases of HD |
Clinical/genetic testing |
NA |
| Kabore
[138], 2000 |
Burkina-Faso |
Hospital |
Case series |
4 cases of HD; age at diagnosis 33–43 y. |
Clinical/genetic testing |
0.04/100,000 |
| Bardien
[139], 2007 |
South Africa |
Hospital |
Case series 2001-2005 |
A family with HD like 2 |
Clinical/genetic testing |
1 |
| Total 39 family members | ||||||
| 13 had the disease | ||||||
| Magazi [140], 2008 | South Africa | Hospital | Case series | 12 cases (men 6); age 25–52 y. | Clinical/genetic testing | NA |
HD; Huntington disease; MRI: magnetic resonance imaging; NA: not applicable; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; y: year.
Discussion
This review represents an unprecedented effort to summarize epidemiological data on neurodegenerative diseases in SSA. However, this being a large diverse multicultural and multiethnic region, it is difficult to reliably quantify and compare the burden of neurodegenerative disorders across countries. Although mostly based on prevalent cases and on retrospective data, from studies that have essentially included urban populations, findings summarized in the current review are very informative.
The most widely investigated and prevalent neurodegenerative condition appeared to be dementia with most cases being of Alzheimer disease type. Major risk factors of AD include an advanced age (higher after the age of 60), female sex, a low schooling (less than 6 year of education), family background and rural residence. Unlike North America, Australia, Europe, and Japan where several population-based studies have been conducted on dementia, good quality epidemiological studies (prospective, population-based, using standardized criteria) are scanty in SSA, with methodological issues hampering any meaningful comparison with other regions of the world. The reported prevalence in one collaborative good quality study in Nigeria about 20 years ago among those aged >60 years was 2.3%. This was lower than the reported prevalence in developing countries, but within the range of reports from developing countries in Asia and Latin America where reported prevalence range from 1.9 to 3.8% [155]. The anticipated ageing of the population (which is the main driver of dementia figures) in Africa may translate in a higher prevalence and absolute number of people living with dementia as observed in other developing regions. However, caution is needed when interpreting findings from studies conducted in different settings by different investigators. Our overview tends to suggest that the projected increase in the prevalence of dementia in SSA is likely, based on the comparison of findings from three recent studies with those from the study above conducted in Nigeria 20 years ago [55-57]. Furthermore, with the large scale implementation of antiretroviral therapy and related improved survival, it is expected that the number of patients with the diagnosis of HIV-related neurocognitive impairment may increase as suggested by the increasing number of related-publications. Such trends will need to be confirmed by large scale prospective observational studies which will also assess the putative accelerating effect of HIV-related neurocognitive impairment on other types of prevalent dementia and neurodegeneration.
For Parkinsonism, the wide prevalence range observed both in population and hospital-based studies might also be a consequence of differences in methodologies for case ascertainment, diagnostic criteria, or age distributions of the study populations. These heterogeneities in PD prevalence are not unique to SSA as these have also been observed in Europe where prevalence of PD ranged from 66 to 12,500/100,000 [156]. There have been provisional set of minimal scientific criteria for conducting epidemiological studies on PD which, when adopted at a large scale will improve comparison within SSA and between SSA and other regions of the world [156]. Prevalence rates reported in population-based studies in the continent are limited to two studies and cases were ascertained through screening and neurological exam in one study, thus making any comparison with other region difficult. In ALS and Huntington disease, the picture is less clear as the majority of studies were hospital-based, retrospective in nature, with a final diagnosis not always based on pathology or genetics and the risk factors not properly assessed; thus making comparisons and inferences inaccurate. For these two conditions therefore, important gaps remain to be filled, without which the issues of prevention and control will not be efficiently addressed in the African context.
The comparatively higher number of population-based investigations of dementia relative to other neurodegenerative conditions in SSA, may at least in part be explained by the availability of standardized and widely accepted screening and diagnostic tools/criteria which facilitate epidemiological studies of dementia [157] as compared with other conditions where existing tools have not always been validated in different settings and therefore remain unpopular [158,159], or which, by the virtue of their low prevalence makes any assessment in the general population difficult and very expensive. There are context-specific challenges to obtaining key epidemiological data on neurodegenerative conditions in SSA including the low level of patient education, the need to accurately translate available screening and diagnostic tools to local languages, limited number of scientists and clinicians in neurosciences, and competing health interest in the setting of limited financial resources [5,16].
Needs in terms of epidemiological data
In order to improve the knowledge base of each of the neurodegenerative conditions addressed in this review, two main types of epidemiological studies appear necessary and feasible in SSA. A population-based prevalence and incidence study including both urban and rural populations, in order to capture the real variability in socio-economic status and possibility in other factors that may exist in the population. Such a study may serve a dual purpose, providing information on disease rate and identification of key risk factors, as it would permit to establish the sequence of events. Given that such an undertaking could be planned beforehand, it offers the possibility of addressing multiple questions and/or diseases at a reduced cost. Inclusion of a large enough but manageable number of participants would be necessary to ensure adequate precision around the estimates generated. As many patients with possible neurodegenerative conditions would be tempted to consult traditional healers rather than accessing health facilities in SSA, special efforts would be required to ensure that these people are captured by such a study. Also, ascertaining cases of neurodegenerative conditions in a population-based sample may be costly and logistically challenging, particularly with regard to the asymptomatic or mildly symptomatic nature of early stages of some of the diseases, and the lack of validated instruments and appropriate expertise.
A second type of epidemiological study is a multicenter, hospital-based, registry investigation. The latter has several advantages over a single large-scale cohort study. Large numbers of cases could potentially be collected over a relatively short period of time, with the possibility of comparing resources and outcomes within and across countries. However, the major limitations of this approach include the costs associated with the effort and infrastructure for coordination and communication between centers, as well as data capture and ongoing monitoring and quality control. In addition, there are biases inherent to any such hospital-based study, especially given that in SSA there is major access and cost barriers to care, with a sizeable proportion of patients with neurodegenerative conditions who are never seen by health care providers thus limiting the scope of registries. The degree of such selection bias is likely to vary considerably across centers, affecting both case mix and outcomes. The approach would therefore not provide a study population fully representative of incident cases and the natural history of disease and its management.
For both types of studies, the definition of the pool of people ‘at-risk’ population could be challenging in the SSA context, given the lack of formal census of the population in many countries; thus making reliable estimation of the effect of individual risk factors difficult. Other methodological issues relate to the assessment of the outcome in a reliable fashion in the African context as discussed above. Hence, a combination of the aforementioned study approaches would probably overcome some of their respective limitations and improve the quality of estimates generated.
The challenges to performing high quality incidence and prevalence studies of neurodegenerative diseases are well known [159]. Cases of most neurodegenerative conditions are difficult to define and ascertain reliably in population-based sample, and there are problems in relating events and the effects of different exposures to defined ‘at-risk’ populations. With the ageing of the population in SSA, the importance of HIV/AIDS, as well as the surge in risk factors such as hypertension and diabetes that have been linked to dementia [157,160,161] and possibly to Parkinson diseases [162,163], the importance of neurodegenerative disorders would considerably increase over time. Indeed, by 2025, the numbers of people aged 60 years and over will more than double in many countries [164]. With this rapid demographic and nutritional transition, neurodegenerative conditions would become an important public health problem in SSA. Critical investments are therefore necessary to improve surveillance and program-relevant research to provide an evidence base for policy development and effective control and prevention of neurodegenerative diseases. Precise identification of risk factors other than ageing would allow proper prevention effort spanning from primordial to secondary and event tertiary prevention, given that most of those conditions are associated with higher levels of disability and increased risk of death. Community-based risk factor control, combined with high risk approaches and realignment of health systems to incorporate the chronic management of neurodegenerative diseases are needed.
Strengths and limitations of the review
Our review is the first of its kind on neurodegenerative conditions in SSA. It is more up-to-date and broader than previous attempts to summarize evidence on single diseases in this setting [4-8]. By systematically assessing all published articles on these conditions, we aimed to draw the attention on the importance of the conditions in the region, and identify the research priorities. A limitation of this review is inherent to the limitations of the individual studies included. We relied on clinic-based studies where necessary in this systematic review; but such studies have limitations, particularly with regard to the generalization of their results data. However, we have tried to convey a clear understanding of the current burden and risk factors of each condition by examining all published papers across a broad range of clinical, biology, public health, and psychosocial literature, incorporating various types of evidence. By the nature of the disease, the age range for participants in studies on ALS and HIV-related neurocognitive impairment extended to the pediatric age for some studies. It is of note that large number of studies are realized in hospital in Africa, often published in local journals or reported in thesis. It the absence of straightforward strategies for capturing this sort of evidence in a systematic way, we did not account for them, which may have lowered the number of results found in some countries. Finally, the many sources of heterogeneity precluded any meaningful assessed of the quality of the included studies.
Conclusion
This review summarizes the body of literature on neurodegenerative disorders in SSA, which is large with regard to Dementia and HIV-related neurocognitive disorders but limited for other neurodegenerative disorders. In addition, it emphasizes some of the challenges in conducting good quality, population-based studies on the continent including the lack of standardized criteria for some neurodegenerative disorders, with most studies limited to few regions/countries on the continent. High-quality prospective cohort studies, which would use internationally- validated criteria, wide catchment areas in several geographic regions, and adjust for the projected ageing of the continent population, by compensating for the imprecise nature of the available data, will help map the epidemiology of neurodegenerative diseases in SSA and improve comparisons with the rest of the world.
Competing interest
The authors declare that they have no competing interests.
Authors’ contribution
All authors equally contributed. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Search terms and strategies.
Contributor Information
Alain Lekoubou, Email: Lekoub77@yahoo.com.
Justin B Echouffo-Tcheugui, Email: justin.basile.echouffo.tcheugui@emory.edu.
Andre P Kengne, Email: andre.kengne@mrc.ac.za.
References
- World Population Prospects: The 2012 Revision. http://esa.un.org/unpd/wpp/unpp/panel_indicators.htm.
- World Population Ageing 2013. http://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeingReport2013.pdf.
- Mateen FJ, Mills EJ. Aging and HIV-related cognitive loss. Jama. 2012;308(4):349–350. doi: 10.1001/jama.2012.8538. [DOI] [PubMed] [Google Scholar]
- Okubadejo NU, Bower JH, Rocca WA, Maraganore DM. Parkinson's disease in Africa: A systematic review of epidemiologic and genetic studies. Mov Disord. 2006;21(12):2150–2156. doi: 10.1002/mds.21153. [DOI] [PubMed] [Google Scholar]
- Cilia R, Akpalu A, Cham M, Bonetti A, Amboni M, Faceli E, Pezzoli G. Parkinson’s disease in sub-Saharan Africa: step-by-step into the challenge. Neurodegener Dis Manag. 2011;1(3):194–202. [Google Scholar]
- Ineichen B. The epidemiology of dementia in Africa: a review. Soc Sci Med. 2000;50(11):1673–1677. doi: 10.1016/s0277-9536(99)00392-5. [DOI] [PubMed] [Google Scholar]
- George-Carey R, Adeloye D, Chan KY, Paul A, Kolcic I, Campbell H, Rudan I. An estimate of the prevalence of dementia in Africa: A systematic analysis. J Glob Health. 2012;2(2):20401. doi: 10.7189/jogh.02.020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin B, Kacem I, Diagana M, Boulesteix M, Gouider R, Preux PM, Couratier P. Juvenile and adult-onset ALS/MND among Africans: incidence, phenotype, survival: a review. Amyotroph Lateral Scler. 2012;13(3):276–283. doi: 10.3109/17482968.2011.648644. [DOI] [PubMed] [Google Scholar]
- Hendrie HC, Murrell J, Gao S, Unverzagt FW, Ogunniyi A, Hall KS. International studies in dementia with particular emphasis on populations of African origin. Alzheimer Dis Assoc Disord. 2000;20:S42–S46. doi: 10.1097/00002093-200607001-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JH, Teshome M, Melaku Z, Zenebe G. Frequency of movement disorders in an Ethiopian university practice. Mov Disord. 2005;20(9):1209–1213. doi: 10.1002/mds.20567. [DOI] [PubMed] [Google Scholar]
- Akinyemi RO, Okubadejo NN, Akinyemi JO, Owolabi MO, Owolabi LF, Ogunniyi A. Cognitive dysfunction in Nigerians with Parkinson's disease. Mov Disord. 2008;23(10):1378–1383. doi: 10.1002/mds.22087. [DOI] [PubMed] [Google Scholar]
- Cosnett JE, Bill PL. Parkinson's disease in blacks. Observations on epidemiology in Natal. S Afr Med J. 1988;73(5):281–283. [PubMed] [Google Scholar]
- Dotchin C, Msuya O, Kissima J, Massawe J, Mhina A, Moshy A, Aris E, Jusabani A, Whiting D, Masuki G, Walker R. The prevalence of Parkinson's disease in rural Tanzania. Mov Disord. 2008;23(11):1567–1672. doi: 10.1002/mds.21898. [DOI] [PubMed] [Google Scholar]
- Schoenberg BS, Osuntokun BO, Adeuja AO, Bademosi O, Nottidge V, Anderson DW, Haerer AF. Comparison of the prevalence of Parkinson's disease in black populations in the rural United States and in rural Nigeria: door-to-door community studies. Neurology. 1988;38(4):645–646. doi: 10.1212/wnl.38.4.645. [DOI] [PubMed] [Google Scholar]
- Winkler AS, Tutuncu E, Trendafilova A, Meindl M, Kaaya J, Schmutzhard E, Kassubek J. Parkinsonism in a population of northern Tanzania: a community-based door-to-door study in combination with a prospective hospital-based evaluation. J Neurol. 2010;257(5):799–805. doi: 10.1007/s00415-009-5420-z. [DOI] [PubMed] [Google Scholar]
- Kengne AP, Dzudie A, Dongmo L. Epidemiological features of degenerative brain diseases as they occurred in Yaounde referral hospitals over a 9-year period. Neuroepidemiology. 2006;27(4):208–211. doi: 10.1159/000096609. [DOI] [PubMed] [Google Scholar]
- Lombard A, Gelfand M. Parkinson's disease in the African. Cent Afr J Med. 1978;24(1):5–8. [PubMed] [Google Scholar]
- Osuntokun BO, Bademosi O. Parkinsonism in the Nigerian African: a prospective study of 217 patients. East Afr Med J. 1979;56(11):597–607. [PubMed] [Google Scholar]
- Osuntokun BO, Adeuja AO, Schoenberg BS, Bademosi O, Nottidge VA, Olumide AO, Ige O, Yaria F, Bolis CL. Neurological disorders in Nigerian Africans: a community-based study. Acta Neurol Scand. 1987;75(1):13–21. doi: 10.1111/j.1600-0404.1987.tb07883.x. [DOI] [PubMed] [Google Scholar]
- Haylett WL, Keyser RJ, du Plessis MC, van der Merwe C, Blanckenberg J, Lombard D, Carr J, Bardien S. Mutations in the parkin gene are a minor cause of Parkinson's disease in the South African population. Parkinsonism Relat Disord. 2012;18(1):89–92. doi: 10.1016/j.parkreldis.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Ekenze OS, Onwuekwe IO, Ezeala Adikaibe BA. Profile of neurological admissions at the University of Nigeria Teaching Hospital Enugu. Niger J Med. 2010;19(4):419–422. doi: 10.4314/njm.v19i4.61967. [DOI] [PubMed] [Google Scholar]
- Owolabi LF, Shehu MY, Shehu MN, Fadare J. Pattern of neurological admissions in the tropics: Experience at Kano, Northwestern Nigeria. Ann Indian Acad Neurol. 2010;13(3):167–170. doi: 10.4103/0972-2327.70875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubadejo NU, Ojini FI, Danesi MA. Longitudinal study of mortality predictors in Parkinson's disease in Nigerians. Afr J Med Med Sci. 2005;34(4):365–369. [PubMed] [Google Scholar]
- Okubadejo NU, Danesi MA. Frequency and predictors of autonomic dysfunction in Parkinson's disease: a study of African patients in Lagos, Nigeria. Niger Postgrad Med J. 2004;11(1):45–49. [PubMed] [Google Scholar]
- Okubadejo NU, Ojo OO, Oshinaike OO. Clinical profile of parkinsonism and Parkinson's disease in Lagos, Southwestern Nigeria. BMC neurology. 2010;10:1. doi: 10.1186/1471-2377-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser RJ, Lesage S, Brice A, Carr J, Bardien S. Assessing the prevalence of PINK1 genetic variants in South African patients diagnosed with early- and late-onset Parkinson's disease. Biochem Biophys Res Commun. 2010;398(1):125–129. doi: 10.1016/j.bbrc.2010.06.049. [DOI] [PubMed] [Google Scholar]
- van der Merwe C, Haylett W, Harvey J, Lombard D, Bardien S, Carr J. Factors influencing the development of early- or late-onset Parkinson's disease in a cohort of South African patients. S Afr Med J. 2012;102(11 Pt 1):848–851. doi: 10.7196/samj.5879. [DOI] [PubMed] [Google Scholar]
- Femi OL, Ibrahim A, Aliyu S. Clinical profile of parkinsonian disorders in the tropics: Experience at Kano, northwestern Nigeria. J Neurosci Rural Pract. 2012;3(3):237–241. doi: 10.4103/0976-3147.102589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilia R, Sironi F, Akpalu A, Cham M, Sarfo FS, Brambilla T, Bonetti A, Amboni M, Goldwurm S, Pezzoli G. Screening LRRK2 gene mutations in patients with Parkinson's disease in Ghana. J Neurol. 2012;259(3):569–570. doi: 10.1007/s00415-011-6210-y. [DOI] [PubMed] [Google Scholar]
- Amoo G, Akinyemi RO, Onofa LU, Akinyemi JO, Baiyewu O, Ogunlesi AO, Ogunniyi A. Profile of clinically-diagnosed dementias in a neuropsychiatric practice in Abeokuta, South-Western Nigeria. Afr J Psychiatry. 2011;14(5):377–382. doi: 10.4314/ajpsy.v14i5.5. [DOI] [PubMed] [Google Scholar]
- Ndiaye D, Sylla A, Toure K, Thiam MH, Gueye M. Bilan de fonctionnement d’une clinique de la memoire senegalaise. AJNS. 2011;30(1):1–9. [Google Scholar]
- Hendrie HC, Osuntokun BO, Hall KS, Ogunniyi AO, Hui SL, Unverzagt FW, Gureje O, Rodenberg CA, Baiyewu O, Musick BS. Prevalence of Alzheimer's disease and dementia in two communities: Nigerian Africans and African Americans. Am J Psychiatry. 1995;152(10):1485–1492. doi: 10.1176/ajp.152.10.1485. [DOI] [PubMed] [Google Scholar]
- Ogeng'o JA, Cohen DL, Sayi JG, Matuja WB, Chande HM, Kitinya JN, Kimani JK, Friedland RP, Mori H, Kalaria RN. Cerebral amyloid beta protein deposits and other Alzheimer lesions in non-demented elderly east Africans. Brain pathology. 1996;6(2):101–107. doi: 10.1111/j.1750-3639.1996.tb00790.x. [DOI] [PubMed] [Google Scholar]
- Hall K, Gureje O, Gao S, Ogunniyi A, Hui SL, Baiyewu O, Unverzagt FW, Oluwole S, Hendrie HC. Risk factors and Alzheimer's disease: a comparative study of two communities. Aust N Z J Psychiatry. 1998;32(5):698–706. doi: 10.3109/00048679809113126. [DOI] [PubMed] [Google Scholar]
- Hendrie HC, Ogunniyi A, Hall KS, Baiyewu O, Unverzagt FW, Gureje O, Gao S, Evans RM, Ogunseyinde AO, Adeyinka AO, Musick B, Hui SL. Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA. 2001;285(6):739–747. doi: 10.1001/jama.285.6.739. [DOI] [PubMed] [Google Scholar]
- Perkins AJ, Hui SL, Ogunniyi A, Gureje O, Baiyewu O, Unverzagt FW, Gao S, Hall KS, Musick BS, Hendrie HC. Risk of mortality for dementia in a developing country: the Yoruba in Nigeria. Int J Geriatr Psychiatry. 2002;17(6):566–573. doi: 10.1002/gps.643. [DOI] [PubMed] [Google Scholar]
- Lane KA, Gao S, Hui SL, Murrell JR, Hall KS, Hendrie HC. Apolipoprotein E and mortality in African-Americans and Yoruba. J Alzheimers Dis. 2003;5(5):383–390. doi: 10.3233/jad-2003-5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuntokun BOOA, Junaid TA, Lekwauwa UG. Autopsy survey for Alzheimer's disease in Nigerian Africans: a preliminary report. Afr J Med Med Sci. 1995;24(1):75–79. [PubMed] [Google Scholar]
- Ben-Arie OSL, Teggin AF, Elk R. The coloured elderly in Cape Town--a psychosocial, psychiatric and medical community survey. Part II. Prevalence of psychiatric disorders. S Afr Med J. 1983;64(27):1056–1061. [PubMed] [Google Scholar]
- Ogunniyi AO, Osuntokun BO, Lekwauwa UB, Falope ZF. Rarity of dementia (by DSM-III-R) in an urban community in Nigeria. East Afr Med J. 1992;69(2):64–68. [PubMed] [Google Scholar]
- Osuntokun BO, Sahota A, Ogunniyi AO, Gureje O, Baiyewu O, Adeyinka A, Oluwole SO, Komolafe O, Hall KS, Unverzagt FW, Hui SL, Yang M, Hendrie HC. Lack of an association between apolipoprotein E epsilon 4 and Alzheimer's disease in elderly Nigerians. Ann Neurol. 1995;38(3):463–465. doi: 10.1002/ana.410380319. [DOI] [PubMed] [Google Scholar]
- Ogunniyi A, Gureje O, Baiyewu O, Unverzagt F, Hall KS, Oluwole S, Osuntokun BO, Hendrie HC. Profile of dementia in a Nigerian community–types, pattern of impairment, and severity rating. J Natl Med Assoc. 1997;89(6):392–396. [PMC free article] [PubMed] [Google Scholar]
- Ogunniyi A, Baiyewu O, Gureje O, Hall KS, Unverzagt F, Siu SH, Gao S, Farlow M, Oluwole OS, Komolafe O, Hendrie HC. Epidemiology of dementia in Nigeria: results from the Indianapolis-Ibadan study. Eur J Neurol. 2000;7(5):485–490. doi: 10.1046/j.1468-1331.2000.00124.x. [DOI] [PubMed] [Google Scholar]
- Baiyewu O, Unverzagt FW, Ogunniyi A, Hall KS, Gureje O, Gao S, Lane KA, Hendrie HC. Cognitive impairment in community-dwelling older Nigerians: clinical correlates and stability of diagnosis. Eur J Neurol. 2002;9(6):573–580. doi: 10.1046/j.1468-1331.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- Ogunniyi A, Hall KS, Baiyewu O, Gureje O, Unverzagt FW, Gao S, Hendrie HC. Caring for individuals with dementia: the Nigerian experience. West Afr J Med. 2005;24(3):259–262. doi: 10.4314/wajm.v24i3.28211. [DOI] [PubMed] [Google Scholar]
- Gureje O, Ogunniyi A, Kola L. The profile and impact of probable dementia in a sub-Saharan African community: Results from the Ibadan Study of Aging. J Psychosom Res. 2006;61(3):327–333. doi: 10.1016/j.jpsychores.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochayi B, Thacher TD. Risk factors for dementia in central Nigeria. Aging Ment Health. 2006;10(6):616–620. doi: 10.1080/13607860600736182. [DOI] [PubMed] [Google Scholar]
- Hall K, Murrell J, Ogunniyi A, Deeg M, Baiyewu O, Gao S, Gureje O, Dickens J, Evans R, Smith-Gamble V, Unverzagt FW, Shen J, Hendrie H. Cholesterol, APOE genotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba. Neurology. 2006;66(2):223–227. doi: 10.1212/01.wnl.0000194507.39504.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerchet M, M'Belesso P, Mouanga AM, Bandzouzi B, Tabo A, Houinato DS, Paraiso MN, Cowppli-Bony P, Nubukpo P, Aboyans V, Clément JP, Dartigues JF, Preux PM. Prevalence of dementia in elderly living in two cities of Central Africa: the EDAC survey. Dement Geriatr Cogn Disord. 2010;30(3):261–268. doi: 10.1159/000320247. [DOI] [PubMed] [Google Scholar]
- Guerchet M, Houinato D, Paraiso MN, von Ahsen N, Nubukpo P, Otto M, Clement JP, Preux PM, Dartigues JF. Cognitive impairment and dementia in elderly people living in rural Benin, west Africa. Dement Geriatr Cogn Disord. 2009;27(1):34–41. doi: 10.1159/000188661. [DOI] [PubMed] [Google Scholar]
- Gureje O, Ogunniyi A, Kola L, Abiona T. Incidence of and risk factors for dementia in the Ibadan study of aging. J Am Geriatr Soc. 2011;59(5):869–874. doi: 10.1111/j.1532-5415.2011.03374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunniyi A, Gao S, Unverzagt FW, Baiyewu O, Gureje O, Nguyen JT, Smith-Gamble V, Murrell JR, Hake AM, Hall KS, Hendrie HC. Weight loss and incident dementia in elderly Yoruba Nigerians: a 10-year follow-up study. Int Psychogeriatr. 2011;23(3):387–394. doi: 10.1017/S1041610210001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunniyi A, Lane KA, Baiyewu O, Gao S, Gureje O, Unverzagt FW, Murrell JR, Smith-Gamble V, Hall KS, Hendrie HC. Hypertension and incident dementia in community-dwelling elderly Yoruba Nigerians. Acta Neurol Scand. 2011;124(6):396–402. doi: 10.1111/j.1600-0404.2011.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiyewu O, Unverzagt FW, Ogunniyi A, Smith-Gamble V, Gureje O, Lane KA, Gao S, Hall KS, Hendrie HC. Behavioral symptoms in community-dwelling elderly Nigerians with dementia, mild cognitive impairment, and normal cognition. Int J Geriatr Psychiatry. 2012;27(9):931–939. doi: 10.1002/gps.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerchet M, Mouanga AM, M'Belesso P, Tabo A, Bandzouzi B, Paraiso MN, Houinato DS, Cowppli-Bony P, Nubukpo P, Aboyans V, Clément JP, Dartigues JF, Preux PM. Factors associated with dementia among elderly people living in two cities in Central Africa: the EDAC multicenter study. J Alzheimers Dis. 2012;29(1):15–24. doi: 10.3233/JAD-2011-111364. [DOI] [PubMed] [Google Scholar]
- Paraiso MN, Guerchet M, Saizonou J, Cowppli-Bony P, Mouanga AM, Nubukpo P, Preux PM, Houinato DS. Prevalence of Dementia among Elderly People Living in Cotonou, an Urban Area of Benin (West Africa) Neuroepidemiology. 2011;36(4):245–251. doi: 10.1159/000328255. [DOI] [PubMed] [Google Scholar]
- Longdon AR, Paddick SM, Kisoli A, Dotchin C, Gray WK, Dewhurst F, Chaote P, Teodorczuk A, Dewhurst M, Jusabani AM, Walker R. The prevalence of dementia in rural Tanzania: a cross-sectional community-based study. Int J Geriatr Psychiatry. 2013;28(7):728–737. doi: 10.1002/gps.3880. [DOI] [PubMed] [Google Scholar]
- Lambo TA. Psychiatric disorders in the aged: epidemiology and preventive measures. West Afr Med J. 1966;15(3):121–124. [PubMed] [Google Scholar]
- Makanjuola RO. Psychiatric disorders in elderly Nigerians. Trop Geogr Med. 1985;37(4):348–351. [PubMed] [Google Scholar]
- Gureje O, Osuntokun BO, Makanjuola JD. Neuropsychiatric disorders in Nigerians: 1914 consecutive new patients seen in 1 year. Afr J Med Med Sci. 1989;18(3):203–209. [PubMed] [Google Scholar]
- Osuntokun BO, Ogunniyi A, Akang EE, Aghadiuno PU, Ilori A, Bamgboye EA, Beyreuther K, Masters C. Beta A4-amyloid in the brains of non-demented Nigerian Africans. Lancet. 1994;343(8888):56. doi: 10.1016/s0140-6736(94)90910-5. [DOI] [PubMed] [Google Scholar]
- Sayi JG, Patel NB, Premkumar DR, Adem A, Winblad B, Matuja WB, Mtui EP, Gatere S, Friedland RP, Koss E, Kalaria RN. Apolipoprotein E polymorphism in elderly east Africans. East Afr Med J. 1997;74(10):668–670. [PubMed] [Google Scholar]
- Baiyewu O, Adeyemi JD, Ogunniyi A. Psychiatric disorders in Nigerian nursing home residents. Int J Geriatr Psychiatry. 1997;12(12):1146–1150. doi: 10.1002/(sici)1099-1166(199712)12:12<1146::aid-gps679>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Uwakwe R. Satisfaction with dementia care–giving in Nigeria–a pilot investigation. Int J Geriatr Psychiatry. 2006;21(3):296–297. doi: 10.1002/gps.1500. [DOI] [PubMed] [Google Scholar]
- Chen CH, Mizuno T, Elston R, Kariuki MM, Hall K, Unverzagt F, Hendrie H, Gatere S, Kioy P, Patel NB, Friedland RP, Kalaria RN. A comparative study to screen dementia and APOE genotypes in an ageing East African population. Neurobiol Aging. 2010;31(5):732–740. doi: 10.1016/j.neurobiolaging.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toure KCM, Ndiaye M, Zunzunegui MV, Bacher Y, Diop AG, Ndiaye MM. Risk factors for dementia in a senegalese elderly population aged 65 years and over. Dement Geriatr Cogn Dis. 2012;2:160–168. doi: 10.1159/000332022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toure K, Coume M, Ndiaye ND, Thiam MH, Zunzunegui MV, Bacher Y, Tal DA, Gueye L, Sene-Diouf F, Ndiaye M, Thiam A, Amadou GD, Ndiaye MM. Facteur de risque de démence dans une population de personne âgées sénégalaises. Afr J Neurol Sci. 2009;28(1):1–15. [Google Scholar]
- Napon CTI, Niakara A, Ouango JG, Kabre A, Kabore J. Les demences en Afrique sub-Saharienne : Aspects cliniques et etiologiques en milieu hospitalier a Ouagadougou (Bourkina Fasso) AJNS. 2009;28(1):37–43. [Google Scholar]
- Siddiqi OK, Atadzhanov M, Birbeck GL, Koralnik IJ. The spectrum of neurological disorders in a Zambian tertiary care hospital. J Neurol Sci. 2009;290(1–2):1–5. doi: 10.1016/j.jns.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwakwe R. Psychiatric morbidity in elderly patients admitted to non-psychiatric wards in a general/teaching hospital in Nigeria. Int J Geriatr Psychiatry. 2000;15(4):346–354. doi: 10.1002/(sici)1099-1166(200004)15:4<346::aid-gps125>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Gureje O, Ogunniyi A, Baiyewu O, Price B, Unverzagt FW, Evans RM, Smith-Gamble V, Lane KA, Gao S, Hall KS, Hendrie HC, Murrell JR. APOE epsilon4 is not associated with Alzheimer's disease in elderly Nigerians. Ann Neurol. 2006;59(1):182–185. doi: 10.1002/ana.20694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunniyi A, Hall KS, Gureje O, Baiyewu O, Gao S, Unverzagt FW, Smith-Gamble V, Evans RE, Dickens J, Musick BS, Hendrie C. Risk factors for incident Alzheimer's disease in African Americans and Yoruba. Metab Brain Dis. 2006;21(2–3):235–240. doi: 10.1007/s11011-006-9017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwakwe R, Ibeh CC, Modebe AI, Bo E, Ezeama N, Njelita I, Ferri CP, Prince MJ. The epidemiology of dependence in older people in Nigeria: prevalence, determinants, informal care, and health service utilization. A 10/66 dementia research group cross-sectional survey. J Am Geriatr Soc. 2009;57(9):1620–1627. doi: 10.1111/j.1532-5415.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- Yusuf AJ, Baiyewu O, Sheikh TL, Shehu AU. Prevalence of dementia and dementia subtypes among community-dwelling elderly people in northern Nigeria. Int Psychogeriatr. 2011;23(3):379–386. doi: 10.1017/S1041610210001158. [DOI] [PubMed] [Google Scholar]
- Coume M, Toure K, Thiam MH, Zunzunegui MV, Bacher Y, Diop TM, Ndiaye MM. Estimate of the prevalence of cognitive impairment in an elderly population of the health center of Senegalese national retirement institution. Geriatr Psychol Neuropsychiatr Vieil. 2012;10(1):39–46. doi: 10.1684/pnv.2012.0334. [DOI] [PubMed] [Google Scholar]
- Onwuekwe I. Assessment of mild cognitive impairment with mini mental state examination among adults in southeast Nigeria. Ann Med Health Sci Res. 2012;2(2):99–102. doi: 10.4103/2141-9248.105653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belec L, Testa J, Vohito MD, Gresenguet G, Martin MI, Tabo A, Di Costanzo B, Georges AJ. Neurologic and psychiatric manifestations of AIDS in Central African Republic. Bull Soc Pathol Exot Filiales. 1989;82(3):297–307. [PubMed] [Google Scholar]
- Howlett WP, Nkya WM, Mmuni KA, Missalek WR. Neurological disorders in AIDS and HIV disease in the northern zone of Tanzania. AIDS. 1989;3(5):289–296. doi: 10.1097/00002030-198905000-00007. [DOI] [PubMed] [Google Scholar]
- Turnbull O, Saling MM, Kaplan-Solms K, Cohn R, Schoub B. Neuropsychological deficit in haemophiliacs with human immunodeficiency virus. J Neurol Neurosurg Psychiatry. 1991;54(2):175–177. doi: 10.1136/jnnp.54.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perriens JH, Mussa M, Luabeya MK, Kayembe K, Kapita B, Brown C, Piot P, Janssen R. Neurological complications of HIV-1-seropositive internal medicine inpatients in Kinshasa, Zaire. J Acquir Immune Defic Syndr. 1992;5(4):333–340. [PubMed] [Google Scholar]
- Maj M, Satz P, Janssen R, Zaudig M, Starace F, D'Elia L, Sughondhabirom B, Mussa M, Naber D, Ndetei D, Schulte G, Sartorius N. WHO Neuropsychiatric AIDS study, cross-sectional phase II. Neuropsychological and neurological findings. Arch Gen Psychiatry. 1994;51(1):51–61. doi: 10.1001/archpsyc.1994.03950010051007. [DOI] [PubMed] [Google Scholar]
- Carson AJ, Sandler R, Owino FN, Matete FO, Johnstone EC. Psychological morbidity and HIV in Kenya. Acta Psychiatr Scand. 1998;97(4):267–271. doi: 10.1111/j.1600-0447.1998.tb09998.x. [DOI] [PubMed] [Google Scholar]
- Sebit MB. Neuropsychiatric HIV-1 infection study: in Kenya and Zaire cross-sectional phase I and II. Cent Afr J Med. 1995;41(10):315–322. [PubMed] [Google Scholar]
- Sacktor N, Nakasujja N, Skolasky R, Robertson K, Wong M, Musisi S, Ronald A, Katabira E. Antiretroviral therapy improves cognitive impairment in HIV + individuals in sub-Saharan Africa. Neurology. 2006;67(2):311–314. doi: 10.1212/01.wnl.0000225183.74521.72. [DOI] [PubMed] [Google Scholar]
- Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, Robertson K, McArthur JC, Ronald A, Katabira E. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS. 2005;19(13):1367–1374. [PubMed] [Google Scholar]
- Modi G, Hari K, Modi M, Mochan A. The frequency and profile of neurology in black South African HIV infected (clade C) patients - a hospital-based prospective audit. J Neurol Sci. 2007;254(1–2):60–64. doi: 10.1016/j.jns.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Clifford DB, Mitike MT, Mekonnen Y, Zhang J, Zenebe G, Melaku Z, Zewde A, Gessesse N, Wolday D, Messele T, Teshome M, Evans S. Neurological evaluation of untreated human immunodeficiency virus infected adults in Ethiopia. J Neurovirol. 2007;13(1):67–72. doi: 10.1080/13550280601169837. [DOI] [PubMed] [Google Scholar]
- Odiase FE, Ogunrin OA, Ogunniyi AA. Memory performance in HIV/AIDS–a prospective case control study. Can J Neurol Sci. 2007;34(2):154–159. doi: 10.1017/s0317167100005977. [DOI] [PubMed] [Google Scholar]
- Wong MH, Robertson K, Nakasujja N, Skolasky R, Musisi S, Katabira E, McArthur JC, Ronald A, Sacktor N. Frequency of and risk factors for HIV dementia in an HIV clinic in sub-Saharan Africa. Neurology. 2007;68(5):350–355. doi: 10.1212/01.wnl.0000252811.48891.6d. [DOI] [PubMed] [Google Scholar]
- Robertson KR, Nakasujja N, Wong M, Musisi S, Katabira E, Parsons TD, Ronald A, Sacktor N. Pattern of neuropsychological performance among HIV positive patients in Uganda. BMC Neurol. 2007;7:8. doi: 10.1186/1471-2377-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salawu FK, Bwala SA, Wakil MA, Bani B, Bukbuk DN, Kida I. Cognitive function in HIV-seropositive Nigerians without AIDS. J Neurol Sci. 2008;267(1–2):142–146. doi: 10.1016/j.jns.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Singh D, Sunpath H, John S, Eastham L, Gouden R. The utility of a rapid screening tool for depression and HIV dementia amongst patients with low CD4 counts- a preliminary report. Afr J Psychiatry. 2008;11(4):282–286. [PubMed] [Google Scholar]
- Sall L, Salamon E, Allgulander C, Owe-Larsson B. Psychiatric symptoms and disorders in HIV infected mine workers in South Africa. A retrospective descriptive study of acute first admissions. Afr J Psychiatry. 2009;12(3):206–212. doi: 10.4314/ajpsy.v12i3.48495. [DOI] [PubMed] [Google Scholar]
- Ganasen KA, Fincham D, Smit J, Seedat S, Stein D. Utility of the HIV Dementia Scale (HDS) in identifying HIV dementia in a South African sample. J Neurol Sci. 2008;269(1–2):62–64. doi: 10.1016/j.jns.2007.12.027. [DOI] [PubMed] [Google Scholar]
- Njamnshi AKDVP, Fonsah JY, Yepnjio FN, Njamnshi DM, Muna WE. The International HIV Dementia Scale is a useful screening tool for HIV-associated dementia/cognitive impairment in HIV-infected adults in Yaoundé-Cameroon. J Acquir Immune Defic Syndr. 2008;49(4):393–397. doi: 10.1097/qai.0b013e318183a9df. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Nakasujja N, Skolasky RL, Robertson K, Musisi S, Ronald A, Katabira E, Clifford DB. Benefits and risks of stavudine therapy for HIV-associated neurologic complications in Uganda. Neurology. 2009;72(2):165–170. doi: 10.1212/01.wnl.0000339042.96109.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njamnshi AKBA, Ongolo-Zogo P, Tabah EN, Lekoubou AZ, Yepnjio FN, Fonsah JY, Kuate CT, Angwafor SA, Dema F, Njamnshi DM, Kouanfack C, Djientcheu Vde P, Muna WF, Kanmogne GD. Risk factors for HIV-associated neurocognitive disorders (HAND) in sub-Saharan Africa: The case of Yaoundé-Cameroon. J Neurol Sci. 2009;285(1–2):149–153. doi: 10.1016/j.jns.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, Nakasujja N, Skolasky RL, Rezapour M, Robertson K, Musisi S, Katabira E, Ronald A, Clifford DB, Laeyendecker O, Quinn TC. HIV subtype D is associated with dementia, compared with subtype A, in immunosuppressed individuals at risk of cognitive impairment in Kampala, Uganda. Clin Infec Dis. 2009;49(5):780–786. doi: 10.1086/605284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasujja N, Skolasky RL, Musisi S, Allebeck P, Robertson K, Ronald A, Katabira E, Clifford DB, Sacktor N. Depression symptoms and cognitive function among individuals with advanced HIV infection initiating HAART in Uganda. BMC Psychiatry. 2010;10:44. doi: 10.1186/1471-244X-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinyanda E, Hoskins S, Nakku J, Nawaz S, Patel V. Prevalence and risk factors of major depressive disorder in HIV/AIDS as seen in semi-urban Entebbe district, Uganda. BMC Psychiatry. 2011;11:205. doi: 10.1186/1471-244X-11-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Townend J, Vincent T, Zaidi I, Sarge-Njie R, Jaye A, Clifford DB. Neurologic manifestations of human immunodeficiency virus-2: dementia, myelopathy, and neuropathy in West Africa. J Neurovirol. 2011;17(2):166–175. doi: 10.1007/s13365-011-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbeck GL, Kvalsund MP, Byers PA, Bradbury R, Mang'ombe C, Organek N, Kaile T, Sinyama AM, Sinyangwe SS, Malama K, Malama C. Neuropsychiatric and socioeconomic status impact antiretroviral adherence and mortality in rural Zambia. Am J Trop Med Hyg. 2011;85(4):782–789. doi: 10.4269/ajtmh.2011.11-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joska JA, Fincham DS, Stein DJ, Paul RH, Seedat S. Clinical correlates of HIV-associated neurocognitive disorders in South Africa. AIDS Behav. 2010;14(2):371–378. doi: 10.1007/s10461-009-9538-x. [DOI] [PubMed] [Google Scholar]
- Kanmogne GD, Kuate CT, Cysique LA, Fonsah JY, Eta S, Doh R, Njamnshi DM, Nchindap E, Franklin DR Jr, Ellis RJ, McCutchan JA, Binam F, Mbanya D, Heaton RK, Njamnshi AK. HIV-associated neurocognitive disorders in sub-Saharan Africa: a pilot study in Cameroon. BMC neurology. 2010;10:60. doi: 10.1186/1471-2377-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler K, Mosepele M, Ratcliffe S, Seloilwe E, Steele K, Nthobatsang R, Steenhoff A. Neurocognitive impairment among HIV-positive individuals in Botswana: a pilot study. J Int AIDS Soc. 2010;13:15. doi: 10.1186/1758-2652-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel VN, Mungwira RG, Tarumbiswa TF, Heikinheimo T, van Oosterhout JJ. High prevalence of suspected HIV-associated dementia in adult Malawian HIV patients. Int J STD AIDS. 2010;21(5):356–358. doi: 10.1258/ijsa.2010.009554. [DOI] [PubMed] [Google Scholar]
- Holguin A, Banda M, Willen EJ, Malama C, Chiyenu KO, Mudenda VC, Wood C. HIV-1 effects on neuropsychological performance in a resource-limited country, Zambia. AIDS and behavior. 2011;15(8):1895–1901. doi: 10.1007/s10461-011-9988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joska JA, Westgarth-Taylor J, Hoare J, Thomas KG, Paul R, Myer L, Stein DJ. Validity of the International HIV Dementia Scale in South Africa. AIDS Patient Care STDS. 2011;25(2):95–101. doi: 10.1089/apc.2010.0292. [DOI] [PubMed] [Google Scholar]
- Obiabo YO, Ogunrin OA, Ogun AS. Effects of highly active antiretroviral therapy on cognitive functions in severely immune-compromised HIV-seropositive patients. J Neurol Sci. 2012;313(1–2):115–122. doi: 10.1016/j.jns.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Joska JA, Westgarth-Taylor J, Myer L, Hoare J, Thomas KG, Combrinck M, Paul RH, Stein DJ, Flisher AJ. Characterization of HIV-Associated Neurocognitive Disorders among individuals starting antiretroviral therapy in South Africa. AIDS Behav. 2011;15(6):1197–1203. doi: 10.1007/s10461-010-9744-6. [DOI] [PubMed] [Google Scholar]
- Robertson K, Kumwenda J, Supparatpinyo K, Jiang JH, Evans S, Campbell TB, Price RW, Murphy R, Hall C, Marra CM, Marcus C, Berzins B, Masih R, Santos B, Silva MT, Kumarasamy N, Walawander A, Nair A, Tripathy S, Kanyama C, Hosseinipour M, Montano S, La Rosa A, Amod F, Sanne I, Firnhaber C, Hakim J, Brouwers P. AIDS Clinical Trials Group. A multinational study of neurological performance in antiretroviral therapy-naive HIV-1-infected persons in diverse resource-constrained settings. J Neurovirol. 2011;17(5):438–447. doi: 10.1007/s13365-011-0044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins RN, Remien RH, Mellins CA, Joska JA, Stein DJ. Screening for HIV-associated dementia in South Africa: potentials and pitfalls of task-shifting. AIDS patient care and STDs. 2011;25(10):587–593. doi: 10.1089/apc.2011.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwasa J, Cettomai D, Lwanya E, Osiemo D, Oyaro P, Birbeck GL, Price RW, Bukusi EA, Cohen CR, Meyer AC. Lessons learned developing a diagnostic tool for HIV-associated dementia feasible to implement in resource-limited settings: pilot testing in Kenya. PLoS One. 2012;7(3):e32898. doi: 10.1371/journal.pone.0032898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies G, Fennema-Notestine C, Archibald SL, Cherner M, Seedat S. Neurocognitive deficits in HIV-infected women and victims of childhood trauma. AIDS Care. 2012;24(9):1126–1135. doi: 10.1080/09540121.2012.687813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestad KA, Menon JA, Silalukey-Ngoma M, Franklin DR Jr, Imasiku ML, Kalima K, Heaton RK. Sex differences in neuropsychological performance as an effect of human immunodeficiency virus infection: a pilot study in Zambia. Africa. J Nerv Ment Dis. 2012;200(4):336–342. doi: 10.1097/NMD.0b013e31824cc225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhe T, Melkamu Y, Amare A. The pattern and predictors of mortality of HIV/AIDS patients with neurologic manifestation in Ethiopia: a retrospective study. AIDS Res Ther. 2012;9:11. doi: 10.1186/1742-6405-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joska JA, Westgarth-Taylor J, Hoare J, Thomas KG, Paul R, Myer L, Stein DJ. Neuropsychological outcomes in adults commencing highly active anti-retroviral treatment in South Africa: a prospective study. BMC infectious diseases. 2012;12:39. doi: 10.1186/1471-2334-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer E, Stoloff K, Myer L, Seedat S, Stein DJ, Joska J. Reliability of the Lay Adherence Counsellor Administered Substance Abuse and Mental Illness Symptoms Screener (SAMISS) and the International HIV Dementia Scale (IHDS) in a Primary care HIV Clinic in Cape Town, South Africa. AIDS and behavior. 2012;16(6):1464–1471. doi: 10.1007/s10461-011-0067-z. [DOI] [PubMed] [Google Scholar]
- Hoare J, Westgarth-Taylor J, Fouche JP, Combrinck M, Spottiswoode B, Stein DJ, Joska JA. Relationship between apolipoprotein E4 genotype and white matter integrity in HIV-positive young adults in South Africa. Eur Arch Psychiatry Clin Neurosci. 2013;263(3):189–195. doi: 10.1007/s00406-012-0341-8. [DOI] [PubMed] [Google Scholar]
- Oshinaike OO, Akinbami AA, Ojo OO, Ojini IF, Okubadejo UN, Danesi AM. Comparison of the Minimental State Examination Scale and the International HIV Dementia Scale in Assessing Cognitive Function in Nigerian HIV Patients on Antiretroviral Therapy. AIDS Res Treat. 2012;2012:581531. doi: 10.1155/2012/581531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal W 3rd, Cherner M, Carr J, Habib AG, Akomolafe A, Abimiku A, Charurat M, Farley J, Oluyemisi A, Mamadu I, Johnson J, Ellis R, McCutchan JA, Grant I, Blattner WA. Clinical features and preliminary studies of virological correlates of neurocognitive impairment among HIV-infected individuals in Nigeria. J Neurovirol. 2012;18(3):191–199. doi: 10.1007/s13365-012-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekle-Haimanot R, Abebe M, Gebre-Mariam A, Forsgren L, Heijbel J, Holmgren G, Ekstedt J. Community-based study of neurological disorders in rural central Ethiopia. Neuroepidemiology. 1990;9(5):263–277. doi: 10.1159/000110783. [DOI] [PubMed] [Google Scholar]
- Jacquin-Cotton L, Dumas M, Girard PL. Paraplegia in Senegal. Bull Soc Med Afr Noire Lang Fr. 1970;15(2):206–220. [PubMed] [Google Scholar]
- Piquemal M, Beugre K, Boa Y, Giordano C. Etude de 30 observations de Syndrome de sclérose latérale Amyotrophique observés en Côte d'Ivoire. Afr J Neurol Sci. 1982;1:31–40. [Google Scholar]
- Cosnett JE, Bill PL, Bhigjee AI. Motor neuron disease in blacks. Epidemiological observations in Natal. S Afr Med J. 1989;76(4):155–157. [PubMed] [Google Scholar]
- Osuntokun BO, Adeuja AO, Bademosi O. The prognosis of motor neuron disease in Nigerian africans. A prospective study of 92 patients. J Neurol. 1974;97(2):385–394. doi: 10.1093/brain/97.1.385. [DOI] [PubMed] [Google Scholar]
- Abdulla MN, Sokrab TE, el Tahir A, Siddig HE, Ali ME. Motor neurone disease in the tropics: findings from Sudan. East Afr Med J. 1997;74(1):46–48. [PubMed] [Google Scholar]
- Sene DF, Ndiaye M, Toure K, Ndao AK, Thiam A, Diop AG, Ndiaye IP. Epidemiological and clinical aspects of amyotrophic lateral sclerosis in neurological clinic of Dakar. Dakar Med. 2004;49(3):167–171. [PubMed] [Google Scholar]
- Adam AM. Unusual form of motor neuron disease in Kenya. East Afr Med J. 1992;69(2):55–57. [PubMed] [Google Scholar]
- Wall DW, Gelfand M. Motor neuron disease in Rhodesian Africans. J Neurol. 1972;95(3):517–520. doi: 10.1093/brain/95.3.517. [DOI] [PubMed] [Google Scholar]
- Imam I, Ogunniyi A. What is happening to motor neuron diseases in Nigeria? Ann African Medicine. 2004;3(1):1–3. [Google Scholar]
- Harries JR. Amyotrophic lateral sclerosis in Africans. East Afr Med J. 1955;32(8):333–335. [PubMed] [Google Scholar]
- Collomb H, Virieu R, Dumas M, Lemercier G. Maladie de Charcot et syndromes de sclérose latérale amyotrophique au sénégal: Etude clinique de 27 observations 1968; 13(4):785–804. Bull Soc Med Noire Lgue Fr. 1968;13(4):785–804. [Google Scholar]
- Mutesa L, Pierquin G, Segers K, Vanbellinghen JF, Gahimbare L, Bours V. Spinocerebellar ataxia type 2 (SCA2): clinical features and genetic analysis. J Trop Pediatr. 2008;54(5):350–352. doi: 10.1093/tropej/fmn034. [DOI] [PubMed] [Google Scholar]
- Traore H, Diagana M, Prieux PM, Debrock C, Ba A, Algadi B,MD. Pathologie hérédodégénérative du système nerveux central dans un service de neurologie à Nouakchott. Afr J Neurol Sci. 1998;17:30–36. [Google Scholar]
- Joubert J, Botha MC. Huntington disease in South African blacks. A report of 8 cases. S Afr Med J. 1988;73(8):489–494. [PubMed] [Google Scholar]
- Silber E, Kromberg J, Temlett JA, Krause A, Saffer D. Huntington's disease confirmed by genetic testing in five African families. Mov Disord. 1998;13(4):726–730. doi: 10.1002/mds.870130420. [DOI] [PubMed] [Google Scholar]
- Kabore J, Ouedraogo A. Huntington disease in Burkina Faso. Rev Neurol (Paris) 2000;156(12):1157–1158. [PubMed] [Google Scholar]
- Bardien S, Abrahams F, Soodyall H, van der Merwe L, Greenberg J, Brink T, Carr J. A South African mixed ancestry family with Huntington disease-like 2: clinical and genetic features. Mov Disord. 2007;22(14):2083–2089. doi: 10.1002/mds.21672. [DOI] [PubMed] [Google Scholar]
- Magazi DS, Krause A, Bonev V, Moagi M, Iqbal Z, Dludla M, van der Meyden CH. Huntington's disease: genetic heterogeneity in black African patients. S Afr Med J. 2008;98(3):200–203. [PubMed] [Google Scholar]
- Hayden MR, Beighton P. Huntington's chorea in the Cape coloured community of South Africa. S Afr Med J. 1977;52(22):886–888. [PubMed] [Google Scholar]
- Hayden MR, MacGregor JM, Beighton PH. The prevalence of Huntington's chorea in South Africa. S Afr Med J. 1980;58(5):193–196. [PubMed] [Google Scholar]
- Hayden MR, Berkowicz AL, Beighton PH, Yiptong C. Huntington's chorea on the island of Mauritius. S Afr Med J. 1981;60(26):1001–1002. [PubMed] [Google Scholar]
- Hayden MR, MacGregor JM, Saffer DS, Beighton PH. The high frequency of juvenile Huntington's chorea in South Africa. J Med Genet. 1982;19(2):94–97. doi: 10.1136/jmg.19.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiyesimoju AB, Osuntokun BO, Bademosi O, Adeuja AO. Hereditary neurodegenerative disorders in Nigerian Africans. Neurology. 1984;34(3):361–362. doi: 10.1212/wnl.34.3.361. [DOI] [PubMed] [Google Scholar]
- Stephany F, Mbaye PS, Jacquin-Cotton L, Ndiaye IP. Huntington chorea in Senegal. Dakar Med. 1984;29(1):75–83. [PubMed] [Google Scholar]
- Samuels BL, Gelfand M. Huntington's chorea in a black Rhodesian family. S Afr Med J. 1978;54(16):648–651. [PubMed] [Google Scholar]
- Glass J, Saffer DS. Huntington's chorea in a black family: a report of 2 cases. S Afr Med J. 1979;56(17):685–688. [PubMed] [Google Scholar]
- Scrimgeour EM. Huntington's disease in Tanzania. J Med Genet. 1981;18(3):200–203. doi: 10.1136/jmg.18.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MR, Beighton P. Genetic aspects of Huntington's chorea: results of a national survey. Am J Med Genet. 1982;11(2):135–141. doi: 10.1002/ajmg.1320110203. [DOI] [PubMed] [Google Scholar]
- Scrimgeour EM. The Huntington's chorea register of Tanzania. East Afr Med J. 1982;59(4):280–282. [PubMed] [Google Scholar]
- Scrimgeour EM, Pfumojena JW. Huntington disease in black Zimbabwean families living near the Mozambique border. Am J Med Genet. 1992;44(6):762–766. doi: 10.1002/ajmg.1320440610. [DOI] [PubMed] [Google Scholar]
- Scrimgeour EM, Samman Y, Brock DJ. Huntington's disease in a Sudanese family from Khartoum. Human genetics. 1995;96(5):624–625. doi: 10.1007/BF00197424. [DOI] [PubMed] [Google Scholar]
- Grunitzky EK, Gnamey DR, Nonon SA, Balogou A. Huntington disease in a large family in southern Togo. Ann Med Interne (Paris) 1995;146(8):581–583. [PubMed] [Google Scholar]
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M. Alzheimer's Disease International. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Campenhausen BB S, Regina W, Kai B, Cristina S, Werner P, Wolfgang O, Uwe S, Karin Berger RD. Prevalence and incidence of Parkinson’s disease in Europe. Eur Neuropsychopharmacol. 2005;15:473–490. doi: 10.1016/j.euroneuro.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Ashford JW. Screening for mental disorders, dementia and Alzheimer's disease. Aging health. 2008;4(4):399–432. [Google Scholar]
- Kim JH, Cheong HK, Lee CS, Yi SE, Park KW. The validity and reliability of a screening questionnaire for Parkinson's disease in a community. J Prev Med Public Health. 2010;43(1):9–17. doi: 10.3961/jpmph.2010.43.1.9. [DOI] [PubMed] [Google Scholar]
- Sarangmath N, Rattihalli R, Ragothaman M, Gopalkrishna G, Doddaballapur S, Louis ED, Muthane UB. Validity of a modified Parkinson's disease screening questionnaire in India: effects of literacy of participants and medical training of screeners and implications for screening efforts in developing countries. Mov Disord. 2005;20(12):1550–1556. doi: 10.1002/mds.20576. [DOI] [PubMed] [Google Scholar]
- Breteler MM, Bots ML, Ott A, Hofman A. Risk factors for vascular disease and dementia. Haemostasis. 1998;28(3–4):167–173. doi: 10.1159/000022428. [DOI] [PubMed] [Google Scholar]
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368(9533):387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- Elbaz A, Moisan F. Update in the epidemiology of Parkinson's disease. Curr Opin Neurol. 2008;21(4):454–460. doi: 10.1097/WCO.0b013e3283050461. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Crowe SM, Landay A, Cysique LA, Guillemin G. Neurodegeneration and ageing in the HAART era. J Neuroimmune Pharmacol. 2009;4(2):163–174. doi: 10.1007/s11481-008-9143-1. [DOI] [PubMed] [Google Scholar]
- Population Division, DESA, United Nations, World Population Ageing 1950-2050. http://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeingReport2013.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search terms and strategies.


