Abstract
Aims
Low androgen levels have been linked with an increased risk of cardiovascular disease in men. Previous studies have suggested that androgens directly inhibit atherosclerotic lesion formation although the underlying mechanisms for this remain unclear. This study addressed the hypothesis that endogenous androgens inhibit arterial remodelling by a direct action on the androgen receptor (AR) in the vascular wall.
Methods and results
We studied a series of novel mouse lines with cell-specific deletion of the AR in either the endothelium or in smooth muscle cells or both cell types. Findings were compared with a model of global androgen deficiency in wild-type mice (castrated). We characterized the cardiovascular phenotype, vascular pharmacology and histology, and assessed neointimal lesion formation following vascular injury to the femoral artery. Cell-specific AR deletion did not alter body weight, circulating testosterone levels or seminal vesicle weight, but caused limited alterations in arterial contractility and blood pressure. Neointimal lesion formation was unaltered by selective deletion of AR from the vascular endothelium, smooth muscle, or both cell types. Castration in wild-type mice increased neointimal lesion volume (Sham vs. Castration: 2.4 × 107 ± 4.5 × 106 vs. 3.9 × 107 ± 4.9 × 106 µm3, P = 0.04, n = 9–10).
Conclusion
Vascular cell-specific AR deletion had no effect on neointimal lesion formation, while low systemic androgen levels adversely affect neointimal lesion size. These findings suggest that the cardio-protective effects of androgens are mediated either by AR outside the vasculature or by AR-independent mechanisms.
Keywords: Androgen receptor, Testosterone, Arterial injury, Neointima
1. Introduction
Male sex hormones have traditionally been linked to the greater risk of cardiovascular disease (CVD) in men.1,2 However, this view is increasingly being challenged, with considerable recent evidence that testosterone may, in fact, be cardio-protective. Cross-sectional studies have associated low testosterone levels with increased cardiovascular risk factors (diabetes mellitus, the metabolic syndrome, abnormal lipid profile) and increased cardiovascular risk in men.2–5 This is particularly important given the progressive population-level decline in serum testosterone concentrations in men from developed countries6–8 which has resulted in a dramatic increase in the use of androgen replacement therapy (ART). Indeed, there has been a 10-fold increase in prescribed ART in the USA9 and nearly a 3-fold increase in the UK10 in the past decade. ART improves the muscle/fat mass ratio, bone mineral density, and blood lipid profile6,11,12 in hypogonadal men. It has also been suggested that ART could provide a novel strategy to reduce cardiovascular risk. More recently, however, concerns have been raised about safety and the Food and Drug Administration in the USA has announced an investigation into the risk of stroke, heart attack, and death in men taking testosterone products.13 This follows recent reports demonstrating an excess of cardiovascular events in apparently hypogonadal men using ART.14–16 Given the inconsistent findings from clinical studies, there is a clear need for additional pre-clinical studies to improve our understanding of how endogenous androgens and pharmacological androgen supplements influence CVD. Previous pre-clinical studies have focused on pharmacological testosterone supplementation and/or deficiency (by castration) and have largely supported a cardio-protective role for androgens, with pharmacological testosterone replacement in castrated animals reducing atherosclerotic plaque formation.17–19 However, the mechanism of this effect is not clear. It may be indirect, following modification of conventional cardiovascular risk factors, and/or due to direct modulation of vascular remodelling. Furthermore, it has not been established whether androgens alter vascular remodelling by direct stimulation of the androgen receptor (AR), by testosterone-mediated AR-independent actions, or, indirectly, via aromatase-mediated conversion of testosterone to oestrogens. In models of arterial injury that lack elevated systemic cardiovascular risk factors, the findings are contradictory, with studies showing that androgens either reduce20 (possibly by inhibiting arterial smooth muscle proliferation),21 or have no effect on22 neointimal lesion formation.
Endogenous androgens play a complex role in determining cardiovascular risk and thus investigation of their mechanism of action is challenging. The influence of AR stimulation on vascular lesion formation has been investigated previously using the testicular feminised (Tfm) mouse, which lacks a functional AR.19 However, interpretation of results from this animal is confounded by the fact that it lacks AR in all tissues, has low (∼10%) circulating testosterone, and, consequently, has sub-physiological concentrations of oestradiol. Generation of a similar total AR knockout mouse on an atherosclerosis-prone (apoE−/−) background suggested that androgens reduce total serum cholesterol via an AR-dependent mechanism but implicated both AR-dependent and AR-independent mechanisms in the observed anti-atherosclerotic effects.23 Recognizing the limitations of these models and the complex role of androgens in influencing a number of aspects of cardiovascular risk, we generated mice with vascular cell-specific deletions of AR in order to address the hypothesis that endogenous testosterone inhibits neointimal proliferation by stimulation of AR in the vascular wall.
2. Methods
See Supplementary material, Online Data for detailed materials and methods related to this study.
2.1. Mice
Animal experiments were performed in accordance both with Directive 2010/63/EU of the European Parliament and with the UK Home Office Animal (Scientific Procedures) Act 1986.
C57Bl/6J mice were supplied by the University of Edinburgh Biomedical Research Facility. Mice with selective ablation of AR from vascular endothelial (VE-ARKO)24 or smooth muscle cells (SM-ARKO)25 were established in our laboratory as previously described. In this study, these two lines were mated to generate stud males hemizygous for both Tie2-Cre and SM22-Cre, which were then mated with female ARfl/fl mice. The mouse line was maintained by breeding male SM22-Cre+/−:Tie2-Cre+/−:ARfl/y mice with female ARfl/fl mice. Four genotypes were identified in the resultant offspring at expected Mendelian ratios (∼25% for each genotype in male pups):
WT: SM22-Cre−/−:Tie2-Cre−/−:ARfl/y. Used as controls.
SM-ARKO: SM22-Cre+/−:Tie2-Cre−/−:ARfl/y. Smooth muscle cell (SMC) ARKO.
VE-ARKO: SM22-Cre−/−:Tie2-Cre+/−:ARfl/y. Endothelial cell (EC) ARKO.
SM/VE-ARKO: SM22-Cre+/−:Tie2-Cre+/−:ARfl/y. Smooth muscle and EC double ARKO.
In this study, only male mice were used for onward analysis.
2.2. Determination of genomic ablation of AR and genotyping of mice
To verify AR ablation in target cells, genomic DNA was extracted immediately from freshly isolated aortic EC and SMC cells (without any culturing) and subjected to PCR amplification using primers GCTGATCATAGGCCTCTCTC and TGCCCTGAAAGCAGTCCTCT. An amplicon of 1142 bp indicated the presence of a floxed AR, while an amplicon of 612 bp indicated recombination between loxP sites and deletion of AR exon 2.24
Inheritance of Cre Recombinase was used to determine genotype. Genomic DNA from ear clips was amplified using primers CGCATAACCAGTGAAACAGCATTGC and CCCTGTGCTCAGACAGAAATGAGA for Tie2-cre;26 and CGCATAACCAGTGAAACAGCATTGC and CAGACACCGAAGCTACTCTCCTTCC for SM22-cre.27 An amplicon of 608 bp indicated the inheritance of the Cre Recombinase transgene in EC under control of the Tie2 promoter, while an amplicon of 575 bp for the Cre Recombinase transgene in SMC under control of SM22 promoter.
2.3. Vascular cell isolation and culture
Mice were euthanized by CO2 and aortic EC and SMC isolated, by collagenase digestion, and cultured, as described.28 Isolated cells were either used directly for DNA extraction, or cultured (EC, 7 days in endothelial culture medium; SMC, 14 days in DMEM/F12 GlutaMAX™) for investigation of AR expression. Testosterone (1 × 10−7 M), DHT (1 × 10−8 M), or vehicle (100% ethanol, 0.1% in final culture medium) was added from the 3rd day of culture and media were replenished twice weekly.
2.4. Phenotyping mice with cell-specific AR deletion
2.4.1. Blood pressure measurement
Systolic blood pressure was assessed in conscious, restrained mice using tail-cuff plethysmography (Harvard Apparatus, UK).
2.4.2. Assay for plasma testosterone, total cholesterol, and triglyceride
Plasma testosterone (DEMEDITEC Diagnostics GmbH, Kiel-Wellsee, Germany), total plasma cholesterol (Olympus Diagnostics Ltd, Watford, UK), and plasma triglyceride (Alpha Laboratories Ltd., Eastleigh, UK) were measured using commercially available kits, in accordance with manufacturer's instructions.
2.4.3. Myographic assessment of arterial function
Mice (12–16 weeks) were euthanized by CO2. Femoral and mesenteric arteries were isolated for functional analysis, as described previously.29 A linear relationship between the increment of cyclic force and the increment of diameter was used to describe arterial compliance.30 Arteries were then exposed to high (125 mM) potassium physiological saline solution (KPSS), phenylephrine (PhE, 10−9–10−5 M), acetylcholine (ACh; 10−9–10−5 M), and sodium nitroprusside (SNP; 10−9–10−5 M). A further set of arteries from the same animals were exposed to testosterone (10−9–10−4 M) and endothelin-1 (ET-1, 10−11–10−7 M).
2.5. Surgical procedures
Surgical procedures were performed in mice under isoflurane-induced anaesthesia with analgesic cover.
2.6. Castration
In C57Bl/6J mice, a small incision was made in the mid-line of the scrotum and both testes externalized and removed (castration) or returned to the scrotum (Sham). The mice were allowed to recover for 1 week prior to induction of femoral artery injury.
2.7. Femoral artery injury
Wire injury was performed by inserting a guidewire using the method of Sata et al.31 Ligation injury was performed on the common femoral artery immediately proximal to the femoropopliteal bifurcation. Wounds were sutured and mice were allowed to recover (21 days) to allow lesion development.
2.8. Optical projection tomography (OPT)
Mice were killed (sodium pentobarbital) and plasma harvested and stored (−20°C). Mice were then perfusion-fixed, femoral arteries excised from the femoropopliteal branch to the bifurcation with the iliac artery, and then processed for optical projection tomography (OPT), as described.32 Longitudinal lesion distribution and total neointimal volume in the first 1.2 mm segment of the artery were used to describe the overall neointima formation (Supplementary material online, Figure S1). The maximum cross-sectional neointimal area was determined from serial histological sections indicated the level of stenosis (Supplementary material online, Figure S1).
2.9. Histology and immuno-fluorescent staining
After OPT scanning, tissues were processed for histology, sectioned (5 µm) and stained with Masson's trichrome. Intimal and luminal area were measured using Image Pro Plus 7.0 rom images obtained using a CoolSNAP camera (Photometrics, UK). Immuno-florescent staining was with Tyramide Signal Amplification (TSA™, PerkinElmer) was applied using primary antibodies against: AR (SantaCruz; 1:400), CD31 (Abcam; 1:300), von Willebrand factor (vWF, Dako; 1:2000), and smooth muscle alpha-actin (SMA, Sigma; 1:1000). Fluorescent images were analysed using confocal microscopy.
For cultured cells, samples were fixed, stained without antigen retrieval, and images were captured using a Zeiss Axiovert 200M epi-fluorescent microscope (Carl Zeiss Ltd., Welwyn, UK).
2.10. Statistics
Data are mean ± standard error of the mean (SEM) for n mice, unless indicated otherwise. Analysis was performed (GraphPad Prism v5.0) using Student's t-test, one-way or two-way ANOVA with a Bonferroni post-hoc test, as appropriate; P < 0.05 indicated statistical significance.
3. Results
3.1. AR localization in vascular tissues
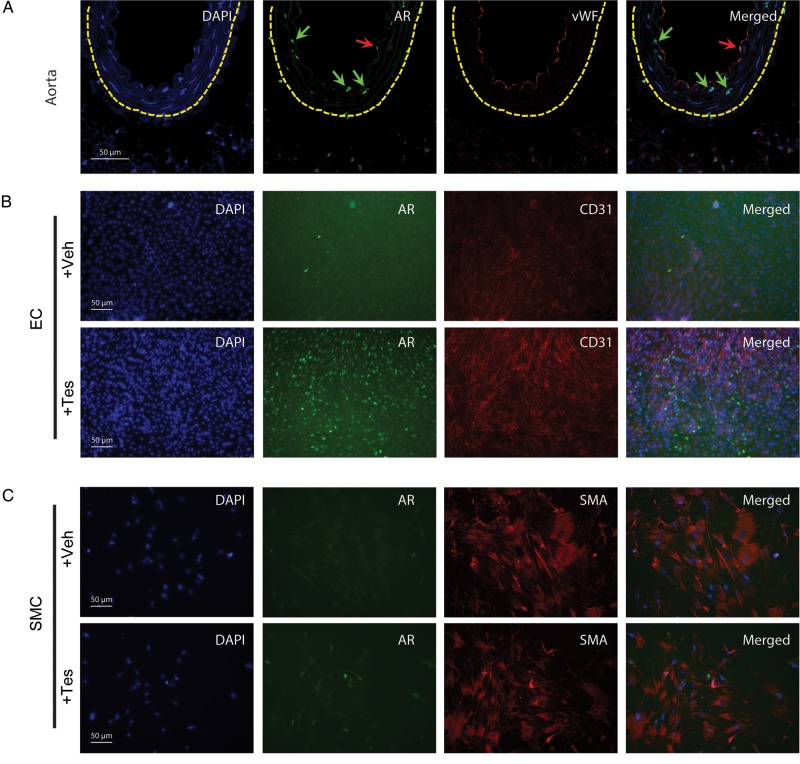
Immunohistochemistry confirmed AR expression in intact mouse aorta (Figure 1A) and in cultured aortic EC (Figure 1B) and SMC (Figure 1C). AR expression in mouse aortic EC and SMC was increased following incubation with testosterone (1 × 10−7 M) (Figure 1B and C).
Figure 1.
Identification of AR in murine vascular cells. AR is expressed in EC (red arrows) and SMC (green arrows) in healthy mouse aorta (A). The dashed yellow line indicates the external elastic lamina. AR expression was up-regulated by testosterone (1 × 10−7 M) in cultured mouse aortic EC (B) and SMC (C). EC were identified using antibodies against vWF and CD31; SMCs were identified using an antibody against SMA. Nuclei were counter-stained with DAPI.
3.2. Establishment of vascular cell-specific AR knockout mice (ARKO)
Three vascular cell-specific AR-ablated mice were generated using the cre-loxP system; SM-ARKO (smooth muscle ARKO, generated using SM22-Cre), VE-ARKO (EC ARKO, generated using Tie2-Cre), and SM/VE-ARKO (smooth muscle and ECs, generated from inter-crossing both Cre lines), and floxed-AR mice (WT), which were used as controls.
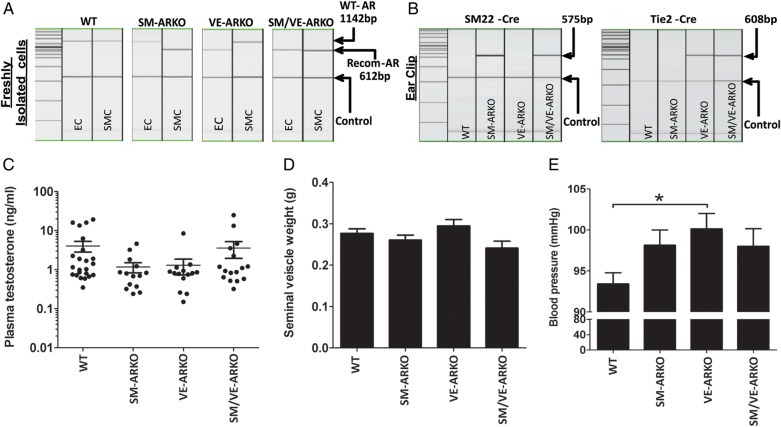
In order to confirm deletion of AR in targeted cell types, genomic DNA from freshly isolated aortic EC and SMC were subjected to PCR analysis. An 1142 bp band representing the wild-type AR allele was only observed in WT EC and SMC, SM-ARKO EC and VE-ARKO SMC (Figure 2A). A recombined 612 bp band representing the recombined non-functional AR allele was only observed in SM-ARKO SMC, VE-ARKO EC, and SM/VE-ARKO EC/SMC. Genomic DNA samples isolated from ear biopsies were used for genotyping. The SM22-cre amplicon consistently correlated with AR ablation in SMC and the Tie2-cre amplicon with AR ablation in EC (Figure 2B).
Figure 2.
Characterization of mice with vascular cell-specific AR deletion. Cell-specific AR deletion was confirmed (A; n = 4 for each genotype) using PCR on genomic DNA from freshly isolated aortic EC and SMC. Mouse genotypes were confirmed (B) with PCR using genomic DNA from ear clip samples. Deletion of vascular AR did not alter circulating testosterone levels (C; n = 14–23) or seminal vesicle weight (D; n = 8–15) but deletion of AR from EC (VE-ARKO) produced a small increase in systolic blood pressure (E). *P < 0.05 (n = 7–12) by one-way ANOVA plus Bonferroni post-hoc test. (WT = wild-type litter mates carrying floxed-AR; SM-ARKO = AR ablated in SMC, VE-ARKO = AR ablated in EC, SM/VE-ARKO = AR ablated in both EC and SMC.)
3.3. Characterization of SM-ARKO/VE-ARKO and SM/VE-ARKO mice
Mice of all four genotypes were healthy. In contrast to Tfm or global ARKO mice,19,23 SM-ARKO, VE-ARKO, and SM/VE-ARKO mice had normal circulating testosterone concentrations (Figure 2C) and seminal vesicle weights (Figure 2D). Total plasma cholesterol and triglyceride were not affected by vascular ARKO (Supplementary material online, Figure S2). Tail-cuff plethysmography revealed a small but significant increase in blood pressure in VE-ARKO mice (Figure 2E).
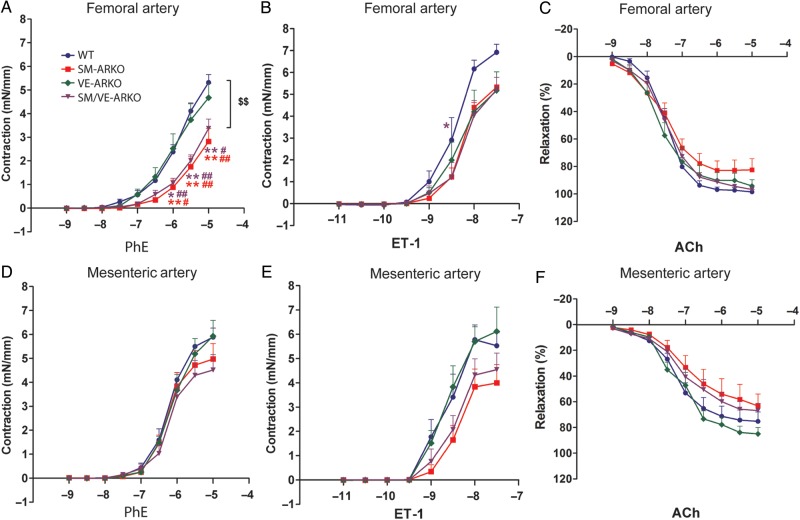
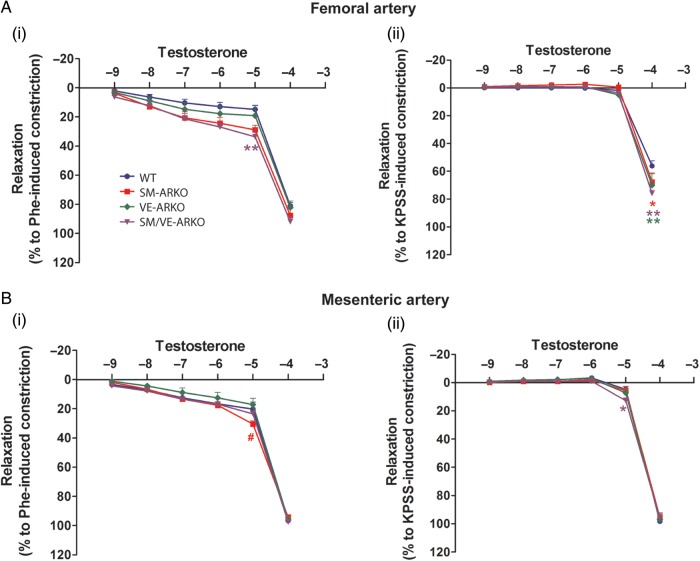
Ex vivo myography was used to determine whether vascular AR deletion was associated with functional changes in smooth muscle contraction or endothelium-dependent relaxation. Vascular AR deletion did not alter femoral or mesenteric arterial compliance (Supplementary material online, Figure S3). PhE-induced contraction, however, was reduced in femoral arteries that lack smooth muscle cell AR from both SM-ARKO and SM/VE-ARKO mice (Figure 3A), while there was a small reduction in ET-1 mediated contraction in all vascular ARKOs (Figure 3B). Vascular AR ablation did not alter PhE- (Figure 3C) or ET-induced (Figure 3D) constriction in mesenteric arteries. KPSS-induced receptor-independent constriction (Supplementary material online, Figure S4A(i) and B(i)), ACh-induced endothelium-mediated dilation (Figure 3E and F), and SNP-induced endothelium-independent dilation (Supplementary material online, Figure S4A(ii) and B(ii)) were not affected by vascular ARKO. Testosterone-induced dilation, which occurred at supra-physiological concentrations (1 × 10−4 M), showed no dramatic alterations following deletion of AR from vascular EC and/or SMC, despite some small differences in response at specific concentrations (Figure 4).
Figure 3.
Agonist-dependent vascular dysfunction in mice with selective deletion of vascular AR. In isolated femoral (A, B, E) and mesenteric (C, D, F) arteries cumulative concentration-response curves were produced using phenylephrine (PhE; A and C) or endothelin-1 (ET-1; B and D). Acetylcholine (ACh; E and F) induced-relaxation was obtained after contraction with a sub-maximal concentration of PhE (3 × 10−6 M). $$P < 0.01 vs. WT; *P < 0.05, **P < 0.01 vs. corresponding WT concentration; #P < 0.05, ##P < 0.01 vs. corresponding VE-ARKO concentration; two-way ANOVA with the Bonferroni post-hoc test. (WT = wild-type litter mates carrying floxed-AR; SM-ARKO = AR ablated in SMC, VE-ARKO = AR ablated in EC, SM/VE-ARKO = AR ablated in both EC and SMC. n = 5–9.)
Figure 4.
Testosterone induces relaxation in vascular ARKO arteries. Supra-physiological concentration of testosterone induced vascular relaxation both in femoral (A) and mesenteric (B) arteries from all genotypes, which was independent of the type of pre-constriction. Vascular ARKO produced no dramatic changes in testosterone-mediated relaxation despite some small differences in relaxation at specific concentrations. *P < 0.05, **P < 0.01 vs. corresponding WT concentration; #P < 0.05 vs. corresponding VE-ARKO concentration; two-way ANOVA plus Bonferroni post-hoc test. (WT = wild-type litter mates carrying floxed-AR; SM-ARKO = AR ablated in SMC, VE-ARKO = AR ablated in EC, SM/VE-ARKO = AR ablated in both EC and SMC. n = 7–9.)
3.4. Influence of castration on neointimal lesion formation
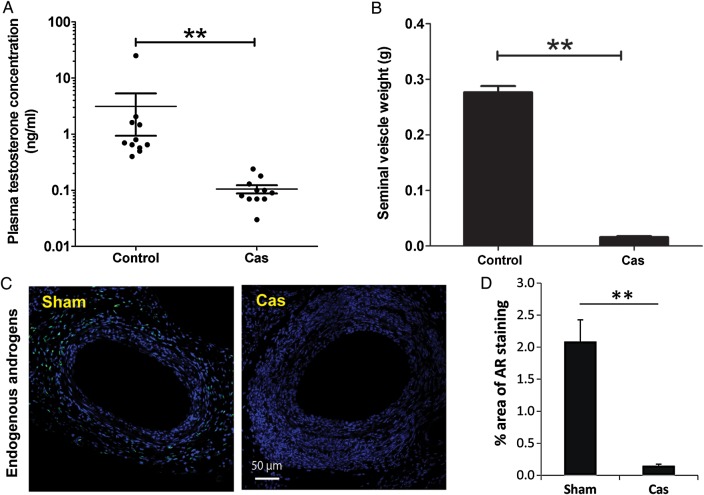
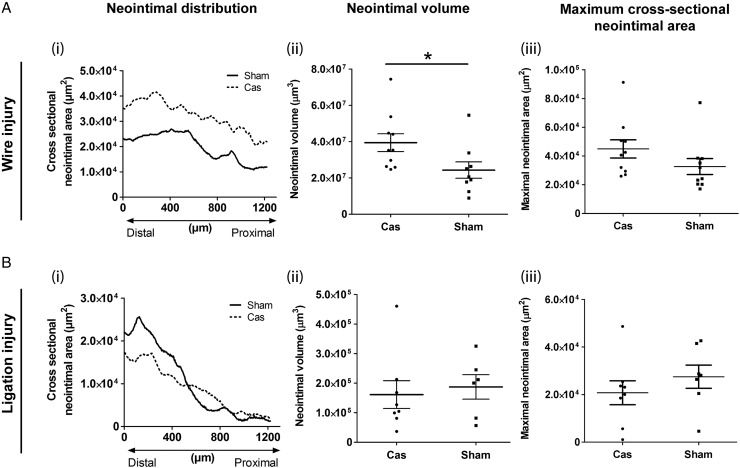
Castration reduced circulating testosterone concentrations (Figure 5A) and seminal vesicle weight (Figure 5B) and decreased AR expression in femoral arteries (Figure 5C). Body weights following surgery were lower in castrated mice than in controls (Supplementary material online, Figure S5). Castration also increased neointimal lesion formation following wire injury (Figure 6A), resulting in increased lesion volume but without increasing the maximal cross-sectional area. In contrast, castration had no effect on the neointimal lesion formation following arterial ligation (Figure 6B).
Figure 5.
Castration reduces vascular AR expression. Plasma testosterone concentrations (A), seminal vesicle weight (B), and AR expression in lesion-bearing femoral arteries (C) were reduced following castration. **P < 0.01. Data were analysed by Student's t-test. (A: n = 9–11; B: n = 8–15; C: n = 9).
Figure 6.
Castration increases neointimal lesion formation following wire injury but not following arterial ligation. In arteries subjected to either (A) wire-induced injury or (B) ligation, neointimal lesion distribution (i) and neointimal volume (ii) were determined by OPT. Maximal cross-sectional narrowing (iii) was measured in serial sections stained with Masson's trichrome. Panels A(i) and B(i) show mean neointimal lesion volumes for each group; error bars have been omitted for clarity. Panels A(ii & iii) and B(ii & iii) show individual data points from each animal in the group with lines and error bars indicating mean ± SEM. *P < 0.05, by Student's t-test (A: n = 8–10; B: n = 6–8 ).
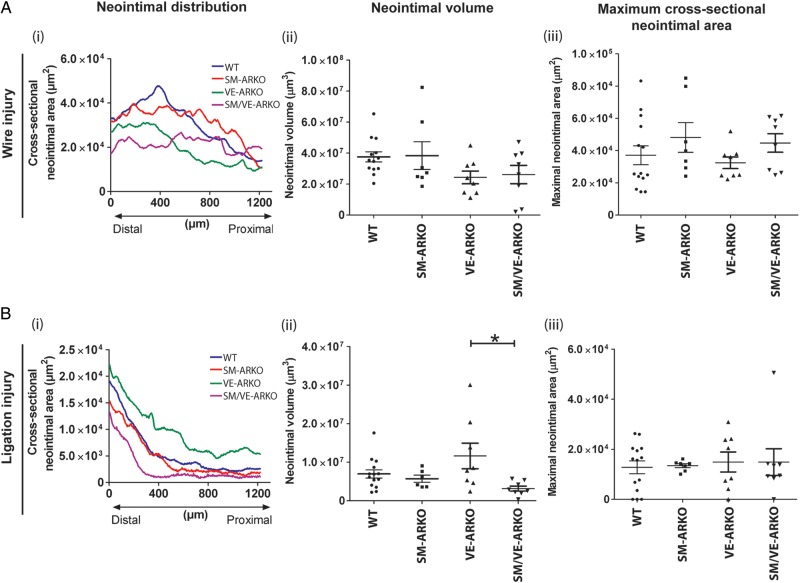
3.5. Effect of vascular ARKO on neointimal lesion formation
Body-weight changes after arterial injury were similar among the four genotypes of mice (Supplementary material online, Figure S6). Vascular ARKO did not alter the profile (Figure 7A(i)), volume (Figure 7A(ii)), or cross-sectional area (Figure 7A(iii)) of lesions induced following wire injury. Similarly, SM-ARKO and SM/VE-ARKO had no effect on lesion size following arterial ligation (Figure 7B). In contrast, deletion of AR from vascular ECs alone (VE-ARKO) resulted in an altered lesion profile (Figure 7B(i)) and a small increase in lesion volume (Figure 7B(ii)), but not cross-sectional area (Figure 7B(iii)), following ligation.
Figure 7.
Effect of vascular-specific AR ablation on neointimal lesion formation. Lesion formation following (A) wire-induced injury (n = 7–14) or (B) ligation-induced injury (n = 6–14) was determined by optical projection tomography (OPT) and histology. Panels A(i) and B(i) show mean neointimal lesion volumes for each genotype; error bars have been omitted for clarity. Panels A(ii and iii) an B(ii and iii) show individual data points from each animal with lines and error bars indicating mean ± SEM. Vascular AR deletion had no effect on lesion formation in response to wire-induced injury. Selective deletion of AR from ECs produced a small increase in neointimal lesion volume following ligation injury (*P < 0.05 by one-way ANOVA plus Bonferroni post-hoc test) but did not alter maximal cross-sectional narrowing. (WT = wild-type litter mates carrying floxed-AR; SM-ARKO = AR ablated in SMC, VE-ARKO = AR ablated in EC, SM/VE-ARKO = AR ablated in both EC and SMC.)
4. Discussion
Declining testosterone levels in men, combined with increased ART, may both have an impact on the development of CVD possibly by affecting the development of atherosclerotic lesions. Precisely how endogenous androgens influence the formation of vascular lesions remains unknown. This investigation addressed the hypothesis that androgen-induced stimulation of AR in the vascular wall inhibits neontimal lesion development. This was addressed using a series of novel vascular-cell-specific mouse lines with AR deleted from EC and/or SMC. Cell-specific deletion of vascular AR did not alter neointimal lesion formation, while castration in wild-type mice did result in adverse neointimal lesion formation. These contrasting findings suggest that the effects of androgens on vascular lesion formation are mediated by one or more of the following mechanisms: (i) activation of AR-independent pathways, (ii) secondary to aromatization of androgen to oestrogen, (iii) stimulation of AR in other cell types.
The generation of vascular-specific ARKO mice was central to this investigation. The functional role of AR has been investigated previously using Tfm19 mice. Interpretation of data from these animals is complicated, however, by low endogenous levels of testosterone and oestradiol (requiring pharmacological replacement). In contrast, consistent with previous demonstrations,22,23 vascular cell-specific deletion of AR did not reduce circulating testosterone levels and, therefore, no exogenous androgen administration was required. This is important since pharmacological administration of androgens does not satisfactorily ‘replace’ endogenous hormone. The pharmacokinetics and tissue accumulation of pharmacologically administered androgen are not fully understood (thus overdosing may occur if the serum/plasma testosterone level is the only clinical parameter for androgen prescription). This may explain (i) the unexpected increase in adverse cardiovascular events associated with androgen treated in a clinical trial involving elderly (>65 years old) hypogonadal men,16 and (ii) the increased death and incidence of stroke and myocardial infarction (regardless of the pre-existing CVD) associated with clinical androgen replacement in hypogonadal men, as demonstrated in a large scale observational study.14
Androgens contribute to elevation of blood pressure by acting on catecholamines in the brain, independent of classical AR; blood pressure in wild-type and Tfm rats is reduced by castration and restored by administration of testosterone.33 This contrasts with vascular selective ARKO which showed either no change or, in the case of VE-ARKO, a small elevation in blood pressure. The implication, therefore, is that testosterone-mediated elevation of blood pressure is not mediated by vascular AR. The increased blood pressure in VE-ARKO mice suggests slight EC dysfunction, but this was not supported by our findings in isolated arteries.
Disruption of systemic androgen/AR signalling impairs normal vascular function.34 In Tfm mice, contraction in response to high potassium (but not to noradrenaline) was reduced in femoral arteries, suggesting altered smooth muscle cell function.34 Relaxation in response to ACh was also impaired, suggesting EC dysfunction.34 Vascular AR ablation had no effect on passive arterial compliance and did not alter endothelium-dependent or -independent relaxation, confirming normal activity of the endothelium-derived nitric oxide system. It was notable that EC function was maintained in VE-ARKO mice, despite the (small) increase in blood pressure in these animals. Unlike the Tfm, smooth muscle AR deletion impaired agonist-mediated contraction without altering the response to potassium, suggesting a change in receptor-dependent signal transduction pathways. Reduced contraction was agonist dependent (being more evident in response to noradrenaline than to ET-1) and tissue-specific (more obvious in the femoral, than in the mesenteric, artery). An androgen-mediated alteration of adrenoceptor activation would be consistent with androgen-induced production and release of noradrenaline in rodents.35 The differences between the current results and those reported for Tfm suggest that AR expressed outside the vasculature contributes to regulation of vascular function. The low testosterone in Tfm could also lead to a loss of rapid, non-genomic androgen signalling. Alternatively, since endogenous oestrogen regulates EC function in males,36,37 the impaired endothelium-mediated dilation in the Tfm mice could be a result of its low oestrogen levels.
As shown previously,38 testosterone caused relaxation of arteries, although only at supra-physiological concentrations. The maintenance of this response in the vascular-specific ARKO mice is consistent with data from Tfm mice.34 This indicates, therefore, that vascular AR does not mediate this response. Similar arterial relaxation has been observed with high concentrations (≥1 × 10−4 M) of corticosteroids, oestrogen, and cholesterol39,40 and may be due to direct alteration of the cell membrane. Given the very high concentrations required to produce this response, testosterone-induced vasodilation is very unlikely to have any physiological relevance.
Evidence for androgen-mediated inhibition of vascular neointimal lesion formation under normolipidemic conditions is less conclusive than for inhibition of atherosclerosis.20,22 This investigation addressed the influence of androgens/AR on neointimal lesion formation using models of denuding (wire) and non-denuding (ligation)41 injury. Wire injury denudes the endothelium and produces lesions formed predominantly from circulating bone marrow-derived progenitor cells. On the other hand, femoral artery ligation does not introduce direct endothelial damage. The disturbed blood flow and altered shear stress may induce endothelial dysfunction and stimulate neointima formation by migration and proliferation of media-derived mural smooth muscle cells.42 Increased lesion volume in castrated mice following wire-injury, but not following ligation, is consistent with evidence that the influence of androgens is dependent on the type of lesion.20 Endothelial denudation is one of the major differences between the wire injury and ligation injury models. Androgens promote endothelial proliferation and migration in vitro43 and improve angiogenesis in vivo following ischaemic injury.44,45 It is possible, therefore, that endothelial regeneration following wire injury was impaired by systemic androgen deprivation, thus favouring formation of lesions. In addition, blocking AR signalling improves self-renewal and migration of bone marrow-derived stem cells46 which may promote lesion formation following wire injury. The well-recognized androgen/AR mediated immune-suppression47 may also modulate adventitial inflammation and subsequent neointima formation and could explain the increased lesion volume following castration.
Following arterial injury, vascular AR ablation had very little impact in neointima formation. Deletion of AR from the endothelium increased lesion volume following arterial ligation, but it was notable that no similar increase was detected in mice with double knockout of AR in EC and SMC. This may suggest that deletion of AR in the smooth muscle opposes the effect of deletion from the endothelium. However, given the variability of the data in the VE-ARKO following ligation, it seems more likely that this result is an anomaly, and that, as proposed, AR in the vascular EC and SMC does not contribute to regulation of neointimal lesion formation. Excluding a role for the EC and SMC AR suggests that any influence of androgens on neointimal lesion formation may be mediated by AR-independent mechanisms, or by conversion to oestrogen by aromatization either systemically or locally in the vascular wall, or by AR expressed in other cell types in the vascular wall. It should be noted, however, that AR expression in the vasculature is not restricted to EC and SMC. In healthy arteries, cells bearing strong AR expression were shown to be present in the adventitia, while the population of AR-positive adventitial cells was increased in injured arteries. It was notable that expression of AR in the adventitia of injured arteries was dramatically reduced following castration. However, the identity of these AR positive cells and their pathophysiological function require further investigation. Interestingly, androgens were recently reported to increase ischaemia-induced angiogenesis, indicating a direct association between androgen and ECs.43,44 Our VE-ARKO mice will be a useful tool to address the question whether endothelial AR truly regulates endothelial function or behaviour using endothelial-specific models.
The use of vascular selective ARKO mice has shown that AR in the arterial wall have little role in regulating androgen-dependent neointimal lesion formation. These results suggest that any protective effects of androgens in atherosclerosis are likely to be mediated by conversion of testosterone to oestrogens, by effects on classical cardiovascular risk factors such as cholesterol, or by AR outside the vascular wall. Future investigations should try to determine whether androgens inhibit atherosclerosis through direct modulation of non-vascular AR or following conversion to oestrogens.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
Funded by a British Heart Foundation Project Grant award to L.B.S., P.W.F.H., and M.A.D. (PG/11/72/29334), an MRC Programme Grant award to L.B.S. (G1100354), and the BHF Centre for Research Excellence. W.G.L. received a BHF CoRE Summer Studentship. I.M. received a Study-abroad Fellowship of the German National Academic Foundation. Funding to pay the Open Access publication charges for this article was provided by The British Heart Foundation.
Supplementary Material
Acknowledgements
Thanks to Laura Milne, Lyndsey Cruickshanks, Nathan Jeffery, and Mike Dodds for technical support.
Conflict of interest: none declared.
References
- 1. Townsend N, Wickramasinghe K, Bhatnagar P, Smolina K, Nichols M, Leal J, Luengo-Fernandez R, Rayner M. Coronary Heart Disease Statistics 2012 Edition. London: British Heart Foundation, 2012.
- 2.Malkin CJ, Pugh PJ, Jones RD, Jones TH, Channer KS. Testosterone as a protective factor against atherosclerosis—immunomodulation and influence upon plaque development and stability. J Endocrinol. 2003;178:373–380. doi: 10.1677/joe.0.1780373. [DOI] [PubMed] [Google Scholar]
- 3.Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, Pols HA. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002;87:3632–3639. doi: 10.1210/jcem.87.8.8762. [DOI] [PubMed] [Google Scholar]
- 4.English KM, Mandour O, Steeds RP, Diver MJ, Jones TH, Channer KS. Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms. Eur Heart J. 2000;21:890–894. doi: 10.1053/euhj.1999.1873. [DOI] [PubMed] [Google Scholar]
- 5.Morris PD, Channer KS. Testosterone and cardiovascular disease in men. Asian J Androl. 2012;14:428–435. doi: 10.1038/aja.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isidori AM, Giannetta E, Gianfrilli D, Greco EA, Bonifacio V, Aversa A, Isidori A, Fabbri A, Lenzi A. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf) 2005;63:381–394. doi: 10.1111/j.1365-2265.2005.02350.x. [DOI] [PubMed] [Google Scholar]
- 7.Travison TG, Araujo AB, O'Donnell AB, Kupelian V, McKinlay JB. A population-level decline in serum testosterone levels in American men. J Clin Endocrinol Metab. 2007;92:196–202. doi: 10.1210/jc.2006-1375. [DOI] [PubMed] [Google Scholar]
- 8.Perheentupa A, Makinen J, Laatikainen T, Vierula M, Skakkebaek NE, Andersson AM, Toppari J. A cohort effect on serum testosterone levels in Finnish men. Eur J Endocrinol. 2013;168:227–233. doi: 10.1530/EJE-12-0288. [DOI] [PubMed] [Google Scholar]
- 9.Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465–1466. doi: 10.1001/jamainternmed.2013.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gan EH, Pattman S, HSP S, Quinton R. A UK epidemic of testosterone prescribing, 2001–2010. Clin Endocrinol (Oxf) 2013;79:564–570. doi: 10.1111/cen.12178. [DOI] [PubMed] [Google Scholar]
- 11.Traish AM, Abdou R, Kypreos KE. Androgen deficiency and atherosclerosis: The lipid link. Vascul Pharmacol. 2009;51:303–313. doi: 10.1016/j.vph.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Coates P. Androgen insufficiency in ageing men: how is it defined and should it be treated? Clin Biochem Rev. 2005;26:37–41. [PMC free article] [PubMed] [Google Scholar]
- 13.2014. FDA evaluating risk of stroke, heart attack and death with FDA-approved testosterone products http://www.fda.gov/Drugs/DrugSafety/ucm383904.htm .
- 14.Vigen R, O'Donnell CI, Baron AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, Rumsfeld JS, Ho PM. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 15.Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, Fraumeni JF, Jr, Hoover RN. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS ONE. 2014;9:e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruck B, Brehme U, Gugel N, Hanke S, Finking G, Lutz C, Benda N, Schmahl FW, Haasis R, Hanke H. Gender-specific differences in the effects of testosterone and estrogen on the development of atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol. 1997;17:2192–2199. doi: 10.1161/01.atv.17.10.2192. [DOI] [PubMed] [Google Scholar]
- 18.Alexandersen P, Haarbo J, Byrjalsen I, Lawaetz H, Christiansen C. Natural androgens inhibit male atherosclerosis: a study in castrated, cholesterol-fed rabbits. Circ Res. 1999;84:813–819. doi: 10.1161/01.res.84.7.813. [DOI] [PubMed] [Google Scholar]
- 19.Nettleship JE, Jones TH, Channer KS, Jones RD. Physiological testosterone replacement therapy attenuates fatty streak formation and improves high-density lipoprotein cholesterol in the Tfm mouse: an effect that is independent of the classic androgen receptor. Circulation. 2007;116:2427–2434. doi: 10.1161/CIRCULATIONAHA.107.708768. [DOI] [PubMed] [Google Scholar]
- 20.Tharp DL, Masseau I, Ivey J, Ganjam VK, Bowles DK. Endogenous testosterone attenuates neointima formation after moderate coronary balloon injury in male swine. Cardiovasc Res. 2009;82:152–160. doi: 10.1093/cvr/cvp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanke H, Lenz C, Hess B, Spindler KD, Weidemann W. Effect of testosterone on plaque development and androgen receptor expression in the arterial vessel wall. Circulation. 2001;103:1382–1385. doi: 10.1161/01.cir.103.10.1382. [DOI] [PubMed] [Google Scholar]
- 22.Chen SJ, Li H, Durand J, Oparil S, Chen YF. Estrogen reduces myointimal proliferation after balloon injury of rat carotid artery. Circulation. 1996;93:577–584. doi: 10.1161/01.cir.93.3.577. [DOI] [PubMed] [Google Scholar]
- 23.Bourghardt J, Wilhelmson AS, Alexanderson C, De Gendt K, Verhoeven G, Krettek A, Ohlsson C, Tivesten A. Androgen receptor-dependent and independent atheroprotection by testosterone in male mice. Endocrinology. 2010;151:5428–5437. doi: 10.1210/en.2010-0663. [DOI] [PubMed] [Google Scholar]
- 24.O'Hara L, Smith LB. Androgen receptor signalling in Vascular Endothelial cells is dispensable for spermatogenesis and male fertility. BMC Res Notes. 2012;5:16. doi: 10.1186/1756-0500-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welsh M, Sharpe RM, Moffat L, Atanassova N, Saunders PT, Kilter S, Bergh A, Smith LB. Androgen action via testicular arteriole smooth muscle cells is important for Leydig cell function, vasomotion and testicular fluid dynamics. PLoS ONE. 2010;5:e13632. doi: 10.1371/journal.pone.0013632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 27.Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci USA. 2002;99:7142–7147. doi: 10.1073/pnas.102650499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi M, Inoue K, Warabi E, Minami T, Kodama T. A simple method of isolating mouse aortic endothelial cells. J Atheroscler Thromb. 2005;12:138–142. doi: 10.5551/jat.12.138. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Wadsworth RM, Kennedy S. Inhibition of inducible nitric oxide synthase promotes vein graft neoadventitial inflammation and remodelling. J Vasc Res. 2011;48:141–149. doi: 10.1159/000316968. [DOI] [PubMed] [Google Scholar]
- 30.Jones RD, Morice AH, Emery CJ. Effects of perinatal exposure to hypoxia upon the pulmonary circulation of the adult rat. Physiol Res. 2004;53:11–17. [PubMed] [Google Scholar]
- 31.Sata M, Maejima Y, Adachi F, Fukino K, Saiura A, Sugiura S, Aoyagi T, Imai Y, Kurihara H, Kimura K, Omata M, Makuuchi M, Hirata Y, Nagai R. A mouse model of vascular injury that induces rapid onset of medial cell apoptosis followed by reproducible neointimal hyperplasia. J Mol Cell Cardiol. 2000;32:2097–2104. doi: 10.1006/jmcc.2000.1238. [DOI] [PubMed] [Google Scholar]
- 32.Kirkby NS, Low L, Seckl JR, Walker BR, Webb DJ, Hadoke PW. Quantitative 3-dimensional imaging of murine neointimal and atherosclerotic lesions by optical projection tomography. PLoS ONE. 2011;6:e16906. doi: 10.1371/journal.pone.0016906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ely D, Toot J, Salisbury R, Ramirez R. Androgens alter brain catecholamine content and blood pressure in the testicular feminized male rat. Clin Exp Hypertens. 2011;33:124–132. doi: 10.3109/10641963.2010.531840. [DOI] [PubMed] [Google Scholar]
- 34.Jones RD, Pugh PJ, Hall J, Channer KS, Jones TH. Altered circulating hormone levels, endothelial function and vascular reactivity in the testicular feminised mouse. Eur J Endocrinol. 2003;148:111–120. doi: 10.1530/eje.0.1480111. [DOI] [PubMed] [Google Scholar]
- 35.Dart AM, Du XJ, Kingwell BA. Gender, sex hormones and autonomic nervous control of the cardiovascular system. Cardiovasc Res. 2002;53:678–687. doi: 10.1016/s0008-6363(01)00508-9. [DOI] [PubMed] [Google Scholar]
- 36.Sun D, Yan C, Jacobson A, Jiang H, Carroll MA, Huang A. Contribution of epoxyeicosatrienoic acids to flow-induced dilation in arteries of male ERalpha knockout mice: role of aromatase. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1239–R1246. doi: 10.1152/ajpregu.00185.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lew R, Komesaroff P, Williams M, Dawood T, Sudhir K. Endogenous estrogens influence endothelial function in young men. Circ Res. 2003;93:1127–1133. doi: 10.1161/01.RES.0000103633.57225.BC. [DOI] [PubMed] [Google Scholar]
- 38.Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013;217:R47–R71. doi: 10.1530/JOE-12-0582. [DOI] [PubMed] [Google Scholar]
- 39.English KM, Jones RD, Jones TH, Morice AH, Channer KS. Gender differences in the vasomotor effects of different steroid hormones in rat pulmonary and coronary arteries. Horm Metab Res. 2001;33:645–652. doi: 10.1055/s-2001-18689. [DOI] [PubMed] [Google Scholar]
- 40.Alvarez E, Cairrao E, Morgado M, Morais C, Verde I. Testosterone and cholesterol vasodilation of rat aorta involves L-type calcium channel inhibition. Adv Pharmacol Sci. 2010;2010:534184. doi: 10.1155/2010/534184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holt AW, Tulis DA. Experimental rat and mouse carotid artery surgery: injury & remodeling studies. ISRN Minim Invasive Surg. 2013 doi: 10.1155/2013/167407. 167407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka K, Sata M, Hirata Y, Nagai R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ Res. 2003;93:783–790. doi: 10.1161/01.RES.0000096651.13001.B4. [DOI] [PubMed] [Google Scholar]
- 43.Sieveking DP, Lim P, Chow RW, Dunn LL, Bao S, McGrath KC, Heather AK, Handelsman DJ, Celermajer DS, Ng MK. A sex-specific role for androgens in angiogenesis. J Exp Med. 2010;207:345–352. doi: 10.1084/jem.20091924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida S, Aihara K, Ikeda Y, Sumitomo-Ueda Y, Uemoto R, Ishikawa K, Ise T, Yagi S, Iwase T, Mouri Y, Sakari M, Matsumoto T, Takeyama K, Akaike M, Matsumoto M, Sata M, Walsh K, Kato S. Androgen receptor promotes sex-independent angiogenesis in response to ischemia and is required for activation of vascular endothelial growth factor receptor signaling. Circulation. 2013;128:60–71. doi: 10.1161/CIRCULATIONAHA.113.001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Fu L, Han Y, Teng Y, Sun J, Xie R, Cao J. Testosterone replacement therapy promotes angiogenesis after acute myocardial infarction by enhancing expression of cytokines HIF-1a, SDF-1a and VEGF. Eur J Pharmacol. 2012;684:116–124. doi: 10.1016/j.ejphar.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 46.Huang CK, Lee SO, Lai KP, Ma WL, Lin TH, Tsai MY, Luo J, Chang C. Targeting androgen receptor in bone marrow mesenchymal stem cells leads to better transplantation therapy efficacy in liver cirrhosis. Hepatology. 2013;57:1550–1563. doi: 10.1002/hep.26135. [DOI] [PubMed] [Google Scholar]
- 47.Lai JJ, Lai KP, Zeng W, Chuang KH, Altuwaijri S, Chang C. Androgen receptor influences on body defense system via modulation of innate and adaptive immune systems: lessons from conditional AR knockout mice. Am J Pathol. 2012;181:1504–1512. doi: 10.1016/j.ajpath.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.