Abstract
Spotted leaf mutant belongs to a class of mutants that can produce necrotic lesions spontaneously in plants without any attack by pathogens. These mutants have no beneficial effect on plant productivity but provide a unique opportunity to study programmed cell death in plant defense responses. A novel rice spotted leaf mutant (spl30) was isolated through low-energy heavy ion irradiation. Lesion expression was sensitive to light and humidity. The spl30 mutant caused a decrease in chlorophyll and soluble protein content, with marked accumulation of reactive oxygen species (ROS) around the lesions. In addition, the spl30 mutant significantly enhanced resistance to rice bacterial blight (X. oryzae pv. oryzae) from China (C1–C7). The use of SSR markers showed that the spl30 gene was located between markers XSN2 and XSN4. The genetic distance between the spl30 gene and XSN2 and between spl30 and XSN4 was 1.7 cM and 0.2 cM, respectively. The spl30 gene is a new gene involved in lesion production and may be related to programmed cell death in rice. The ability of this mutant to confer broad resistance to bacterial blight provides a model for studying the interaction between plants and pathogenic bacteria.
Keywords: gene mapping, reactive oxygen species, rice (Orzya sativa), rice bacterial blight, spotted leaf mutant
Introduction
Plants that develop spontaneous necrotic lesions in the absence of infection by pathogens, environmental stress and mechanical damage are known as lesion mimic mutants (Wang, 2005). Such mutants have been reported in many plants, including maize (Hoisington et al., 1982), Arabidopsis (Lorrain et al., 2003), soybean (Badigannavar et al., 2002) and rice (Takahashi et al., 1999). The phenotype of these mutants resembles cell death induced by the hypersensitive response (HR), which suggests that the lesion mimic mutation results in the activation of defense gene expression. In addition, several reports have shown that cell death in lesion mimic mutants is regulated by environmental factors such as temperature (Xiao et al., 2003), light (Arase et al., 2000), humidity (Yoshioka et al., 2001) and photoperiod (Ishikawa et al., 2001). Lesion mimic phenotypes have also been reported in a number of transgenic plants expressing foreign or modified genes.
In plants, reactive oxygen species (ROS) such as superoxide (O2−), hydrogen peroxide (H2O2) and hydroxyl radicals (-OH) are produced under environmental stress or as byproducts of normal biochemical processes (Jabs et al., 1996). These ROS usually adversely affect cellular metabolism and membrane structure (Lorrain et al., 2003). Plants have several physiological mechanisms and structural adaptations for detoxifying ROS and for protecting themselves from different types of stress, including pathogens.
Numerous lesion mimic mutants have been identified and cloned, e.g., acd2, acd11 and lsd1 in Arabidopsis, mlo in barley and Lls1 in maize, that were cloned in the 1990s. These genes encode proteins that regulate plant defense to pathogens and/or cell death (Dietrich et al., 1994, 1997; Buschges et al., 1997; Gray et al., 1997, 2002). In rice, spl7 was the first lesion mimic mutant gene to be cloned and encodes a heat stress transcription factor (Yamanouchi et al., 2002). Other mutants cloned shortly after spl7 included spl5, spl11, spl18 and spl28 (Zeng et al., 2004; Mori et al., 2007; Qiao et al., 2010). Recent studies have shown that the spl5 gene encodes a putative splicing factor 3b subunit 3 (SF3b3) and may be involved in the splicing of pre-mature RNAs, thereby participating in the regulation of cell death and plant resistance responses (Chen et al., 2012). The cloning of these genes has contributed to our understanding of the molecular mechanisms of lesion formation, cell development and apoptosis in plants (Wu et al., 2008). Many of the rice lesion mimic mutants identified so far, including spl4, spl5, spl7, spl10, spl11, spl12, spl13, spl14, spl15, spl17, spl18, spl26 and spl28, show enhanced resistance to rice blast and/or bacterial blight pathogen (Mizobuchi et al., 2002; Mori et al., 2007; Yin et al., 2000).
In this report, we describe the isolation and characterization of a novel spotted leaf mutant (spl30) in rice irradiated by low-energy heavy ion irradiation. SSR markers were used to determine the location of the spl30 gene. The resistance of the mutant to rice bacterial blight from China (C1–C7) was also examined.
Materials and Methods
Isolation and phenotypic analysis of spotted leaf mutant
A mutant (spl30) showing lesion mimic phenotypes on leaves was identified in M2 lines of 9311 rice mutagenized with low-energy heavy ions. The mutant displayed irregular brown spots that usually occurred on the outer margin of leaves. For phenotypic analysis, the spl30 mutant and wild-type plants were grown under natural field conditions and in a greenhouse. The time required for spot formation as well as the color, structure and arrangement of spots throughout the leaves at different developmental stages were investigated. Scanning electron microscopy (SEM) was used to examine the surface of the lesions. Some agronomic traits, including plant height (PH), panicle length (PL), tiller number per panicle (TN), grains per panicle (GP), seed fertility (SF) and 1000-grain weight (GW), were evaluated in spl mutant and wild-type plants. Most of the agronomic data were evaluated during the eighth week after transplantation, which coincided with the end of vegetative growth in wild-type rice plants. Ten plants of each accession were evaluated for each agronomic trait.
Biochemical analyses
The chlorophyll content and total soluble protein content of leaves were measured once a week after transplanting until the eighth week. Chlorophyll was extracted from the same position on leaves of wild-type and spl30 plants using ice-cold 80% acetone, and the chlorophyll content was determined according to Lichtenthaler (1987). Soluble proteins were extracted with 10 mM Tris-HCl, pH 6.8, followed by centrifugation (10,000 rpm, 15 min, 4 °C) to obtain a soluble protein-rich extract. Soluble protein content was determined with a modified Lowry protein assay kit (Sangon, Shanghai, CN).
Histochemical analysis
Trypan blue was used to detect dead cells in leaves after transplant. The samples were immersed in trypan blue solution (2.5 mg/ml in 25% lactic acid, 23% water-saturated phenol and 25% glycerol in H2O) and heated in boiling water for 10 min followed by cooling for 2 h. The samples were subsequently placed in chloral hydrate solution (25 g in 10 mL of H2O) and destained for 2–3 d (Yin et al., 2000). After destaining, the samples were stored in 70% glycerol until examination. Superoxide anion (O2−) was detected by immersing the leaf samples in a solution of nitroblue tetrazolium (NBT, 0.5 mg/ml dissolved in 10 mM potassium phosphate buffer, pH 8.0, for 12 h in the dark. Hydrogen peroxide (H2O2) was detected by immersing the leaf samples in a solution of 3,3′-diaminobenzidine (DAB, 1 mg/ml, in 10 mM MES, pH 7.0, for 12 h in the dark. In both cases, the samples were destained in 95% ethanol heated in boiling water until chlorophyll was completely removed. The samples were then stored in 70% glycerol until observation (Kariola et al., 2005; Mahalingam et al., 2006).
Resistance to rice bacterial blight disease
Rice plants (30 days after transplantation) were inoculated with bacterial suspensions (OD600 = 0.5 in 10 mM MgCl2) of seven X. oryzae pv. oryzae strains from China (C1–C7). Leaves of wild-type and spl30 plants were cut with scissors ∼2 cm from the leaf opex and drenched with bacterial suspension (Ogawa and Sekizawa, 1980). The length of the lesions was measured 20 days after inoculation.
Genetic analysis and gene mapping
For genetic analysis, the spl30 mutant was used as the maternal parent and crossed with japonica-type pollen donors, specifically Balila (japonica) and Zhonghua11 (japonica). The resulting F1 was self-fertilized to produce the F2. Large numbers of F2 progeny were grown in the field and phenotypic data related to the segregation of mutant and wild-type traits were collected. Bulked segregant analysis was done using a DNA mixture contributed equally by 20 homozygous spotted leaf plants derived from the F2 mapping population.
The simple sequence repeats (SSR) technique was used to evaluate the genomic variation between the spl30 mutant and wild-type plants (Ishii et al., 2001). Genomic DNA was extracted from each parent, F1 and F2 individuals using the modified CTAB (cetyltrimethyl ammonium bromide) method (Murray and Thompson, 1980). The primer sequences of the SSR markers were downloaded from http://www.gramene.org/ and the primers were synthesized by Shanghai Sangon Inc. (Shangai, China).
The PCR mixture consisted of 2.0 μL of 10PCR buffer, 2.0 μL of 25 mM MgCl2, 1.0 μL of 2.5 mM dNTPs, 12.0 μL of ddH2O, 2.0 μL of 10 μM primers, 1.0 μL of DNA and 1.0 μL of Taq DNA polymerase (1 U/μL) in a final volume of 20 μL. The amplification conditions consisted of denaturation at 94 °C for 5 min, followed by 39 cycles of denaturation at 94 °C for 30 s, annealing at 55–60 °C (according to the specific primer) for 30 s, extension at 72 °C for 1 min and a final extension at 72 °C for 10 min. The PCR products were subsequently analyzed on 3% agarose-ethidium bromide gels (Liu et al., 2008).
Linkage groups were determined using MAPMAKER/EXP 3.0 (Lander et al., 1987). The Kosambi mapping function was used to transform the recombination frequency to mapping distance (cM) (Kosambi, 1944). Linkage maps were constructed based on a limit of detection (LOD) threshold of 3.0 and a maximum genetic distance of 30 cM.
Semi-quantitative RT-PCR
Total RNA was extracted from leaves of 9311 plants and spl 30 mutants with TRIzol reagent (Invitrogen, USA). The first-strand synthesis of cDNA was done with a TransScriptTM one-step gDNA removal and cDNA synthesis SuperMix (TransGen, China) according to the manufacturer’s instructions. The first-strand cDNA was used as a template for amplification by PCR (30 cycles for spl5, spl11 and spl28 and 25 cycles for the actin control). The reaction mixture was cycled through the following temperature profiles: 94 °C for 210 s for one cycle, followed by 94 °C for 45 s, 60 °C for 45 s, and 72 °C for 60 s for 30 cycles, and a final incubation at 72 °C for 5min. The primers for RT-PCR were as follows: spl5-Forward (5′-TTGCAG CAAACTTCATCAGGAC-3′) and spl5-Reverse (5′-GAGGGACTCCAAGGAAAGTGTTAT-3′) for spl5, spl11-Forward (5′-GATGCTTGCCTTATTGTCCTCA--3′) and spl11-Reverse (5′-ACGGATTGATATGCCTGA CGAT-3′) for spl11, spl28-Forward (5′-GTGAAAGCA AGAAGTCAGTTTAAGG-3′) and spl28-Reverse (CTAACAAGATGAACAACGAGACAGA-3′) for spl28, actin-Forward (5′-TGTCATGGTTGGAATGGGCCA-3′) and actin-Reverse (5′-AGGCAGTCAGTCAGATCA CGA-3′) for actin. Each analysis was repeated independently three times using different biological samples each time.
Results
Phenotypic analysis of the spl30 mutant
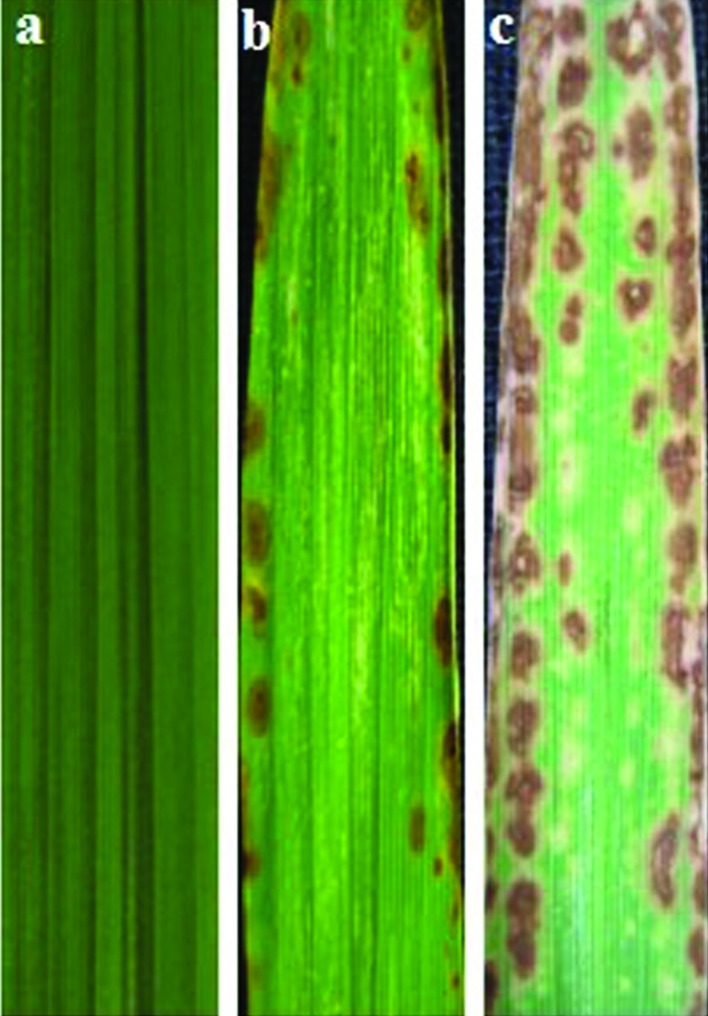
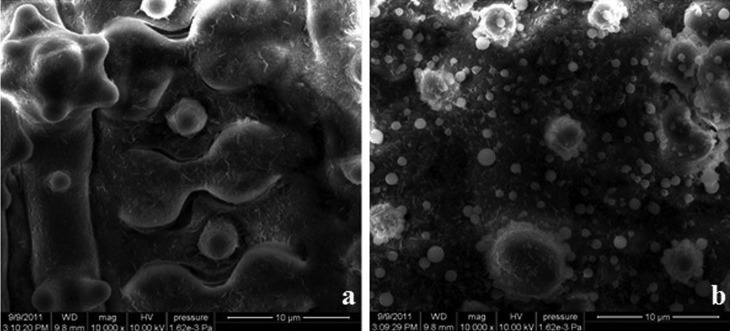
Typical spots (brown lesions) appeared on the leaves of spl30 mutants after 15–20 days. The number and size of the spots increased with growth but stopped expanding at the tillering stage. The distribution of spots differed between plants grown in natural field conditions (average temperature 25.6 °C, humidity 81.1% and light intensity 3241.8 lx) and those grown in a greenhouse (average temperature 25.9 °C, humidity 36.1% and light intensity 1108.1 lx) (Figure 1). SEM showed that the surface of wild-type leaves was very clean while that of spl30 mutants containing lesions had many spheres ∼1 μm in diameter (Figure 2); these spheres were suspected to be apoptotic bodies formed during apoptosis.
Figure 1.
Phenotype of wild-type and mutant leaves. (a) Leaves of wild-type plant grown in the field, (b) Leaves of spl30 mutant grown in the field and (c) Leaves of spl30 mutant grown in a glasshouse.
Figure 2.
Scanning electron micrographs of the surface of wild-type (a) and spl30 mutant (b) leaves.
To understand the effects of the lesions on the agronomic characteristics of the spl30 mutants, various agronomic traits of the mutants were analyzed and compared with those of the wild-type. Ten plants of each accession were evaluated for each agronomic trait. The mean ± SE of the values are given in Table 1. The spl30 mutant showed abnormal developmental phenotypes with varying degrees of agronomic characteristics and significantly lower trait values than those of wild-type plants. The plant height, seed fertility rate and 1000-grain weight of spl30 mutants were significantly lower than those of wild-type plants. These results indicate that the formation of spots and plant growth are interrelated processes.
Table 1.
Agronomic traits of wild-type and spl30 mutant plants.
| Material | pH (cm) | PL (cm) | TN | GP | SF (%) | GW(g) |
|---|---|---|---|---|---|---|
| Wild-type | 126.2 ± 6.01 | 22.1 ± 0.69 | 4.4 ± 1.14 | 340.4 ± 17.64 | 87.1 ± 1.49 | 31.1 ± 0.19 |
| spl30 | 109.8 ± 3.70** | 20.0 ± 0.52 | 4.4 ± 1.14 | 244.8 ± 35.91 | 55.9 ± 7.99** | 28.3 ± 0.23* |
GP = grains per panicle, GW = weight of 1000 grains, pH = plant height, PL = panicle length, SF = setting fertility, TN = tiller number per panicle. The values are the mean ± SD of 10 plant determinations.
p < 0.05 and p < 0.01, respectively, compared to wild-type plants.
Senescence indicators in spl30 mutants
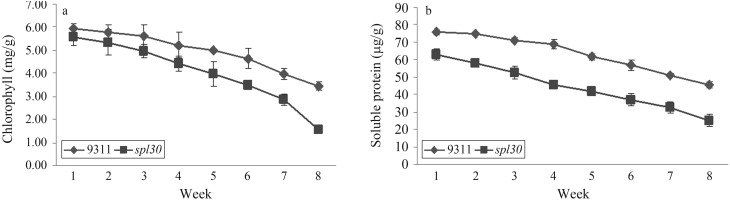
Since chlorophyll and soluble protein contents generally reflect the degree of leaf senescence, the levels of these two indicators was measured at the same position in leaves from wild-type plants and spl30 mutants once a week up to the eight week after transplant. The chlorophyll content in spl30 mutants decreased from 5.57 mg/g in the first week to 1.56 mg/g at the eighth week (Figure 3A). In contrast, in wild-type plants, the chlorophyll content decreased slowly, from 5.93 mg/g in the first week to 3.45 mg/g in the eighth week. The soluble protein content showed a similar pattern of changes to that observed for chlorophyll. The soluble protein content of spl30 mutants decreased from 62.75 μg/g in the first week to 24.98 μg/g in the eighth week, whereas in wild-type plants the corresponding values were 75.75 μg/g and 45.3 μg/g, respectively (Figure 3B). These results indicated that the early senescence in mutant leaves may be caused by the formation of spots.
Figure 3.
Analysis of chlorophyll content (a) and soluble protein (b) content, two indicators of senescence.
Histochemical analysis
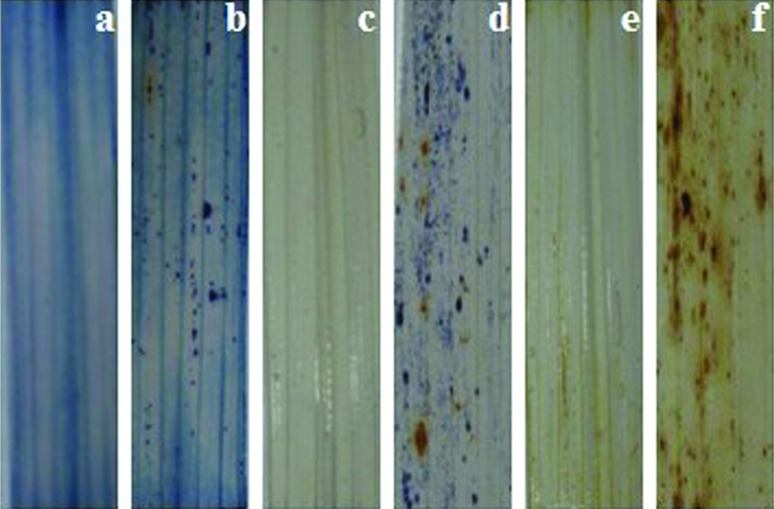
Trypan blue is a vital stain used to selectively stain dead tissues or cells (stained cells have irreversibly damaged membranes that permit dye entry). The spl30 mutants showed strong blue staining of cells in the spots whereas wild-type plants had no trypan blue staining (Figure 4A,B). Furthermore, the pattern of NBT staining, which reflects the formation of blue formazan precipitates and indicates O2− accumulation, correlated strongly with lesion formation in cleared spl30 mutant leaves; there was no evidence for ROS accumulation in wild-type leaves (Figure 4C,D). Similar results were obtained when DAB staining was used to assess H2O2 accumulation (Figure 4E,F). These findings suggest that ROS accumulation in the early and middle periods could account for spot formation.
Figure 4.
Photographs of leaves stained with Trypan blue, NBT and DAN. Trypan blue: (a) wild-type and (b) spl30; NBT: (c) wild-type and (d) spl30; DAB: (e) wild-type and (f) spl30.
Resistance to bacterial blight pathogens
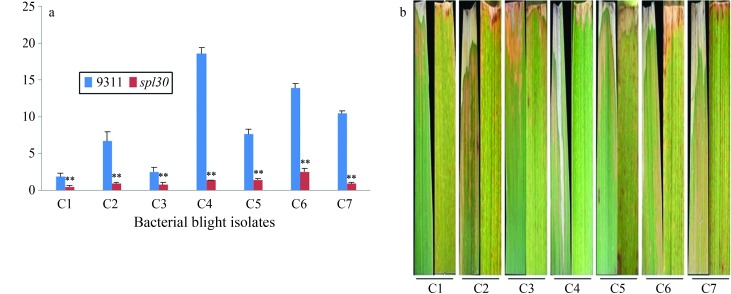
The resistance of spl30 to bacterial blight was assessed using seven strains of bacterial blight (X. oryzae pv. oryzae). Twenty days after inoculation, the lesion lengths were measured as an indicator of the severity of symptoms in the mutants and wild-type controls. The lesion length for the different bacterial isolates ranged from 0.4 to 2.5 cm in spl30 plants and from 1.9 to 18.5 cm in wild-type plants (Figure 5A). The spotted leaf mutant was significantly more resistant to three X. oryzae pv. oryzae (C4, C6 and C7) isolates than were wild-type plants (Figure 5B). These results indicate that the spl30 mutant is resistant to bacterial blight pathogens from China (C1–C7).
Figure 5.
Analysis of lesions in leaves of wild-type and spl30 plants after inoculation with bacterial blight isolates. (a) Lesion length in wild-type and spl30 plants. (b) Phenotypes of leaves from wild-type and spl30 plants (from left).
Genetic analysis and gene mapping
The pattern of inheritance of the mutant gene in spl30 was deduced by observing the segregation of the parental phenotypes in the F1 and F2 generations after crosses between mutant and wild-type cultivars, as indicated in Table 2. The traits examined were inherited in a 3:1 ratio, which indicates that the spl30 mutant is controlled by single recessive genes (Table 2).
Table 2.
Segregation of wild-type and mutant plants in F2 populations of spl30.
| Hybrid combination | F1 phenotype | F2 Population
|
χ2 3:1 χ20.05 = 3.84 |
|||
|---|---|---|---|---|---|---|
| Population | Wild-type | Mutant | Wild-type/ mutant ratio | |||
| spl30/Balila | WT | 180 | 137 | 43 | 3.19 | 0.0395 |
| spl30/Zhonghua11 | WT | 3977 | 3025 | 952 | 3.18 | 2.3938 |
WT = wild-type.
PCR was done with DNA from two parents; F1 and F2 mixing pool (DNA from 20 random mutants equivalent mixed in F2 population) with the polymorphic SSR markers. The chromosomal location of spl30 was determined by observing the genotypes of the spl30 mutant and the spl30 locus was then mapped to chromosome 9, with genetic distances of 8.5 cM and 0.6 cM for RM6543 and RM7697, respectively. For finer mapping of the target gene, ten new SSR markers (XSN1-XSN10) between RM6543 and RM7697 were designed, with four of them being polymorphic. The spl30 gene was mapped between markers XSN2 and XSN4 and the genetic distances of the two markers were 1.7 cM and 0.2 cM, respectively (Figure 6). The results indicated that the spl30 locus was located on the long arm of chromosome 9.
Figure 6.
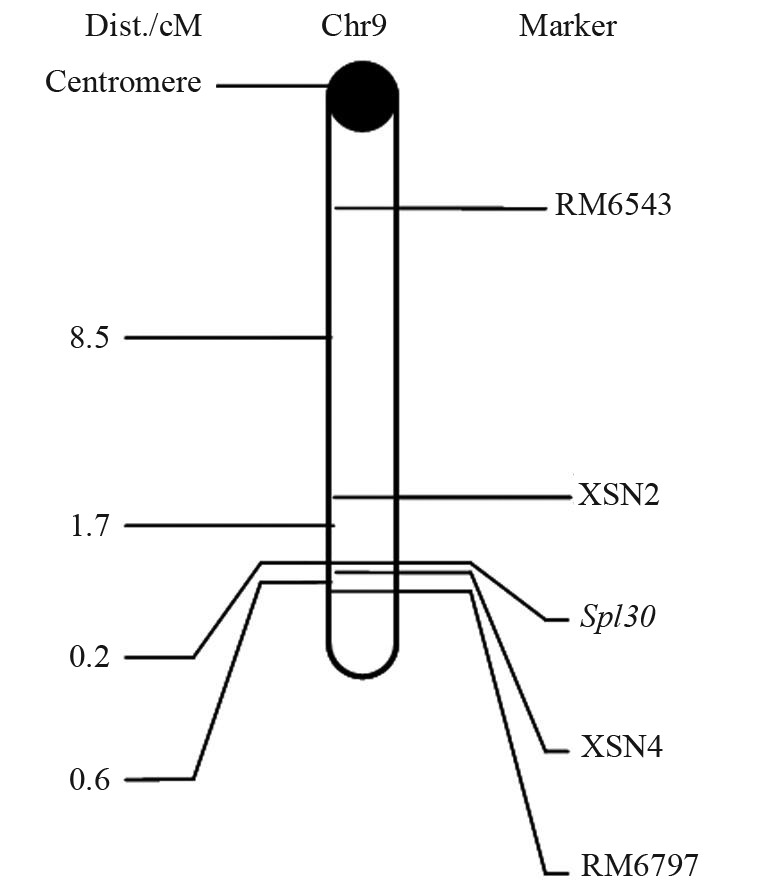
Linkage relationships of spl30 with its markers on chromosome 9 of rice.
Expression of known spotted leaf genes
Some spl mutants can enhance resistance to rice bacterial blight pathogens, and spl5, spl11 and spl28 have been cloned. RT-PCR was used to assess the levels of spl5, spl11 and spl28 mRNA in spl30 mutant and wild-type plants. Approximately 20 days after sowing (DAS), lesion mimics began to appear on the leaves of mutant plants and at 30 DAS, the leaves were used for mRNA detection. Figure 7 shows that the mRNA levels of spl5, spl11 and spl28 were similar in wild-type plants and spl30 mutants. Hence, the resistance to bacterial blight pathogens observed in spl30 mutants was not attributable to these spl genes and spl30 was apparently a new gene capable of conferring bacterial resistance to rice.
Figure 7.
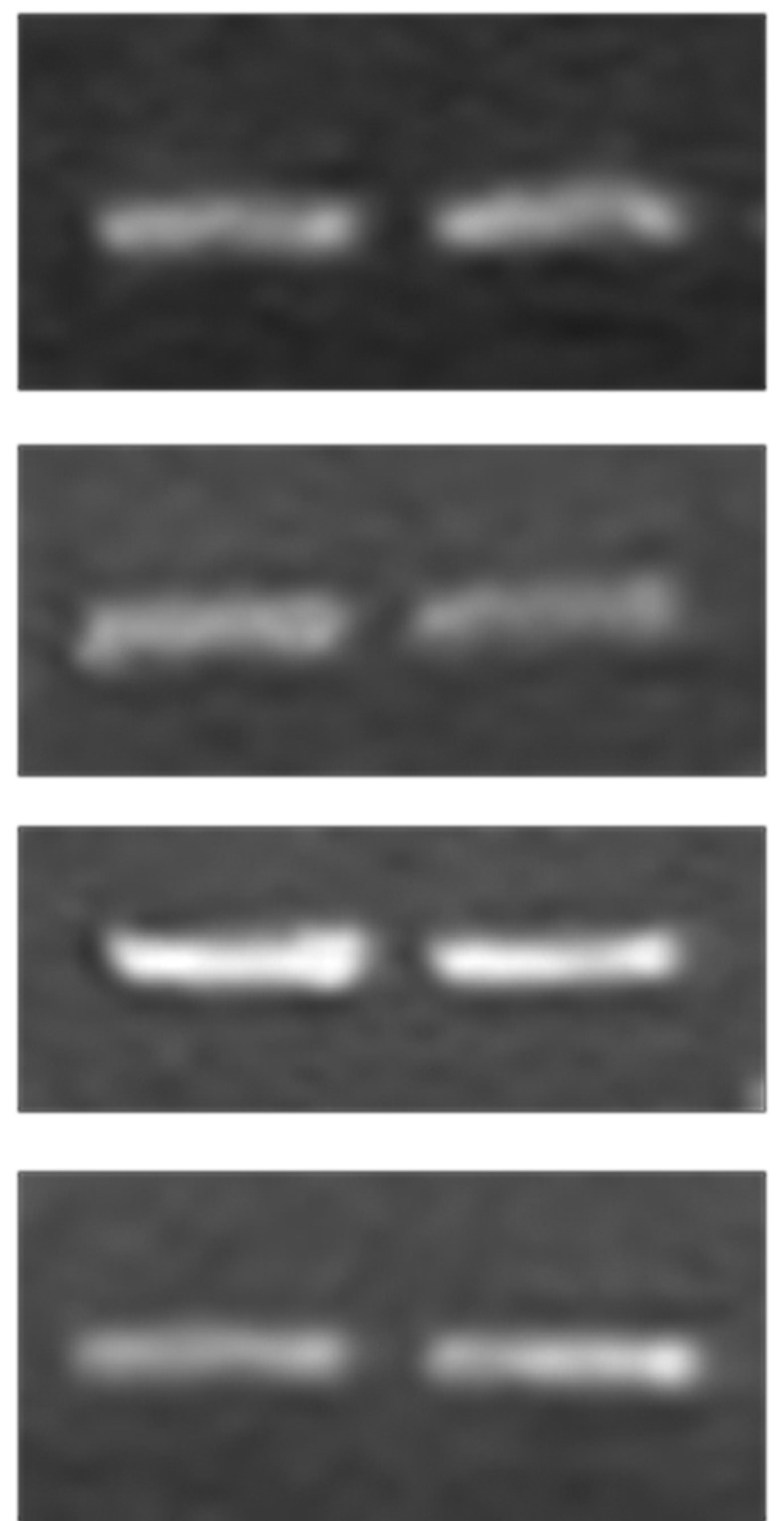
Expression analysis of Spl5, Spl11 and Spl28 by assessed by RT-PCR.
Discussion
Programmed cell death (PCD) is a physiological process involved in the selective elimination of unwanted cells (Yeung and Meinke, 1993). In plants, selective cell death is necessary for growth and survival and can occur on a small or large scale. The formation of plant lesion mimics correlates with disturbances in the regulation of metabolic pathways mediated by mutated genes and associated with various environmental factors, including light, temperature, humidity and nutrition. The size of the lesion in spl1, spl3 and spl4 mutants increases with increasing temperature and light intensity (Matin et al., 2010). The spl7 mutant shows variable lesion density at different temperatures, with the density decreasing at low temperature (Yamanouchi et al., 2002). In physiology, high levels of ROS around the lesions have been identified in slms mutants and ROS production is considered to be the causal agent of cell death (Lorrain et al., 2003). In spl30 mutants, lesion development is unrelated to growth conditions, which suggests that the lesions may result from spontaneous cell death. The expression of lesion spots is sensitive to light and humidity. The spl30 mutants showed an excessive accumulation of O2− and H2O2. The staining of wild-type and mutant leaves with trypan blue, NBT and DAB revealed a hypersensitivity-like reaction without the involvement of distinct external factors. This finding suggested that spl30 spot formation and development probably resulted from PCD in leaves.
In Arabidopsis, lsd1 mutants are resistant to the fungal pathogen Peronospora parasitica and the bacterial pathogen Pseudomonas syringae (Dietrich et al., 1997). In rice, spotted leaf mutants such as spl1, spl11, cdr (cell death and resistance) and blm (blast lesion mimic mutants) show broad-spectrum resistance to rice blast fungus (Magnaporthe grisea) and rice bacterial blight (Xanthomonas oryzae pv. oryzae), while others show specific resistance to certain diseases or races. As shown here, the spl30 mutation conferred broad-spectrum resistance to various strains of rice bacterial blight from China (C1–C7). This mutant could therefore be useful for studying the mechanism of disease resistance in rice. Based on this conclusion, we reasoned that investigation of the genetics of spotted leaf mutants could be useful in elucidating the molecular mechanisms involved in plant defense against pathogens and in understanding the resistance of the spl30 mutant to rice blast.
Gene mapping indicated that the spl30 locus was located on the long arm of chromosome 9, a location not previously reported for spotted leaf mutant genes. RT-PCR showed that the expression of some spl genes related to resistance was not unaltered. These findings indicate that we have uncovered new genes involved in mimicking lesions; these genes could be useful for studying the mechanisms underlying leaf death and disease resistance. We are currently expanding the size of the mapped population and are developing new markers for fine-mapping, cloning and functional analysis of spl30.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (grant no. 10975153), the Knowledge Innovative Program of the Chinese Academy of Sciences (grant nos. KJCX2-EW-N05 and KJCX2-YXN34), the National Natural Science Foundation of Anhui (grant no. 11040606Q58), the Science and Technology Key Project of Anhui Province (grant no. 1101031008) and the Talent Special Funds of Anhui.
Footnotes
Associate Editor: Marcia Pinheiro Margis
References
- Arase S, Fujita K, Uehara T, Honda Y, Isota J. Light-enhanced resistance to Magnaporthe grisea infection in the rice Sekiguchi lesion mutants. J Physiol. 2000;148:197–203. [Google Scholar]
- Badigannavar AM, Kale DM, Eapen S, Murty GSS. Inheritance of disease lesion mimic leaf trait in groundnut. J Hered. 2002;93:50–52. doi: 10.1093/jhered/93.1.50. [DOI] [PubMed] [Google Scholar]
- Buschges R, Hollricher H, Pansteruga R, Simons G, Wolter M, Frijters A, Van-Daelen R, Vander-Lee T, Diergaarde P, Groenendijk J. The barley Mlo gene: A novel control element of plant pathogen resistance. Cell. 1997;88:695–705. doi: 10.1016/s0092-8674(00)81912-1. [DOI] [PubMed] [Google Scholar]
- Chen XF, Liang H, Pan JW, Zheng XX, Jiang GH, Jin Y, Gu ZM, Qian Q, Zhai WX, Ma BJ. SPL5, a cell death and defense-related gene, encodes a putative splicing factor 3b subunit 3 (SF3B3) in rice. Mol Breed. 2012;30:939–949. [Google Scholar]
- Cheong YH, Moon BC, Kim JK, Kim CY, Kim MC, Kim IH, Park CY, Kim JC, Park BO, Koo SC, et al. BWMK1, a rice mitogen-activated protein kinase, locates in the nucleus and mediates pathogenesis - related gene expression by activation of a transcription factor. Plant Physiol. 2003;132:1961–1972. doi: 10.1104/pp.103.023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich RA, Delanry TP, Uknes SJ, Ward ER, Ryals JA, Dangl JL. Arabidopsis mutants simulating disease resistance response. Cell. 1994;77:565–577. doi: 10.1016/0092-8674(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Dietrich RA, Richberg MH, Schmidt R, Dean C, Dangl JL. A novel zinc finger protein is encoded by the Arabidopsis LSD1 gene and functions as a negative regulator of plant cell death. Cell. 1997;88:685–694. doi: 10.1016/s0092-8674(00)81911-x. [DOI] [PubMed] [Google Scholar]
- Gray J, Close PS, Briggs SP, Johal GS. A novel suppressor of cell death in plants encoded by the Lls1 gene of maize. Cell. 1997;89:25–31. doi: 10.1016/s0092-8674(00)80179-8. [DOI] [PubMed] [Google Scholar]
- Gray J, Janick-Buckner D, Buckner B, Close PS, Johal GS. Light-dependent death of maize lls1 cells is mediated by mature chloroplasts. Plant Physiol. 2002;130:1894–1907. doi: 10.1104/pp.008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoisington DA, Neuffer MG, Walbot V. Disease lesion mimics in maize. I. Effect of genetic background, temperature, developmental age, and wounding on necrotic spot formation with Les1. Dev Biol. 1982;93:381–388. doi: 10.1016/0012-1606(82)90125-7. [DOI] [PubMed] [Google Scholar]
- Ishii T, Xu Y, McCouch SR. Nuclear- and chloroplast-microsatellite variation in A-genome species of rice. Genomics. 2001;44:658–666. [PubMed] [Google Scholar]
- Ishikawa A, Okamoto H, Iwasaki Y, Asahi T. A deficiency of coproporphyrinogen III oxidase causes lesion formation in Arabidopsis. Plant J. 2001;27:89–99. doi: 10.1046/j.1365-313x.2001.01058.x. [DOI] [PubMed] [Google Scholar]
- Jabs T, Dietrich RA, Dangl JL. Initiation of runaway cell death in an Arabidopsis mutant by extracellular super-oxide. Science. 1996;273:1853–1856. doi: 10.1126/science.273.5283.1853. [DOI] [PubMed] [Google Scholar]
- Kariola T, Brader G, Li J, Palva ET. Chlorophyllase 1, a damage control enzyme, affects the balance between defense pathways in plants. Plant Cell. 2005;17:282–294. doi: 10.1105/tpc.104.025817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosambi DD. The estimation of map distance from recombination values. Ann Eugen. 1944;12:172–175. [Google Scholar]
- Lander ES, Green P, Abrahamason J, Barlow A, Daly MJ, Lincoln SE, Newburg L. Mapmaker: An interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987;1:174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method Enzymol. 1987;148:350–382. [Google Scholar]
- Liu BM, Wu YJ, Fu XD, Qian Q. Characterizations and molecular mapping of a novel dominant semi-dwarf gene Sdd(t) in rice (Oryza sativa) Plant Breed. 2008;127:125–130. [Google Scholar]
- Lorrain S, Vailleau F, Balague C, Roby D. Lesion mimic mutants: Keys for deciphering cell death and defense pathways in plants. Trends Plant Sci. 2003;8:263–271. doi: 10.1016/S1360-1385(03)00108-0. [DOI] [PubMed] [Google Scholar]
- Mahalingam R, Jambunathan N, Gunjan SK, Faustin E, Weng H, Ayoubi P. Analysis of oxidative signaling induced by ozone in Arabidopsis thaliana. Plant Cell Environ. 2006;29:1357–1371. doi: 10.1111/j.1365-3040.2006.01516.x. [DOI] [PubMed] [Google Scholar]
- Matin MN, Pandeya D, Baek KH, Lee DS, Lee JH, Kang H, Cheong YH, Moon BC, Kim JK, Kim CY, et al. BWMK1, a rice mitogen-activated protein kinase, locates in the nucleus and mediates pathogenesis-related gene expression by activation of a transcription factor. Plant Physiol. 2003;132:1961–1972. doi: 10.1104/pp.103.023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin MN, Pandeya D, Baek KH, Lee DS, Lee JH, Kang H, Kang SG. Phenotypic and genotypic analysis of rice lesion mimic mutants. Plant Pathol J. 2010;26:159–169. [Google Scholar]
- Mizobuchi R, Hirabayshi H, Kaji R. Isolation and characterization of rice lesion-mimic mutants with enhanced resistance to rice blast and bacterial blight. Plant Sci. 2002;163:345–353. [Google Scholar]
- Mori M, Tomita C, Sugimoto K, Hasegawa M, Hayashi N, Dubouzet JG, Ochiai H, Sekimoto H, Hirochika H, Kikuchi S. Isolation and molecular characterization of a spotted leaf 18 mutant by modified activation tagging in rice. Plant Mol Biol. 2007;63:847–860. doi: 10.1007/s11103-006-9130-y. [DOI] [PubMed] [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Sekizawa K. Studies on the breeding of rice varieties resistant to bacterial leaf blight. 2. Test of the quantitative resistance of rice native varieties in Japan by clipping inoculation method. Bull Chugoku Nat Agric Exp Stn A. 1980;27:19–36. [Google Scholar]
- Qiao YL, Jiang WZ, Lee JH, Park BS, Choi MS, Piao R, Woo MO. SPL28 encodes a clathrin-associated adaptor protein complex 1, medium subunit l1 (AP1M1) and is responsible for spotted leaf and early senescence in rice (Oryza sativa) New Phytol. 2010;185:258–274. doi: 10.1111/j.1469-8137.2009.03047.x. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Kawasaki T, Henmi K, Shi IK, Kodama O, Satoh H, Shimamoto K. Lesion mimic mutants of rice with alterations in early signaling events of defense. Plant J. 1999;17:535–545. doi: 10.1046/j.1365-313x.1999.00405.x. [DOI] [PubMed] [Google Scholar]
- Wang ZH. Induction and mutation mechanism of plant lesion mimic mutants. Chin J Cell Biol. 2005;27:530–534. [Google Scholar]
- Wu C, Bordeos A, Madamba MR, Baraoidan M, Ramos M, Wang GL, Leach JE, Leung H. Rice lesion mimic mutants with enhanced resistance to diseases. Mol Genet Genomics. 2008;279:605–619. doi: 10.1007/s00438-008-0337-2. [DOI] [PubMed] [Google Scholar]
- Xiao S, Brown S, Patrick E, Brearley C, Turner JG. Enhanced transcription of the Arabidopsis disease resistance genes RPW8.1 and RPW8.2 via a salicylic acid dependent amplification circuit is required for hypersensitive cell death. Plant Cell. 2003;15:33–45. doi: 10.1105/tpc.006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanouchi U, Yano M, Lin H, Ashikari M, Yamada K. A rice spotted leaf gene, Spl7, encodes a heat stress transcription factor protein. Proc Natl Acad Sci USA. 2002;99:7530–7535. doi: 10.1073/pnas.112209199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung EC, Meinke DW. Embryogenesis in angiosperms: Development of the suspensor. Plant Cell. 1993;5:1371–1381. doi: 10.1105/tpc.5.10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Chen J, Zeng L, Goh M, Leung H, Khush GS, Wang GL. Characterizing rice lesion mimic mutants and identifying a mutant with broad-spectrum resistance to rice blast and bacterial blight. Mol Plant Microbe Interact. 2000;13:869–876. doi: 10.1094/MPMI.2000.13.8.869. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Kachroo P, Tsui F, Sharma SB, Shah J, Klessig DF. Environmentally sensitive, SA-dependent defense responses in the cpr22 mutant of Arabidopsis. Plant J. 2001;26:447–459. doi: 10.1046/j.1365-313x.2001.2641039.x. [DOI] [PubMed] [Google Scholar]
- Zeng LR, Qu S, Bordeos A, Yang C, Baraoidan M, Yan H, Xie Q, Nahm BH, Leung H, Wang GL. Spotted leaf11, a negative regulator of plant cell death and defense, encodes a U-box/armadillo repeat protein endowed with E3 ubiquitin ligase activity. Plant Cell. 2004;16:2795–2808. doi: 10.1105/tpc.104.025171. [DOI] [PMC free article] [PubMed] [Google Scholar]