Abstract
Chymotrypsin is one of the serine proteases families that have various biological functions. A chymotrypsin gene was isolated from hepatopancreas of the mud crab, Scylla paramamosain (designated SpCHY) in this study. The full-length cDNA of SpCHY contained 942 nucleotides with a polyadenylation sequence and encoded a peptide of 270 amino acids with a signal peptide of 17 amino acids. The SpCHY gene contains seven exons, six introns, a TATA box and several transcription factor binding sites that were found in 5’-promoter region which is 1221 bp in length. Real-time quantitative PCR analysis indicated that the expression level of SpCHY mRNA in hepatopancreas was significantly higher than that in other tissues. Immunocytochemistry and in situ hybridization exhibited the CHY-like reactivity presented in resorptive cells of the hepatopancreas. After bacterial challenge with Vibrio alginolyticus, the expression level of SpCHY mRNA was extremely up-regulated at 3 h in hepatopancreas. Our results suggest that SpCHY might play an important role in the mud crab’s immune response.
Keywords: chymotrypsin, Scylla paramamosain, immune response, immunocytochemistry, in situ hybridization
Introduction
Belonging to one of the largest gene family in the animal kingdom, serine proteases (SP) have a tryp-spc domain, which is conserved with the catalytic triad (His, Asp and Ser), part of an extensive hydrogen bonding network (Szabo and Bugge, 2008; Zhou et al., 2012). In the human genome, approximate 500 protease-encoding genes have been identified, of which about 30% are SP or SP homologues (SPH) (Southan, 2001). In Drosophila melanogaster, around 200 SP- and SPH-encoding genes have been identified (Ross et al., 2003). SPs participate in various biological processes, including protein digestion (Mazumdar and Broadway, 2001; Broehan et al., 2008), immune response (Jiang et al., 2003a, b), and molting (Samuel and Reynolds, 1993; He et al., 2009).
As one of the SP, the chymotrypsin family includes chymotrypsin A and chymotrypsin B, two structurally related, but phylogenetically distinct subfamilies (Rawlings et al., 2008). Chymotrypsin B plays an important role in intracellular protein turnover, while chymotrypsin A is prevalent in the extracellular space and performs different functions (Broehan et al., 2010). The chymotrypsin A sub-family contains a variety of enzymes, such as chymotrypsin, trypsin, elastase, granzyme and different matrix peptidases, with different cleavage specificities. The substrate-binding pocket near the catalytic site determines these types of specificity (Perona and Craik, 1995). These proteins are all synthesized as inactive zymogens, which can be activated by specific proteolytic cleavage. The canonical catalytic triad residues (Ser, His and Asp) form the active site (Hedstrom, 2002).
In invertebrates, studies on chymotrypsin are mostly focused on the digestive system of some pest insects. In the lepidopteran, Spodoptera exigua, chymotrypsin was found likely to mediate the proteolytic remodeling in the gut during larval-pupal transition (Herrero et al., 2005). The injection of dsRNA for chymotrypsin 5C/6C in the red flour beetle, Tribolium castaneum, resulted in severe molting defects, which indicate that chymotrypsin plays an important role in molting process (Broehan et al., 2010). In addition, chymotrypsin was associated with immune defense reactions against bacteria in D. melanogaster (de Morais et al., 2005). In crustaceans, only few studies report on chymotrypsin (Sellos and Wormhoudt, 1992; Shi et al., 2008; Serrano, 2013), and only few chymotrypsin cDNA and genomic DNA sequences have been cloned and characterized. The polymorphism and evolution of this gene have been analyzed in the pacific white shrimp, Litopenaeus vannamei (Sellos and Wormhoudt, 1992, 1999). Chymotrypsin in Chinese shrimp, Fenneropenaeus chinensis, was observed to be involved in innate immune reactions after bacterial and viral challenges (Shi et al., 2008).
The mud crabs of the genus Scylla are important cultured crustaceans that live in intertidal and subtidal sheltered soft-sediment habitats (Keenan, 1999). In Southeast Asia, mud crabs are a valuable source of income for coastal communities (Le Vay, 2001; Ye et al., 2011). The bacterium, Vibrio alginolyticus, can cause many diseases (such as exoskeleton ulcer disease, black gill disease) that seriously affect crustacean aquaculture and thus receive increasing attention in recent years (Zhu et al., 2008).
In this study, we first cloned the cDNA, 5-promoter region and genomic DNA of a chymotrypsin gene from the mud crab, Scylla paramamosain (designated SpCHY), and investigated its expression in various tissues by real-time quantitative PCR. The localization of chymotrypsin protein and mRNA in hepatopancreas was detected by immunocytochemistry and in situ hybridization. The temporal responses of SpCHY to the bacterium V. alginolyticus were investigated to study the role of SpCHY in the immune response.
Materials and Methods
Sample collection
Vigorous female crabs (∼250 g), with both claws intact and antennae in movement, were purchased from a local fish market in Xiamen city, China. Brain, thoracic ganglion, heart, gill, hepatopancreas, stomach, muscle, and ovary tissues were dissected and immediately preserved in liquid nitrogen. Total RNA was extracted using Trizol re-agent (Invitrogen, USA) according to the manufacturer’s protocol and potential genomic contamination was removed by DNase I treatment. RNA quality was determined by agarose gel electrophoresis and quantification was done with an ND-1000 NanoDrop UV spectrophotometer (NanoDrop Technologies, USA). RNA aliquots of 1 μg were reversely transcribed using a reversed first strand cDNA synthesis kit (Fermentas, USA) and stored at −20 °C.
Cloning of full-length SpCHY cDNA
The degenerate primers CHYf1 and CHYr1 (Table 1), directed to highly conserved sequences of various chymotrypsin orthologs, were used to amplify a partial chymotrypsin-like sequence of S. paramamosain. The SpCHY sequence was completed by 3’ and 5’ rapid amplification of cDNA ends (RACE) by means of a 3’, 5’ full race kit (Takara, Dalian, China). The specific primers CHY3’ and CHY5’ are listed in Table 1.
Table 1.
Summary of primers used in this study.
| Primer name | Primer Sequence (5’- 3’) | Purpose | Amplified fragment length |
|---|---|---|---|
| CHYf1 | GGYGTYGTYTGCATYGACGGHRC | fragment amplication | 203 bp |
| CHYr1 | GCTCAGGGWKTGACRCCRGTCTT | fragment amplication | |
| CHY3’ | CTCGCTCTGCTCCTTGTCTG | 3’ amplication | 904 bp |
| CHY5’ | GAAAGATGTGATGCCGTAGGTC | 5’ amplication | 728 bp |
| CHYf2 | ATGATTGCCAAGCTCGCTCTG | genomic DNA amplify | 1994 bp |
| CHYr2 | TCAGGGGGTGACACCGGTC | genomic DNA amplify | |
| CHYf3 | ACGAGCAGGGACTTCTTCACC | real-time RT-PCR for SpCHY | 286 bp |
| CHYr3 | AGACGACGCCACTTCCAACA | real-time RT-PCR for SpCHY | |
| CHY5-1 | CAGCAACGCAGACAAGGAGCA | promoter region clone | 1261 bp |
| CHY5-2 | TGGGGAAAGAAGGAAAGTGGC | promoter region clone | 1155 bp |
| CHY5-3 | GCAAAACATCTACGACCACAGCA | promoter region clone | 974 bp |
| TCHYf1 | GCCAGAACGAGCCCTCTCAG | riboprobe amplication clone | 314 bp |
| TCHYr1 | GACGACGCCACTTCCAACAAT | riboprobe amplication clone | |
| T7 | TAATACGACTCACTATAGGG | riboprobe amplication clone | |
| M13–47 | CGCCAGGGTTTTCCCAGTCACG | colony PCR | |
| RV-M | GAGCGGATAACAATTTCACACA | colony PCR | |
| β-actin F | GAGCGAGAAATCGTTCGTGAC | internal control | 183 bp |
| β-actin R | GGAAGGAAGGCTGGAAGAGAG | internal control |
Polymerase chain reactions (PCR) were carried out in a total volume of 25 μL that contained 1 μL of cDNA template, 2.5 μL of 10xPCR buffer (containing Mg2+), 1 μL of each primer (10 μM), 2.5 μL of dNTP (2.5 mM), 0.2 μL (2.5 U) of LA Taq polymerase (Takara, Dalian, China) and 16.8 μL of PCR-grade water. PCR conditions were as follows: 94 °C for 3 min; 32 cycles of 94 °C for 30 s, 58 °C for 30 s and 72 °C for 1 min; followed by a final extension at 72 °C for 10 min. After agarose gel electrophoresis, the DNA fragment of expected size was ligated into pMD19-T vectors (Takara, Dalian, China) and then used to transform competent cells of Escherichia coli. Positive recombinant clones were sequenced using the specific primers RV-M and M13–47 (Table 1) at Sangon Biotech Co, Ltd (China). Finally, the full-length of SpCHY cDNA was assembled from 3’ end and 5’ end sequences.
Genomic DNA and promoter cloning of SpCHY
Genomic DNA was extracted from muscle tissue of the mud crab by means of a DNA extraction kit (Takara, Dalian, China) PCR amplified by two specific primers CHYf2 and CHYr2 (Table 1) PCR and cloned as described above. The promoter region was cloned by genome walking using the Universal Genome Walker kit (Takara, Dalian, China). Nested PCR was performed with primers CHY5-1, CHY5-2, CHY5-3 (Table 1) according to the manufacturer’s protocol. The PCR product was purified and sequenced as before.
Phylogenetic and sequence analysis of SpCHY
A homology analysis of SpCHY with CHY genes of other species was performed using the Blastp algorithm. Characteristics of the protein were predicted using algorithms.of the Expasy site. The putative signal peptide was identified with SignalP software (Nielsen et al., 1997), and the ClustalW program was used to perform multiple sequence alignments. The neighbor-joining method implemented in MEGA3.1 software was used to construct the phylogenetic tree based on protein sequences (Kumar et al., 2004), with a bootstrapping replication of 1000. SSRHunter software was used to search for microsatellite sequences.
Tissue expression of SpCHY
mRNA transcripts of SpCHY in different tissues were examined by real-time quantitative PCR (Applied Biosystems 2770 Thermal Cycle, New York, USA). The reactions were performed in a 20 μL reaction volume containing 10 μL of SYBR premix, 2 μL of cDNA template (1/10x dilution of cDNA), 0.8 μL of each primer (10 μM CHYf3 and CHYr3; Table 1) which amplify a product of 286 bp, and 6.4 μL of PCR-grade water. PCR conditions were as follows: 94 °C for 10 min; 40 cycles of 94 °C for 20 s, 56 °C for 30 s and 72 °C for 40 s; final extension at 72 °C for 10 min. A 183 bp β-actin (GU99242) fragment of S. paramamosain was amplified as the internal control. Standard curves were run for each primer and the cDNA templates were tested in a graded dilution series (1, 1/10, 1/100, 1/1000). Based on these analyses, PCR efficiency was calculated to be > 96% (according to the PCR amplification formula E = 10(−1/slope)−1; where E is the PCR efficiency). The negative control was performed with PCR-grade water replacing the cDNA template. All samples were run in triplicate and relative expression was calculated as 2−ΔΔCt (Livak and Schmittgen, 2001).
Immunocytochemistry
Hepatopancreas tissue removed from adult female crabs was fixed in Bouin’s fixative overnight, dehydrated, embedded in paraffin, and then sectioned at 7 μm thickness. The sections were immunocytochemically stained by the streptavidin-peroxidase method with a primary antiserum generated in mouse against CHY (1:100 dilution, Abcam, UK) following an immunocytochemical protocol of the supplier (Transgen, China). The presence of CHY-like immunoreactivity in the tissues was visualized by a DAB enhanced liquid substrate system (Sigma-Aldrich, USA). Thereafter, the sections were dehydrated and observed on an Olympus multifunction microscope BX51 (Olympus, Japan). Control sections were prepared simultaneously by substituting PBS buffer solution in place of the primary antibody.
In situ hybridization
Digoxigenin-labeled cRNA riboprobes were synthesized with a DIG-RNA labeling Kit (Roche, Switzerland) using a 314 bp template of SpCHY that was ligated into the pGEM-T easy vector (Promega, USA). Hepatopancreas tissue was dissected and immediately fixed overnight in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) made in diethypyrocarbonate (DEPC) water. Tissue sections of 7 μm thickness were hybridized with the digoxigenin-labeled riboprobes at 57 °C overnight followed by incubation in an anti-DIG alkaline phosphatase-conjugated antibody (Roche, Switzerland). Hybridization signals were visualized with the colorimetric substrates nitroblue tetrazolium/4-bromo-4-chloro-30-indolylphosphate (NBT/BCIP). The riboprobe templates for SpCHY were generated by RT-PCR from hepatopancreas cDNA using the specific primers TCHYf1, TCHYr1 containing T7 adapters. Photographs were taken on an Olympus multifunction microscope BX51 (Olympus, Japan).
Temporal expression of SpCHY in hepatopancreas after immune challenge
In an attempt to determine whether SpCHY was involved in innate immune reactions, the expression profiles of SpCHY after bacterial challenge were measured and compared to the unchallenged (control group). The bacterium V. alginolyticus was prepared and washed for animal challenge. A dose of 1×107 CFU mixed with 20 μL crab saline was injected at the base of the last pereiopods into each of 30 vigorous female crabs (∼250 g) of the experimental group (Cheng et al., 2004). Another 30 vigorous female crabs (∼250 g) composing the control group were injected with 20 μL saline. These two groups were reared separately in culture tanks under the same conditions with seawater at a temperature between 26–28 °C, salinity at 26 ppt, and with continuous aeration. For real-time quantitative PCR assays, three crabs each were sampled at 0, 3, 6, 12, 24, 48 and 72 h post-injection and their hepatopancreas tissues were dissected and preserved in RNAsafer Stabilizer Reagent (Takara, Dalian, China). Total RNA extraction, first-strand cDNA synthesis and real-time quantitative assays were performed according to the procedures described above.
Statistical analysis
One-way analysis of variance (ANOVA) and Student’s t-test done with SPSS 11.5 software were used to determine the statistical significance of SpCHY expression in different tissues and challenge experiment respectively (SPSS, Chicago, IL, USA). Before the comparisons, Kolmogorov-Smirnov and Cochran tests were run to test for normality and homogeneity of variances. P values of < 0.05 were considered statistically significant.
Results
Cloning of the SpCHY gene
A 942 bp cDNA sequence of SpCHY (GenBank accession number: JF831535.1) was obtained in this study. It comprises an 813 bp open reading frame (ORF) encoding 270 amino acids with a signal peptide of 17 amino acids, an 115 bp 3’-untranslated region (UTR) with a polyA tail, and a 14 bp 5’UTR (Figure 1). The deduced molecular weight of mature SpCHY protein was 28.5 kDa and its isoelectric point 6.11. Conserved domain analysis done online in NCBI showed that SpCHY contained a trypsin-like SP domain including one cleavage site I-45, three active site (H-85, D-131, S-222), three substrate binding sites (S-216, S-237, G-239), and six cysteine residues, which were similar to other chymotrypsin members.
Figure 1.
Nucleotide and deduced amino acid sequences of the SpCHY gene (GenBank accession no. JF831535.1). The nucleotides are numbered on the right, and the amino acids on the left. The putative signal peptide is underlined. The trypsin-like SP domain is wave underlined. The catalytic triad (H, D, and S) is gray shadowed. The boxed letters are the polyadenylation signal. The asterisk (*) indicates the stop codon and arrows indicate the location of introns.
Similar to other chymotrypsin genes, SpCHY is composed of seven exons interrupted by six introns. In addition, all the intron-exon boundaries conformed to the GT-AG rule, which belonged to a 0-type intron/exon junction. Moreover, a 36 CA repeat microsatellite sequence was found by screening with SSRHunter software (Figure 2).
Figure 2.

Organization of the SpCHY gene. The positions of the exons (open boxes 1–7), introns (A–F), and CA repeat sequence (filled box) are denoted.
In order to study the regulation of SpCHY expression in the mud crab, we used a cloned 1221 bp fragment of the 5’ flanking region of the SpCHY gene. Using the program Promoter 2.0, we found a putative TATA box that was located at 45 bp upstream of the translation start site. In addition, several putative transcriptional factor binding sites or cis-regulatory elements including HSF, Hb, Dfd, SP1, Bcd, CF1 and Ubx were also identified.
Phylogenetic analysis of SpCHY
Blastp data showed that the deduced amino acid sequence shared high similarity with chymotrypsins of L. vannamei CHYA (GenBank accession no. CAA71672, 82%), F. chinensis (ACC68669.1, 80%), M. japonicus (BAI49929.1, 79%), L. vannamei CHYB (CAA71673.1, 79%). The phylogenetic analysis suggested that three different groups were formed, representing CHYs from invertebrates, vertebrates and urochordates respectively. The vertebrate CHY group could be further separated into three distinct and well-supported clades: CHYA, CHYB, and CHYC (caldecrin). The invertebrate group contained 2 subgroups. As showed in Figure 3, crustacean CHY was well separated from insect CHY and formed a separate cluster.
Figure 3.
Phylogenetic analysis of SpCHY with other chymotrypsins. A NJ tree was produced with Mega3.1 software. One thousand bootstraps were carried out to check the repeatability of the result. L. vannamei-CHYA (CAA71672), L. vannamei-CHYB (CAA71673), F. chinensis-CHY (ACC68669), P. humanus corporis-CHY (AAV68346), P. cochleariae-CHY (CAA76928), T. molitor-CHY (DQ356031.1), M. sexta-CHY (2120321A), A. grandis-CHY (AAT09847.1), A. gambiae-CHY1 (CAA79325), A. gambiae-CHY2 (CAA79326), B. Taurus - CHYB (P00767), G. morhua-CHYB (P80646), S. aurata-CHYB (AAT45258), M. musculus-CHY (AAL11034), H. sapiens-CHY (CAA74031.1), B. taurus-CHYC(AAI51507.1), X. laevis-CHYC(NP_001085458), B. taurus-CHYB (NP_001098800.1), D. rerio-CHYB1 (NP_997783.1), and O. dioica-CHYB (AAT47850).
Tissue distribution of SpCHY mRNA
Real-time quantitative PCR showed that SpCHY mRNA is expressed in a wide variety of tissues, including brain, thoracic ganglion, heart, gill, hepatopancreas, stomach, muscle, and ovary. The mRNA expression level in hepatopancreas was considerably higher than that of other tissues, with the expression level in muscle being the lowest (Figure 4).
Figure 4.
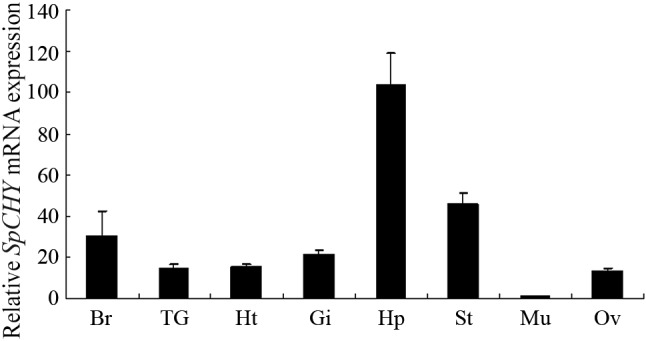
The results of quantitative real-time PCR analysis of SpCHY expression in various tissues. Expression of a β-actin gene was used as control. Values were shown as means ±S.E. (N = 3). Abbreviations: Br, brain; TG, thoracic ganglion; Ht, heart; Gi, gill; Hp, hepatopancreas; St, stomach; Mu, muscle; Ov, ovary.
Immunocytochemistry and in situ hybridization
The histological results showed that hepatopancreas of S. paramamosain consists of many blind ending tubules (hepatopancreatic tubules). The hepatopancreas cells could be classified into four types: embryonic cells, fibrillar cells, resorptive cells, and blister cells (Figure 5A).
Figure 5.
Location of SpCHY by immunocytochemistry and in situ hybridization in hepatopancreas of S. paramamosain. (A) histological observation; R resorptive cells, B blister cells, E embryonic cells, F fibrillar cells, Nu nucleus. (B) immunocytochemistry results; the arrows point to immunocytochemical positive signals. (C) in situ hybridization results; arrows indicate the specific SpCHY mRNA hybridization signal with the antisense riboprobe. (D) The negative control with the sense riboprobe showed no specific signal. Scale bars: 50 μm.
Using immunocytochemistry, SpCHY protein was detected in resorptive cells of the hepatopancreas, and the positive signals were mottled (Figure 5B). SpCHY gene expression was determined by in situ hybridization. Positive hybridization signals with the antisense SpCHY riboprobe were also mainly localized in resorptive cells (Figure 5C). However, specific signals were also detected in some small cells around the blind ending tubules. No positive signal was detected with the sense SpCHY riboprobe in hepatopancreas (Figure 5D).
Expression of SpCHY in hepatopancreas following bacterial challenge
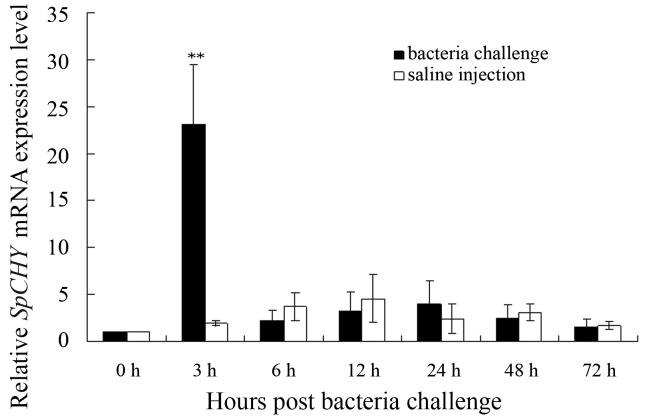
In order to determine whether SpCHY may be involved in innate immune reactions, the expression profiles of SpCHY after bacterial challenge were evaluated. Total hepatopancreas RNA was extracted from control and bacterial challenged crab at 0, 3, 6, 12, 24, 48 and 72 h. Compared to the control group, in crabs injected with the bacterium V. alginolyticus the SpCHY mRNA expression level increased distinctly about 20-fold at3h(p< 0.01) and then decreased to normal level (Figure 6). During this 72 h time interval SpCHY expression levels in the control group fluctuated slightly but not significantly.
Figure 6.
Transcript profiles of SpCHY in hepatopancreas of S. paramamosain following challenge with V. alginolyticus,. The relative SpCHY transcript levels in crabs challenged with V. alginolyticus were compared to those of saline injected animals. The expression of a β-actin gene was used as endogenous control. Significant differences of SpCHY expression between the challenged and the control group are indicated with asterisks. **p < 0.01.
Discussion
In present study, a new chymotrypsin gene was identified from the mud crab, S. paramamosain, and was designated as SpCHY. The full-length cDNA contained an 813 bp open reading frame which encoded a putative chymotrypsin of 270 amino acids. The putative amino acids sequence has high identity with the other known crustacean chymotrypsins such as L. vannamei and F. chinensis. ClustalX alignment of the CHY sequence revealed that the tryp-spc domain was conserved among arthropod chymotrypsins. In addition, the catalytic triad (H, D, S) characteristic of chymotrypsins was observed in the deduced amino sequence. Furthermore, three disulfide bonds formed by six cysteines were found at the same location as in other chymotrypsins. This indicates the importance of secondary structure conservation for the enzymatic activity of this family. Another free cysteine residue found in the signal peptide was also identical to chymotrypsins from other invertebrates. The high similarity, together with the conservation of tryp-spc domain and catalytic triad, indicated that SpCHY is a true member of the chymotrypsin family.
The genomic sequence of SpCHY, here first reported in crabs, is composed of seven exons and six introns, with the first intron inserted near the end of the putative signal peptide. The locations of introns were almost the same as in the white shrimp, L. vannamei, chymotrypsin gene (Sellos and Wormhoudt, 1992). The active site residues (His85, Asp131 and Ser222) involved in catalysis, as well as the residues (Ser216, Ser237 and Gly239) forming the binding pocket to interact with the hydrophobic side chains of the substrate, were encoded by separate exons. These functionally important amino acids and binding regions in separate exons are typical for the SP genes that have been described (Swift et al., 1984; Craik et al., 1984). Hence, the joining of different exons, encoding intrinsically catalytically inactive protein segments, resulted in the substrate specificity and catalytic activity of the enzyme. Moreover, the similarity between SpCHY and other SP genes in the number and location of intron/exon junctions revealed an evolutionary conservation of chymotrypsin gene.
In our study, SpCHY expression was detected in various tissues and strongly so in hepatopancreas. The high expression level of SpCHY in hepatopancreas was consistent with the role of the hepatopancreas as the main site for synthesizing digestive enzymes in crustaceans (Shi et al., 2008). Furthermore, crustacean hepatopancreas plays important roles in initiating humoral immunity and mediating cellular immune responses performed by certain specialized cells and phagocytes (Gross et al., 2001), which is supported by the discovery of several immunity-related genes in crustacean hepatopancreas post bacterial infection (Pan et al., 2005; Zhao et al., 2007).
The results obtained by immunocytochemistry and in situ hybridization indicated that the hepatopancreas is the site of expression and translation of SpCHY. CHY-immunoreactivity was found in resorptive cells, supplying morphological evidence for the secretory function of resorptive cells. The localization of SpCHY mRNA in resorptive cells by in situ hybridization further strengthens this conclusion. All these findings indicated that SpCHY is synthesized in resorptive cells and might be secreted to implement the digestive and immune roles.
Lacking an acquired specific immune system, the innate immune system in crustaceans is considered as the major microbial infection defense mechanism (Chaikeeratisak et al., 2012; Kiruthiga et al., 2012). In recent years, non-specific immune system has been found to be of equal importance as a specific immune system, especially for the production of anti-bacterial and anti-viral proteins (Liu et al., 2010). Pathogen molecules can trigger these immune responses by pattern recognition proteins (PRPs) (Medzhitov and Janeway, 1997). These PRPs bind to microbes and then activate the prophenoloxidase system (proPO-system), stimulate the release of antimicrobial peptides (AMPs), or initiate other biological defense processes. Recently, the clip domain SP was demonstrated to be cofactor for the activation of the proPO cascade in invertebrates (Cerenius and Söderhäll, 2004; Gai et al., 2009). For example, in Sydney rock oysters, Saccostrea glomerata, the increase in chymotrypsin could activate ProPO to PO (Aladaileh et al., 2007).
The immune function of chymotrypsin has been reported in F. chinensis (Shi et al., 2008). However, little research has focused on the function of innate immunity in crabs. In this study, SpCHY was strongly up-regulated in S. paramamosain at 3 h after infection with the bacterium V. alginolyticus. In appropriate hosts, this kind of bacteria could proliferate unceasingly. The infection caused by un-ceasing reproduction of bacteria could induce the formation of reactive oxygen species (ROS) and severely destroy the functionality of crab cells (Li et al., 2011). Similar results showing that SpCHY expression is significantly shortly after bacterial infection were also obtained in other crustaceans (Amparyup et al., 2007; Qin et al., 2009; Cui et al., 2010). Hence we hypothesize that increasing the expression of SpCHY could activate PO production triggering an immune response and killing the bacteria.
In conclusion, our data suggest clearly for the first time that SpCHY is involved in the immune reaction against invading bacteria in the mud crab, S. paramamosain. The result should be helpful to understand the antibacterial defense mechanisms of crabs and provide biological information for mitigating crab diseases. Notwithstanding, the exact role of SpCHY in the activation of the immune response cascade needs further investigation.
Acknowledgments
This work was funded by grants from the National Natural Science Foundation of China (No. 41076081, 31272632) and the Innovative Research Funds in Xiamen University (No. 201112G009).
Footnotes
Associate Editor: Juan Lucas Argueso Almeida
References
- Amparyup P, Jitvaropas R, Pulsook N, Tassanakajon A. Molecular cloning, characterization and expression of a masquerade-like serine proteinase homologue from black tiger shrimp Penaeus monodon. Fish Shellfish Immunol. 2007;22:535–546. doi: 10.1016/j.fsi.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Aladaileh S, Rodney P, Nair SV, Raftos DA. Characterization of phenoloxidase activity in Sydney rock oysters (Saccostrea glomerata) Comp Biochem Physiol B. 2007;148:470–480. doi: 10.1016/j.cbpb.2007.07.089. [DOI] [PubMed] [Google Scholar]
- Broehan G, Kemper M, Driemeier D, Vogelpohl I, Merzendorfer H. Cloning and expression analysis of midgut chymotrypsin-like proteinases in the tobacco hornworm. J Insect Physiol. 2008;54:1243–1252. doi: 10.1016/j.jinsphys.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Broehan G, Arakane Y, Beeman RW, Kramer KJ, Muthukrishnan S, Merzendorfer H. Chymotrypsin-like peptidases from Tribolium castaneum: A role in molting revealed by RNA interference. Insect Biochem Mol Biol. 2010;40:274–283. doi: 10.1016/j.ibmb.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Cerenius L, Söderhäll K. The prophenoloxidase-activating system in invertebrates. Immunol Rev. 2004;1981:16–26. doi: 10.1111/j.0105-2896.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- Chaikeeratisak V, Somboonwiwat K, Wang HC, Lo CF, Tassanakajon A. Proteomic analysis of differentially expressed proteins in the lymphoid organ of Vibrio harveyiinfected Penaeus monodon. Mol Biol Rep. 2012;39:6367–6377. doi: 10.1007/s11033-012-1458-6. [DOI] [PubMed] [Google Scholar]
- Cheng W, Liu CH, Ye ST, Chen JC. The immune stimulatory effect of sodium alginate on the white shrimp Litopenaeus vannamei and its resistance against Vibrio alginolyticus. Fish Shellfish Immunol. 2004;17:41–51. doi: 10.1016/j.fsi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Craik CS, Choo QL, Swift GH, Quinto C, MacDonald RJ, Rutter WJ. Structure of two related rat pancreatic trypsin genes. J Biol Chem. 1984;259:14255–14264. [PubMed] [Google Scholar]
- Cui ZX, Liu Y, Wu DH, Luan WS, Wang SY, Li QQ, Song CW. Molecular cloning and characterization of a serine proteinase homolog prophenoloxidase-activating factor in the swimming crab Portunus trituberculatus. Fish Shellfish Immunol. 2010;29:679–686. doi: 10.1016/j.fsi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Gai YC, Qiu LM, Wang LL, Song LS, Mu CK, Zhao JM, Zhang Y, Li L. A clip domain serine protease (cSP) from the Chinese mitten crab Eriocheir sinensis: cDNA characterization and mRNA expression. Fish Shellfish Immunol. 2009;27:670–677. doi: 10.1016/j.fsi.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Gross PS, Bartlett CL, Browdy CL, Chapman RW, Warr GW. Immune gene discovery by expressed sequence tag analysis of hemocytes and hepatopancreas in the pacific white shrimp, Litopenaeus vannamei, and the atlantic white shrimp, L. setiferus. Dev Comp Immunol. 2001;25:565–577. doi: 10.1016/s0145-305x(01)00018-0. [DOI] [PubMed] [Google Scholar]
- Hedstrom L. Serine protease mechanism and specificity. Chem Rev. 2002;102:4501–4524. doi: 10.1021/cr000033x. [DOI] [PubMed] [Google Scholar]
- Herrero S, Combes E, Van OMM, Vlak JM, deMaagd RA, Beekwilder J. Identification and recombinant expression of a novel chymotrypsin from Spodoptera exigua. Insect Biochem Mol Biol. 2005;35:1073–1082. doi: 10.1016/j.ibmb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- He WY, Zheng YP, Tang L, Zheng SC, Beliveau C, Doucet D, Cusson M, Feng QL. Cloning, expression and localization of a trypsin-like serine protease in the spruce budworm, Choristoneura fumiferana. Insect Sci. 2009;16:455–464. [Google Scholar]
- Jiang H, Wang Y, Yu XQ, Zhu Y, Kanost MR. Prophenoloxidase-activating proteinase-2 (PAP-2) from hemolymph of Manduca sexta: A bacteria-inducible serine proteinase containing two clip domains. J Biol Chem. 2003a;278:3552–3561. doi: 10.1074/jbc.M205743200. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Yu XQ, Zhu Y, Kanost MR. Prophenoloxidase-activating proteinase-3 (PAP-3) from Manduca sexta hemolymph: A clip-domain serine proteinase regulated by serpin-1J and serine proteinase homologs. Insect Biochem Mol Biol. 2003b;33:1049–1060. doi: 10.1016/s0965-1748(03)00123-1. [DOI] [PubMed] [Google Scholar]
- Keenan C. Aquaculture of mud crab, genus Scylla- past, present and future. In: Keenan C, Blackshaw, editors. A Mud Crab Aquaculture and Biology. Watson Ferguson and Company; Canberra: 1999. pp. 9–13. ACIAR Proceedings, No. 78. [Google Scholar]
- Kiruthiga C, Rajesh S, Rashika V, Priya R, Narayanan RB. Molecular cloning, expression analysis and characterization of peroxiredoxin during WSSV infection in shrimp Fenneropenaeus indicus. J Invert Pathol. 2012;109:52–58. doi: 10.1016/j.jip.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Kumar S, Tamura K, Nei M. MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- Le Vay L. Ecology and management of mud-crab Scylla spp. Asian Fish Sci. 2001;14:101–112. [Google Scholar]
- Li JT, Chen P, Liu P, Gao BQ, Wang QY, Li J. Molecular characterization and expression analysis of extracellular copper-zinc superoxide dismutase gene from swimming crab Portunus trituberculatus. Mol Biol Rep. 2011;38:2107–2115. doi: 10.1007/s11033-010-0337-2. [DOI] [PubMed] [Google Scholar]
- Liu HP, Chen RY, Zhang M, Wang KJ. Isolation, gene cloning and expression profile of a pathogen recognition protein: A serine proteinase homolog (Sp-SPH) involved in the antibacterial response in the crab Scylla paramamosain. Dev Comp Immunol. 2010;34:741–748. doi: 10.1016/j.dci.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time quantitative PCR and the 2 (-Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mazumdar LS, Broadway RM. Identification of six chymotrypsin cDNAs from larval midguts of Helicoverpa zea and Agrotis ipsilon feeding on the soybean Kunitz trypsin inhibitor. Insect Biochem Mol Biol. 2001;31:633–644. doi: 10.1016/s0965-1748(00)00168-5. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA. Innate immunity: The virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- de Morais GS, Vitorino R, Domingues R, Tomer K, Correia AJF, Amado F, Domingues P. Proteomics of immune-challenged Drosophila melanogaster larvae hemolymph. Biochem Biophys Res Commun. 2005;328:10–15. doi: 10.1016/j.bbrc.2004.12.135. [DOI] [PubMed] [Google Scholar]
- Nielsen H, Engelbrecht J, Brunak S, vonHeijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng Des Sel. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- Pan D, He N, Yang Z, Liu H, Xu X. Differential gene expression profile in hepatopancreas of WSSV-resistant shrimp (Penaeus japonicus) by suppression subtractive hybridization. Dev Comp Immunol. 2005;29:103–112. doi: 10.1016/j.dci.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Perona JJ, Craik CS. Structural basis of substrate specificity in the serine proteases. Protein Sci. 1995;4:337–360. doi: 10.1002/pro.5560040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Chen L, Qin JG, Zhao D, Zhang H, Wu P. Characterization of a serine proteinase homologous (SPH) in Chinese mitten crab Eriocheir sinensis. Dev Comp Immunol. 2009;34:14–18. doi: 10.1016/j.dci.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Rawlings ND, Morton FR, Kok CY, Kong J, Barrett AJ. MEROPS: The peptidase database. Nucleic Acids Res. 2008;36:320–325. doi: 10.1093/nar/gkm954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J, Jiang H, Kanost MR, Wang Y. Serine proteases and their homologs in the Drosophila melanogaster genome: An initial analysis of sequence conservation and phylogenetic relationships. Gene. 2003;304:117–131. doi: 10.1016/s0378-1119(02)01187-3. [DOI] [PubMed] [Google Scholar]
- Samuel RI, Reynolds SE. Molting fluid enzymes of the tobacco hornworm Manduca sexta: Timing of proteolytic and chitinolytic activity in relation to preecdysial development. Arch Insect Biochem. 1993;24:33–44. [Google Scholar]
- Sellos D, Wormhoudt A. Molecular cloning of a cDNA that encodes a serine protease with chymotryptic and collagenolytic activities in the hepatopancreas of the shrimp Penaeus vanameii (Crustacea, Decapoda) FEBS Lett. 1992;309:219–224. doi: 10.1016/0014-5793(92)80777-e. [DOI] [PubMed] [Google Scholar]
- Sellos D, Wormhoudt A. Polymorphism and evolution of collagenolytic serine protease genes in crustaceans. Biochim Biophys Acta. 1999;1432:419–424. doi: 10.1016/s0167-4838(99)00121-1. [DOI] [PubMed] [Google Scholar]
- Serrano AE. Ontogenetic changes in the activity of chymotrypsin and carboxypeptidases A and B in mud crab, Scylla serrata. Isr J Aquacult-Bamid. 2013;65:1–6. [Google Scholar]
- Shi XZ, Zhao XF, Wang JX. Molecular cloning and expression analysis of chymotrypsin-like serine protease from the Chinese shrimp, Fenneropenaeus chinensis. Fish Shellfish Immunol. 2008;25:589–597. doi: 10.1016/j.fsi.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Southan C. A genomic perspective on human proteases as drug targets. Drug Discov Today. 2001;6:681–688. doi: 10.1016/s1359-6446(01)01793-7. [DOI] [PubMed] [Google Scholar]
- Swift GH, Craik CS, Stary SJ, Quinto C, Lahaie RG, Rutter WJ, MacDonald RJ. Structure of the two related elastase genes expressed in the rat pancreas. J Biol Chem. 1984;259:14271–14278. [PubMed] [Google Scholar]
- Szabo R, Bugge TH. Type II transmembrane serine pro-teases in development and disease. Int J Biochem Cell B. 2008;40:1297–1316. doi: 10.1016/j.biocel.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Ye HH, Tao Y, Wang GZ, Lin QW, Chen XL, Li SJ. Experimental nursery culture of the mud crab Scylla paramamosain (Estampador) in China. Aquacult Int. 2011;19:313–321. [Google Scholar]
- Zhao ZY, Yin ZX, Weng SP, Guan HJ, Li SD, Xing K, Chan SM, He JG. Profiling of differentially expressed genes in hepatopancreas of white spot syndrome virus-resistant shrimp (Litopenaeus vannamei) by suppression subtractive hybridization. Fish Shellfish Immunol. 2007;22:520–534. doi: 10.1016/j.fsi.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Zhou LM, Wu SG, Liu DC, Xu B, Zhang XF, Zhao BS. Characterization and expression analysis of a trypsin-like serine protease from planarian Dugesia japonica. Mol Biol Rep. 2012;39:7041–7047. doi: 10.1007/s11033-012-1535-x. [DOI] [PubMed] [Google Scholar]
- Zhu L, Song LS, Mao YZ, Zhao JM, Li CH, Xu W. A novel serine protease with clip domain from scallop Chlamys farreri. Mol Biol Rep. 2008;35:257–264. doi: 10.1007/s11033-007-9078-2. [DOI] [PubMed] [Google Scholar]
Internet Resources
- ORF Finder, http://www.ncbi.nlm.nih.gov/gorf (July 3, 2013).
- NCBI, http://www.ncbi.nlm.nih.gov (July 3, 2013).
- Expasy, http://www.expasy.ch/ (July 3, 2013).
- SignalP 4.0 software, http://www.cbs.dtu.dk/services/SignalP (July 3, 2013).
- ClustalW, http://www.ebi.ac.uk/Tools/msa/clustalw2/.